ABSTRACT
Kaposi's sarcoma-associated herpesvirus (KSHV), like other herpesviruses, replicates within the nuclei of its human cell host and hijacks host machinery for expression of its genes. The activities that culminate in viral DNA synthesis and assembly of viral proteins into capsids physically concentrate in nuclear areas termed viral replication compartments. We sought to better understand the spatiotemporal regulation of viral RNAs during the KSHV lytic phase by examining and quantifying the subcellular localization of select viral transcripts. We found that viral mRNAs, as expected, localized to the cytoplasm throughout the lytic phase. However, dependent on active viral DNA replication, viral transcripts also accumulated in the nucleus, often in foci in and around replication compartments, independent of the host shutoff effect. Our data point to involvement of the viral long noncoding polyadenylated nuclear (PAN) RNA in the localization of an early, intronless viral mRNA encoding ORF59-58 to nuclear foci that are associated with replication compartments.
IMPORTANCE Late in the lytic phase, mRNAs from Kaposi's sarcoma-associated herpesvirus accumulate in the host cell nucleus near viral replication compartments, centers of viral DNA synthesis and virion production. This work contributes spatiotemporal data on herpesviral mRNAs within the lytic host cell and suggests a mechanism for viral RNA accumulation. Our findings indicate that the mechanism is independent of the host shutoff effect and splicing but dependent on active viral DNA synthesis and in part on the viral noncoding RNA, PAN RNA. PAN RNA is essential for the viral life cycle, and its contribution to the nuclear accumulation of viral messages may facilitate propagation of the virus.
KEYWORDS: KSHV, nuclear foci, DNA replication compartments, viral noncoding PAN RNA, mRNA localization
INTRODUCTION
Herpesviruses are large double-stranded DNA viruses that undergo a biphasic life cycle and replicate within the nucleus (1). Their predominantly latent (dormant) state within the host cell is characterized by limited viral gene expression and is a hallmark of persistent infection. To produce viral progeny and thus enable spread to new hosts, herpesviruses trigger a cascade of viral gene expression and enter the lytic (active) phase. For Kaposi's sarcoma-associated herpesvirus (KSHV), a gammaherpesvirus, the progression of KSHV-related cancers is attributed to apoptotic and angiogenic viral activities in both the latent and lytic phases (2). KSHV is the causative agent of several human cancers, such as Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease.
The KSHV lytic phase culminates in viral DNA replication and virion assembly and is temporally separated into three subphases: immediate early, early, and late. The RTA/ORF50 gene (replication and transcription activator) and other immediate-early genes activate expression of the early genes, which facilitate immune evasion and viral DNA replication. Viral DNA replication ensues, driving late lytic gene expression. Late genes encode immune evasion proteins, the viral capsid, and the virion tegument. Collectively, late lytic proteins promote the final events of KSHV's lytic phase, virion assembly and release. Historically, late genes have been classified as genes that are not expressed in the presence of an inhibitor of herpesviral DNA replication and early genes as those that are expressed. However, next-generation RNA-sequencing studies of KSHV-infected cells revealed a more fluid timeline of lytic activation, with some “early” genes reaching maximum expression after the onset of viral DNA replication and some “late” genes being expressed before viral DNA replication begins (3). Moreover, the expression timelines for KSHV noncoding RNAs, such as the long noncoding polyadenylated nuclear (PAN) RNA and microRNAs, which regulate virion assembly and latency, respectively (3–7), have been recently characterized.
During the lytic phase, KSHV establishes replication factories within host nuclei called replication compartments, which are home to viral transcription, viral DNA synthesis, and virion assembly (1). These virus-induced nuclear structures emerge as small foci early in the lytic phase and then coalesce into DAPI (4′,6-diamidino-2-phenylindole)-reduced, kidney-shaped areas characterized by high levels of DNA synthesis and concentrations of viral and host proteins (8, 9). The core viral proteins of KSHV replication compartments include DNA polymerase (POL/ORF9), single-stranded DNA binding protein (SSB/ORF6), primase (PRI/ORF56), primase-associated factor (PAF/ORF40-41), helicase (HEL/ORF44), and polymerase processivity factor (PPF/ORF59) (9). Viral gene regulators, such as RTA/ORF50, MTA/ORF57 (mRNA transport and accumulation), and the early DNA-binding K8/K-bZIP proteins also collect in these regions (9, 10). To facilitate viral transcription and protein folding for capsid assembly, herpesviruses recruit to their replication compartments host machinery, such as RNA polymerase (Pol) II (Ser5 phosphorylated), 20S proteasomes, Hsc70, and other chaperone proteins (11–15). Moreover, nascent, intron-containing transcripts from the KSHV RTA/ORF50 gene localize to regions of Ser5-phosphorylated RNA Pol II near replication compartments (14). Although many studies have investigated the components of replication compartments, little is known regarding the behavior of viral transcripts in relation to these viral foci.
In this study, we sought to better understand the spatiotemporal regulation of KSHV RNA localization with respect to viral replication. We found that KSHV RNAs localize in foci within and on the edges of viral replication compartments, underscoring the central role of replication compartments in the production of viral progeny. Furthermore, the viral noncoding PAN RNA contributes to the accumulation of some viral mRNAs in nuclear foci, expanding our understanding of the roles of noncoding RNAs in the viral life cycle.
RESULTS
FISH detects select lytic viral transcripts.
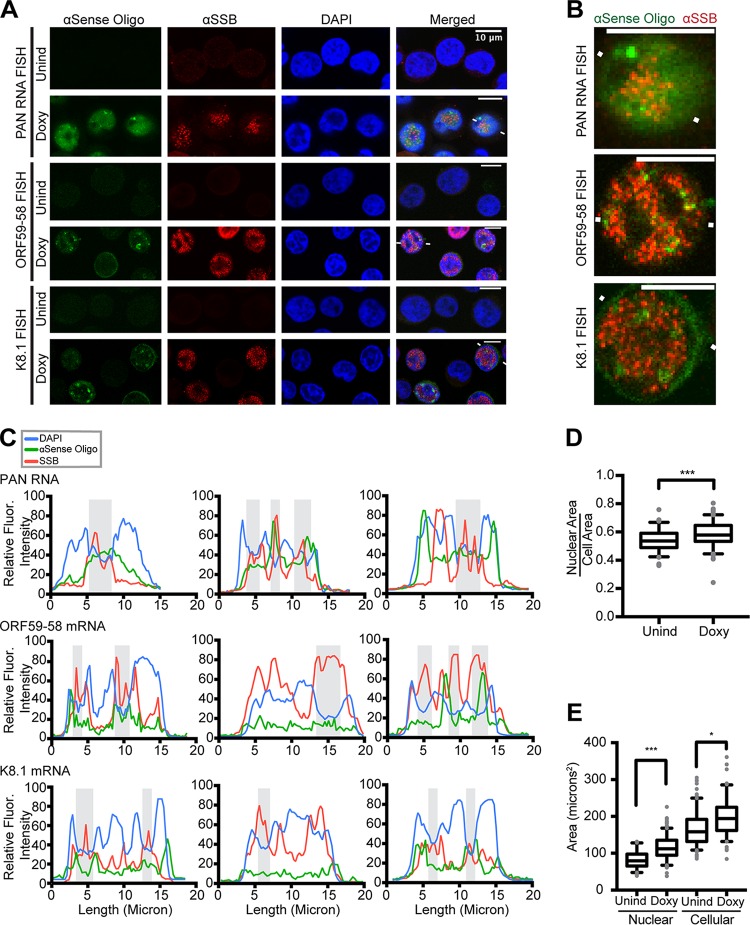
Throughout this study, we used PAN RNA as a marker of lytic activation and SSB/ORF6, a component of the viral replication complex, as a marker of viral replication compartments (1). To activate the lytic phase in B cells harboring KSHV, TREx RTA BCBL-1 cells were treated with 1 μg/ml doxycycline (Doxy) for 24 h to induce expression of RTA/ORF50, KSHV's master transcription factor for the lytic phase (16). At 24 h, expression of PAN RNA and SSB/ORF6 was evident by fluorescence in situ hybridization (FISH) and immunofluorescence (IF), respectively, confirming activation of the lytic phase (Fig. 1A).
FIG 1.
Viral RNAs accumulate at late times in nuclear foci in and near viral replication compartments. Shown are confocal images of TREx RTA BCBL-1 cells that were induced into the lytic phase for 24 h with Doxy. (A) FISH for viral RNAs (green) and IF for viral single-stranded DNA binding protein (ORF6/SSB) (red) revealed that viral transcripts localize in the cytoplasm, nucleus, and nuclear foci outside ORF6/SSB-enriched areas, also known as replication compartments. The white lines flanking cells depict the line path of fluorescence intensities for the FISH and IF signals, plotted in the first column of panel C. αSense, antisense; Unind, uninduced. (B) Digitally enlarged images of the cells in panel A flanked by white lines. For simplicity, the blue DAPI channel was omitted. Scale bars, 10 μm. (C) Plots showing the relative fluorescent intensities for each stain along the same line: αSSB (red), a viral transcript (indicated on the plot) (green), and DAPI (blue). The shaded areas indicate DAPI-reduced regions that correspond to viral replication compartments or SSB/ORF6-enriched areas. Line traces for two additional cells for each of the three transcripts are shown. (D) The ratio of nuclear area to cellular area changes, and thus the fluorescence intensity ratio used throughout this study was normalized for area. (E) Nuclear and cellular areas measured for TREx RTA BCBL-1 cells with and without undergoing lytic activation. Statistically significant changes were seen compared to uninduced cells. *, P < 0.05; ***, P < 0.0005.
To explore the subcellular distribution of viral transcripts, we developed and tested oligonucleotides for FISH analyses of specific viral transcripts. We attempted to detect mRNAs of the genes encoding MCP/ORF25 (major capsid protein), ORF26, gB/ORF8 (glycoprotein B), ORF59-58, ORF47-45, MTA/ORF57, K8.1, and SOX/ORF37 (shutoff exonuclease). We were successful in developing specific, low-background FISH strategies for (i) the early, intronless bicistronic ORF59-58 gene transcript, which encodes a viral PPF and a putative transmembrane protein that facilitates virion attachment to specific cell types, and (ii) a late intron-containing transcript encoding the K8.1 virion glycoprotein (17, 18). The FISH probes for ORF59-58 mRNA can also detect a monocistronic transcript encoding ORF58; however, in KSHV-infected JSC-1 cells, this mRNA is at least 18-fold less abundant than the bicistronic transcript and likely contributes only a minor portion of the total fluorescent signal observed (17). The FISH oligonucleotides for the K8.1 transcript detect both the long and short spliced isoforms, in addition to the unspliced transcript (19, 20). FISH signals for the ORF59-58 and K8.1 transcripts are seen in lytic KSHV-infected cells (Fig. 1A, Doxy), but not in latent, uninduced KSHV-infected cells (Fig. 1A, Unind).
