Abstract
Allergic asthma is a complex inflammatory disease that leads to significant healthcare costs and reduction in quality of life. Although many cell types are implicated in the pathogenesis of asthma, CD4+ T-helper cell type 2 (Th2) cells are centrally involved. We previously reported that the asthma phenotype is virtually absent in ovalbumin-sensitized and -challenged mice that lack global expression of β-arrestin (β-arr)-2 and that CD4+ T cells from these mice displayed significantly reduced CCL22–mediated chemotaxis. Because CCL22-mediated activation of CCR4 plays a role in Th2 cell regulation in asthmatic inflammation, we hypothesized that CCR4-mediated migration of CD4+ Th2 cells to the lung in asthma may use β-arr–dependent signaling. To test this hypothesis, we assessed the effect of various signaling inhibitors on CCL22-induced chemotaxis using in vitro–polarized primary CD4+ Th2 cells from β-arr2–knockout and wild-type mice. Our results show, for the first time, that CCL22-induced, CCR4-mediated Th2 cell chemotaxis is dependent, in part, on a β-arr2–dependent signaling pathway. In addition, we show that this chemotactic signaling mechanism involves activation of P-p38 and Rho-associated protein kinase. These findings point to a proinflammatory role for β-arr2–dependent signaling and support β-arr2 as a novel therapeutic target in asthma.
Keywords: CCR4, T-helper type 2 cell, chemotaxis, β-arrestin-2–dependent signaling, asthma
Clinical Relevance
Pharmacological modulation of G protein-coupled receptor activity is a well-established treatment approach that is pervasive in clinical medicine. The results reported herein emphasize the importance of considering the effect of potential pharmacologic agents on dual signaling pathways. Our study points to a proinflammatory role for β-arrestin-2–dependent signaling in T-helper type 2 cells. If, like the murine cells, human T-helper type 2 cells use a β-arrestin-2–dependent chemotactic signaling pathway, then this pathway could be selectively targeted in patients with asthma.
Allergic asthma is a chronic inflammatory syndrome characterized by airway inflammation, hyperresponsiveness, remodeling, and reversible airflow obstruction that leads to significant healthcare costs and reduction in quality of life (1). In a sensitized individual, antigen exposure triggers a coordinated interaction among several immune (2) and lung structural cell types (3), resulting in lung inflammation. Allergen-specific T lymphocytes differentiate into various T-helper (Th) cell subtypes that migrate to the lung, where they participate in the allergic inflammatory response (2). Studies using RAG (recombinase-activating gene) 1 knockout (KO) mice show that T lymphocytes are both necessary and sufficient for the development of allergic airway inflammation (4, 5). Similarly, activated CD4+ T cells are localized in the bronchial mucosa of patients with asthma, and allergen challenge of patients with asthma induces a selective recruitment of CD4+ T cells into the airways (6, 7). Although many Th cell subsets (including Th1 and Th17) are implicated in the pathogenesis of asthma, CD4+ T-helper type 2 (Th2) cells are centrally involved (reviewed in References 8–12).
Directed migration of immune cells from secondary lymphoid tissue to the site of inflammation, is initiated by chemokine receptor activation. One outcome of allergen-induced Th2 polarization is elevated cell surface expression of CCR4, which enables Th2 cells to preferentially respond to inflammatory, rather than homeostatic, chemokines (13, 14). Evidence that CCR4 is implicated in asthma pathogenesis comes from human studies, where increased lung production of C-C motif chemokine (CCL) 17 and CCL22, chemokines that selectively activate CCR4, occurs after segmental allergen challenge (15–17). Despite an early study using CCR4-KO mice that showed no change in ovalbumin-induced airway inflammation (18), the importance of the CCL17/22–CCR4 axis to murine asthma models has been demonstrated in several subsequent studies, where genetic, pharmacologic, and immunologic inhibition of CCR4 activation significantly impairs both Th2 cell extravasation into the lung and asthma phenotype development (19–22).
We previously showed that mice genetically devoid of β-arrestin (β-arr)-2 are significantly protected from developing the asthma phenotype (23). In addition, CD4+ T cells harvested from the lungs of these mice displayed a significant impairment in CCL22-mediated chemotaxis in vitro. We concluded that β-arr2 regulates CCR4-mediated chemotaxis in Th2 cells; however, the mechanism by which β-arr2 exerts this regulation is unknown.
β-arr2, a ubiquitously expressed scaffold protein, regulates and transduces 7 transmembrane receptor (7TMR) signaling, including chemokine receptors (24). β-arr2 was first shown to play a role in chemokine receptor–mediated chemotaxis in a 2002 report where naive T lymphocytes derived from β-arr2–knockout (β-arr2–KO) mice demonstrated significantly impaired CXCR4-mediated migration (25). Although canonical signaling pathways associated with CCR4-mediated chemotaxis in human Th2 cells have been examined (26), contribution by β-arr–dependent signaling was not considered.
For many reasons, delineation of the mechanistic role for β-arrs in chemotactic signaling is difficult to unravel. First, β-arrs are known to both arrest G protein–mediated, and transduce β-arr–dependent, signaling at a single chemokine receptor, and each of these dual signaling pathways may contribute to migration (reviewed in References 27 and 28). Second, ligand activation of chemokine receptors induces cell chemotaxis as well as several other cellular functions, including adhesion, proliferation, and gene expression (16). Thus, cellular levels of signaling intermediates must be proven to be associated with chemotaxis. Third, signaling intermediates, such as second messengers or mitogen-activated protein kinases (MAPKs), associated with G protein–mediated signaling may be identical to those activated by the β-arr–dependent pathway. Currently, the signaling source of these intermediates is only distinguishable by their spatiotemporal localization (29–32). Finally, chemotactic signaling pathways are cell type– and context-specific and, thus, must be individually examined.
The goal of the current study was to carefully dissect the role for β-arr2 in regulating CCR4-mediated chemotaxis in primary murine CD4+ Th2 cells.
Methods
Animals
Animal care protocols were approved by Duke University Institutional Animal Care and Use Committee and performed in accordance with U.S. Animal Welfare Act standards and NIH guidelines. Male β-arr2–KO (23) and littermate wild-type (WT) mice were used for all experiments at 8–16 weeks of age.
Chemicals
SB203580, Y27632, LY294002, and 227013 (CCR4 antagonist) were purchased from Calbiochem Corp. MK2206 was purchased from SelleckChem. Pertussis toxin (PTX) was purchased from Sigma Aldrich. Murine CCL22 and CCL17 were purchased from R&D system.
Polarization of CD4+ T Cells to Th2
Naive CD4+ T cells, purified and enriched (EasySep; StemCell Technologies) from spleens, were cultured in RPMI 1640 and polarized to Th2 using standard methods (see the data supplement). Th2 skewing was assessed by GATA-3 or IL-4 measurement using anti–GATA3–Phycoerythrin (PE) antibody (no. 12-9966-42; Ebioscience) or anti–IL-4–PE antibody (no. 554436; BD Pharmingen). Cells that underwent IL-4 staining were restimulated before surface staining. FACSVantage (Becton Dickinson) and FlowJo software (Tree Star Inc.) were used for flow cytometric analysis.
