ABSTRACT
Hepatitis E virus (HEV) causes liver disease in humans and is thought to be a zoonotic infection, with domestic animals, including swine and rabbits, being a reservoir. One of the proteins encoded by the virus is the capsid protein. This is likely the major immune-dominant protein and a target for vaccination. Four monoclonal antibodies (MAbs), three novel, 1E4, 2C7, and 2G9, and one previously characterized, 1B5, were evaluated for binding to the capsid protein from genotype 4 swine HEV. The results indicated that 625DFCP628, 458PSRPF462, and 407EPTV410 peptides on the capsid protein comprised minimal amino acid sequence motifs recognized by 1E4, 2C7, and 2G9, respectively. The data suggested that 2C7 and 2G9 epitopes were partially exposed on the surface of the capsid protein. Truncated genotype 4 swine HEV capsid protein (sp239, amino acids 368 to 606) can exist in multimeric forms. Preincubation of swine HEV with 2C7, 2G9, or 1B5 before addition to HepG2 cells partially blocked sp239 cell binding and inhibited swine HEV infection. The study indicated that 2C7, 2G9, and 1B5 partially blocked swine HEV infection of rabbits better than 1E4 or normal mouse IgG. The cross-reactivity of antibodies suggested that capsid epitopes recognized by 2C7 and 2G9 are common to HEV strains infecting most host species. Collectively, MAbs 2C7, 2G9, and 1B5 were shown to recognize three novel linear neutralizing B-cell epitopes of genotype 4 HEV capsid protein. These results enhance understanding of HEV capsid protein structure to guide vaccine and antiviral design.
IMPORTANCE Genotype 3 and 4 HEVs are zoonotic viruses. Here, genotype 4 HEV was studied due to its prevalence in human populations and pig herds in China. To improve HEV disease diagnosis and prevention, a better understanding of the antigenic structure and neutralizing epitopes of HEV capsid protein are needed. In this study, the locations of three novel linear B-cell recognition epitopes within genotype 4 swine HEV capsid protein were characterized. Moreover, the neutralizing abilities of three MAbs specific for this protein, 2C7, 2G9, and 1B5, were studied in vitro and in vivo. Collectively, these findings reveal structural details of genotype 4 HEV capsid protein and should facilitate development of applications for the design of vaccines and antiviral drugs for broader prevention, detection, and treatment of HEV infection of diverse human and animal hosts.
KEYWORDS: hepatitis E virus, swine HEV, epitope mapping, neutralization
INTRODUCTION
Hepatitis E virus (HEV) infects more than 20 million people worldwide each year, resulting in 14 million symptomatic cases and 300,000 deaths annually (1). Generally, HEV infections do not frequently lead to fulminant hepatitis in humans (1 to 4%), but mortality rates in pregnant women can approach 25% in some areas where HEV is endemic (2). Meanwhile, many recent chronic cases of hepatitis E have been reported in immunosuppressed individuals (3, 4). Therefore, HEV infection is an important public health concern worldwide.
Swine HEV, the first identified animal HEV strain, shares 80% to 90% nucleotide sequence identity with human HEV from the same geographical location (5, 6). The known host range of HEV is continually expanding, as various distinct HEV isolates have been detected in domestic pigs (6) and in wild boar (7), deer (8, 9), chicken (10, 11), rat (12), ferret (13), rabbit (14), mongoose (15), camel (16), and bat (17) species. At present, genotype 3 and 4 HEVs are regarded as potential zoonotic viruses, with a main natural reservoir that includes pigs, wild boar, and rabbits (18–21). HEV belongs to the family Hepeviridae, which includes two genera, Orthohepevirus and Piscihepevirus (22). Orthohepevirus includes four HEV species (A to D), of which the Orthohepevirus A, C, and D species mainly infect mammalian hosts while the Orthohepevirus B species mainly infects chickens (22). Within Orthohepevirus A species, HEV genotypes 1 and 2 are restricted to humans only, while HEV genotypes 3, 4, and 7 are zoonotic (1). So far, isolates of both HEV genotypes 3 and 4 have been isolated from pigs and hence are commonly known as swine HEV (23). In China, genotype 4 is the predominant HEV genotype found in pig herds (24, 25).
HEV is a nonenveloped, positive-sense, single-stranded RNA virus (23, 26) with a genome that is approximately 7.2 kb in size and that contains three open reading frames (ORFs), ORF1, ORF2, and ORF3 (27). ORF2 encodes the main viral structural protein, the viral capsid protein (designated ORF2 protein), which is composed of approximately 660 amino acids (aa). The capsid protein harbors both immunodominant and neutralization epitopes of viral particles and is the target of a protective humoral immune response (28). To date, six neutralizing epitopes, including two conformational and four linear epitopes, have been identified within the ORF2 protein of genotype 1 HEV (29). Almost all neutralizing epitopes studied to date (with one exception) reside mainly in the E2 (protruding) domain spanning capsid protein aa 459 to 602. Interestingly, although all four major HEV genotypes share a single serotype, a monoclonal antibody (MAb) recognizing a conformational neutralizing epitope could neutralize only genotype 1 HEV, not other HEV genotypes (30, 31). This result suggested that slight structural diversity exists among ORF2 proteins of the four major HEV genotypes. In addition, few published reports to date have described antibody-neutralizing epitopes located within the genotype 4 swine HEV ORF2 protein.
In the present study, three epitopes recognized by three novel MAbs, 1E4, 2C7, and 2G9, were characterized within the capsid protein of genotype 4 swine HEV. Of these, 2C7 and 2G9 could partially inhibit genotype 4 swine HEV infection of HepG2 cells in vitro and of rabbits in vivo. In addition, MAb 1B5, recognizing an epitope common to HEV isolates infecting various host species, also showed partial neutralizing ability both in vitro and in vivo. These results should help us better understand the antigenic structure of the HEV capsid protein and provide a foundation for future HEV vaccine and antiviral drug design.
RESULTS
Precise definition of minimal motifs recognized by three novel MAbs.
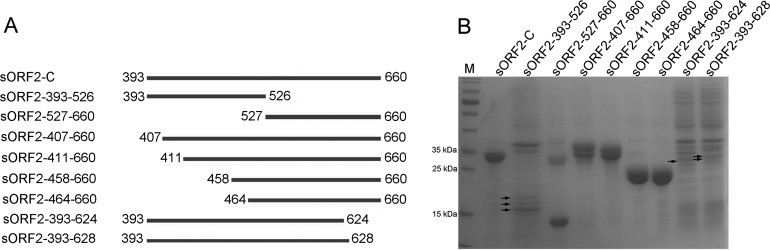
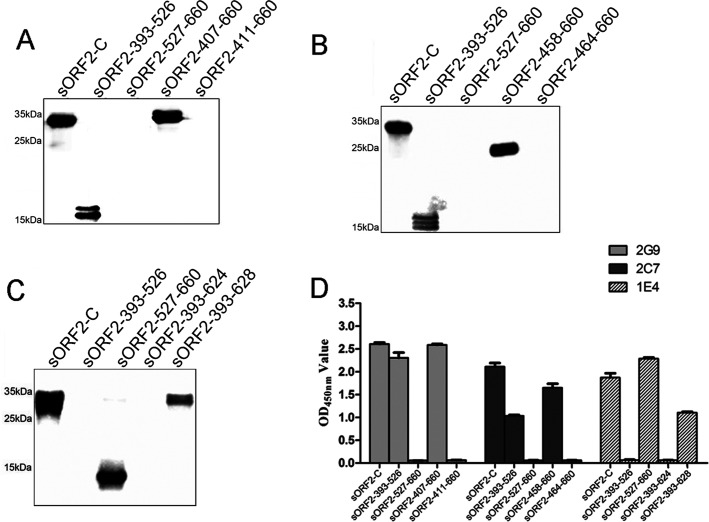
To determine the minimal antigenic domains recognized by three novel MAbs, 2G9, 2C7, and 1E4, various truncated and overlapping sORF2-C fragments were designed (Fig. 1A) and expressed using a bacterial system. SDS-PAGE results showed that truncated fragments of aa 393 to 526 (15.6 kDa), 527 to 660 (14.7 kDa), 407 to 660 (27.8 kDa), 411 to 660 (27.2 kDa), 458 to 660 (21.9 kDa), 464 to 660 (21.4 kDa), 393 to 624 (25.1 kDa), and 393 to 628 (25.6 kDa) were successfully expressed at predicted sizes in the prokaryotic expression system (Fig. 1B). All fusion proteins were expressed predominantly as inclusion bodies in bacterial cells. Subsequently, Western blotting and indirect enzyme-linked immunosorbent assay (ELISA) methods were used to determine immune reactivity between the three MAbs and these ORF2 fragments. Western blotting results showed that MAb 2G9 reacted with fragments spanning aa 393 to 660 (sORF2-C), 393 to 526, and 407 to 660 but not aa 527 to 660 or 411 to 660, suggesting that the epitope recognized by 2G9 was located within aa 407 to 410 of swine HEV capsid protein (Fig. 2A). By use of the same methods, epitopes recognized by MAbs 2C7 and 1E4 were mapped to aa 458 to 464 and aa 625 to 628, respectively (Fig. 2B and C). The fragments comprising aa 393 to 526, 393 to 624, and 393 to 628 showed different sizes (Fig. 1B), which were possible due to the partial degradation of these proteins and then different forms of aggregation. In fact, these different forms of proteins were reactive with MAbs (Fig. 2A to C). When the reactivities of the three MAbs were tested to determine binding to various fragments using indirect ELISA, the results were in agreement with Western blotting results (Fig. 2D).
FIG 1.
Schematic diagram (A) and SDS-PAGE analysis (B) of various sORF2-C truncated fragments expressed using a prokaryotic system. Recombinant plasmid containing the gene sequence encoding the C-terminal 268 amino acids of genotype 4 swine HEV was used as the template for construction of eight truncated ORF2 fragments, as described in the text. Overlapping amino acid regions of the various truncated fragments are shown in the schematic diagram in panel A.
