Abstract
Loss of secretory IgA is common in the small airways of patients with chronic obstructive pulmonary disease and may contribute to disease pathogenesis. Using mice that lack secretory IgA in the airways due to genetic deficiency of polymeric Ig receptor (pIgR−/− mice), we investigated the role of neutrophils in driving the fibrotic small airway wall remodeling and emphysema that develops spontaneously in these mice. By flow cytometry, we found an increase in the percentage of neutrophils among CD45+ cells in the lungs, as well as an increase in total neutrophils, in pIgR−/− mice compared with wild-type controls. This increase in neutrophils in pIgR−/− mice was associated with elastin degradation in the alveolar compartment and around small airways, along with increased collagen deposition in small airway walls. Neutrophil depletion using anti-Ly6G antibodies or treatment with broad-spectrum antibiotics inhibited development of both emphysema and small airway remodeling, suggesting that airway bacteria provide the stimulus for deleterious neutrophilic inflammation in this model. Exogenous bacterial challenge using lysates prepared from pathogenic and nonpathogenic bacteria worsened neutrophilic inflammation and lung remodeling in pIgR−/− mice. This phenotype was abrogated by antiinflammatory therapy with roflumilast. Together, these studies support the concept that disruption of the mucosal immune barrier in small airways contributes to chronic obstructive pulmonary disease progression by allowing bacteria to stimulate chronic neutrophilic inflammation, which, in turn, drives progressive airway wall fibrosis and emphysematous changes in the lung parenchyma.
Keywords: chronic obstructive pulmonary disease, emphysema, mucosal immunity, secretory IgA, polymeric Ig receptor
Inflammation in the lungs is a hallmark of chronic obstructive pulmonary disease (COPD) (1–3), and inflammatory cells remain elevated in the lungs of patients with COPD long after smoking cessation (4–6). Although innate immune cells, such as neutrophils and macrophages, are critical for pathogen eradication during acute infection, evidence from animal models suggests that proteases produced by these cells may overwhelm endogenous antiproteases and injure the lung, resulting in emphysematous destruction of alveolar tissue (7, 8).
Recent evidence suggests that localized loss of secretory IgA (SIgA) in small airways may play an important role in COPD progression after smoking cessation (2, 9, 10). SIgA normally lines mucosal surfaces throughout the body where it agglutinates bacteria and other antigens, facilitating their clearance by the mucociliary escalator and preventing activation of inflammatory signaling cascades in epithelial cells through immune exclusion (11–13). Consistent with a role in airway defense, loss of airway surface SIgA in individual small airways in lungs of patients with COPD is associated with markers of latent viral reactivation, bacterial invasion into the mucosa, NF-κB activation in airway epithelial cells, and accumulation of immune/inflammatory cells (2, 10). Similar findings occur in the lungs of polymeric Ig receptor null (pIgR−/−) mice, which lack SIgA in the airways and develop fibrotic remodeling of small airways and emphysema with aging (14).
In these studies, we sought to investigate the role of neutrophils in determining small airway pathology and emphysema in SIgA-deficient (pIgR−/−) mice. We found that chronic neutrophilic inflammation develops as a consequence of pIgR deficiency and contributes to small airway fibrosis and emphysema. Furthermore, we showed that bacterial products are the primary stimulus for neutrophilic inflammation in pIgR−/− mice. These data help to define the mechanisms of persistent inflammation in COPD, and suggest that impaired mucosal immunity in the airways results in airway wall remodeling and emphysematous destruction of the adjacent alveolar compartment.
Methods
Additional material and methods information is provided in the data supplement.
Animal Model
pIgR−/− mice, backcrossed onto a C57Bl/6 background for a minimum of eight generations (15), were obtained from the Mutant Mouse Resource Research Center at the University of Missouri. Wild-type (WT) and pIgR−/− mice were housed in standard microisolator cages in a centralized animal care facility and provided food and water ad libitum. All procedures involving mice were approved by the Institutional Care and Use Committee of Vanderbilt University.
Morphometry
Airway wall remodeling was assessed by morphometric evaluation of small airway wall thickness, as previously described (1, 2). Emphysematous changes of lung parenchyma were quantified using alveolar septal perimeter measurements on 10 randomly chosen fields of alveolar tissue at 200× magnification, according to American Thoracic Society recommendations (16). For measurement of collagen content in the lamina propria of small airways, we measured the area occupied by collagen content (based on picosirius red staining) divided by the length of the basement membrane in small airways. For quantification of elastin in the alveolar compartment, elastin immunofluorescence was evaluated on five consecutive fields of lung parenchyma (captured at ×40 objective) and quantified as actual pixel counts per field (airspace normalized to zero). All morphometric measurements were made using Image-Pro Plus software (Media Cybernetics).
Neutrophil Depletion
100 μg of anti-Ly6G antibodies (Clone 1A8, cat. BP0075–1) from BioXCell (West Lebanon, NH) or rat IgG2a isotype control antibodies (BioXCell) were delivered by intraperitoneal injection twice weekly for 16 weeks as previously described (17).
Antibiotics Administration
Vancomycin (0.5 mg/ml), neomycin (1 mg/ml), ampicillin (1 mg/ml), and metronidazole (1 mg/ml) (VNAM) were dissolved in autoclaved drinking water and provided to animals ad libitum for 3 months. Water was changed twice weekly. Control animals received autoclaved water only.
Nebulization Treatments
WT or pIgR−/− mice were placed in a whole-body nebulization chamber (inExpose; Scireq) and exposed to 10 mg of bacterial lysate aerosolized by a 5 L/min pump. Control animals were treated with nebulized sterile PBS. Mice were treated with nebulized bacterial lysates once weekly for 4 months. All animals were harvested 4 days after the final nebulization treatment.