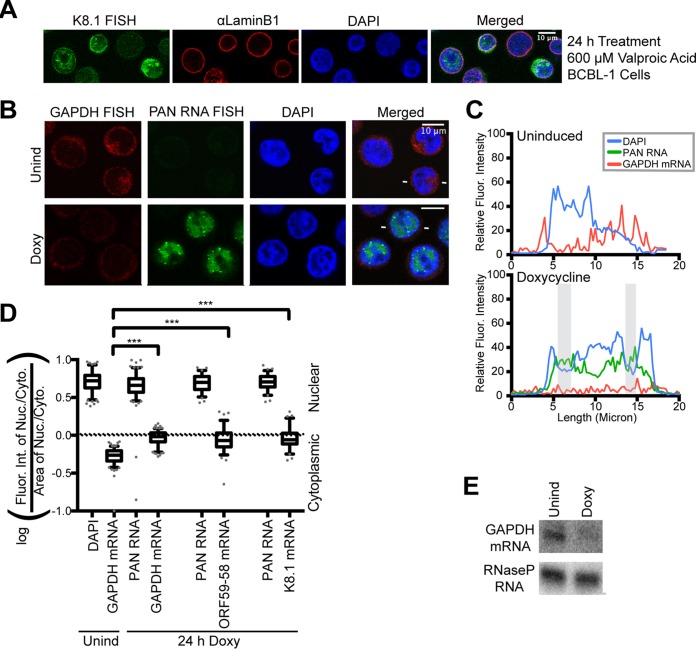
As a host transcript control for the FISH studies, we obtained validated FISH probes (Stellaris) for GAPDH (glyceraldehyde-3-phosphate dehydrogenase) mRNA. Figure 2B to D shows that the GAPDH message localizes to the cytoplasm in latent KSHV-infected cells. As previously reported (21), GAPDH mRNA is subject to the viral host shutoff effect (Fig. 2E), and accordingly, its FISH signal is greatly reduced upon lytic induction (Fig. 2B, Doxy versus Unind). Attempts to develop a FISH strategy for the well-documented host shutoff escapee host interleukin 6 (IL-6) mRNA were unsuccessful (21).
FIG 2.
Host transcript GAPDH does not accumulate in nuclear foci during the KSHV lytic phase. (A) Immunofluorescent staining of BCBL-1 cells treated with 600 μM valproic acid for 24 h showing concentrated nuclear foci of viral transcript K8.1. (B) FISH for host GAPDH mRNA (red) and for the viral lncRNA PAN (green) and DAPI nuclear staining (blue) showing that host GAPDH mRNA does not localize to nuclear foci in lytic cells. (C) Fluorescence intensities along a line (indicated by white lines flanking lytic cells in panel B and Fig. 1A). The shaded areas are as in Fig. 1. (D) Quantification of the fluorescence intensities of cells represented in panel B (n = 150 for each GAPDH sample; n = 75 for the ORF59-58 or K8.1 samples) was performed for three biological replicates (see Materials and Methods). The values for ORF59-58 and K8.1 transcripts were from the experiments shown in Fig. 3 and 4D. ***, P < 0.0005. (E) Representative Northern blot of RNA from TREx RTA BCBL-1 cells 24 h after Doxy treatment.
Focal concentrations of KSHV mRNA localize in and around nuclear viral replication compartments.
To better understand the spatiotemporal regulation of KSHV RNAs with respect to viral replication, we examined the localization of PAN, ORF59-58, and K8.1 viral transcripts in 24 h Doxy-induced lytic TREx RTA BCBL-1 cells. To quantitatively represent the subcellular distribution trends exhibited by the FISH and IF signals, we measured the raw fluorescence intensity along a line bisecting the cell (Fig. 1C; indicated by white lines flanking representative cells in Fig. 1A). The line traces quantitatively depict the raw fluorescence across the channels, indicating corresponding valleys and peaks among the fluorescent signals and thus RNA- or SSB-containing areas. The line paths were chosen to pass a central axis of the cell, capturing some RNA foci while avoiding foci that might saturate the quantification and thus hide subtleties in the signals (Fig. 1B shows enlarged images of the cell) (see Materials and Methods).
Plots of fluorescence intensity (Fig. 1C) revealed that nuclear concentrations of SSB/ORF6 protein occurred in DAPI-reduced regions (gray shading). This observation supports previous findings that the virus reorganizes the host nucleus and chromosomes to generate replication compartments (22, 23). The viral mRNAs for ORF59-58 and K8.1 are present in the cytoplasm, as well as diffusely within the nucleus (Fig. 1A). PAN, ORF59-58, and K8.1 RNAs collect within nuclear foci in and around concentrations of viral SSB/ORF6 (Fig. 1A to C). Similar localization patterns were seen in BCBL-1 cells induced with 600 μM valproic acid for 24 h (Fig. 2A).
To investigate whether viral transcripts selectively accumulate in nuclear foci, we examined the subcellular localization of host GAPDH mRNA. Plots of fluorescence intensity along a line crossing the nuclei of representative cells revealed strong cytoplasmic localization of GAPDH mRNA in latent cells and weak overall signal in lytic cells (Fig. 2C). GAPDH mRNA was notably not concentrated in nuclear foci (Fig. 2B), in contrast to the behavior of viral PAN RNA and the ORF59-58 and K8.1 mRNAs (Fig. 1A) (21).
We then asked if the distribution of the viral transcript favored nuclear localization, considering the appearance of foci. We developed a calculation method with a cytoplasmic and nuclear control to ascertain the predominant cellular localization of the viral transcripts. We quantified the raw fluorescence intensities in the cytoplasm and the nucleus and then calculated a ratio that captures the fluorescence intensity in the nucleus compared to the cytoplasm within a given cell (see Materials and Methods). This value also controls for changes in cellular area, which varies between the different drug treatments used throughout the study (Fig. 1D and E). Notably, this ratio does not indicate the strength of fluorescence, only the subcellular distribution of the fluorescence intensity. For each cell, DAPI or PAN RNA was quantified to serve as a nuclear-localized control (positive values) for our calculation method. We capitalized on the cytoplasmic localization of GAPDH mRNA as a cytoplasmic control (negative values) to test our calculation method for ORF59-58 and K8.1 RNAs (Fig. 2D). The box-and-whisker plots of ORF59-58 and K8.1 FISH data display a broader distribution than that of either the cytoplasmic (GAPDH) or nuclear (PAN) controls (Fig. 2D) and span both positive (nuclear) and negative (cytoplasmic) values. This indicates that within the population of cells examined, these viral mRNAs are present in both the cytoplasm and nucleus, but the nucleocytoplasmic distribution shows large cell-to-cell variability.
Together, data from this combined semiquantitative FISH methodology on lytic KSHV-infected cells indicate that the viral transcripts ORF59-58, PAN RNA, and K8.1, but not the host GAPDH mRNA, concentrate in nuclear foci in and near replication compartments (Fig. 1 and 2).
Accumulation of KSHV PAN RNA in nuclear foci is detected at 6 h, while ORF59-58 and K8.1 mRNAs concentrate in nuclear foci at 18 h.
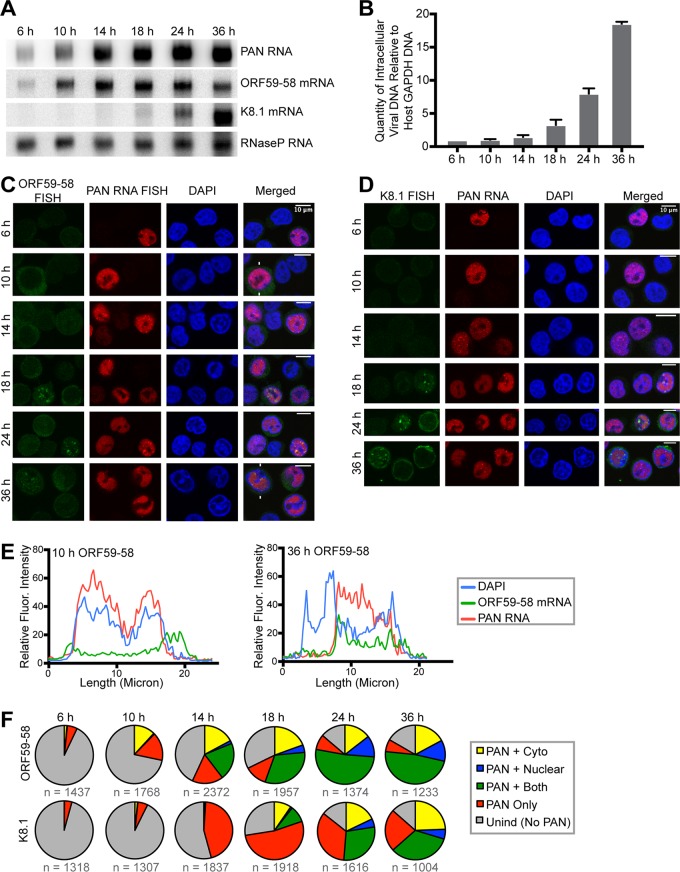
To track when viral transcripts localize to nuclear foci, we collected cells at 6 h, 10 h, 14 h, 18 h, 24 h, and 36 h after lytic induction by Doxy. Because cell viability decreases dramatically at 36 h postinduction, later time points were not surveyed. Northern blotting of total RNA demonstrated that ORF59-58 and K8.1 mRNAs exhibited expression patterns expected for early and late lytic transcripts, respectively (Fig. 3A). The ORF59-58 transcript was detected at 6 h, peaking at 14 to 18 h. In contrast, viral DNA replication accelerated at 18 h and continued well beyond 24 h (Fig. 3B). Expression of the late gene K8.1 mRNA correlated with the rise of viral DNA replication (Fig. 3A and B). Thus, the time points in this experiment were sufficiently frequent to capture subphases of the lytic cycle.
FIG 3.
