Abstract
Streptococcus pneumoniae is an important bacterial pathogen that causes a range of noninvasive and invasive diseases. The mechanisms underlying variability in the ability of S. pneumoniae to transition from nasopharyngeal colonization to disease-causing pathogen are not well defined. Mucosal-associated invariant T (MAIT) cells are prevalent in mucosal tissues such as the airways and are believed to play an important role in the early response to infection with bacterial pathogens. The ability of MAIT cells to recognize and contain infection with S. pneumoniae is not known. In the present study, we analyzed MAIT-cell responses to infection with clinical isolates of S. pneumoniae serotype 19A, a serotype linked to invasive pneumococcal disease. We found that although MAIT cells were capable of responding to human dendritic and airway epithelial cells infected with S. pneumoniae, the magnitude of response to different serotype 19A isolates was determined by genetic differences in the expression of the riboflavin biosynthesis pathway. MAIT-cell release of cytokines correlated with differences in the ability of MAIT cells to respond to and control S. pneumoniae in vitro and in vivo in a mouse challenge model. Together, these results demonstrate first that there are genetic differences in riboflavin metabolism among clinical isolates of the same serotype and second that these likely determine MAIT-cell function in response to infection with S. pneumoniae. These differences are critical when considering the role that MAIT cells play in early responses to pneumococcal infection and determining whether invasive disease will develop.
Clinical Relevance
The mechanisms leading Streptococcus pneumoniae to become invasive are not fully understood. A recently described and prevalent subset of T cells known as mucosal-associated invariant T cells are believed to play an early role in response to bacterial infection. Differences in the ability of mucosal-associated invariant T cells to recognize various clinical isolates of S. pneumoniae may play a role in the ability of this pathogen to become invasive.
Streptococcus pneumoniae is the most common bacterial cause of community-acquired pneumonia (1). Despite the use of multivalent capsular polysaccharide conjugate vaccines, there are individuals who remain at risk for disease and increasing incidence of disease caused by nonvaccine serotypes (2, 3). In addition, there is considerable variation in the ability of isolates of S. pneumoniae to cause invasive disease, which is not fully explained by differences in capsular protein expression (4). These factors underscore the need to better understand cellular responses induced by infection with S. pneumoniae.
CD8+ T cells are important contributors to host defense against pathogens. CD8+ T cells produce IFN-γ, TNF-α, and cytotoxic mediators such as perforin and granzyme in response to microbes, and thus they could play an important role in pathogen control or promotion of a proinflammatory environment. CD8+ T cells are most commonly studied for their role in control of viral or intracellular bacterial infections such as Mycobacterium tuberculosis (5, 6). Increasing evidence demonstrates, however, that CD8+ T cells also play a role in the cellular immune response to extracellular bacteria, including S. pneumoniae. For example, mice lacking CD8, perforin, or IFN-γ have increased bacterial dissemination, lung inflammation, and mortality after infection with S. pneumoniae (7), and low CD8+ T-cell counts are associated with increased risk of bacterial pneumonia (8). Increased understanding of the role of CD8+ T cells is critical to the continued development of therapeutics and vaccines for S. pneumoniae.
Mucosal-associated invariant T (MAIT) cells are a conserved subset of donor-unrestricted and predominantly CD8+ T cells that are capable of releasing cytokines and other cytolytic factors immediately upon maturation and emigration from the thymus (9). MAIT cells are highly prevalent in peripheral blood, with frequencies between 1 and 10% of circulating T cells, and they are also abundant in certain tissues, including the lung (10–14). MAIT cells recognize small molecules derived from the riboflavin metabolic pathway presented by MR1, a nonpolymorphic major histocompatibility complex class I–like molecule (15, 16). Many fungal and bacterial pathogens, including S. pneumoniae (17), encode the enzymes required for biosynthesis of these small molecules. This suggests that S. pneumoniae produces ligands capable of activating MAIT cells; however, the ability of MAIT cells to recognize and respond to S. pneumoniae–infected cells has not yet been reported. In the present study, we tested a panel of clinical isolates of S. pneumoniae serotype 19A, which has emerged as one of the most prevalent causes of invasive pneumococcal disease (18, 19), to determine their ability to activate MAIT cells. We demonstrate that MAIT cells are capable of recognizing and responding to S. pneumoniae–infected cells and that S. pneumoniae isolates recognized by MAIT cells were controlled in an in vitro killing assay. Together, our results suggest that MAIT cells may play a critical role in the control of invasive pneumococcal infections.
Methods
Bacteria and Cells
Clinical isolates of S. pneumoniae or S. pneumoniae serotype 3 URF918 strain were cultured as described in the data supplement. Monocyte-derived dendritic cells (DCs) were prepared as previously described (20) and as described in the data supplement. Primary normal human bronchial airway epithelial cells (AECs) were obtained from Lonza and cultured as recommended. The MR1-restricted MAIT cell clone D426 G11 (21) was expanded as previously described (22).
Ethics Statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. Study participants, protocols, and consent forms were approved by the Oregon Health & Science University Institutional Review Board (IRB00000186). Written informed consent was obtained from all participants. Healthy adults were recruited from among employees at Oregon Health & Science University as previously described (23).
Mice
Vα19iTgCα−/−MR1+/+ mice were described previously (24) and were a gift of Dr. M. Brigl (Brigham and Women’s Hospital, Boston, MA). All animal experiments were approved and performed according to the guidelines of the institutional animal care and use committee of the La Jolla Institute for Allergy and Immunology (San Diego, CA).
Enzyme-linked Immunospot Assay
Isolates of S. pneumoniae were added to DCs or AECs as described in the data supplement. Infected DCs were used as APCs in an IFN-γ enzyme-linked immunospot (ELISPOT) assay as previously described (25). For experiments with MR1 blocking, the 26.5 α-MR1 blocking antibody (a kind gift from Dr. Ted Hansen, Washington University, St. Louis, MO) was added to the ELISPOT assay.
