Abstract
In this article, the authors review the literature related to long-term outcome of pediatric moyamoya disease, focusing on late cerebrovascular events and social outcome of pediatric patients once they reach adulthood. Late-onset de novo hemorrhage is rare but more serious than recurrence of ischemic stroke. Long-term follow-up data on Asian populations suggest that the incidence of de novo hemorrhage might increase at age 20 or later, even more than 10 years after bypass surgery. Social adaptation difficulty, possibly related to cognitive impairment caused by frontal ischemia, continues in 10–20% of patients after they reach adulthood, even if no significant disability is present in daily life. A treatment strategy aimed at improving long-term outcome and careful follow-up might be required.
Keywords: moyamoya disease, pediatrics, follow-up studies, review
Introduction: Long-term Outcome of Pediatric Moyamoya Disease: It’s Time to Investigate
Do children with moyamoya disease enjoy a satisfactory social life once they reach adulthood? Are they able to study and work as well as their healthier counterparts? Do they enjoy long lives free from concerns about stroke after bypass surgery? All these questions are relevant to parents of a child with moyamoya disease. However, these questions, also important to clinicians,1) which have remained unanswered.
The time has come for researchers to address the issues of social outcome and late cerebrovascular events after adolescence in pediatric moyamoya disease. The first successful surgical treatment for moyamoya disease – superficial temporal artery (STA) to middle cerebral artery (MCA) anastomosis, also described as “direct bypass” – was reported in late 1970s by Karasawa et al.2) In the early 1980s, Matsushima et al.3) introduced an indirect bypass known as encephalo-duro-arterio-synangiosis (EDAS). It is only since the 1980s, therefore, that surgical treatment has commonly been applied to the disease. Many children who had undergone surgery at that time have been entering adulthood.
Indeed, some recent studies have accumulated more than a decade of follow-up data.4–8) Such extremely long-term data might provide important clues to solve the above questions. Accumulation of reliable data is not always easy, however, because of the heterogeneity of surgical procedures and loss of follow-up, both of which could introduce critical bias to the study results.
The purpose of the present article is to review literature on the long-term outcome of pediatric moyamoya disease, focusing on late cerebrovascular events and social outcome after patients have entered adulthood.
Late Cerebrovascular Events, Especially De Novo Hemorrhage
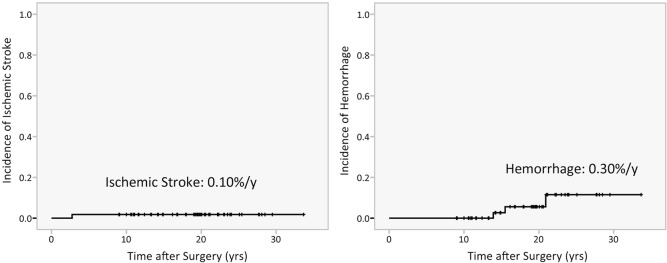
Bypass surgery seems to prevent ischemic symptoms, the most common symptoms in pediatric patients, for years. Follow-up studies in pediatric moyamoya disease demonstrate the eventual elimination of transient ischemic attacks (TIA) after surgery in 93–98% of patients.7,9–13) Recurrence of ischemic stroke is also rare; only a small proportion (0–6.3%) of patients experience late ischemic stroke (Table 1).4,6,8–10,14–18) In the authors’ data, only one case experienced ischemic stroke 2 years after surgery, the event occurring after craniotomy for a severe head injury from a traffic accident.8) The incidence of late-onset ischemic stroke in our data was as low as 0.10% per year (Fig. 1).
Table 1.
