Abstract
Hostile conflict in marriage can increase risks for disease and mortality. Physiological synchrony between partners—e.g., the linkage between their autonomic fluctuations—appears to capture engagement, or an inability to disengage from an exchange, and thus may amplify the health risks of noxious interactions such as marital conflict. Prior work has not examined the unique health correlates of this physiological signature. To test associations between couples’ heart rate variability (HRV) synchrony during conflict and inflammation, 43 married couples engaged in a marital problem discussion while wearing heart monitors and provided four blood samples; they repeated this protocol at a second visit. When couples’ moment-to-moment HRV changes tracked more closely together during conflict, they had higher levels of three inflammatory markers (i.e., IL-6, stimulated TNF-α, and sVCAM-1) across the day. Stronger HRV synchrony during conflict also predicted greater negative affect reactivity. Synchrony varied within couples, and was related to situational factors rather than global relationship traits. These data highlight partners’ HRV linkage during conflict as a novel social-biological pathway to inflammation-related disease.
Keywords: couples, marital conflict, health, inflammation, synchrony
1. Introduction
A bad marriage can increase risks for age-related disease and death (Liu and Waite, 2014; Robles et al., 2014). Chronic, low-grade inflammation is a hallmark of many age-related chronic conditions (Franceschi and Campisi, 2014), and thus may link marital discord to poorer health. For example, wives who felt less supported by their husbands had higher baseline levels of inflammatory markers, C-reactive protein (CRP) and interleukin-6 (IL-6) (Donoho et al., 2013; Whisman and Sbarra, 2012). In a lab study, couples with more hostile interaction styles had larger increases in proinflammatory cytokines IL-6 and tumor necrosis factor-alpha (TNF-α) compared to less negative couples; increases were more pronounced after marital disagreement than support (Kiecolt-Glaser et al., 2005). In another study, couples whose disagreements were more hostile had higher inflammation throughout the day (Kiecolt-Glaser et al., 2015). Indeed, more discordant, hostile couples tend to have higher circulating inflammation, and their fights may trigger larger inflammatory increases compared to less hostile couples.
Couples’ physiological synchrony during interaction, or the degree to which partners’ autonomic fluctuations track together, appears to capture an important interpersonal process. This physiological signature, also termed physiological linkage, coregulation, and covariation, emerges when partners are in close proximity (Palumbo et al., 2017; Timmons et al., 2015). Synchrony has been studied in many physiological systems, across time scales, with disparate statistical approaches, and in a range of social contexts, all with varying results. Nevertheless, three excellent synthetic reviews converged on the working conclusion that the relational implications of synchrony depend on its context (Butler, 2015; Palumbo et al., 2017; Timmons et al., 2015). For instance, synchrony may be stronger when unhappy couples have upsetting interactions. In one study, couples’ synchrony scores were calculated for a neutral task and a marital problem discussion using a combination of four indices—interbeat interval (IBI), pulse transmission time, skin conductance, and movement (Levenson and Gottman, 1983). Couples who synchronized more closely during disagreement had poorer marital quality than those who were less tightly synced, but synchrony during neutral conversation did not relate to marital satisfaction. Synchrony during conflict explained more than half of the variance in marital satisfaction and was independent of couples’ negative affect reciprocity, suggesting that it captured an important feature of marital satisfaction, and uniquely characterized the experience of being “locked into [a] destructive interaction” (p. 596, Levenson and Gottman, 1983). A study of skin conductance synchrony replicated these findings in two samples (Chaspari et al., 2015).
Other studies have demonstrated the link between greater synchrony and poorer marital quality, even for tasks intended to be neutral or positive. For example, more discordant couples had more synchronous high-frequency heart rate variability (HF-HRV) during free play with their child (Gates et al., 2015). The respiration cycles of dissatisfied couples fell into sync when they imitated each other, but not at rest or when sharing eye contact (Helm et al., 2012). In a notable exception, more satisfied couples showed stronger HF-HRV linkage, when positive, negative, and neutral topics were aggregated (Helm et al., 2014); the authors attributed the divergent pattern, in part, to the couples’ ability to maintain low arousal across the 3-minute tasks.
Though it is difficult to draw strong conclusions when the emotional tone of couples’ interactions is unclear, it appears that joint increases in arousal during difficult interactions may reflect poorer relationship quality, and that linkage during positive, warm interactions may index the opposite (Timmons et al., 2015). Synchrony may also vary over time (Palumbo et al., 2017; Reed et al., 2013). Additional work is needed to compare synchrony across occasions in well-defined emotional contexts; no research to date has addressed whether synchrony during upsetting interactions independently predicts poorer health.
To assess the health relevance of synchrony during conflict, we examined couples’ HF-HRV synchrony during marital disagreement as a predictor of inflammation on two occasions, controlling for relevant inflammatory confounds. We focused on HF-HRV synchrony over other autonomic measures because there was a validated method for calculating synchrony scores with these data (Gates et al., 2015), and according to two excellent reviews, synchrony’s interpersonal significance does not appear to systematically differ across physiological streams (Palumbo et al., 2017; Timmons et al., 2015). Thus, we developed predictions based on prior work that was most similar to our study’s time course and marital interaction paradigm. Further, vagal activity, indexed by HF-HRV, plays a role in threat appraisal and emotion regulation (Thayer and Lane, 2000), and dampens inflammation (Pavlov and Tracey, 2005). Namely, HF-HRV decreases with stress or potential threat (Thayer and Lane, 2000) and increases with attempts to regulate emotions and engage socially (Butler et al., 2006; Porges, 2007). Thus, HF-HRV synchrony would include moments of coactivation (Timmons et al., 2015), or joint vagal withdrawal, when both partners feel threatened or fail to regulate.
Because greater marital hostility predicts higher inflammation on average (Kiecolt-Glaser et al., 2015), we hypothesized that stronger conflict-based HF-HRV synchrony would be associated with higher inflammation across the day. Consistent with hostile couples’ greater inflammatory responsiveness to marital conflict (Kiecolt-Glaser et al., 2005), we also expected stronger synchrony to predict greater inflammatory reactivity to conflict. Specifically, we examined a panel of six inflammatory markers—serum IL-6 and TNF-α, lipopolysaccharide (LPS) stimulated IL-6 and TNF-α, and serum soluble intercellular adhesion molecule 1 (sICAM-1) and vascular cell adhesion molecule 1 (sVCAM-1)—to minimize the likelihood of relying on a single spurious finding. Based on the preliminary evidence that stronger synchrony in negative emotional contexts relates to poorer marital quality (Timmons et al., 2015), we predicted that in follow-up analyses, synchronizing more closely during conflict would be associated with greater negative affect reactivity to the task. We also expected stronger synchrony to predict dampened HF-HRV reactivity, a direct route to heightened inflammation (Pavlov and Tracey, 2005).
With a unique two-visit study design, we aimed to characterize synchrony’s stability across occasions. We also examined its associations with a range of interpersonal factors, stable and interaction-specific, with the expectation that stronger synchrony during conflict would track with poorer relationship quality and more ineffective conflict strategies.
2. Method
2.1 Participants
Couples were recruited for a parent study of immune responses to high-fat meals (Kiecolt-Glaser et al., 2015). An initial online screen and follow-up in-person screen determined eligibility. Couples married fewer than 3 years and those who had sensory impairments that would interfere with study completion were excluded. Couples were not considered if either partner had a chronic health problem including anemia or diabetes (HbA1c > 6.5), smoked, abused substances, or used prescription medication other than birth control (n = 5) or levothyroxine (n = 3). Participants fit our exercise criteria if they engaged in a minimum of 2 hours of vigorous activity per week for those with a BMI of < 24.99 (normal weight) and 5 hours per week for BMI > 25 (overweight or obese).
In the online screen, potential participants completed the 16-item version of the Couples Satisfaction Index; the full version was given at the end of the first visit (Funk and Rogge, 2007). Happier couples were overrepresented among applicants, a general challenge for marital research. Accordingly, in terms of both inclusion and scheduling, we prioritized dissatisfied couples to represent the full range of marital discord. We also spent considerable time and effort to recruit people who were healthy but overweight to address aims relevant to the parent study’s meal component. A total of 350 interested individuals were excluded because either they or their spouse did not meet our stringent health criteria.
The sample consisted of 86 participants (43 couples). Participants were 38 years old on average (SD = 8.2, range = 24–61) and primarily White (81%). All couples were married, and the average length of marriage was 11.5 years (SD = 6.6, range = 3 – 27). Most were employed full-time (70%). Table 1 provides additional sample characteristics.
Table 1.