Chemotaxis Assays
Chemotaxis assays were performed in 24-well transwells as described previously (23). The bottom chamber was filled with 0.6 ml of RPMI 10% (vol/vol) FBS media with or without various concentrations of murine CCL17 or CCL22 (R&D Systems). Murine Th2 cells (1 × 106) in 0.1 ml of media were added to the top chamber. After 90 minutes of incubation at 37°C, the transmigrated cells in the bottom chamber were stained and counted by flow cytometry. The number of cells that transmigrated to media alone (chemokinesis) was subtracted from the number of cells found in chemokine-containing lower chambers. The difference represents the number of cells that underwent agonist-induced chemotaxis. This value was divided by the total number of cells placed on the upper transwells, and expressed as “percentage directed migration.” Enumeration of cells using flow cytometry was based on sample collection time, not percentage of cells.
Pharmacologic Cell Signaling Inhibitors
The effect of various signaling inhibitors on CCL22-induced chemotaxis was assessed in Th2 cells replete with, and devoid of, β-arr2. Th2 cells were preincubated with inhibitors for 30 minutes before chemotaxis stimulation with 10 nM CCL22, except for PTX, which required 16-hour incubation.
CCR4 Expression
Radioligand binding and RT-PCR were used to assess CCR4 expression in Th2 cells. Saturation binding technique was employed where Th2 cells were preincubated (5 min) with 1-μM cold murine CCL17 followed by 0.3 nM [125I]-labeled CCL17 (Perkin Elmer) for 60 minutes on ice. Maximal CCR4 binding (Bmax) was calculated and normalized to WT maximal binding (see the data supplement). Real-time PCR was performed to measure the expression of CCR4 relative to the housekeeping gene, GAPDH. The fold change in CCR4 expression over the labortory standard is presented. cDNA was synthesized using total cellular RNA isolated from Th2 cells.
Western Blot Analysis
CD4+ Th2 cells were serum starved for 18–24 hours before stimulation with 10-nM CCL22. Cells were harvested at different time points after stimulation and resuspended in RIPA buffer containing protease inhibitors. Lysates were mixed with sample buffer and proteins were separated on 10% Tris-glycine gels, transferred to polyvinylidene difluoride (PVDF) membranes and probed using antibodies against p38, P-p38, extracellular signal–regulated kinase (ERK), and P-ERK (see the data supplement). Using chemiluminescent detection (Pierce), each band in the immunoblots was quantified by densitometry using the GeneTools program (SynGene).
Statistical Analysis
Data are expressed as mean (±SEM). Statistical calculations were performed using GraphPad Prism (GraphPad Software, Inc.) or SPSS software. A two-tailed Student’s t test was applied as appropriate, and repeated measures analysis was used for dose–response comparisons. A P value of less than or, where noted, equal to, 0.05 was considered statistically significant.
Results
Effect of β-arr2 on Th2 Cell Migration
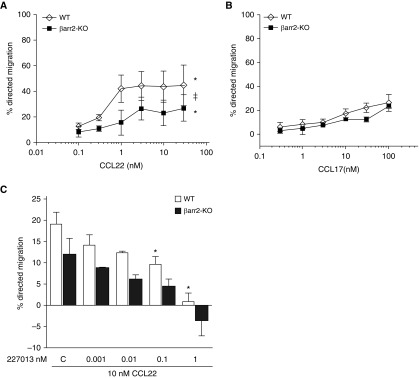
The β-arr–dependent and -independent signaling pathways can be distinguished by distinct temporal signatures, where signaling via the former pathway is delayed and prolonged compared with the latter (29–32). Thus, we chose to examine chemotaxis at 90 minutes. Chemokinesis of WT and β-arr2–KO Th2 cells at 90 minutes was not different (16.5 ± 1.6% and 18.7 ± 2.4%, respectively), indicating that β-arr2 does not regulate baseline Th2 cell migration. CCL22-stimulated Th2 cell chemotaxis occurs in a dose-dependent manner, but the magnitude of the chemotactic response was significantly impaired in cells lacking β-arr2 (β-arr2–KO) (Figure 1A). Given that CCL17 is a CCR4-specific ligand (33) and has been shown to have different signaling effects than CCL22 (34, 35), we tested its chemotactic effect on Th2 cells. WT Th2 cells migrated less well to CCL17 than to CCL22 (Figure 1A versus 1B), and the chemotactic response to CCL17 was not different between WT and β-arr2–KO Th2 cells (Figure 1B). Directed migration in response to CCL17 was dose dependent, but even at the highest CCL17 concentration, the directed migration was only around twice that observed in the absence of chemokine. Taken together, these results suggest that CCL17 does not promote β-arr2–dependent chemotaxis, in contrast to CCL22. Therefore, to study the role for β-arr2–dependent signaling in CCR4-mediated chemotaxis, we chose to use CCL22 in our experiments. Antagonists of CCR4 blocked CCL22-induced chemotaxis, suggesting that CCR4 is the operative chemokine receptor for CCL22-induced responses in Th2 cells (Figure 1C).
Figure 1.
Effect of β-arrestin (β-arr)-2 on CCL22– and CCL17-induced T-helper cell type 2 (Th2) migration. Migration of in vitro polarized wild-type (WT) and β-arr2–knockout (KO) Th2 cells was measured in response to a 90-minute exposure to increasing doses of (A) CCL22 or (B) CCL17. (C) Before stimulation with 10 nM CCL22, Th2 cells underwent a 30-minute pretreatment with the CCR4 antagonist, 227013. Data are presented as the mean (±SEM) of four independent experiments. ‡P < 0.05 effect of genotype and *P < 0.05 effect of dose according to repeated measures ANOVA for WT versus β-arr2–KO.
Effect of β-arr2 on CCR4 Expression
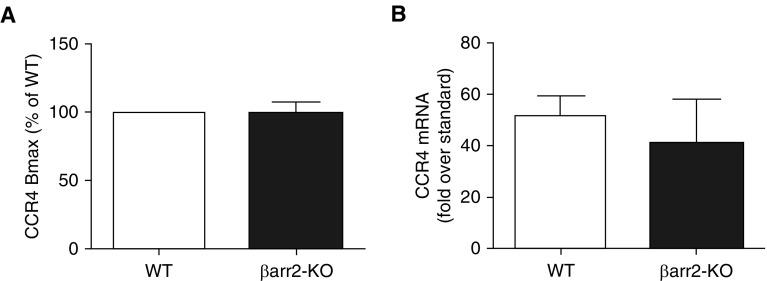
Because β-arr can regulate internalization of several chemokine receptors, we measured surface expression of CCR4 in WT and β-arr2–KO Th2 cells. CCR4 binding efficiency and expression levels in β-arr2–KO Th2 cells were similar to that in WT Th2 cells, as shown by measurement of radioligand binding (Figure 2A) and receptor mRNA levels using the RT-PCR method (Figure 2B). The fact that chemotaxis to CCL17 was the same in WT and β-arr2–KO cells also supports the notion that the two cell types have equal levels of CCR4 expression.