Incidence of late cerebrovascular events* after surgery in the literature of pediatric moyamoya disease, stratified by population size and length of follow-up
| Size† | F/u length‡ | Authors | Treatment | No. of cases | Mean F/u period (years) | F/u rate (%) | No. of late ischemic strokes (%) | No. of late hemorrhagic strokes (%) | Overall incidence of stroke (% per year) |
|---|---|---|---|---|---|---|---|---|---|
| Large | Extremely-long | Mukawa et al.6) | Indirect | 172 | 14.3 | 83 | 3 (1.7) | 3 (1.7) | 0.24 |
| Funaki et al.8)§ | Direct (combined) | 58 | 18.1 | 96.6 | 1 (1.7) | 3 (5.2) | 0.41 | ||
| Imaizumi et al.4) | Various | 25 | 18.8 | 80.6 | 1 (4.0) | 3 (12.0) | 0.85 | ||
| Large | Long | Bao et al.9) | Indirect | 288 | 4.4 | N/A | 8 (2.8) | 2 (0.7) | 9.00‖ |
| Scott et al.16) | Indirect | 126 | 5.1 | 99.2 | 4 (3.2) | 0 | NA | ||
| Kuroda et al.10) | Direct (combined) | 28 | 6.1 | N/A | 0 | 0 | 0 | ||
| Rashad et al.14) | Direct (combined) | 23 | 6.4 | 95.7 | 0 | 0 | 0 | ||
| Small | Extremely-long | Isono et al.18) | Indirect | 11 | 12.8 | N/A | 0 | 0 | 0 |
| Goda et al.17) | Indirect | 6 | 15.2 | N/A | 0 | 0 | 0 | ||
| Small | Long | Darvish et al.15) | Various | 16 | 7.3 | N/A | 1 (6.3) | 0 | N/A |
Excludes perioperative strokes,
“Large” indicates number of patients exceeds 20,
“Extremely long” indicates a mean F/u period exceeding 10 years,
The authors’ group,
Includes perioperative strokes.
Fig. 1.
Kaplan–Meier Curves showing incidence of late-onset ischemic stroke (left) and hemorrhage (right) following direct bypass surgery for pediatric moyamoya disease in the authors’ group.
De novo intracranial hemorrhage, a rather unique transformation from ischemic to hemorrhagic moyamoya disease, might be the more serious issue in adulthood, according to data on Asian populations.4,6,8) Hemorrhage is the factor most severely affecting outcomes in moyamoya disease and is often fatal.19) The incidence of such de novo hemorrhage is estimated at 0.30% per year after surgery according to the authors’ data, higher than that of late ischemic stroke (Fig. 1).7) The incidence is quite low, however, compared with the de novo hemorrhage rate in asymptomatic adult patients (2–3% per year);20,21) hence, surgery in childhood is probably beneficial for primary prevention of hemorrhage. Yet the risk of de novo intracranial hemorrhage cannot be underestimated, given that it increases with age.
The data support this hypothesis. As shown in Table 1, late onset hemorrhagic events were reported in all three extremely long-term follow-up studies, the follow-up period of which exceeds 10 years.4,6,8) Two of these studies described the initial presentation at disease onset,4,8) and all late-onset hemorrhages included in the two studies occurred in the patients who had not initially presented with intracranial hemorrhage at disease onset. We calculated the frequency of late cerebrovascular events using data pooled from previous studies4,6,8–10,14–18) and stratified by the length of study period (Fig. 2). More hemorrhages are observed in studies exceeding 10 years than in those of less than 10 years. In the authors’ data, all de novo hemorrhages occurred more than 10 years after bypass surgery (Fig. 1). This finding is similar to other extremely long-term follow-up studies.4,6) De novo hemorrhage tends to occur at age 20 or later;8) in authors’ series, all hemorrhages occurred in patients in their 20s and 30s. This is consistent with epidemiological findings that hemorrhagic presentation in the disease increases at age 20.22)
Fig. 2.