Correlations of study variables and summary statistics
| Wife | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | Husband M (SD) |
Wife M (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Husband | ||||||||||||||
| 1. HRV synchrony during conflict | 1.00* | −0.16 | 0.08 | −0.14 | 0.23 | −0.05 | 0.02 | 0.16 | 0.20 | −0.10 | −0.04 | −0.11 | −0.001 (0.095) | −0.001 (0.095) |
| 2. Baseline HRV (self) | 0.08 | −0.07 | −0.07 | 0.69* | 0.00 | −0.16 | 0.12 | 0.02 | −0.42* | −0.25 | 0.17 | −0.16 | 5.67 (1.28) | 6.33 (1.08) |
| 3. Baseline HRV (partner) | −0.16 | −0.07 | −0.07 | 0.21 | 0.78* | 0.11 | −0.23* | −0.16 | −0.19 | 0.24* | 0.03 | 0.17 | 6.33 (1.08) | 5.67 (1.28) |
| 4. Conflict HRV (self) | 0.23 | 0.78* | 0.00 | 0.24 | 0.24 | 0.06 | 0.04 | −0.16 | −0.39* | −0.15 | 0.06 | −0.02 | 5.03 (1.12) | 5.50 (1.06) |
| 5. Conflict HRV (partner) | −0.14 | 0.21 | 0.69* | 0.24 | 0.24 | 0.13 | −0.13 | 0.02 | −0.27* | 0.26* | 0.14 | 0.21 | 5.50 (1.06) | 5.03 (1.12) |
| 6. Marital satisfaction | −0.06 | 0.04 | −0.13 | −0.02 | −0.03 | 0.76* | −0.22* | −0.42* | −0.07 | 0.20 | −0.13 | 0.70* | 125.11 (31.20) | 123.91 (34.39) |
| 7. Morning negative affect | −0.09 | 0.00 | 0.20 | 0.10 | 0.30* | −0.26* | 0.20 | 0.23* | −0.04 | 0.02 | 0.03 | −0.05 | 5.49 (0.91) | 5.95 (2.04) |
| 8. Post-conflict neg. affect | 0.14 | −0.15 | −0.10 | −0.19 | −0.12 | −0.23* | 0.17 | 0.23* | 0.01 | 0.19 | 0.13 | −0.24 | 5.53 (1.28) | 6.40 (1.89) |
| 9. Age | 0.13 | −0.29* | −0.40* | −0.31* | −0.43* | −0.09 | −0.05 | 0.28* | 0.90* | −0.23* | −0.31* | 0.13 | 39.26 (9.12) | 37.19 (6.96) |
| 10. Trunk fat (kg) | −0.16 | −0.21 | 0.39* | −0.23* | 0.30* | −0.05 | 0.17 | 0.03 | 0.11 | −0.07 | 0.24* | 0.39* | 19.50 (7.72) | 19.38 (7.34) |
| 11. Education | −0.16 | −0.15 | −0.13 | −0.14 | −0.15 | 0.24* | −0.11 | −0.07 | 0.02 | −0.06 | 0.23* | −0.11 | – | – |
| 12. IOS | 0.00 | 0.08 | −0.35* | 0.06 | −0.23 | 0.61* | −0.02 | −0.02 | −0.03 | −0.17 | 0.20 | 0.48* | 4.86 (1.45) | 4.91 (1.59) |
Note.
p < .05. Husband correlations fall below the diagonal, wife correlations above the diagonal, and correlations between husbands and wives along the diagonal (bolded for readability). All coefficients reflect Spearman’s correlations to account for non-normality in some study variables. Baseline and conflict HRV variables were natural-log-transformed. Of the 86 participants, 12 (14%) had a high school degree or less, 37 (43%) had at least some college, and 37 (43%) had graduate or professional training.
The sample size of the parent study was planned based on the expected power for a hypothesized three-way interaction (Kiecolt-Glaser et al., 2015). Given that lower-order interactions should be adequately powered for similarly sized effects, it was concluded that the primary hypotheses for the present study were sufficiently powered. Nevertheless, post-hoc power estimates were also calculated. As noted, 86 participants in 43 couples completed study visits on two separate occasions. Based on previous analyses using these data, the intraclass correlation (ICC) for inflammatory markers was estimated to be small, ICC = .06, giving an effective N of 81. Power calculations were based on the ability to detect an increase in R2 due to the addition of the HRV synchrony predictor to the regression model. Studies of marital conflict behavior and post-conflict immunological changes have shown small to medium effects (e.g., Kiecolt-Glaser et al., 2015; Kiecolt-Glaser et al., 2005). With n = 81, α = .05, and a two-tailed test, there was 80% power to detect a small to medium effect (Cohen’s f2 = .10). Because each partner provided two sessions of data and multiple samples within each session, this is a conservative power estimate for our models.
2.2 Data Collection Procedure
This research was approved by the Ohio State University (OSU) Institutional Review Board; participants provided written informed consent before participating. Participants completed two full-day study visits at the Clinical Research Center (CRC), a hospital research unit. During this double-blind randomized crossover study, couples ate a high saturated fat meal at the beginning of one visit and a high oleic sunflower oil meal at the beginning of the other (in random order to test the parent study’s key aims). Couples were told to avoid alcohol and caffeine use within 1 day prior and strenuous physical activity within 2 days prior to both study visits. Participants were also instructed to stop taking aspirin, vitamins (except multivitamins), antioxidants, and any other dietary supplements for 7 days prior to each admission. On the day before each visit, participants received three standardized meals from the CRC’s metabolic kitchen, reducing any variability in inflammation associated with recent food intake. They began a 12-h fast at 7:30 p.m. the evening before each visit.
At each admission, both members of a couple arrived at 7:30 a.m., at which time a catheter was inserted into each person’s arm and each person was fitted with a heart rate monitor to obtain heart rate variability (HRV) throughout the visit. Following a 5-minute resting period where baseline HRV was measured, each member of the couple ate either the high saturated fat or high oleic sunflower oil meal; the husband and wife received the same meal and both were required to eat the entire meal.
Couples also engaged in a marital problem discussion on the morning of each visit. To initiate the discussion, an experimenter conducted a 10- to 20-min interview to identify the most contentious topics within the marriage for both partners. These topics were selected from an inventory each spouse completed about their relationship problems. Couples were then asked to discuss and try to resolve one or more marital issues that the experimenter judged to be the most conflict-producing (e.g., money, communication, or in-laws). The research team remained out of sight while videotaping the subsequent 20-min problem discussion.
Blood samples for inflammatory assays were collected upon arrival (8:30 a.m.), 2 hours after the meal (11:00 a.m.), and approximately 1 and 4 hours after the conflict discussion (1:00 p.m. and 4:45 p.m., respectively). The two study visits occurred 1–25 weeks apart (mean = 4.45, SD = 4.76). Although 55% of visits occurred within 3 weeks, some were more widely spaced as a consequence of participants’ work schedules.
2.3 Primary self-report measures
Primary self-report measures were included as covariates in models with inflammation as an outcome due to their known associations with inflammation.
2.3.1 Marital quality
Administered at the first full-day visit, the 32-item Couples Satisfaction Index (CSI) assessed marital satisfaction (Funk and Rogge, 2007). Developed using item response theory, the CSI can discriminate between satisfied and dissatisfied couples with greater precision than other commonly used marital scales (Funk and Rogge, 2007). Marital satisfaction was included as a covariate to account for the association of general relationship quality with inflammation (Donoho et al., 2013; Whisman and Sbarra, 2012).
2.3.2 Negative affect
Negative affect was assessed throughout each study visit, including at the beginning of the day and immediately after the disagreement task, using the 10-item subscale of the Positive and Negative Affect Schedule (PANAS, Watson et al., 1988). Negative mood and negative affect reactivity to daily stressors are associated with elevated inflammation (Sin et al., 2015); thus, negative affect served as a covariate in inflammatory models. Post-conflict negative affect was also tested as an outcome of synchrony in negative affect reactivity models. We focused specifically on negative affect because it corresponded to the expected increase in negative valence and negative behavior associated with the marital disagreement task, both of which are consistently related to poorer marital quality and health. Also, the PANAS positive affect subscale in particular is biased toward the high-arousal dimension, which is unusual for the interaction context and also shares an equivocal link to inflammation (Marsland et al., 2007; Pressman and Cohen, 2005). Further, in our past work, we had noticed that baseline and post-conflict positive affect have been highly correlated, as was the case here (r = 0.59, p < 0.0001).
2.4 Secondary self-report measures
Secondary self-report measures were used in supplemental correlational analysis to assess the interpersonal and behavioral correlates of HRV synchrony.