ELISA
Supernatants were collected from infected DCs after overnight incubation and used directly (TNF-α) or frozen at −80°C (IL-12[p70], IL-6, IL-18) as described in the data supplement.
Colony-Forming Unit Assay
Colony-forming units were enumerated from infected DCs or AECs as described in the data supplement.
Generation of SP9ΔribD Mutant
The ribD gene was replaced with a chloramphenicol acetyltransferase cassette. The cat gene was amplified from vector pDC123 (a kind gift from Dr. Victor Nizet, University of California, San Diego) and inserted in between the upstream and downstream fragments of ribD by overlap extension PCR. The amplicon was cloned into pCR-Blunt vector (Invitrogen) and used to transform SP9.
RNA Isolation, cDNA Synthesis, and qRT-PCR
RNA isolation, cDNA synthesis, and qRT-PCR were performed as described in the data supplement. Expression data were normalized to the gyrA gene, and relative expression levels were determine using comparative cycle threshold method (26).
Mass Spectrometry Sample Preparation and Analysis
Lysates from S. pneumoniae isolates SP9 and SP37 were analyzed for known MAIT-cell ligands, riboflavin, and flavin mononucleotide (FMN) as described in the data supplement.
In Vitro Killing Assay
AECs (Lonza) were infected with SP9 or SP37, then cultured with D426 G11 (MAIT) or D466 A10 (HLA-B45) T-cell clones as described in the data supplement. Infected cells cultured overnight were then harvested, and serial dilutions were plated to determine colony-forming units.
In Vivo Infection and Cytokine Analysis
S. pneumoniae isolates SP9 and SP37, or URF918 as a virulent control, were used to intrapharyngeally infect mice with 4 × 106 to 7 × 106 cfu. Lungs were harvested 19 to 22 hours later and analyzed as described in the data supplement.
Results
Clinical Isolates of S. pneumoniae Induce Differential MAIT-Cell Activation
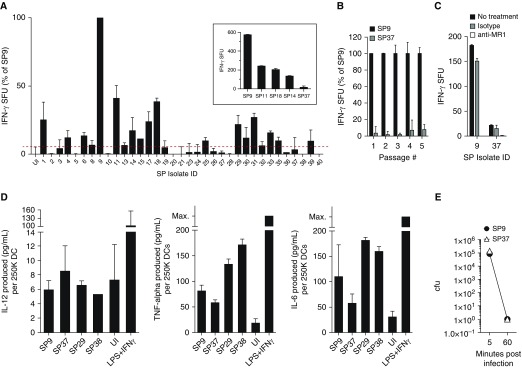
Because S. pneumoniae encodes genes in the riboflavin biosynthesis pathway, we hypothesized that cells infected with S. pneumoniae would be capable of stimulating MAIT cells. To test this hypothesis, we obtained 35 clinical isolates of S. pneumoniae serotype 19A (Table E1). The clinical isolates were characterized either as colonizers cultured from the nasopharynx or invasive strains cultured from otherwise sterile sites such as the blood or pleural fluid. Human monocyte-derived DCs were infected with each isolate at the same multiplicity of infection and tested by ELISPOT assay for their ability to induce IFN-γ production by a previously described MAIT-cell clone, D426 G11 (21), which expresses the canonical MAIT-cell TCR. There was a wide range of IFN-γ production by the MAIT-cell clone in response to DCs infected with the clinical isolates. Infection with one isolate, 08AR0009 (“SP9”), consistently resulted in the most robust production of IFN-γ by the MAIT cells. As a result, we normalized the MAIT-cell responses to all other isolates to this isolate for comparison (Figure 1A). The MAIT-cell clone produced different amounts of IFN-γ in response to DCs infected with 18 of the 35 isolates, and it did not produce IFN-γ above background in response to other isolates. The difference in the ability of DCs infected with different clinical isolates to induce distinct MAIT-cell responses persisted over multiple rounds of in vitro bacterial passage, as demonstrated by the responses to DCs infected with SP9 and a representative isolate inducing a low response, 02AR0813 (“SP37”), from successive cultures (Figure 1B).
Figure 1.
(A) Dendritic cells (DCs) were infected with each Streptococcus pneumoniae (SP) isolate at a multiplicity of infection of 5:1 for 1 hour. Infected DCs were used as APCs in an enzyme-linked immunospot (ELISPOT) assay with IFN-γ production by the D426 G11 human mucosal-associated invariant T–cell clone as a readout. As control cells, uninfected DCs and DCs infected with SP9 were used in each experiment, and responses were normalized to IFN-γ production by D426 G11 in response to SP9. The inset is shown to demonstrate D426 G11 responses to DCs infected with five SP isolates in a representative experiment, where the error bars represent the mean and SD from replicate ELISPOT wells. Each SP isolate was tested in at least two experiments, and in each experiment, the response to SP9-infected DCs was used to normalize the data. (B) DCs infected with SP9 and SP37 passaged in successive cultures were tested by ELISPOT assay as described in A. (C) The MR1 blocking antibody (26.5) or isotype control was added to the ELISPOT assay with infected DCs before the addition of D426 G11. (D) Supernatants from DCs infected with indicated SP isolates at a multiplicity of infection of 5:1 overnight were analyzed by ELISA for IL-12, TNF-α, or IL-6. The concentration of each cytokine was determined using interpolation from a standard curve and was then normalized to a concentration per 2.5E5 DCs for each condition. (E) Colony-forming units in infected DCs were determined by plating serial dilutions 5 and 60 minutes postinfection. For B–E, the results are the mean and SD derived from at least two experiments per isolate. Max. = maximum; SFU = spot-forming units; UI = uninfected.
