Abstract
Objective
Advanced stage epithelial ovarian cancer (AEOC) can be treated with either neoadjuvant chemotherapy (NACT) or primary cytoreductive surgery (PCS). Although randomized controlled trials show that NACT is non-inferior in overall survival compared to PCS, there may be improvement in short-term morbidity. We sought to investigate the cost-effectiveness of NACT relative to PCS for AEOC from the US Medicare perspective.
Methods
A cost-effectiveness analysis using a Markov model with a 7-month time horizon comparing (1) 3 cycles of NACT with carboplatin and paclitaxel (CT), followed by interval cytoreductive surgery, then 3 additional cycles of CT, or (2) PCS followed by 6 cycles of CT. Input parameters included probability of chemotherapy complications, surgical complications, treatment completion, treatment costs, and utilities. Model outcomes included costs, life-years gained, quality-adjusted life-years (QALYs) gained, and incremental cost-effectiveness ratios (ICER), in terms of cost per life-year gained and cost per QALY gained. We accounted for differences in surgical complexity by incorporating the cost of additional procedures and the probability of undergoing those procedures. Probabilistic sensitivity analysis (PSA) was performed via Monte Carlo simulations.
Results
NACT resulted in a savings of $7,034 per patient with a 0.035 QALY increase compared to PCS; therefore, NACT dominated PCS in the base case analysis. With PSA, NACT was the dominant strategy more than 99% of the time.
Conclusions
In the short-term, NACT is a cost-effective alternative compared to PCS in women with AEOC. These results may translate to longer term cost-effectiveness; however, data from randomized control trials continues to mature.
Introduction
Epithelial ovarian cancer (EOC) remains the most aggressive and lethal of all gynecologic malignancies. In 2016, there were 22,280 new cases of EOC and 14,240 deaths in the United States (US).[1] Due to its insidious nature, most women present with advanced stage epithelial ovarian cancer (AEOC). Patients with AEOC are traditionally treated with primary cytoreductive surgery followed by adjuvant chemotherapy (PCS). The goal of primary surgery is to achieve minimal or no residual tumor burden; this often requires complex surgeries and carries associated surgical morbidity.[2–5] In order to achieve higher rates of optimal cytoreduction, an alternate treatment modality has been explored consisting of neoadjuvant chemotherapy (NACT) followed by interval cytoreductive surgery (ICS) and further adjuvant chemotherapy.
The treatment decision between NACT and PCS remains controversial. Multiple randomized control trials (RCTs) found that NACT was non-inferior to PCS in terms of overall survival. These same RCTs revealed decreased morbidity in patients initiating treatment with NACT; however, the trials have been criticized due to lower than expected median overall survival and lower than expected rates of optimal cytoreduction. [6–9] Based on these data, the Society of Gynecologic Oncology (SGO) and the American Society of Clinical Oncology (ASCO) recently released clinical practice guidelines regarding the management of AEOC.[10] All women with suspected AEOC should undergo evaluation by a gynecologic oncologist prior to initiation of therapy in order to determine if they are candidates for PCS. The recommendations state that women who are not surgical candidates or in whom optimal cytoreduction cannot be achieved should undergo NACT. The recommendations for patients who are fit for PCS with potentially optimally resectable disease are less clear. These women can be offered either NACT or PCS. Though the impact of choosing either treatment modality remains unclear in regards to overall survival, NACT may be less costly, since it results in less extensive surgery. Moreover, NACT may result in improved short term morbidity, but caution must prevail until long term survival data from remaining RCTs matures.