2.4.1 Overall closeness
Each partner completed the 1-item Inclusion of Other in Self (IOS) Scale—seven pairs of circles with varying overlap, meant to represent oneself and one’s partner (Aron et al., 1992). The individual is asked to choose the pair of circles that best describes the relationship. This is a well-established, reliable measure of partners’ subjective feelings of interpersonal closeness (Aron et al., 1992).
2.4.2 Momentary closeness
Immediately following the disagreement task, each partner completed the four-item Current Closeness to Partner scale, to capture their feelings of interpersonal closeness in the moment (Jaremka and Collins, 2011). On a scale of 0 to 6, subjects responded to items such as “Right now I feel emotionally connected to my partner” and “Right now I feel emotionally distant from my partner” (reverse scored). The items were averaged, with higher scores indicating greater feelings of closeness in the moment.
2.5 Word use
Transcribed problem discussions were processed with Linguistic Inquiry and Word Count (Pennebaker, Austin, TX), text analysis software that produces percentages of words, adjusted for total word count, in psychologically relevant categories based on a standardized dictionary. Of interest for the current study were cognitive processing words, use of singular and plural personal pronouns, and use of past and present tense. Use of more cognitive words (e.g., think, consider, realize, why, because) during marital conflict has been associated with blunted inflammatory responses (Graham et al., 2009). Speaking in past tense more than the present tense reflects psychological distancing (Tausczik and Pennebaker, 2009). Using more we-talk and less I-talk during marital conflict have been linked to lower cardiovascular reactivity, and to more positive, less negative behavior (Seider et al., 2009).
2.6 Problem Discussion Behavior
Marital disagreement discussions were coded using the Rapid Marital Interaction Coding System (RMICS), which discriminates well between distressed and nondistressed couples (Heyman, 2004). Each conversational turn was assigned one of several codes, with priority given to more noxious behaviors: psychological abuse (e.g., disgust, contempt, belligerence, as well as nonverbal behaviors like glowering), distress-maintaining attributions (e.g., “You’re only being nice so I’ll have sex with you tonight” or “You were being mean on purpose”), hostility (e.g., criticism, hostile voice tone, or rolling the eyes dramatically), withdrawal (behaviors that suggest pulling back from the interaction or not listening), acceptance (e.g., attempts at active listening, expressing concern), relationship-enhancing attributions (e.g., “You’re short with me because you’ve had a hard day”), self-disclosure (e.g., any expression of feelings, wishes, or beliefs not considered hostile toward the partner, “I felt uncomfortable at your parents’ house” or “I think children should respect their parents”), humor (e.g. playful joking, teasing, or sarcasm), and constructive problem discussion (e.g., “Let’s stop eating out so often” or “I think you’re right about that”).
Distressed marriages are characterized by negative affect, conflictual communication, and poor listening skills (Snyder et al., 2005). Accordingly, the negative composite index summed four RMICS codes: psychological abuse, distress-maintaining attributions, hostility, and withdrawal. Holley and Gilford’s G was used to quantify inter-rater agreement for the RMICS hostility composite. Interrater agreement was high, with a G index of 0.88. Distressed couples also exhibit more negative affect reciprocity, where hostilities are perpetuated and escalated in a tit-for-tat exchange (Snyder et al., 2005). We captured negative reciprocity by summing all sequences in the discussion that included a negative behavior (any of the four behaviors in the negative composite) followed by another negative behavior.
2.7 HRV
For HRV measurement, intervals between wrist pulses (which we refer to as IBIs, from interbeat intervals, for brevity) were continuously measured noninvasively with the Polar s810 wristwatch and Wearlink 31 belt band at a standard 1000 Hz sampling rate. Before HRV analysis, visual artifact correction was performed on the raw IBI series.
We used different approaches for estimating the HRV synchrony score (which requires a time-varying measure) versus summarizing HRV levels at baseline and during conflict. However, both methods applied spectral analysis to IBIs to extract the variance within the high frequency band (HF-HRV), e.g., 0.12 – 0.40 Hz (Gates et al., 2015; Hansson and Jönsson, 2006), and resulting values are very highly correlated across the methods (r = 0.95 in the current study). For simplicity, HRV is used synonymously with HF-HRV throughout the report.
2.7.1 HRV synchrony during disagreement
We estimated time-varying HRV for the entire 20-min disagreement period using the program RSAseconds (Gates et al., 2015). Each partner’s IBI series during the disagreement task was extracted and interpolated at 250 ms using a cubic spline to create equal intervals. The data were then tapered using Peak Matched Multiple Windows (Hansson & Jönsson, 2006). A short-time Fourier transform (STFT) was applied to produce 1-s estimates of HRV from 32-s windows.
Next, we differenced each HRV series to remove any linear trends in the data and to enable conclusions about how changes in one partner’s HRV related to changes in the other partner’s (Gates et al., 2015). Using a difference of lag-1 for the synchrony calculation, prior research found that more discordant couples synchronized more closely during interaction (Gates et al., 2015). A subsequent study proposed using a longer lag to align with the time course of the adult respiration cycle, 4 - 7 s (Fisher et al., 2016). To both maintain consistency with a validated approach and match a plausible respiration cycle, we differenced series at lag-4, i.e., HRVlag 4(t*) = HRV(t) − HRV(t − 4). Then we cross-correlated these differenced time series within each couple and, to stabilize the variance of the correlations, transformed each correlation using the Fisher z-transformation (Gates et al., 2015).
2.7.2 HRV baseline and conflict levels
To capture HRV levels during the 5-minute rest period and during conflict, we extracted the corresponding IBI series for those windows and applied spectral analysis using Fast Fourier transform (FFT) to produce a single value for each with Kubios software (Kubios, Kuopio, Finland). FFT was chosen to maintain consistency with the approach used to calculate HRV synchrony. We used a 5-minute subset of the conflict discussion (10 minutes in) to match the 5-minute window of the rest period (Laborde et al., 2017).
2.8 Assays
2.8.1 Serum cytokines
Serum IL-6 and TNF-α were multiplexed and measured using an electrochemilluminescence method with Meso Scale Discovery kits, and read using the Meso Scale Discovery Sector Imager 2400, following kit instructions (Meso Scale Discovery, Rockville, MD). Each subject’s samples were assayed for all cytokine markers in one run, thus using the same controls for all time points (Kiecolt-Glaser et al., 2015). Sensitivity for these serum cytokines was 0.3 pg/mL. The intra-assay coefficient of variation for IL-6 was 3.42%, and the inter-assay coefficient of variation was 8.43%; corresponding values for TNF-α were 2.59% and 8.14%.
2.8.2 Stimulated cytokines
Peripheral blood mononuclear cells (PBMCs) were treated with 1.0 μg/mL lipopolysaccharide (LPS) for 24 hours to measure the production of IL-6 and TNF-α in response to a pathogen. After 24 hours, the cells were pelleted by centrifugation (2000 rpm for 5 minutes) and the supernatant removed, aliquoted and frozen at −80C until assayed. All samples, including each person’s control cell cultures incubated in media alone, were run at the same time for each subject. Sensitivity was 0.7 pg/mL for stimulated IL-6 and 1.0 pg/mL for stimulated TNF-α. The intra-assay coefficient of variation for IL-6 was 2.76%, and the inter-assay coefficient of variation was 13.57%; corresponding values for TNF-α were 3.40% and 11.91%.
2.8.3 Serum adhesion molecules
sVCAM-1 and sICAM-1 were measured in duplicate with the Vascular Injury Panel 2 Multispot Kit on an MSD Imager 2400, following kit instructions. Both samples for each subject were analyzed within the same plate, and all plates were from the same kit and lot. Assay sensitivity was 0.07 ng/mL for sVCAM-1 and 0.01 ng/mL for sICAM-1. For sVCAM-1, the intra-assay coefficient of variation was 2.14% and inter-assay coefficient of variation was 5.16%; corresponding values for sICAM-1 were 4.19% and 6.92%.
2.9 Analytic Plan
Research questions were addressed using linear mixed models, which allowed explicit modeling of the within-subject correlations across visits and accounted for couple-level correlation. Time effects were treated as categorical (i.e., rather than continuous as in a growth curve framework) due to the varying nonlinear trajectories of the inflammatory markers. Where possible, cytokine models also included a random effect for assay plate to account for plate-to-plate variability and to increase precision of estimates. In some of the models, random effects could not be estimated for subject, couple, and plate factors due to a lack of sufficient variability; we report in Table 2 which random effects were modeled for each outcome.
Table 2.