We next determined whether the response of the MAIT-cell clone to S. pneumoniae isolates inducing high or low MAIT-cell responses was completely MR1 dependent. First, we performed the ELISPOT assay in the context of blocking with an α-MR1 antibody. For isolates inducing both high (SP9) and low (SP37) MAIT-cell responses, the IFN-γ response was completely blocked with the α-MR1 antibody (Figure 1C). Second, we examined whether infected DCs were making cytokines, including IL-12 and IL-18, that could activate MAIT cells in an antigen-independent manner (27). Supernatants from DCs infected with a selection of S. pneumoniae isolates inducing distinct MAIT-cell responses were collected and analyzed for IL-12, IL-18, TNF-α, or IL-6 production after overnight infection. Whereas the DCs were capable of making IL-12, S. pneumoniae–infected DCs did not make more IL-12 than uninfected DCs (Figure 1D). Both TNF-α and IL-6 were produced by S. pneumoniae–infected DCs (Figure 1D). However, for all cytokines, there was no correlation between the production of cytokines by DCs infected with different isolates and the MAIT-cell responses. IL-18 was not detected above the limit of detection for any conditions (data not shown). Together, these data demonstrate that whereas MAIT cells are capable of MR1-dependent recognition and response to cells infected with S. pneumoniae, the clinical serotype 19A isolates were not equivalently recognized.
S. pneumoniae Isolates Are Phagocytosed and Killed by DCs Equivalently
The different MAIT-cell responses could be explained by variation in phagocytosis or killing by DCs. The structure of the S. pneumoniae polysaccharide capsule, which delineates distinct serotypes, can protect against phagocytosis (28, 29). Although all of our isolates were of the same serotype (19A), we tested if phagocytosis was similar for different isolates. DCs were infected by an isolate inducing low (SP37) or high (SP9) MAIT-cell responses. After 5 minutes, infected DCs were extensively washed, and the number of intracellular bacteria was determined by plating and enumerating colony-forming units. At 5 minutes postinfection, enumeration of colony-forming units demonstrated that each of the isolates was phagocytosed by the DCs equivalently (Figure 1E). Because the infected DCs were plated in the ELISPOT assay with MAIT cells 1 hour after infection, we also enumerated colony-forming units at this time point, and we found that there were no viable bacteria remaining for either isolate at 1 hour postinfection (Figure 1E). These results demonstrate that differential MAIT-cell responses cannot be explained by different rates of phagocytosis or killing by DC.
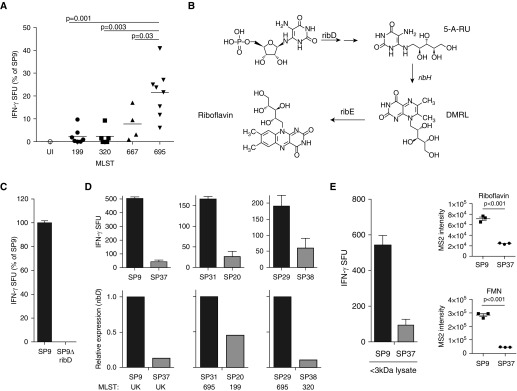
Differences in Regulation of Genes for Riboflavin Metabolism and Transport
In addition to serotype, S. pneumoniae isolates can also be grouped on the basis of genetic background characterized through multilocus sequence typing (MLST) (30). Further analysis of the variation in IFN-γ production by MAIT cells revealed that responses to DCs infected with isolates with known MLST grouped together and were significantly different between MLST groups (Figure 2A). These results suggested a correlation between MAIT-cell response and genetic background, possibly through differences in the production of MAIT-cell ligands. Known MAIT-cell ligands are produced during bacterial riboflavin metabolism and include a precursor of riboflavin, the small molecule 6,7-dimethyl-8-ribityllumazine (DMRL) (16), which is downstream of 5-amino-6-ribityluracil (5-A-RU). In addition, 5-A-RU can combine with glyoxal or methylglyoxal from the host or bacterial cells to form 5-(2-oxoethylideneamino)-6-d-ribitylaminouracil (5-OE-RU) or 5-(2-oxopropylideneamino)-6-d-ribityluracil (5-OP-RU), two additional MAIT-cell ligands (15). The enzymes responsible for riboflavin metabolism (RibD, RibE, RibBA, and RibH) are encoded on a single operon in the S. pneumoniae genome (31). ribD encodes a pyrimidine deaminase/reductase that generates 5-A-RU, the precursor to DMRL, 5-OP-RU, and 5-OE-RU (Figure 2B). To determine whether S. pneumoniae MAIT-cell ligands require 5-A-RU, we generated a ribD-knockout strain of SP9 (SP9ΔribD). DCs infected with SP9ΔribD were not able to activate MAIT cells (Figure 2C), demonstrating that MAIT-cell ligands from S. pneumoniae require RibD activity and the production of 5-A-RU. We therefore hypothesized that isolates which induce strong MAIT-cell responses would have higher expression of ribD than isolates that do not result in MAIT-cell activation. Using qRT-PCR, we quantified the expression of ribD in isolates from different MLST groups. As shown in Figure 2D, expression of ribD in S. pneumoniae isolates correlated with the observed MAIT-cell response in ELISPOT assays. These data indicate that S. pneumoniae isolates with higher expression of genes in the riboflavin metabolic pathway may be capable of expressing increased 5-A-RU and thus increased MAIT-cell ligands DMRL, 5-OP-RU, and 5-OE-RU.
Figure 2.