The proportion of spending on health care, relative to GDP, is projected to continue to increase within the next decade.[11] Therefore, the rising cost of health care delivery will likely influence US policy makers and insurers making decisions about reimbursement; in turn, changes to cancer-specific healthcare reimbursement will impact the decision-making processes for physicians. While survival and quality of life (QOL) are of utmost importance, the cost-effectiveness of either treatment modality will become a significant factor in the decision-making process. Indeed, ASCO and other national organizations have embraced the concept of value-based clinical decision making by endorsing the creation of value-based oncology pathways to guide treatment decisions.[12] In order to better inform this discussion in the setting of AEOC, we performed a cost-effectiveness analysis using a Markov model that was populated with data from the four RCTs cited in the SGO/ASCO guidelines, and a prospective study on the use of NACT compared to PCS in National Cancer Institute-designated cancer centers.[6–9,13] Our objective was to evaluate the cost-utility implications of treatment with NACT compared to treatment with PCS for patients with AEOC.
Materials and Methods
Overview
We created a Markov model to compare the immediate health and economic impacts of two primary treatment modalities for advanced stage epithelial ovarian cancer: PCS and NACT. Treatment strategies were compared in a hypothetical cohort of US patients with AEOC – defined in our model as stage III or stage IV disease. Costs were applied using a US Medicare perspective, and outcomes of interest include average cost per patient, life-years, and quality-adjusted life-years (QALYs). Both strategies were compared by estimating their incremental cost-effectiveness ratio (ICER), which is defined as the ratio of the incremental cost to the incremental effect (in terms of life-years and QALYs gained) of NACT compared to PCS. In comparing the cost-effectiveness of either strategy, a “dominant” strategy means that an intervention costs less and is more effective than the comparator; a “dominated” strategy is one that is costlier and less effective than the comparator.
Model structure
The Markov model was constructed using Microsoft® Excel® 2016 for Windows®. Its structure (see figure 1) was informed by the RCTs included in the SGO and ASCO practice guidelines published in 2016.[6–10] For every set of chemotherapy cycles (3 for NACT – before and after ICS — and 6 for PCS), patients could experience grade 3 or 4 chemotherapy-related adverse events (AE) (defined by the Common Terminology Criteria for Adverse Events [CTCAE] v 4.0), or die. If patients experienced AEs from either surgery or chemotherapy, they could either recover or die from the event. Patients who underwent NACT received an initial 3 cycles of chemotherapy. Survivors then underwent ICS, and could experience grade 3 or 4 surgical AE (as defined within the RCTs), or die. Three additional rounds of chemotherapy were administered.
Figure 1.
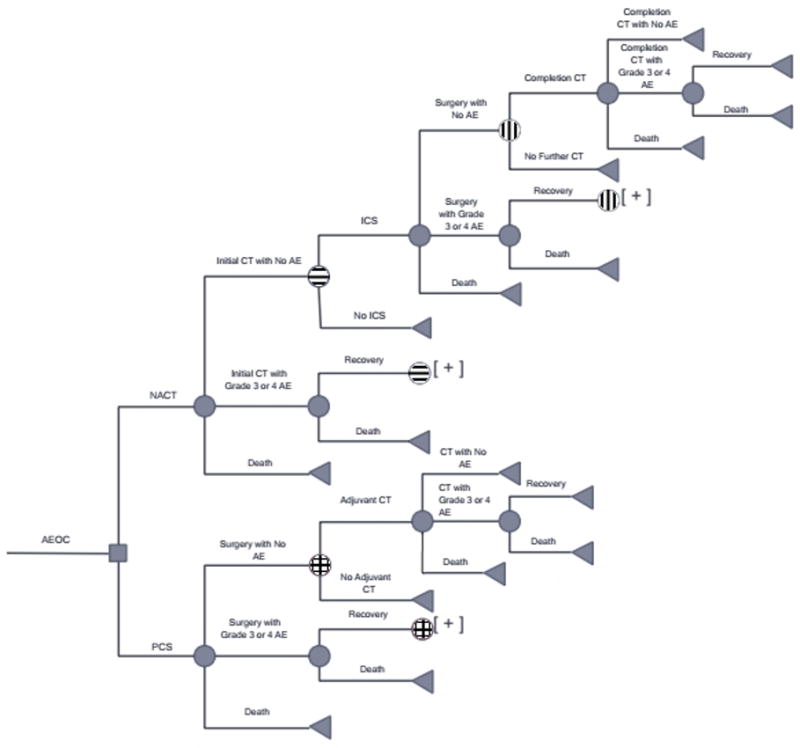
Markov model of patients undergoing neoadjuvant chemotherapy compared to primary cytoreductive surgery. Abbreviations: AEOC: advanced stage epithelial ovarian cancer, NACT: neoadjuvant chemotherapy, PCS: primary cytoreductive surgery, CT: chemotherapy, AE: adverse event
Patients undergoing PCS could experience grade 3 or 4 AE (as defined within the RCTs), or die. Survivors then received six rounds of chemotherapy.