Viral message accumulation in nuclear foci correlates with viral DNA replication. TREx RTA BCBL-1 cells were treated with Doxy and examined at 6 h, 10 h, 14 h, 18 h, 24 h, and 36 h post-lytic induction. The data are from two biological replicates. (A) Northern blot of total RNA collected at the indicated time points showing relative levels of select viral transcripts. Host RNase P RNA, which is not subject to the host shutoff effect, served as the loading control. (B) qPCR of total intracellular viral DNA at the indicated time points, normalized to the promoter region of the host GAPDH gene in four biological replicates, showing that viral DNA replication increases significantly at 18 h. The error bars indicate standard deviations. (C and D) Representative FISH images of the lytic viral PAN RNA (red) and either early, intronless ORF59-58 mRNA (green) (C) or late, spliced K8.1 mRNA (green) (D) at the indicated time points after induction. DAPI staining (blue) was used to visualize the nucleus. (E) Fluorescence intensities along lines drawn on cells (indicated by white lines in panel C). Three additional cells each for the 10-h and 36-h time points are shown in Fig. 4A and B. (F) Cells were categorized for induction into the lytic phase by the presence of PAN RNA and then examined for localization of ORF59-58 or K8.1 transcripts. The cells induced into the lytic phase but with no visible FISH signal for ORF59-58 or K8.1 transcripts were categorized as PAN only. Cells with either ORF59-58 or K8.1 transcripts in the nucleus were categorized as PAN plus nuclear, in the cytoplasm as PAN plus Cyto, and in both compartments as PAN plus both. n, number of cells examined to produce the pie charts.
FISH analysis of cells from the time course revealed accumulation of PAN RNA in DAPI-rich nuclear foci as early as 6 h post-lytic induction (Fig. 3C to E), while viral messages appeared in DAPI-reduced foci only later in the lytic phase, at 18 h (Fig. 3C to E), coincident with the rise of viral DNA replication (Fig. 3B). ORF59-58 transcripts appeared first in the cytoplasm at 6 h and 10 h, started to accumulate in nuclei at 14 h, and increased in nuclear foci at 18 h and 24 h (Fig. 3C and F). Meanwhile, the late K8.1 transcript concentrated in nuclear foci even when it was first detectable at 18 h (Fig. 3D and F).
Cells from two biological replicates represented by the images in Fig. 3C and D were categorized with respect to their lytic activation and the subcellular localization of either ORF59-58 or K8.1 mRNA (Fig. 3F). The cell population for each time point was first classified by the absence (Unind [latent]) or presence (lytic) of PAN RNA. Lytic cells were further categorized as cells expressing only PAN RNA (PAN Only) or as cells expressing PAN RNA as well as the protein-coding transcript in the cytoplasm (PAN + Cyto), the protein-coding transcript in the nucleus (PAN + Nuclear), or the protein-coding transcript in both the nucleus and cytoplasm (PAN + Both) (Fig. 3F). For the ORF59-58 transcript, the fraction of cells with cytoplasmic localization remained constant from 10 h to 36 h. The fraction of cells with nuclear signal increased from 10 h to 36 h, as well as the fraction of cells with ORF59-58 mRNA in both the cytoplasm and nucleus (Fig. 3C, E, and F and 4A and B). On the other hand, the fraction of cells with nuclear signal for K8.1 mRNA was constant through time points 18 h to 36 h (Fig. 3F).
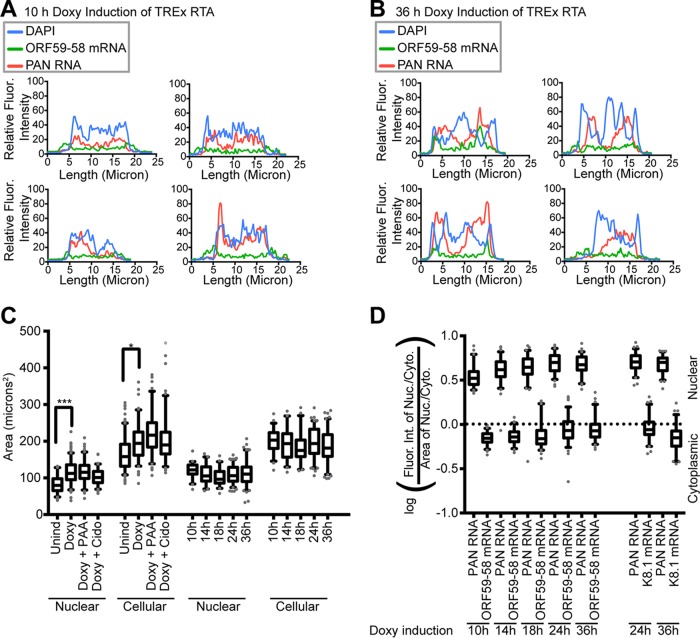
FIG 4.
Semiquantitative analysis revealed late lytic appearance of nuclear foci and widening of the nucleocytoplasmic distribution of viral mRNAs. (A and B) Line traces of four TREx RTA BCBL-1 cells induced with Doxy for 10 h (A) or 36 h (B) and stained with ORF59-58 FISH and PAN RNA FISH, as shown in Fig. 3C. (C) Areas were measured for TREx RTA BCBL-1 cells and nuclei undergoing lytic activation with or without viral DNA replication inhibition. Statistically significant changes were seen compared to uninduced cells. The statistics of the graph are shown in Table 2. (D) Plot of fluorescence intensity ratios for ORF59-58 and K8.1 mRNAs from three biological replicates (n = 75). PAN RNA served as the nuclear control for the calculation. *, P < 0.05; ***, P < 0.0005.
Analysis of the fluorescence intensity ratio (see Materials and Methods) revealed that the nucleocytoplasmic distribution of the ORF59-58 mRNA became broader over time in the box-and-whisker plots after controlling for differences in cell size (Fig. 4C and D) and had a greater nuclear presence after 18 h than at 10 h (Fig. 3F), although the difference was not statistically significant. We attribute this to the heterogeneity of lytic activation; lytically induced KSHV-infected cell cultures are not synchronous and contain cells in different lytic subphases. Combining these observations with the timeline of viral DNA replication, the accumulation of viral messages in the nucleus and/or in nuclear foci appears to be coincident with viral DNA replication.
Accumulation of ORF59-58 RNA in the nucleus requires active viral DNA synthesis.
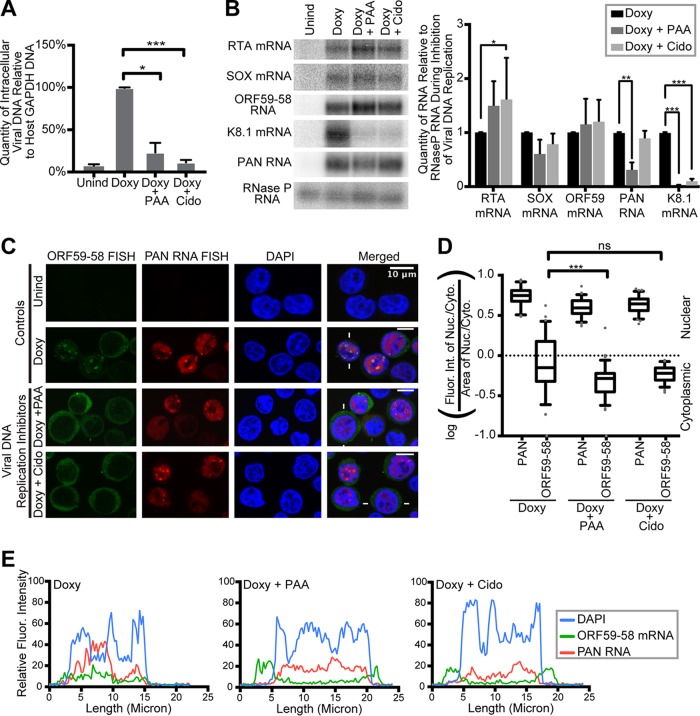
We explored the relationship of viral DNA synthesis to the accumulation of the ORF59-58 message in the nucleus and nuclear foci by inhibiting viral DNA replication. We treated TREx RTA BCBL-1 cells simultaneously with Doxy to induce the lytic phase and with an inhibitor of herpesviral DNA replication for 24 h. Two different herpesviral DNA replication inhibitors, phosphonoacetic acid (PAA) and cidofovir (Cido), were used to minimize the likelihood that observed changes could be off-target effects of the drug. Both drugs act as nucleotide analog inhibitors of the herpesviral DNA polymerase and do not affect host DNA replication (24, 25). Figure 5A shows that treatment with Doxy plus PAA and Doxy plus Cido reduced intracellular viral DNA to ∼23.5% and ∼11.8%, respectively, compared to viral DNA replication in cells treated with Doxy alone. As expected, the levels of viral immediate-early (RTA) and early (SOX, ORF59-58, and PAN) transcripts were impacted only partially by inhibition of viral DNA replication (Fig. 5B). In contrast, late K8.1 gene expression was reduced about 10-fold. Therefore, K8.1 was excluded from subsequent analyses of inhibition; we attribute any residual expression of K8.1 to incomplete inhibition of viral DNA replication (Fig. 5A and B).
FIG 5.
Accumulation of ORF59-58 message in the nucleus depends on viral DNA replication. TREx RTA BCBL-1 cells were treated for 24 h with no drug (Unind), doxycycline only (Doxy), or doxycycline and one inhibitor of herpesviral DNA replication, phosphonoacetic acid (Doxy plus PAA) or cidofovir (Doxy plus Cido). Panels A to C show data from samples collected from three biological replicates. (A) qPCR values for viral intracellular DNA during inhibition of viral DNA replication were normalized to the quantity of promoter DNA of the host cell GAPDH gene. (B) Northern blot (left) and quantification (right) showing total RNA levels during inhibition of viral DNA replication. Uninduced levels of all RNAs were undetectable. The error bars indicate standard deviations. (C) Representative FISH images for viral ORF59-58 transcripts (green) and PAN RNA (red) upon inhibition of viral DNA replication. DAPI (blue) was the nuclear stain. (D) Quantification of the fluorescence intensities of cells represented in panel C (n = 75 each) done on biological triplicates (see Materials and Methods). The quantification controls for changes in area represented in Fig. 7F. (E) Fluorescence intensities along lines drawn across cells (indicated by white lines in panel C). ns, P > 0.05; *, P < 0.05; **, P < 0.005; ***, P < 0.0005.
We examined the localization of the early ORF59-58 transcript to determine whether viral DNA replication contributes to its nuclear accumulation. We observed that cells with inhibited herpesviral DNA replication had predominantly cytoplasmic localization of ORF59-58 mRNA at 24 h while PAN RNA still accumulated in nuclear foci (Fig. 5C to E).
We conclude that nuclear accumulation of ORF59-58 mRNAs depends on viral DNA replication. This is consistent with the close physical association of these mRNAs with replication compartments under normal conditions and the reduced nuclear localization of ORF59-58 signal upon inhibition of viral DNA replication (Fig. 1A and 5A and C). The localization of PAN RNA in nuclear foci as early as 6 h despite the presence of viral DNA replication inhibitors suggests that PAN RNA might influence the accumulation of viral messages in nuclear foci.