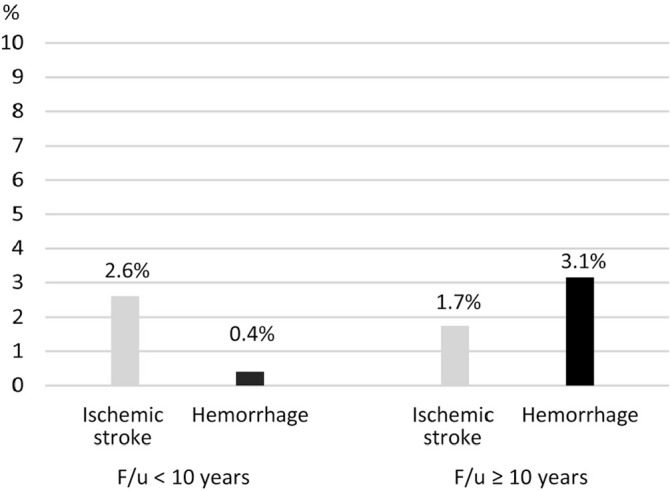
Graph showing frequency of late cerebrovascular events (ischemic stroke and hemorrhage) in pediatric moyamoya disease using data pooled from previous studies,4,6,8–10,14–18) stratified by length of study period.
Long-term Management Focusing on De Novo Hemorrhage
The above findings suggest that the pediatric patients should be monitored carefully for more than 10 years after surgery in light of the risk of de novo hemorrhage after adulthood. Follow-up for a period of less than 10 years might underestimate the risk (Table 1).9,10,13–16,23) For female patients in particular, an examination might be recommended before pregnancy considering the risk of intracranial hemorrhage during pregnancy and delivery, although most patients diagnosed with moyamoya disease are able to give birth safely when carefully managed.24)
Antiplatelet agents are often administrated in medical treatment and perioperative management of pediatric moyamoya disease.14,22,25) These agents should be discontinued when possible before adulthood in light of their adverse effect on hemorrhage. In the authors’ data, administration of antiplatelet agents was discontinued after surgery in 92.7% of pediatric patients before adulthood. Risk factor control, especially blood pressure management, is also important in preventing de novo hemorrhage. Hypertension can occur even in young patients because renal artery stenosis can accompany moyamoya disease.26) Lifestyle advice, including efforts to discourage smoking in adulthood, might also be advisable to prevent de novo hemorrhage.
The mechanism of de novo hemorrhage remains unclear. In our cases of de novo hemorrhage, collaterals from the choroidal artery or thalamic perforators had been identified before hemorrhage despite the good patency of the bypass.8) Recent studies on hemorrhagic moyamoya disease suggest that such collaterals are at higher risk of rupture than are moyamoya vessels.27) Further studies focusing on angiographic risk factors of de novo hemorrhage might be required.
Social Outcome after Adolescence
While most pediatric patients are capable of independent daily activity for a long time after surgery,6,7,14,16) recent studies have revealed that 10–20% of patients have difficulty with social adaptation after adolescence. Nakashima et al.28) reported that about 10% of patients had severe difficulty in social or school life because of intellectual impairment. Miyamoto et al.1) reported in their questionnaire-based study that 13.3% of patients had difficulty carrying on an independent social life because of minor intellectual delays, although the study included both pediatric and adult patients. Phi et al.5) also conducted a long-term follow-up study with questionnaire and reported that the prevalence of pediatric patients who eventually entered college or university was 80% and that 62% of patients found employment in adulthood. They also reported, however, 20% of the responders were dissatisfied with their treatment outcomes. In the authors’ study, 17.9% of pediatric patients with moyamoya disease continued to suffer from social adaptation difficulties, such as difficulty with regular school attendance or obtaining regular employment.7)
Several risk factors associated with unfavorable social or functional outcome have been reported to date4,5,7,12,13,29–32) (Table 2). Among them, preoperative neurological deficit, preoperative infarct, involvement of the posterior cerebral artery, and indirect bypass with small craniotomy have been identified as risk factors through multivariate analyses.5,7,12) Preoperative neurological impairment and major stroke are probably the strongest factors associated with unfavorable social outcome.4,5,7,13,30,32) On the other hand, a recent study demonstrates that some TIA-type pediatric patients, exhibiting no focal intracranial lesion, have specific cognitive impairments.33) Several studies on adult patients support this finding. Karzmark et al.34) demonstrated that executive functioning, classified as frontal lobe function, could be impaired in the absence of ischemic stroke. The study of the authors’ group also demonstrated that those suffering social adaptation difficulty with minimum brain lesion showed significantly lower scores in several frontal lobe assessment tasks.35) Nakagawara et al.36) demonstrated that significant cortical neuron loss, detected with [123I]-iomazenil single photon emission computed tomography, had occurred in the frontal lobes of patients with higher brain dysfunction.