Figure 2.
CCR4 expression in Th2 cells. Expression of CCR4, as assessed by (A) radioligand binding and (B) RT-PCR, was the same in WT and β-arr2–KO Th2 cells. Data are presented as the mean (±SEM) of three independent experiments. Bmax = maximum specific binding.
β-arr2 Does Not Regulate CD4+ T Cell Differentiation
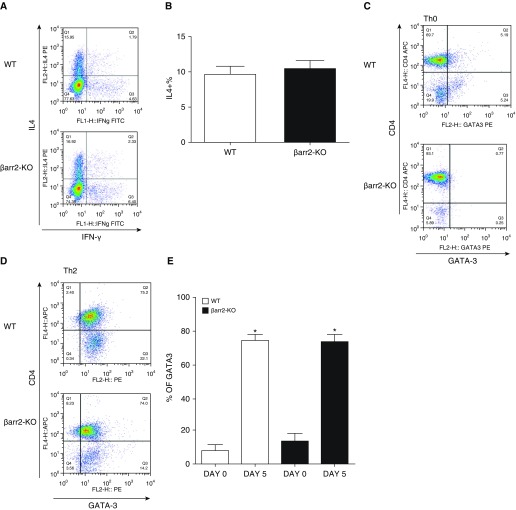
GATA-3 expression and IL-4 secretion are distinguishing features of Th2 cells (36, 37). As shown in Figure 3, naive WT and β-arr2–KO T cells similarly polarized to the Th2 phenotype as assessed by IL-4 (Figures 3A and 3B) and GATA-3 expression (Figures 3C–3E). These results demonstrate that Th2 cell expression of GATA-3 and production of IL-4 are independent of β-arr2.
Figure 3.
Effect of β-arr2 deficiency on CD4+ Th2 cell polarization. (A) Dot displays and (B) summary bar graphs show the proportion of CD4+ cells that express IL-4 (representative of 10 separate experiments). (C and D) Dot displays show the proportion of total WT (top) and β-arr2–KO (bottom) CD4+ cells that are GATA-binding protein 3 (GATA3) positive before (C) and after (D) Th2 cell polarization. (E) Summary bar graphs show the percent of CD4+ GATA3+ cells. Data are presented as the mean (±SEM) of 3–10 experiments. *P < 0.05 according to Student’s t test versus Day 0.
Effect of Signaling Inhibitors on CCL22-induced Th2 Cell Chemotaxis
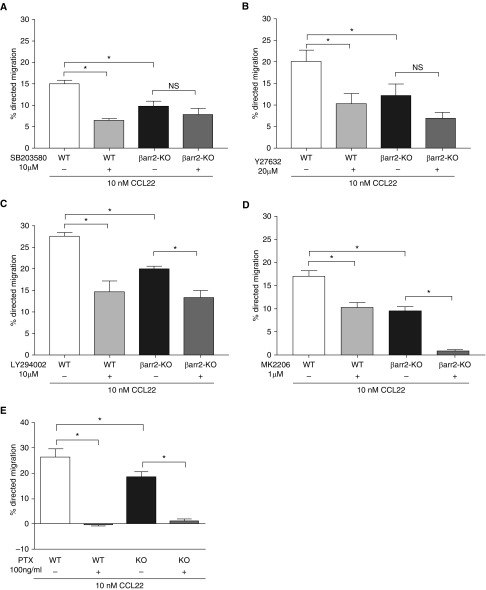
To determine the signaling molecules that mediate chemotaxis, Th2 cells were preincubated with inhibitors of p38 MAPK (SB203580) and Rho-associated protein kinase (ROCK; Y27632) before stimulation with CCL22. Inhibition of either p38 or ROCK significantly reduced chemotaxis in WT Th2 cells, but had no significant effect on that of β-arr2–KO Th2 cells (Figures 4A and 4B), suggesting that a significant proportion of Th2 cell chemotaxis is mediated by a β-arr2–dependent signaling pathway involving these signaling intermediates. In contrast, inhibitors of PI3K (LY294002) or Akt (MK2206) similarly reduced CCL22-induced chemotaxis in WT and β-arr2–KO Th2 cells (Figures 4C and 4D, respectively), suggesting that the PI3K/Akt signaling pathway to chemotaxis is operative even in the absence of β-arr2. Interestingly, the heterotrimeric Gi protein inhibitor, PTX, completely abolished CCL22-induced chemotaxis in WT and β-arr2–KO Th2 cells (Figure 4E) without affecting chemokinesis (data not shown). These data suggest that the CCR4 dual signaling pathways (β-arr2–dependent and -independent) are both downstream of Gi protein.
Figure 4.
Effect of cell signaling inhibitors on CCL22–induced Th2 cell chemotaxis. (A–E) Shown is the percent directed migration of WT and β-arr2–KO CD4+/Th2 cells that were pretreated, or not, with indicated concentrations of cell signaling inhibitors (A) SB203580, (B) Y27632, (C) LY294002, (D) MK2206, and (E) pertussis toxin (PTX) before incubation with 10-nM CCL22. All inhibitor incubations were for 30 minutes, except PTX, which was 16 hours. Data are presented as the mean (±SEM) of three to five experiments. *P ≤ 0.05 according to paired t test for within-subject effect of inhibitor or Student’s t test for WT versus β-arr2–KO. NS = not significant.
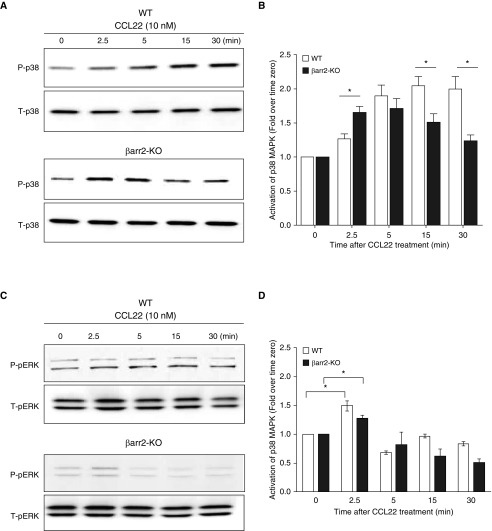
To further verify that p38 MAPK is involved in β-arr2–dependent signaling downstream of CCR4 activation, we measured cellular levels of P-p38. Because β-arr–dependent MAPK signaling is characteristically delayed and prolonged relative to that of β-arr–independent signaling (29), we measured MAPKs at early and late time points after CCL22 stimulation. CCL2-induced activation of P-p38 was similar in WT and β-arr2–KO Th2 cells at 5 minutes. In contrast, at the 15- and 30-minute time points, the P-p38 signal was significantly less in the β-arr2–KO Th2 cells compared with the WT cells in which the P-p38 signal remained elevated. These results suggest that the MAPK signal at later time points (15 and 30 min) results from β-arr2–dependent signaling. Because ERK1/2 signaling is known to be involved in β-arr–dependent signaling in other cellular contexts (38), we measured CCR4-mediated activation of P-ERK1/2 (Figures 5D and 5E). P-ERK1/2 was induced weakly, but similarly, in WT and β-arr2–KO Th2 cells at the early time points and absent in both genotypes at the late time point (30 min), indicating that, although there is precedence for β-arr–dependent P-ERK1/2 signaling to chemotaxis, this pathway/effector combination is not used by Th2 cells in response to CCR4 activation. Taken together, our observations are consistent with the inhibitor data, which suggest that the MAPK effector molecule associated with CCR4-mediated β-arr–dependent signaling to chemotaxis is P-p38, not P-ERK1/2.