Roflumilast Administration
A 200-μl aliquot of 0.5 mg/ml suspension of roflumilast or vehicle (4% methylcellulose, 1.3% polyethylene glycol 400, ∼5 μg drug/mg animal weight) was administered by oral gavage once daily, 5 d/wk for the duration of treatment, as previously described (14). The roflumilast suspension was freshly prepared each week and stored at 4°C.
Statistical Analysis
Mice were randomly assigned to the study groups and, where possible, researchers were blinded to the study groups until the time of statistical analysis. All animals were included in each analysis. Error bars reflect mean (±SEM). Pair-wise comparisons were made using t tests, whereas multiple comparisons were made using two-way ANOVA followed by Tukey’s test for multiple comparisons. Prism 7 software (GraphPad Software Inc.) was used for all statistical calculations.
Results
Neutrophilic Inflammation Is Associated with Small Airway Remodeling and Emphysema in pIgR−/− Mice
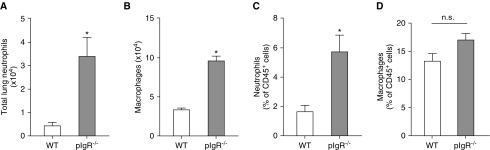
To evaluate inflammation in the lungs of pIgR−/− mice (C57Bl6/J background), we measured lung neutrophils and macrophages by flow cytometry in lung tissue from 9-month-old pIgR−/− mice and WT controls (see Figure E1 in the data supplement). We found increased numbers of neutrophils (CD45+/CD11b+/Ly6G+) and alveolar macrophages (CD45+/CD11chi/F4/80hi/CD103−/CD11b−) in pIgR−/− mice compared with age-matched WT controls (Figures 1A and 1B). The percentage of neutrophils among CD45+ immune/inflammatory cells was also increased in pIgR−/− mice, but there was no difference in the percentage of alveolar macrophages (Figures 1C and 1D).
Figure 1.
Increased neutrophils and macrophages in lungs of polymeric Ig receptor null (pIgR−/−) mice. Absolute number of (A) neutrophils and (B) macrophages in lungs of wild-type (WT) and pIgR−/− mice; n = 3–6 mice/group; *P < 0.05 (t test). Percentage of (C) neutrophils and (D) macrophages relative to CD45+ immune/inflammatory cells in lungs of 8- to 9-month-old WT and pIgR−/− mice; n = 3–6 mice/group; *P < 0.05 (t test). From CD45+ cells, alveolar macrophages were defined as CD11chiF4/80hiCD103-CD11b− and neutrophils were defined as CD11b+/Ly6G+. n.s. = not significant.
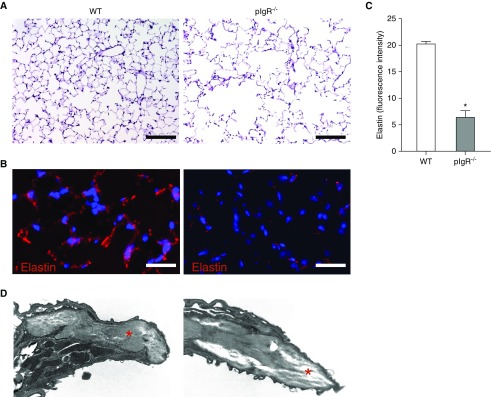
Next, we investigated the relationship between inflammation and lung remodeling in pIgR−/− mice using stains for elastin and collagen, as well as transmission electron microscopy. As shown in Figure 2A, 12-month-old pIgR−/− mice developed marked emphysemous destruction of the distal lung parenchyma. Consistent with evidence of emphysema on hematoxylin and eosin staining, we found that elastin content was markedly reduced in interalveolar septae of pIgR−/− mice (Figures 2B and 2C). In addition, transmission electron microscopy micrographs showed increased lucency of the interalveolar septum, suggesting extracellular matrix degradation in pIgR−/− mice (Figure 2D, red stars).
Figure 2.
Elastin degradation in lung parenchyma of pIgR−/− mice. (A) Hematoxylin and eosin staining of lung sections shows emphysematous lung destruction in a pIgR−/− mouse (12-mo-old) relative to an age-matched WT control. Scale bars: 50 μm. (B) Immunostaining for elastin from a WT mouse (12-mo-old) and a pIgR−/− mouse. Scale bars: 100 μm. (C) Quantification of fluorescent intensity of elastin staining reported as actual pixel density for each field of lung parenchyma (captured at ×40 objective); n = 6 mice/group; *P < 0.0001 (t test). (D) Transmission electron microscopy image of an interalveolar septum from a WT and pIgR−/− mouse (×50,000). The red stars denote extracellular matrix.
