Abstract
This review argues for a central role of the lateral hypothalamus in those deviant forms of aggression, which result from chronic glucocorticoid deficiency. Currently, this nucleus is considered a key region of the mechanisms that control predatory aggression. However, recent findings demonstrate that it is strongly activated by aggression in subjects with a chronically downregulated hypothalamus-pituitary-adrenocortical (HPA) axis; moreover, this activation is causally involved in the emergence of violent aggression. The review has two parts. In the first part, we review human findings demonstrating that under certain conditions, strong stressors downregulate the HPA-axis on the long run, and that the resulting glucocorticoid deficiency is associated with violent aggression including aggressive delinquency and aggression-related psychopathologies. The second part addresses neural mechanisms in animals. We show that the experimental downregulation of HPA-axis function elicits violent aggression in rodents, and the activation of the brain circuitry that originally subserves predatory aggression accompanies this change. The lateral hypothalamus is not only an integral part of this circuitry, but can elicit deviant and violent forms of aggression. Finally, we formulate a hypothesis on the pathway that connects unfavorable social conditions to violent aggression via the neural circuitry that includes the lateral hypothalamus.
Keywords: violence, aggression, hypothalamus, humans, rodents
Introduction
Early studies by Hess (1928) attributed the hypothalamus a central role in aggression control, by showing that the electrical stimulation of particular hypothalamic sites rapidly induces biting attacks. This methodology contributed to aggression research by delimitating aggression-related hypothalamic regions, and by elucidating the major components of aggression-related neural networks (for reviews see Kruk, 1991; Siegel et al., 1999). Initially, hypothalamic regions involved in attack behavior were divided into two separate mechanisms. Mediobasal aspects of the hypothalamus (the mediobasal hypothalamus in cats, the hypothalamic attack area in rats, and the ventrolateral part of the ventromedial hypothalamus in mice) were shown to control intraspecific aggression. Albeit disparate early studies suggested that the electric stimulation of the lateral hypothalamus might also promote intraspecific aggression (Woodworth, 1971; Koolhaas, 1978), the effect remained somewhat elusive and occurred at rather large current intensities. Consequently, the consensus is that the lateral hypothalamus (in both cats and rats) controls predatory aggression (Smith et al., 1970; Kruk et al., 1979; Shaikh et al., 1987; Lin et al., 2011). In all the species studied so far (including humans) hypothalamic areas located medioventrally to the fornix were associated with intraspecific aggression, whereas hypothalamic areas located laterally to the fornix were shown to control interspecific (predatory) aggression (Haller, 2013).
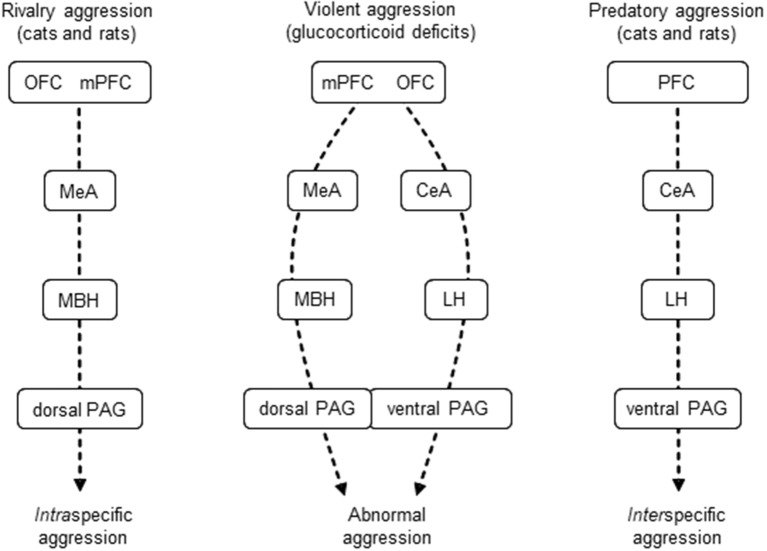
Our recent studies, however, indicated that the lateral hypothalamus is involved also in the control of intraspecific aggression, particularly in its violent forms. We found that the lateral hypothalamus was strongly activated during resident-intruder conflicts, but only if subjects were submitted to the glucocorticoid hypofunction model of abnormal aggression (Tulogdi et al., 2010, 2015). In this model of abnormal aggression, rats attack vulnerable body parts of opponents (head, throat band belly) without appropriately signaling attack intentions by social signals. We also showed that a subpopulation of prefrontal neurons specifically project to, and influence violent aggression controlled by the lateral hypothalamus (Biro et al., 2017, 2018). We recently proposed that the control mechanisms of violent forms of aggression are combinations of the mechanisms that subserve intraspecific (rivalry) and interspecific (predatory) aggressions (Haller, 2017; Figure 1).
Figure 1.
Mechanisms of glucocorticoid deficit-induced aggression are combinations of those subserving intraspecific and predatory aggressions. For explanations, see section Introduction. Dashed arrows indicate hypothetical information flow. CeA, central amygdala; LH, lateral hypothalamus; MBH, mediobasal hypothalamus (hypothalamic attack area); MeA, medial amygdala; mPFC, medial prefrontal cortex; OFC, orbitofrontal cortex; PAG, periaqueductal gray. Dashed arrows, hypothetical flow of information.
The present review argues in favor of the role of the lateral hypothalamus in violent aggression, specifically in aggression associated with glucocorticoid deficits. The first part of the review is devoted to the role of glucocorticoid deficits in human aggression. The ultimate aim of this section is to clarify the translational value of the mechanisms discussed in the second part of the review. This latter section will in fact provide explanations for, and arguments supporting the mechanisms schematically represented in Figure 1. We suggest that glucocorticoid deficits are important contributors of deviant aggression in humans, and that animal findings with translational value suggest that the lateral hypothalamus has an important role in mediating the violence-related roles of glucocorticoid deficits.
Glucocorticoid deficits and aggression in humans
Virkunnen reported in 1985 that cortisol secretion by habitually violent offenders with antisocial personality disorder was considerably lower than that of all three antisocial personality disordered subjects without habitual aggression, recidivist arsonists, and male clinic personnel. This report was published at a time when current thought associated aggression with increased rather than decreased stress responses (Posner and Conway, 1981; Public Health Report, 1983; Mason and Blankenship, 1987; Susman et al., 1988). Not surprisingly, reference to the now classical work by Virkkunen (1985) had a slow start. The first independent citation (a review) was published 4 years later (Zuckerman, 1989), and it required about a decade till the first confirmatory studies were published (Vanyukov et al., 1993; van Goozen et al., 1998; McBurnett et al., 2000; Dolan et al., 2001; Pajer et al., 2001; Kariyawasam et al., 2002). More than a decade elapsed until the first laboratory model of the condition was developed (Haller et al., 2001, 2004), albeit associations between low glucocorticoid levels and aggression were observed earlier (Poole and Brain, 1974).
Taken together, these findings suggested that certain types of human aggression and aggression-related psychopathologies are associated with downregulated hypothalamus- pituitary-adrenocortical (HPA) axis function, and that mimicking this condition in rodents leads to the development of abnormal forms of aggression (Haller, 2014). Several authors questioned the assumption that low glucocorticoid levels are associated with aggression problems (Klimes-Dougan et al., 2001; von der Pahlen, 2005; Marsman et al., 2008), and indeed, findings are often contradictory, especially in children diagnosed with various aggression-related psychopathologies (Table 1). Nevertheless, the findings presented in Table 1 clearly demonstrate that such psychopathologies are associated with downregulated HPA-axis function in certain study populations at least, which raises the question of how such conditions develop.
Table 1.
Cross sectional studies in children: associations between aggression-related psychological conditions and cortisol plasma levels.
| Condition | Cortisol measurement | Association | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary | Secondary | Single | Multiple | Diurnal | Total | Awakening | Stress response | ||
| CD | X | ↑ | van Bokhoven et al., 2005 | ||||||
| DIS | X | → | Scerbo and Kolko, 1994 | ||||||
| ADHD | ODD | X | ↓ | Kariyawasam et al., 2002 | |||||
| CD | X | ↓ | Vanyukov et al., 1993 | ||||||
| CD | CU | X | ↓ | Loney et al., 2006 | |||||
| CD | ODD, AGG | X | ↓ | Oosterlaan et al., 2005 | |||||
| CD | X | ↓ | Pajer et al., 2001 | ||||||
| CD | X | ↓ | Pajer et al., 2006 | ||||||
| DIS | ANX | X | X | ↓** | Schoorl et al., 2016 | ||||
| CD | X | → | Fairchild et al., 2008 | ||||||
| UNR | AGG | X | → | Van den Bergh et al., 2008 | |||||
| UNR | AGG | X | ↓ | Oberle et al., 2017 | |||||
| UNR | EXT | X | ↓ | Martin et al., 2014 | |||||
| DIS | X | → | Kruesi et al., 1989 | ||||||
| UNR | EXT | X | ↓ | Puetz et al., 2017 | |||||
| UNR | EXT | X | ↑ | Marsman et al., 2008 | |||||
| UNR | EXT | X | → | Klimes-Dougan et al., 2001 | |||||
| CD | CU | X | ↓ | von Polier et al., 2013 | |||||
| UNR | EXT | X | ↓ | Cicchetti and Rogosch, 2001 | |||||
| DIS | AGG | X | ↓ | Van de Wiel et al., 2004 | |||||
| UNR | EXT | X | ↓ | Puetz et al., 2016 | |||||
| DIS | X | ↑‡ | McBurnett et al., 2005 | ||||||
| ADHD | X | ↓‡ | Pesonen et al., 2011 | ||||||
| ADHD | CD | X | ↓* | Northover et al., 2016 | |||||
| CD | X | ↓† | Yang et al., 2007 | ||||||
| DIS | X | ↓ | van Goozen et al., 1998 | ||||||
| DIS | X | ↓‡ | van Goozen et al., 2000 | ||||||
| ODD | X | ↓$ | Snoek et al., 2002 | ||||||
| ADHD | CU | X | ↓€ | Stadler et al., 2011 | |||||
Psychiatric/psychological conditions. ADHD, attention deficit hyperactivity disorder; AGG, aggression; ANX, anxiety; CD, conduct disorder; CU, callous emotional traits, DIS, disruptive behavior; EXT, externalizing behavior; ODD, oppositional-defiant disorder; UNR, unreferred. Cortisol measurements. X, type of measurement performed; single, condition associated with a single cortisol measurement; multiple, condition associated with averaged multiple cortisol measurements; diurnal, condition associated with altered diurnal secretion rhythm; total, 24 h urinary cortisol or equivalent; awakening, diurnal increase in cortisol measured in the morning; stress response, stress response independent from aggression. Association with cortisol. ↓, condition associated with downregulated HPA-axis function; → , condition not associated with changes in HPA-axis function; ↑, condition associated with upregulated HPA-axis function;
association with CD traits;
complex interaction with anxiety;
anticipation of stress (e.g., public speaking);
psychological challenge;
pharmacological challenge;
psychological stress, association with CU traits.
Conditions leading to glucocorticoid deficits in humans
Yehuda et al. (1990) were the first to suggest that on the long-run stressors may decrease HPA-axis function. They observed that cortisol secretion was significantly lower in post-traumatic stress disorder (PTSD) patients than in controls; moreover, cortisol levels correlated with PTSD symptomatology. Importantly, a recent paper from the same lab suggested that glucocorticoid treatments can ameliorate the symptoms of PTSD, suggesting causal relationships between the hormonal response and psychiatric symptoms (Yehuda et al., 2015). Despite these early findings in PTSD, more than a decade later Gunnar and Vazquez (2001) still felt the need to encourage those who observed similar phenomena. They wrote “Lower than expected cortisol values should not necessarily be relegated to the file drawer because they contradict the central dogma that stress must be associated with elevations in cortisol” (Gunnar and Vazquez, 2001). Scientific opinion has changed considerably currently; an increasing number of stressors that lower HPA-axis activity have been reported since.
A brief account of the findings
Detrimental conditions acquired via the mother such as exposure to various illicit drugs, caffeine, nicotine, alcohol, as well as synthetic glucocorticoids (administered as medications) were all shown to inhibit HPA-axis function in adulthood (Kapoor et al., 2008; Vázquez et al., 2012; Zhang et al., 2014; Buckingham-Howes et al., 2016).
Deficient parental care also inhibits HPA-axis function on the long run (Van der Vegt et al., 2009; Koss et al., 2014; Martin et al., 2014; McLaughlin et al., 2015); moreover, this effect becomes irreversible if “treatment” by adoption comes too late e.g., after the age of 2–3 years (McLaughlin et al., 2015). Family tradition of aggressiveness (Saxbe et al., 2012; Arbel et al., 2016) as well as parental psychopathology (antisocial personality disorder, and post-traumatic stress disorder) also decrease HPA-axis function on the long run (Vanyukov et al., 1993; Cordero et al., 2017). Various forms of early maltreatment e.g., neglect, and all forms of abuse (emotional, physical, sexual abuse) as well as traumatic stressors have a similar effect (Weissbecker et al., 2006; Elzinga et al., 2008; Carpenter et al., 2009; Bosch et al., 2012; Lovallo et al., 2012; Doom et al., 2014; Trickett et al., 2014; Peckins et al., 2015; Puetz et al., 2016).
Extra-familial conditions, e.g., violent neighborhoods and community violence in general, bullying in schools, the proximity of pubs were all negatively associated with indices of HPA-axis function in the adulthood of subjects (blunted stress responses: Busso et al., 2017; blunted cortisol responses: Ouellet-Morin et al., 2011; Janusek et al., 2017; steeper diurnal decline in cortisol levels during the day: Theall et al., 2017; low basal cortisol levels: Vaillancourt et al., 2008).
Finally, adversities suffered in adulthood also decrease HPA-axis function on the long run (Carsia and McIlroy, 1998; Dayan et al., 2016; Pinto et al., 2016; Ewing-Cobbs et al., 2017). Such stressors include malnutrition, intimate partner violence, traumatic brain injury, and traumatic stress. HPA-axis function also showed chronic deficits in certain psychopathologies e.g., antisocial personality disorder, atypical depression, chronic fatigue syndrome, cocaine addiction, post-traumatic stress disorder, and schizophrenia (Buydens-Branchey et al., 1997; Yehuda, 1999; Strous et al., 2004; Bührsch et al., 2009; Flory et al., 2009; Nijhof et al., 2014; Berger et al., 2016; Juruena et al., 2017; Pruessner et al., 2017; Das et al., 2018). While the association was likely expected with certain disorders (e.g., antisocial personality and post-traumatic stress disorders), others may need some clarification. Depression overall is clearly associated with chronic stress; yet depressed patients who suffered strong stressful events in their early life showed decreased awakening cortisol in adulthood (Strous et al., 2004). Noteworthy, the awakening-induced increase in cortisol production is believed to reflect best the overall functional status of the HPA-axis as it is free of confounds from daily events (Kudielka and Wüst, 2010). Decreased HPA-axis function was also observed in atypical depression, a condition that may be associated with aggressiveness (Hasler et al., 2004; Juruena et al., 2017). Although stressors were earlier associated with the development of schizophrenia, it was recently shown that the disorder per se is associated with low cortisol plasma levels throughout the day–possibly as a response to early stress exposure (Strous et al., 2004; Berger et al., 2016; Pruessner et al., 2017; Das et al., 2018). Moreover, decreased cortisol levels were shown to contribute to aggression in psychosis. Finally, cocaine addiction per se was not associated with decreased plasma levels of cortisol, but aggressive addicts had a blunted cortisol response to meta-chlorophenylpiperazine challenge (Buydens-Branchey et al., 1997).