Figure 5.
Effect of β-arr2 on CCL22–induced activation of mitogen-activated protein kinases (MAPKs) in Th2 cells. The time course of CCL22-induced Th2 cell production of (A and B) P-p38 and (C and D) P-ERK1/2 was assessed by immunoblot and quantified by densitometry. Open bars, WT Th2 cells; solid bars, β-arr2–KO Th2 cells. Western blot is representative of three separate experiments. Data are expressed as mean (±SEM). *P < 0.05 significant difference between WT and β-arr2–KO Th2 cells at the indicated time point according to Student’s t test. Readers may view the uncut gels for C in the data supplement.
Discussion
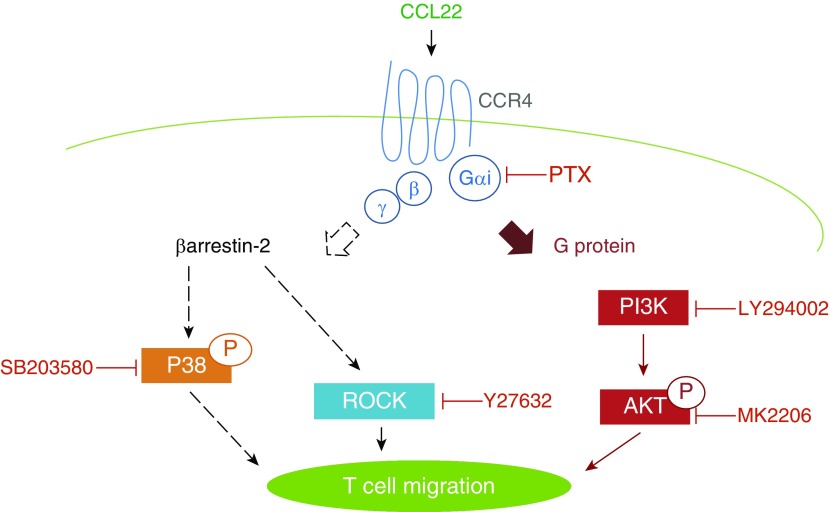
In this study, we reveal that, in addition to the classical G protein–dependent/β-arr–independent signaling pathway, CCR4-mediated Th2 cell chemotaxis is also transduced by a β-arr–dependent mechanism. Furthermore, we delineated that β-arr–dependent signaling uses P-p38 and ROCK effector molecules, whereas PI3K/Akt are associated with the β-arr–independent pathway (Figure 6).
Figure 6.
Role for β-arr2–dependent signaling in Th2 cell chemotaxis. CCL22–induced Th2 cell chemotaxis is promoted by both β-arr2–dependent and β-arr2–independent signaling pathways, both of which are downstream of Gαi activation. The β-arr2–dependent signaling arm uses P-p38 and Rho-associated protein kinase (ROCK), whereas the β-arr2–independent arm uses PI3K and Akt.
To characterize the CCR4-mediated chemotactic signaling pathways in Th2 cells, we reasoned that inhibition of signaling intermediates downstream of the β-arr–dependent chemotactic signaling pathway would reduce chemotaxis only in WT cells, because the β-arr–dependent pathway is absent in β-arr2–KO cells. Our results show that the β-arr–dependent signaling pathway uses both P-p38 and ROCK to initiate cell migration.
ROCK is a downstream effector of small, GTP-binding proteins, or GTPases, of the Rho family, such as rho, Rac, and Cdc42. These GTPases are central regulators of actin-based cytoskeletal reorganization (39). Our finding that T lymphocytes use ROCK signaling to chemotax is consistent with that of others. For example, using human peripheral blood mononuclear cells (PBMCs) and a lymphoblastoid CD4+ T cell line (CEM cells), Bardi and colleagues (40) showed that lymphocyte migration to homing chemokines CCL19 and CCL21 is dependent upon the activation of ROCK. Similarly, the ROCK pathway has been implicated in promoting lymphocyte chemotaxis to CXCL12 (41). Precedence for β-arr involvement in ROCK activation and stress fiber formation came from a study by Barnes and colleagues (42) who used angiotensin II type 1A receptor (AT1aR) stably expressing HEK293 cells. A report that linked ROCK-mediated chemotaxis to asthma came from Cronshaw and colleagues (43), who showed that migration of human CD4+ Th2 cells to CCL22 was reduced by the ROCK inhibitor, Y-27632. Their finding is consistent with the murine results presented herein. Moreover, in the current study, we have further delineated that ROCK-mediated chemotaxis is β-arr dependent. Although there was a trend for a small inhibitory effect of Y27632 on chemotaxis of β-arr2–KO Th2 cells, statistical significance was not reached even with a sample size of five experiments. However, it is possible that the β-arr2–independent, or an as-yet undiscovered, signaling pathway downstream of CCR4 and independent of β-arr2 uses ROCK to contribute, modestly, to Th2 cell chemotaxis.
In addition to ROCK, we show that the MAPK, P-p38, is also involved in CCR4-mediated, β-arr–dependent signaling to chemotaxis. Several previous studies using immortalized cell lines have demonstrated a role for P-p38 in mediating 7TMR-induced chemotaxis (33, 38, 44). For example, in HEK 293 cells, knockdown of β-arr2 blocked CXCR4- or AT1aR-mediated chemotaxis by a mechanism involving the activation of p38 MAPK (38, 44). In a separate study, where a role for β-arr was not examined, Lee and colleagues (45) showed that activation of the lysophosphatidic acid receptor, a 7TMR, by lysophosphatidylserine causes chemotaxis of a human glioma cell line (U87) via a P-p38–dependent mechanism. The work presented herein is the first to demonstrate that a primary immune cell uses a β-arr–dependent P-p38 signaling cascade to migrate toward an inflammatory chemokine. Whether or not P-p38 and ROCK directly interact to facilitate β-arr–dependent chemotaxis is unknown and was not tested in this study.