FIG 2.
Reactivity of three novel MAbs with various sORF2-C truncated fragments using Western blotting and indirect ELISA. (A) Western blotting of MAb 2G9 binding to truncated fragments; (B) Western blotting of MAb 2C7 binding to truncated fragments; (C) Western blotting of MAb 1E4 binding to truncated fragments; (D) indirect ELISA analysis showing binding of three MAbs, 2G9, 2C7, and 1E4, to truncated fragments.
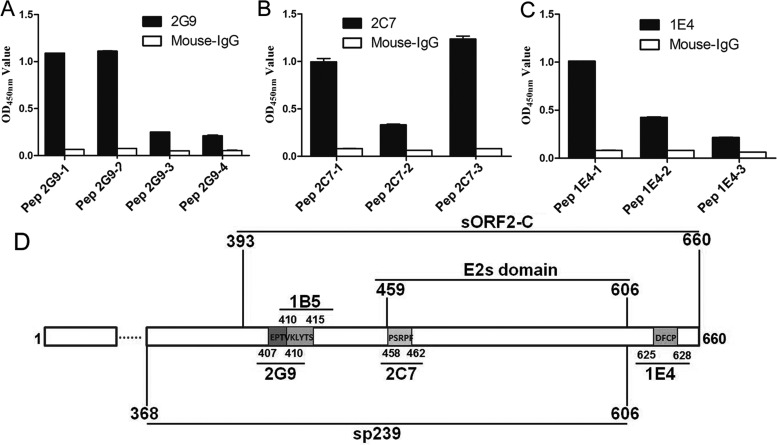
To further precisely define the minimal motifs required for binding by MAbs 2G9, 2C7, and 1E4, three panels of peptides were synthesized based on the aforementioned identified antigenic domains. The core amino acid sequences of peptides were then determined to be EPTVK for 2G9, PSRPFS for 2C7, and DFCP for 1E4. Furthermore, truncated and extended peptides were designed and synthesized based on these core amino acid sequences (see Table 3). Synthetic peptides were next used as indirect ELISA coating antigens to test the antigen reactivities of MAbs 2G9, 2C7, and 1E4. For binding to MAb 2G9, Pep2G9-1 (containing EPTVK) and Pep2G9-2 (containing EPTV) showed higher reactivities than did Pep2G9-3 (containing PTV) and 2G9-4 (containing EPT) (Fig. 3A). For binding to MAb 2C7, Pep2C7-1 (containing PSRPFS) and Pep2C7-3 (containing PSRPF) showed higher reactivities than did Pep2C7-2 (containing SRPFS), which showed no reactivity (Fig. 3B). For binding to MAb 1E4, Pep1E4-1 (containing DFCP) showed higher reactivity than did Pep1E4-2 (containing DFC), but no reactivity was observed for Pep1E4-3 (containing DF) (Fig. 3C). These results confirmed that the minimal amino acid sequences required for epitope recognition by MAb 2G9, 2C7, and 1E4 were 407EPTV410, 458PSRPF462, and 625DFCP628, respectively.
TABLE 3.
Sequences of the synthetic peptides used to map the minimal MAb binding motifs
| Short peptide | No. of amino acids | Purity (%) | Sequence (N terminus to C terminus)a | Interact with relative MAb (OD450) |
|---|---|---|---|---|
| Pep2G9-1 | 6 | 96.5 | GEPTVK | 1.09 |
| Pep2G9-2 | 6 | 95.7 | NGEPTV | 1.11 |
| Pep2G9-3 | 6 | 98.0 | PTVKLY | 0.25 |
| Pep2G9-4b | 6 | 95.6 | ANGEPT | 0.21 |
| Pep2C7-1 | 6 | 95.6 | PSRPFS | 1.00 |
| Pep2C7-2 | 6 | 95.2 | SRPFSV | 0.33 |
| Pep2C7-3 | 6 | 98.8 | APSRPF | 1.24 |
| Pep1E4-1 | 6 | 95.6 | FDDFCP | 1.01 |
| Pep1E4-2 | 6 | 98.6 | TFDDFC | 0.42 |
| Pep1E4-3 | 6 | 96.5 | HTFDDF | 0.21 |
Bold letters indicate key amino acids determined by truncated sORF2-C protein.
Pep2G9-4 was used to identify whether V470 is a common amino acid in the epitopes shared by 2G9 and 1B5.
FIG 3.
Fine mapping of epitopes recognized by three MAbs by indirect ELISA using synthetic peptides as coating antigens. Minimal amino acids bound by MAbs 2G9 (A), 2C7 (B), and 1E4 (C) were determined using synthetic peptides determined using indirect ELISA. (D) The linear locations of sORF2-C, sp239, E2s domain (42), and genotype 4 swine HEV capsid protein epitopes which are recognized by these MAbs (1B5 was reported by Wang et al. [30]) are shown.
Collectively, epitopes recognized by MAbs 2G9, 2C7, and 1E4 were localized within the swine HEV capsid protein model (Fig. 3D). In addition, MAb 1B5 recognized the common HEV epitope shared by diverse HEVs, as identified previously (28). This epitope, shown in Fig. 3D, prompted us to test 1B5 for its ability to neutralize swine HEV infection in host cells in vitro and in rabbits in vivo as part of this study.
Epitopes recognized by MAbs 2G9 and 2C7 on the surface of the swine HEV particle.
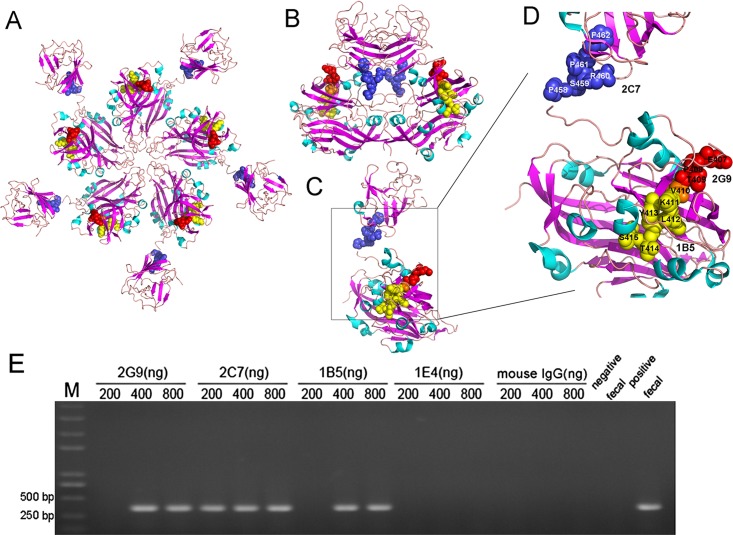
Protein modeling studies of the HEV capsid protein were undertaken to spatially localize capsid antigenic motifs bound by 2G9 and 2C7. In a previous study, MAbs 1B5 and 3E8 were shown to recognize two common epitopes of HEVs with tropisms for various host species (28). Subsequently, these epitopes were mapped to the 2ZTN HEV capsid protein structure obtained from the RCSB Protein Data Bank (PDB) using the protein modeling program PyMOL (28). Comparison of epitopes recognized by MAbs 2G9 and 1B5 spanning aa 407 to 410 and aa 410 to 415, respectively, revealed that these epitopes shared a common amino acid, V410. V410 is located within the M domain (aa 320 to 455) that partially protrudes from the capsid protein surface (Fig. 4A) as well as the dimeric form (Fig. 4B). The epitope recognized by MAb 2C7, spanning aa 458 to 462, is located within the E2s domain (aa 459 to 602) and almost entirely protrudes from the surface of the molecule in both pentameric (Fig. 4A) and dimeric forms (Fig. 4B). All epitopes are located within the loop region between two beta folding sheets and on the outer edge of the HEV capsid protein (Fig. 4C and D). The epitope of MAb 1E4 (aa 625 to 628) is not visible in the 2ZTN HEV capsid protein structure, and therefore its spatial location was not included in Fig. 4.
FIG 4.
Spatial locations of epitopes within the HEV capsid protein recognized by MAbs 2C7, 2G9, and 1B5. Overall structures of HEV capsid protein pentamer (A), dimer (B), and monomer (C) and locations of epitopes 2C7, 2G9, and 1B5 in the monomer (D) are shown. The spatial locations of the MAb 2G9-binding epitope (yellow), 1B5-binding epitope (red) (30), and 2C7-binding epitope (blue) protruding from the HEV capsid protein are highlighted. Binding epitopes of 1B5 and 2G9 share an amino acid, V410 (orange). The three-dimensional capsid protein structure (2ZTN) was obtained from the RCSB PDB. (E) Immune capture analysis of native swine HEV particles by the four MAbs, 1E4, 2C7, 2G9, and 1B5, followed by viral detection using nested RT-PCR. Negative and positive fecal samples were used as negative and positive controls, respectively, and mouse IgG was used as an irrelevant MAb control.
After speculation regarding the spatial locations of HEV capsid protein epitopes, immune capture assays were carried out to determine whether epitopes recognized by MAbs were localized to the surface of natural swine HEV particles by using a sensitive nested reverse transcription (RT)-PCR procedure to detect swine HEV particles captured by the MAbs. As shown in Fig. 4E, 2C7 coated at a concentration of 200, 400, and 800 ng/well, and both 2G9 and 1B5 coated at concentrations of 400 and 800 ng/well could capture native swine HEV particles from feces. Conversely, neither 1E4 nor control mouse IgG could capture virus at any concentration (Fig. 4E).
Collectively, the epitopes recognized by MAbs 2C7, 2G9, and 1B5 were localized to the surface of natural swine HEV particles by using predictions based on the capsid protein crystal structure and immune capture assay results. However, the data suggested that the epitope recognized by MAb 1E4 might not be on the surface.
sp239 proteins assembled into polymers.