Overall evaluation of findings
HPA-axis deficits evidenced by the studies briefly reviewed above may take many forms, from decreased morning cortisol and blunted awakening response, trough decreased levels over the day to blunted cortisol responses to various types of challenges. Although the relative importance of such individual events is unclear at present, it should be noted that one and same stressful event elicited the full range of changes albeit in different studies. For example, early adversity decreased cortisol stress responses in some studies (Elzinga et al., 2008; Trickett et al., 2014), decreased basal levels or flattened the diurnal rhythm of cortisol secretion in others (Sánchez et al., 2005; Bosch et al., 2012; Peckins et al., 2015), and decreased morning cortisol in yet other studies (Weissbecker et al., 2006; Puetz et al., 2017). As such, effects may cover a variety of indices of HPA-axis deficits, even if one particular aspect was addressed in a particular study. Taken together, the findings demonstrate that various stressors suffered from prenatal to adult ages can inhibit the function of the HPA-axis on the long run.
Albeit the studies reviewed above were primarily endocrinological in their scope, some of them did investigate the behavior of subjects. In all such studies, low HPA-axis function was associated with indices of aggression (bullying: Ouellet-Morin et al., 2011; cocaine addicts with low stress responses: Buydens-Branchey et al., 1997; early life stress: Puetz et al., 2016; poor parental monitoring: Martin et al., 2014; prenatal drug exposure: Buckingham-Howes et al., 2016; prenatal synthetic glucocorticoids: Kapoor et al., 2008; schizophrenia: Strous et al., 2004; Das et al., 2018). In these studies, aggression was conceptualized as problem behavior, social and behavioral problems, externalizing behavior, high scores on aggression inventories, the display of aggression-related psychopathologies, and aggression histories. Particular behavioral descriptions were usually not provided. Thus, long-term decreases in HPA-axis function was associated with various indices of aggressiveness.
Comparison of HPA-axis down- and upregulating factors
Stressful events that led to a downregulated HPA-axis on the long run (see above) are surprisingly similar to those, which augmented HPA-axis function in other studies (see the reviews by Kuhlman et al., 2017; Raymond et al., 2017). This leads to the surprising conclusion that one and same stressor may down—or upregulate the stress system on the long run, depending on an unknown array of circumstances. While such circumstances cannot be identified at present, the available data provide some hints toward the solution of the contrasting findings.
The first aspect to be considered is related to the temporal evolution of events. It has been repeatedly shown that stressors that upregulate the HPA-axis may ultimately lead to its downregulation. For instance, caffeine, nicotine or alcohol consumed by the mother enhanced cortisol secretion in both the mother and the infant; blunted stress responses were observed only when subjects reached adulthood (Zhang et al., 2014). In the study by Bosch et al. (2012), pre- and post-natal stressors also elicited a cortisol response acutely. Subsequently, the adversity was not associated with cortisol outcomes up to the age of 5, the upregulation of the HPA-axis was observed up to the age of 11, whereas a few years thereafter low cortisol levels were observed in subjects. Although not detailed to a similar degree, the trajectory of HPA-axis changes followed the same pattern in a number of cases. Acute and (sub)chronic stress responses were followed over time by a downregulation of the HPA-axis (Gunnar and Vazquez, 2001; Fries et al., 2005; Sánchez et al., 2005; Doom et al., 2014; Zhang, 2017). These findings show that the glucocorticoid deficit develops over time, and subjects frequently reach this phase after a period of HPA-axis upregulation. It also occurs that the process may take years.
Another factor that should be taken into account is the variability in HPA-axis responses. Peckins et al. (2015) categorized their subjects with respect to stress responses rather than calculating overall averages. They identified three cortisol profiles emerging after stress exposure (blunted, moderate, and elevated stress responses), and found that maltreated youth were more likely to show a blunted cortisol profile on the long run, but some of them fell into the other two categories. Van der Vegt et al. (2009) found that the magnitude of HPA-axis dysregulation depended largely on the severity of early maltreatment. Individual variability in HPA-axis responses to stress was observed in other studies as well (Weissbecker et al., 2006; Flory et al., 2009). Thus, the direction of HPA-axis changes (up- or down-regulation) and the magnitude of the effect strongly depend on the individual features of subjects, and/or to life events subsequent to the initial stressor. Naturally, other factors e.g., genetic susceptibility, and the type of stress are also important (see Daskalakis et al., 2013 for a review).
Conclusions
The long-term decrease in HPA-axis function that develops in response to strong stressors may be best conceptualized as an “allostatic crash,” a term coined by Van Houdenhove et al. (2009). This concept suggests that severe stressors, when associated with low individual resilience lead to an allostatic load that overpasses the adaptive capacities of the HPA-axis. This—metaphorically saying—results in the “fatigue” or “burnout” of the system. Such a “burnout” may be facilitated by the transient phase of upregulation (see above) or by subsequent stressors. Collectively, the excessive allostatic load leads to a failure of the adrenals to produce adequate quantities of glucocorticoids under basal conditions, and/or to failures of the system to adequately respond to acute stressors.
The likely factors that differentiate conditions that lead to the chronic upregulation or the chronic downregulation of the HPA-axis are the severity and duration of the triggering event (the initial stressor), individual susceptibility to stress effects, and life events that follow the initial stressor. Their relationship may place the HPA-axis on opposing trajectories and may lead either to the up- or to the downregulation of the HPA-axis.
Glucocorticoid deficits and human aggression
The study by Virkkunen (1985) implicitly suggests that glucocorticoid deficiency is associated with severe aggression; moreover, superimposed deficits e.g., antisocial personality disorder and habitual offending. Some recent findings also imply that HPA-axis hypoactivity characterizes a particularly severe subgroup of subjects who show both chronic antisocial behavior and callous-unemotional traits (Hawes et al., 2009). Other evidence, however, suggests that glucocorticoid deficits can be associated with less severe forms of aggression.
A brief account on findings
By a thorough Medline search using various keywords, we identified 76 studies on the interaction between HPA-axis function and aggressiveness. We summarized findings in Tables 1–4. Individual tables refer to studies in particular subjects; Table 1 for instance shows cross-sectional studies performed in children who showed various aggression-related conditions. Findings were arranged according to the method of cortisol measurements to visualize possible confound resulting from this variable. As argued elsewhere (Haller et al., 1998, 2008; Haller, 2014), types of measurement are not equivalent. Single measurements appear to be the least reliable due to accidental variations in plasma cortisol levels. Averaged multiple measurements eliminate such confounds, whereas awakening cortisol as well as indices of total cortisol production (e.g., 24 h urinary cortisol) can be considered general indices of HPA-axis function. Diurnal variations provide detailed information, which may reveal alterations specific to particular phases of the day. Finally, the magnitude of the stress response may vary independently from basal levels, by this providing a yet different measure of HPA-axis function.
Out of the 29 cross-sectional studies performed in children, 21 (72.4%) revealed a negative association between HPA-axis function and conditions associated with aggressiveness; 5 (17.3%) found no association, whereas the opposite relationship was reported in the remaining 3 studies (10.3%) (Table 1). The method of glucocorticoid measurement does not seem to have a role in discrepancies, because all the 6 ways of HPA-axis evaluation was found in all the three categories of interactions. Out of the primary conditions (diagnoses) investigated, attention deficit hyperactivity disorder (ADHD) showed the negative association consistently, whereas findings in disruptive behavior were the least consistent. Findings in unreferred subjects evaluated for externalizing behavior were rather inconsistent as well. Out of the secondary conditions, callous-unemotional traits showed the negative association consistently whereas findings in externalizing behavior were the least consistent. Studies lacking a secondary moderating factor also provided inconsistent findings.
Taken together, the findings summarized in Table 1 show that the association between aggression-related conditions and HPA-axis function are conflicting in cross-sectional studies performed in children. Albeit the overwhelming majority of studies reported a negative association, a significant proportion of studies are at variance with this conclusion. No contradictions were found with certain conditions e.g., ADHD and callous unemotional traits, but the number of relevant studies is too small to draw definite conclusions regarding these conditions.
In sharp contrast to cross-sectional studies, longitudinal ones performed in children appear highly consistent (Table 2). In the majority of these studies, unreferred subjects were studied at time-point 1, when there was no interaction between cortisol levels and behavior. Note that no problem behavior was identified at this time-point. Low levels of cortisol at time-point 1, however, predicted the development of problem behaviors at the 2nd time-point i.e., more than 2 years later (up to 6 years in some studies). There were only two exceptions, but neither produced conflicting findings. In one study, childhood internalizing behavior predicted downregulated HPA-axis function in adolescence; no similar association was found for externalizing behavior (Ruttle et al., 2011). In the second study, the negative association was observed at the first time-point already, and this was maintained over time (Salis et al., 2016). Noteworthy, however, the subjects of this study were adolescents at the first time-point. One can hypothesize that the prediction of problem behavior by low cortisol was valid for these subjects as well, but they were over the critical age. Again, the method of cortisol measurement did not affect the relationship between cortisol and behavior.
Table 2.
Longitudinal studies in children: associations between cortisol plasma levels and long-term changes in aggression-related conditions.
| Condition | Cortisol measurement | Association | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Concurrent | Longitudinal | Single | Multiple | Diurnal rhythm | Daily production | Awakening response | Stress response | Concurrent | Longitudinal | |
| UNR | DIS | X | → | ↓ | Sondeijker et al., 2008 | |||||
| UNR | DIS | X | → | ↓ | McBurnett et al., 2000 | |||||
| UNR | DIS | X | → | ↓ | Alink et al., 2012 | |||||
| UNR | EXT | X | → | ↓ | Shirtcliff and Essex, 2008 | |||||
| UNR | EXT | X | → | ↓ | Shirtcliff et al., 2005 | |||||
| UNR | AGG | X | → | ↓ | Shoal et al., 2003 | |||||
| INT | INT | X | → | ↓* | Ruttle et al., 2011 | |||||
| UNR | AGG | X | → | ↓ | Salis et al., 2016 | |||||
| AGG | AGG | X | ↓ | ↓** | Platje et al., 2013 | |||||
Temporal relationships. Concurrent, first time point of the study; longitudinal, subsequent time-points of the study (usually years after “current”). Psychological conditions. AGG, aggression; DIS, disruptive behavior; EXT, externalizing behavior; INT, internalizing behavior; UNR, unreferred. Cortisol measurements. X, type of measurement performed; single, condition associated with a single cortisol measurement; multiple, condition associated with averaged multiple cortisol measurements; diurnal, condition associated with altered diurnal secretion rhythm; total, 24 h urinary cortisol or equivalent; awakening, diurnal increase in cortisol measured in the morning; stress response, stress response independent from aggression. Association with cortisol. → , condition not associated with changes in HPA-axis function concurrently (i.e., during the first time-point of the study); ↓, the condition associated with downregulated HPA-axis function over time (cortisol levels at the first time point were compared with psychiatric conditions observed in subsequent time-points);
increase in internalizing behavior predicted the decrease in cortisol plasma levels over time;
study subjects were adolescents (16–19 years old).
Out of the 19 studies performed in adolescents and adults, low HPA-axis functioning was associated with various indices of aggressiveness in 18 studies (Table 3). The only exception was a study performed in antisocial personality-disordered subjects, where psychopathic traits did not correlate with the diurnal patterns of cortisol secretion (Loomans et al., 2016). The lack of interaction cannot be attributed to the way of cortisol measurement, as two other studies employed the same approach; moreover, one of these found associations with psychopathic traits (Vaillancourt and Sunderani, 2011; Das et al., 2018). This discrepancy is difficult to explain. However, the rest of the studies are remarkably consistent as it regards the association between aggressiveness and cortisol, despite the large variation in subject types (from unreferred through psychotic and drug addiction to antisocial personality disorder) and the various ways of HPA-axis evaluations.
Table 3.
Studies in adolescents and adults: associations between plasma cortisol and aggression-related psychiatric conditions.
| Condition | Cortisol measurement | Interaction | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primary | Secondary | Single | Multiple | Diurnal rhythm | Daily production* | Awakening | Stress responses | ||
| UNR | AGG | X | ↓ | Yu and Shi, 2009 | |||||
| ALC | VIOL | X | ↓ | Bergman and Brismar, 1994 | |||||
| UNR | PP | X | ↓* | Glenn et al., 2011 | |||||
| SCH | AGG | X | ↓ | Strous et al., 2004 | |||||
| UNR | CU | X | ↓** | Fanti and Kimonis, 2017 | |||||
| UNR | AGG | X | ↓ | Victoroff et al., 2011 | |||||
| UNR | PP1 | X | ↓† | Vaillancourt and Sunderani, 2011 | |||||
| PSYCH | VIOL | X | X | ↓ | Das et al., 2018 | ||||
| APD | PP | X | → | Loomans et al., 2016 | |||||
| UNR | AGG | X | ↓* | Grotzinger et al., 2018 | |||||
| UNR | AGG | X | ↓‡ | Böhnke et al., 2010 | |||||
| UNR | AGG | X | ↓$ | Gordis et al., 2006 | |||||
| HERadd (ABST) | AGG | X | ↓‡ | Gerra et al., 2004 | |||||
| HERadd (METH) | AGG | X | ↓‡ | Gerra et al., 2001 | |||||
| APD | - | X | ↓€ | Almeida et al., 2010 | |||||
| UNR | EXT | X | ↓* | Portnoy et al., 2015 | |||||
| UNR | PP | X | ↓ | O'Leary et al., 2007 | |||||
| COCadd | AGG | X | ↓€ | Buydens-Branchey et al., 1997 | |||||
| PDE | AGG, EXT | X | ↓ | Buckingham-Howes et al., 2016 | |||||
Psychiatric/psychological conditions. ABST, abstinent at the time of the study; AGG, aggression; ALC, alcoholic; APD, antisocial personality disorder; CU, callous emotional traits, EXT, externalizing behavior; HERadd, heroin addiction; METH, on methadone at the time of the study; PDE, prenatal drug exposure; PP, psychopathic traits; PP1, psychopathy type 1; PSYCH, psychosis; SCH, schizophrenia; UNR, unreferred; VIOL, violence. Cortisol measurements. X, type of measurement performed; single, condition associated with a single cortisol measurement; multiple, condition associated with averaged multiple cortisol measurements; diurnal, condition associated with altered diurnal secretion rhythm; total, 24 h urinary cortisol or equivalent; awakening, diurnal increase in cortisol measured in the morning; stress response, stress response independent from aggression. Association with cortisol. ↓, condition associated with downregulated HPA-axis function; → , condition not associated with changes in HPA-axis function; ↑, condition associated with upregulated HPA-axis function;
association with testosterone/cortisol ratio;
association with externalizing and internalizing behaviors;
females only;
experimental provocation unrelated to aggressiveness;
interaction dependent on alpha amylase activity;
pharmacological challenge.