The molecular mechanism by which β-arr mediates chemotaxis is poorly understood; in part owing to the complexity of β-arr–mediated regulation of 7TMR signaling. In general, a cell is able to directionally migrate to an area of high chemokine concentration due, in part, to a greater ligand occupancy of chemokine receptors on the leading edge of the cell than on the trailing edge (27, 46). This differential activation of chemokine receptors leads to gross reorganization of the actin cytoskeleton, cell shape change, and cell chemotaxis (27, 39). Acting in a classical role (24, 47), β-arr may regulate chemotaxis by promoting chemokine receptor desensitization and recycling; a collective mechanism by which cells maintain their ability to sense a chemoattractant gradient (27). On the other hand, acting in a signal transduction role (24, 47), β-arr may scaffold multiple kinases that ultimately regulate actin cytoskeletal rearrangement (28). Our P-p38 data suggest that the latter role for β-arr is at work in CCR4-mediated migration of Th2 cells. Progress in delineation of the chemotactic cytoskeletal substrates activated by β-arr–dependent signaling complexes is an area of active investigation, and the reader is directed to reports from DeFea’s group (28, 48) for a thorough review.
The absence of β-arr2 does not completely impair the CCR4-induced chemotaxis of CD4+ Th2 cells, illustrating the existence of a β-arr–independent signaling pathway to chemotaxis. PI3K, and its substrate, Akt, are well known as chemotactic signaling intermediates in many cell types (49–51). Thus, as anticipated, inhibition of PI3K or Akt resulted in a significant decline in WT Th2 cell chemotaxis. The observation that β-arr2–KO Th2 cell chemotaxis was also significantly impaired in the presence of LY294002 or MK2206 places PI3K and Akt on the β-arr–independent arm of the chemotactic signaling pathways (Figure 6). Our results are in agreement with the finding by Xue and colleagues (52) who used human CD4+ Th2 cells to show that chemotaxis induced by agonist activation of the 7TMR CRTH2 (chemoattractant receptor-homologous molecule expressed on Th2 cells) was significantly inhibited by 100 μM LY294002 as were actin polymerization and Akt phosphorylation. Interestingly, although in their study LY294002 completely blocked Akt phosphorylation, it only partially inhibited chemotaxis. Thus, we speculate that a β-arr–dependent signaling pathway may also contribute to chemotaxis in human CD4+ Th2 cells. Although we did not measure intracellular Ca2+ concentration in the present studies, Cronshaw and colleagues (26) showed that CCR4-mediated Th2 cell chemotaxis was not impaired in the presence of 2-aminoethoxydiphenyl borane, which blocks Ca2+ release from intracellular stores (53).
Interestingly, the β-arr2–dependent, CCR4-mediated chemotactic signaling pathway is downstream of Gi family heterotrimeric proteins (Figure 6), as PTX completely abrogated CCL22-induced migration in WT (β-arr–replete) Th2 cells. This finding is consistent with that of Cronshaw and colleagues (43) who showed that human Th2 cell migration to CCL22 was abolished by prior treatment with PTX. To date, β-arr–dependent 7TMR signaling has been shown to be completely independent of Gs or Gq protein family member–mediated signaling (32, 44, 47, 54, 55), and Cheung and colleagues (56) showed that CCR5-mediated, β-arr–dependent chemotaxis is independent of Gi. Thus, it may seem curious that our study demonstrates that β-arr–dependent signaling to chemotaxis is downstream of Gi protein. However, Kohout and colleagues (57) showed that CCR7-mediated, β-arr–dependent chemotaxis is PTX sensitive, and speculated that β-arr translocation to a chemokine receptor at the cell surface, which is required for β-arr–dependent signaling, is triggered by free β-γ Gi protein subunits. Using human Th2 cells, Cronshaw and colleagues (26, 43) showed that CCR4-mediated Th2 cell chemotaxis requires Gi signaling, and uses both a Rho-dependent signaling pathway, as well as a phospholipase C(PLC)/PKC-δ signaling cascade that is calcium independent and diacylglycerol (DAG) dependent. Although at the time of the Cronshaw studies it was known that β-arr signaling is significantly involved in naive and allergen-activated CD4+ T cell migration (23, 25), as well as the migration of multiple transformed cell lines (24, 38, 57), tools for examining β-arr–dependent signaling in primary human cells with endogenous levels of receptor expression were lacking. Thus, future studies may show that CCR4-activated, Rho-dependent signaling to chemotaxis in human Th2 cells is, like murine cells, mediated by β-arr–dependent signaling.
Several lines of evidence suggest that CCL17 does not trigger β-arr–dependent signaling and is, therefore, a biased CCR4 ligand (8, 35, 58). Using human immortalized cells (HUT78 cells) transfected with CCR4, Ajram and colleagues (34) showed that CCL22, but not CCL17, triggered β-arr recruitment to the CCR4 receptor as assessed by DiscoveRx. Current knowledge indicates that translocation of β-arr to the cell surface receptor is required for β-arr–dependent signaling. The data presented herein are consistent with the notion that CCL17 is biased toward G protein signaling. Our observation, that WT Th2 cells migrate less well to CCL17 relative to CCL22 and about the same as cells lacking β-arr2, suggests that the β-arr–dependent signaling is not activated by CCL17 in WT Th2 cells.
β-arr2–mediated regulation of cell chemotaxis has gained prominence in the study of cancer (38, 59, 60; reviewed in References 28 and 39), atherosclerosis (61), and inflammatory disorders (reviewed in Reference 62), where it appears that β-arr is a proinflammatory/prometastatic protein (reviewed in Reference 27). Collectively, our prior work shows that β-arr2 plays a similar proinflammatory role in controlling cell migration crucial to asthma pathogenesis (23, 63). An example of the immunopathologic relevance of β-arr–dependent chemokine receptor chemotactic signaling is highlighted in WHIM (warts, hypogammaglobulinemia, infection, and myelokathexis) syndrome (64). Genetic mutations that cause truncation of the CXCR4 cytoplasmic tail underlie the highly varied disease characteristics observed in patients afflicted with WHIM syndrome. The truncation of CXCR4 augments and prolongs β-arr–dependent, CXCL12-induced signal transduction leading to enhanced leukocyte chemotaxis. Similarly, truncation of the CCR4 carboxy terminal cytoplasmic domain observed in adult T cell leukemia/lymphoma cells results in enhanced CCR4-mediated chemotaxis (65). If the parallel to WHIM syndrome holds true, where the CCR4 truncation enhances β-arr–dependent chemotaxis, then β-arr2 regulation of CCR4 may play a role in the pathogenesis of adult T cell leukemia/lymphoma.