HRV synchrony predicting inflammation across the day in reduced models
| Ln(IL-6) | Ln(TNF-α) | Ln(IL-6 LPS) | Ln(TNF-α LPS) | Ln(sICAM-1) | Ln(sVCAM-1) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | B | 95% CI | |
| Intercept | 0.889* | [0.35, 1.42] | 1.566* | [1.38, 1.75] | 11.027* | [10.72,11.33] | 7.071* | [6.66, 7.48] | 12.647* | [12.49, 12.81] | 13.157* | [12.98, 13.33] |
| Time (1) | −1.037* | [−1.17, −0.90] | 0.018 | [−0.01, 0.05] | 0.168* | [0.08, 0.25] | 0.165* | [0.08, 0.25] | 0.032* | [0.01, 0.06] | 0.024* | [0.00, 0.04] |
| Time (2) | −0.627* | [−0.76, −0.49] | 0.000 | [−0.03, 0.03] | 0.093* | [0.01, 0.18] | 0.083 | [−0.00, 0.17] | 0.051* | [0.03, 0.08] | 0.029* | [0.01, 0.05] |
| Time (3) | −0.357* | [−0.49, −0.22] | −0.002 | [−0.03, 0.03] | 0.194* | [0.11, 0.28] | 0.122* | [0.04, 0.21] | 0.045* | [0.02, 0.07] | 0.016 | [−0.00, 0.04] |
| Visit | 0.171* | [0.06, 0.29] | −0.022 | [−0.05, 0.00] | −0.035 | [−0.11, 0.04] | −0.029 | [−0.10, 0.04] | 0.035* | [0.01, 0.06] | 0.031* | [0.01, 0.05] |
| Meal | −0.018 | [−0.13, 0.10] | 0.023 | [−0.00, 0.05] | 0.055 | [−0.02, 0.13] | −0.004 | [−0.07, 0.07] | 0.007 | [−0.02, 0.03] | −0.005 | [−0.02, 0.01] |
| Female | 0.323* | [0.03, 0.62] | 0.008 | [−0.08, 0.10] | 0.075 | [−0.07, 0.22] | 0.087 | [−0.07, 0.25] | 0.041 | [−0.05, 0.13] | −0.022 | [−0.12, 0.08] |
| Education | −0.034 | [−0.25, 0.18] | −0.012 | [−0.09, 0.06] | −0.044 | [−0.16, 0.07] | −0.071 | [−0.22, 0.07] | 0.007 | [−0.06, 0.07] | −0.006 | [−0.07, 0.06] |
| Age | −0.000 | [−0.02, 0.02] | −0.004 | [−0.01, 0.00] | −0.003 | [−0.01, 0.01] | −0.019 | [−0.04, 0.00] | −0.000 | [−0.01, 0.01] | 0.001 | [−0.01, 0.01] |
| Trunk fat (kg) | 0.022* | [0.00, 0.04] | 0.012* | [0.01, 0.02] | −0.000 | [−0.01, 0.01] | 0.004 | [−0.01, 0.02] | 0.008* | [0.00, 0.01] | 0.005 | [−0.00, 0.01] |
| Baseline HRV | 0.105 | [−0.00, 0.21] | −0.008 | [−0.03, 0.02] | 0.047 | [−0.02, 0.11] | −0.036 | [−0.10, 0.03] | −0.017 | [−0.04, 0.01] | −0.026* | [−0.05, −0.01] |
| HRV synchrony | 1.479* | [0.59, 2.37] | −0.135 | [−0.32, 0.05] | 0.869* | [0.29, 1.45] | 0.672* | [0.11, 1.23] | 0.036 | [−0.13, 0.21] | 0.190* | [0.05, 0.33] |
Note. For the meal covariate, high saturated fat was the reference category. Due to differential variability, subject-level intercepts, couple-specific effects, and random plate effects could not be estimated simultaneously for every outcome. Serum IL-6 models included subject-level intercepts and a random plate effect. Serum TNF-α and LPS-stimulated IL-6 models included all three random terms. LPS-stimulated TNF-α models included subject and couple random intercepts. sICAM-1 and sVCAM-1 models included subject-specific intercepts.
Cytokine data were natural-log (ln) transformed to better approximate normality of residuals. Residual plots were also inspected for outliers. The Kenward-Roger degrees of freedom adjustment was used to control type I error. Continuous variables were grand-mean-centered for ease of interpretation. Correlation matrices report Spearman’s coefficients to account for nonnormality in some of the variables and to maintain consistency within the matrix; Pearson’s and Spearman’s coefficients converge when both variables are normally distributed. To further define synchrony’s validity, we constructed a heatmap to broadly characterize the correlates of synchrony during conflict with secondary measures not included in inflammatory models—self-reported relationship metrics (marital satisfaction, overall closeness, and momentary closeness), coded behaviors (distress, hostility, withdrawal, acceptance, relationship-enhancing attributions, self-disclosure, humor, and constructive problem discussion, as well as the negative composite and negative reciprocity), word use (cognitive processing, past vs. present, I vs. we), number of times the disagreement topic had been previously discussed, as well as baseline and conflict negative mood and HRV levels.
2.9.1 Synchrony and inflammation
To minimize the number of statistical tests, associations between synchrony and inflammation were examined in a nested sequence of models. First, we examined whether synchrony predicted each inflammatory marker in a reduced model, with standard health and demographic covariates including sex, age, abdominal trunk fat, education, and baseline HRV. Both meal type (high saturated fat or high oleic sunflower oil) and visit (first or second) were also included as fixed effects. Because each inflammatory marker has a different trajectory across the day, measurement time was included as a categorical predictor using the final time point as the reference level.
Next, for outcomes where synchrony had significant effects in the reduced model, we included additional psychosocial variables in fully-adjusted models: marital quality and negative affect, as well as the partner’s baseline HRV, a potential contributor to HRV synchrony between the partners. To prevent overinterpretation, we explored synchrony’s interaction with time to determine whether effects arose at particular points or persisted throughout the day, only for inflammatory markers that synchrony predicted above and beyond these additional psychosocial variables.
2.9.2 Synchrony and reactivity
We also hypothesized that HRV synchrony during conflict would predict reactivity to the task, with inflammation, negative affect, and HRV as separate outcomes. Inflammatory reactivity models treated post-disagreement inflammation as the outcome, and included all covariates from the fully-adjusted models described above, in addition to the respective inflammatory marker at baseline. To avoid redundancy, we did not test reduced models for inflammatory reactivity. The negative mood reactivity model treated post-conflict negative affect as the outcome, controlling for baseline negative mood, visit (first or second), sex, marital quality, and negative behavior during the conflict. Due to the remarkably high rank-order stability of HRV at baseline and during conflict (Spearman’s r = .78, p < .0001), the HRV reactivity model was not tested.
3. Results
3.1 Descriptives
Synchrony scores ranged from −0.25 to 0.24 (M = −0.001, SD = 0.095); see supplemental material for the frequency distribution. Negative scores (with increases in one partner’s HRV corresponding to decreases in the other’s) denote anti-phase, rather than in-phase, linkage, which may reflect the turn-taking pattern of a neutral conversation, or that one partner is successfully regulating while the other feels threatened (Reed et al., 2013). Figure 1 depicts two couples’ HRV series during their problem discussions. Couple B (score = 0.21) spent more time in synchrony (shaded red) compared to Couple A (score = −0.23), who spent most of the conversation in a neutral pattern.
Figure 1.