Finally, findings obtained in delinquents also appear rather consistent (Table 4). Out of the 18 studies, delinquents had downregulated HPA-axes in 15 (83%). Interestingly, the association did not depend on the type of delinquency, as low HPA-axis function was observed in both violent and nonviolent offender populations. In two studies, no association was observed (Feilhauer et al., 2013; Gostisha et al., 2014). However, the impact of antisocial and psychopathic traits was investigated in these studies. If HPA-axis deficits were associated with delinquency per se (as the rest of the studies suggest), the lack of an additional impact by such traits becomes explainable. The only finding that was discrepant indeed reported that violent delinquents show enhanced stress responses (Soderstrom et al., 2004). The discrepancy cannot be due to the way of HPA-axis evaluation as four other studies found decreased stress responses in delinquent populations; these were done either in violent or non-violent offenders (Moss et al., 1995; Popma et al., 2006; Couture et al., 2008; Johnson et al., 2015).
Table 4.
Studies in delinquent populations: associations between plasma cortisol and delinquency.
| Condition | Cortisol measurement | Interaction | References | |||||
|---|---|---|---|---|---|---|---|---|
| Delinquent type | Single | Multiple | Diurnal rhythm | Daily production* | Awakening response | Stress responses | ||
| M/nV | X | ↓* | Popma et al., 2007b | |||||
| M/nV | X | ↓** | Poustka et al., 2010 | |||||
| M/nV | X | ↓ | Dolan et al., 2001 | |||||
| M/nV | X | ↓† | Horn et al., 2014 | |||||
| M/nV | X | X | ↓‡ | Couture et al., 2018 | ||||
| PP | X | ↓* | Dabbs et al., 1991 | |||||
| PP | X | → | Feilhauer et al., 2013 | |||||
| ANTS | X | →₳ | Gostisha et al., 2014 | |||||
| M/nV | X | ↓ | Popma et al., 2007a | |||||
| VIOL | X | ↓ | Brewer-Smyth et al., 2004 | |||||
| PP | X | ↓ | Cima et al., 2008 | |||||
| VIOL | X | ↓ | Virkkunen, 1985 | |||||
| VIOL | X | ↓$ | Holi et al., 2006 | |||||
| M/nV | X | ↓€ | Popma et al., 2006 | |||||
| VIOL | X | ↓€ | Moss et al., 1995 | |||||
| VIOL | X | ↑€ | Soderstrom et al. (2004) | |||||
| M/nV | X | ↓¥ | Johnson et al., 2015 | |||||
| M/nV | X | ↓‡ | Couture et al., 2008 | |||||
Delinquent type. ANTS, delinquents with antisocial traits; M / nV, mixed delinquent population, committing mostly non-violent crime; PP, delinquents with psychopathic traits; VIOL, violent crime. Cortisol measurements. X, type of measurement performed; single, condition associated with a single cortisol measurement; multiple, condition associated with averaged multiple cortisol measurements; diurnal, condition associated with altered diurnal secretion rhythm; total, 24 h urinary cortisol or equivalent; awakening, diurnal increase in cortisol measured in the morning; stress response, stress response independent from aggression. Association with cortisol. ↓, delinquency associated with downregulated HPA-axis function; ↑, delinquency associated with upregulated HPA-axis function;
interaction with testosterone/cortisol ratio;
males only;
interactions mediated by personality and substance use disorders;
interaction mediated by risk taking;
, complex interactions with stress exposure;
interaction with psychopathic features;
anticipation of stress (e.g., public speaking);
interaction with the number of incarcerations.
Taken together, conditions associated with aggressiveness or violence show a more reliable association with downregulated HPA-axis than generally believed (Klimes-Dougan et al., 2001; von der Pahlen, 2005; Marsman et al., 2008). The findings briefly reviewed above reveal that the negative association is neither restricted to extreme violence nor to callus-unemotional traits as previously suggested (Virkkunen, 1985; Hawes et al., 2009). Moreover, it may be valid for delinquents in general, irrespective to the violent nature of offenses committed. At the same time, however, there are a series of discrepant findings as well. Although these represent a rather small share of the studies, there are no obvious reasons to neglect them.
Discrepant findings—possible explanations
One possible explanation to the discrepant findings may reside in the putative long-term association of glucocorticoid deficits and aggressiveness. Such findings were summarized in Table 2. One can hypothesize that the negative association between glucocorticoid production and aggressiveness may not be obvious in some cases because the behavioral change is subsequent to the decrease in glucocorticoid production, and the temporal difference between the two can be measured in years. This may at least partly explain discrepant findings in children. However, discrepant findings were reported at older ages too. Another explanation may reside in the type of aggression performed by subjects, which is considered rather rarely.
Aggression is often divided into two types: instrumental (proactive) and emotional (reactive). The former is non-impulsive and gain oriented, whereas the latter is a response to threat or provocation, is impulsive, and is not performed for gain (Feshbach, 1971; Blair, 2001; Kempes et al., 2005; Raine et al., 2006; van Honk et al., 2010). The two forms of aggression appear to have a differential association with cortisol. Instrumental (proactive) aggression appears associated with blunted, whereas emotional (reactive) aggression seems to be associated with upregulated HPA-axis function (McBurnett et al., 2003, 2005; Kempes et al., 2005; van Bokhoven et al., 2005; Lopez-Duran et al., 2009; O'Neal et al., 2010; Poustka et al., 2010; van Honk et al., 2010; Geniole et al., 2011; Stoppelbein et al., 2014). Noteworthy, a similar dichotomy was found in animal models (Haller, 2016).
Evaluating the relationship between aggression and aggression-induced changes in cortisol production, as well as the relationship between reactive aggression and enhanced glucocorticoid production is outside the scope of this review, which aims at evaluating the role of the lateral hypothalamus in aggression performed under conditions of downregulated HPA-axis. However, studies where reactive and proactive forms of aggression were directly compared as it regards their glucocorticoid background strongly suggest that the two types of aggression substantially differ in this respect, and may explain discrepancies in the studies reviewed above.
Overall evaluation of human findings
The studies briefly reviewed above show that on the long run, a large number of stressors elicits HPA-axis deficits in humans. These stressors coincide largely with those, which on the long run may also elicit a chronic enhancement of HPA-axis function. There are no explanations for such contrasting long-term trajectories of HPA-axis responses to stress, but disparate findings suggest that the severity of the “initial” stressor, as well as subsequent life events, together with individual vulnerabilities may have an important role. The long-term decrease in HPA-axis function may result from an “allostatic crash” i.e., from an allostatic load that overpasses the adaptive capacities of the HPA-axis. HPA-axis deficits develop slowly over time, and subjects often go through a phase of HPA-axis hyperfunction before the “final” glucocorticoid deficit develops, albeit this assumption requires further evidence.
Although initially suspected to be related to extreme aggression, a series of studies suggest that glucocorticoid deficiency is associated with a wide variety of aggressive behaviors. Overall, the findings suggest that the “prime suspects” are proactive (instrumental) forms of aggression. Psychopathic e.g., callous-unemotional traits strengthen the association. Glucocorticoid deficiency was repeatedly associated violent crime as well.
The available findings suggest that glucocorticoid deficiency precedes behavioral changes. The vast amount of evidence on the association of glucocorticoid deficiency with aggression as well as the precedence of hormonal over behavioral changes suggest a causal relationship. However, this assumption is based on indirect evidence only, and the nature of the phenomenon precludes the obtaining of direct evidence in humans. The issue requires animal experimentation.
The role of the lateral hypothalamus
Findings in humans were tentatively summarized in Figure 2. In addition to factors that were based on the findings reviewed above, we added “other factors” to indicate that hormonal conditions are likely embedded in a wider array of biological, psychological, and social factors (Dodge et al., 1990; Loeber and Hay, 1997; van Honk et al., 2010).
Figure 2.
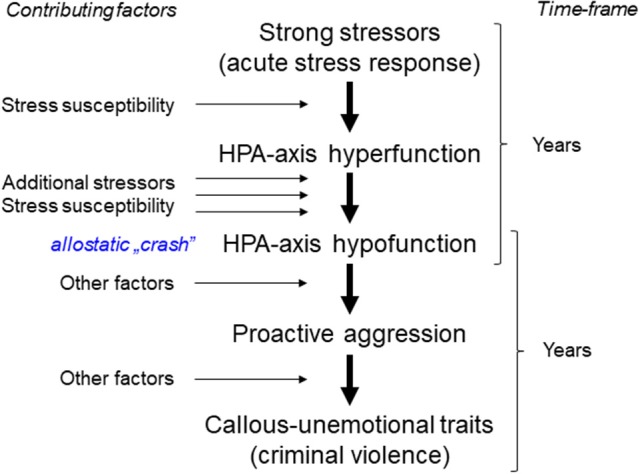
Hypothetical pathway from strong stressors to violent aggression through HPA-axis hypofunction. The latter is attributed to an “allostatic crash” (see section Glucocorticoid Deficits and Aggression in Humans). “Other factors” were included to indicate that hormonal conditions are likely embedded in a wider array of biological, psychological, and social factors. The time frame is based on a number of longitudinal studies performed in late childhood—early adolescence.
Despite the clear involvement of the hypothalamus in the control of aggression, little effort was invested in understanding its role in humans. The available studies either focus on the “triangle of Sano” (a hypothalamic region, the destruction of which strongly inhibits aggressive behavior; Sano et al., 1970) or the technologies employed were unable to differentiate hypothalamic nuclei, and consequently considered it as a unit (Rosa et al., 2012; Van den Stock et al., 2015; Mutic et al., 2017). As the triangle of Sano includes parts of the lateral hypothalamus, but is not restricted to it, and the term “hypothalamus” is not sufficiently precise for the purposes of this study, we have to turn to animal models.
The concept of abnormal aggression in animals
Over the last decades, rodent abnormal aggression models have increasingly been used to mimic symptoms of aggression-related psychopathologies. Such models (1) mimic in rodents etiological factors of human aggression problems; (2) study the resulting aggressiveness by focusing on the form of aggression and its behavioral contexts, and (3) strive at investigating aggression-independent features (e.g., autonomic responses, anxiety, and social behaviors beyond aggressiveness) which are important concomitants of aggression-related psychopathologies (for reviews see Haller et al., 2004, 2014; Miczek et al., 2013).
The overall conclusion deriving from such studies is that the etiological factors of human aggression problems result in abnormal forms of aggression in rodents. Human etiological factors modeled so far to study their effects on animal aggression include but are not restricted to alcohol, early social isolation, frustration by the omission of scheduled reward, glucocorticoid deficiency, peripubertal social or physical stressors, repeated maternal separation, repeated treatment with cocaine, amphetamine, or anabolic steroids during adolescence, and selection for extremes in aggression and anxiety to mimic the genetic component of human aggression (Benus et al., 1991; Miczek et al., 1992; Melloni and Ferris, 1996; Delville et al., 1998; Haller et al., 2001; de Almeida and Miczek, 2002; Ricci et al., 2005; Veenema et al., 2006; Tóth et al., 2008; Gobrogge et al., 2009; Neumann et al., 2010; Márquez et al., 2013). All these etiological factors elicit abnormal manifestations of aggression in rodents, which are perceived as models for the symptoms of aggression-related psychopathologies. As an example, the glucocorticoid deficiency model changed the behavior and physiology of subjects in the following ways. (1) Attack targeting shifted from less vulnerable body parts (back and flanks) to more vulnerable ones (head, throat, belly, and occasionally paws and testicles); (2) The propensity of signaling attack intentions by social signals (threats) decreased; (3) Attacks were performed under conditions of low autonomic arousal; (4) The social behavior of subjects showed important deficits (beyond those related to aggression). This array of features mimics important aspects of human proactive (antisocial) aggressiveness (Haller et al., 2004; Haller, 2017).
Aggression models associated with glucocorticoid deficits
The glucocorticoid deficit model of abnormal aggression was purposefully developed to mimic the human condition discussed in this review (see above). In addition to this model, there are several other abnormal aggression models, where the induction of glucocorticoid deficits was not an aim, but deficient HPA-axis function was observed later on. This was observed in the early subjugation model of aggression (Ferris, 2003), in mouse lines selected for aggressiveness, particularly the short attack latency mice (Veenema et al., 2004; Natarajan and Caramaschi, 2010), and rats selected for extremes in anxiety (Neumann et al., 2010). In the pubertal stress model, corticosterone secretion was not decreased overall, but was decreased relative to testosterone secretion (Márquez et al., 2013).
Maternal aggression is also associated with low glucocorticoid stress responses (Neumann, 2001; Neumann et al., 2001); moreover, acute stressors suffered before the aggressive encounter and increased stress responsiveness (as an individual feature) decrease maternal aggression (Gammie et al., 2005; Gammie and Stevenson, 2006). Albeit maternal aggression cannot be considered abnormal, it shares several features with the glucocorticoid deficit model of abnormal aggression. When protecting their pups, dams attack vulnerable body part of their opponents, and fail to signal their attack intentions by social signals (Parmigiani et al., 1988). Finally, predatory aggression is also performed under low arousal, and consist of lethal attacks on vulnerable body parts (Siegel et al., 1999).
The studies briefly reviewed above suggest that in certain abnormal aggression models as well as in two models of natural aggressiveness (maternal and predatory aggressions) violent forms of aggression are associated with decreased HPA-axis function.