Despite the importance of CCR4-mediated, β-arr–dependent Th2 cell migration to the development of asthma, it is doubtful that the absence of the β-arr–dependent arm of the chemotactic signaling cascade in Th2 cells is solely responsible for the striking lack of asthma phenotype observed in β-arr2–KO mice (23). In fact, β-arr–dependent regulation of nonchemokine 7TMRs plays a role in asthma pathogenesis. For example, protease-activated receptor-2 stimulation has been shown to stimulate β-arr2–dependent actin cytoskeletal rearrangement (reviewed in References 27 and 28) and CD4+ T cell migration, which may underlie much of the protection from asthma phenotype development observed in protease-activated receptor-2–KO mice (66). Another mechanism by which β-arr–mediated regulation of 7TMRs, other than chemokine receptors, may influence the asthma phenotype is through regulation of β2-adrenoceptor (β2AR) signaling. Endogenous activation of β2ARs is required for full development (67–69) and exacerbation (70) of the asthma phenotype, and β-arrs are well known to regulate β2AR signaling and function (32, 53).
Although in vitro models of chemotaxis may not always predict the in vivo migration of cells, our prior work (23, 63) suggests that β-arr–dependent signaling downstream of CCR4 in Th2 cells is indeed pathophysiologically relevant. We previously showed that WT mice that received hematopoietic cells from β-arr2–KO donors were significantly protected from development of the inflammatory characteristics of the asthma phenotype (63). Although transplanted marrow contains dendritic cell and B lymphocyte precursors that could impact asthma phenotype development, it is unlikely that function of these cell types in asthma is mediated by β-arr2, as our original study (in β-arr2–KO mice) showed that responses to allergen sensitization and challenge mediated by these cells were normal (23). Transplant of β-arr2–null eosinophils could potentially confound the interpretation of the role for Th2 cells in promoting asthma inflammation in chimeric mice, as eosinophil migration to the lung in allergic inflammatory airway disease is β-arr2 dependent; however, eosinophil activation is secondary to chemokine release by Th2 cells (71), supporting the importance of β-arr2 regulation of Th2 cell migration in allergic inflammation.
In summary, the in vitro work presented herein demonstrates, for the first time, that β-arr2–dependent chemotactic signaling occurs in Th2 cells, and that this signaling pathway is downstream of CCL22-mediated activation of CCR4. Although in vivo experiments will be required to unravel exactly where, or at what time point in Th2 cell migration, β-arr2–dependent chemotaxis is important for asthma pathogenesis, this study provides a better understanding of the nature of signaling pathways that underlie cell chemotaxis. Such information will help generate more specific pharmacological tools with which to treat pathologies characterized by aberrant cell migration. In fact, CCR4 is currently being targeted for antiasthma therapy (57, 72, 73). An advantage of antimigration-based treatment is reduced risk of toxicity compared with immunosuppressive therapy. Because CCR4-mediated signaling pathways can now be additionally distinguished by dependence, or not, on β-arr2, the potential exists to further reduce therapeutic side effects by searching for and testing orthosteric ligands or allosteric modulators of CCR4 that selectively modulate one signaling pathway over the other. Selective manipulation of CCR4-mediated chemotactic signaling pathways is highly feasible given that CCL17 is an endogenous biased ligand, and that the receptor has distinct conformational binding sites for CCL17 and CCL22 (35), which can be selectively inhibited by existing monoclonal antibodies and allosteric antagonists.
Acknowledgments
Acknowledgment
The authors are grateful to Ms. Barbara Theriot (Duke University Division of Pulmonary Medicine) for mouse colony management.
Footnotes
This work was supported by National Institutes of Health (NIH) grants R01 HL84123 and R01 AI110007 (J.K.L.W.).
R.L. is currently affiliated with the Division of Food Safety, Florida Department of Agriculture and Consumer Services, Tallahassee, FL 32399.
Y.h.C. is currently affiliated with the Institute for Clinical Research, CHA University, Sungnam, Republic of Korea.
Author Contributions: Conception and design of experiments—J.K.L.W., R.L., Y.h.C., and D.A.Z.; acquisition, analysis, and interpretation of data—J.K.L.W., R.L., and Y.h.C.; drafting of the manuscript for important intellectual content—J.K.L.W., R.L., Y.h.C., and D.A.Z.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0240OC on January 23, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. American Thoracic Society/European Respiratory Society Task Force on Asthma Control and Exacerbations. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med. 2009;180:59–99. doi: 10.1164/rccm.200801-060ST. [DOI] [PubMed] [Google Scholar]
- 2.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18:705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holgate ST, Lackie PM, Davies DE, Roche WR, Walls AF. The bronchial epithelium as a key regulator of airway inflammation and remodelling in asthma. Clin Exp Allergy. 1999;29:90–95. doi: 10.1046/j.1365-2222.1999.00016.x. [DOI] [PubMed] [Google Scholar]
- 4.Corry DB, Grünig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell–deficient mice. Mol Med. 1998;4:344–355. [PMC free article] [PubMed] [Google Scholar]
- 5.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, et al. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–2261. doi: 10.1126/science.282.5397.2258. [DOI] [PubMed] [Google Scholar]
- 6.Azzawi M, Bradley B, Jeffery PK, Frew AJ, Wardlaw AJ, Knowles G, et al. Identification of activated T lymphocytes and eosinophils in bronchial biopsies in stable atopic asthma. Am Rev Respir Dis. 1990;142:1407–1413. doi: 10.1164/ajrccm/142.6_Pt_1.1407. [DOI] [PubMed] [Google Scholar]
- 7.Gerblich AA, Salik H, Schuyler MR. Dynamic T-cell changes in peripheral blood and bronchoalveolar lavage after antigen bronchoprovocation in asthmatics. Am Rev Respir Dis. 1991;143:533–537. doi: 10.1164/ajrccm/143.3.533. [DOI] [PubMed] [Google Scholar]
- 8.Arima M, Fukuda T. Prostaglandin D2 and T(H)2 inflammation in the pathogenesis of bronchial asthma. Korean J Intern Med. 2011;26:8–18. doi: 10.3904/kjim.2011.26.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Zhang Y, Sun B. Current understanding of Th2 cell differentiation and function. Protein Cell. 2011;2:604–611. doi: 10.1007/s13238-011-1083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: the ins and outs. Annu Rev Immunol. 2008;26:205–232. doi: 10.1146/annurev.immunol.26.021607.090312. [DOI] [PubMed] [Google Scholar]