Knockdown of KSHV PAN RNA affects accumulation in nuclear foci of the early, intronless ORF59-58, but not of late, spliced K8.1 mRNA.
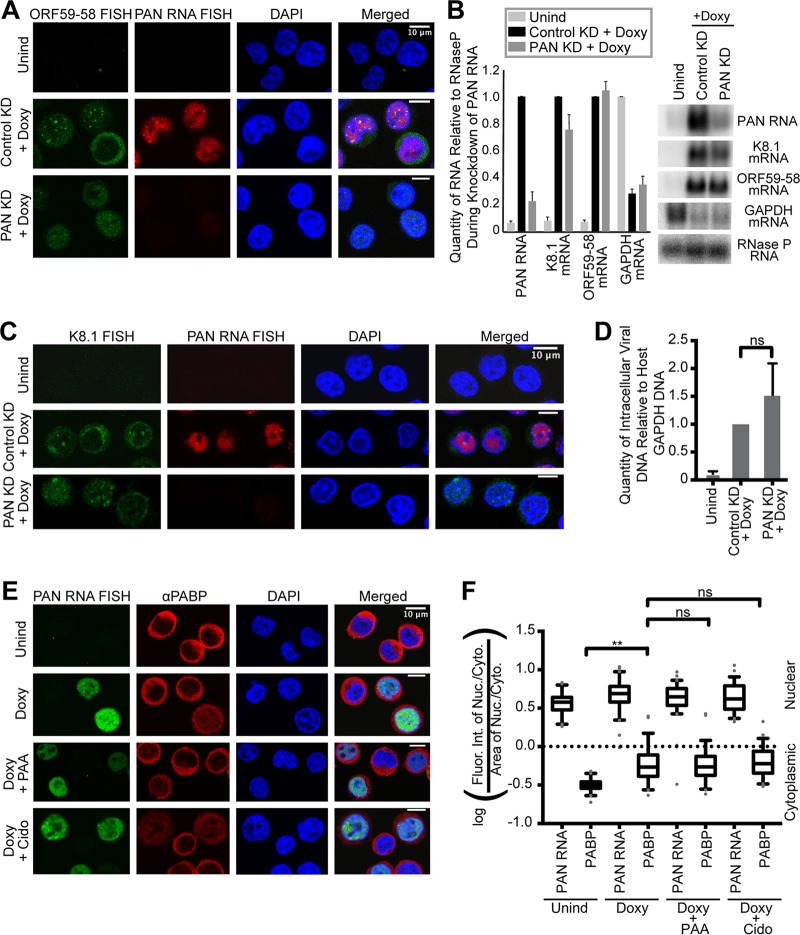
To assess the possible role of PAN RNA in the accumulation of viral messages in nuclear foci, we knocked down PAN RNA by electroporating an antisense oligonucleotide into TREx RTA BCBL-1 cells prior to Doxy induction (see Materials and Methods). As reported previously (26–28), viral DNA replication was not inhibited upon elimination of PAN RNA (Fig. 6B) compared to cells treated with a control antisense oligonucleotide targeting green fluorescent protein (GFP) (Fig. 6D). PAN RNA knockdown, therefore, does not act indirectly by impeding viral DNA synthesis or the formation of replication compartments (Fig. 7E). Knockdown of PAN RNA also did not affect the expression of the early ORF59-58 mRNA but decreased the K8.1 mRNA levels by approximately 20% (Fig. 6B).
FIG 6.
Knockdown of KSHV PAN RNA abolishes focal accumulation of early, intronless ORF59-58 transcript but not of late, spliced K8.1 mRNA, while nuclear accumulation of viral messages is independent of the host shutoff effect. (A and C) TREx RTA BCBL-1 cells were treated for 24 h with no drug (Unind), a control antisense oligonucleotide for GFP and doxycycline (Control KD plus Doxy), or an antisense oligonucleotide for PAN RNA and doxycycline (PAN KD plus Doxy) in biological triplicates. Shown are representative images of cells stained by FISH for viral transcripts encoding ORF59-58 (A) (green) or K8.1 (C) (green) and for PAN RNA in cells (red). (B) Northern blot (right) and quantification (left) of total RNA collected from knockdown samples showing relative levels of select viral transcripts. Host RNase P RNA served as the loading control. The error bars indicate standard deviations. (D) qPCR showing that viral intracellular DNA levels are unaffected upon PAN RNA knockdown. (E and F) Herpesviral DNA replication was inhibited in the same manner as for Fig. 5 by cotreatment with Doxy and one inhibitor of viral DNA replication, either phosphonoacetic acid (Doxy plus PAA) or cidofovir (Doxy plus Cido) (biological triplicates). (E) Representative images of FISH for KSHV PAN RNA and IF for PABP during inhibition of herpesviral DNA replication. (F) Quantification of fluorescence intensity in cells like those shown in panel E (n = 60). ns, P > 0.05; **, P < 0.005.
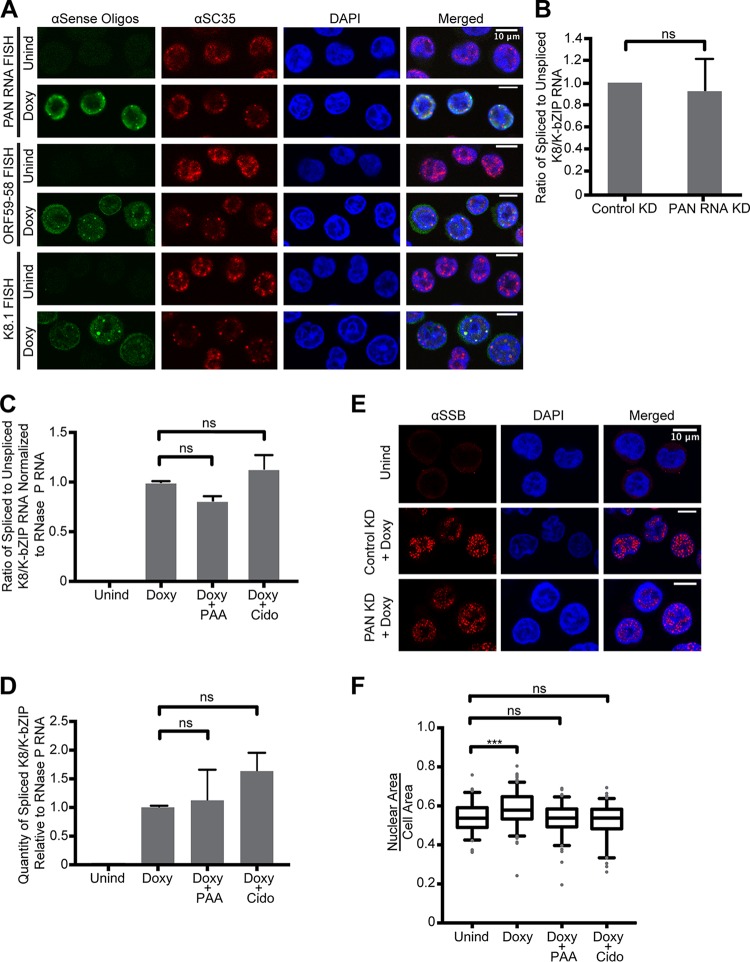
FIG 7.
Accumulation of KSHV messages in nuclear foci appears to be unrelated to splicing. (A) Latent (Unind) and 24-h lytic (Doxy) cells were stained for viral RNA (FISH) and for the host splicing factor, SC35 (IF). (B) Ratio of RT-qPCR values of spliced to unspliced viral K8/K-bZIP RNA normalized to host 18S rRNA following either control or PAN RNA knockdown (KD). The error bars indicate standard deviations. (C and D) RT-qPCR quantification of K8/K-bZIP RNA following viral DNA replication inhibition. Shown are ratios of spliced to unspliced viral K8/K-bZIP RNA (C) and quantities of spliced K8/K-bZIP RNA normalized to host RNase P RNA (D). The amplicon for the spliced K8/K-bZIP transcript spanned the splice junction and shared the same upstream primer as the unspliced amplicon, which spanned the intron. The RT-qPCR values are from biological triplicates. (E) Twenty-four-hour-induced TREx BCBL-1 cells were treated as described for Fig. 6 to knock down KSHV PAN RNA (PAN KD) and then stained for the viral single-stranded DNA binding protein (SSB/ORF6; red) and for the nucleus (DAPI; blue) in biological duplicates. (F) The ratio of nuclear area to cellular area also changes, and thus, the fluorescence intensity ratio used throughout this study was normalized for area (square micrometers). Unind and Doxy values are the same as in Fig. 1D. Doxy plus PAA and Doxy plus Cido correspond to Fig. 5C and D. ns, P > 0.05; ***, P < 0.0005.
Knockdown of KSHV PAN RNA did not reduce nuclear accumulation of the ORF59-58 message; however, at this resolution, the mRNA signal appeared to be distributed diffusely in the nucleoplasm rather than concentrated in nuclear foci (Fig. 6A). The foci of PAN RNA and ORF59-58 mRNA—when present—often overlap, suggesting a mechanistic link between the concentration of PAN RNA and this viral message (Fig. 3C and 5C). In contrast, the late K8.1 transcript accumulates in nuclear foci that rarely overlap those containing PAN RNA (Fig. 3D). Moreover, K8.1 foci persist in the absence of PAN RNA (Fig. 6C), despite a slight reduction in levels. Thus, PAN RNA appears to facilitate the accumulation of early, intronless ORF59-58 in common nuclear foci, whereas PAN RNA does not affect the localization of late, spliced K8.1 mRNA.
Accumulation of KSHV messages in nuclear foci is independent of the host shutoff effect and appears to be unrelated to splicing.
Another major KSHV phenomenon coincident with the appearance of viral RNA in nuclear foci is the host shutoff effect (29). Under the host shutoff effect, a viral endonuclease, SOX/ORF37, degrades both host and, to a lesser extent, viral transcripts in the cytoplasm (30). Following the dramatic reduction in host mRNA, cytoplasmic poly(A) binding protein (PABP) relocalizes to the nucleus (31). Thus, the observed accumulation of viral messages in the nucleus could simply be due to cytoplasmic mRNA degradation by SOX/ORF37. If this is true, then upon inhibition of viral DNA replication, when viral mRNAs become localized to the cytoplasm, a corresponding loss of PABP relocalization to the nucleus should be observed. To test this hypothesis, we assessed the nuclear relocalization of PABP in the presence of viral DNA replication inhibitors. Immunofluorescence revealed that PABP relocalizes to the nucleus despite inhibition of viral DNA replication (Fig. 6D to F), which indicates that the host shutoff effect is preserved (31). Because viral messages are present predominantly in the cytoplasm (Fig. 5C) only when viral DNA replication is inhibited, we conclude that the phenomena of PABP relocalization and viral mRNA nuclear accumulation are not linked. Together, this suggests that the accumulation of viral messages in the nucleus or nuclear foci is not influenced by the host shutoff effect.