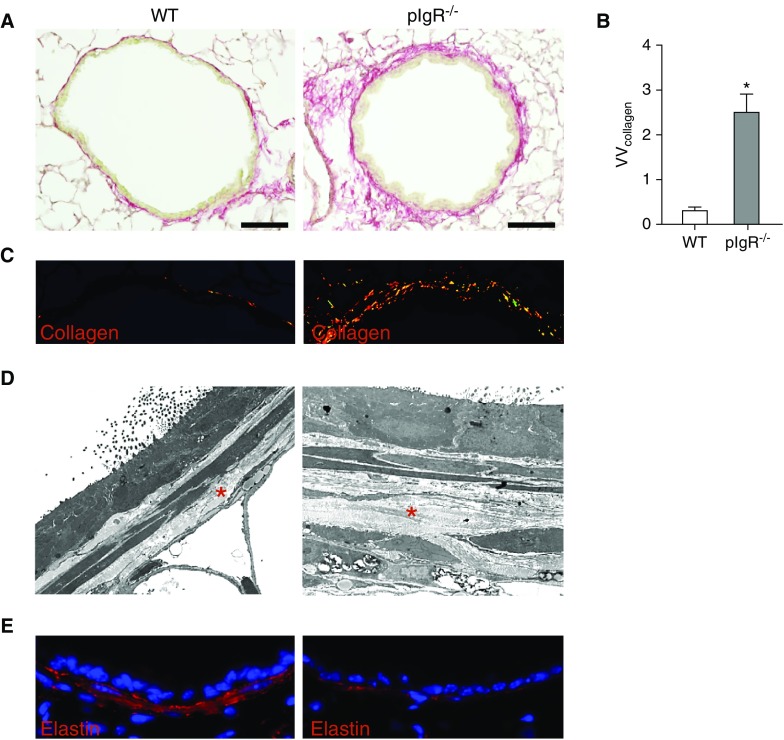
In the small airways of 12-month-old pIgR−/− mice, we observed thickening of the airway wall and increased collagen deposition in the lamina propria compared with age-matched WT mice (Figures 3A–3D). Despite increased collagen deposition, elastin destruction and reduced elastin content was observed in small airways of pIgR−/− mice (Figure 3E). Together, these data indicate that lungs of pIgR−/− mice are characterized by diffuse elastin degradation and fibrotic remodeling of small airway walls in association with neutrophil accumulation.
Figure 3.
Collagen deposition and subepithelial elastin degradation in small airways of pIgR−/− mice. (A) Picosirius red staining to detect collagen in a small airway from a WT mouse (12-mo-old) and an age-matched pIgR−/− mouse. Scale bars: 50 μm. (B) Quantification of collagen content in small airway walls normalized to basement membrane length (VVcollagen); n = 6 mice/group; *P < 0.001 (t test). (C) High-power magnification of picosirius red staining under polarized light in the same airways. (D) Transmission electron microscopy image of a small airway wall in a WT mouse and an age-matched pIgR−/− mouse (×10,000). The red stars denote subepithelial collagen. (E) Immunostaining for elastin in small airway wall from a WT mouse (12-mo-old) and an age-matched pIgR−/− mouse.
Because destructive changes in the lungs of pIgR−/− mice were present in both small airways and lung parenchyma, we sought to determine whether these pathological changes were a direct result of localized pIgR deficiency or occurred as a consequence of loss of pIgR only in small airways. By immunostaining, pIgR was readily observable in the airway epithelium, where it could be seen in close proximity to SIgA on the airway surface; however, pIgR expression was not identified in alveolar tissue (Figure E2A). In vitro, murine tracheal epithelial cells grown in air–liquid interface culture to stimulate differentiation had robust pIgR expression, as determined by immunostaining, which colocalized with α-tubulin expression in multiciliated cells (Figure E2B). In addition, PIGR mRNA expression was significantly higher in murine tracheal epithelial cells compared with isolated type II alveolar epithelial cells (Figure E2C). Together, these findings suggest that emphysematous destruction in the alveolar compartment of pIgR−/− mice occurs as a downstream result of the impaired mucosal immune barrier in small airways.
Lung Remodeling in pIgR−/− Mice Is Abrogated by Depletion of Neutrophils or Airway Bacteria
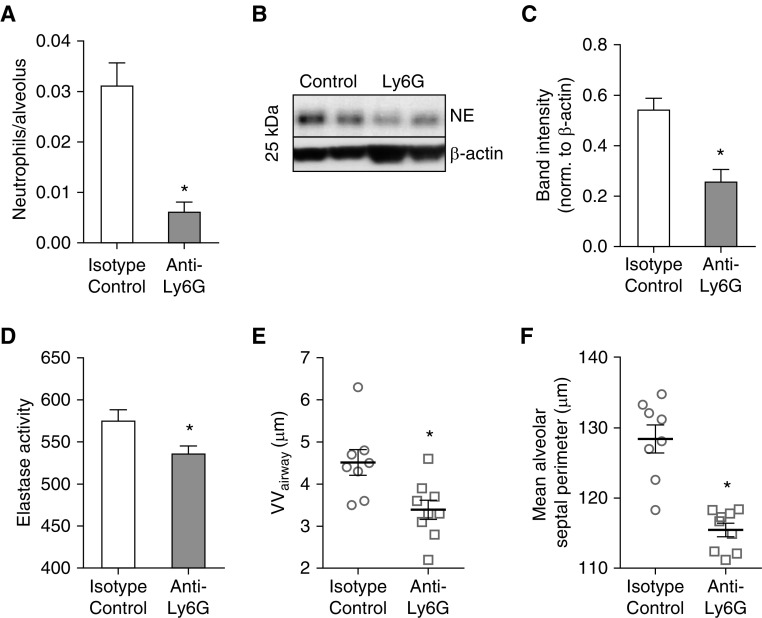
To determine whether neutrophilic inflammation plays an important role in lung remodeling in pIgR−/− mice, we treated pIgR−/− mice via intraperitoneal injection with anti-neutrophil–specific antibodies (anti-Ly6G, clone 1A8) (18) or rat isotype control IgG twice weekly for 4 months, according to a protocol previously shown to reduce neutrophil numbers in the lung (17). Compared with treatment with isotype control IgG, pIgR−/− mice treated with anti-Ly6G antibodies had a fivefold reduction in parenchymal neutrophil numbers (Figure 4A), as well as decreased neutrophil elastase and total elastase activity in lung lysates (Figures 4B–4D). These mice displayed a marked reduction in fibrotic remodeling around small airways and emphysema in the lung parenchyma as assessed by morphometric evaluation (Figures 4E and 4F).
Figure 4.