(A) Relative IFN-γ production by D426 G11 in response to SP isolates grouped by multilocus sequence typing (MLST). Statistical significance between MLST group 695 and MLST group 199, 320, or 667 was determined by an unpaired t test with Welch’s correction. (B) SP riboflavin biosynthesis pathway. RibD, RibH, and RibE enzymes are encoded on a single operon. (C) DCs were infected with SP9 or SP9ΔribD and used in an ELISPOT assay as previously described. (D) Top: Indicated SP isolates were cultured, and mucosal-associated invariant T–cell response was analyzed as described in Figure 1. Bottom: At the time the ELISPOT assay was performed, ribD expression in paired isolates was determined by RT-PCR analysis. Isolates were tested in pairs, determined by similar gyrA expression. For C and D, results shown are representative of three independent experiments; error bars are the mean and SD derived from replicate wells. (E) DCs were incubated with the less than 3 kD lysate fractions from SP9 and SP37 in an ELISPOT assay with IFN-γ production by D426 G11 as a readout. Relative ion intensities of riboflavin and flavin mononucleotide (FMN) in less than 3 kD lysate fractions from SP9 and SP37 are shown. Solid circles in the graphs in E represent data derived from triplicate injections. 5-A-RU = 5-amino-6-ribitylaminouracil; DMRL = 6,7-dimethyl-8-ribityllumazine; MS2 = MS/MS fragment spectra; UK = unknown MLST.
To confirm that differences in expression of the ribD gene resulted in different amounts of MR1 ligands, we used mass spectrometry to quantify relative amounts of molecules in the riboflavin biosynthesis pathway in isolates of S. pneumoniae. SP9 and SP37 were grown to equivalent optical densities, and bacterial pellets were lysed and passed through a 3 kD size exclusion spin filter. The fraction less than 3 kD was analyzed by liquid chromatography–mass spectrometry. Although DCs incubated with the lysate fraction were able to activate MAIT cells (Figure 2E), demonstrating the presence of ligand(s), we were unable to detect DMRL, 5-OP-RU, or 5-OE-RU, likely owing to the labile nature of these ligands (15, 32). Instead, we analyzed the relative amounts of riboflavin and the downstream molecule FMN. As shown in Figure 2E, the ion intensity of both riboflavin and FMN was significantly higher in SP9 than in SP37, suggesting that SP9 is synthesizing higher quantities of MAIT-cell ligands than SP37.
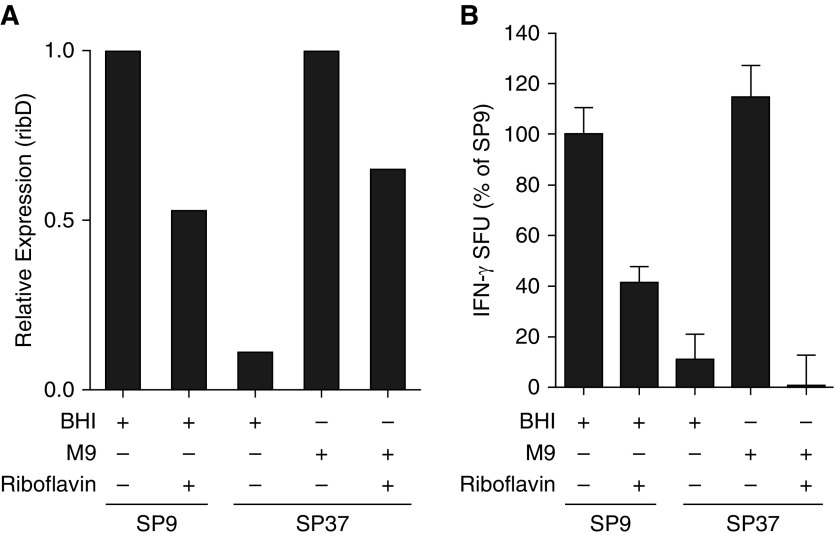
MAIT-Cell Activation by S. pneumoniae Is Modified by Altering Riboflavin Availability
We hypothesized that altering the amount of riboflavin available in the medium would result in changes to expression of genes in the riboflavin biosynthesis pathway and thus of MAIT-cell ligands. SP9 and SP37 were grown in optimal brain heart infusion (BHI) medium, BHI medium supplemented with excess riboflavin, minimal medium (M9), or M9 supplemented with excess riboflavin. qRT-PCR of ribD expression in each of the cultures revealed that addition of riboflavin to SP9 cultured in BHI medium or SP37 cultured in minimal essential medium resulted in downregulation of the ribD gene (Figure 3A). We next infected DCs with the isolates and determined whether the altered expression of the enzymes in the riboflavin metabolic pathway resulted in altered IFN-γ production by the MAIT-cell clone. As we previously observed, cells infected with SP9 grown in BHI medium elicited higher MAIT-cell responses than DCs infected with SP37 grown in the same medium (Figure 3B). When DCs infected with SP9 grown in BHI medium supplemented with excess riboflavin were used in the ELISPOT assay, the response by the MAIT-cell clone was decreased to 41% of the control using bacteria grown in BHI medium without riboflavin. In contrast, when DCs infected with SP37 grown in minimal M9 medium, which does not contain riboflavin, were used in the ELISPOT assay, there was a 10-fold increase in response by the MAIT-cell clone (Figure 3B). This increase was sufficient to bring the response to DCs infected with M9-grown SP37 up to the level of DCs infected with BHI medium–grown SP9. DCs infected with the SP37 isolate grown in M9 medium supplemented with riboflavin were unable to elicit an IFN-γ response from the MAIT-cell clone. These results demonstrate that changes in exogenous riboflavin availability can alter expression of riboflavin enzymes and subsequent availability of MR1 ligands. Together, our results suggest that clinical isolates of S. pneumoniae have different genetic requirements for riboflavin metabolism, resulting in differential activation of MAIT cells.
Figure 3.
(A) Relative expression of ribD in SP9 or SP37 isolates cultured in complete (brain heart infusion [BHI]) or minimal (M9) medium supplemented or not with riboflavin was evaluated by RT-PCR using gyrA as an internal control. Relative expression was determined separately for bacteria grown in BHI (SP9 + BHI) or M9 (SP39 + M9) medium because gyrA expression was different in BHI versus M9 medium. The results shown are representative of three independent experiments. (B) Relative IFN-γ production by D426 G11 in response to SP9 or SP37 isolates grown in complete (BHI) or minimal medium supplemented or not with riboflavin was evaluated by enzyme-linked immunospot assay as previously described. The results shown are derived from two independent experiments. Error bars indicate the mean and SD observed in both experiments.