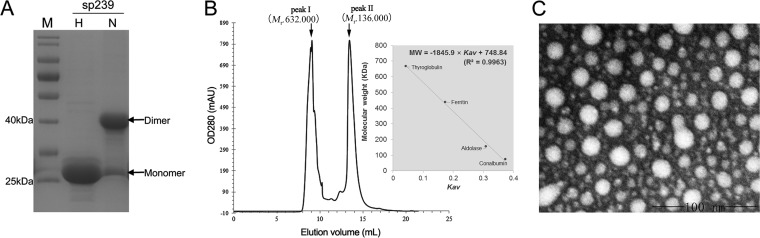
Previously, it was reported that a fragment (designated p239) spanning the region of aa 368 to 606 of genotype 1 HEV capsid protein expressed in a bacterial system could self-assemble into virus-like particles (VLPs) (32). In addition, p239 could adsorb to and enter host cells in vitro, mimicking the action of native HEV particles. Here, the region corresponding to the truncated capsid protein (sp239) of genotype 4 swine HEV was expressed and analyzed to determine if it could self-assemble into a polymer for use in MAb neutralization testing in vitro. SDS-PAGE results showed that after dialysis and renaturation, genotype 4 HEV sp239 formed dimers in phosphate buffer at pH 7.5 (PB7.5) (Fig. 5A). To determine whether sp239 could self-assemble into a polymer, size exclusion chromatography (SEC) and electron microscopy were applied to investigate the final physical states of sp239 in PB7.5. SEC results showed that two peaks emerged during gel filtration (Fig. 5B). Based on the molecular weights of the protein standards used for gel filtration, peak I represented a polymeric form (Mr, 632,000) and peak II represented a pentameric form (Mr, 136,000). Electron microscopy results demonstrated that sp239 collection peak I represented polymers with a diameter of approximately 23 nm (Fig. 5C). Taken together, these results demonstrated that sp239 could self-assemble into polymers that exhibited good homogeneity.
FIG 5.
Analysis of assembly of sp239 protein from genotype 4 swine HEV to form polymers. (A) Monomers and dimers of sp239 with or without prior heat treatment in PB buffer were observed using modified SDS-PAGE. Lane M, molecular size markers; lane H, heated samples under reducing conditions (samples were heated to 100°C for 3 min with BME); lane N, unheated samples under nonreducing conditions (0.1% SDS in the absence of BME). (B) Size exclusion chromatography of sp239 purified using a Superdex 200 Increase 10/300 GL column. The inset shows the calibration of the column and the equation used for calculating the size of the sp239 complex. MW, molecular weight. Accordingly, Mr 632,000 polymeric and Mr 136,000 pentameric forms of sp239 were detected in peak I and peak II, respectively, during gel filtration using an ÄKTA purifier (GE Healthcare). (C) Bacterially expressed sp239 protein particles in PB7.5 were visualized using electron microscopy and exhibited diameters of approximately 23 nm.
MAbs blocked sp239 attachment and swine HEV infection of HepG2 cells.
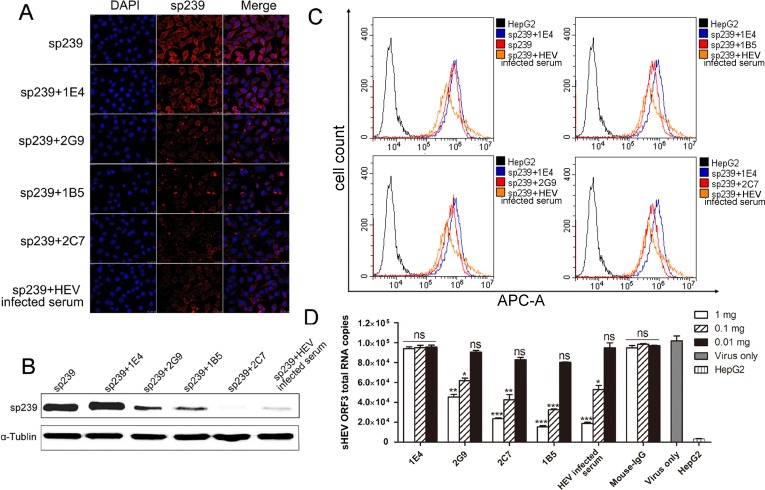
Neutralization abilities of MAbs in vitro were tested using sp239 in lieu of intact swine HEV particles. First, as shown in the top row of immunofluorescence focus assay (IFA) results (Fig. 6A), the first lane of Western blotting results (Fig. 6B), and flow cytometry neutralization (FCM) data (Fig. 6C), sp239 could bind to and penetrate untreated HepG2 cells. Western blotting, IFA, and FCM assays could all detect differences in sp239 adsorption to HepG2 cells after sp239 preincubation with various MAbs. Because the epitope recognized by 1E4 is not located within sp239, 1E4 served as an ideal negative MAb control. In addition, pig serum pooled from specific-pathogen-free (SPF) pigs challenged with strain CHN-SD-sHEV at 42 days postinoculation (dpi) served as a positive control. Western blotting results showed that the amounts of sp239 attached to HepG2 cells were significantly reduced after incubation with MAbs 2C7, 2G9, and 1B5 relative to the results for 1E4 (Fig. 6B). Consistent with the Western blotting results, IFA also showed that the amounts of sp239 attached to cells after sp239 preincubation with MAb 2C7, 2G9, or 1B5 or with HEV-infected serum were significantly less than for cells incubated with only sp239 or sp239 preincubated with MAb 1E4 (Fig. 6A). In addition, FCM results also showed that sp239 preincubated with MAb 2C7, 2G9, or 1B5 (red curve in each group) or with HEV-infected serum exhibited less binding to HepG2 cells than did sp239 after preincubation with MAb 1E4 (blue curve) (Fig. 6C). However, in comparison with results of Western blotting and IFA, differences in FCM results among MAbs 2C7, 2G9, and 1B5 regarding blocking of sp239 cell attachment were not obvious.
FIG 6.
MAbs block sp239 attachment and swine HEV infection of HepG2 cells. Analysis of blocking of sp239 attachment to HepG2 cells by MAbs 1E4, 2C7, 2G9, and 1B5, followed by detection using IFA (A), Western blotting (B), and FCM (C) assays. Mouse IgG served as an irrelevant MAb control, and HEV-infected serum served as a positive control. APC-A, allophycocyanin. (D) Analysis of blocking of swine HEV infection of HepG2 cells by different amounts of MAbs and HEV-infected serum using real-time RT-PCR. Data are the mean ± SD of results based on three independent experiments. P values were calculated using Student's t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
Second, the abilities of MAbs to block swine HEV infection of HepG2 cells were also evaluated using real-time RT-PCR. A dose-dependent study was performed to validate the ability of MAbs to block virus infection of HepG2 cells. MAbs 2C7, 2G9, 1E4, and 1B5, mouse IgG at 1, 0.1, and 0.01 mg, and HEV-infected serum undiluted and at 1/10 and 1/100 dilutions were separately mixed with 105 copies of swine HEV. Each mixture was then added to HepG2 cells (106 cells/well) and incubated at 37°C for 30 min. Swine HEV RNA copy numbers in the cells were then detected by real-time RT-PCR. As shown in Fig. 6D, compared with the virus-only group, viral RNA copy numbers were significantly decreased in the presence of MAbs 2C7, 2G9, 1B5, and normal mouse IgG at 1 and 0.1 mg, and with undiluted and 1/10 diluted HEV-infected serum. Therefore, the data indicated that the three MAbs blocked swine HEV infection of HepG2 cells in a dose-dependent manner.
MAbs partially neutralize swine HEV infection in rabbits.
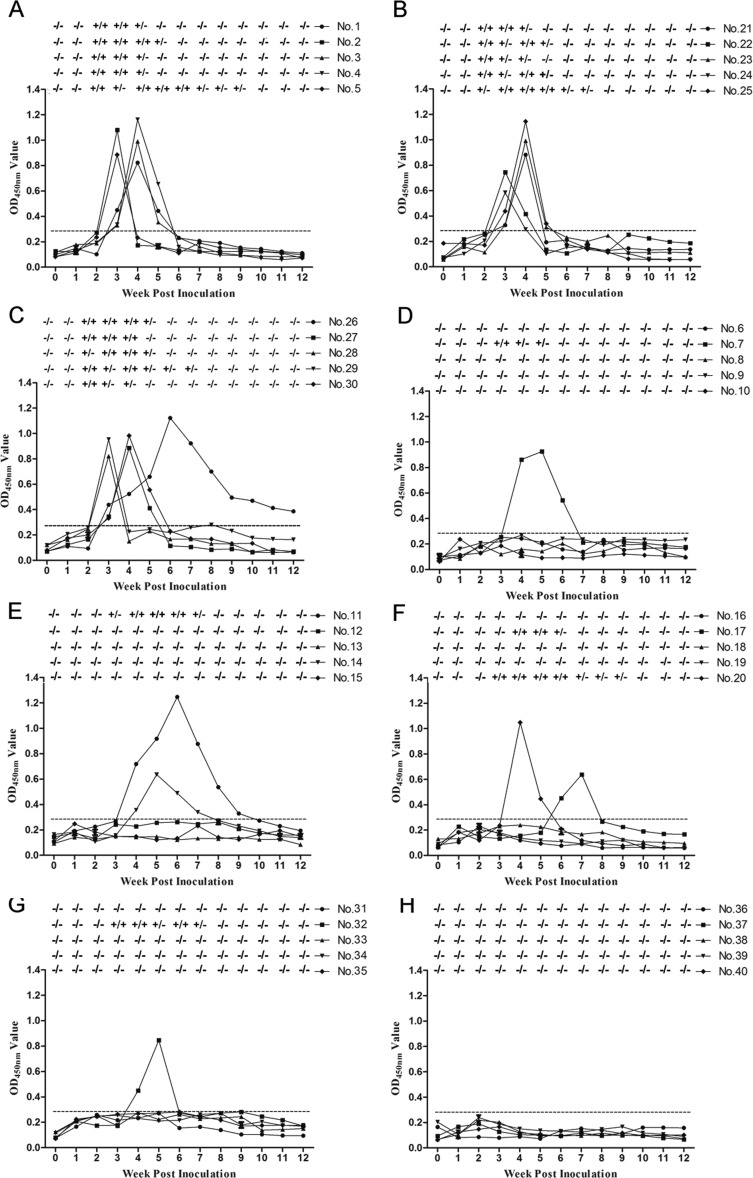
Previous studies have demonstrated that genotype 4 swine HEV could cause robust infection in rabbits (33–36). To avoid high expense and experimental difficulties associated with large-animal models, rabbits were used in this study to conduct in vivo MAb neutralization experiments. Prior to inoculation, all rabbits were shown to be negative for anti-swine HEV IgG antibodies and HEV RNA. Swine HEV stock (104 genomic equivalents [GE], 1 ml) was incubated with each MAb (2 mg, 1 ml), normal mouse IgG, or HEV-infected serum (1 ml). Mixtures were incubated for 1 h at 25°C and then at 4°C for 12 h. After inoculation, all five rabbits in the group inoculated with virus only (Fig. 7A), the group inoculated with virus preincubated with MAb 1E4 (Fig. 7B), and the group inoculated with virus preincubated with normal mouse IgG (Fig. 7C) seroconverted to positive anti-swine HEV IgG antibody titers at 3 weeks postinoculation (wpi), as based on the indirect ELISA cutoff value of 0.29. In contrast, in groups inoculated with virus that had been preincubated with MAb 2C7, 1B5, or 2G9 or with HEV-infected serum, three or four rabbits exhibited no detectable seroconversion throughout the study (Fig. 7D to G).