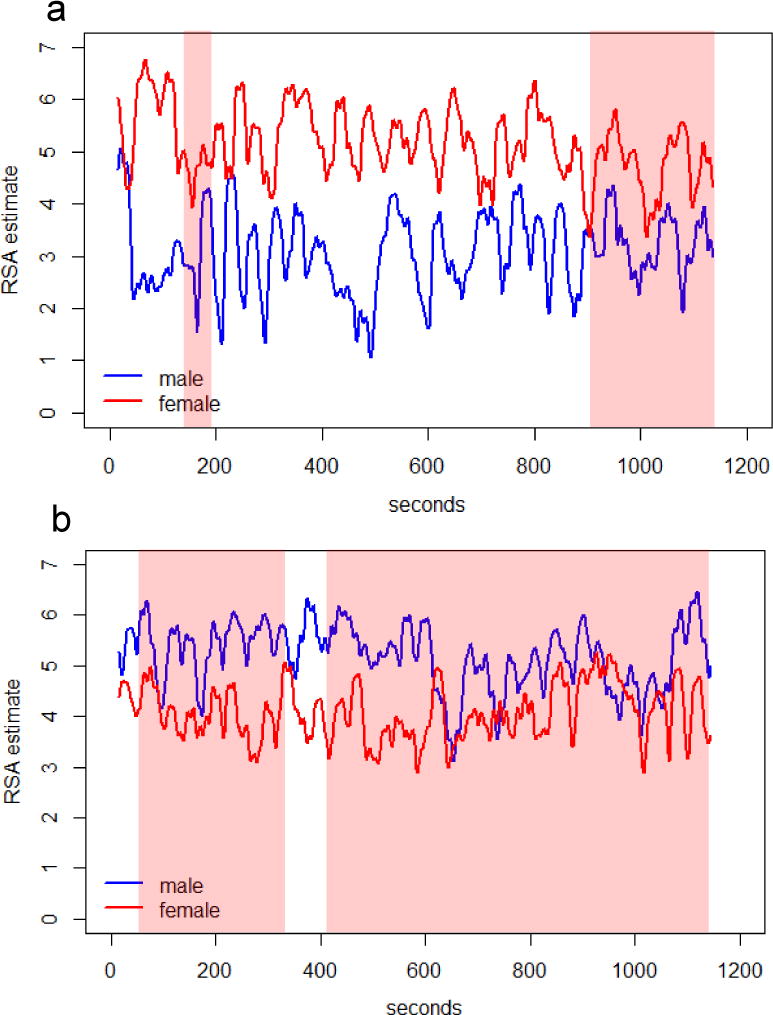
Two couples’ HF-HRV series during the marital problem discussion. Highlighted in red are periods when the two partners were in synchrony (i.e., in-phase linkage, when one partner increased or decreased, the other partner followed); the unhighlighted are neutral periods, where partners were not linked or were in anti-phase synchrony (i.e., when one increased or decreased, the other partner did the opposite). To illustrate, Panel A depicts a couple who spent relatively little time in synchrony (z = −.23); Panel B shows a couple who spent the majority of time synchronized (z = .21). Synchrony estimates were based on cross-correlation and did not quantify the exact amount of time spent in each pattern; see the Method for a detailed description of the approach.
3.2 Is synchrony stable across visits?
In a first effort to characterize synchrony across occasions, we examined the intraclass correlation coefficient (ICC) of HRV synchrony as well as the correlation between couples’ synchrony scores at visits 1 and 2. Both indicated low correspondence between couples’ scores across visits (ICC = 0.01; rVisit1, Visit 2 = −0.01, p > 0.250), suggesting that synchronizing during disagreement is not necessarily a stable, couple-level characteristic, but rather a process that any couple can experience. A paired-samples t-test indicated that there were no systematic increases or decreases in synchrony from one visit to the next (t = 0.52, SE = 0.03, p > 0.250).
3.3 What stable and transient characteristics does synchrony relate to?
Table 1 depicts the full correlation matrix for continuous primary predictors; see supplemental material for a comparison of demographics between partners, within couples. Contrary to expectation, HRV synchrony did not relate to self-reported marital quality (Spearman’s r = −0.05, p > .250). Nevertheless, as expected, lower baseline HRV was associated with older age and greater abdominal adiposity (Table 1). As shown in Figure 2, we examined synchrony’s associations with other conflict-specific factors and relationship constructs in supplemental correlational analysis. Stronger synchrony was associated with using more cognitive-mechanism language (Spearman’s r = 0.26, p = 0.004), speaking more in the past tense than the present (Spearman’s r = 0.18, p = 0.042), feeling less close to the partner in the moment (Spearman’s r = −0.46, p < .0001), self-disclosing more (Spearman’s r = 0.28, p = 0.002), and, at a trend level, using less humor (Spearman’s r = −0.18, p = .054), having previously discussed the chosen topic more times (Spearman’s r = 0.17, p = 0.061), and reporting greater post-conflict negative affect (Spearman’s r = 0.14, p = 0.114). Contrary to expectation, synchrony did not track with behaving more negatively during the disagreement (p = 0.242), or with negative reciprocity (p > .250); hostile behavior and negative reciprocity were highly correlated (Spearman’s r = 0.86, p < 0.0001). To explore the link between synchrony during conflict and processes that occurred prior to the study visits, supplemental analyses examined the correlation between synchrony and sleep duration in the past two nights. Indeed, when partners had slept fewer hours in prior nights, HRV synchrony during conflict was stronger (Spearman’s r = −0.20, p = 0.025).
Figure 2.
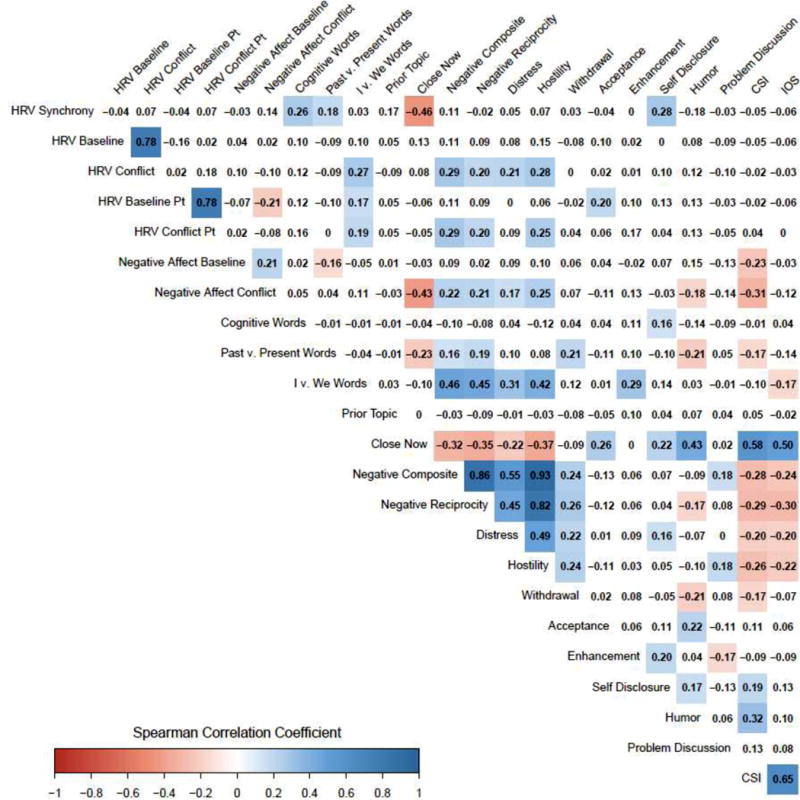
Heatmap of couple and disagreement characteristics. All correlations are Spearman’s coefficients. Statistically significant (p < .05) positive correlations are colored blue. Significant negative correlations are colored red. Darker saturation reflects larger magnitude. Couples Satisfaction Index (CSI) reflects marital satisfaction; Inclusion of Other in Self (IOS) measures general closeness to the partner.
3.4 Does synchrony predict inflammation across the day?
3.4.1 Reduced models
As shown in Table 2, when couples synchronized more closely during conflict they also had higher inflammation throughout the day in four of the six inflammatory markers: serum IL-6 (B = 1.48, SE = 0.45, p = 0.001, 95% CI [0.591, 2.367]), LPS-stimulated IL-6 (B = 0.87, SE = 0.29, p = 0.003, 95% CI [0.292, 1.446]), LPS-stimulated TNF-α (B = 0.67, SE = 0.29, p = 0.019, 95% CI [0.112, 1.232]), and sVCAM-1 (B = 0.19, SE = 0.07, p = 0.008, 95% CI [0.049, 0.330]). The effect of synchrony was non-significant for the other two inflammatory markers, serum TNF-α (p = 0.161, 95% CI [−0.323, 0.054]) and sICAM-1 (p = .679, 95% CI [−0.134, 0.206]).
3.4.2 Fully adjusted models
With the addition of psychosocial covariates—partner baseline HRV, negative affect, and marital satisfaction—synchrony remained a significant predictor of inflammation in three of the four models: serum IL-6 (B = 1.29, SE = 0.62, p = 0.037, 95% CI [0.076, 2.513]), LPS-stimulated TNF-α (B = 1.12, SE = 0.42, p = 0.009, 95% CI [0.284, 1.949]), and sVCAM-1 (B = 0.27, SE = 0.10, p = 0.009, 95% CI [0.069, 0.467]). The association between synchrony and LPS-stimulated IL-6 was not significant in the fully adjusted model (p = 0.433, 95% CI [−0.450, 1.046]).