The target population
We used a hypothetical cohort of 16,500 women with AEOC in the US, aged 65 years, 162.1 cm tall, weighing 75.39 kg, and with a serum creatinine concentration of 0.7mg/dL. Our cohort size was calculated from the 2016 incidence of ovarian cancer (roughly 22,000 women) and the knowledge that approximately 75 percent of women currently living with ovarian carcinoma present with AEOC.[1] The clinical parameters used reflect median measures for US women, and were used to calculate doses of chemotherapy.[14] Carboplatin is administered with a target area under the curve (AUC) of 6 using the Calvert formula and estimated GFR from the Cockcroft-Gault formula, while paclitaxel is administered at 175mg/m2 of body surface area.[6–9,15]
Model inputs
Input probabilities (see table 1) were informed by four RCTs (EORTC 55971, CHORUS, SCORPION, and JCOG0602)[6–9] and a multi-institutional observational study from women treated at six National Cancer Institute-designated cancer centers.[13] Some of these input probabilities include the probability of patients having serious AE (grade 3 or 4) from surgery or chemotherapy, the probability of recovering from those AE, and the probability of an additional surgical procedure (e.g. small bowel resection, large bowel resection, or upper abdominal surgery). We normalized these probabilities (and their respective measures of uncertainty) by aggregating data from these five studies in a random-effects meta-analytic model, to generate more representative estimates that better accommodate heterogeneity across the studies (see supplemental digital content).
Table 1.
Base case probabilities, minimum and maximum values, for all events within the model.
| Probabilities of Clinical Parameters | Base Case | Min | Max |
|---|---|---|---|
| PCS | |||
| PCS with No AE*,†, ‡, §, ‖ | 0.716 | 0.64 | 0.792 |
| PCS with Grade 3 or 4 AE*,†, ‡, §, ‖ | 0.262 | 0.199 | 0.326 |
| Recovery from a Surgical Grade 3 or 4 AE*,†, ‡, §, ‖ | 0.995 | 0.988 | 1 |
| Death from a Surgical Grade 3 or 4 AE*,†, ‡, §, ‖ | 0.005 | 0 | 0.012 |
| Death from Surgery*,†, ‡, §, ‖ | 0.022 | 0.007 | 0.037 |
| Surgery to Adjuvant Chemotherapy*,†, ‡, §, ‖ | 0.925 | 0.879 | 0.97 |
| Surgery without Adjuvant Chemotherapy*,†, ‡, §, ‖ | 0.075 | 0.03 | 0.121 |
| Chemotherapy with No AE*, ‡, ‖ | 0.592 | 0.448 | 0.736 |
| Chemotherapy with Grade 3 or 4 AE*, ‡, ‖ | 0.398 | 0.256 | 0.539 |
| Recovery from a Chemotherapy Grade 3 or 4 AE*, ‡, ‖ | 0.994 | 0.982 | 1 |
| Death from a Chemotherapy Grade 3 or 4 AE*, ‡, ‖ | 0.006 | 0 | 0.018 |
| Death From Chemotherapy*, ‡, ‖ | 0.01 | 0 | 0.02 |
| NACT | |||
| NACT with No AE*, ‡, ‖ | 0.793 | 0.756 | 0.83 |
| NACT with Grade 3 or 4 AE*, ‡, ‖ | 0.202 | 0.165 | 0.239 |
| Recovery from a NACT Grade 3 or 4 AE*, ‡, ‖ | 0.988 | 0.965 | 1 |