Violent aggression and the lateral hypothalamus
Where the lateral hypothalamus was investigated, glucocorticoid deficit-associated violent aggression was also associated with a marked activation of this hypothalamic region (glucocortioid deficit model: Tulogdi et al., 2010; mouse lines selected for aggressiveness: Haller et al., 2006; rats selected for extremes in anxiety: Beiderbeck et al., 2012; predatory aggression: Tulogdi et al., 2015; maternal aggression: Hasen and Gammie, 2006). In these models, the mediobasal hypothalamus was also activated, with the exception of the predatory aggression model. A detailed analysis of activation patterns observed in aggression models associated with glucocorticoid deficit and those associated with normal or enhanced glucocorticoid responses revealed three different brain activation patterns (Haller, 2014, 2017). The overall conclusion of these studies was that: (1) regularly performed resident-intruder test activate the medial amygdala-mediobasal hypothalamus-dorsal periaqueductal gray pathway. (2) In abnormal aggression models associated with increased glucocorticoid stress responses, the same pathway was activated, but the medial amygdala and mediobasal hypothalamus together with certain areas of the prefrontal cortex and the basolateral amygdala showed increased activations. (3) Abnormal aggression models associated with decreased glucocorticoid stress responses activate the same pathway but in addition they also activate the central amygdala-lateral hypothalamus-ventral periaqueductal gray pathway. Finally (4) predatory aggression activates exclusively the central amygdala-lateral hypothalamus-ventral periaqueductal gray pathway (Figure 1).
Naturally, correlations between behavioral, endocrine and brain activation patterns are indicative of, but not proofs of causal relationships. Recent studies, however, do suggest such causal relationships. These studies were prompted by the finding that hypothalamic areas involved in aggression control receive direct inputs from the prefrontal cortex, a brain area tightly involved in aggression control (Sesack et al., 1989; Siegel et al., 2007; Toth et al., 2010). Blair (2001) attributed a large role of such direct prefrontal cortex–hypothalamus connections in his model of human reactive aggression, albeit subsequent studies by the same author suggest that the prefrontal cortex–hypothalamus link is not direct but is mediated by the amygdala (Blair, 2012, 2016). Recent rodent findings, however, appear to re-establish the notion that the prefrontal cortex controls aggression by direct effects on the hypothalamus, a mechanism that may complements the more established prefrontal cortex–amygdala–hypothalamus pathway (Biro et al., 2018). We established in a recent study that the hypothalamus is directly (mono-synaptically) innervated by the prefrontal cortex; moreover, the mediobasal and lateral hypothalamus received inputs from dominantly distinct prefrontal neuron populations. These neurons were located in the prelimbic and infralimbic region of the medial prefrontal cortex. Prefrontal projecting neurons were dispersed in the layers III–V of the infralimbic and prelimbic cortices, and their dense axon terminals at hypothalamic sites exclusively contained vesicular glutamate transporter 1, i.e., these neurons were glutamatergic. The optogenetic stimulation of mPFC terminals in the MBH distinctively increased bite counts in resident/intruder conflicts, whereas stimulation of similar terminals in LH specifically resulted in violent bites. These were aimed at vulnerable targets of opponents (head, throat and belly, occasionally paws), and/or were not preceded by social signals (threats). No other behavior was affected, suggesting that dedicated prefrontal neurons control particular aspects of aggressiveness via the hypothalamus in a highly selective manner. Besides shedding light on the way in which the prefrontal cortex controls aggressive behavior (e.g., by highly dedicated subcortical efferents), these findings are to our knowledge the first proof of a causal involvement of the lateral hypothalamus in intraspecific aggression. These findings are in agreement with the role of this brain area in violent aggression, which was assumed earlier based on brain activation patterns, but surprisingly show that subpopulations of prefrontal neurons may increase aggressiveness; moreover, may make it more violent. In principle, this finding is at variance with the prefrontal deficit theory of aggression (Blair, 2001; Siegel et al., 2007). The role of the prefrontal cortex in aggression control is outside the scope of this review, therefore this aspect of the findings will not be discussed here in detail. We showed earlier, however, that aggressiveness is associated with both chronic prefrontal deficits and increased acute aggression-induced prefrontal activation in abnormal aggression models (Biro et al., 2017), and repeatedly argued elsewhere, that the involvement of the prefrontal cortex in aggression control should be revised (Haller, 2014, 2017). Albeit chronic prefrontal deficits increase the likelihood and severity of aggressive behavior, this brain area is activated by aggressive behavior acutely; moreover, activation is stronger in models of abnormal aggression and in subjects with aggression-related psychopathologies. Based on these findings, one can hypothesize that HPA-axis hypofunction affects the aggression circuitry at least partly at the level of the prefrontal cortex.
The impact of glucocorticoid deficits on aggression: putative mechanisms
The basic question raised by the findings reviewed above is how the mechanisms controlling aggression are shifted from the ones that control rivalry aggression to the ones that control predatory aggression under normal circumstances. Low HPA-axis activity after allostatic overload or experimental manipulations is not likely to cause behavioral maladaptation by itself. Glucocorticoids affect the properties of neurons rather than elicit membrane depolarizations or activate brain circuitries. Consequently, the basic question is how the absence of well-timed, adaptive adrenocortical response in the face of a social challenge or emergency alters the properties of aggression-controlling networks.
Some time ago we speculated that the dynamics of the endocrine responses accompanying social challenges and aggression,—most notably the dynamics of the HPA-axis—, would rapidly affect the way animals cope with actual and future conflicts. We also suggested that studying the underlying mechanisms at the level of the hypothalamus and its interactions with frontal systems could possibly contribute to our understanding of inadequate, maladaptive conflict behavior in humans (Kruk et al., 1998). We also suggested that the hypothalamus serves as a crucial link between frontal areas involved in memory, the appraisal of the environments the recognition of conspecifics and the execution of specific aggressive responses. Such frontal areas, as we suggested at that time could either facilitate or inhibit hypothalamic aggression in rats (Kruk et al., 1998). Since then animal studies have provided strong support for these ideas reviewed e.g., in Kruk (2014). The dynamics of the adrenocortical response facilitates hypothalamic,- as well as territorial aggression within the time frame of one single conflict (Kruk et al., 2004; Mikics et al., 2004) by an initially non-genomic rapid mechanism. In the absence of such a response inadequate conflict handling takes over. Rapid adrenocortical signaling via the mineralocorticoid receptor also enables the first emergence of an adaptive aggressive response in a novel environment (Kruk et al., 2013). More recent studies clearly suggested the involvement of structures “up-stream” from the hypothalamus, such as the amygdala and the frontal cortex, in such behavioral effects of the adrenocortical response (Biro et al., 2017, 2018; Mikics et al., 2018).
These experimental studies suggest that the precise timing of the adrenocortical response with respect to the timing of a social challenge determines the adaptive nature of the aggressive response. Therefore, it stands to reason to predict that the absence of a well-timed adrenocortical response as a consequence of repeated severe stressors and the ensuing allostatic crash, could also be a major factor in inadequate conflict handling in humans. Recent results from animal experiments summarized here, suggest that the behavioral changes accompanying an allostatic crash in humans could be due to a reorganization of hypothalamic connections with “upstream,” modulating circuits, resulting in a change from affective social conflict handling toward a predatory like responding in conflicts.
Albeit acute deficits in the regulatory roles of glucocorticoids may have effects per se via the so-called non-genomic effects (Haller et al., 2008), the consequences of HPA-axis downregulation for aggression appear to develop slowly. This renders slow glucocorticoid actions better candidates for mediating the behavioral effects of glucocorticoid deficits, even if such slow alterations interact with rapid effects (Joëls et al., 2013).
A typical example of slow glucocorticoid effects are the ones that are mediated by epigenetic mechanisms. For example, early adversity was shown to durably alter the epigenetic regulation of glucocorticoid signaling genes and to induce DNA methylation in various brain areas (Lutz and Turecki, 2014; Tyrka et al., 2016). Importantly, glucocorticoids control gene expression in the prefrontal cortex, the brain site that shapes behavioral strategies during social conflict (Costin et al., 2013; Biro et al., 2018). The assumption that epigenetic changes in the prefrontal cortex elicit abnormal aggression is supported by recent findings showing that such behaviors largely depend on prefrontal neuronal plasticity, particularly plasticity in the prelimbic and infralimbic cortex (Mikics et al., 2018).
In line with these findings, it was recently shown that there is a complex interplay between early stressors, plasma glucocorticoid levels, and decision-making at the level of the prefrontal cortex (Fan et al., 2014; Quevedo et al., 2017; Joëls et al., 2018). A key component of this interaction is HPA-axis functioning in adulthood, that depends to a large extent on early adversities (Raymond et al., 2017; Kaiser et al., 2018). Albeit the issue clearly needs further studies, findings obtained so far suggest that the link between early adversity, adult glucocorticoid deficits and abnormal aggression is established by epigenetic changes, and one of the major loci of these processes is the prefrontal cortex.
Conclusions and hypothesis
Ample human evidence suggests that strong stressors downregulate the HPA-axis on the long run. Stressors that have this outcome largely coincide with those that have an opposite effect i.e., which upregulate the HPA-axis on the long run. The factors that underlie these opposite trajectories of HPA-axis responses are largely unknown at present, but disparate findings suggest that individual differences in stress responsiveness, and exposure to subsequent stressors may play an important role. The stress-induced decrease in glucocorticoid secretion capacities develop slowly. Low HPA-axis function on its turn was consistently associated with increases in aggressiveness and aggressive delinquency in humans. Findings suggest that the association is valid primarily for proactive, antisocial aggressiveness. Longitudinal studies indicate that the decrease in glucocorticoid secretion precedes the increase in aggressiveness by years, raising the possibility of a causal relationship. These human findings demonstrate the relevance of the relationship between glucocorticoid hypofunction and aggressiveness.
Hypothalamic mechanisms possibly underlying this relationship are difficult to study in humans, for which such mechanisms need to be investigated in animal models.
In rodents, the deliberate limitation of glucocorticoid secretion as well as conditions that on the long run decrease HPA-axis function result in increased aggression. This was observed in models of abnormal aggression, i.e., models that mimic the etiological factors of pathologic human aggression and where aggressive behavior strongly deviates from species-typical patterns. Recent findings suggest that an alternate neural route of aggression control mediates the effects of glucocorticoid deficits on aggression. Particularly, neural mechanisms of predatory aggression are activated in conditions associated with glucocorticoid deficits. Within this alternate mechanism, the lateral hypothalamus, especially the prefrontal cortex-lateral hypothalamic pathway seems to play an important role.
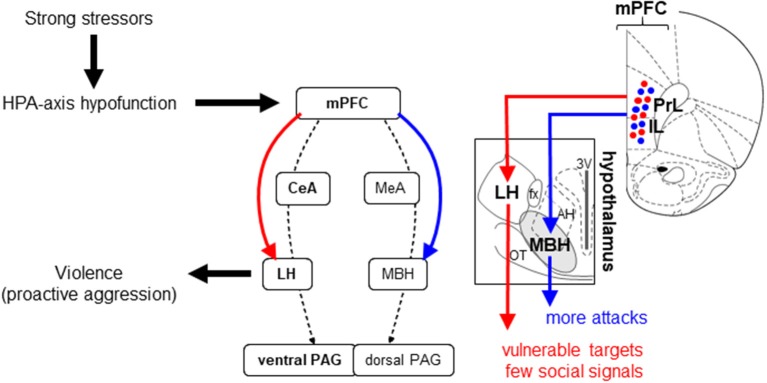
By corroborating human and animal findings, we hypothesize the following (Figure 3):
Figure 3.
Brain mechanisms that mediate the effects of glucocorticoid deficits on aggression. Dashed arrows indicate hypothetical information flow based on earlier studies. Continuous arrows indicate the pathways discovered by Biro et al. (2018). The left hand panel is a simplified version of Figure 2. The arrows to the right and then to the left indicate the likely neural mechanism that link the hormonal to the behavioral event. The middle panel shows the “mixed” mechanism of glucocorticoid hypofunction-induced aggression complemented with two pathways that directly connect the medial prefrontal cortex to hypothalamic centers of aggression. The right hand panel shows on Paxinos and Watson slides (Paxinos and Watson, 1998) two subpopulations of medial prefrontal neurons, which project either to the lateral or mediobasal hypothalamus. The latter is an electrophysiologically defined area of the hypothalamus that covers several hypothalamic nuclei (Kruk, 1991). The optogenetic stimulation of axon terminals in the lateral hypothalamus increases the share of attacks on vulnerable targets that are poorly signaled socially. When stimulations are aimed at the mediobasal hypothalamus, the number of bites increases. Both effects are highly selective behaviorally (Biro et al., 2018). 3V, the third ventricle; AH, anterior hypothalamic nucleus; fx, fornix; IL, infralimbic cortex; OT, optic tract; PrL, prelimbic cortex. For other abbreviations, see Figure 1.
Strong stressors associated with subsequent stress exposure and high individual stress sensitivity decrease the glucocorticoid secretion capacity of the HPA-axis by a slowly developing process (“allostatic crash.”)
HPA-axis hypofunction alters the function of the aggression circuitry. One of the primary targets of this change is the prefrontal cortex. This process also develops slowly.
The ultimate consequence of brain alterations is that social challenges elicit the activation of brain mechanisms that originally control predatory aggression. This results in violent, harmful aggression.
The lateral hypothalamus plays a key role in eliciting violent forms of aggression. When the aggression circuitry is altered by glucocorticoid hypofunction, this brain region is activated by the prefrontal cortex and possibly by the central amygdala upon social challenge, and its activation leads to aggression patterns that are substantially more damaging than species-typical ones.
Ultimately, this hypothetical sequence of events explains how unfavorable (stressful) social conditions lead to endocrine and later to neural alterations that result in proneness to violence, which on its turn may restart the cycle of violence by creating social tensions that elicit hyporesponsive HPA-axes in others.
Author contributions
The author confirms being the sole contributor of this work and approved it for publication.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by NKFI grant No. 112907, and the KÖFOP-2.1.2-VEKOP-15-2016-00001 grant.