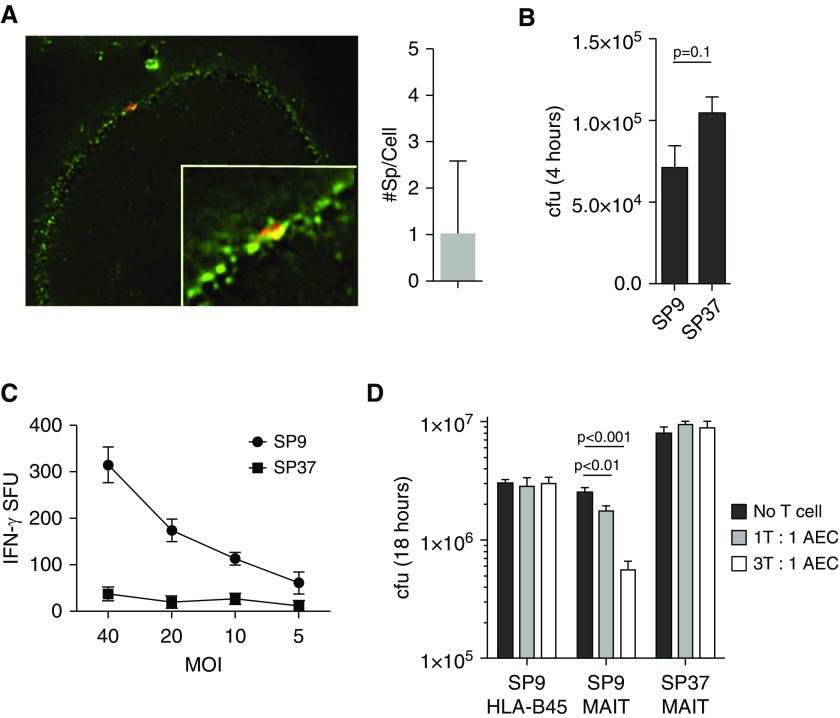
MAIT-Cell Recognition of S. pneumoniae Controls Bacterial Growth
S. pneumoniae is an airway pathogen that is likely to first interact with AECs. We have previously shown that primary human airway large epithelial cells can present antigen from M. tuberculosis and other microbial pathogens to MAIT cells (33–35). To determine if MAIT cells also respond to S. pneumoniae–infected AECs, we cultured primary human large airway epithelial cells from a healthy lung and infected them with S. pneumoniae labeled with Alexa Fluor 647 dye (Life Technologies). Using high-resolution deconvolution microscopy, we demonstrate that S. pneumoniae associates with AECs by binding to the surface of the cells (Figure 4A), as expected on the basis of previous literature (36). When infected with a multiplicity of infection of 5:1 for 4 hours, there was an average of one bacterium per cell, ranging from 0 to 8 per cell (Figure 4A). Although DCs exhibited the ability to kill all bacteria within 1 hour, we observed that S. pneumoniae isolates survived for at least 4 hours after infection of AECs (Figure 4B). We then tested the ability of MAIT cells to produce IFN-γ in response to AECs infected with SP9 or SP37. MAIT cells responded to AECs infected with SP9 (Figure 4C). Similar to what we observed for DCs, the MAIT cells responded poorly to SP37 (Figure 4C).
Figure 4.
(A) Primary airway epithelial cells (AECs) were infected with SP9 (multiplicity of infection [MOI] of 5:1) that had been labeled with Alexa Fluor 647 dye and stained with a cell surface mask. At least 100 cells were imaged in three independent experiments, and the number of bacteria per cell was counted using Imaris software (Bitplane). (B) cfu were enumerated by plating serial dilutions from primary AECs infected with SP9 or SP37 for 4 hours. (C) IFN-γ production by D426 G11 in response to SP9- or SP37-infected AECs was measured by ELISPOT assay as previously described. (D) Cells infected with SP9 or SP37 for 4 hours were extensively washed and incubated for an additional 14 hours with the D426 G11 mucosal-associated invariant T (MAIT)-cell clone or the HLA-B45–restricted D466 A10 T-cell clone. cfus were enumerated by plating serial dilutions. Data are representative of four independent experiments.
We hypothesized that MAIT cells would be capable of controlling S. pneumoniae in AECs exposed to this microbe. Because there was a distinct difference in the IFN-γ response by the MAIT cells to cells infected with different isolates, we further hypothesized that there would be a difference in the ability of MAIT cells to control SP9 and SP37. To test this, we added MAIT cells or an irrelevant T-cell clone overnight to AECs that were infected with SP9 or SP37. In cultures with no T cells or in the context of the irrelevant HLA-B45–restricted T-cell clone D466 A10, there was no effect on the survival of S. pneumoniae. However, the MAIT cells were able to control SP9 growth with increasing efficiency at higher MAIT-cell/AEC ratios. In contrast, the addition of MAIT cells had no impact on the survival of SP37 (Figure 4D). These in vitro data suggest that the ability of MAIT cells to recognize infection with different clinical isolates could play a critical role in controlling infection.