Table 2.
Risk factors associated with unfavorable social or functional outcome in pediatric moyamoya disease
| Type | Factor |
|---|---|
| Unchangeable risk factors | Preoperative neurological impairment4,5,32)* Preoperative major stroke/infarct2,7,30)* |
| Indicator of early surgical intervention | Onset at younger age2,4) Posterior cerebral artery involvement7)* Homozygous RNF213 p.R4810K variant29) |
| Changeable risk factors | Longer duration after onset without surgery31) Small craniotomy12)* |
Statistically significant in multivariate analyses.
These results reasonably lead to the hypothesis that longstanding frontal ischemia since childhood can cause impairment of cognitive function, resulting in unfavorable social outcome in adulthood. Although attractive, this hypothesis remains to be tested. The correlation between cognitive dysfunction and frontal ischemia has rarely been addressed, and it is a subject of controversy. Calviere et al.37) demonstrated that dysexecutive cognitive syndrome is related to the frontal cerebrovascular reserve measured with perfusion magnetic resonance imaging, although their study included only 10 adult patients. They also demonstrated that elevation of the apparent diffusion coefficient in normal-appearing frontal white matter of adult patients was associated with executive dysfunction.38) On the other hand, Mogensen et al.39) demonstrated that similar executive dysfunction was associated with a posterior, not anterior, stroke. Recent studies using resting state functional magnetic resonance imaging reveal that patients with cognitive and executive dysfunction exhibit a disrupted functional connectivity network.40–42)
Social Outcome: How Can It Be Managed?
Elimination of known risk factors associated with unfavorable social outcome is a reasonable preventive approach, although not all factors are changeable (Table 2). Younger age at onset4,13) – especially age under 3 years – and posterior cerebral artery involvement7) are considered indicators of early surgical intervention, because patients with these factors are at high risk of future unfavorable social outcome.
Kuroda et al reported that direct bypass with a large craniotomy extending to the frontal area was associated with better intellectual outcome.12) Bypass surgery targeting the frontal area supplied by the anterior cerebral artery (ACA) seems an attractive approach for improving social outcome, although the reason for this is not completely clear. Several bypass procedures targeting the ACA territory have been reported to date, and these are classified into four types: simultaneous indirect revascularization of both ACA and MCA territories;43–45) simultaneous direct and indirect revascularization of the MCA territory and ACA territory, respectively (or vice versa);10,46) simultaneous direct revascularization of both ACA and MCA territories;47) and additional direct revascularization of the ACA territory after conventional STA–MCA anastomosis.48) As the number of patients included in these series was relatively small, no evidence exists regarding indication and optimal procedure for the ACA territory bypass. Controversy might also arise regarding the universal strategy for targeting the ACA territory; a recent study revealed hemodynamic improvement of the ACA territory after conventional MCA territory bypass alone.49) Whether bypass for the ACA territory is beneficial to cognitive function should be validated in further studies.