Neutrophil depletion in pIgR−/− mice blocks small airway wall fibrosis and emphysema. pIgR−/− mice were treated with anti-Ly6G or anti-IgG2a isotype control antibodies between 4 and 8 months of age. (A) Quantification of parenchymal neutrophil numbers after immunostaining lung sections with neutrophil elastase–specific antibodies; n = 8–9 mice/group; *P < 0.001 (t test). (B) Western blot and (C) densitometry for neutrophil elastase (NE; 26 kD) in lung tissue, normalized to β-actin; n = 6–7 mice/group; *P < 0.001 (t test). (D) Elastase activity in whole-lung lysates; n = 4–5 mice/group; *P < 0.05 (t test). (E) Morphometric analysis of small airway wall thickness (VVairway); n = 8–9 mice/group; *P < 0.01 (t test). (F) Morphometric analysis of emphysema (mean alveolar septal perimeter); n = 8–9 mice/group; *P < 0.0001 (t test).
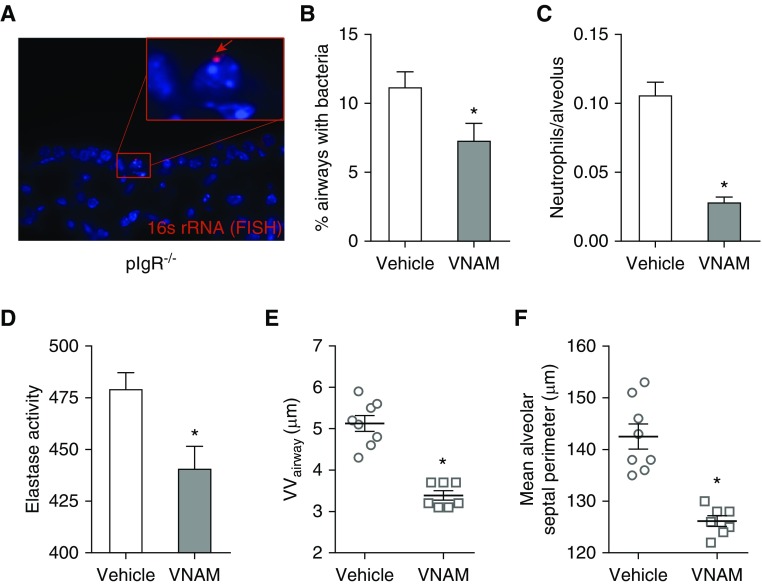
Next, we wondered whether airway bacteria were responsible for the neutrophil influx that contributes to small airway wall remodeling and emphysema in pIgR−/− mice. For these studies, we treated pIgR−/− mice for 3 months with a broad-spectrum antibiotic cocktail (VNAM) dissolved in drinking water, which has previously been shown to reduce endogenous bacterial flora in mice (19). pIgR−/− mice treated with the VNAM cocktail showed a reduction in the percentage of airways with bacterial invasion across epithelial barrier, indicating that the VNAM regimen partially depleted endogenous airway bacteria (Figures 5A and 5B). Reduced bacterial burden was associated with reduced numbers of neutrophils in the lung parenchyma and reduced total elastase activity (Figures 5C and 5D). Treatment with the VNAM cocktail led to significant reductions in small airway wall thickening and emphysema (Figures 5E and 5F). Together with our previous study (14), these data strongly implicate airway bacteria as a key driver of lung remodeling in this model.
Figure 5.
Treatment with broad-spectrum antibiotics inhibits small airway wall remodeling and emphysema in pIgR−/− mice. pIgR−/− mice received an antibiotics cocktail dissolved in drinking water (vancomycin, neomycin, ampicillin, and metronidazole [VNAM]) or regular drinking water only between 9 and 12 months of age. (A) Representative image of a bacterium invading the mucosa in a small airway from an untreated pIgR−/− mouse. The bacterium (red arrow) is labeled by a fluorescent in situ hybridization (FISH) probe targeting prokaryotic 16s rRNA. (B) Quantification of the percentage of airways/mouse with luminal bacteria visualized by FISH staining for bacterial 16s rRNA; n = 7–8 mice/group; *P < 0.05 (t test). (C) Quantification of neutrophil numbers in lung parenchyma after immunostaining with neutrophil elastase–specific antibodies; n = 7–8 mice/group; *P < 0.0001 (t test). (D) Elastase activity in whole-lung lysates; n = 6 mice/group; *P < 0.05 (t test). (E) Morphometric analysis of VVairway; n = 7–8 mice/group; *P < 0.0001 (t test). (F) Morphometric analysis of emphysema (mean alveolar septal perimeter); n = 7–8 mice/group; *P < 0.0001 (t test).
Exogenous Bacterial Lysates Exacerbate Small Airway Remodeling and Emphysema in pIgR−/− Mice through Increased Lung Inflammation
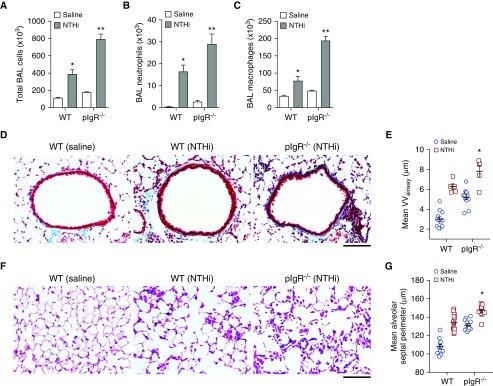
Patients with COPD may develop acute worsening of respiratory symptoms due to bacterial infection or acquisition of a new bacterial species (20, 21), and these exacerbations are associated with increased inflammation (22–25). To investigate whether loss of SIgA in the airways contributes to increased inflammation and lung remodeling in response to a bacterial challenge, we adapted a previously reported model using repetitive nebulization with nontypeable Haemophilus influenzae (NTHi) (26), which is the most common bacterium isolated during COPD exacerbations (27). We found that a single dose of nebulized NTHi lysate resulted in a significant accumulation of neutrophils and increased macrophages in BAL fluid after 24 hours in WT mice (Figures E3A and E3B). We then treated 2-month-old WT or pIgR−/− mice with NTHi lysate via nebulization once weekly for 16 weeks. After NTHi treatment, we observed significantly higher cell counts in BAL fluid, including both neutrophils and macrophages, from pIgR−/− mice compared with WT mice (Figures 6A–6C). As shown in Figures 6D–6G, NTHi treatment exacerbated fibrotic thickening of the small airway walls and caused emphysematous parenchymal destruction in both WT and pIgR−/− mice. Among all groups, emphysema was most severe in NTHi-treated pIgR−/− mice and small airway wall thickening tended to be most severe in NTHi-treated pIgR−/− mice (P = 0.05 compared with NTHi-treated WT mice).