- 11.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 12.Weaver JM, Sant AJ. Understanding the focused CD4 T cell response to antigen and pathogenic organisms. Immunol Res. 2009;45:123–143. doi: 10.1007/s12026-009-8095-8. [DOI] [PubMed] [Google Scholar]
- 13.Bonecchi R, Bianchi G, Bordignon PP, D’Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, et al. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 15.Pilette C, Francis JN, Till SJ, Durham SR. CCR4 ligands are up-regulated in the airways of atopic asthmatics after segmental allergen challenge. Eur Respir J. 2004;23:876–884. doi: 10.1183/09031936.04.00102504. [DOI] [PubMed] [Google Scholar]
- 16.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation—therapeutic opportunities and pharmacological challenges. Pharmacol Rev. 2013;65:47–89. doi: 10.1124/pr.111.005074. [DOI] [PubMed] [Google Scholar]
- 17.Velazquez JR, Teran LM. Chemokines and their receptors in the allergic airway inflammatory process. Clin Rev Allergy Immunol. 2011;41:76–88. doi: 10.1007/s12016-010-8202-6. [DOI] [PubMed] [Google Scholar]
- 18.Chvatchko Y, Hoogewerf AJ, Meyer A, Alouani S, Juillard P, Buser R, et al. A key role for CC chemokine receptor 4 in lipopolysaccharide-induced endotoxic shock. J Exp Med. 2000;191:1755–1764. doi: 10.1084/jem.191.10.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalo JA, Pan Y, Lloyd CM, Jia GQ, Yu G, Dussault B, et al. Mouse monocyte-derived chemokine is involved in airway hyperreactivity and lung inflammation. J Immunol. 1999;163:403–411. [PubMed] [Google Scholar]
- 20.Mikhak Z, Fukui M, Farsidjani A, Medoff BD, Tager AM, Luster AD. Contribution of CCR4 and CCR8 to antigen-specific T(h)2 cell trafficking in allergic pulmonary inflammation. J Allergy Clin Immunol. 2009;123:67–73.e63. doi: 10.1016/j.jaci.2008.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64:995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 22.Schuh JM, Power C, Proudfoot AE, Kunkel SL, Lukacs NW, Hogaboam CM. Airway hyperresponsiveness, but not airway remodeling, is attenuated during chronic pulmonary allergic responses to Aspergillus in CCR4−/− mice. FASEB J. 2002;16:1313–1315. doi: 10.1096/fj.02-0193fje. [DOI] [PubMed] [Google Scholar]
- 23.Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, et al. β-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by β-arrestins. Science. 2005;308:512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- 25.Fong AM, Premont RT, Richardson RM, Yu YR, Lefkowitz RJ, Patel DD. Defective lymphocyte chemotaxis in β-arrestin2– and GRK6-deficient mice. Proc Natl Acad Sci USA. 2002;99:7478–7483. doi: 10.1073/pnas.112198299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cronshaw DG, Kouroumalis A, Parry R, Webb A, Brown Z, Ward SG. Evidence that phospholipase-C–dependent, calcium-independent mechanisms are required for directional migration of T-lymphocytes in response to the CCR4 ligands CCL17 and CCL22. J Leukoc Biol. 2006;79:1369–1380. doi: 10.1189/jlb.0106035. [DOI] [PubMed] [Google Scholar]
- 27.DeFea KA. Stop that cell! β-arrestin–dependent chemotaxis: a tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 28.Min J, Defea K. β-arrestin–dependent actin reorganization: bringing the right players together at the leading edge. Mol Pharmacol. 2011;80:760–768. doi: 10.1124/mol.111.072470. [DOI] [PubMed] [Google Scholar]
- 29.Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of β-arrestin and G protein–mediated ERK activation by the angiotensin II receptor. J Biol Chem. 2004;279:35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 30.Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, et al. Distinct β-arrestin– and G protein–dependent pathways for parathyroid hormone receptor–stimulated ERK1/2 activation. J Biol Chem. 2006;281:10856–10864. doi: 10.1074/jbc.M513380200. [DOI] [PubMed] [Google Scholar]
- 31.Ren X-R, Reiter E, Ahn S, Kim J, Chen W, Lefkowitz RJ. Different G protein–coupled receptor kinases govern G protein and β-arrestin–mediated signaling of V2 vasopressin receptor. Proc Natl Acad Sci USA. 2005;102:1448–1453. doi: 10.1073/pnas.0409534102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shenoy SK, Drake MT, Nelson CD, Houtz DA, Xiao K, Madabushi S, et al. β-arrestin–dependent, G protein–independent ERK1/2 activation by the β2 adrenergic receptor. J Biol Chem. 2006;281:1261–1273. doi: 10.1074/jbc.M506576200. [DOI] [PubMed] [Google Scholar]
- 33.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell–directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 34.Ajram L, Begg M, Slack R, Cryan J, Hall D, Hodgson S, et al. Internalization of the chemokine receptor CCR4 can be evoked by orthosteric and allosteric receptor antagonists. Eur J Pharmacol. 2014;729:75–85. doi: 10.1016/j.ejphar.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viney JM, Andrew DP, Phillips RM, Meiser A, Patel P, Lennartz-Walker M, et al. Distinct conformations of the chemokine receptor CCR4 with implications for its targeting in allergy. J Immunol. 2014;192:3419–3427. doi: 10.4049/jimmunol.1300232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 37.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Cheng Z, Ma L, Pei G. β-arrestin2 is critically involved in CXCR4-mediated chemotaxis, and this is mediated by its enhancement of p38 MAPK activation. J Biol Chem. 2002;277:49212–49219. doi: 10.1074/jbc.M207294200. [DOI] [PubMed] [Google Scholar]
- 39.Cotton M, Claing A. G protein–coupled receptors stimulation and the control of cell migration. Cell Signal. 2009;21:1045–1053. doi: 10.1016/j.cellsig.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Bardi G, Niggli V, Loetscher P. Rho kinase is required for CCR7-mediated polarization and chemotaxis of T lymphocytes. FEBS Lett. 2003;542:79–83. doi: 10.1016/s0014-5793(03)00351-x. [DOI] [PubMed] [Google Scholar]
- 41.Vicente-Manzanares M, Cabrero JR, Rey M, Perez-Martinez M, Ursa A, Itoh K, et al. A role for the Rho–p160 Rho coiled-coil kinase axis in the chemokine stromal cell–derived factor-1α–induced lymphocyte actomyosin and microtubular organization and chemotaxis. J Immunol. 2002;168:400–410. doi: 10.4049/jimmunol.168.1.400. [DOI] [PubMed] [Google Scholar]
- 42.Barnes WG, Reiter E, Violin JD, Ren X-R, Milligan G, Lefkowitz RJ. β-arrestin 1 and Gαq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J Biol Chem. 2005;280:8041–8050. doi: 10.1074/jbc.M412924200. [DOI] [PubMed] [Google Scholar]
- 43.Cronshaw DG, Owen C, Brown Z, Ward SG. Activation of phosphoinositide 3-kinases by the CCR4 ligand macrophage-derived chemokine is a dispensable signal for t lymphocyte chemotaxis. J Immunol. 2004;172:7761–7770. doi: 10.4049/jimmunol.172.12.7761. [DOI] [PubMed] [Google Scholar]