FIG 7.
Time course of seroconversion, viremia, and fecal shedding in SPF rabbits inoculated with various virus mixtures. (A to C) Rabbits inoculated with only swine HEV (strain CHN-SD-sHEV), MAb 1E4, and normal mouse IgG; (D to F) rabbits inoculated with virus mixed separately with MAbs 2C7, 1B5, and 2G9; (G) rabbits inoculated with virus preincubated with HEV-infected serum; (H) rabbits inoculated with PBS only. Serum and fecal samples were collected before inoculation and at different weeks postinoculation. The dotted line represents the cutoff value of 0.29 of indirect ELISA. +/−, positive or negative HEV RNA in fecal or serum samples.
Fecal virus shedding and viremia after inoculation were observed in all five rabbits in the virus-only group (Fig. 7A), while the rabbits in groups inoculated with virus preincubated with either MAb 1E4 (Fig. 7B) or with normal mouse IgG (Fig. 7C) developed fecal virus shedding and viremia at 2 or 3 wpi that lasted for 2 to 8 weeks. In contrast, of the rabbits inoculated with virus preincubated with MAb 2C7, 1B5, or 2G9 or with HEV-infected serum, three or four remained negative throughout the experiment, while the remainder developed signs of infection that were delayed by 1 or more weeks (Fig. 7D to G). These results suggest that 2C7, 1B5, and 2G9 can at least partially neutralize swine HEV infection in rabbits. The breakthrough isolates in the MAb 2C7, 1B5, and 2G9 and HEV-infected serum groups were sequenced for the HEV complete genome. The results showed that these isolates shared 97% to 100% identities with the inoculated virus without amino acid mutation in the ORF2 and ORF3 proteins (data not shown).
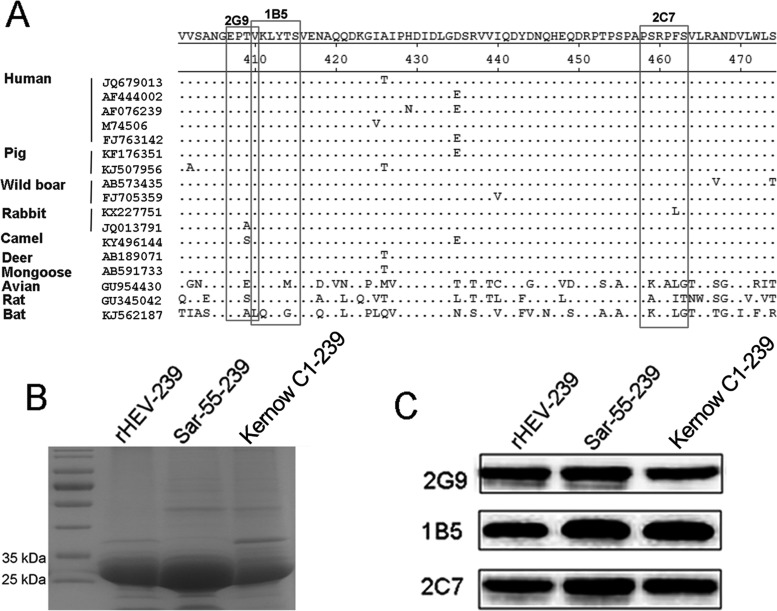
Motifs recognized by the three MAbs common to HEVs of various host species.
To analyze the conservation of novel neutralizing epitopes recognized by MAbs 2G9 and 2C7, amino acid sequences of capsid proteins from 17 HEV isolates, including human, swine, boar, rabbit, mongoose, deer, camel, avian, rat, and bat, were aligned using the Clustal W module of Lasergene 7.1 (DNASTAR, Inc.). Alignment analysis revealed that both epitopes are highly conserved (Fig. 8A) among HEVs with diverse host tropisms. In addition, the epitope recognized by MAb 1B5 also demonstrated a sequence that is shared among HEV strains infecting avian, rat, and bat species (Fig. 8A), as reported previously (28). Subsequently, MAbs 2C7, 2G9, and 1B5 were tested to react with capsid proteins (aa 368 to 606) of two human HEV strains (Sar-55 [genotype 1] and Kernow C1 [genotype 3]) and one rabbit HEV strain (CHN-SX-rHEV [genotype 3]). SDS-PAGE and Western blotting results showed that the three truncated capsid proteins with predicted sizes of about 35 kDa (Fig. 8B) were bound with each of the three MAbs (Fig. 8C). In addition, indirect ELISA results showed that antisera from human, swine, and rabbit naturally infected with human HEV, swine HEV, and rabbit HEV also interacted with the capsid 239 protein of swine HEV (sp239) and three synthetic peptides derived from MAb-reactive HEV capsid core sequences, i.e., Pep2G9-2, Pep2C7-3, and Pep1B5-1, but not with the control peptide, Pep3E8-PH (Table 1). Taken together, the results of this study suggest that two newly identified HEV capsid epitopes discovered here are common to swine, human, and rabbit HEVs, exist in native viral particles, and can induce an immune response in the host.
FIG 8.
Epitopes recognized by MAbs 2G9 and 2C7 are common to various distinct HEVs isolated from various host species. (A) Conservation of amino acid sequences within two common epitopes among various HEV strains isolated from different species. Amino acid alignments of capsid proteins from 17 HEV isolates were performed using the Clustal W module of the MegAlign program of Lasergene 7.1 (DNASTAR, Inc.). Key amino acid residues within epitopes recognized by 2C7 and 2G9 are shown in boxes, with dots indicating sequence identity. The epitope recognized by MAb 1B5 described in a previous study is also shown (30). (B) SDS-PAGE analysis of rHEV-239, Sar-55-239, and Kernow C1-239 proteins, showing approximate sizes of 30 kDa. (C) Western blotting results of binding of three MAbs, 2G9, 1B5, and 2C7, with three distinct p239 proteins.
TABLE 1.
Indirect ELISA results of human, swine, and rabbit antisera interacting with HEV capsid proteins and synthetic peptides
| Antiserum | OD450 valuea |
|||||||
|---|---|---|---|---|---|---|---|---|
| sp239 | Sar-55-239 | Kernow C1-239 | rHEV-239 | Pep2G9-2 | Pep2C7-3 | Pep1B5-1 | Pep3E8-PHb | |
| Human | 0.83 ± 0.26b | 1.43 ± 0.33 | 1.33 ± 0.26 | 1.37 ± 0.30 | 0.59 ± 0.31 | 0.630 ± 0.28 | 0.61 ± 0.23 | 0.15 ± 0.06 |
| Pig | 1.12 ± 0.32 | 1.26 ± 0.29 | 1.11 ± 0.36 | 1.48 ± 0.34 | 0.08 ± 0.23 | 0.81 ± 0.31 | 0.81 ± 0.31 | 0.16 ± 0.02 |
| Rabbit | 1.21 ± 0.32 | 1.14 ± 0.31 | 1.10 ± 0.31 | 1.56 ± 0.29 | 0.57 ± 0.27 | 0.60 ± 0.25 | 0.57 ± 0.29 | 0.17 ± 0.08 |
The data are the mean OD450 value for 10 sera ± SD.
Pep3E8-PH was used as the negative control (27).
DISCUSSION
HEV belongs to the Hepeviridae family, which contains two genera: Orthohepevirus and Piscihepevirus (22). Within the Orthohepevirus genus, four species designated A to D include HEV isolates that infect a variety of hosts (22). In Orthohepevirus HEV species A, four major genotypes have been identified that all belong to a single serotype (37). Genotypes 1 and 2 are restricted to only humans, while genotypes 3 and 4 are zoonotic (1). Previously, variable distributions of antigenic epitopes in capsid proteins have been documented, even though the capsid proteins among the four HEV genotypes shared high amino acid sequences identities (38). While the antigenicity of the capsid protein from genotype 1 HEV has been characterized in detail (29, 39, 40), few studies have focused on the capsid protein from genotype 4 HEV. Due to a recently observed shift in HEV prevalence from genotype 1 to genotype 4 in China (24, 25), detailed studies of genotype 4 HEV antigenicity are urgently needed to facilitate development of serological diagnostic testing and vaccine design. In the present study, three neutralizing epitopes located within the capsid protein of genotype 4 HEV were characterized. In addition, amino acid sequence alignments of capsid proteins from various HEV genotypes demonstrated that these three epitopes may be common to HEV strains with diverse host tropisms. Such studies should help to further the understanding of HEV capsid protein antigenic structure toward the design of vaccines for prevention of genotype 4 HEV infection.