Figure 6.
Increased lung inflammation in pIgR−/− mice treated with nontypeable Haemophilus influenzae (NTHi). WT and pIgR−/− mice were treated with NTHi lysates via nebulization once weekly from 2 to 6 months of age. (A–C) Total cells, neutrophils, and macrophages in BAL fluid in WT and pIgR−/− mice treated with NTHi or saline only, as indicated; n = 5–7 mice/group; *P < 0.05 compared with saline-treated WT mice; **P < 0.05 compared with all other groups (ANOVA). (D) Representative images of subepithelial collagen (stained blue) around the small airways of a saline-treated WT mouse or NTHi-treated WT or pIgR−/− mouse as indicated. Masson’s trichrome stain; scale bar: 50 μm. (E) Morphometric analysis of VVairway in WT and pIgR−/− mice as shown in D; n = 5–12 mice/group; *P = 0.05 compared with NTHi-treated WT mice and P < 0.0001 compared with all other groups (ANOVA). (F) Representative images of emphysema in the lungs of a saline-treated WT mouse or NTHi-treated WT or pIgR−/− mouse, as indicated. Hematoxylin and eosin; scale bar: 50 μm. (G) Morphometric analysis of emphysema (mean alveolar septal perimeter) in WT and pIgR−/− mice as shown in F; n = 11–12 mice/group; *P < 0.01 compared with all other groups (ANOVA).
Bacterial LPS is a major constituent of outer membrane of gram-negative bacteria, such as NTHi, and repetitive exposure to LPS has previously been shown to cause emphysema in mice (28). To determine whether bacterial products other than LPS could augment inflammatory responses and lung remodeling in pIgR−/− mice, we treated mice with lysates prepared from the nonpathogenic, gram-positive organism, Bacillus badius, once weekly for 16 weeks. In these studies, we found that pIgR−/− mice treated with B. badius lysates had higher levels of neutrophils in BAL and more severe airway wall fibrosis and emphysema than similarly treated WT mice (Figure E4). Together, these data indicate that exogenous bacterial challenge can further exacerbate small airway fibrosis and emphysema in pIgR−/− mice.
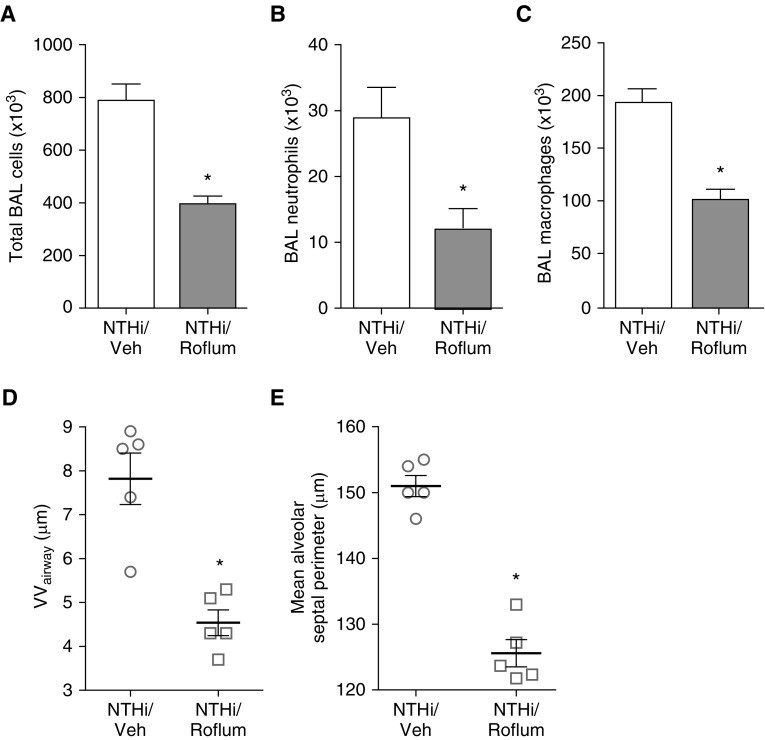
Roflumilast is a phosphodiesterase-4 inhibitor that has been shown to decrease neutrophilic inflammation in animal models of COPD (29), and can reduce exacerbations in patients with severe disease and symptoms of chronic bronchitis (30). To determine whether roflumilast protects pIgR−/− mice from NTHi-induced inflammation and lung remodeling, we treated pIgR−/− mice daily by oral gavage with 100 μg of roflumilast (5 μg/g) or vehicle (5% methylcellulose, 1.3% polyethylene glycol 400) for 4 months concurrent with weekly NTHi nebulization. Roflumilast treatment significantly reduced the number of BAL cells, both neutrophils and macrophages, in pIgR−/− mice after repetitive NTHi lysate treatment (Figures 7A–7C). Relative to animals treated with vehicle, roflumilast treatment resulted in significant reductions in both small airway wall fibrosis and emphysema (Figures 7D and 7E). These data indicate that exaggerated inflammation drives lung remodeling in pIgR−/− mice after repetitive exposure to bacterial products.
Figure 7.