References
- Alink L. R., Cicchetti D., Kim J., Rogosch F. A. (2012). Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Dev. Psychol. 48, 224–236. 10.1037/a0024892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M., Lee R., Coccaro E. F. (2010). Cortisol responses to ipsapirone challenge correlate with aggression, while basal cortisol levels correlate with impulsivity, in personality disorder and healthy volunteer subjects. J. Psychiatr. Res. 44, 874–880. 10.1016/j.jpsychires.2010.02.012 [DOI] [PubMed] [Google Scholar]
- Arbel R., Rodriguez A. J., Margolin G. (2016). Cortisol reactions during family conflict discussions: influences of Wives' and Husbands' exposure to family-of-origin aggression. Psychol. Viol. 6, 519–528. 10.1037/a0039715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiderbeck D. I., Reber S. O., Havasi A., Bredewold R., Veenema A. H., Neumann I. D. (2012). High and abnormal forms of aggression in rats with extremes in trait anxiety - involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology 37, 1969–1980. 10.1016/j.psyneuen.2012.04.011 [DOI] [PubMed] [Google Scholar]
- Benus R. F., Bohus B., Koolhaas J. M., van Oortmerssen G. A. (1991). Behavioural differences between artificially selected aggressive and non-aggressive mice: response to apomorphine. Behav. Brain Res. 43, 203–208. 10.1016/S0166-4328(05)80072-5 [DOI] [PubMed] [Google Scholar]
- Berger M., Kraeuter A. K., Romanik D., Malouf P., Amminger G. P., Sarnyai Z. (2016). Cortisol awakening response in patients with psychosis: systematic review and meta-analysis. Neurosci. Biobehav. Rev. 68, 157–166. 10.1016/j.neubiorev.2016.05.027 [DOI] [PubMed] [Google Scholar]
- Bergman B., Brismar B. (1994). Hormone levels and personality traits in abusive and suicidal male alcoholics. Alcohol. Clin. Exp. Res. 18, 311–316. 10.1111/j.1530-0277.1994.tb00019.x [DOI] [PubMed] [Google Scholar]
- Biro L., Sipos E., Bruzsik B., Farkas I., Zelena D., Balazsfi D., et al. (2018). Task division within the prefrontal cortex: distinct neuron populations selectively control different aspects of aggressive behavior via the hypothalamus. J. Nerosci. 38, 3234–17. 10.1523/JNEUROSCI.3234-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro L., Toth M., Sipos E., Bruzsik B., Tulogdi A., Bendahan S., et al. (2017). Structural and functional alterations in the prefrontal cortex after post-weaning social isolation: relationship with species-typical and deviant aggression. Brain Struct. Funct. 222, 1861–1875. 10.1007/s00429-016-1312-z [DOI] [PubMed] [Google Scholar]
- Blair R. J. (2001). Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J. Neurol. Neurosurg. Psychiatry. 71, 727–731. 10.1136/jnnp.71.6.727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R. J. (2016). The neurobiology of impulsive aggression. J. Child. Adolesc. Psychopharmacol. 26, 4–9. 10.1089/cap.2015.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair R. J. R. (2012). Considering anger from a cognitive neuroscience perspective. Wiley Interdiscip. Rev. Cogn. Sci. 3, 65–74. 10.1002/wcs.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhnke R., Bertsch K., Kruk M. R., Naumann E. (2010). The relationship between basal and acute HPA axis activity and aggressive behavior in adults. J. Neural. Transm. 117, 629–637. 10.1007/s00702-010-0391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch N. M., Riese H., Reijneveld S. A., Bakker M. P., Verhulst F. C., Ormel J., et al. (2012). Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents' cortisol stress response. The TRAILS study. Psychoneuroendocrinology 37, 1439–1447. 10.1016/j.psyneuen.2012.01.013 [DOI] [PubMed] [Google Scholar]
- Brewer-Smyth K., Burgess A. W., Shults J. (2004). Physical and sexual abuse, salivary cortisol, and neurologic correlates of violent criminal behavior in female prison inmates. Biol. Psychiatry 55, 21–31. 10.1016/S0006-3223(03)00705-4 [DOI] [PubMed] [Google Scholar]
- Buckingham-Howes S., Mazza D., Wang Y., Granger D. A., Black M. M. (2016). Prenatal drug exposure and adolescent cortisol reactivity: association with behavioral concerns. J. Dev. Behav. Pediatr. 37, 565–572. 10.1097/DBP.0000000000000338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bührsch N. C., Swoboda U., Quante A., Zeugmann S., Bajbouj M., Anghelescu I., et al. (2009). Cortisol awakening reaction in depressed patients with and without early life stress. Pharmacopsychiatry 42:214 10.1055/s-0029-1240094 [DOI] [Google Scholar]
- Busso D. S., McLaughlin K. A., Sheridan M. A. (2017). Dimensions of adversity, physiological reactivity, and externalizing psychopathology in adolescence: deprivation and threat. Psychosom. Med. 79, 162–171. 10.1097/PSY.0000000000000369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buydens-Branchey L., Branchey M., Fergeson P., Hudson J., McKernin C. (1997). The meta-chlorophenylpiperazine challenge test in cocaine addicts: hormonal and psychological responses. Biol. Psychiatry. 41, 1071–1086. 10.1016/S0006-3223(96)00182-5 [DOI] [PubMed] [Google Scholar]
- Carpenter L. L., Tyrka A. R., Ross N. S., Khoury L., Anderson G. M., Price L. H. (2009). Effect of childhood emotional abuse and age on cortisol responsivity in adulthood. Biol. Psychiatry 66, 69–75. 10.1016/j.biopsych.2009.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsia R. V., McIlroy P. J. (1998). Dietary protein restriction stress in the domestic turkey (Meleagris gallopavo) induces hypofunction and remodeling of adrenal steroidogenic tissue. Gen. Comp. Endocrinol. 109, 140–153. 10.1006/gcen.1997.7016 [DOI] [PubMed] [Google Scholar]
- Cicchetti D., Rogosch F. A. (2001). The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev. Psychopathol. 13, 783–804. [PubMed] [Google Scholar]
- Cima M., Smeets T., Jelicic M. (2008). Self-reported trauma, cortisol levels, and aggression in psychopathic and non-psychopathic prison inmates. Biol. Psychol. 78, 75–86. 10.1016/j.biopsycho.2007.12.011 [DOI] [PubMed] [Google Scholar]
- Cordero M. I., Moser D. A., Manini A., Suardi F., Sancho-Rossignol A., Torrisi R., et al. (2017). Effects of interpersonal violence-related post-traumatic stress disorder (PTSD) on mother and child diurnal cortisol rhythm and cortisol reactivity to a laboratory stressor involving separation. Horm. Behav. 90, 15–24. 10.1016/j.yhbeh.2017.02.007 [DOI] [PubMed] [Google Scholar]
- Costin B. N., Wolen A. R., Fitting S., Shelton K. L., Miles M. F. (2013). Role of adrenal glucocorticoid signaling in prefrontal cortex gene expression and acute behavioral responses to ethanol. Alcohol. Clin. Exp. Res. 37, 57–66. 10.1111/j.1530-0277.2012.01841.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couture S., Brown T. G., Ouimet M. C., Gianoulakis C., Tremblay J., Carbonneau R. (2008). Hypothalamic-pituitary-adrenal axis response to stress in male DUI recidivists. Accid. Anal. Prev. 40, 246–253. 10.1016/j.aap.2007.06.003 [DOI] [PubMed] [Google Scholar]
- Couture S., Ouimet M. C., Dedovic K., Laurier C., Plusquellec P., Brown T. G. (2018). Blunted cortisol reactivity and risky driving in young offenders - a pilot study. Int. J. Adolesc. Med. Health. 10.1515/ijamh-2017-0123 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Dabbs J. M., Jr, Jurkovic G. J., Frady R. L. (1991). Salivary testosterone and cortisol among late adolescent male offenders. J. Abnorm. Child Psychol. 19, 469–478. 10.1007/BF00919089 [DOI] [PubMed] [Google Scholar]
- Das S., Sengupta S., Pathak K., Sah D., Mehta S., Avinash P. R., et al. (2018). Aggression as an independent entity even in psychosis - The role of cortisol. Psychiatry Res. 259, 405–411. 10.1016/j.psychres.2017.11.002 [DOI] [PubMed] [Google Scholar]
- Daskalakis N. P., Bagot R. C., Parker K. J., Vinkers C. H., de Kloet E. R. (2013). The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38, 1858–1873. 10.1016/j.psyneuen.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan J., Rauchs G., Guillery-Girard B. (2016). Rhythms dysregulation: A new perspective for understanding PTSD? J. Physiol. Paris 110(4 Pt B), 453–460. 10.1016/j.jphysparis.2017.01.004 [DOI] [PubMed] [Google Scholar]
- de Almeida R. M., Miczek K. A. (2002). Aggression escalated by social instigation or by discontinuation of reinforcement (frustration) in mice: inhibition by anpirtoline: a 5-HT1B receptor agonist. Neuropsychopharmacology 27, 171–181. 10.1016/S0893-133X(02)00291-9 [DOI] [PubMed] [Google Scholar]
- Delville Y., Melloni R. H., Jr., Ferris C. F. (1998). Behavioral and neurobiological consequences of social subjugation during puberty in golden hamsters. J. Neurosci. 18, 2667–2672. 10.1523/JNEUROSCI.18-07-02667.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge K. A., Bates J. E., Pettit G. S. (1990). Mechanisms in the cycle of violence. Science 250, 1678–1683. 10.1126/science.2270481 [DOI] [PubMed] [Google Scholar]
- Dolan M., Anderson I. M., Deakin J. F. (2001). Relationship between 5-HT function and impulsivity and aggression in male offenders with personality disorders. Br. J. Psychiatry 178, 352–359. 10.1192/bjp.178.4.352 [DOI] [PubMed] [Google Scholar]
- Doom J. R., Cicchetti D., Rogosch F. A. (2014). Longitudinal patterns of cortisol regulation differ in maltreated and nonmaltreated children. J. Am. Acad. Child Adolesc. Psychiatry 53, 1206–1215. 10.1016/j.jaac.2014.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga B. M., Roelofs K., Tollenaar M. S., Bakvis P., van Pelt J., Spinhoven P. (2008). Diminished cortisol responses to psychosocial stress associated with lifetime adverse events a study among healthy young subjects. Psychoneuroendocrinology 33, 227–237. 10.1016/j.psyneuen.2007.11.004 [DOI] [PubMed] [Google Scholar]
- Ewing-Cobbs L., Prasad M. R., Cox C. S., Jr, Granger D. A., Duque G., Swank P. R. (2017). Altered stress system reactivity after pediatric injury: Relation with post-traumatic stress symptoms. Psychoneuroendocrinology 84, 66–75. 10.1016/j.psyneuen.2017.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairchild G., van Goozen S. H., Stollery S. J., Brown J., Gardiner J., Herbert J., et al. (2008). Cortisol diurnal rhythm and stress reactivity in male adolescents with early-onset or adolescence-onset conduct disorder. Biol. Psychiatry 64, 599–606. 10.1016/j.biopsych.2008.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y., Herrera-Melendez A. L., Pestke K., Feeser M., Aust S., Otte C., et al. (2014). Early life stress modulates amygdala-prefrontal functional connectivity: implications for oxytocin effects. Hum. Brain Mapp. 35, 5328–5339. 10.1002/hbm.22553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanti K. A., Kimonis E. (2017). Heterogeneity in externalizing problems at age 3: Association with age 15 biological and environmental outcomes. Dev. Psychol. 53, 1230–1241. 10.1037/dev0000317 [DOI] [PubMed] [Google Scholar]
- Feilhauer J., Cima M., Korebrits A., Nicolson N. A. (2013). Salivary cortisol and psychopathy dimensions in detained antisocial adolescents. Psychoneuroendocrinology 38, 1586–1595. 10.1016/j.psyneuen.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Ferris C. F. (2003). Using an animal model to assess the long-term behavioral and biological consequences of adolescent abuse and exposure to alcohol. Ann. N.Y. Acad. Sci. 1008, 69–78. 10.1196/annals.1301.008 [DOI] [PubMed] [Google Scholar]
- Feshbach S. (1971). The function of aggression and the regulation of aggressive drive. Psychol. Rev. 71, 257–272. 10.1037/h0043041 [DOI] [PubMed] [Google Scholar]
- Flory J. D., Yehuda R., Grossman R., New A. S., Mitropoulou V., Siever L. J. (2009). Childhood trauma and basal cortisol in people with personality disorders. Compr. Psychiatry 50, 34–37. 10.1016/j.comppsych.2008.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries E., Hesse J., Hellhammer J., Hellhammer D. H. (2005). A new view on hypocortisolism. Psychoneuroendocrinology 30, 1010–1016. 10.1016/j.psyneuen.2005.04.006 [DOI] [PubMed] [Google Scholar]
- Gammie S. C., Hasen N. S., Stevenson S. A., Bale T. L., D'Anna K. L. (2005). Elevated stress sensitivity in corticotropin-releasing factor receptor 2 deficient mice decreases maternal, but not intermale aggression. Behav. Brain Res. 160, 169–177. 10.1016/j.bbr.2004.11.026 [DOI] [PubMed] [Google Scholar]
- Gammie S. C., Stevenson S. A. (2006). Effects of daily and acute restraint stress during lactation on maternal aggression and behavior in mice. Stress 9, 171–180. 10.1080/10253890600969106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geniole S. N., Carré J. M., McCormick C. M. (2011). State, not trait, neuroendocrine function predicts costly reactive aggression in men after social exclusion and inclusion. Biol. Psychol. 87, 137–145. 10.1016/j.biopsycho.2011.02.020 [DOI] [PubMed] [Google Scholar]
- Gerra G., Zaimovic A., Moi G., Bussandri M., Bubici C., Mossini M., et al. (2004). Aggressive responding in abstinent heroin addicts: neuroendocrine and personality correlates. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 129–139. 10.1016/j.pnpbp.2003.09.029 [DOI] [PubMed] [Google Scholar]
- Gerra G., Zaimovic A., Raggi M. A., Giusti F., Delsignore R., Bertacca S., et al. (2001). Aggressive responding of male heroin addicts under methadone treatment: psychometric and neuroendocrine correlates. Drug Alcohol. Depend. 65, 85–95. 10.1016/S0376-8716(01)00152-1 [DOI] [PubMed] [Google Scholar]