The HEV capsid protein is likely to contain the major immunodominant epitopes of viral particles (41, 42). Crystal structure analysis enabled the identification of three major capsid protein domains, including shell (S) (aa 129 to 319), middle (M) (aa 320 to 455), and protruding (P) (aa 456 to 604) domains (43). Of the three domains, M and P are located on the viral particle surface, whereby the P domain forms a spike that acts as the predominant antigenic domain (44). To date, all reported neutralization epitopes of genotype 1 HEV are present within the P domain (29, 45). In this study, the novel neutralizing epitope (aa 458 to 462) recognized by MAb 2C7 also localizes to the P domain. However, the other two neutralizing epitopes recognized by MAbs 2G9 (aa 407 to 410) and 1B5 (aa 410 to 415) are located within the M domain. In a previous study, an epitope located within aa 403 to 417 of the M domain lacked neutralizing ability (39). However, two sequential epitopes, aa 407 to 410 and aa 410 to 415, do account for the neutralization observed in the present study. These results suggest that the antigenicity of capsid proteins of diverse HEV genotypes may be complicated, warranting the need for future research to elucidate biological functions of various epitopes within the protein.
Previously it was documented that immunization of SPF chickens with peptide Pep1B5-1, which contains the epitope recognized by MAb 1B5, was not protective against avian HEV challenge (28). In the present study, MAb 1B5 can block the attachment of sp239 and swine HEV infection of HepG2 cells and partially inhibits swine HEV infection in rabbits, indicating that MAb 1B5 recognizes a neutralizing epitope of swine HEV. There may be several possible explanations for these contrasting results. This includes the possibility that the epitope in avian HEV, recognized by MAb 1B5, may not be exposed on the virus surface. Or the epitope is not completely linear, and consequently the synthetic peptide immunogen does not present this epitope in its native structure. However, further investigation is needed to elucidate the nature of this epitope.
Previously, rabbits had been shown to be susceptible to experimental infection by genotype 4 HEV isolates from human and pig animal hosts. Moreover, the advantages of rabbits as an animal model for researching HEV pathogenicity have been described previously (46, 47) and include lower cost and easier manipulation than with large animals, prompting the use of rabbits here for in vivo MAb neutralization assays. Notably, our results demonstrated that CHN-SD-sHEV (genotype 4 swine HEV) could cause robust infection of rabbits. Furthermore, results using rabbits inoculated with swine HEV preincubated with MAbs or HEV-infected serum suggested that rabbits can serve as an effective animal model for the evaluation of neutralization abilities of MAbs specific for swine HEV. There were one or two rabbits in the groups treated with MAbs or HEV-infected serum that showed active infection, but in comparison with the virus-only group, the times of fecal virus shedding and viremia were delayed (Fig. 7). Since the breakthrough isolates from these rabbits showed no amino acid mutations in the ORF2 and ORF3 proteins (data not shown), the possible reason for the single breakthrough infection may be the inefficient neutralization by a MAb recognizing a single epitope and insufficient prophylactic levels of neutralizing antibodies in the HEV-infected serum.
In summary, the locations of three novel linear epitopes, aa 407 to 410, 458 to 462, and 625 to 628, recognized by 2G9, 2C7, and 1E4, respectively, were localized to the HEV capsid protein in this study. MAbs 2G9 and 2C7 exhibited neutralizing activities in vitro and in vivo, as did MAb 1B5, which was developed in a previous study (28). In addition, these three novel neutralizing linear epitopes are common to HEV isolates from various host species and differ from previously reported neutralizing epitopes. Ultimately, such studies should provide a foundation for the design of vaccines, antiviral drugs, and effective antigens for the serodiagnosis of HEV.
MATERIALS AND METHODS
Virus, cell line, and monoclonal antibodies.
Swine HEV (strain CHN-SD-sHEV, genotype 4, GenBank accession no. KF176351) was isolated from a bile sample of a 32-week-old pig from a slaughterhouse in China. Viral stock containing 104 genomic equivalents (GE)/ml was then prepared from suspensions of fecal and bile samples collected from specific-pathogen-free (SPF) pigs infected with CHD-SD-sHEV, as previously described (28).
HepG2 cells (HB-8065; ATCC, MD, USA) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (GIBCO, Gaithersburg, MD, USA) and antibiotics (100 U/ml ampicillin and 100 U/ml streptomycin) (Lukang, Shandong, China) and maintained at 37°C with 5% CO2.
MAbs 1E4, 2C7, and 2G9 were generated by immunization of BALB/c mice with sORF2-C, a protein fragment containing the C-terminal 268 amino acids of CHN-SD-sHEV ORF2 protein, as described previously (48). In addition, MAbs 1B5 and 3E8 (also studied here) had been produced previously using ORF2-C containing the C-terminal 268 amino acids of avian HEV ORF2 protein as an immunogen (49). Epitopes recognized by MAbs 1B5 and 3E8 are shared by most HEV isolates obtained from various host species. Convalescent-phase pig serum positive for anti-swine HEV antibodies (designated HEV-infected serum) served as the positive control and was collected from pigs at 42 days postinoculation (dpi) with CHN-SD-sHEV. Mouse IgG (Santa Cruz, CA, USA) served as the negative MAb control.
Serum samples.
Antisera against human HEV were collected from human subjects naturally infected with genotype 4 human HEV. Antisera against swine HEV were collected from pigs naturally infected by genotype 4 swine HEV in Shandong Province, as previously reported (28). Antisera against rabbit HEV were collected from specific-pathogen-free (SPF) rabbits infected experimentally with genotype 3 rabbit HEV (CHN-SX-rHEV), as previously described (50).
Expression of different truncated capsid proteins from swine HEV.
In a previous study, three MAbs, 2C7, 1E4, and 2G9, were produced using sORF2-C immunogen, followed by hybridoma production, as reported previously (48). Mapping of epitopes recognized by the three MAbs was performed using a series of truncated and overlapping fragments from the sORF2-C protein that were expressed using a bacterial system. The truncation of sORF2-C protein was designed as follows. First, sORF2-C protein was divided into two equal fragments which were used to interact with MAbs. The interactive fragments were then further truncated in half until four to six key amino acids recognized by each MAb were identified. These fragments containing sequences comprising aa 393 to 526, 527 to 660, 407 to 660, 411 to 660, 458 to 660, 464 to 660, 393 to 624, and 393 to 628 are shown in Fig. 1A. Sequences encoding these fragments were amplified with primer pairs designed using the EditSeq program of Lasergene 7.1 (DNASTAR Inc., Madison, WI) (Table 2) and then were separately cloned into the pET-21b vector using BamHI and HindIII restriction sites. After confirmation by DNA sequencing, positive recombinant plasmids were transformed into Escherichia coli BL21(DE3) (TransGen Biotech, Beijing, China). Expression of protein fragments was induced by incubation with 1.0 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 6 to 8 h at 37°C. Bacterial cells were harvested and lysed by sonication. Inclusion bodies remained after the removal of other cellular debris by washing with 2% Triton X-100 and were dissolved in 8 M urea and gradually dialyzed and renatured in renaturation buffer (1 mM EDTA, pH 7.5 phosphate buffer [PB7.5], and a urea gradient of 4 M, 2 M, and 0 M). Analysis of the expressed truncated proteins was performed using SDS-PAGE, and the proteins were stained with Coomassie brilliant blue and by Western blotting.
TABLE 2.
Synthetic oligonucleotide primers used in the studya
| Oligonucleotide | Sequenceb |
|---|---|
| sORF2-393F | CGCGGATCCGCAGTTATTTTACTCC |
| sORF2-407F | CGCGGATCCGGAGCCGACAGTAAAA |
| sORF2-411F | CGCGGATCCGCTCTACACTTCAGTC |
| sORF2-458F | CGCGGATCCGTCTCGTCCTTTCTCT |
| sORF2-464F | CGCGGATCCGTCCTTCGTGCCAAT |
| sORF2-527F | CGCGGATCCGACCATCCAGCAGTAT |
| sORF2-526R | CCCAAGCTTTCAAGTGAGCGGGCGACC |
| sORF2-660R | CCCAAGCTTTCAATACTCCCGGGT |
| sORF2-624R | CCCAAGCTTTCAATCAAAAGTATGGGC |
| sORF2-628R | CCCAAGCTTTCAAGGGCAGAAATCATC |
| sp239-F | CCCCCATATGATAGCGCTAACTTTGT |
| sp239-R | TTCCAAGCTTTCACGCAGTATGGGGGGCG |
| Sar-55-239-F | CCCCCATATGATAGCGCTTACCCTGT |
| Sar-55-239-R | TTCCAAGCTTTCACACAGAGTGGGGGGCT |
| Kernow C1-239-F | CCCCCATATGATCGCCCTGACACTGT |
| Kernow C1-239-R | TTCCAAGCTTTCAGGCCGAGTGTGGGGCT |
| rHEV-239-F | CCCCCATATGATAGCCCTGACGCTGT |
| rHEV-239-R | TTCCGGATCCTCAGACAGAATGTGGAGCG |
The oligonucleotide primers were used for cloning and expression of different truncated fragments of genotype 4 swine HEV capsid protein and target regions of amino acids 368 to 606 of capsid proteins from various swine, human, and rabbit HEV strains.
Underlining indicates BamHI, NdeI, or HindIII sequences.
Expression of aa 368 to 606 of swine HEV capsid protein.