Nuclear accumulation of transcripts might instead be attributed to inefficient splicing or inhibition of splicing (32–34). Although only a small subset (16 to 20%) of KSHV transcripts undergo splicing (3), we tested whether a change in splicing efficiency in infected cells with and without herpesviral DNA replication inhibitors could account for the observed changes in the nuclear accumulation of viral messages. We reasoned that if inefficient splicing were the primary cause of nuclear accumulation of viral message, then the splicing efficiency should increase in cells treated with Doxy plus PAA or Doxy plus Cido in order for the viral messages to appear predominantly in the cytoplasm under these conditions (Fig. 5D). We detected no difference in the splicing efficiency of early K8/K-bZIP pre-mRNA between Doxy- and Doxy-plus-PAA- or Doxy-plus-Cido-treated cells (Fig. 7C and D). Likewise, knockdown of PAN RNA did not affect splicing of K8/K-bZIP (Fig. 7B). We did, however, observe that the host splicing factor SC35 localizes just outside the nuclear foci of PAN, K8.1, and ORF59-58 transcripts (Fig. 7A).
DISCUSSION
Viruses create replication compartments, disrupting host nuclear structures and accumulating host and viral proteins at sites of active viral DNA synthesis. We have shown that certain viral transcripts likewise collect in foci at the peripheries of replication compartments (Fig. 1). In contrast, the host GAPDH transcript does not accumulate in nuclear foci (Fig. 2). From a time course of lytic induction (Fig. 3), we observed that the viral noncoding PAN RNA appears early (6 h after Doxy) in nuclear foci whereas ORF59-58 and K8.1 mRNAs accumulate in nuclear foci only at the onset of viral DNA synthesis. The early appearance of PAN RNA foci may be attributable to its much higher abundance. Inhibition of viral DNA replication (Fig. 5) further revealed that accumulation of ORF59-58 mRNA in nuclear foci requires active DNA synthesis whereas that of the viral noncoding RNA, PAN RNA, is not affected. Semiquantitative analysis of our time course data to determine if cells accumulate viral messages in the nucleus over the lytic cycle revealed a clear shift in their nucleocytoplasmic distribution.
By knocking down PAN RNA with an antisense oligonucleotide (Fig. 6), we demonstrated that the accumulation of ORF59-58 mRNA in nuclear foci also depends on the expression of PAN RNA, whereas that of K8.1 mRNA does not. Although K8.1 is a spliced mRNA and ORF59-58 is not, splicing does not appear to be the determining difference, because splicing of K8/K-bZIP is not affected by PAN RNA knockdown or viral DNA replication inhibition (Fig. 7) (26). Likewise, the relocalization of PABP after these treatments (Fig. 6) indicates that the host shutoff effect is not pivotal to changes in viral transcript localization.
Our data suggest that viral DNA replication is important for the initial nuclear accumulation of late viral-protein-encoding transcripts. Perhaps PAN RNA acts as a scaffold for enriching viral early transcripts in nuclear bodies. This would identify PAN RNA as an architectural RNA (arcRNA), which is defined as a long noncoding RNA (lncRNA) that functions as a platform to scaffold nuclear bodies, similar to NEAT1 for paraspeckles (35). PAN RNA possesses three characteristics typical of arcRNAs, namely, PAN RNA contains repetitive sequences; its expression is upregulated only during the specific disease state of lytic infection; and it binds regulatory RNA binding proteins, such as PABPC1 and hnRNPs (26). Recently, a SHAPE-MaP study by Sztuba-Solinska and colleagues (60) demonstrated that in the cell and in the virion, regions of KSHV PAN RNA are protected from chemical probing, supporting the idea that PAN RNA may act as a scaffold that interacts with RNA and proteins (36). Although we observed only PAN RNA and ORF59-58 mRNA colocalization, another arcRNA could be facilitating the formation of viral RNA nuclear bodies containing late transcripts, such as K8.1. Further studies on this alternative mechanism of accumulation of late and spliced viral mRNAs are needed, including investigation of more viral transcripts.
A previous report (37) pointed to the possibility that accumulation of late messages in nuclear foci depends on the presence of a newly synthesized viral DNA template. In cells infected by another gammaherpesvirus, Epstein-Barr virus (EBV), viral transcripts appeared in the cytoplasm of infected cells and concentrated in nuclear foci near viral proteins associated with replication compartments. Sugimoto and colleagues studied five EBV transcripts, two early and three late mRNAs, and found that early transcripts, such as the homologue of KSHV ORF59-58 (BMRF1) mRNA, form “small globular regions” just outside replication compartments, while late mRNAs, including some for glycoproteins, concentrate inside replication compartments (37). They attributed the differences in localization to the source of the template, since late genes have been shown to be transcribed from newly synthesized viral DNA. Some of the observations by Sugimoto and colleagues are consistent with our findings on KSHV messages and suggest a potential mechanism for accumulation of late herpesviral transcripts in nuclear foci. Our study similarly identifies differences in nuclear localization dynamics for an early and late transcript but provides further insight by pointing to a function for the KSHV long noncoding RNA, PAN RNA. Although an EBV homologue of PAN RNA is unknown at this time, it might be interesting to knock down the EBV lncRNA, BHLF1, to ask if this noncoding RNA affects nuclear accumulation of viral transcripts (38, 39).
The functional significance of accumulation of viral messages in nuclear foci remains unclear, but the literature points to a possibility related to virion structure. Mature KSHV virions contain viral RNAs that can be acquired during capsid assembly, tegument building, or particle envelopment (40–44). Viral RNAs concentrated in the nucleus would be incorporated into the virion at the capsid assembly stage. Perhaps packaging such transcripts into virions enables immediate viral protein production during de novo infection, offering the virus an advantage over host defenses. Alternatively, packaged RNAs may serve as structural or organizational elements of stable viral particles, as has been suggested for murine leukemia virus (MuLV) and HIV-1 virus-like particles (45–47). Whether the virus includes these transcripts by an active mechanism or the viral transcript population within virions merely reflects the transcript population at the site of viral particle assembly is debated (48). However, the literature on KSHV virions indicates that not all transcripts are incorporated into virions (40, 41). For HSV-1 and human cytomegalovirus (hCMV), RNA can be incorporated into virions through nonspecific interactions with tegument proteins (42, 44). Therefore, nuclear accumulation of viral messages may be one of the means, but not the only means, by which KSHV packages RNAs into virions.
Our findings suggest that KSHV, and possibly other herpesviruses, like EBV (37), may accumulate viral messages near replication compartments to aid the incorporation of viral messages into capsids. Alternatively, accumulation of viral messages in nuclear foci may enhance m6A RNA modification, which has been shown to promote RTA/ORF50 splicing and thus lytic activation (49). Focal accumulation of viral messages may also serve as a mechanism for controlling transcriptional bursts (50). In a wide range of animals, including mammals, some cellular mRNAs are produced in transcriptional pulses, followed by periods of transcriptional quiescence (51–54). Cells are able to accumulate mRNAs in the nucleus to reduce burst-associated fluctuations in cytoplasmic mRNA levels, thereby permitting steady protein production (50). Because viruses coevolve with their hosts and herpesviruses rely on host transcription machinery, KSHV may have adopted a similar mechanism to accumulate or compartmentalize viral mRNAs in the nucleus. This would facilitate sustained, robust production of viral proteins during the lytic phase. Studies definitively calculating the total number of nuclear foci through the lytic phase may prove valuable for mechanistic insights in the future.
MATERIALS AND METHODS
Cell lines, lytic induction, and viral DNA replication inhibition.
TREx RTA BCBL-1 cells were a gift from Jae Jung (16) and were maintained in RPMI medium supplemented with 1% penicillin/streptomycin (pen/strep), 1% l-glutamine, 20 μg/ml hygromycin, and 20% tetracycline-compatible fetal bovine serum (FBS) at 37°C and 5% CO2. BCBL-1 cells were maintained in RPMI medium supplemented with 1% pen/strep, 1% l-glutamine, and 20% FBS at 37°C and 5% CO2. To induce the KSHV lytic phase, cells were grown to a density of 0.8 million to 1.0 million cells/ml and then induced with 1 μg/ml Doxy for 24 h (TREx RTA BCBL-1 cells) or 600 μM valproic acid for 48 h (BCBL-1 cells), unless otherwise indicated. For viral DNA replication inhibition, cells were treated with a viral DNA replication inhibitor and doxycycline simultaneously for 24 h. The two viral DNA replication inhibitors used were PAA at 500 μM (55) and Cido at 100 μM (56).
FISH protocol.
FISH was performed as previously described (57) with minor changes. Eight-chamber slides (Nunc Lab-Tek II; catalog number 154453) were pretreated with 1:10 poly-l-lysine (Sigma; P8920) for 5 min and left to dry overnight at room temperature or for 1 h at 65°C. The cells were incubated in the chambered slides (800 μl per chamber) for 30 min at 37°C and 5% CO2 and then fixed with 4% formaldehyde-PBS (phosphate-buffered saline) on ice for 30 min. Total RNA and genomic-DNA samples were taken at the point of fixation to ensure consistency between FISH slides (see below for processing of nucleic acids). After three 5-min PBS washes, the fixed cells were permeabilized with 0.5% Triton-X–PBS for 10 min on ice or 70% ethanol at 4°C for 1 h (minimum) to 7 days (maximum). FISH probes were labeled with digoxigenin (DIG)-dUTP or AlexaFluor 594-5-dUTP (PAN probes only) (Life Technologies; C1100) using a DIG-Oligonucleotide tailing kit (Sigma Roche; 2nd generation; 03353583910), and terminal transferase (Roche; 003333574001), respectively. Table 1 lists the antisense oligonucleotide sequences. GAPDH probes, purchased from Stellaris, were used with the protocol, which is very similar to the recommended Stellaris RNA FISH protocol. The chambers were removed, and the cells were washed with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) once and then prehybridized with 50% formamide, 10% dextran sulfate, 2× SSC, 0.1% bovine serum albumin (BSA), 500 μg/ml salmon sperm DNA, 125 μg/ml Escherichia coli tRNA, and 1 mM vanadyl ribonucleoside complexes (NEB) for 1 h at 37°C. DIG- and Alexa Fluor 594-labeled oligonucleotides were denatured at 95°C for 5 min and then added to the hybridization solution. The samples were left overnight in a humidity chamber at 37°C. The next day, the cells were washed twice with 2× SSC for 10 min each time at 37°C and then twice with 1× SSC for 10 min each time at 25°C. The cells were fixed with 4% formaldehyde for 10 to 15 min on ice, washed with PBS three times, and permeabilized for 1 h with 70% ethanol or for 10 min with 0.5% Triton-X–PBS at 4°C. Then, the cells were incubated with 1:200 anti-DIG fluorescein isothiocyanate (FITC) (Jackson Lab Immunologicals) in 0.1% BSA-PBS for 1 h at 4°C. After three PBS washes, the cells were fixed, washed, and incubated with 0.4 μg/ml DAPI in 0.5% Triton-X–PBS for 15 min on ice. After washing three times, the slides were mounted with Vectashield (Vector Laboratories, Inc.). Images were taken with a Leica SP5 confocal microscope and analyzed with ImageJ (NIH, Bethesda, MD [http;//rsb.info.nih.gov/ij/]).