- Glenn A. L., Raine A., Schug R. A., Gao Y., Granger D. A. (2011). Increased testosterone-to-cortisol ratio in psychopathy. J. Abnorm. Psychol. 120, 389–399. 10.1037/a0021407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobrogge K. L., Liu Y., Young L. J., Wang Z. (2009). Anterior hypothalamic vasopressin regulates pair-bonding and drug-induced aggression in a monogamous rodent. Proc. Natl. Acad. Sci. U.S.A. 106, 19144–19149. 10.1073/pnas.0908620106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordis E. B., Granger D. A., Susman E. J., Trickett P. K. (2006). Asymmetry between salivary cortisol and alpha-amylase reactivity to stress: relation to aggressive behavior in adolescents. Psychoneuroendocrinology 31, 976–987. 10.1016/j.psyneuen.2006.05.010 [DOI] [PubMed] [Google Scholar]
- Gostisha A. J., Vitacco M. J., Dismukes A. R., Brieman C., Merz J., Shirtcliff E. A. (2014). Beyond physiological hypoarousal: the role of life stress and callous-unemotional traits in incarcerated adolescent males. Horm. Behav. 65, 469–479. 10.1016/j.yhbeh.2014.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grotzinger A. D., Mann F. D., Patterson M. W., Tackett J. L., Tucker-Drob E. M., Harden K. P. (2018). Hair and salivary testosterone, hair cortisol, and externalizing behaviors in adolescents. Psychol. Sci. 29, 688–699. 10.1177/0956797617742981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M. R., Vazquez D. M. (2001). Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 13, 515–538. 10.1017/S0954579401003066 [DOI] [PubMed] [Google Scholar]
- Haller J. (2013). The neurobiology of abnormal manifestations of aggression–a review of hypothalamic mechanisms in cats, rodents, and humans. Brain Res. Bull. 93, 97–109. 10.1016/j.brainresbull.2012.10.003 [DOI] [PubMed] [Google Scholar]
- Haller J. (2014). The glucocorticoid/aggression relationship in animals and humans: an analysis sensitive to behavioral characteristics, glucocorticoid secretion patterns, and neural mechanisms. Curr. Top. Behav. Neurosci. 17, 73–109. 10.1007/7854_2014_284 [DOI] [PubMed] [Google Scholar]
- Haller J. (2016). Preclinical models of conduct disorder - principles and pharmacologic perspectives. Neurosci. Biobehav. Rev. 10.1016/j.neubiorev.2016.05.032 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Haller J. (2017). Studies into abnormal aggression in humans and rodents: Methodological and translational aspects. Neurosci. Biobehav. Rev. 76(Pt A), 77–86. 10.1016/j.neubiorev.2017.02.022 [DOI] [PubMed] [Google Scholar]
- Haller J., Halasz J., Makara G. B., Kruk M. R. (1998). Acute effects of glucocorticoids: behavioral and pharmacological perspectives. Neurosci. Biobehav. Rev. 23, 337–344. 10.1016/S0149-7634(98)00035-9 [DOI] [PubMed] [Google Scholar]
- Haller J., Halasz J., Mikics E., Kruk M. R. (2004). Chronic glucocorticoid deficiency-induced abnormal aggression, autonomic hypoarousal, and social deficit in rats. J. Neuroendocrinol. 16, 550–557. 10.1111/j.1365-2826.2004.01201.x [DOI] [PubMed] [Google Scholar]
- Haller J., Harold G., Sandi C., Neumann I. D. (2014). Effects of adverse early-life events on aggression and anti-social behaviours in animals and humans. J Neuroendocrinol. 26, 724–738. 10.1111/jne.12182 [DOI] [PubMed] [Google Scholar]
- Haller J., Mikics E., Makara G. B. (2008). The effects of non-genomic glucocorticoid mechanisms on bodily functions and the central neural system. A critical evaluation of findings. Front. Neuroendocrinol. 29, 273–291. 10.1016/j.yfrne.2007.10.004 [DOI] [PubMed] [Google Scholar]
- Haller J., Toth M., Halasz J., De Boer S. F. (2006). Patterns of violent aggression-induced brain c-fos expression in male mice selected for aggressiveness. Physiol. Behav. 88, 173–182. 10.1016/j.physbeh.2006.03.030 [DOI] [PubMed] [Google Scholar]
- Haller J., Van De Schraaf J., Kruk M. R. (2001). Deviant forms of aggression in glucocorticoid hyporeactive rats: a model for 'pathological' aggression? J. Neuroendocrinol. 13, 102–107. 10.1046/j.1365-2826.2001.00600.x [DOI] [PubMed] [Google Scholar]
- Hasen N. S., Gammie S. C. (2006). Maternal aggression: new insights from Egr-1. Brain Res. 1108, 147–156. 10.1016/j.brainres.2006.06.007 [DOI] [PubMed] [Google Scholar]
- Hasler G., Pine D. S., Gamma A., Milos G., Ajdacic V., Eich D., et al. (2004). The associations between psychopathology and being overweight: a 20-year prospective study. Psychol. Med. 34, 1047–1057. 10.1017/S0033291703001697 [DOI] [PubMed] [Google Scholar]
- Hawes D. J., Brennan J., Dadds M. R. (2009). Cortisol, callous-unemotional traits, and pathways to antisocial behavior. Curr. Opin. Psychiatry 22, 357–362. 10.1097/YCO.0b013e32832bfa6d [DOI] [PubMed] [Google Scholar]
- Hess W. R. (1928). Stammganglien-reizversuche. Berichte Gesamt. Physiol. 42, 554–555. [Google Scholar]
- Holi M., Auvinen-Lintunen L., Lindberg N., Tani P., Virkkunen M. (2006). Inverse correlation between severity of psychopathic traits and serum cortisol levels in young adult violent male offenders. Psychopathology 39, 102–104. 10.1159/000091021 [DOI] [PubMed] [Google Scholar]
- Horn M., Potvin S., Allaire J. F., Côté G., Gobbi G., Benkirane K., et al. (2014). Male inmate profiles and their biological correlates. Can. J. Psychiatry 59, 441–449. 10.1177/070674371405900807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janusek L. W., Tell D., Gaylord-Harden N., Mathews H. L. (2017). Relationship of childhood adversity and neighborhood violence to a proinflammatory phenotype in emerging adult African American men: An epigenetic link. Brain Behav. Immun. 60, 126–135. 10.1016/j.bbi.2016.10.006 [DOI] [PubMed] [Google Scholar]
- Joëls M., Karst H., Sarabdjitsingh R. A. (2018). The stressed brain of humans and rodents. Acta Physiol. 233:e13066. 10.1111/apha.13066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joëls M., Pasricha N., Karst H. (2013). The interplay between rapid and slow corticosteroid actions in brain. Eur. J. Pharmacol. 719, 44–52. 10.1016/j.ejphar.2013.07.015 [DOI] [PubMed] [Google Scholar]
- Johnson M. M., Mikolajewski A., Shirtcliff E. A., Eckel L. A., Taylor J. (2015). The association between affective psychopathic traits, time incarcerated, and cortisol response to psychosocial stress. Horm. Behav. 72, 20–27. 10.1016/j.yhbeh.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juruena M. F., Bocharova M., Agustini B., Young A. H. (2017). Atypical depression and non-atypical depression: is HPA axis function a biomarker? A systematic review. J. Affect. Disord. 233, 45–67. 10.1016/j.jad.2017.09.052 [DOI] [PubMed] [Google Scholar]
- Kaiser R. H., Clegg R., Goer F., Pechtel P., Beltzer M., Vitaliano G., et al. (2018). Childhood stress, grown-up brain networks: corticolimbic correlates of threatrelated early life stress and adult stress response. Psychol. Med. 48, 1157–1166. 10.1017/S0033291717002628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapoor A., Petropoulos S., Matthews S. G. (2008). Fetal programming of hypothalamic-pituitary-adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res. Rev. 57, 586–595. 10.1016/j.brainresrev.2007.06.013 [DOI] [PubMed] [Google Scholar]
- Kariyawasam S. H., Zaw F., Handley S. L. (2002). Reduced salivary cortisol in children with comorbid Attention deficit hyperactivity disorder and oppositional defiant disorder. Neuroendocrinol. Lett. 23, 45–48. Available online at: https://europepmc.org/abstract/MED/11880861 [PubMed] [Google Scholar]
- Kempes M., Matthys W., de Vries H., van Engeland H. (2005). Reactive and proactive aggression in children–a review of theory, findings and the relevance for child and adolescent psychiatry. Eur. Child. Adolesc. Psychiatry 14, 11–19. 10.1007/s00787-005-0432-4 [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B., Hastings P. D., Granger D. A., Usher B. A., Zahn-Waxler C. (2001). Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev. Psychopathol. 13, 695–719. 10.1017/S0954579401003157 [DOI] [PubMed] [Google Scholar]
- Koolhaas J. M. (1978). Hypothalamically induced intraspecific aggressive behaviour in the rat. Exper. Brain Res. 32, 365–375. 10.1007/BF00238708 [DOI] [PubMed] [Google Scholar]
- Koss K. J., Hostinar C. E., Donzella B., Gunnar M. R. (2014). Social deprivation and the HPA axis in early development. Psychoneuroendocrinology 50, 1–13. 10.1016/j.psyneuen.2014.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruesi M. J., Schmidt M. E., Donnelly M., Hibbs E. D., Hamburger S. D. (1989). Urinary free cortisol output and disruptive behavior in children. J. Am. Acad. Child Adolesc. Psychiatry 28, 441–443. 10.1097/00004583-198905000-00024 [DOI] [PubMed] [Google Scholar]
- Kruk M. R. (1991). Ethology and pharmacology of hypothalamic aggression in the rat. Neurosci. Biobehav. Rev. 15, 527–538. 10.1016/S0149-7634(05)80144-7 [DOI] [PubMed] [Google Scholar]
- Kruk M. R. (2014). Hypothalamic attack: a wonderful artifact or a useful perspective on escalation and pathology in aggression? A viewpoint. Curr. Top. Behav. Neurosci. 17, 143–188. 10.1007/7854_2014_313 [DOI] [PubMed] [Google Scholar]
- Kruk M. R., Halász J., Meelis W., Haller J. (2004). Fast positive feedback between the adrenocortical stress response and a brain mechanism involved in aggressive behavior. Behav. Neurosci. 118, 1062–1070. 10.1037/0735-7044.118.5.1062 [DOI] [PubMed] [Google Scholar]
- Kruk M. R., Haller J., Meelis W., de Kloet E. R. (2013). Mineralocorticoid receptor blockade during a rat's first violent encounter inhibits its subsequent propensity for violence. Behav. Neurosci. 127, 505–514. 10.1037/a0033553 [DOI] [PubMed] [Google Scholar]
- Kruk M. R., van der Poel A. M., de Vos-Frerichs T. P. (1979). The induction of aggressive behaviour by electrical stimulation in the hypothalamus of male rats. Behaviour 70, 292–322. 10.1163/156853979X00106 [DOI] [PubMed] [Google Scholar]
- Kruk M. R., Westphal K. G., Van Erp A. M., van Asperen J., Cave B. J., Slater E., et al. (1998). The hypothalamus: cross-roads of endocrine and behavioural regulation in grooming and aggression. Neurosci. Biobehav. Rev. 23, 163–177. 10.1016/S0149-7634(98)00018-9 [DOI] [PubMed] [Google Scholar]
- Kudielka B. M., Wüst S. (2010). Human models in acute and chronic stress: assessing determinants of individual hypothalamus-pituitary-adrenal axis activity and reactivity. Stress 13, 1–14. 10.3109/10253890902874913 [DOI] [PubMed] [Google Scholar]
- Kuhlman K. R., Chiang J. J., Horn S., Bower J. E. (2017). Developmental psychoneuroendocrine and psychoneuroimmune pathways from childhood adversity to disease. Neurosci. Biobehav. Rev. 80, 166–184. 10.1016/j.neubiorev.2017.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D., Boyle M. P., Dollar P., Lee H., Lein E. S., Perona P., et al. (2011). Functional identification of an aggression locus in the mouse hypothalamus. Nature 470, 221–226. 10.1038/nature09736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeber R., Hay D. (1997). Key issues in the development of aggression and violence from childhood to early adulthood. Annu. Rev. Psychol. 48, 371–410. 10.1146/annurev.psych.48.1.371 [DOI] [PubMed] [Google Scholar]
- Loney B. R., Butler M. A., Lima E. N., Counts C. A., Eckel L. A. (2006). The relation between salivary cortisol, callous-unemotional traits, and conduct problems in an adolescent non-referred sample. J. Child Psychol. Psychiatry 47, 30–36. 10.1111/j.1469-7610.2005.01444.x [DOI] [PubMed] [Google Scholar]
- Loomans M. M., Tulen J. H., de Rijke Y. B., van Marle H. J. (2016). A hormonal approach to anti-social behaviour. Crim. Behav. Ment. Health 26, 380–394. 10.1002/cbm.1968 [DOI] [PubMed] [Google Scholar]
- Lopez-Duran N. L., Olson S. L., Hajal N. J., Felt B. T., Vazquez D. M. (2009). Hypothalamic pituitary adrenal axis functioning in reactive and proactive aggression in children. J. Abnormal Child Psychol. 37, 169–182. 10.1007/s10802-008-9263-3 [DOI] [PubMed] [Google Scholar]
- Lovallo W. R., Farag N. H., Sorocco K. H., Cohoon A. J., Vincent A. S. (2012). Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma family health patterns project. Biol. Psychiatry. 71, 344–349. 10.1016/j.biopsych.2011.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz P. E., Turecki G. (2014). DNA methylation and childhood maltreatment: from animal models to human studies. Neuroscience 264, 142–156. 10.1016/j.neuroscience.2013.07.069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez C., Poirier G. L., Cordero M. I., Larsen M. H., Groner A., Marquis J., et al. (2013). Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry 3:e216. 10.1038/tp.2012.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsman R., Swinkels S. H., Rosmalen J. G., Oldehinkel A. J., Ormel J., Buitelaar J. K. (2008). HPA-axis activity and externalizing behavior problems in early adolescents from the general population: the role of comorbidity and gender The TRAILS study. Psychoneuroendocrinology 33, 789–798. 10.1016/j.psyneuen.2008.03.005 [DOI] [PubMed] [Google Scholar]
- Martin C. G., Kim H. K., Bruce J., Fisher P. A. (2014). Child diurnal cortisol rhythms, parenting quality, and externalizing behaviors in preadolescence. Psychoneuroendocrinology 40, 170–180. 10.1016/j.psyneuen.2013.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason A., Blankenship V. (1987). Power and affiliation motivation, stress, and abuse in intimate relationships. J. Pers. Soc. Psychol. 52, 203–210. 10.1037/0022-3514.52.1.203 [DOI] [PubMed] [Google Scholar]
- McBurnett K., King J., Scarpa A. (2003). The HPA and the development of aggressive, antisocial, and substance abuse disorders, in Neurodevelopmental Mechanisms in Psychopathology, eds Cicchetti D., Walker E. (New York, NY: Cambridge University Press; ), 324–344. [Google Scholar]