Roflumilast treatment inhibits inflammation and blocks lung remodeling in pIgR−/− mice after repetitive exposure to NTHi lysate. pIgR−/− mice were treated by oral gavage with daily roflumilast or vehicle (Veh) concurrent with weekly NTHi nebulizations from 2 to 6 months of age. (A–C) Total cells, neutrophils, and macrophages in BAL fluid; n = 6–7 mice/group; *P < 0.05 (t test). (D) Morphometric analysis of small VVairway; n = 5 mice/group; *P < 0.01 (t test). (E) Morphometric analysis of emphysema (mean alveolar septal perimeter); n = 5 mice/group; *P < 0.0001.
Discussion
These studies demonstrate that, in a murine model of SIgA deficiency, neutrophils accumulate in the lung and promote injury and remodeling of small airways and alveolar parenchyma. Treatment with broad-spectrum antibiotics reduces numbers of neutrophils in the lungs and inhibits small airway and parenchymal remodeling, implicating airway bacteria as the primary driver of these COPD-like phenotypes in the lungs of pIgR−/− mice. Conversely, exposure to exogenous bacterial products from pathogenic and nonpathogenic bacteria amplifies inflammation and remodeling above that caused by endogenous bacteria. Treatment with the antiinflammatory drug, roflumilast, blocks inflammatory cell recruitment and remodeling in pIgR−/− mice, suggesting that neutrophil-derived proteases, rather than bacterial products per se, are the stimulus for small airway wall fibrosis and emphysema in these mice. Together, these data show that airway bacteria drive chronic neutrophilic inflammation and progressive lung destruction in pIgR−/− mice where the mucosal immune barrier is compromised.
Neutrophilic inflammation is a hallmark of COPD (1, 31–33), and neutrophils are known to contribute to lung destruction in cigarette smoke–exposed mice (8). This shows that neutrophils also accumulate in the lungs of pIgR−/− mice, which lack SIgA in the lung and develop small airway and parenchymal remodeling independent of cigarette smoke exposure (14). We found that increased numbers of neutrophils in the lungs of pIgR−/− mice are associated with decreased elastin content in small airways and the lung parenchyma, consistent with elastin degradation by neutrophil-derived proteases. In addition to elastin breakdown, we observed increased collagen deposition around the airways of pIgR−/− mice similar to the small airways of patients with COPD (1). In vivo neutrophil depletion using neutrophil-specific antibodies protected pIgR−/− mice from small airway remodeling and emphysema, providing direct evidence that neutrophils promote lung pathology in this model. Given that SIgA is known to be widespread in the airways of patients with COPD (2, 9, 10), our work suggests that loss of SIgA in the airways of former smokers with COPD drives chronic neutrophilic inflammation, protease–antiprotease imbalance, and ongoing injury and remodeling of small airways and alveolar tissue.
Under basal conditions in WT mice, we found robust expression of pIgR in the airway epithelium, but minimal expression of pIgR in the alveolar epithelium. This finding suggests that an impaired mucosal immunobarrier in the small airways initiates a pathologic cycle of inflammation and protease production that ultimately damages both the small airways and the lung parenchyma. These data provide a potential biologic explanation for the observation that small airways disease precedes the development of emphysema in many patients, and that emphysematous lung destruction is most severe near terminal bronchioles (34, 35). Although our studies suggest that neutrophils are a key mediator of lung damage in SIgA-deficient airways, these data do not exclude contributions from other cell types. Macrophages are also increased in the lungs of patients with COPD (1, 10), and elastase and matrix metalloproteinase production by these cells is known to contribute to emphysema in animal models (7, 36). Further investigation into a role for macrophages in lung destruction in pIgR−/− mice is warranted. In addition, loss of SIgA may activate the adaptive immune system, which could potentiate lung damage through direct cytolytic effects, by stimulating epithelial cell apoptosis or by augmenting innate immune responses.
Multiple studies have suggested that airway bacteria play a role in COPD progression (20, 37–39). We previously reported that loss of SIgA in small airways is associated with increased bacterial invasion into the airway mucosa, and that pIgR−/− mice raised in germ-free conditions are protected from the lung remodeling phenotype (10). Here, we show that broad-spectrum antibiotic treatment ameliorates neutrophilic inflammation and lung remodeling in pIgR−/− mice, providing additional evidence that airway bacteria drive lung remodeling in this model. Despite these findings, it is unlikely that long-term antibiotics would be effective as a therapeutic strategy in COPD. Expression of pIgR is regulated by the presence of airway bacteria (40, 41), and thus suppression of endogenous bacteria with antibiotics might paradoxically suppress pIgR expression and SIgA transcytosis. Furthermore, some bacteria are known to degrade SIgA (19, 42–44), and alterations in bacterial community structure induced by antibiotics could favor outgrowth of SIgA-degrading bacteria.
Exposure to NTHi or B. badius lysates resulted in more severe neutrophilic inflammation and lung remodeling in pIgR−/− mice. This finding indicates that loss of SIgA may result in an exaggerated inflammatory response to bacteria, consistent with observations in patients with COPD (45). Furthermore, these data may help explain the observation that frequent COPD exacerbations, which are often caused by bacteria (20), are associated with an accelerated rate of decline in forced expiratory volume in 1 second (37). Our studies, therefore, provide additional rationale for aggressive pharmacotherapy to reduce exacerbations, as recommended by current GOLD (Global Initiative for Chronic Obstructive Lung Disease) guidelines (46). In addition, we found that treatment with roflumilast could ameliorate neutrophilic inflammation and lung remodeling in pIgR−/− mice treated with NTHi lysates. Roflumilast is known to reduce numbers of inflammatory cells, including neutrophils and macrophages, in the lungs of cigarette smoke–exposed mice (29). Our study provides additional evidence supporting the use of this treatment to reduce exacerbations in patients with COPD.