For children suffering from neurological or cognitive dysfunction due to pre-existing infarct, educational support addressing the cognitive characteristics of each patient and cooperation between hospital and school staff might also be made mandatory for improving quality of life.50)
Conclusion
Recent long-term follow-up studies of pediatric moyamoya disease have revealed two salient issues for patients who have reached adulthood: late-onset de novo intracranial hemorrhage and social adaptation difficulty. The risk of de novo hemorrhage might increase after age 20 or later, even more than 10 years after bypass surgery, according to data on Asian populations. Careful long-term follow-up and management focusing on hemorrhage prevention in adulthood might be required. Social adaptation difficulty, possibly related to cognitive impairment caused by frontal ischemia, continues in 10–20% of patients after they reach adulthood, even if they do not have significant disability in daily life. Early surgical intervention might be considered, especially for patients at high risk of unfavorable social outcome. Although bypass procedures targeting the ACA territory are an attractive approach for preventing unfavorable social outcome, the benefit should be validated in further studies.
Acknowledgment
This work was supported by JSPS KAKENHI Grant Number JP 26861147.
Footnotes
Conflicts of Interest Disclosure
All authors have no conflict of interest with regard to the article. All authors have registered online Self-reported COI Disclosure Statement Forms through the website for JNS members.
References
- 1). Miyamoto S, Akiyama Y, Nagata I, et al. : Long-term outcome after STA–MCA anastomosis for moyamoya disease. Neurosurg Focus 5: e5, 1998. [DOI] [PubMed] [Google Scholar]
- 2). Karasawa J, Kikuchi H, Furuse S, Kawamura J, Sakaki T: Treatment of moyamoya disease with STA-MCA anastomosis. J Neurosurg 49: 679– 688, 1978. [DOI] [PubMed] [Google Scholar]
- 3). Matsushima Y, Fukai N, Tanaka K, et al. : A new surgical treatment of moyamoya disease in children: a preliminary report. Surg Neurol 15: 313– 320, 1981. [DOI] [PubMed] [Google Scholar]
- 4). Imaizumi T, Hayashi K, Saito K, Osawa M, Fukuyama Y: Long-term outcomes of pediatric moyamoya disease monitored to adulthood. Pediatr Neurol 18: 321– 325, 1998. [DOI] [PubMed] [Google Scholar]
- 5). Phi JH, Wang KC, Cho BK, et al. : Long-term social outcome in children with moyamoya disease who have reached adulthood. J Neurosurg Pediatr 8: 303– 309, 2011. [DOI] [PubMed] [Google Scholar]
- 6). Mukawa M, Nariai T, Matsushima Y, et al. : Long-term follow-up of surgically treated juvenile patients with moyamoya disease. J Neurosurg Pediatr 10: 451– 456, 2012. [DOI] [PubMed] [Google Scholar]
- 7). Funaki T, Takahashi JC, Takagi Y, et al. : Impact of posterior cerebral artery involvement on long-term clinical and social outcome of pediatric moyamoya disease. J Neurosurg Pediatr 12: 626– 632, 2013. [DOI] [PubMed] [Google Scholar]
- 8). Funaki T, Takahashi JC, Takagi Y, et al. : Incidence of late cerebrovascular events after direct bypass among children with moyamoya disease: a descriptive longitudinal study at a single center. Acta Neurochir (Wien) 156: 551– 559; discussion 559, 2014. [DOI] [PubMed] [Google Scholar]
- 9). Bao XY, Duan L, Yang WZ, et al. : Clinical features, surgical treatment, and long-term outcome in pediatric patients with moyamoya disease in China. Cerebrovasc Dis 39: 75– 81, 2015. [DOI] [PubMed] [Google Scholar]