TABLE 1.
Oligonucleotides used in the study
| Oligonucleotide | Gene | Sequence | Positiona |
|
|---|---|---|---|---|
| In gene | In reference NC_009333 genome | |||
| Northern oligonucleotides | ||||
| KORF50 | KSHV RTA/ORF50 | CGCATTGCGGTGGTTGAAATTGCTGG | 1284–1309 | 73936–73961 |
| JBW249 | KSHV SOX/ORF37 | TAACCTGACACCACCAAACACACGGTCCAC | 262–291 | 57633–57662 |
| tkv379 | KSHV ORF59-58 | TGGAGTCCGGTATAGAATCGGGAACCT | 941–967 (ORF59) | 95879–95905 |
| tkv13 | KSHV K8.1 | AAGGCATAGGATTAGGAGCGCCACAGGGATTTCTGTGCGAAT | 16–57 | 76029–76070 |
| SB2 | KSHV PAN RNA | ACAAATGCCACCTCACTTTGTCGC | 664–687 | 29496–29519 |
| RNase P | Human RNase P | TGGGCGGAGGAGAGTAGTCTG | 319–339 | NA |
| FISH probes | ||||
| SB2 | KSHV PAN RNA | ACAAATGCCACCTCACTTTGTCGC | 664–687 | 29496–29519 |
| SB85 | KSHV PAN RNA | CGCTGCTTTCCTTTCACATT | 373–392 | 29205–29224 |
| SB88 | KSHV PAN RNA | GTGAAGCGGCAGCCAAGGTGACTGG | 1–22 | 28830–28854 |
| tkv13 | KSHV K8.1 | AAGGCATAGGATTAGGAGCGCCACAGGGATTTCTGTGCGAAT | 16–57 | 76029–76070 |
| tkv14 | KSHV K8.1 | TGATATTAAGGCATCGGTCAGTTCTGTGGTGGCCTGGA | 377–414 | 76390–76427 |
| tkv15 | KSHV K8.1 | GTAAGGTTACGCTTTATCCCTACACACCGACGGTTTACCC | 461–500 | 76474–76513 |
| tkv16 | KSHV K8.1 | GGACAAGTCCCAGCAATAAACCCACAGCCCATAGTATG | 688–725 | 76701–76738 |
| tkv376 | KSHV ORF59-58 | TAATGTGTTCATTGACCCTCCTGATT | 54–79 | 96767–96792 |
| tkv377 | KSHV ORF59-58 | GCCGATCCGTGCACTTGCACTACTCCGGTT | 93–122 | 96724–96753 |
| tkv378 | KSHV ORF59-58 | AAGGCTATGCCAGCGTCGAGTACATTCGCA | 300–329 | 96517–96546 |
| tkv379 | KSHV ORF59-58 | TGGAGTCCGGTATAGAATCGGGAACCT | 941–967 | 95879–95905 |
| tkv380 | KSHV ORF59-58 | AAAGAGTGTGAACGAGTACAGGGCCTT | 1289–1315 | 95531–95557 |
| tkv381 | KSHV ORF59-58 | AAACACTGCTGACGCGCAGATCCATTCC | 1423–1450 | 95396–95423 |
| tkv382 | KSHV ORF59-58 | TACCTGTGTACTATTGGCGGCGCCTGATACAC | 1571–1602 | 95244–95275 |
| tkv383 | KSHV ORF59-58 | GGGTCGAGATTCAGCTAATTAGGCGAAAACTCCACAGG | 2136–2173 | 94673–94710 |
| Stellaris | GAPDH | Premade by Stellaris | NA | NA |
| qPCR primers | ||||
| tkv458 | GAPDH promoter | CTGCACCACCAACTGCTTAG | NA | NA |
| tkv459 | GAPDH promoter | GTCTTCTGGGTGGCAGTGAT | NA | NA |
| tkv319 | KSHV ORF39 (gM) | GTGAGGTGCTTCGCTGAGTT | NA | 60075–60094 |
| tkv320 | KSHV ORF39 (gM) | CCTGGGTCAAGCTGTTGTTT | NA | 60218–60237 |
| RT-qPCR primers | ||||
| tkv 455 | K8/K-bZIP Forward RT qPCR primer | CGAAAGCAAGGCAGATACG | 655–673 | 75603–75621 |
| tkv 456 | K8/K-bZIP Reverse RT qPCR primer for unspliced | GCCATTGTTCCCATTTGAGT | 755–774 | 75703–75722 |
| tkv 457 | K8/K-bZIP Reverse RT qPCR primer for spliced | CATCAGCATGTCGCGAAG | 871–888 | 75819–75836 |
| JBW479 | Human RNase P Forward | AGCTTGGAACAGACTCACGG | 238–257 | NA |
| JBW480 | Human RNase P Reverse | GCGGAGGAGAGTAGTCTGAA | 317–336 | NA |
| PAN RNA knockdown oligonucleotide | ||||
| JBW659 | KSHV PAN RNA | mC*mC*mA*mC*mA*T*T*C*A*G*A*C*A*C*G*mU*mU*mA*mA*mG | 362–381 | 29180–29199 |
The numbers do not reflect direction or strand. NA, not applicable.
IF and FISH.
Cells were incubated with polyclonal primary antibodies before the FISH procedure and with monoclonal primary antibodies after the FISH procedure. For polyclonal rabbit PABP IF, cells were fixed and permeabilized as described above. Then, the cells were rinsed with PBS and blocked with 4% BSA-PBS for 30 min at 4°C after removing the slide chambers. Following the blocking step, the cells were incubated with 1:200 anti-PABP (Abcam; ab21060) in 0.1% BSA-PBS for 1 h at 4°C and then washed three times with PBS. The cells were then incubated with the secondary antibody, anti-rabbit Alexa Fluor 594, for 1 h at 4°C; washed three times with PBS; fixed; and then permeabilized before proceeding with FISH. The same procedure was used for anti-SSB antibody (rabbit polyclonal; a gift from G. Hayward) (58) at 1:200 dilution and anti-lamin B1 (rabbit polyclonal; Abcam; ab16048) at 1:500 dilution. For anti-SC35 antibody (mouse monoclonal; Abcam; ab11826), the same IF protocol was performed after FISH at 1:1,000 dilution.
Quantification of FISH and IF images.
Image analysis was performed with ImageJ on an assembled stack of the different fluorescent stains and the merged image. Measurements were first scaled according to the scale bar provided by Leica software. To measure fluorescence intensity along a line, we used the line tool and then the Plot Profile function to determine the raw fluorescence intensity along the length of a representative cell. The line traces quantitatively depict the raw fluorescence across all channels, revealing valleys and peaks among the fluorescent signals and thus providing a topograph. The path of the line trace was chosen to bisect the cell at random locations while capturing some nuclear foci of viral RNA but avoiding any fluorescent signals that might be oversaturated. The line path avoided foci with strong signals so that subtleties in the signal would not be masked by saturation. Additional factors that influenced path selection were avoidance of dead or dividing cells and the distance from other cells, so that the traces included background signal and detected signal from only one cell. Sample paths were indicated by white dashes at the approximate starting points and endpoints of the line. Data for the different stains were exported to Microsoft Excel and compiled on the same plot. For the line plot, the raw intensities were plotted without correction.
To measure the raw fluorescence intensity of the nucleus and cytoplasm, we used the polygon tool and the Measure function. For nuclear and cellular measurements, the polygon selection tool was used to draw boundaries around DAPI in the nucleus and the IF or FISH staining in the cytoplasm. The data were exported to Microsoft Excel and analyzed with the following equation, giving the fluorescence intensity ratio: log10 ([background-subtracted nuclear raw intensity/{background-subtracted cellular raw intensity − background-subtracted nuclear raw intensity}]/[nuclear area/{cellular area − nuclear area}]).
To normalize fluorescence intensity to background, an area comparable to an average cell was drawn in a region devoid of cells at three different locations on each image. To normalize the fluorescence intensity value for each cell, the individual cell area was multiplied by the average background mean, a value from ImageJ that represents the average fluorescence intensity over a defined area. This value was then subtracted from the raw fluorescence intensity value of the particular cell area for each stain or fluorescent signal.
The nuclear and cytoplasmic areas changed depending on the treatments, as did the ratio of the nuclear area to the total cellular area (Fig. 1D and E, 4C and D, and 7F and Table 2). To control for this changing area, each fluorescence intensity ratio was divided by its corresponding area ratio. Then, to represent both the cytoplasmic and nuclear values of the ratio equally, we calculated the logarithm base 10 of the fluorescence intensity relative to the area.
TABLE 2.