Cytokine Production by Lung MAIT Cells in S. pneumoniae–infected Mice
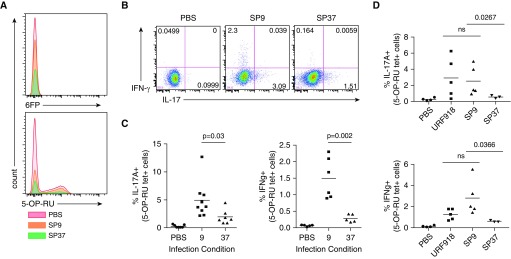
The in vitro MAIT-cell killing assay suggested that MAIT cells are capable of controlling S. pneumoniae and that this control is dependent on MR1-dependent recognition of the infection. To determine whether these differences are observed in vivo, we analyzed cytokine production by MAIT cells after intrapharyngeal infection in mice. The infection was induced in Vα19-transgenic mice on the Cα-deficient background to increase the number of tetramer-positive cells in the lungs (Figure 5A). S. pneumoniae serotype 19A has been described to be avirulent in mice (37, 38), and in agreement with this, there were no detectable SP9 or SP37 colony-forming units in the lungs of mice infected 2 days earlier. This was in contrast to the outcome after infection with a virulent serotype 3 control S. pneumoniae strain, URF918 (data not shown). Although the mice were able to completely control the growth of either SP9 and SP37, lung MAIT cells isolated from mice infected with SP9 produced more IL-17 and IFN-γ when analyzed directly ex vivo compared with those from mice infected with SP37 (Figures 5B and 5C). MAIT cells from mice infected with the virulent URF918 strain also produced both IL-17 and IFN-γ (Figure 5D). These data are in agreement with our in vitro results using human cells, and they suggest that the ability of MAIT cells to respond to S. pneumoniae in vivo is sensitive to quantitative differences in the clinical isolates to synthesize antigen.
Figure 5.
(A) Representative MR1/6FP and MR1/5-(2-oxopropylideneamino)-6-d-ribityluracil (5-OP-RU) tetramer staining for the plots shown in B–D. (B) IFN-γ and IL-17 staining of MR1 tetramer–positive cells harvested from lungs of three Vα19-transgenic mice infected with either SP9 or SP37, or with a PBS control, 20 hours earlier. (C) Pooled data from three (IL-17) or two (IFN-γ) independent experiments with two or three individual mice analyzed per group. (D) Pooled data from two independent experiments including SP serotype 3 URF918. Statistical significance was determined using an unpaired t test. 6FP = 6-formylpterin; ns = not significant.
Discussion
MAIT cells are a high-frequency, innate-like, lung-resident CD8+ T-cell subset in humans that is capable of responding to a broad group of bacterial and fungal pathogens. This finding has led to numerous efforts to demonstrate the importance of MAIT cells in response to human pathogens, including M. tuberculosis (12, 39), Klebsiella pneumoniae (40), Francisella tularensis (41), and Helicobacter pylori (42). The prevailing view is that infection with bacterial or fungal organisms that are capable of riboflavin biosynthesis results in antigen-dependent activation of MAIT cells. This was first observed in seminal studies in which researchers reported MAIT-cell responses to panels of bacterial, fungal, and viral species (12, 39). These studies included group A Streptococcus species, which do not encode riboflavin metabolic enzymes and did not elicit MAIT-cell responses (39), but they did not include group B Streptococcus species, which do encode riboflavin metabolic enzymes. To our knowledge, the present study is the first to demonstrate that MAIT cells are capable of responding to cells infected with S. pneumoniae. However, in our analysis of MAIT-cell responses to a panel of clinical isolates of S. pneumoniae that are of the same serotype, we also demonstrated that clinical isolates produce very different amounts of the riboflavin metabolites. These data may help to explain why only some isolates of S. pneumoniae are invasive, and they could have implications for the design of therapeutics or vaccines that target MAIT-cell activity.
There are multiple explanations for the diminished expression of riboflavin metabolic enzymes and concentrations of riboflavin metabolites in clinical isolates of S. pneumoniae with the same serotype. Isolates with low levels of riboflavin metabolism may be natural auxotrophs unable to synthesize their own riboflavin; they may have more efficient transport mechanisms for obtaining riboflavin from the environment; or they may have different metabolic needs for riboflavin and the downstream molecule FMN. Riboflavin biosynthesis is believed to be regulated through the FMN riboswitch, where FMN represses expression of genes in the riboflavin biosynthesis operon (43). Because riboflavin is converted to FMN, the availability of riboflavin, whether synthesized de novo or obtained from the environment, plays a role in the regulation of expression of the riboflavin biosynthesis operon. Our data suggest that the clinical isolate SP37 is not an auxotroph, because it is capable of expression of enzymes and metabolites in the riboflavin biosynthesis pathway in conditions of riboflavin starvation. In contrast, our data support a hypothesis proposing that clinical isolates of S. pneumoniae have different abilities to obtain environmental riboflavin through transporters or have different metabolic needs for riboflavin. The S. pneumoniae genome encodes at least one riboflavin transporter, RibU. Although expression of ribU was not higher in the SP37 isolate than in SP9, we did find that ribU expression was higher in SP2, SP11, and SP11 (data not shown), other clinical isolates in which infected DCs did not elicit as high a MAIT-cell response as SP9.
These data suggest that some clinical isolates of S. pneumoniae may have more efficient transport of environmental riboflavin. We observed downregulation of ribD expression and decreased presence of riboflavin metabolites, as measured by MAIT-cell immune responses, when either SP37 or SP9 was grown with excess riboflavin. Interestingly, regulation of riboflavin metabolism by the SP9 isolate was less sensitive to changes in environmental riboflavin than that by SP37. These data further suggest that riboflavin metabolism in S. pneumoniae clinical isolates is regulated in response to the environmental availability of riboflavin. Together, our data demonstrate that clinical isolates have different requirements for riboflavin, reflected by expression of both riboflavin biosynthesis and transport genes. Furthermore, among isolates, different factors can be responsible for regulation of riboflavin metabolism. In mice, our data suggest that riboflavin availability in vivo is similar to what was observed in vitro. It is not known, however, how this relates to the availability of riboflavin in the human lung environment, which is likely to be influenced by diet. Further study is required to determine the regulation of de novo riboflavin biosynthesis by S. pneumoniae in vivo and the impact that this has on MAIT-cell recognition and invasiveness of the pathogen.