- 10). Kuroda S, Houkin K, Ishikawa T, Nakayama N, Iwasaki Y: Novel bypass surgery for moyamoya disease using pericranial flap: its impacts on cerebral hemodynamics and long-term outcome. Neurosurgery 66: 1093– 1101; discussion 1101, 2010. [DOI] [PubMed] [Google Scholar]
- 11). Kim SK, Cho BK, Phi JH, et al. : Pediatric moyamoya disease: an analysis of 410 consecutive cases. Ann Neurol 68: 92– 101, 2010. [DOI] [PubMed] [Google Scholar]
- 12). Kuroda S, Houkin K, Ishikawa T, et al. : Determinants of intellectual outcome after surgical revascularization in pediatric moyamoya disease: a multivariate analysis. Childs Nerv Syst 20: 302– 308, 2004. [DOI] [PubMed] [Google Scholar]
- 13). Karasawa J, Touho H, Ohnishi H, Miyamoto S, Kikuchi H: Long-term follow-up study after extracranial-intracranial bypass surgery for anterior circulation ischemia in childhood moyamoya disease. J Neurosurg 77: 84– 89, 1992. [DOI] [PubMed] [Google Scholar]
- 14). Rashad S, Fujimura M, Niizuma K, Endo H, Tominaga T: Long-term follow-up of pediatric moyamoya disease treated by combined direct-indirect revascularization surgery: single institute experience with surgical and perioperative management. Neurosurg Rev 39: 615– 623, 2016. [DOI] [PubMed] [Google Scholar]
- 15). Darwish B, Besser M: Long term outcome in children with Moyamoya disease: experience with 16 patients. J Clin Neurosci 12: 873– 877, 2005. [DOI] [PubMed] [Google Scholar]
- 16). Scott RM, Smith JL, Robertson RL, Madsen JR, Soriano SG, Rockoff MA: Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 100: 142– 149, 2004. [DOI] [PubMed] [Google Scholar]
- 17). Goda M, Isono M, Ishii K, Kamida T, Abe T, Kobayashi H: Long-term effects of indirect bypass surgery on collateral vessel formation in pediatric moyamoya disease. J Neurosurg 100: 156– 162, 2004. [DOI] [PubMed] [Google Scholar]
- 18). Isono M, Ishii K, Kamida T, Inoue R, Fujiki M, Kobayashi H: Long-term outcomes of pediatric moyamoya disease treated by encephalo-duro-arterio-synangiosis. Pediatr Neurosurg 36: 14– 21, 2002. [DOI] [PubMed] [Google Scholar]
- 19). Han DH, Kwon OK, Byun BJ, et al. : A co-operative study: clinical characteristics of 334 Korean patients with moyamoya disease treated at neurosurgical institutes (1976–1994). The Korean Society for Cerebrovascular Disease. Acta Neurochir (Wien) 142: 1263– 1273; discussion 1273–1274, 2000. [DOI] [PubMed] [Google Scholar]
- 20). Cho WS, Chung YS, Kim JE, et al. : The natural clinical course of hemodynamically stable adult moyamoya disease. J Neurosurg 122: 82– 89, 2015. [DOI] [PubMed] [Google Scholar]
- 21). Kuroda S, Hashimoto N, Yoshimoto T, Iwasaki Y, Research Committee on Moyamoya Disease in Japan : Radiological findings, clinical course, and outcome in asymptomatic moyamoya disease: results of multicenter survey in Japan. Stroke 38: 1430– 1435, 2007. [DOI] [PubMed] [Google Scholar]
- 22). Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis. Health Labour Sciences Research Grant for Research on Measures for Intractable Diseases : Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurol Med Chir (Tokyo) 52: 245– 266, 2012. [DOI] [PubMed] [Google Scholar]