Statistics on areas shown in Fig. 4C
| Cell treatmenta | No. of cells | Area (μm2) |
SD | |||||
|---|---|---|---|---|---|---|---|---|
| Minimum | 25th percentile | Median | 75th percentile | Maximum | Mean | |||
| Unind, nuclear | 110 | 38.6 | 65.4 | 79.7 | 97.7 | 131.7 | 81.3 | 22.9 |
| Doxy, nuclear | 160 | 38.0 | 94.9 | 12.2 | 135.5 | 225.9 | 115.6 | 30.9 |
| Doxy + PAA, nuclear | 110 | 44.0 | 98.4 | 115.3 | 134.1 | 209.2 | 116.7 | 30.5 |
| Doxy + Cido, nuclear | 110 | 57.8 | 87.1 | 100.9 | 117.5 | 164.8 | 102.3 | 21.1 |
| Unind, cellular | 160 | 84.9 | 131.5 | 57.9 | 191.7 | 305.1 | 166.2 | 45.3 |
| Doxy, cellular | 110 | 84.7 | 161.7 | 194.1 | 224.9 | 361.2 | 198.3 | 49.1 |
| Doxy + PAA, cellular | 110 | 106.6 | 183.8 | 216.6 | 250.4 | 381.1 | 221.4 | 56.7 |
| Doxy + Cido, cellular | 110 | 113.9 | 165.3 | 190.0 | 224.5 | 467.7 | 201.8 | 58.4 |
| 10 h nuclear | 50 | 68.7 | 110.3 | 121.8 | 135.1 | 173.1 | 120.9 | 19.0 |
| 14 h nuclear | 51 | 65.0 | 91.3 | 105.3 | 130.1 | 165.8 | 110.0 | 25.3 |
| 18 h nuclear | 50 | 57.6 | 85.8 | 96.2 | 117.4 | 160.3 | 101.4 | 22.9 |
| 24 h nuclear | 101 | 63.4 | 91.0 | 106.0 | 128.8 | 162.4 | 108.8 | 23.4 |
| 36 h nuclear | 100 | 32.9 | 89.9 | 110.1 | 29.6 | 206.8 | 111.5 | 32.3 |
| 10 h cellular | 50 | 120.8 | 180.1 | 202.9 | 223.3 | 274.8 | 200.0 | 30.7 |
| 14 h cellular | 51 | 115.5 | 156.5 | 193.3 | 220.5 | 282.1 | 193.4 | 42.7 |
| 18 h cellular | 50 | 119.3 | 158.0 | 174.7 | 203.0 | 291.8 | 184.8 | 39.7 |
| 24 h cellular | 102 | 106.0 | 166.7 | 193.5 | 232.8 | 291.2 | 95.0 | 39.6 |
| 36 h cellular | 100 | 98.1 | 158.5 | 181.1 | 217.9 | 283.7 | 186.2 | 42.8 |
Unind, uninduced.
The ratios are shown in box-and-whisker plots. The edges of the boxes depict the 25th and 75th percentiles of the data, with the center lines indicating the median. The extended bars (whiskers) indicate the 5th and 95th percentiles. Data points beyond the 5th and 95th percentiles are shown as light-gray circles.
Primer design, qPCR, Northern blotting, and RT-qPCR.
Quantitative-PCR (qPCR) and reverse transcription (RT)-qPCR primers were designed using Primer3 (http://biotools.umassmed.edu/bioapps/primer3_www.cgi) with settings of 60°C Tm, 100- to 200-bp amplicon, and GC content around 50%. The amplicon was purified using the Wizard SV Gel and PCR Clean-Up system (Promega) and then run on a 1% agarose ethidium bromide (EtBr) gel to verify the presence of a single amplicon.
RNA was purified with TRIzol and genomic DNA with DNAzol according to the manufacturers' protocols. Eight nanograms of DNA per reaction was analyzed with qPCR using Fast Essential Mix containing SYBR green (Roche) on a Roche LightCycler 96 in 15-μl reaction mixtures according to the manufacturer's protocol. The primers are shown Table 1. Northern blots were performed as described previously (59). DNA oligonucleotides were labeled with [γ-32P]ATP and T4 PNK (NEB) and then hybridized at 37°C in a hybridization oven as outlined previously (59). Northern blots were quantified using ImageQuant. For RT-qPCR, the TRIzol-purified total RNA was treated with DNase RQ1 (Promega) according to the manufacturer's instructions and then phenol-chloroform extracted and ethanol precipitated with the addition of glycogen. Two micrograms of RNA was reverse transcribed with Superscript III (Invitrogen) using 200 ng random hexamers. The cDNA was then diluted 3-fold and added to SYBR green Fast Essential Mix at 0.75 μl/15-μl reaction mixture with 3 pmol forward primer and 3 pmol reverse primer.
KSHV PAN RNA knockdown.
TREx RTA BCBL-1 cells (107) were pelleted at 900 rpm for 4 min, washed once with 5 ml of RPMI medium lacking serum, and then resuspended in 400 μl of RPMI medium lacking serum and containing 2 nmol of RNase H-verified knockdown oligonucleotide or control GFP oligonucleotide. The cell suspension was pulsed in a 0.4-cm electroporation cuvette (Bio-Rad) at 975 μF, 210 mV and immediately added gently to 500 μl 20% FBS RPMI medium prewarmed to 37°C. The cell solution was incubated for 5 min at room temperature and transferred to 7 ml 20% FBS RPMI medium with 20 μg/ml hygromycin and, when appropriate, 1 μg/ml doxycycline for lytic activation, prewarmed to 37°C. The cells were then placed in a 37°C, 5% CO2 incubator for 24 h.
ACKNOWLEDGMENTS
We thank Jonathan Rodenfels for advice on data analysis and Kazimierz Tycowski, George Miller, and Richard Park for critical review of the manuscript.
This work was supported by the Hope Funds for Cancer Research (J.B.W.), grants T32GM007223 and T32AI055403 from the National Institutes of Health (to T.K.V.), and NIH grant (CA16038) (to J.A.S.). J.A.S. is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Schmid M, Speiseder T, Dobner T, Gonzalez RA. 2014. DNA virus replication compartments. J Virol 88:1404–1420. doi: 10.1128/JVI.02046-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dissinger NJ, Damania B. 2016. Recent advances in understanding Kaposi's sarcoma-associated herpesvirus. F1000Res 5:F1000 Faculty-Rev-740. doi: 10.12688/f1000research.7612.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias C, Weisburd B, Stern-Ginossar N, Mercier A, Madrid AS, Bellare P, Holdorf M, Weissman JS, Ganem D. 2014. KSHV 2.0: a comprehensive annotation of the Kaposi's sarcoma-associated herpesvirus genome using next-generation sequencing reveals novel genomic and functional features. PLoS Pathog 10:e1003847. doi: 10.1371/journal.ppat.1003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cai X, Lu S, Zhang Z, Gonzalez CM, Damania B, Cullen BR. 2005. Kaposi's sarcoma-associated herpesvirus expresses an array of viral microRNAs in latently infected cells. Proc Natl Acad Sci U S A 102:5570–5575. doi: 10.1073/pnas.0408192102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pfeffer S, Sewer A, Lagos-Quintana M, Sheridan R, Sander C, Grasser FA, van Dyk LF, Ho CK, Shuman S, Chien M, Russo JJ, Ju J, Randall G, Lindenbach BD, Rice CM, Simon V, Ho DD, Zavolan M, Tuschl T. 2005. Identification of microRNAs of the herpesvirus family. Nat Methods 2:269–276. doi: 10.1038/nmeth746. [DOI] [PubMed] [Google Scholar]
- 6.Samols MA, Hu J, Skalsky RL, Renne R. 2005. Cloning and identification of a microRNA cluster within the latency-associated region of Kaposi's sarcoma-associated herpesvirus. J Virol 79:9301–9305. doi: 10.1128/JVI.79.14.9301-9305.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottwein E. 2012. Kaposi's sarcoma-associated herpesvirus microRNAs. Front Microbiol 3:165. doi: 10.3389/fmicb.2012.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quinlan MP, Chen LB, Knipe DM. 1984. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell 36:857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- 9.Wu FY, Ahn JH, Alcendor DJ, Jang WJ, Xiao J, Hayward SD, Hayward GS. 2001. Origin-independent assembly of Kaposi's sarcoma-associated herpesvirus DNA replication compartments in transient cotransfection assays and association with the ORF-K8 protein and cellular PML. J Virol 75:1487–1506. doi: 10.1128/JVI.75.3.1487-1506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Tang Q, Maul GG, Yuan Y. 2006. Kaposi's sarcoma-associated herpesvirus ori-Lyt-dependent DNA replication: dual role of replication and transcription activator. J Virol 80:12171–12186. doi: 10.1128/JVI.00990-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baquero-Perez B, Whitehouse A. 2015. Hsp70 isoforms are essential for the formation of Kaposi's sarcoma-associated herpesvirus replication and transcription compartments. PLoS Pathog 11:e1005274. doi: 10.1371/journal.ppat.1005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burch AD, Weller SK. 2004. Nuclear sequestration of cellular chaperone and proteasomal machinery during herpes simplex virus type 1 infection. J Virol 78:7175–7185. doi: 10.1128/JVI.78.13.7175-7185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burch AD, Weller SK. 2005. Herpes simplex virus type 1 DNA polymerase requires the mammalian chaperone hsp90 for proper localization to the nucleus. J Virol 79:10740–10749. doi: 10.1128/JVI.79.16.10740-10749.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CP, Lyu Y, Chuang F, Nakano K, Izumiya C, Jin D, Campbell M, Izumiya Y. 2017. Kaposi's sarcoma-associated herpesvirus hijacks RNA polymerase II to create a viral transcriptional factory. J Virol 91:e02491-16. doi: 10.1128/JVI.02491-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li L, Johnson LA, Dai-Ju JQ, Sandri-Goldin RM. 2008. Hsc70 focus formation at the periphery of HSV-1 transcription sites requires ICP27. PLoS One 3:e1491. doi: 10.1371/journal.pone.0001491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura H, Lu M, Gwack Y, Souvlis J, Zeichner SL, Jung JU. 2003. Global changes in Kaposi's sarcoma-associated virus gene expression patterns following expression of a tetracycline-inducible Rta transactivator. J Virol 77:4205–4220. doi: 10.1128/JVI.77.7.4205-4220.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majerciak V, Yamanegi K, Zheng ZM. 2006. Gene structure and expression of Kaposi's sarcoma-associated herpesvirus ORF56, ORF57, ORF58, and ORF59. J Virol 80:11968–11981. doi: 10.1128/JVI.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xiao J, Palefsky JM, Herrera R, Tugizov SM. 2007. Characterization of the Epstein-Barr virus glycoprotein BMRF-2. Virology 359:382–396. doi: 10.1016/j.virol.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 19.Chandran B, Bloomer C, Chan SR, Zhu L, Goldstein E, Horvat R. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249:140–149. doi: 10.1006/viro.1998.9316. [DOI] [PubMed] [Google Scholar]
- 20.Tang S, Zheng ZM. 2002. Kaposi's sarcoma-associated herpesvirus K8 exon 3 contains three 5′-splice sites and harbors a K8.1 transcription start site. J Biol Chem 277:14547–14556. doi: 10.1074/jbc.M111308200. [DOI] [PubMed] [Google Scholar]
- 21.Clyde K, Glaunsinger BA. 2011. Deep sequencing reveals direct targets of gammaherpesvirus-induced mRNA decay and suggests that multiple mechanisms govern cellular transcript escape. PLoS One 6:e19655. doi: 10.1371/journal.pone.0019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monier K, Armas JC, Etteldorf S, Ghazal P, Sullivan KF. 2000. Annexation of the interchromosomal space during viral infection. Nat Cell Biol 2:661–665. doi: 10.1038/35023615. [DOI] [PubMed] [Google Scholar]