Next, we examined whether synchrony predicted inflammation at specific times throughout the day, displayed in Figure 3. When couples synchronized more strongly during conflict, they had higher stimulated TNF-α one hour after the conflict (B = 1.59, SE = 0.50, p = 0.002, 95% CI [0.607, 2.577]) and four hours afterward (B = 1.02, SE = 0.50, p = 0.042, 95% CI [0.037, 2.011]), with non-significant trends at baseline (B = 0.96, SE = 0.50, p = 0.054, 95% CI [−0.017, 1.944]) and post-meal (B = 0.91, SE = 0.50, p = 0.069, 95% CI [−0.070, 1.880]). When synchrony was stronger, they also had higher sVCAM-1 throughout the day: at the morning baseline (B = 0.26, SE = 0.12, p = 0.042, 95% CI [0.009, 0.501]), after the study meal (B = 0.32, SE = 0.12, p = 0.011, 95% CI [0.072, 0.562]), and one hour after the disagreement (B = 0.25, SE = 0.13, p = 0.049, 95% CI [0.001, 0.495]), with a non-significant trend four hours after the disagreement task (B = 0.25, SE = 0.13, p = 0.050, 95% CI [−0.000, 0.496]). Stronger synchrony predicted higher serum IL-6 at baseline (B = 2.03, SE = 0.82, p = 0.013, 95% CI [0.425, 3.636]) and post-meal (B = 1.80, SE = 0.81, p = 0.028, 95% CI [0.194, 3.397]), but not after the disagreement (ps > .250).
Figure 3.
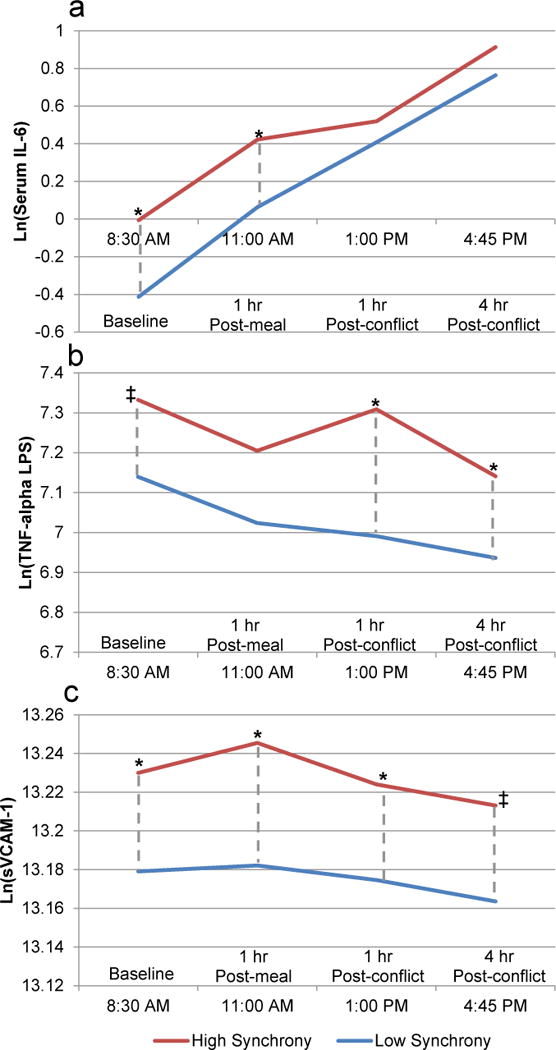
Three of the six inflammatory markers showed significant effects for synchrony in fully adjusted models: serum IL-6 (Panel A), LPS-stimulated TNF-α (Panel B), and sVCAM-1 (Panel C). High synchrony (1 SD above the mean) is depicted in red; low synchrony (1 SD below the mean) is in blue. The dashed vertical grey lines depict differences at respective time points (*, p < .05; ‡, p < .06).
Supplemental analyses further tested the association between synchrony during conflict and inflammation across the day controlling for additional covariates: past-month sleep quality as assessed by the Pittsburgh Sleep Quality Index (Buysse et al., 1989), sleep duration in prior nights, and depressive symptoms assessed by the Center for Epidemiological Studies-Depression (Radloff, 1977); all significant associations between synchrony and inflammatory markers described above were unchanged.
3.5 Does synchrony predict reactivity to conflict?
3.5.1 Inflammatory reactivity
We tested the association between synchrony and inflammatory reactivity to conflict by treating post-conflict inflammation as the outcome and controlling for baseline levels of inflammation. Synchrony was non-significant in all models (ps > .250).
3.5.2 Negative affect reactivity
Controlling for morning negative affect, visit, sex, and marital satisfaction, greater synchrony during conflict predicted larger negative affect reactivity to the task (B = 2.86, SE = 1.41, p = 0.044, 95% CI [0.078, 5.648]). This association held with the addition of couples’ hostile behavior to the model (B = 2.90, SE = 1.42, p = 0.044, 95% CI [0.079, 5.716]). To further evaluate this effect, we investigated in supplemental analyses the association between synchrony at one visit with negative reactivity at the opposite visit, and found no significant relationship (p > 0.250), thus providing support for the link between synchrony during a particular marital disagreement and negative emotional responsiveness at that visit.
3.5.3 HRV reactivity
We predicted that stronger HRV synchrony would be associated with larger decreases in HRV levels, but due to the remarkably high rank-order stability, i.e., redundancy, of HRV at baseline and during conflict (Spearman’s r = .78), this test was not conducted. Nevertheless, in supplemental analyses, change score models revealed significant decreases in HRV from baseline to conflict (B = −0.62, SE = 0.09, p < 0.0001).
4. Discussion
Stronger HF-HRV synchrony between disagreeing partners predicted a heightened inflammatory profile, beyond the effects of marital quality, negative affect, and other health and demographic factors known to influence inflammation. Synchronized partners also had greater negative emotional responsiveness, magnifying the emotional impact of the conflict. Couples’ degree of synchrony varied across disagreements, and tracked more closely with conversation characteristics than with global factors such as marital quality. Thus, the current study provides evidence of synchrony’s plasticity. These data also highlight partners’ HRV linkage during conflict as a novel social-biological pathway that may help to explain couples’ risks for inflammation-related disease.
Couples completed the conflict protocol twice, approximately four weeks apart, which provided the opportunity to examine the stability of synchrony across occasions. A small ICC and non-significant correlation between synchrony scores at the first and second visits indicate that synchrony was not a stable, couple-level tendency, but a process that any couple could experience. Becoming physiologically linked in disagreement one day did not dictate whether a couple would become ‘locked in’ on another day. This is consistent with other work showing changes in synchrony across tasks (Helm et al., 2012; Liu et al., 2016) and with a prior description of synchrony as a “transient state” (p. 26, Palumbo et al., 2017). This may explain why one prior study found that couples’ physiological synchrony predicted poorer marital quality concurrently, but not three years later (Levenson and Gottman, 1985). The construct may require multiple assessments to reveal long-term risk.
Accordingly, synchrony tracked more closely with interaction-specific factors than overall relationship characteristics. Indeed, synchrony was strongest when couples had more frequently discussed the chosen topic. When couples were synchronized, they also felt more distant in the moment, talked more about the past than the present, used more cognitive words, and spent more time self-disclosing. Although thought to be constructive, cognitive word use and self-disclosure did not track consistently with positive characteristics in our study. Contrary to some past work (Chaspari et al., 2015; Gates et al., 2015; Levenson and Gottman, 1983), synchrony was unrelated to poor marital quality, hostile behavior, and negative reciprocity, null associations which may emerge when synchrony is measured repeatedly. Indeed, our results align with another study that found minute-to-minute linkage in mean blood pressure was stronger in moments when couples were demanding or withdrawing, but unrelated to trait-level marital conflict (Reed 2013). Unhappier couples may synchronize during disagreement more frequently or for longer periods compared to happier couples; future studies must measure synchrony repeatedly to better understand synchrony in relation to both situational and trait factors. Taken together, results seem to suggest that couples synchronized during disagreement when they were rehashing long-lived disputes in ways that left them feeling distant from their partner. Therefore, this linkage appeared to capture the quality of being stuck, or jointly engaged in the conflict, consistent with prior theorizing (Levenson and Gottman, 1983; Timmons et al., 2015).