- McBurnett K., Lahey B. B., Rathouz P. J., Loeber R. (2000). Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Arch. Gen. Psychiatry 57, 38–43. 10.1001/archpsyc.57.1.38 [DOI] [PubMed] [Google Scholar]
- McBurnett K., Raine A., Stouthamer-Loeber M., Loeber R., Kumar A. M., Kumar M., et al. (2005). Mood and hormone responses to psychological challenge in adolescent males with conduct problems. Biol. Psychiatry 57, 1109–1116. 10.1016/j.biopsych.2005.01.041 [DOI] [PubMed] [Google Scholar]
- McLaughlin K. A., Sheridan M. A., Tibu F., Fox N. A., Zeanah C. H., Nelson C. A., et al. (2015). Causal effects of the early caregiving environment on development of stress response systems in children. Proc. Natl. Acad. Sci. U.S.A. 112, 5637–5642. 10.1073/pnas.1423363112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melloni R. H., Jr., Ferris C. F. (1996). Adolescent anabolic steroid use and aggressive behavior in golden hamsters. Ann. N.Y. Acad. Sci. 794, 372–375. 10.1111/j.1749-6632.1996.tb32546.x [DOI] [PubMed] [Google Scholar]
- Miczek K. A., de Boer S. F., Haller J. (2013). Excessive aggression as model of violence: a critical evaluation of current preclinical methods. Psychopharmacology 226, 445–458. 10.1007/s00213-013-3008-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek K. A., Weerts E. M., Tornatzky W., DeBold J. F., Vatne T. M. (1992). Alcohol and bursts of aggressive behavior: ethological analysis of individual differences in rats. Psychopharmacology 107, 551–563. 10.1007/BF02245270 [DOI] [PubMed] [Google Scholar]
- Mikics É., Guirado R., Umemori J., Tóth M., Biró L., Miskolczi C., et al. (2018). Social learning requires plasticity enhanced by fluoxetine through prefrontal Bdnf-TrkB signaling to limit aggression induced by post-weaning social isolation. Neuropsychopharmacology 43, 235–245. 10.1038/npp.2017.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikics E., Kruk M. R., Haller J. (2004). Genomic and non-genomic effects of glucocorticoids on aggressive behavior in male rats. Psychoneuroendocrinology 29, 618–635. 10.1016/S0306-4530(03)00090-8 [DOI] [PubMed] [Google Scholar]
- Moss H. B., Vanyukov M. M., Martin C. S. (1995). Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biol. Psychiatry 38, 547–555. 10.1016/0006-3223(94)00382-D [DOI] [PubMed] [Google Scholar]
- Mutic S., Brünner Y. F., Rodriguez-Raecke R., Wiesmann M., Freiherr J. (2017). Chemosensory danger detection in the human brain: body odor communicating aggression modulates limbic system activation. Neuropsychologia 99, 187–198. 10.1016/j.neuropsychologia.2017.02.018 [DOI] [PubMed] [Google Scholar]
- Natarajan D., Caramaschi D. (2010). Animal violence demystified. Front. Behav. Neurosci. 4:9. 10.3389/fnbeh.2010.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I. D. (2001). Alterations in behavioral and neuroendocrine stress coping strategies in pregnant, parturient and lactating rats. Prog. Brain Res. 133, 143–152. 10.1016/S0079-6123(01)33011-X [DOI] [PubMed] [Google Scholar]
- Neumann I. D., Toschi N., Ohl F., Torner L., Kromer S. A. (2001). Maternal defence as an emotional stressor in female rats: correlation of neuroendocrine and behavioural parameters and involvement of brain oxytocin. Eur. J. Neurosci. 13, 1016–1024. 10.1046/j.0953-816x.2001.01460.x [DOI] [PubMed] [Google Scholar]
- Neumann I. D., Veenema A. H., Beiderbeck D. I. (2010). Aggression and anxiety: social context and neurobiological links. Front. Behav. Neurosci. 4:12. 10.3389/fnbeh.2010.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijhof S. L., Rutten J. M., Uiterwaal C. S., Bleijenberg G., Kimpen J. L., Putte E. M. (2014). The role of hypocortisolism in chronic fatigue syndrome. Psychoneuroendocrinology 42, 199–206. 10.1016/j.psyneuen.2014.01.017 [DOI] [PubMed] [Google Scholar]
- Northover C., Thapar A., Langley K., Fairchild G., van Goozen S. H. M. (2016). Cortisol levels at baseline and under stress in adolescent males with attention-deficit hyperactivity disorder, with or without comorbid conduct disorder. Psychiatry Res. 242, 130–136. 10.1016/j.psychres.2016.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberle E., McLachlan K., Catherine N. L. A., Brain U., Schonert-Reichl K. A., Weinberg J., et al. (2017). Afternoon cortisol provides a link between self-regulated anger and peer-reported aggression in typically developing children in the school context. Dev. Psychobiol. 59, 688–695. 10.1002/dev.21522 [DOI] [PubMed] [Google Scholar]
- O'Leary M. M., Loney B. R., Eckel L. A. (2007). Gender differences in the association between psychopathic personality traits and cortisol response to induced stress. Psychoneuroendocrinology 32, 183–191. 10.1016/j.psyneuen.2006.12.004 [DOI] [PubMed] [Google Scholar]
- O'Neal C. R., Brotman L. M., Huang K. Y., Gouley K. K., Kamboukos D., Calzada E. J., et al. (2010). Understanding relations among early family environment, cortisol response, and child aggression via a prevention experiment. Child Dev. 81, 290–305. 10.1111/j.1467-8624.2009.01395.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterlaan J., Geurts H. M., Knol D. L., Sergeant J. A. (2005). Low basal salivary cortisol is associated with teacher-reported symptoms of conduct disorder. Psychiatry Res. 134:1–10. 10.1016/j.psychres.2004.12.005 [DOI] [PubMed] [Google Scholar]
- Ouellet-Morin I., Odgers C. L., Danese A., Bowes L., Shakoor S., Papadopoulos A. S., et al. (2011). Blunted cortisol responses to stress signal social and behavioral problems among maltreated/bullied 12-year-old children. Biol. Psychiatry 70, 1016–1023. 10.1016/j.biopsych.2011.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajer K., Gardner W., Rubin R. T., Perel J., Neal S. (2001). Decreased cortisol levels in adolescent girls with conduct disorder. Arch. Gen. Psychiatry 58, 297–302. 10.1001/archpsyc.58.3.297 [DOI] [PubMed] [Google Scholar]
- Pajer K., Tabbah R., Gardner W., Rubin R. T., Czambel R. K., Wang Y. (2006). Adrenal androgen and gonadal hormone levels in adolescent girls with conduct disorder. Psychoneuroendocrinology 31, 1245–1256. 10.1016/j.psyneuen.2006.09.005 [DOI] [PubMed] [Google Scholar]
- Parmigiani S., Brain P. F., Mainardi D., Brunoni V. (1988). Different patterns of biting attack employed by lactating female mice (Musdomesticus) in encounters with male and female conspecific intruders. J. Comp. Psychol. 102, 287–293. 10.1037/0735-7036.102.3.287 [DOI] [PubMed] [Google Scholar]
- Paxinos G., Watson C. (1998). The Rat Brain in Stereotaxic Coordinates, 4th Edn. San Diego, CA: Academic Press. [Google Scholar]
- Peckins M. K., Susman E. J., Negriff S., Noll J., Trickett P. K. (2015). Cortisol profiles: a test for adaptive calibration of the stress response system in maltreated and nonmaltreated youth. Dev. Psychopathol. 27, 1461–1470. 10.1017/S0954579415000875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen A. K., Kajantie E., Jones A., Pyhälä R., Lahti J., Heinonen K., et al. (2011). Symptoms of attention deficit hyperactivity disorder in children are associated with cortisol responses to psychosocial stress but not with daily cortisol levels. J. Psychiatr. Res. 45, 1471–1476. 10.1016/j.jpsychires.2011.07.002 [DOI] [PubMed] [Google Scholar]
- Pinto R. J., Correia-Santos P., Costa-Leite J., Levendosky A. A., Jongenelen I. (2016). Cortisol awakening response among women exposed to intimate partner violence. Psychoneuroendocrinology 74, 57–64. 10.1016/j.psyneuen.2016.08.024 [DOI] [PubMed] [Google Scholar]
- Platje E., Jansen L. M., Raine A., Branje S. J., Doreleijers T. A., de Vries-Bouw M., et al. (2013). Longitudinal associations in adolescence between cortisol and persistent aggressive or rule-breaking behavior. Biol. Psychol. 93, 132–137. 10.1016/j.biopsycho.2013.01.002 [DOI] [PubMed] [Google Scholar]
- Poole A. E., Brain P. (1974). Effects of adrenalectomy and treatments with ACTH and glucocorticoids on isolation-induced aggressive behavior in male albino mice. Prog. Brain Res. 41, 465–472. 10.1016/S0079-6123(08)61926-3 [DOI] [PubMed] [Google Scholar]
- Popma A., Doreleijers T. A., Jansen L. M., Van Goozen S. H., Van Engeland H., Vermeiren R. (2007a). The diurnal cortisol cycle in delinquent male adolescents and normal controls. Neuropsychopharmacology 32, 1622–1628. 10.1038/sj.npp.1301289 [DOI] [PubMed] [Google Scholar]
- Popma A., Jansen L. M., Vermeiren R., Steiner H., Raine A., Van Goozen S. H., et al. (2006). Hypothalamus pituitary adrenal axis and autonomic activity during stress in delinquent male adolescents and controls. Psychoneuroendocrinology 31, 948–957. 10.1016/j.psyneuen.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Popma A., Vermeiren R., Geluk C. A., Rinne T., van den Brink W., Knol D. L., et al. (2007b). Cortisol moderates the relationship between testosterone and aggression in delinquent male adolescents. Biol. Psychiatry 61, 405–411. 10.1016/j.biopsych.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Portnoy J., Raine A., Glenn A. L., Chen F. R., Choy O., Granger D. A. (2015). Digit ratio (2D:4D) moderates the relationship between cortisol reactivity and self-reported externalizing behavior in young adolescent males. Biol. Psychol. 112, 94–106. 10.1016/j.biopsycho.2015.09.013 [DOI] [PubMed] [Google Scholar]
- Posner I., Conway K. M. (1981). Arousal of sexual and aggressive contact by exposure to stressors in albino rats. J. Gen. Psychol. 104, 145–151. [DOI] [PubMed] [Google Scholar]
- Poustka L., Maras A., Hohm E., Fellinger J., Holtmann M., Banaschewski T., et al. (2010). Negative association between plasma cortisol levels and aggression in a high-risk community sample of adolescents. J. Neural Transm. 117, 621–627. 10.1007/s00702-010-0386-7 [DOI] [PubMed] [Google Scholar]
- Pruessner M., Cullen A. E., Aas M., Walker E. F. (2017). The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci. Biobehav. Rev. 73, 191–218. 10.1016/j.neubiorev.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Public Health Report . (1983). Health promotion: Control of stress and violent behavior. Public Health Rep. (Suppl.), 167–176. [PMC free article] [PubMed] [Google Scholar]
- Puetz V. B., Zweerings J., Dahmen B., Ruf C., Scharke W., Herpertz-Dahlmann B., et al. (2016). Multidimensional assessment of neuroendocrine and psychopathological profiles in maltreated youth. J. Neural Transm. 123, 1095–1106. 10.1007/s00702-016-1509-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz V. B., Parker D., Kohn N., Dahmen B., Verma R., Konrad K. (2017). Altered brain network integrity after childhood maltreatment: A structural connectomic DTI-study. Hum. Brain Mapp. 38, 855–868. 10.1002/hbm.23423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo K., Doty J., Roos L., Anker J. J. (2017). The cortisol awakening response and anterior cingulate cortex function in maltreated depressed versus non-maltreated depressed youth. Psychoneuroendocrinology 86, 87–95. 10.1016/j.psyneuen.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A., Dodge K., Loeber R., Gatzke-Kopp L., Lynam D., Reynolds C., et al. (2006). The reactive-proactive aggression questionnaire: differential correlates of reactive and proactive aggression in adolescent boys. Aggress Behav. 32, 159–171. 10.1002/ab.20115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond C., Marin M. F., Majeur D., Lupien S. (2017). Early child adversity and psychopathology in adulthood: HPA axis and cognitive dysregulations as potential mechanisms. Prog. Neuropsychopharmacol. Biol. Psychiatry 85, 152–160. 10.1016/j.pnpbp.2017.07.015 [DOI] [PubMed] [Google Scholar]
- Ricci L. A., Grimes J. M., Knyshevski I., Melloni R. H., Jr. (2005). Repeated cocaine exposure during adolescence alters glutamic acid decarboxylase-65 (GAD65) immunoreactivity in hamster brain: correlation with offensive aggression. Brain Res. 1035, 131–138. 10.1016/j.brainres.2004.11.049 [DOI] [PubMed] [Google Scholar]
- Rosa M., Franzini A., Giannicola G., Messina G., Altamura A. C., Priori A. (2012). Hypothalamic oscillations in human pathological aggressiveness. Biol. Psychiatry. 72, e33–e35. 10.1016/j.biopsych.2012.06.007 [DOI] [PubMed] [Google Scholar]
- Ruttle P. L., Shirtcliff E. A., Serbin L. A., Fisher D. B., Stack D. M., Schwartzman A. E. (2011). Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm. Behav. 59, 123–132. 10.1016/j.yhbeh.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salis K. L., Bernard K., Black S. R., Dougherty L. R., Klein D. (2016). Examining the concurrent and longitudinal relationship between diurnal cortisol rhythms and conduct problems during childhood. Psychoneuroendocrinology 71, 147–154. 10.1016/j.psyneuen.2016.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez M. M., Noble P. M., Lyon C. K., Plotsky P. M., Davis M., Nemeroff C. B., et al. (2005). Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol. Psychiatry 57, 373–381. 10.1016/j.biopsych.2004.11.032 [DOI] [PubMed] [Google Scholar]
- Sano K., Mayanagi Y., Sekino H., Ogashiwa M., Ishijima B. (1970). Results of stimulation and destruction of the posterior hypothalamus in man. J. Neurosurg. 33, 689–707. 10.3171/jns.1970.33.6.0689 [DOI] [PubMed] [Google Scholar]
- Saxbe D. E., Margolin G., Spies Shapiro L. A., Baucom B. R. (2012). Does dampened physiological reactivity protect youth in aggressive family environments? Child Dev. 83, 821–830. 10.1111/j.1467-8624.2012.01752.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbo A. S., Kolko D. J. (1994). Salivary testosterone and cortisol in disruptive children: relationship to aggressive, hyperactive, and internalizing behaviors. J. Am. Acad. Child Adolesc. Psychiatry 33, 1174–1184. 10.1097/00004583-199410000-00013 [DOI] [PubMed] [Google Scholar]