- 23.Taylor TJ, McNamee EE, Day C, Knipe DM. 2003. Herpes simplex virus replication compartments can form by coalescence of smaller compartments. Virology 309:232–247. doi: 10.1016/S0042-6822(03)00107-7. [DOI] [PubMed] [Google Scholar]
- 24.Huang ES. 1975. Human cytomegalovirus. IV. Specific inhibition of virus-induced DNA polymerase activity and viral DNA replication by phosphonoacetic acid. J Virol 16:1560–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neyts J, Snoeck R, Schols D, Balzarini J, De Clercq E. 1990. Selective inhibition of human cytomegalovirus DNA synthesis by (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine [(S)-HPMPC] and 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG). Virology 179:41–50. doi: 10.1016/0042-6822(90)90271-R. [DOI] [PubMed] [Google Scholar]
- 26.Borah S, Darricarrere N, Darnell A, Myoung J, Steitz JA. 2011. A viral nuclear noncoding RNA binds re-localized poly(A) binding protein and is required for late KSHV gene expression. PLoS Pathog 7:e1002300. doi: 10.1371/journal.ppat.1002300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell M, Kim KY, Chang PC, Huerta S, Shevchenko B, Wang DH, Izumiya C, Kung HJ, Izumiya Y. 2014. A lytic viral long noncoding RNA modulates the function of a latent protein. J Virol 88:1843–1848. doi: 10.1128/JVI.03251-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossetto CC, Pari G. 2012. KSHV PAN RNA associates with demethylases UTX and JMJD3 to activate lytic replication through a physical interaction with the virus genome. PLoS Pathog 8:e1002680. doi: 10.1371/journal.ppat.1002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Covarrubias S, Gaglia MM, Kumar GR, Wong W, Jackson AO, Glaunsinger BA. 2011. Coordinated destruction of cellular messages in translation complexes by the gammaherpesvirus host shutoff factor and the mammalian exonuclease Xrn1. PLoS Pathog 7:e1002339. doi: 10.1371/journal.ppat.1002339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abernathy E, Clyde K, Yeasmin R, Krug LT, Burlingame A, Coscoy L, Glaunsinger B. 2014. Gammaherpesviral gene expression and virion composition are broadly controlled by accelerated mRNA degradation. PLoS Pathog 10:e1003882. doi: 10.1371/journal.ppat.1003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee YJ, Glaunsinger BA. 2009. Aberrant herpesvirus-induced polyadenylation correlates with cellular messenger RNA destruction. PLoS Biol 7:e1000107. doi: 10.1371/journal.pbio.1000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bhatt DM, Pandya-Jones A, Tong AJ, Barozzi I, Lissner MM, Natoli G, Black DL, Smale ST. 2012. Transcript dynamics of proinflammatory genes revealed by sequence analysis of subcellular RNA fractions. Cell 150:279–290. doi: 10.1016/j.cell.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandya-Jones A, Bhatt DM, Lin CH, Tong AJ, Smale ST, Black DL. 2013. Splicing kinetics and transcript release from the chromatin compartment limit the rate of lipid A-induced gene expression. RNA 19:811–827. doi: 10.1261/rna.039081.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shalgi R, Hurt JA, Lindquist S, Burge CB. 2014. Widespread inhibition of posttranscriptional splicing shapes the cellular transcriptome following heat shock. Cell Rep 7:1362–1370. doi: 10.1016/j.celrep.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 35.Chujo T, Yamazaki T, Hirose T. 2016. Architectural RNAs (arcRNAs): A class of long noncoding RNAs that function as the scaffold of nuclear bodies. Biochim Biophys Acta 1859:139–146. doi: 10.1016/j.bbagrm.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 36.Sztuba-Solinska J, Rausch JW, Smith R, Miller JT, Whitby D, Le Grice SFJ. 2017. Kaposi's sarcoma-associated herpesvirus polyadenylated nuclear RNA: a structural scaffold for nuclear, cytoplasmic and viral proteins. Nucleic Acids Res 45:6805–6821. doi: 10.1093/nar/gkx241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugimoto A, Sato Y, Kanda T, Murata T, Narita Y, Kawashima D, Kimura H, Tsurumi T. 2013. Different distributions of Epstein-Barr virus early and late gene transcripts within viral replication compartments. J Virol 87:6693–6699. doi: 10.1128/JVI.00219-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeang KT, Hayward SD. 1983. Organization of the Epstein-Barr virus DNA molecule. III. Location of the P3HR-1 deletion junction and characterization of the NotI repeat units that form part of the template for an abundant 12-O-tetradecanoylphorbol-13-acetate-induced mRNA transcript. J Virol 48:135–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rennekamp AJ, Lieberman PM. 2011. Initiation of Epstein-Barr virus lytic replication requires transcription and the formation of a stable RNA-DNA hybrid molecule at OriLyt. J Virol 85:2837–2850. doi: 10.1128/JVI.02175-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bechtel J, Grundhoff A, Ganem D. 2005. RNAs in the virion of Kaposi's sarcoma-associated herpesvirus. J Virol 79:10138–10146. doi: 10.1128/JVI.79.16.10138-10146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossetto CC, Tarrant-Elorza M, Verma S, Purushothaman P, Pari GS. 2013. Regulation of viral and cellular gene expression by Kaposi's sarcoma-associated herpesvirus polyadenylated nuclear RNA. J Virol 87:5540–5553. doi: 10.1128/JVI.03111-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terhune SS, Schroer J, Shenk T. 2004. RNAs are packaged into human cytomegalovirus virions in proportion to their intracellular concentration. J Virol 78:10390–10398. doi: 10.1128/JVI.78.19.10390-10398.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sciortino MT, Taddeo B, Poon AP, Mastino A, Roizman B. 2002. Of the three tegument proteins that package mRNA in herpes simplex virions, one (VP22) transports the mRNA to uninfected cells for expression prior to viral infection. Proc Natl Acad Sci U S A 99:8318–8323. doi: 10.1073/pnas.122231699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Greijer AE, Dekkers CA, Middeldorp JM. 2000. Human cytomegalovirus virions differentially incorporate viral and host cell RNA during the assembly process. J Virol 74:9078–9082. doi: 10.1128/JVI.74.19.9078-9082.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Muriaux D, Mirro J, Harvin D, Rein A. 2001. RNA is a structural element in retrovirus particles. Proc Natl Acad Sci U S A 98:5246–5251. doi: 10.1073/pnas.091000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muriaux D, Mirro J, Nagashima K, Harvin D, Rein A. 2002. Murine leukemia virus nucleocapsid mutant particles lacking viral RNA encapsidate ribosomes. J Virol 76:11405–11413. doi: 10.1128/JVI.76.22.11405-11413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang SW, Aldovini A. 2002. RNA incorporation is critical for retroviral particle integrity after cell membrane assembly of Gag complexes. J Virol 76:11853–11865. doi: 10.1128/JVI.76.23.11853-11865.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amen MA, Griffiths A. 2011. Packaging of non-coding RNAs into herpesvirus virions: comparisons to coding RNAs. Front Genet 2:81. doi: 10.3389/fgene.2011.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye F, Chen ER, Nilsen TW. 2017. Kaposi's sarcoma-associated herpesvirus utilizes and manipulates RNA N(6)-adenosine methylation to promote lytic replication. J Virol 91:e00466-17. doi: 10.1128/JVI.00466-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bahar Halpern K, Caspi I, Lemze D, Levy M, Landen S, Elinav E, Ulitsky I, Itzkovitz S. 2015. Nuclear retention of mRNA in mammalian tissues. Cell Rep 13:2653–2662. doi: 10.1016/j.celrep.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dar RD, Razooky BS, Singh A, Trimeloni TV, McCollum JM, Cox CD, Simpson ML, Weinberger LS. 2012. Transcriptional burst frequency and burst size are equally modulated across the human genome. Proc Natl Acad Sci U S A 109:17454–17459. doi: 10.1073/pnas.1213530109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Darzacq X, Shav-Tal Y, de Turris V, Brody Y, Shenoy SM, Phair RD, Singer RH. 2007. In vivo dynamics of RNA polymerase II transcription. Nat Struct Mol Biol 14:796–806. doi: 10.1038/nsmb1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Larson DR, Zenklusen D, Wu B, Chao JA, Singer RH. 2011. Real-time observation of transcription initiation and elongation on an endogenous yeast gene. Science 332:475–478. doi: 10.1126/science.1202142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suter DM, Molina N, Gatfield D, Schneider K, Schibler U, Naef F. 2011. Mammalian genes are transcribed with widely different bursting kinetics. Science 332:472–474. doi: 10.1126/science.1198817. [DOI] [PubMed] [Google Scholar]
- 55.Sun R, Lin SF, Staskus K, Gradoville L, Grogan E, Haase A, Miller G. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol 73:2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu M, Suen J, Frias C, Pfeiffer R, Tsai MH, Chuang E, Zeichner SL. 2004. Dissection of the Kaposi's sarcoma-associated herpesvirus gene expression program by using the viral DNA replication inhibitor cidofovir. J Virol 78:13637–13652. doi: 10.1128/JVI.78.24.13637-13652.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pawlicki JM, Steitz JA. 2008. Primary microRNA transcript retention at sites of transcription leads to enhanced microRNA production. J Cell Biol 182:61–76. doi: 10.1083/jcb.200803111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chiou CJ, Poole LJ, Kim PS, Ciufo DM, Cannon JS, ap Rhys CM, Alcendor DJ, Zong JC, Ambinder RF, Hayward GS. 2002. Patterns of gene expression and a transactivation function exhibited by the vGCR (ORF74) chemokine receptor protein of Kaposi's sarcoma-associated herpesvirus. J Virol 76:3421–3439. doi: 10.1128/JVI.76.7.3421-3439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown JA, Steitz JA. 2016. Intronless beta-globin reporter: a tool for studying nuclear RNA stability elements. Methods Mol Biol 1428:77–92. doi: 10.1007/978-1-4939-3625-0_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Siegfried NA, Busan S, Rice GM, Nelson JA, Weeks KM. 2014. RNA motif discovery by SHAPE and mutational profiling (SHAPE-MaP). Nat Methods 11:959–965. doi: 10.1038/nmeth.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]