Synchronizing around conflict predicted higher inflammation across the day, beyond marital quality and negative affect, as well as other consistent proinflammatory factors such as abdominal adiposity, age, and lower education. The wide array of covariates provided a strict test of synchrony, and broadly suggests that this emergent dyadic mechanism may be uniquely important for couples’ health. In reduced models, greater synchrony was related to higher IL-6, sVCAM-1, and LPS-stimulated TNF-α and IL-6; additional psychosocial factors in fully adjusted models attenuated the LPS-stimulated IL-6 effect. These inflammatory outcomes reflect distinct biological processes. Higher serum IL-6 indexes more circulating proinflammatory cytokines in the periphery, and thus greater inflammatory load. A proinflammatory phenotype is a common precondition for developing many age-related diseases (e.g., cardiovascular disease, cancer, diabetes, dementia), frailty, and functional decline (Franceschi and Campisi, 2014). LPS-stimulated cytokine levels reflect how robustly mononuclear cells respond to a pathogen (De Groote et al., 1992): thus, synchronized couples’ TNF-α exhibited greater responsiveness to a pathogen than that of unsynchronized couples. sVCAM-1’s direct role in arterial plaque accumulation and tumor proliferation (Blankenberg et al., 2003; Wu, 2007) means that synchrony’s links to this adhesion molecule may promote the progression of specific diseases, atherosclerosis and cancer, over time.
Synchrony was associated with inflammation both before and after the disagreement; patterns across the day differed by inflammatory marker. When couples were synchronized, all three significant markers (IL-6, stimulated TNF-α, and sVCAM-1) were elevated prior to the disagreement itself, suggesting that synchrony or related processes may have been at work before the task. Certainly, couples can serve as stressors to each other outside the lab (Levenson and Gottman, 1985; Snyder et al., 2005), and arriving promptly at 7:30 a.m. to commence a lengthy study protocol may have induced tension before recording began. Moreover, the way couples disagree in the lab mirrors how they argue in daily life (Snyder et al., 2005), and though synchrony differed across visits, there would be no closer resemblance than between the couples’ afternoon disagreement and their unrecorded morning interactions. Indeed, morning experiences set the tone for the rest of the day (Golder and Macy, 2011) more commonly than they spill over from one day to the next (Marco and Suls, 1993). Furthermore, when partners slept fewer hours before the visit, their HRV changes also synchronized more closely during the marital disagreement. This is consistent with previous studies showing that when couples sleep poorly one night, they have more conflictual interactions on the next day (Gordon and Chen, 2014; Hasler and Troxel, 2010; Wilson et al., 2017). Thus, this pattern also converges with our working hypothesis that couples may have synchronized around interpersonal tension before their arrival, though future studies must evaluate this hypothesis directly and we caution against overinterpreting this particular result. Also, both the ability to regulate emotions and marital interaction quality fluctuate day-to-day (August et al., 2013; Robinson et al., 2016). Further, the quality of these fleeting moments matters for health: people who had worse daily marital quality had thicker carotid artery walls than people with happier daily marital interactions, whereas general self-reported marital satisfaction did not relate to this marker of subclinical cardiovascular disease (Joseph et al., 2014).
Significant effects on post-disagreement sVCAM-1 and stimulated TNF-α are consistent with synchrony’s links to conflict characteristics. However, the association with IL-6 weakened across the day due to its steady incline—perhaps a function of its circadian rhythm and catheter placement (Gudmundsson et al., 1997; Nilsonne et al., 2016). Synchrony did not predict inflammatory reactivity to conflict, likely because it was related to baseline inflammation. Synchrony also did not predict serum TNF-α or sICAM-1, even in reduced models. The null effect for TNF-α may be due to a mismatch between the sampling frame (1, 4 h post-conflict) and the time course of its response to psychosocial stress (31-50 min) (Marsland et al., 2017). The evidence of sICAM-1 and acute psychosocial stress in humans is too sparse to explain this null effect.
Synchrony magnified the emotional impact of the disagreement, a potential mechanism to health risks. Indeed, more exaggerated negative affect reactivity in daily life is linked to elevated inflammation—additional evidence that fleeting experiences are important for health (Sin et al., 2015). Like synchrony, negative emotional responsiveness to the disagreement varied from one visit to the next, and synchrony at one visit did not predict negative emotional reactivity at the other visit, further confirmation that the link was not spurious. However, negative affect did not explain synchrony’s association with inflammation, suggesting that the intermediate pathways extend beyond self-reported emotion. We expected HRV synchrony to reduce HRV (Pavlov and Tracey, 2005), but the very strong correspondence of baseline and conflict HRV ruled out this mechanism as operationalized in the current study. However, conflict did induce overall decreases in HRV on average, suggesting that synchrony occurred in an aroused state. The exact functional forms of these associations over time are not well understood, and any sampling mismatch may have obscured the dynamics.
All participants completed a disagreement task; without a control condition, we can neither generalize to synchrony during other interactions (e.g., imitation, mutual gaze) nor conclude that the pattern only emerges during marital conflict, one limitation. Synchrony in other contexts may have different implications for inflammatory responses and long-term health. Similarly, synchrony over longer time scales, such as cortisol coupling (e.g., Liu et al., 2013), may have different determinants and sequelae. Though we would not predict that higher inflammation drives stronger HRV synchrony between partners during conflict, the findings prevent strong directional conclusions. Because couples may or may not have shared eye contact or conversed during our HRV baseline measurement, we could not calculate synchrony at baseline. Prior work has found consonant results across synchrony indices in different physiological systems (e.g., Gates et al., 2015; Helm et al., 2012; Levenson and Gottman, 1983) with a notable exception (Helm et al., 2014); nevertheless, the association between HRV synchrony during conflict and inflammation must be replicated in other physiological channels.
The current study provides the first evidence of synchrony’s potential relevance for couples’ physical health. It also captured variations in synchrony that tracked with partners’ momentary feelings and suggests HRV linkage during conflict as one possible social-biological pathway to inflammatory health risks.
Supplementary Material
Highlights.
HRV synchrony is the degree to which partners’ HRV fluctuations track together.
Stronger HRV synchrony during marital conflict predicted heightened inflammation.
Stronger HRV synchrony during conflict also predicted greater mood reactivity.
Partners’ synchrony varied from one disagreement to the next.
Acknowledgments
This work was supported in part by NIH grants K99 AG056667, R21 CA158868, UL1TR001070, K05 CA172296, and T32 DE014320; a Pelotonia Postdoctoral Fellowship from Ohio State University’s Comprehensive Cancer Center; and American Cancer Society Postdoctoral Fellowship Grant 121911-PF-12-040-01-CPPB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- Aron A, Aron EN, Smollan D. Inclusion of other in the self scale and the structure of interpersonal closeness. Interpersonal Relations and Group Processes. 1992;63:596–612. [Google Scholar]
- August KJ, Rook KS, Franks MM, Stephens MAP. Spouses ‘involvement in their partners’ diabetes management: Associations with spouse stress and perceived marital quality. Journal of Family Psychology. 2013;27:712–721. doi: 10.1037/a0034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- Butler EA. Interpersonal Affect Dynamics: It Takes Two (and Time) to Tango. Emotion Review. 2015;7:336–341. [Google Scholar]
- Butler EA, Wilhelm FH, Gross JJ. Respiratory sinus arrhythmia, emotion, and emotion regulation during social interaction. Psychophysiology. 2006;43:612–622. doi: 10.1111/j.1469-8986.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chaspari T, Baucom B, Timmons A, Tsiartas A, Borofsky Del Piero L, Baucom K, Georgiou P, Margolin G, Narayanan SS. Proceedings of IEEE International Conference on Audio, Speech and Signal Processing (ICASSP) Brisbane, AU: 2015. Quantifying eda synchrony through joint sparse representation: A case-study of couples’ interactions; pp. 817–821. [Google Scholar]