Together, our data support the conclusion that loss of SIgA results in chronic neutrophilic inflammation that contributes to progressive small airway fibrosis and emphysema. In the future, strategies aimed at reducing chronic inflammation or restoring the normal SIgA immunobarrier in patients with COPD may be useful to slow disease progression.
Acknowledgments
Acknowledgment
The authors thank Pete Gulleman (Department of Pediatrics, Division of Pulmonary Medicine, Vanderbilt University School of Medicine, Nashville, TN) for his assistance with type II cell isolation and cDNA preparation.
Footnotes
This work was supported by National Institutes of Health National Institute of Child Health and Human Development grant 5 K12 HD 043483, National Heart, Lung, and Blood Institute (NHLBI) grants HL138088, HL1311906, and T32 HL094296, American Lung Association grant RT-309491, Department of Veterans Affairs Merit Review Award 1I01BX002378, and investigator-initiated Forest Pharmaceuticals grant DAL-IT-07.
Author Contributions: B.W.R., V.V.P., and T.S.B. designed the experiments and interpreted the data; B.W.R., Y.Z., and D.-S.C. performed in vivo treatments; R.-H.D., L.G., M.G., A.M., and V.V.P. performed histologic studies, including immunostaining; V.V.P. performed morphometry; R.-H.D. performed Western blot; W.H. performed flow cytometry; J.T.B. and R.v.d.M. performed tissue elastase measurements; B.W.R. and R.-H.D. isolated and cultured murine tracheal epithelial cells; B.W.R. performed qPCR; B.W.R., V.V.P., and T.S.B. drafted the manuscript; V.V.P. and T.S.B. cosupervised the project; all authors approved the final version of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2017-0329OC on January 9, 2018
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 2.Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP, et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;184:317–327. doi: 10.1164/rccm.201010-1629OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polosukhin VV, Richmond BW, Du RH, Cates JM, Wu P, Nian H, et al. Secretory IgA deficiency in individual small airways is associated with persistent inflammation and remodeling. Am J Respir Crit Care Med. 2017;195:1010–1021. doi: 10.1164/rccm.201604-0759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wright JL, Lawson LM, Pare PD, Wiggs BJ, Kennedy S, Hogg JC. Morphology of peripheral airways in current smokers and ex-smokers. Am Rev Respir Dis. 1983;127:474–477. doi: 10.1164/arrd.1983.127.4.474. [DOI] [PubMed] [Google Scholar]
- 5.Rutgers SR, Postma DS, ten Hacken NH, Kauffman HF, van Der Mark TW, Koëter GH, et al. Ongoing airway inflammation in patients with COPD who do not currently smoke. Thorax. 2000;55:12–18. doi: 10.1378/chest.117.5_suppl_1.262s. [DOI] [PubMed] [Google Scholar]
- 6.Willemse BW, ten Hacken NH, Rutgers B, Lesman-Leegte IG, Postma DS, Timens W. Effect of 1-year smoking cessation on airway inflammation in COPD and asymptomatic smokers. Eur Respir J. 2005;26:835–845. doi: 10.1183/09031936.05.00108904. [DOI] [PubMed] [Google Scholar]
- 7.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro SD, Goldstein NM, Houghton AM, Kobayashi DK, Kelley D, Belaaouaj A. Neutrophil elastase contributes to cigarette smoke–induced emphysema in mice. Am J Pathol. 2003;163:2329–2335. doi: 10.1016/S0002-9440(10)63589-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilette C, Godding V, Kiss R, Delos M, Verbeken E, Decaestecker C, et al. Reduced epithelial expression of secretory component in small airways correlates with airflow obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163:185–194. doi: 10.1164/ajrccm.163.1.9912137. [DOI] [PubMed] [Google Scholar]
- 10.Polosukhin VV, Richmond BW, Du RH, Cates JM, Wu P, Nian H, et al. SIgA deficiency in individual small airways is associated with persistent inflammation and remodeling. Am J Respir Crit Care Med. 2017;195:1010–1021. doi: 10.1164/rccm.201604-0759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantis NJ, Rol N, Corthésy B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 2011;4:603–611. doi: 10.1038/mi.2011.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pabst O. New concepts in the generation and functions of IgA. Nat Rev Immunol. 2012;12:821–832. doi: 10.1038/nri3322. [DOI] [PubMed] [Google Scholar]
- 13.Corthésy B. Role of secretory IgA in infection and maintenance of homeostasis. Autoimmun Rev. 2013;12:661–665. doi: 10.1016/j.autrev.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Richmond BW, Brucker RM, Han W, Du RH, Zhang Y, Cheng DS, et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun. 2016;7:11240. doi: 10.1038/ncomms11240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, et al. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–922. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsia CC, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLoed AG, Sherrill TP, Cheng DS, Han W, Saxon JA, Gleaves LA, et al. Neutrophil-derived IL-1β impairs the efficacy of NF-κB inhibitors against lung cancer. Cell Reports. 2016;16:120–132. doi: 10.1016/j.celrep.2016.05.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 19.Moon C, Baldridge MT, Wallace MA, D CA, Burnham, Virgin HW, et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 2015;521:90–93. doi: 10.1038/nature14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sethi S, Evans N, Grant BJ, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002;347:465–471. doi: 10.1056/NEJMoa012561. [DOI] [PubMed] [Google Scholar]