- 23). Tatli B, Ekici B, Sencer A, et al. : Clinical features, prothrombotic risk factors, and long-term follow-up of eight pediatric Moyamoya patients. J Clin Neurol 8: 100– 103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Takahashi JC, Ikeda T, Iihara K, Miyamoto S: Pregnancy and delivery in moyamoya disease: results of a nationwide survey in Japan. Neurol Med Chir (Tokyo) 52: 304– 310, 2012. [DOI] [PubMed] [Google Scholar]
- 25). Zhao Y, Zhang Q, Zhang D, Zhao Y: Effect of aspirin in postoperative management of adult ischemic moyamoya disease. World Neurosurg 105: 728– 731, 2017. [DOI] [PubMed] [Google Scholar]
- 26). Yamada I, Himeno Y, Matsushima Y, Shibuya H: Renal artery lesions in patients with moyamoya disease: angiographic findings. Stroke 31: 733– 737, 2000. [DOI] [PubMed] [Google Scholar]
- 27). Funaki T, Takahashi JC, Houkin K, et al. JAM Trial Investigators : Angiographic features of hemorrhagic moyamoya disease with high recurrence risk: a supplementary analysis of the Japan adult moyamoya trial. J Neurosurg 128: 777– 784, 2018. [DOI] [PubMed] [Google Scholar]
- 28). Nakashima H, Meguro T, Kawada S, Hirotsune N, Ohmoto T: Long-term results of surgically treated moyamoya disease. Clin Neurol Neurosurg 99 Suppl 2: S156– S161, 1997. [DOI] [PubMed] [Google Scholar]
- 29). Kim EH, Yum MS, Ra YS, et al. : Importance of RNF213 polymorphism on clinical features and long-term outcome in moyamoya disease. J Neurosurg 124: 1221– 1227, 2016. [DOI] [PubMed] [Google Scholar]
- 30). Kim SK, Seol HJ, Cho BK, Hwang YS, Lee DS, Wang KC: Moyamoya disease among young patients: its aggressive clinical course and the role of active surgical treatment. Neurosurgery 54: 840– 844; discussion 844–846, 2004. [DOI] [PubMed] [Google Scholar]
- 31). Imaizumi C, Imaizumi T, Osawa M, Fukuyama Y, Takeshita M: Serial intelligence test scores in pediatric moyamoya disease. Neuropediatrics 30: 294– 299, 1999. [DOI] [PubMed] [Google Scholar]
- 32). Matsushima Y, Aoyagi M, Nariai T, Takada Y, Hirakawa K: Long-term intelligence outcome of post-encephalo-duro-arterio-synangiosis childhood moyamoya patients. Clin Neurol Neurosurg 99 Suppl 2: S147– S150, 1997. [DOI] [PubMed] [Google Scholar]
- 33). Hsu YH, Kuo MF, Hua MS, Yang CC: Selective neuropsychological impairments and related clinical factors in children with moyamoya disease of the transient ischemic attack type. Childs Nerv Syst 30: 441– 447, 2014. [DOI] [PubMed] [Google Scholar]
- 34). Karzmark P, Zeifert PD, Bell-Stephens TE, Steinberg GK, Dorfman LJ: Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery 70: 634– 638, 2012. [DOI] [PubMed] [Google Scholar]
- 35). Araki Y, Takagi Y, Ueda K, et al. : Cognitive function of patients with adult moyamoya disease. J Stroke Cerebrovasc Dis 23: 1789– 1794, 2014. [DOI] [PubMed] [Google Scholar]
- 36). Nakagawara J, Osato T, Kamiyama K, et al. : Diagnostic imaging of higher brain dysfunction in patients with adult moyamoya disease using statistical imaging analysis for [123I]iomazenil single photon emission computed tomography. Neurol Med Chir (Tokyo) 52: 318– 326, 2012. [DOI] [PubMed] [Google Scholar]
- 37). Calviere L, Catalaa I, Marlats F, et al. : Correlation between cognitive impairment and cerebral hemodynamic disturbances on perfusion magnetic resonance imaging in European adults with moyamoya disease. Clinical article. J Neurosurg 113: 753– 759, 2010. [DOI] [PubMed] [Google Scholar]
- 38). Calviere L, Ssi Yan Kai G, Catalaa I, Marlats F, Bonneville F, Larrue V: Executive dysfunction in adults with moyamoya disease is associated with increased diffusion in frontal white matter. J Neurol Neurosurg Psychiatry 83: 591– 593, 2012. [DOI] [PubMed] [Google Scholar]