In our present study, we focused on MAIT-cell responses to infected DCs, a professional antigen-presenting and phagocytic cell type, or to infected primary large airway epithelial cells. We demonstrated that DCs are highly efficient at controlling the bacteria, killing all intracellular bacteria within 1 hour after infection. In contrast to DC phagocytosis, S. pneumoniae adhered to the surface of epithelial cells ([44] and Figure 4A) and was not controlled by these cells. CD8+ T cells are typically believed to be important for recognition of viral or intracellular bacterial infections. Our data, however, demonstrate that MAIT cells are capable of recognizing infection of AECs with S. pneumoniae, an extracellular pathogen. MAIT-cell small-molecule ligands are distinct from other CD8+ T-cell peptide or lipid ligands in that they are small, soluble, and often secreted, providing a mechanism for interaction with MR1 antigen-presenting molecules even without intracellular infection. These properties may provide a unique role for MAIT cells as lung-resident sentinels capable of early recognition and response to extracellular infection in the large airways. Further study is required to determine whether lung MAIT cells are also activated in the context of S. pneumoniae infection through antigen-independent mechanisms.
Rapid MAIT-cell recognition and response to S. pneumoniae and subsequent production of antimicrobial factors could result in early control of the bacterium in the airway epithelium, before the initiation of adaptive immune responses. MAIT-cell responses to S. pneumoniae may also provide an early source of cytokines and/or chemokines that recruit additional immune cells such as inflammatory monocytes or neutrophils. Further study is required to determine the mechanisms by which MAIT cells can control the growth of an extracellular pathogen such as S. pneumoniae.
Acknowledgments
Acknowledgment
The authors thank Dr. Stephen I. Pelton (Boston Medical Center) for providing clinical isolates of Streptococcus pneumoniae.
Footnotes
Supported in part by Career Development Award IK2 CX000538 from the U.S. Department of Veterans Affairs Clinical Sciences Research and Development Program (M.J.H.), Merit Award I01 BX000533 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Program (D.M.L.), NIH grant R01 AI105215 (M.K.), NIH grant R01 AI129976 (M.J.H.), and a Deutsche Forschungsgemeinschaft Research Fellowship (N.H.). The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
Author Contributions: Conception and design: N.H., C.M., J.P.M., W.H., M.K., D.M.L., and M.J.H.; acquisition of data: N.H., C.M., M.L.S., M.E.H., R.K., F.T.C., and M.J.H.; analysis and interpretation: N.H., C.M., M.L.S., M.E.H., R.K., W.H., M.K., D.M.L., and M.J.H.; and drafting and approval of the manuscript: N.H., C.M., M.L.S., F.T.C., J.P.M., W.H., M.K., D.M.L., and M.J.H.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0290OC on January 22, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ruiz M, Ewig S, Torres A, Arancibia F, Marco F, Mensa J, et al. Severe community-acquired pneumonia: risk factors and follow-up epidemiology. Am J Respir Crit Care Med. 1999;160:923–929. doi: 10.1164/ajrccm.160.3.9901107. [DOI] [PubMed] [Google Scholar]
- 2.Albrich WC, Baughman W, Schmotzer B, Farley MM. Changing characteristics of invasive pneumococcal disease in Metropolitan Atlanta, Georgia, after introduction of a 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1569–1576. doi: 10.1086/518149. [DOI] [PubMed] [Google Scholar]
- 3.Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196:1346–1354. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 4.File TM. Community-acquired pneumonia. Lancet. 2003;362:1991–2001. doi: 10.1016/S0140-6736(03)15021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grotzke JE, Lewinsohn DM. Role of CD8+ T lymphocytes in control of Mycobacterium tuberculosis infection. Microbes Infect. 2005;7:776–788. doi: 10.1016/j.micinf.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Wong P, Pamer EG. CD8 T cell responses to infectious pathogens. Annu Rev Immunol. 2003;21:29–70. doi: 10.1146/annurev.immunol.21.120601.141114. [DOI] [PubMed] [Google Scholar]
- 7.Weber SE, Tian H, Pirofski LA. CD8+ cells enhance resistance to pulmonary serotype 3 Streptococcus pneumoniae infection in mice. J Immunol. 2011;186:432–442. doi: 10.4049/jimmunol.1001963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gohil SK, Heo M, Schoenbaum EE, Celentano D, Pirofski LA. CD8+ T cells and risk for bacterial pneumonia and all-cause mortality among HIV-infected women. J Acquir Immune Defic Syndr. 2012;60:191–198. doi: 10.1097/QAI.0b013e31824d90fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gold MC, Eid T, Smyk-Pearson S, Eberling Y, Swarbrick GM, Langley SM, et al. Human thymic MR1-restricted MAIT cells are innate pathogen-reactive effectors that adapt following thymic egress. Mucosal Immunol. 2013;6:35–44. doi: 10.1038/mi.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun. 2012;80:3256–3267. doi: 10.1128/IAI.00279-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dusseaux M, Martin E, Serriari N, Péguillet I, Premel V, Louis D, et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- 12.Gold MC, Cerri S, Smyk-Pearson S, Cansler ME, Vogt TM, Delepine J, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol. 2010;8:e1000407. doi: 10.1371/journal.pbio.1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakala IG, Kjer-Nielsen L, Eickhoff CS, Wang X, Blazevic A, Liu L, et al. Functional heterogeneity and antimycobacterial effects of mouse mucosal-associated invariant T cells specific for riboflavin metabolites. J Immunol. 2015;195:587–601. doi: 10.4049/jimmunol.1402545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Treiner E, Duban L, Bahram S, Radosavljevic M, Wanner V, Tilloy F, et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- 15.Corbett AJ, Eckle SB, Birkinshaw RW, Liu L, Patel O, Mahony J, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- 16.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- 17.Tettelin H, Nelson KE, Paulsen IT, Eisen JA, Read TD, Peterson S, et al. Complete genome sequence of a virulent isolate of Streptococcus pneumoniae. Science. 2001;293:498–506. doi: 10.1126/science.1061217. [DOI] [PubMed] [Google Scholar]