- 21.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–796. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhowmik A, Seemungal TA, Sapsford RJ, Wedzicha JA. Relation of sputum inflammatory markers to symptoms and lung function changes in COPD exacerbations. Thorax. 2000;55:114–120. doi: 10.1136/thorax.55.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiu Y, Zhu J, Bandi V, Atmar RL, Hattotuwa K, Guntupalli KK, et al. Biopsy neutrophilia, neutrophil chemokine and receptor gene expression in severe exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:968–975. doi: 10.1164/rccm.200208-794OC. [DOI] [PubMed] [Google Scholar]
- 24.Drost EM, Skwarski KM, Sauleda J, Soler N, Roca J, Agusti A, et al. Oxidative stress and airway inflammation in severe exacerbations of COPD. Thorax. 2005;60:293–300. doi: 10.1136/thx.2004.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hurst JR, Perera WR, Wilkinson TM, Donaldson GC, Wedzicha JA. Systemic and upper and lower airway inflammation at exacerbation of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2006;173:71–78. doi: 10.1164/rccm.200505-704OC. [DOI] [PubMed] [Google Scholar]
- 26.Monsó E, Ruiz J, Rosell A, Manterola J, Fiz J, Morera J, et al. Bacterial infection in chronic obstructive pulmonary disease: a study of stable and exacerbated outpatients using the protected specimen brush. Am J Respir Crit Care Med. 1995;152:1316–1320. doi: 10.1164/ajrccm.152.4.7551388. [DOI] [PubMed] [Google Scholar]
- 27.Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173:1114–1121. doi: 10.1164/rccm.200506-859OC. [DOI] [PubMed] [Google Scholar]
- 28.Brass DM, Hollingsworth JW, Cinque M, Li Z, Potts E, Toloza E, et al. Chronic LPS inhalation causes emphysema-like changes in mouse lung that are associated with apoptosis. Am J Respir Cell Mol Biol. 2008;39:584–590. doi: 10.1165/rcmb.2007-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martorana PA, Lunghi B, Lucattelli M, De Cunto G, Beume R, Lungarella G. Effect of roflumilast on inflammatory cells in the lungs of cigarette smoke–exposed mice. BMC Pulm Med. 2008;8:17. doi: 10.1186/1471-2466-8-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385:857–866. doi: 10.1016/S0140-6736(14)62410-7. [DOI] [PubMed] [Google Scholar]
- 31.Keatings VM, Collins PD, Scott DM, Barnes PJ. Differences in interleukin-8 and tumor necrosis factor-α in induced sputum from patients with chronic obstructive pulmonary disease or asthma. Am J Respir Crit Care Med. 1996;153:530–534. doi: 10.1164/ajrccm.153.2.8564092. [DOI] [PubMed] [Google Scholar]
- 32.Di Stefano A, Capelli A, Lusuardi M, Balbo P, Vecchio C, Maestrelli P, et al. Severity of airflow limitation is associated with severity of airway inflammation in smokers. Am J Respir Crit Care Med. 1998;158:1277–1285. doi: 10.1164/ajrccm.158.4.9802078. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell RA, Peebles C, Ward JA, Daraker A, Angco G, Broberg P, et al. Relationship between peripheral airway dysfunction, airway obstruction, and neutrophilic inflammation in COPD. Thorax. 2004;59:837–842. doi: 10.1136/thx.2003.019349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogg JC, Macklem PT, Thurlbeck WM. Site and nature of airway obstruction in chronic obstructive lung disease. N Engl J Med. 1968;278:1355–1360. doi: 10.1056/NEJM196806202782501. [DOI] [PubMed] [Google Scholar]
- 35.Hogg JC, McDonough JE, Suzuki M. Small airway obstruction in COPD: new insights based on micro-CT imaging and MRI imaging. Chest. 2013;143:1436–1443. doi: 10.1378/chest.12-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Churg A, Wang R, Wang X, Onnervik PO, Thim K, Wright JL. Effect of an MMP-9/MMP-12 inhibitor on smoke-induced emphysema and airway remodelling in guinea pigs. Thorax. 2007;62:706–713. doi: 10.1136/thx.2006.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–852. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson TM, Patel IS, Wilks M, Donaldson GC, Wedzicha JA. Airway bacterial load and FEV1 decline in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167:1090–1095. doi: 10.1164/rccm.200210-1179OC. [DOI] [PubMed] [Google Scholar]
- 39.Desai H, Eschberger K, Wrona C, Grove L, Agrawal A, Grant B, et al. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:303–309. doi: 10.1513/AnnalsATS.201310-350OC. [DOI] [PubMed] [Google Scholar]
- 40.Kaetzel CS. The polymeric immunoglobulin receptor: bridging innate and adaptive immune responses at mucosal surfaces. Immunol Rev. 2005;206:83–99. doi: 10.1111/j.0105-2896.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 41.Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol Lett. 2014;162:10–21. doi: 10.1016/j.imlet.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plaut AG, Gilbert JV, Artenstein MS, Capra JD. Neisseria gonorrhoeae and Neisseria meningitidis: extracellular enzyme cleaves human immunoglobulin A. Science. 1975;190:1103–1105. doi: 10.1126/science.810892. [DOI] [PubMed] [Google Scholar]
- 43.Plaut AG, Genco RJ, Tomasi TB., Jr Isolation of an enzyme from Streptococcus sanguis which specifically cleaves IgA. J Immunol. 1974;113:589–591. [PubMed] [Google Scholar]
- 44.Fujiyama Y, Kobayashi K, Senda S, Benno Y, Bamba T, Hosoda S. A novel IgA protease from Clostridium sp. capable of cleaving IgA1 and IgA2 A2m(1) but not IgA2 A2m(2) allotype paraproteins. J Immunol. 1985;134:573–576. [PubMed] [Google Scholar]
- 45.Bresser P, Out TA, van Alphen L, Jansen HM, Lutter R. Airway inflammation in nonobstructive and obstructive chronic bronchitis with chronic haemophilus influenzae airway infection. Comparison with noninfected patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:947–952. doi: 10.1164/ajrccm.162.3.9908103. [DOI] [PubMed] [Google Scholar]
- 46.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]