- 39). Mogensen MA, Karzmark P, Zeifert PD, et al. : Neuroradiologic correlates of cognitive impairment in adult Moyamoya disease. AJNR Am J Neuroradiol 33: 721– 725, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Sakamoto Y, Okamoto S, Maesawa S, et al. : Default mode network changes in moyamoya disease before and after bypass surgery: preliminary report. World Neurosurg 112: e652– e661, 2018. [DOI] [PubMed] [Google Scholar]
- 41). Kazumata K, Tha KK, Narita H, et al. : Investigating brain network characteristics interrupted by covert white matter injury in patients with moyamoya disease: insights from graph theoretical analysis. World Neurosurg 89: 654– 665. e2, 2016. [DOI] [PubMed] [Google Scholar]
- 42). Christen T, Jahanian H, Ni WW, Qiu D, Moseley ME, Zaharchuk G: Noncontrast mapping of arterial delay and functional connectivity using resting-state functional MRI: a study in moyamoya patients. J Magn Reson Imaging 41: 424– 430, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43). Matsushima T, Inoue T, Ikezaki K, et al. : Multiple combined indirect procedure for the surgical treatment of children with moyamoya disease. A comparison with single indirect anastomosis and direct anastomosis. Neurosurg Focus 5: e4, 1998. [DOI] [PubMed] [Google Scholar]
- 44). Matsushima T, Inoue TK, Suzuki SO, et al. : Surgical techniques and the results of a fronto-temporo-parietal combined indirect bypass procedure for children with moyamoya disease: a comparison with the results of encephalo-duro-arterio-synangiosis alone. Clin Neurol Neurosurg 99 Suppl 2: S123– S127, 1997. [DOI] [PubMed] [Google Scholar]
- 45). Kinugasa K, Mandai S, Kamata I, Sugiu K, Ohmoto T: Surgical treatment of moyamoya disease: operative technique for encephalo-duro-arterio-myo-synangiosis, its follow-up, clinical results, and angiograms. Neurosurgery 32: 527– 531, 1993. [DOI] [PubMed] [Google Scholar]
- 46). Houkin K, Kamiyama H, Takahashi A, Kuroda S, Abe H: Combined revascularization surgery for childhood moyamoya disease: STA-MCA and encephalo-duro-arterio-myo-synangiosis. Childs Nerv Syst 13: 24– 29, 1997. [DOI] [PubMed] [Google Scholar]
- 47). Ishikawa T, Kamiyama H, Kuroda S, Yasuda H, Nakayama N, Takizawa K: Simultaneous superficial temporal artery to middle cerebral or anterior cerebral artery bypass with pan-synangiosis for moyamoya disease covering both anterior and middle cerebral artery territories. Neurol Med Chir (Tokyo) 46: 462– 468, 2006. [DOI] [PubMed] [Google Scholar]
- 48). Iwama T, Hashimoto N, Miyake H, Yonekawa Y: Direct revascularization to the anterior cerebral artery territory in patients with moyamoya disease: report of five cases. Neurosurgery 42: 1157– 1161; discussion 1161–1162, 1998. [DOI] [PubMed] [Google Scholar]
- 49). Cho WS, Kim JE, Paeng JC, et al. : Can combined bypass surgery at middle cerebral artery territory save anterior cerebral artery territory in adult moyamoya disease? Neurosurgery 80: 431– 438, 2017. [DOI] [PubMed] [Google Scholar]
- 50). Aihara Y, Komatsu K, Chiba K, Yamaguchi K, Okada Y, Kawamata T: Clinical features of magnetic resonance imaging and angiography image findings and cognitive dysfunction in pediatric moyamoya disease: psychological evaluation based on cognitive function examination. Nerv Syst Child 42: 355– 363, 2017. (in Japanese) [Google Scholar]