- De Groote D, Zangerle PF, Gevaert Y, Fassotte MF, Beguin Y, Noizat-Pirenne F, Pirenne J, Gathy R, Lopez M, Dehart I, et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine. 1992;4:239–248. doi: 10.1016/1043-4666(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Donoho CJ, Crimmins EM, Seeman TE. Marital Quality, Gender, and Markers of Inflammation in the MIDUS Cohort. Journal of marriage and the family. 2013;75:127–141. doi: 10.1111/j.1741-3737.2012.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AJ, Reeves JW, Chi C. Dynamic RSA: Examining parasympathetic regulatory dynamics via vector-autoregressive modeling of time-varying RSA and heart period. Psychophysiology. 2016;53:1093–1099. doi: 10.1111/psyp.12644. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Funk JL, Rogge RD. Testing the ruler with item response theory: increasing precision of measurement for relationship satisfaction with the Couples Satisfaction Index. J Fam Psychol. 2007;21:572–583. doi: 10.1037/0893-3200.21.4.572. [DOI] [PubMed] [Google Scholar]
- Gates KM, Gatzke-Kopp LM, Sandsten M, Blandon AY. Estimating time-varying RSA to examine psychophysiological linkage of marital dyads. Psychophysiology. 2015;52:1059–1065. doi: 10.1111/psyp.12428. [DOI] [PubMed] [Google Scholar]
- Golder SA, Macy MW. Diurnal and Seasonal Mood Vary with Work, Sleep, and Daylength Across Diverse Cultures. Science. 2011;333:1878–1881. doi: 10.1126/science.1202775. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Chen S. The role of sleep in interpersonal conflict: Do sleepless nights mean worse fights? Social Psychological and Personality. Science. 2014;5:168–175. [Google Scholar]
- Graham JE, Glaser R, Loving TJ, Malarkey WB, Stowell JR, Kiecolt-Glaser JK. Cognitive Word Use During Marital Conflict and Increases in Proinflammatory Cytokines. Health psychology : official journal of the Division of Health Psychology, American Psychological Association. 2009;28:621–630. doi: 10.1037/a0015208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudmundsson A, Ershler WB, Goodman B, Lent SJ, Barczi S, Carnes M. Serum concentrations of interleukin-6 are increased when sampled through an indwelling venous catheter. Clinical chemistry. 1997;43:2199–2201. [PubMed] [Google Scholar]
- Hansson M, Jönsson P. Estimation of HRV spectrogram using multiple window methods focussing on the high frequency power. Med Eng Phys. 2006;28:749–761. doi: 10.1016/j.medengphy.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Troxel WM. Couples’ nighttime sleep efficiency and concordance: Evidence for bidirectional associations with daytime relationship functioning. Psychosomatic medicine. 2010;72:794–801. doi: 10.1097/PSY.0b013e3181ecd08a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm JL, Sbarra D, Ferrer E. Assessing cross-partner associations in physiological responses via coupled oscillator models. Emotion. 2012;12:748–762. doi: 10.1037/a0025036. [DOI] [PubMed] [Google Scholar]
- Helm JL, Sbarra DA, Ferrer E. Coregulation of respiratory sinus arrhythmia in adult romantic partners. Emotion. 2014;14:522–531. doi: 10.1037/a0035960. [DOI] [PubMed] [Google Scholar]
- Heyman RE. Rapid Marital Interaction Coding System (RMICS) In: Kerig PK, Baucom DH, editors. Couple Observational Coding Systems. Lawrence Erlbaum Associates; Nahwah, New Jersey: 2004. pp. 67–94. [Google Scholar]
- Jaremka LM, Collins NL. Current Closeness to Partner Scale. University of California; Santa Barbara: 2011. [Google Scholar]
- Joseph NT, Kamarck TW, Muldoon MF, Manuck SB. Daily marital interaction quality and carotid artery intima-medial thickness in healthy middle-aged adults. Psychosomatic Medicine. 2014;76:347–354. doi: 10.1097/PSY.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Jaremka L, Andridge R, Peng J, Habash D, Fagundes CP, Glaser R, Malarkey WB, Belury MA. Marital discord, past depression, and metabolic responses to high-fat meals: Interpersonal pathways to obesity. Psychoneuroendocrinology. 2015;52:239–250. doi: 10.1016/j.psyneuen.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Loving TJ, Stowell JR, Malarkey WB, Lemeshow S, Dickinson SL, Glaser R. Hostile marital interactions, proinflammatory cytokine production, and wound healing. Arch. Gen. Psychiatry. 2005;62:1377–1384. doi: 10.1001/archpsyc.62.12.1377. [DOI] [PubMed] [Google Scholar]
- Laborde S, Mosley E, Thayer JF. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research – Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Frontiers in Psychology. 2017;8 doi: 10.3389/fpsyg.2017.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: physiological linkage and affective exchange. J Pers Soc Psychol. 1983;45:587–597. doi: 10.1037//0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Physiological and affective predictors of change in relationship satisfaction. J Pers Soc Psychol. 1985;49:85–94. doi: 10.1037//0022-3514.49.1.85. [DOI] [PubMed] [Google Scholar]
- Liu H, Waite L. Bad Marriage, Broken Heart? Age and Gender Differences in the Link between Marital Quality and Cardiovascular Risks among Older Adults. Journal of health and social behavior. 2014;55:403–423. doi: 10.1177/0022146514556893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Rovine MJ, Klein LC, Almeida DM. Synchrony of Diurnal Cortisol Pattern in Couples. Journal of family psychology : JFP : journal of the Division of Family Psychology of the American Psychological Association (Division 43) 2013;27:579–588. doi: 10.1037/a0033735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Zhou Y, Palumbo R, Wang JL. Dynamical correlation: A new method for quantifying synchrony with multivariate intensive longitudinal data. Psychol Methods. 2016;21:291–308. doi: 10.1037/met0000071. [DOI] [PubMed] [Google Scholar]
- Marco CA, Suls J. Daily stress and the trajectory of mood: spillover, response assimilation, contrast, and chronic negative affectivity. J Pers Soc Psychol. 1993;64:1053–1063. doi: 10.1037//0022-3514.64.6.1053. [DOI] [PubMed] [Google Scholar]
- Marsland AL, Pressman S, Cohen S. Positive Affect and Immune Function. In: Ader R, editor. Psychoneuroimmunology. 4. Academic Press; Burlington: 2007. pp. 761–779. [Google Scholar]
- Marsland AL, Walsh C, Lockwood K, John-Henderson NA. The effects of acute psychological stress on circulating and stimulated inflammatory markers: A systematic review and meta-analysis. Brain Behav Immun. 2017;64:208–219. doi: 10.1016/j.bbi.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsonne G, Lekander M, Åkerstedt T, Axelsson J, Ingre M. Diurnal Variation of Circulating Interleukin-6 in Humans: A Meta-Analysis. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0165799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palumbo RV, Marraccini ME, Weyandt LL, Wilder-Smith O, McGee HA, Liu S, Goodwin MS. Interpersonal Autonomic Physiology: A Systematic Review of the Literature. Pers Soc Psychol Rev. 2017;21:99–141. doi: 10.1177/1088868316628405. [DOI] [PubMed] [Google Scholar]
- Pavlov VA, Tracey KJ. The cholinergic anti-inflammatory pathway. Brain Behav Immun. 2005;19:493–499. doi: 10.1016/j.bbi.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull. 2005;131:925–971. doi: 10.1037/0033-2909.131.6.925. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1 [Google Scholar]
- Reed RG, Randall AK, Post JH, Butler EA. Partner influence and in-phase versus anti-phase physiological linkage in romantic couples. International journal of psychophysiology : official journal of the International Organization of Psychophysiology. 2013;88:309–316. doi: 10.1016/j.ijpsycho.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Robinson SA, Rickenbach EH, Lachman ME. Self-regulatory strategies in daily life. International Journal of Behavioral Development. 2016;40:126–136. doi: 10.1177/0165025415592187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Slatcher RB, Trombello JM, McGinn MM. Marital quality and health: A meta-analytic review. Psychological Bulletin. 2014;140:140–187. doi: 10.1037/a0031859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seider BH, Hirschberger G, Nelson KL, Levenson RW. We can work it out: Age differences in relational pronouns, physiology, and behavior in marital conflict. Psychology and aging. 2009;24:604–613. doi: 10.1037/a0016950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin NL, Graham-Engeland JE, Ong AD, Almeida DM. Affective reactivity to daily stressors is associated with elevated inflammation. Health Psychol. 2015;34:1154–1165. doi: 10.1037/hea0000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder DK, Heyman RE, Haynes SN. Evidence-based approaches to assessing couple distress. Psychol Assess. 2005;17:288–307. doi: 10.1037/1040-3590.17.3.288. [DOI] [PubMed] [Google Scholar]
- Tausczik YR, Pennebaker JW. The Psychological Meaning of Words: LIWC and Computerized Text Analysis Methods. Journal of Language and Social Psychology. 2009;29:24–54. [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Timmons AC, Margolin G, Saxbe DE. Physiological linkage in couples and its implications for individual and interpersonal functioning: A literature review. J Fam Psychol. 2015;29:720–731. doi: 10.1037/fam0000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Sbarra DA. Marital adjustment and interleukin-6 (IL-6) Journal of Family Psychology. 2012;26:290–295. doi: 10.1037/a0026902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SJ, Jaremka LM, Fagundes CP, Andridge R, Peng J, Malarkey WB, Habash D, Belury MA, Kiecolt-Glaser JK. Shortened sleep fuels inflammatory responses to marital conflict: Emotion regulation matters. Psychoneuroendocrinology. 2017;79:74–83. doi: 10.1016/j.psyneuen.2017.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TC. The Role of Vascular Cell Adhesion Molecule-1 in Tumor Immune Evasion. Cancer research. 2007;67:6003–6006. doi: 10.1158/0008-5472.CAN-07-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