- Schoorl J., Rijn S. V., Wied M., van Goozen S., Swaab H. (2016). The role of anxiety in cortisol stress response and cortisol recovery in boys with oppositional defiant disorder/conduct disorder. Psychoneuroendocrinology 73, 217–223. 10.1016/j.psyneuen.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Sesack S. R., Deutch A. Y., Roth R. H., Bunney B. S. (1989). Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J. Comp. Neurol. 290, 213–242. 10.1002/cne.902900205 [DOI] [PubMed] [Google Scholar]
- Shaikh M. B., Barrett J. A., Siegel A. (1987). The pathways mediating affective defense and quiet biting attack behavior from the midbrain central gray of the cat: an autoradiographic study. Brain Res. 437, 9–25. 10.1016/0006-8993(87)91522-8 [DOI] [PubMed] [Google Scholar]
- Shirtcliff E. A., Essex M. J. (2008). Concurrent and longitudinal associations of basal and diurnal cortisol with mental health symptoms in early adolescence. Dev. Psychobiol. 50, 690–703. 10.1002/dev.20336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff E. A., Granger D. A., Booth A., Johnson D. (2005). Low salivary cortisol levels and externalizing behavior problems in youth. Dev. Psychopathol. 17, 167–184. 10.1017/S0954579405050091 [DOI] [PubMed] [Google Scholar]
- Shoal G. D., Giancola P. R., Kirillova G. P. (2003). Salivary cortisol, personality, and aggressive behavior in adolescent boys: a 5-year longitudinal study. J. Am. Acad. Child Adolesc. Psychiatry 42, 1101–1107. 10.1097/01.CHI.0000070246.24125.6D [DOI] [PubMed] [Google Scholar]
- Siegel A., Bhatt S., Bhatt R., Zalcman S. S. (2007). The neurobiological bases for development of pharmacological treatments of aggressive disorders. Curr. Neuropharmacol. 5, 135–147. 10.2174/157015907780866929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel A., Roeling T. A., Gregg T. R., Kruk M. R. (1999). Neuropharmacology of brainstimulation-evoked aggression. Neurosci. Biobehav. Rev. 23, 359–389. 10.1016/S0149-7634(98)00040-2 [DOI] [PubMed] [Google Scholar]
- Smith D. E., King M. B., Hoebel B. G. (1970). Lateral hypothalamic control of killing: evidence for a cholinoceptive mechanism. Science 167, 900–901. 10.1126/science.167.3919.900 [DOI] [PubMed] [Google Scholar]
- Snoek H., van Goozen S. H., Matthys W., Sigling H. O., Koppeschaar H. P., Westenberg H. G., et al. (2002). Serotonergic functioning in children with oppositional defiant disorder: a sumatriptan challenge study. Biol. Psychiatry 51, 319–325. 10.1016/S0006-3223(01)01230-6 [DOI] [PubMed] [Google Scholar]
- Soderstrom H., Blennow K., Forsman A., Liesivuori J., Pennanen S., Tiihonen J. (2004). A controlled study of tryptophan and cortisol in violent offenders. J. Neural Transm 111, 1605–1610. 10.1007/s00702-004-0219-7 [DOI] [PubMed] [Google Scholar]
- Sondeijker F. E., Ferdinand R. F., Oldehinkel A. J., Tiemeier H., Ormel J., Verhulst F. C. (2008). HPA-axis activity as a predictor of future disruptive behaviors in young adolescents. Psychophysiology 45, 398–404. 10.1111/j.1469-8986.2008.00639.x [DOI] [PubMed] [Google Scholar]
- Stadler C., Kroeger A., Weyers P., Grasmann D., Horschinek M., Freitag C., et al. (2011). Cortisol reactivity in boys with attention-deficit/hyperactivity disorder and disruptive behavior problems: the impact of callous unemotional traits. Psychiatry Res. 187, 204–209. 10.1016/j.psychres.2010.05.004 [DOI] [PubMed] [Google Scholar]
- Stoppelbein L., Greening L., Luebbe A., Fite P., Becker S. P. (2014). The role of cortisol and psychopathic traits in aggression among at-risk girls: tests of mediating hypotheses. Aggress Behav. 40, 263–272. 10.1002/ab.21513 [DOI] [PubMed] [Google Scholar]
- Strous R. D., Maayan R., Lapidus R., Goredetsky L., Zeldich E., Kotler M., et al. (2004). Increased circulatory dehydroepiandrosterone and dehydroepiandrosterone-sulphate in first-episode schizophrenia: relationship to gender, aggression and symptomatology. Schizophr. Res. 71, 427–434. 10.1016/j.schres.2004.03.005 [DOI] [PubMed] [Google Scholar]
- Susman E. J., Nottelmann E. D., Dorn L. D., Inoff-Germain G., Chrousos G. P. (1988). Physiological and behavioral aspects of stress in adolescence. Adv. Exp. Med. Biol. 245, 341–352. 10.1007/978-1-4899-2064-5_27 [DOI] [PubMed] [Google Scholar]
- Theall K. P., Shirtcliff E. A., Dismukes A. R., Wallace M., Drury S. S. (2017). Association between neighborhood violence and biological stress in children. JAMA Pediatr. 171, 53–60. 10.1001/jamapediatrics.2016.2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth M., Fuzesi T., Halasz J., Tulogdi A., Haller J. (2010). Neural inputs of the hypothalamic “aggression area” in the rat. Behav. Brain Res. 215, 7–20. 10.1016/j.bbr.2010.05.050 [DOI] [PubMed] [Google Scholar]
- Tóth M., Halasz J., Mikics E., Barsy B., Haller J. (2008). Early social deprivation induces disturbed social communication and violent aggression in adulthood. Behav. Neurosci. 122, 849–854. 10.1037/0735-7044.122.4.849 [DOI] [PubMed] [Google Scholar]
- Trickett P. K., Gordis E., Peckins M. K., Susman E. J. (2014). Stress reactivity in maltreatedand comparison male and female young adolescents. Child Maltreat. 19, 27–37. 10.1177/1077559513520466 [DOI] [PubMed] [Google Scholar]
- Tulogdi A., Biro L., Barsvari B., Stankovic M., Haller J., Toth M. (2015). Neural mechanisms of predatory aggression in rats-implications for abnormal intraspecific aggression. Behav. Brain Res. 283, 108–115. 10.1016/j.bbr.2015.01.030 [DOI] [PubMed] [Google Scholar]
- Tulogdi A., Toth M., Halasz J., Mikics E., Fuzesi T., Haller J. (2010). Brain mechanisms involved in predatory aggression are activated in a laboratory model of violent intra-specific aggression. Eur. J. Neurosci. 32, 1744–1753. 10.1111/j.1460-9568.2010.07429.x [DOI] [PubMed] [Google Scholar]
- Tyrka A. R., Ridout K. K., Parade S. H. (2016). Childhood adversity and epigenetic regulation of glucocorticoid signaling genes: associations in children and adults. Dev. Psychopathol. 28, 1319–1331. 10.1017/S0954579416000870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaillancourt T., Sunderani S. (2011). Psychopathy and indirect aggression: the roles of cortisol, sex, and type of psychopathy. Brain Cogn. 77, 170–175. 10.1016/j.bandc.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Vaillancourt T., Duku E., Decatanzaro D., Macmillan H., Muir C., Schmidt L. A. (2008). Variation in hypothalamic-pituitary-adrenal axis activity among bullied and nonbullied children. Aggress. Behav. 34, 294–305. 10.1002/ab.20240 [DOI] [PubMed] [Google Scholar]
- van Bokhoven I., Van Goozen S. H., van Engeland H., Schaal B., Arseneault L., Séguin J. R., et al. (2005). Salivary cortisol and aggression in a population-based longitudinal study of adolescent males. J. Neural Transm. 112, 1083–1096. 10.1007/s00702-004-0253-5 [DOI] [PubMed] [Google Scholar]
- Van de Wiel N., van Goozen S., Matthys W., Snoek H., van Engeland H. (2004). Cortisol and treatment effect in children with disruptive behavior disorders: a preliminary study. J. Am. Acad. Child Adolosc. Psychiatry 43, 1011–1018. 10.1097/01.chi.0000126976.56955.43 [DOI] [PubMed] [Google Scholar]
- Van den Bergh B. R., Van Calster B., Pinna Puissant S., Van Huffel S. (2008). Self-reported symptoms of depressed mood, trait anxiety and aggressive behavior in post-pubertal adolescents: associations with diurnal cortisol profiles. Horm. Behav. 54, 253–257. 10.1016/j.yhbeh.2008.03.015 [DOI] [PubMed] [Google Scholar]
- Van den Stock J., Hortensius R., Sinke C., Goebel R., de Gelder B. (2015). Personality traits predict brain activation and connectivity when witnessing a violent conflict. Sci. Rep. 5:13779. 10.1038/srep13779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Vegt E. J., van der Ende J., Kirschbaum C., Verhulst F. C., Tiemeier H. (2009). Early neglect and abuse predict diurnal cortisol patterns in adults A study of international adoptees. Psychoneuroendocrinology 34, 660–669. 10.1016/j.psyneuen.2008.11.004 [DOI] [PubMed] [Google Scholar]
- van Goozen S. H., Matthys W., Cohen-Kettenis P. T., Buitelaar J. K., van Engeland H. (2000). Hypothalamic-pituitary-adrenal axis and autonomic nervous system activity in disruptive children and matched controls. J. Am. Acad. Child Adolesc. Psychiatry 39, 1438–1445. 10.1097/00004583-200011000-00019 [DOI] [PubMed] [Google Scholar]
- van Goozen S. H., Matthys W., Cohen-Kettenis P. T., Gispen-de Wied C., Wiegant V. M., van Engeland H. (1998). Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol. Psychiatry 43, 531–539. 10.1016/S0006-3223(97)00253-9 [DOI] [PubMed] [Google Scholar]
- van Honk J., Harmon-Jones E., Morgan B. E., Schutter D. J. (2010). Socially explosive minds: the triple imbalance hypothesis of reactive aggression. J. Pers. 78, 67–94. 10.1111/j.1467-6494.2009.00609.x [DOI] [PubMed] [Google Scholar]
- Van Houdenhove B., Van Den Eede F., Luyten P. (2009). Does hypothalamic–pituitary–adrenal axis hypofunction in chronic fatigue syndrome reflect a “crash” in the stress system? Med. Hypotheses 72, 701–705. 10.1016/j.mehy.2008.11.044 [DOI] [PubMed] [Google Scholar]
- Vanyukov M. M., Moss H. B., Plail J. A., Blackson T., Mezzich A. C., Tarter R. E. (1993). Antisocial symptoms in preadolescent boys and in their parents: associations with cortisol. Psychiatry Res. 46, 9–17. 10.1016/0165-1781(93)90003-Y [DOI] [PubMed] [Google Scholar]
- Vázquez D. M., Neal C. R., Patel P. D., Jr, Kaciroti N., López J. F. (2012). Regulation of corticoid and serotonin receptor brain system following early life exposure of glucocorticoids: long term implications for the neurobiology of mood. Psychoneuroendocrinology 37, 421–437. 10.1016/j.psyneuen.2011.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema A. H., Blume A., Niederle D., Buwalda B., Neumann I. D. (2006). Effects of early life stress on adult male aggression and hypothalamic vasopressin and serotonin. Eur. J. Neurosci. 24, 1711–1720. 10.1111/j.1460-9568.2006.05045.x [DOI] [PubMed] [Google Scholar]
- Veenema A. H., Koolhaas J. M., de Kloet E. R. (2004). Basal and stress-induced differences in HPA axis, 5-HT responsiveness, and hippocampal cell proliferation in two mouse lines. Ann. N. Y. Acad. Sci. 1018, 255–265. 10.1196/annals.1296.030 [DOI] [PubMed] [Google Scholar]
- Victoroff J., Quota S., Adelman J. R., Celinska B., Stern N., Wilcox R., et al. (2011). Support for religio-political aggression among teenaged boys in Gaza: part II: neuroendocrinological findings. Aggress. Behav. 37, 121–132. 10.1002/ab.20376 [DOI] [PubMed] [Google Scholar]
- Virkkunen M. (1985). Urinary free cortisol secretion in habitually violent offenders. Acta Psychiatr. Scand. 72, 40–44. 10.1111/j.1600-0447.1985.tb02568.x [DOI] [PubMed] [Google Scholar]
- von der Pahlen B. (2005). The role of alcohol and steroid hormones in human aggression. Vitam. Horm. 70, 415–437. 10.1016/S0083-6729(05)70014-5 [DOI] [PubMed] [Google Scholar]
- von Polier G. G., Herpertz-Dahlmann B., Konrad K., Wiesler K., Rieke J., Heinzel-Gutenbrunner M., et al. (2013). Reduced cortisol in boys with early-onset conduct disorder and callous-unemotional traits. Biomed. Res. Int. 2013:349530. 10.1155/2013/349530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbecker I., Floyd A., Dedert E., Salmon P., Sephton S. (2006). Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology 31, 312–324. 10.1016/j.psyneuen.2005.08.009 [DOI] [PubMed] [Google Scholar]
- Woodworth C. H. (1971). Attack elicited in rats by electrical stimulation of the lateral hypothalamus. Physiol. Behav. 6, 345–353. 10.1016/0031-9384(71)90166-1 [DOI] [PubMed] [Google Scholar]
- Yang S. J., Shin D. W., Noh K. S., Stein M. A. (2007). Cortisol is inversely correlated with aggression for those boys with attention deficit hyperactivity disorder who retain their reactivity to stress. Psychiatry Res. 153, 55–60. 10.1016/j.psychres.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Yehuda R. (1999). Managing anger and aggression in patients with posttraumatic stress disorder. J. Clin. Psychiatry. 60(Suppl.) 15, 33–37. [PubMed] [Google Scholar]
- Yehuda R., Bierer L. M., Pratchett L. C., Lehrner A., Koch E. C., Van Manen J. A., et al. (2015). Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology 51, 589–597. 10.1016/j.psyneuen.2014.08.004 [DOI] [PubMed] [Google Scholar]
- Yehuda R., Southwick S. M., Nussbaum G., Wahby V., Giller E. L., Jr, Mason J. W. (1990). Low urinary cortisol excretion in patients with posttraumatic stress disorder. J. Nerv. Ment. Dis. 178, 366–369. 10.1097/00005053-199006000-00004 [DOI] [PubMed] [Google Scholar]
- Yu Y. Z., Shi J. X. (2009). Relationship between levels of testosterone and cortisol in saliva and aggressive behaviors of adolescents. Biomed. Environ. Sci. 22, 44–49. 10.1016/S0895-3988(09)60021-0 [DOI] [PubMed] [Google Scholar]
- Zhang B. (2017). Consequences of early adverse rearing experience (EARE) on development: insights from non-human primate studies. Zool. Res. 38, 7–35. 10.13918/j.issn.2095-8137.2017.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Xu D., Luo H., Lu J., Liu L., Ping J., et al. (2014). Prenatal xenobiotic exposure and intrauterine hypothalamus-pituitary-adrenal axis programming alteration. Toxicology 325, 74–84. 10.1016/j.tox.2014.08.015 [DOI] [PubMed] [Google Scholar]
- Zuckerman M. (1989). Personality in the third dimension: A psychobiological approach. Pers. Individ. Diff. 10, 391–418. 10.1016/0191-8869(89)90004-4 [DOI] [Google Scholar]