Previously it had been documented that a truncated ORF2 protein (designated p239), spanning aa 368 to 606 of genotype 1 HEV capsid protein and expressed in E. coli, could self-assemble to form a 23-nm virus-like particle (VLP) (32). Notably, this VLP could enter HepG2 cells by mimicking the entry mechanism normally used by complete HEV particles. Here, the corresponding region (aa 368 to 606) of genotype 4 swine HEV capsid protein was generated using similar methods. Briefly, a sequence encoding aa 368 to 606 of genotype 4 swine HEV ORF2 was amplified using the primers listed in Table 2. Recombinant plasmids containing the target sequence were constructed, transformed into E. coli BL21(DE3), and then induced with 1.0 mM IPTG to produce HEV proteins for 6 to 8 h at 37°C. Bacterial cells were harvested from cultures and lysed by sonication. Inclusion bodies containing truncated capsid protein (designated sp239) were isolated and then dissolved in PB7.5. sp239 was then renatured as described above. After concentration, 200 μl (10 mg) of filtered sp239 samples was loaded onto a Superdex 200 Increase 10/300 GL column (28-9909-44; GE Healthcare, USA) connected to an ÄKTA purifier (GE Healthcare) preequilibrated with 2 column bed volumes (48 ml) of PB7.5. The flow rate was 0.4 ml min−1, and 0.5-ml fractions were collected. The column was calibrated using a high-molecular-weight gel filtration calibration kit (28-4038-42; GE Healthcare) containing blue dextran 2000 (to determine void volume), conalbumin (75 kDa), aldolase (158 kDa), ferritin (440 kDa), and thyroglobulin (669 kDa). The gel phase distribution coefficent (KAV) value was calculated as follows: (elution volume − void volume)/(column volume − void volume). Column cleaning was performed in place after every 10 runs using 1 bed volume of 0.5 M sodium hydroxide, followed by column recalibration after cleaning. Column flow rate was maintained at 0.4 ml/min. Purified sp239 was prepared under reducing conditions (heating to 100°C for 3 min in the presence of β-mercaptoethanol [BME]) as well as under nonreducing conditions (0.1% SDS in the absence of BME and not heated) and subjected to SDS-PAGE. In addition, to analyze sp239 polymer formation, dialyzed proteins were examined after negative staining using a JEM-1400 electron microscope (JEOL, Japan) operating at 80 kV.
Peptide synthesis.
To finely map the minimal MAb-binding motifs, a series of six HEV ORF2 peptides designed from the CHN-SD-sHEV capsid protein sequence were commercially synthesized based on the results of MAb reactivities with truncated fragments. All peptides were conjugated to keyhole limpet hemocyanin (KLH), and the amino acid sequences of the peptides are shown in Table 3.
Indirect ELISA.
To test MAb and antiserum immune reactivity to truncated capsid proteins and synthetic peptides, indirect ELISAs were performed using procedures described by Wang et al. (28). Briefly, 96-well ELISA plate wells were coated with truncated capsid proteins or synthesized peptides (200 ng/well) dissolved in phosphate-buffered saline (PBS, pH 7.2) and incubated for 12 h at 4°C, washed. and incubated with blocking buffer consisting of 2.5% dry milk in 0.05% Tween 20 in PBS (PBST) for 1 h at 25°C. The plates were then incubated with MAbs (diluted 1/1,000) or antisera (diluted 1/100) for 1 h at 37°C and washed, and then horseradish peroxidase (HRP)-conjugated goat IgG antibodies against various hosts were added to wells at a dilution of 1:10,000. Color was developed using tetramethylbenzidine (TMB; Sigma Chemical Co., St. Louis, MO, USA) for 15 min, and the reaction was stopped with 3 M H2SO4. The optical density at 450 nm (OD450) was read using an automated ELISA plate reader (Bio-Rad, Hercules, CA, USA).
Western blotting.
Truncated protein samples were heated and then loaded onto separating gels. The gels were then transferred electrophoretically to polyvinylidene difluoride (PVDF) membranes using a Mini Trans-Blot apparatus (Bio-Rad). After incubation with blocking buffer 12 h at 4°C, the membranes were washed and incubated with each corresponding MAb for 1 h at 25°C. After washing, membranes were incubated with HRP-conjugated goat anti-mouse IgG (1:5,000) (Jackson ImmunoResearch, West Grove, PA, USA) in blocking buffer for 1 h. Membranes were next washed three times with PBST and then visualized using an ECL chemiluminescence detection system (Pierce, Rockford, IL, USA).
Determination of spatial locations of epitopes recognized by MAbs.
First, the spatial locations of epitopes recognized by MAbs were predicted using computer analysis based on the established crystal structure of HEV capsid protein, deposited under entry number 2ZTN within the Research Collaboratory for Structural Bioinformatics (RCSB) Protein Data Bank. Areas of interest on the protein were visually highlighted using the PyMOL molecular graphics system (www.pymol.org) (43).
Second, to determine whether the epitopes recognized by the MAbs were located on the surface of natural swine HEV particles, a modified immune capture–RT-PCR method was performed as previously described (51). Briefly, 2, 4, and 8 μg/ml MAbs or normal mouse IgG was used to coat the wells (100 μl/well) of ELISA plates (Nunc). Fecal samples containing 104 GE/ml of swine HEV from infected pigs were added (100 μl per well) and incubated at 25°C for 1 h and then at 4°C for 12 h. After washing six times with PBS, total RNA was extracted from each well using the RNAgents total RNA isolation system (Promega, Madison, WI, USA). A partial helicase gene sequence of swine HEV was then amplified using nested RT-PCR as described by Huang et al. (52) with total RNA as the template. Final PCR products (348 bp) were subjected to electrophoresis on a 1.2% agarose gel containing ethidium bromide and were confirmed by sequencing. Experiments were repeated twice.
Analysis of MAb blocking of sp239 attachment to HepG2 cells.
Due to the absence of a highly effective cell culture system for swine HEV, several in vitro neutralization assays have been described. In one previous study, MAb blocking of p239 attachment to HepG2 cells was used to analyze the neutralizing abilities of MAbs by an assay that exploits the fact that p239 can mimic native HEV particle entry into cells (53). In that study, sp239 prepared using methods described above was used in place of native swine HEV particles for HepG2 cell entry assays. Briefly, 500 μg/ml of MAb 2G9, 2C7, 1E4, or 1B5 or HEV-infected serum was mixed with 70 μg/ml sp239 protein (in PB7.5) and incubated at 37°C for 30 min. Mixtures were then separately added to HepG2 cells and incubated at 4°C for 30 min, followed by washing of cells three times with PBS. HepG2 cells were then lysed in NP-40 lysis buffer (Beyotime, Shanghai, China) supplemented with protease and phosphatase inhibitors, lysates were heated and then separated using a separating gel, and amounts of sp239 protein that had associated with HepG2 cells were detected using Western blotting. Detection of sp239 and cellular proteins was performed using the primary antibodies anti-sp239 MAb 3E8 at a 1:2,000 dilution or anti-α-tubulin at a 1:5,000 dilution (Sigma-Aldrich, St. Louis, MO, USA). HRP-conjugated goat anti-mouse IgG at a 1:2,000 dilution (Jackson ImmunoResearch) was used as a secondary antibody. Western blotting was performed as described above.
IFA and FCM assay.
In addition to Western blotting, the immunofluorescence focus assay (IFA) and flow cytometry neutralization (FCM) assay were used to analyze MAb blocking of sp239 attachment to HepG2 cells. For IFA, the procedures were carried out as reported previously (53). Briefly, HepG2 cells were cultured on coverslips and incubated with sp239 or a mixture of sp239 (70 μg/ml) and either 500 μg/ml of 2C7, 2G9, 1B5, or 1E4 or HEV-infected serum for 30 min at 4°C. After washing with PBS three times, cells were fixed with 4% paraformaldehyde (Sigma-Aldrich) and permeabilized with 0.3% Triton X-100 (Sigma-Aldrich) in PBS. Fixed cells were blocked with 10% goat serum in PBS for 1 h at 25°C, incubated with 3E8 (1:2,000) for 1 h at 25°C, and then labeled with Alexa Fluor 555 goat anti-mouse IgG (1:5,000; Thermofisher Scientific, USA) for 1 h at 25°C. After three washes, cells were stained with 0.5% 4′,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA, USA). Fluorescence signals were detected with a Leica microscope (LSM 780; Carl Zeiss, Germany).
For FCM, after incubation of HepG2 cells with sp239 and mixtures of sp239 with MAb 2C7, 2G9, 1B5, or 1E4 or HEV-infected serum for 30 min at 4°C, cells were collected and subjected to FCM (Beckman Coulter CyAn ADP) using a HyperCyt loader (UNC, USA). Primary and secondary antibodies were the same as those used for IFA. Mock-adsorbed cells were treated equally and used for background gating. Results were analyzed using CytExpert 1.11 software.
Analysis of MAb blocking of natural swine HEV infection in HepG2 cells using real-time RT-PCR.
Although no highly effective cell culture system for HEV propagation has yet been developed, HEV can be propagated at low levels in HepG2, A549, and PLC/PRF/5 cells, as noted previously (54). Subsequently, a neutralization test was developed by Tang et al. based on the low-level HEV propagation in HepG2 cells observed in that study, with HEV quantitation in cells conducted via real-time RT-PCR (39). Using this method, neutralization tests were performed here to evaluate whether the HEV-infected serum and MAbs can block genotype 4 swine HEV propagation in HepG2 cells. Briefly, different amounts of MAbs 2C7, 2G9, 1E4, and 1B5 and mouse IgG or different dilutions of HEV-infected serum were mixed with 105 copies of swine HEV and incubated at 37°C for 30 min. The virus only was used as the positive control. Each mixture was then separately added to HepG2 cells (106 cells per well) and incubated at 37°C for 30 min. After washing three times with PBS, total RNA from HepG2 cells was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Next, TaqMan RT-PCR was carried out to quantify HEV RNA with the extracted total RNA as the template, using the QuantiTect Probe RT-PCR kit master mix (Qiagen, Valencia, CA, USA).
Neutralization assays in rabbits.