- 18.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–e11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kyaw MH, Lynfield R, Schaffner W, Craig AS, Hadler J, Reingold A, et al. Active Bacterial Core Surveillance of the Emerging Infections Program Network. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455–1463. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 20.Romani N, Gruner S, Brang D, Kämpgen E, Lenz A, Trockenbacher B, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold MC, McLaren JE, Reistetter JA, Smyk-Pearson S, Ladell K, Swarbrick GM, et al. MR1-restricted MAIT cells display ligand discrimination and pathogen selectivity through distinct T cell receptor usage. J Exp Med. 2014;211:1601–1610. doi: 10.1084/jem.20140507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewinsohn DM, Grotzke JE, Heinzel AS, Zhu L, Ovendale PJ, Johnson M, et al. Secreted proteins from Mycobacterium tuberculosis gain access to the cytosolic MHC class-I antigen-processing pathway. J Immunol. 2006;177:437–442. doi: 10.4049/jimmunol.177.1.437. [DOI] [PubMed] [Google Scholar]
- 23.Lewinsohn DM, Briden AL, Reed SG, Grabstein KH, Alderson MR. Mycobacterium tuberculosis-reactive CD8+ T lymphocytes: the relative contribution of classical versus nonclassical HLA restriction. J Immunol. 2000;165:925–930. doi: 10.4049/jimmunol.165.2.925. [DOI] [PubMed] [Google Scholar]
- 24.Kawachi I, Maldonado J, Strader C, Gilfillan S. MR1-restricted Vα19i mucosal-associated invariant T cells are innate T cells in the gut lamina propria that provide a rapid and diverse cytokine response. J Immunol. 2006;176:1618–1627. doi: 10.4049/jimmunol.176.3.1618. [DOI] [PubMed] [Google Scholar]
- 25.Heinzel AS, Grotzke JE, Lines RA, Lewinsohn DA, McNabb AL, Streblow DN, et al. HLA-E–dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. 2002;196:1473–1481. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 27.Ussher JE, Bilton M, Attwod E, Shadwell J, Richardson R, de Lara C, et al. CD161++CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur J Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hostetter MK. Serotypic variations among virulent pneumococci in deposition and degradation of covalently bound C3b: implications for phagocytosis and antibody production. J Infect Dis. 1986;153:682–693. doi: 10.1093/infdis/153.4.682. [DOI] [PubMed] [Google Scholar]
- 29.Melin M, Jarva H, Siira L, Meri S, Käyhty H, Väkeväinen M. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun. 2009;77:676–684. doi: 10.1128/IAI.01186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–3060. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 31.Vitreschak AG, Rodionov DA, Mironov AA, Gelfand MS. Regulation of riboflavin biosynthesis and transport genes in bacteria by transcriptional and translational attenuation. Nucleic Acids Res. 2002;30:3141–3151. doi: 10.1093/nar/gkf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reantragoon R, Corbett AJ, Sakala IG, Gherardin NA, Furness JB, Chen Z, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med. 2013;210:2305–2320. doi: 10.1084/jem.20130958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harriff MJ, Cansler ME, Toren KG, Canfield ET, Kwak S, Gold MC, et al. Human lung epithelial cells contain Mycobacterium tuberculosis in a late endosomal vacuole and are efficiently recognized by CD8+ T cells. PLoS One. 2014;9:e97515. doi: 10.1371/journal.pone.0097515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harriff MJ, Karamooz E, Burr A, Grant WF, Canfield ET, Sorensen ML, et al. Endosomal MR1 trafficking plays a key role in presentation of Mycobacterium tuberculosis ligands to MAIT cells. PLoS Pathog. 2016;12:e1005524. doi: 10.1371/journal.ppat.1005524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meermeier EW, Laugel BF, Sewell AK, Corbett AJ, Rossjohn J, McCluskey J, et al. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat Commun. 2016;7:12506. doi: 10.1038/ncomms12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adamou JE, Wizemann TM, Barren P, Langermann S. Adherence of Streptococcus pneumoniae to human bronchial epithelial cells (BEAS-2B) Infect Immun. 1998;66:820–822. doi: 10.1128/iai.66.2.820-822.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azoulay-Dupuis E, Rieux V, Muffat-Joly M, Bédos JP, Vallée E, Rivier C, et al. Relationship between capsular type, penicillin susceptibility, and virulence of human Streptococcus pneumoniae isolates in mice. Antimicrob Agents Chemother. 2000;44:1575–1577. doi: 10.1128/aac.44.6.1575-1577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Bourhis L, Martin E, Péguillet I, Guihot A, Froux N, Coré M, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- 40.Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48:769–775. doi: 10.1016/j.molimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Meierovics A, Yankelevich WJ, Cowley SC. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc Natl Acad Sci USA. 2013;110:E3119–E3128. doi: 10.1073/pnas.1302799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Booth JS, Salerno-Goncalves R, Blanchard TG, Patil SA, Kader HA, Safta AM, et al. Mucosal-associated invariant T cells in the human gastric mucosa and blood: role in Helicobacter pylori infection. Front Immunol. 2015;6:466. doi: 10.3389/fimmu.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mack M, van Loon AP, Hohmann HP. Regulation of riboflavin biosynthesis in Bacillus subtilis is affected by the activity of the flavokinase/flavin adenine dinucleotide synthetase encoded by ribC. J Bacteriol. 1998;180:950–955. doi: 10.1128/jb.180.4.950-955.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El Ahmer OR, Essery SD, Saadi AT, Raza MW, Ogilvie MM, Weir DM, et al. The effect of cigarette smoke on adherence of respiratory pathogens to buccal epithelial cells. FEMS Immunol Med Microbiol. 1999;23:27–36. doi: 10.1111/j.1574-695X.1999.tb01713.x. [DOI] [PubMed] [Google Scholar]