- 44.Hunton DL, Barnes WG, Kim J, Ren XR, Violin JD, Reiter E, et al. β-arrestin 2–dependent angiotensin II type 1A receptor–mediated pathway of chemotaxis. Mol Pharmacol. 2005;67:1229–1236. doi: 10.1124/mol.104.006270. [DOI] [PubMed] [Google Scholar]
- 45.Lee SY, Lee HY, Kim SD, Jo SH, Shim JW, Lee HJ, et al. Lysophosphatidylserine stimulates chemotactic migration in u87 human glioma cells. Biochem Biophys Res Commun. 2008;374:147–151. doi: 10.1016/j.bbrc.2008.06.117. [DOI] [PubMed] [Google Scholar]
- 46.Schwab A, Fabian A, Hanley PJ, Stock C. Role of ion channels and transporters in cell migration. Physiol Rev. 2012;92:1865–1913. doi: 10.1152/physrev.00018.2011. [DOI] [PubMed] [Google Scholar]
- 47.Lefkowitz RJ, Whalen EJ. β-arrestins: traffic cops of cell signaling. Curr Opin Cell Biol. 2004;16:162–168. doi: 10.1016/j.ceb.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 48.DeFea KA. β-arrestins as regulators of signal termination and transduction: how do they determine what to scaffold? Cell Signal. 2011;23:621–629. doi: 10.1016/j.cellsig.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 49.Hawkins PT, Stephens LR, Suire S, Wilson M.Pi3k signaling in neutrophils Rommel C, Vanhaesebroeck B, Vogt PK.editorsPhosphoinositide 3-kinase in health and disease. Vol. 1. Berlin, Heidelberg: Springer Berlin Heidelberg; 2011183–202. [Google Scholar]
- 50.Sotsios Y, Whittaker GC, Westwick J, Ward SG. The CXC chemokine stromal cell–derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J Immunol. 1999;163:5954–5963. [PubMed] [Google Scholar]
- 51.Ward SG, Marelli-Berg FM. Mechanisms of chemokine and antigen-dependent T-lymphocyte navigation. Biochem J. 2009;418:13–27. doi: 10.1042/BJ20081969. [DOI] [PubMed] [Google Scholar]
- 52.Xue L, Gyles SL, Barrow A, Pettipher R. Inhibition of PI3K and calcineurin suppresses chemoattractant receptor-homologous molecule expressed on Th2 cells (CRTH2)-dependent responses of Th2 lymphocytes to prostaglandin D(2) Biochem Pharmacol. 2007;73:843–853. doi: 10.1016/j.bcp.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Braun F-J, Aziz O, Putney JW., Jr 2-aminoethoxydiphenyl borane activates a novel calcium-permeable cation channel. Mol Pharmacol. 2003;63:1304–1311. doi: 10.1124/mol.63.6.1304. [DOI] [PubMed] [Google Scholar]
- 54.DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. β-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 55.Wei H, Ahn S, Shenoy SK, Karnik SS, Hunyady L, Luttrell LM, et al. Independent β-arrestin 2 and G protein–mediated pathways for angiotensin II activation of extracellular signal–regulated kinases 1 and 2. Proc Natl Acad Sci USA. 2003;100:10782–10787. doi: 10.1073/pnas.1834556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheung R, Malik M, Ravyn V, Tomkowicz B, Ptasznik A, Collman RG. An arrestin-dependent multi-kinase signaling complex mediates MIP-1β/CCL4 signaling and chemotaxis of primary human macrophages. J Leukoc Biol. 2009;86:833–845. doi: 10.1189/jlb.0908551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS. Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7. J Biol Chem. 2004;279:23214–23222. doi: 10.1074/jbc.M402125200. [DOI] [PubMed] [Google Scholar]
- 58.Solari R, Pease JE. Targeting chemokine receptors in disease—a case study of CCR4. Eur J Pharmacol. 2015;763:169–177. doi: 10.1016/j.ejphar.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buchanan FG, Gorden DL, Matta P, Shi Q, Matrisian LM, DuBois RN. Role of β-arrestin 1 in the metastatic progression of colorectal cancer. Proc Natl Acad Sci USA. 2006;103:1492–1497. doi: 10.1073/pnas.0510562103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shukla AK, Violin JD, Whalen EJ, Gesty-Palmer D, Shenoy SK, Lefkowitz RJ. Distinct conformational changes in β-arrestin report biased agonism at seven-transmembrane receptors. Proc Natl Acad Sci USA. 2008;105:9988–9993. doi: 10.1073/pnas.0804246105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Zhang L, Peppel K, Wu JH, Zidar DA, Brian L, et al. β-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma D, Parameswaran N. Multifaceted role of β-arrestins in inflammation and disease. Genes Immun. 2015;16:499–513. doi: 10.1038/gene.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hollingsworth JW, Theriot BS, Li Z, Lawson BL, Sunday M, Schwartz DA, et al. Both hematopoietic-derived and non-hematopoietic–derived β-arrestin-2 regulates murine allergic airway disease. Am J Respir Cell Mol Biol. 2010;43:269–275. doi: 10.1165/rcmb.2009-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, et al. CXCR4 dimerization and β-arrestin–mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34–44. doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- 65.Nakagawa M, Schmitz R, Xiao W, Goldman CK, Xu W, Yang Y, et al. Gain-of-function CCR4 mutations in adult T cell leukemia/lymphoma. J Exp Med. 2014;211:2497–2505. doi: 10.1084/jem.20140987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nichols HL, Saffeddine M, Theriot BS, Hegde A, Polley D, El-Mays T, et al. β-arrestin-2 mediates the proinflammatory effects of proteinase-activated receptor-2 in the airway. Proc Natl Acad Sci USA. 2012;109:16660–16665. doi: 10.1073/pnas.1208881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, et al. Effects of acute and chronic administration of β-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Forkuo GS, Kim H, Thanawala VJ, Al-Sawalha N, Valdez D, Joshi R, et al. Phosphodiesterase 4 inhibitors attenuate the asthma phenotype produced by β2-adrenoceptor agonists in phenylethanolamine N-methyltransferase–knockout mice. Am J Respir Cell Mol Biol. 2016;55:234–242. doi: 10.1165/rcmb.2015-0373OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thanawala VJ, Forkuo GS, Al-Sawalha N, Azzegagh Z, Nguyen LP, Eriksen JL, et al. β2-Adrenoceptor agonists are required for development of the asthma phenotype in a murine model. Am J Respir Cell Mol Biol. 2013;48:220–229. doi: 10.1165/rcmb.2012-0364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin R, Degan S, Theriot BS, Fischer BM, Strachan RT, Liang J, et al. Chronic treatment in vivo with β-adrenoceptor agonists induces dysfunction of airway β(2)-adrenoceptors and exacerbates lung inflammation in mice. Br J Pharmacol. 2012;165:2365–2377. doi: 10.1111/j.1476-5381.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walsh ERSK, Stokes K, August A. The role of eosinophils in allergic airway inflammation. Discov Med. 2010;9:357–362. [PubMed] [Google Scholar]
- 72.Castan L, Magnan A, Bouchaud G. Chemokine receptors in allergic diseases. Allergy. 2017;72:682–690. doi: 10.1111/all.13089. [DOI] [PubMed] [Google Scholar]
- 73.Yoshie O, Matsushima K. CCR4 and its ligands: from bench to bedside. Int Immunol. 2015;27:11–20. doi: 10.1093/intimm/dxu079. [DOI] [PubMed] [Google Scholar]