Several previous studies had documented that genotype 4 swine HEVs can cause infection in rabbits without prior adaptation (46) and that they are also a potential zoonotic reservoir of HEV. Therefore, rabbits were selected for animal experiments involving swine HEV infection to avoid experimental challenges associated with the use of pigs as an animal model. A total of 40 2-month-old New Zealand White rabbits with initial body weights averaging 10.7 kg (SD, ±1.74 kg) were divided into eight groups, with five rabbits per group. Before challenge, each rabbit was screened for both serum anti-HEV antibodies using indirect ELISA and fecal HEV RNA using nested RT-PCR. First, the swine HEV stock (104 GE/ml) prepared from a 10% fecal stock was incubated with each MAb, normal mouse IgG, or HEV-infected serum. Mixtures were incubated for 1 h at room temperature and then overnight at 4°C. Next, each mixture was injected into separate groups of five rabbits via ear vein inoculation. PBS-only, virus-only, normal-mouse-IgG, and HEV-infected-serum groups were treated in parallel as normal (uninfected), antibody-negative, irrelevant antibody (non-HEV-binding), and anti-HEV-positive control groups. Serum and fecal samples were collected before inoculation and weekly thereafter for 12 weeks. Viremia and fecal virus shedding were determined for each inoculated rabbit by using a sensitive nested RT-PCR as described previously (52). In addition, serum samples were used to detect anti-swine HEV antibodies by indirect ELISA using sORF2-C as the coating antigen, as described by Chen et al. (48). No rabbits died or became severely ill prior to the experimental endpoint.
Analysis of epitopes common to HEVs of various host species that are recognized by MAbs.
We next determined whether B-cell epitopes represented by the three novel MAbs characterized in this study are common to distinct HEV strains (or genotypes) that infect various host species. To conduct this analysis, known amino acid sequence motifs recognized by MAbs that bind HEV isolates obtained from various host species were aligned using the MegAlign program of Lasergene 7.1 (DNASTAR, Inc.). In addition, sequences spanning the analogous capsid protein coding region (aa 368 to 606) from genotype 1 human HEV (Sar-55, accession no. AF444002), genotype 3 human HEV (Kernow-C1, accession no. JQ679013), and genotype 3 rabbit HEV (CHN-SX-rHEV, accession no. KX227751) were cloned and expressed using the same procedures as described above and were designated Sar-55-239, Kernow C1-239, and rHEV-239, respectively. Primers used to amplify these target gene regions are shown in Table 2. After cloning, SDS-PAGE was used to assess HEV capsid protein expression, followed by Western blotting to detect immune reactivity of MAbs toward the three HEV capsid proteins. In addition, to assess antigenic reactivity and reveal minimal motifs involved in MAb binding, ELISAs were conducted as described above using various 239-amino-acid truncated proteins and synthetic HEV capsid peptides in place of coating antigen, while primary antibodies were replaced with antisera against human, swine, or rabbit HEVs. Synthetic peptide Pep3E8-PH was used as an irrelevant antigen control (negative control) (28). Next, secondary antibodies were replaced with appropriate HRP-conjugated goat anti-human, goat anti-swine, or goat anti-rabbit IgG antibodies.
Ethics statement.
All experiments in this study were designed based on the principles expressed in the Guide for the Care and Use of Laboratory Animals by the National Research Council of the National Academies (55) and Guidance for Experimental Animal Welfare and Ethical Treatment by the Ministry of Science and Technology of China (56). Experimental procedures and animal use and care protocols were carried out in accordance with the guidelines of the Northwest A&F University Institutional Committee for the Care and Use of Laboratory Animals and were approved by the Committee on Ethical Use of Animals of Northwest A&F University.
ACKNOWLEDGMENTS
Y.C. and B.L. performed the research, analyzed data, and drafted the paper. Y.S., H.L., and T.D. contributed to the animal study. Y.N. and J.A.H. contributed to the additional analyses and revised the paper. E.-M.Z. and Q.Z. conceived the study, carried out additional analyses, and finalized the paper.
This study was supported by grants from the National Natural Science Foundation of China (grants 31720103919 and 31372464 to E.-M.Z. and grants 31672583 and 31402233 to Q.Z.). Q.Z. is a Tang Scholar of Northwest A&F University.
REFERENCES
- 1.Perez-Gracia MT, Garcia M, Suay B, Mateos-Lindemann ML. 2015. Current knowledge on hepatitis E. J Clin Transl Hepatol 3:117–126. doi: 10.14218/JCTH.2015.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Labrique AB, Sikder SS, Krain LJ, West KP Jr, Christian P, Rashid M, Nelson KE. 2012. Hepatitis E, a vaccine-preventable cause of maternal deaths. Emerg Infect Dis 18:1401–1404. doi: 10.3201/eid1809.120241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dalton HR, Bendall RP, Keane FE, Tedder RS, Ijaz S. 2009. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med 361:1025–1027. doi: 10.1056/NEJMc0903778. [DOI] [PubMed] [Google Scholar]
- 4.Ollier L, Tieulie N, Sanderson F, Heudier P, Giordanengo V, Fuzibet JG, Nicand E. 2009. Chronic hepatitis after hepatitis E virus infection in a patient with non-Hodgkin lymphoma taking rituximab. Ann Intern Med 150:430–431. doi: 10.7326/0003-4819-150-6-200903170-00025. [DOI] [PubMed] [Google Scholar]
- 5.Mori Y, Matsuura Y. 2011. Structure of hepatitis E viral particle. Virus Res 161:59–64. doi: 10.1016/j.virusres.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Meng XJ, Purcell RH, Halbur PG, Lehman JR, Webb DM, Tsareva TS, Haynes JS, Thacker BJ, Emerson SU. 1997. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci U S A 94:9860–9865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schielke A, Sachs K, Lierz M, Appel B, Jansen A, Johne R. 2009. Detection of hepatitis E virus in wild boars of rural and urban regions in Germany and whole genome characterization of an endemic strain. Virol J 6:58. doi: 10.1186/1743-422X-6-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J, Karlsson M, Olofson AS, Belak S, Malmsten J, Dalin AM, Widen F, Norder H. 2015. High prevalence of hepatitis E virus in Swedish moose—a phylogenetic characterization and comparison of the virus from different regions. PLoS One 10:e0122102. doi: 10.1371/journal.pone.0122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tei S, Kitajima N, Takahashi K, Mishiro S. 2003. Zoonotic transmission of hepatitis E virus from deer to human beings. Lancet 362:371–373. doi: 10.1016/S0140-6736(03)14025-1. [DOI] [PubMed] [Google Scholar]
- 10.Haqshenas G, Shivaprasad HL, Woolcock PR, Read DH, Meng XJ. 2001. Genetic identification and characterization of a novel virus related to human hepatitis E virus from chickens with hepatitis-splenomegaly syndrome in the United States. J Gen Virol 82:2449–2462. doi: 10.1099/0022-1317-82-10-2449. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Q, Zhou EM, Dong SW, Qiu HK, Zhang L, Hu SB, Zhao FF, Jiang SJ, Sun YN. 2010. Analysis of avian hepatitis E virus from chickens, China. Emerg Infect Dis 16:1469–1472. doi: 10.3201/eid1609.100626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Shen Q, Hua X, Cui L. 2011. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg Infect Dis 17:1981–1983. doi: 10.3201/eid1710.101399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raj VS, Smits SL, Pas SD, Provacia LB, Moorman-Roest H, Osterhaus AD, Haagmans BL. 2012. Novel hepatitis E virus in ferrets, the Netherlands. Emerg Infect Dis 18:1369–1370. doi: 10.3201/eid1808.111659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao C, Ma Z, Harrison TJ, Feng R, Zhang C, Qiao Z, Fan J, Ma H, Li M, Song A, Wang Y. 2009. A novel genotype of hepatitis E virus prevalent among farmed rabbits in China. J Med Virol 81:1371–1379. doi: 10.1002/jmv.21536. [DOI] [PubMed] [Google Scholar]
- 15.Nidaira M, Takahashi K, Ogura G, Taira K, Okano S, Kudaka J, Itokazu K, Mishiro S, Nakamura M. 2012. Detection and phylogenetic analysis of hepatitis E viruses from mongooses in Okinawa, Japan. J Vet Med Sci 74:1665–1668. doi: 10.1292/jvms.11-0520. [DOI] [PubMed] [Google Scholar]
- 16.Woo PC, Lau SK, Teng JL, Tsang AK, Joseph M, Wong EY, Tang Y, Sivakumar S, Xie J, Bai R, Wernery R, Wernery U, Yuen KY. 2014. New hepatitis E virus genotype in camels, the Middle East. Emerg Infect Dis 20:1044–1048. doi: 10.3201/eid2006.140140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drexler JF, Geipel A, Konig A, Corman VM, van Riel D, Leijten LM, Bremer CM, Rasche A, Cottontail VM, Maganga GD, Schlegel M, Muller MA, Adam A, Klose SM, Carneiro AJ, Stocker A, Franke CR, Gloza-Rausch F, Geyer J, Annan A, Adu-Sarkodie Y, Oppong S, Binger T, Vallo P, Tschapka M, Ulrich RG, Gerlich WH, Leroy E, Kuiken T, Glebe D, Drosten C. 2013. Bats carry pathogenic hepadnaviruses antigenically related to hepatitis B virus and capable of infecting human hepatocytes. Proc Natl Acad Sci U S A 110:16151–16156. doi: 10.1073/pnas.1308049110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirano M, Ding X, Li TC, Takeda N, Kawabata H, Koizumi N, Kadosaka T, Goto I, Masuzawa T, Nakamura M, Taira K, Kuroki T, Tanikawa T, Watanabe H, Abe K. 2003. Evidence for widespread infection of hepatitis E virus among wild rats in Japan. Hepatol Res 27:1–5. doi: 10.1016/S1386-6346(03)00192-X. [DOI] [PubMed] [Google Scholar]
- 19.Meng XJ. 2010. Hepatitis E virus: animal reservoirs and zoonotic risk. Vet Microbiol 140:256–265. doi: 10.1016/j.vetmic.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng XJ. 2011. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res 161:23–30. doi: 10.1016/j.virusres.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mizuo H, Yazaki Y, Sugawara K, Tsuda F, Takahashi M, Nishizawa T, Okamoto H. 2005. Possible risk factors for the transmission of hepatitis E virus and for the severe form of hepatitis E acquired locally in Hokkaido, Japan. J Med Virol 76:341–349. doi: 10.1002/jmv.20364. [DOI] [PubMed] [Google Scholar]
- 22.Smith DB, Simmonds P, Members of the International Committee on the Taxonomy of Viruses Hepeviridae Study Group, Jameel S, Emerson SU, Harrison TJ, Meng XJ, Okamoto H, Van der Poel WH, Purdy MA. 2015. Consensus proposals for classification of the family Hepeviridae. J Gen Virol 96:1191–1192. doi: 10.1099/vir.0.000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamar N, Bendall R, Legrand-Abravanel F, Xia NS, Ijaz S, Izopet J, Dalton HR. 2012. Hepatitis E. Lancet 379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Li L, Wang L, Bu Q, Fu H, Han J, Zhu Y, Lu F, Zhuang H. 2012. Phylogenetic analysis of 626 hepatitis E virus (HEV) isolates from humans and animals in China (1986-2011) showing genotype diversity and zoonotic transmission. Infect Genet Evol 12:428–434. doi: 10.1016/j.meegid.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Sridhar S, Lo SK, Xing F, Yang J, Ye H, Chan JF, Teng JL, Huang C, Yip CC, Lau SK, Woo PC. 2017. Clinical characteristics and molecular epidemiology of hepatitis E in Shenzhen, China: a shift toward foodborne transmission of hepatitis E virus infection. Emerg Microbes Infect 6:e115. doi: 10.1038/emi.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arends JE, Ghisetti V, Irving W, Dalton HR, Izopet J, Hoepelman AI, Salmon D. 2014. Hepatitis E: an emerging infection in high income countries. J Clin Virol 59:81–88. doi: 10.1016/j.jcv.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad I, Holla RP, Jameel S. 2011. Molecular virology of hepatitis E virus. Virus Res 161:47–58. doi: 10.1016/j.virusres.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Zhao Q, Dang L, Sun Y, Gao J, Liu B, Syed SF, Tao H, Zhang G, Luo J, Zhou EM. 2015. Characterization of two novel linear B-cell epitopes in the capsid protein of avian hepatitis E virus (HEV) that are common to avian, swine, and human HEVs. J Virol 89:5491–5501. doi: 10.1128/JVI.00107-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao M, Li XJ, Tang ZM, Yang F, Wang SL, Cai W, Zhang K, Xia NS, Zheng ZZ. 2015. A comprehensive study of neutralizing antigenic sites on the hepatitis E virus (HEV) capsid by constructing, clustering, and characterizing a tool box. J Biol Chem 290:19910–19922. doi: 10.1074/jbc.M115.649764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X, Yang C, Gu Y, Song C, Zhang X, Wang Y, Zhang J, Hew CL, Li S, Xia N, Sivaraman J. 2011. Structural basis for the neutralization and genotype specificity of hepatitis E virus. Proc Natl Acad Sci U S A 108:10266–10271. doi: 10.1073/pnas.1101309108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gu Y, Tang X, Zhang X, Song C, Zheng M, Wang K, Zhang J, Ng MH, Hew CL, Li S, Xia N, Sivaraman J. 2015. Structural basis for the neutralization of hepatitis E virus by a cross-genotype antibody. Cell Res 25:604–620. doi: 10.1038/cr.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li SW, Zhang J, Li YM, Ou SH, Huang GY, He ZQ, Ge SX, Xian YL, Pang SQ, Ng MH, Xia NS. 2005. A bacterially expressed particulate hepatitis E vaccine: antigenicity, immunogenicity and protectivity on primates. Vaccine 23:2893–2901. doi: 10.1016/j.vaccine.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 33.Cossaboom CM, Cordoba L, Sanford BJ, Pineyro P, Kenney SP, Dryman BA, Wang Y, Meng XJ. 2012. Cross-species infection of pigs with a novel rabbit, but not rat, strain of hepatitis E virus isolated in the United States. J Gen Virol 93:1687–1695. doi: 10.1099/vir.0.041509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halbur PG, Kasorndorkbua C, Gilbert C, Guenette D, Potters MB, Purcell RH, Emerson SU, Toth TE, Meng XJ. 2001. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and a human. J Clin Microbiol 39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meng XJ, Halbur PG, Shapiro MS, Govindarajan S, Bruna JD, Mushahwar IK, Purcell RH, Emerson SU. 1998. Genetic and experimental evidence for cross-species infection by swine hepatitis E virus. J Virol 72:9714–9721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun EC, Ma JN, Liu NH, Yang T, Zhao J, Geng HW, Wang LF, Qin YL, Bu ZG, Yang YH, Lunt RA, Wang LF, Wu DL. 2011. Identification of two linear B-cell epitopes from West Nile virus NS1 by screening a phage-displayed random peptide library. BMC Microbiol 11:160. doi: 10.1186/1471-2180-11-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schlauder GG, Mushahwar IK. 2001. Genetic heterogeneity of hepatitis E virus. J Med Virol 65:282–292. doi: 10.1002/jmv.2031. [DOI] [PubMed] [Google Scholar]
- 38.Behloul N, Wen J, Dai X, Dong C, Meng J. 2015. Antigenic composition and immunoreactivity differences between HEV recombinant capsid proteins generated from different genotypes. Infect Genet Evol 34:211–220. doi: 10.1016/j.meegid.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 39.Tang ZM, Tang M, Zhao M, Wen GP, Yang F, Cai W, Wang SL, Zheng ZZ, Xia NS. 2015. A novel linear neutralizing epitope of hepatitis E virus. Vaccine 33:3504–3511. doi: 10.1016/j.vaccine.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 40.Schofield DJ, Glamann J, Emerson SU, Purcell RH. 2000. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J Virol 74:5548–5555. doi: 10.1128/JVI.74.12.5548-5555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purdy MA, McCaustland KA, Krawczynski K, Spelbring J, Reyes GR, Bradley DW. 1993. Preliminary evidence that a trpE-HEV fusion protein protects cynomolgus macaques against challenge with wild-type hepatitis E virus (HEV). J Med Virol 41:90–94. doi: 10.1002/jmv.1890410118. [DOI] [PubMed] [Google Scholar]
- 42.Tsarev SA, Tsareva TS, Emerson SU, Govindarajan S, Shapiro M, Gerin JL, Purcell RH. 1994. Successful passive and active immunization of cynomolgus monkeys against hepatitis E. Proc Natl Acad Sci U S A 91:10198–10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yamashita T, Mori Y, Miyazaki N, Cheng RH, Yoshimura M, Unno H, Shima R, Moriishi K, Tsukihara T, Li TC, Takeda N, Miyamura T, Matsuura Y. 2009. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc Natl Acad Sci U S A 106:12986–12991. doi: 10.1073/pnas.0903699106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Tang X, Seetharaman J, Yang C, Gu Y, Zhang J, Du H, Shih JW, Hew CL, Sivaraman J, Xia N. 2009. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus-host interaction. PLoS Pathog 5:e1000537. doi: 10.1371/journal.ppat.1000537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meng J, Dai X, Chang JC, Lopareva E, Pillot J, Fields HA, Khudyakov YE. 2001. Identification and characterization of the neutralization epitope (s) of the hepatitis E virus. Virology 288:203–211. doi: 10.1006/viro.2001.1093. [DOI] [PubMed] [Google Scholar]
- 46.Han J, Lei Y, Liu L, Liu P, Xia J, Zhang Y, Zeng H, Wang L, Wang L, Zhuang H. 2014. SPF rabbits infected with rabbit hepatitis E virus isolate experimentally showing the chronicity of hepatitis. PLoS One 9:e99861. doi: 10.1371/journal.pone.0099861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang L, Liu L, Wang L. 2018. An overview: rabbit hepatitis E virus (HEV) and rabbit providing an animal model for HEV study. Rev Med Virol 28:e1961. doi: 10.1002/rmv.1961. [DOI] [PubMed] [Google Scholar]
- 48.Chen Y, Zhao Q, Liu B, Wang L, Sun Y, Li H, Wang X, Syed SF, Zhang G, Zhou EM. 2016. A novel blocking ELISA for detection of antibodies against hepatitis E virus in domestic pigs. PLoS One 11:e0152639. doi: 10.1371/journal.pone.0152639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong S, Zhao Q, Lu M, Sun P, Qiu H, Zhang L, Lv J, Zhou EM. 2011. Analysis of epitopes in the capsid protein of avian hepatitis E virus by using monoclonal antibodies. J Virol Methods 171:374–380. doi: 10.1016/j.jviromet.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 50.Liu B, Sun Y, Du T, Chen Y, Wang X, Huang B, Li H, Nan Y, Xiao S, Zhang G, Hiscox JA, Zhou EM, Zhao Q. 2017. Rabbit hepatitis E virus is an opportunistic pathogen in specific-pathogen-free rabbits with the capability of cross-species transmission. Vet Microbiol 201:72–77. doi: 10.1016/j.vetmic.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 51.Zhou EM, Guo H, Huang FF, Sun ZF, Meng XJ. 2008. Identification of two neutralization epitopes on the capsid protein of avian hepatitis E virus. J Gen Virol 89:500–508. doi: 10.1099/vir.0.83366-0. [DOI] [PubMed] [Google Scholar]
- 52.Huang FF, Haqshenas G, Guenette DK, Halbur PG, Schommer SK, Pierson FW, Toth TE, Meng XJ. 2002. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J Clin Microbiol 40:1326–1332. doi: 10.1128/JCM.40.4.1326-1332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai W, Tang ZM, Wen GP, Wang SL, Ji WF, Yang M, Ying D, Zheng ZZ, Xia NS. 2016. A high-throughput neutralizing assay for antibodies and sera against hepatitis E virus. Sci Rep 6:25141. doi: 10.1038/srep25141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Okamoto H. 2011. Hepatitis E virus cell culture models. Virus Res 161:65–77. doi: 10.1016/j.virusres.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 55.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 56.Ministry of Science and Technology of the People's Republic of China. 2006. Guidance for experimental animal welfare and ethical treatment. http://www.most.gov.cn/fggw/zfwj/zfwj2006/zf06yw/zf06qt/200612/t20061226_39235.htm. [Google Scholar]