| Death from NACT Grade 3 or 4 AE*, ‡, ‖ | 0.012 | 0 | 0.035 |
| Death from NACT*, ‡, ‖ | 0.005 | 0 | 0.011 |
| From NACT to ICS*,†, ‡, §, ‖ | 0.884 | 0.811 | 0.958 |
| No ICS after NACT*,†, ‡, §, ‖ | 0.116 | 0.042 | 0.189 |
| ICS with No AE*,†, ‡, §, ‖ | 0.924 | 0.894 | 0.954 |
| ICS with Grade 3 or 4 AE*,†, ‡, §, ‖ | 0.069 | 0.038 | 0.1 |
| Recovery from ICS Grade 3 or 4 AE*,†, ‡, §, ‖ | 0.976 | 0.942 | 1 |
| Death from ICS Grade 3 or 4 AE*,†, ‡, §, ‖ | 0.024 | 0 | 0.058 |
| Death from ICS*,†, ‡, §, ‖ | 0.007 | 0.002 | 0.012 |
| Completion Chemotherapy after ICS*,†, ‡, ‖ | 0.957 | 0.928 | 0.986 |
| No Further Treatment after ICS*, ‡, ‖ | 0.043 | 0.014 | 0.072 |
| Completion Chemotherapy with No AE*, ‡, ‖ | 0.833 | 0.777 | 0.89 |
| Completion Chemotherapy with Grade 3 or 4 AE*, ‡, ‖ | 0.164 | 0.107 | 0.22 |
| Recovery from Completion Chemotherapy Grade 3 or 4 AE*, ‡, ‖ | 0.987 | 0.962 | 1 |
| Death from Completion Chemotherapy Grade 3 or 4 AE*, ‡, ‖ | 0.013 | 0 | 0.038 |
| Death from Completion Chemotherapy*, ‡, ‖ | 0.003 | 0 | 0.007 |
Aggregate probabilities were taken from a random-effects meta-analysis of the proportions of events within
CHORUS;
Meyer, et al.;
JCOG0602;
EORTC 55971;
SCORPION.[6–9,13] Min and max values represent the 95% confidence intervals from the aggregate probabilities of the random-effects meta-analytic model.
Abbreviations: PCS: primary cytoreductive surgery, AE: adverse event, NACT: neoadjuvant chemotherapy, ICS: interval cytoreductive surgery.
In keeping with best practices, we used methods that have been previously described to generate our cost inputs (see table 2).[16,17] Hospitalization costs were obtained from the Agency for Healthcare Research and Quality’s (AHRQ) Healthcare Cost and Utilization Project (HCUP) data for inpatient stays in 2013. We used Diagnosis Related Group (DRG) codes 736 and 738 to assess costs of surgical procedures for ovarian cancer patients with and without a major complication, respectively. We accounted for providers’ fees using 2015 national figures from the Centers for Medicare and Medicaid Services (CMS) Physician Fee Schedule. We used Current Procedural Terminology (CPT) code 58956 for debulking ovarian cancer (as a proxy for NACT ICS), and CPT code 58953 for radical debulking surgery (as a proxy for PCS). We also accounted for costs of add-on surgical procedures: we used CPT codes 44120 (for small bowel resection), 44140 (for large bowel resection), 45111 (for rectal resection), and 38102 for splenectomy (as a proxy for upper abdominal procedures). We used the risks of these add-on procedures to estimate weighted-average composite cost inputs for PCS and ICS (see table 3).
Table 2.
Cost parameters (in 2015 US dollars) for events within model.
| Costs | ||||
|---|---|---|---|---|
| Base Case | Min | Max | Source | |
| Surgery without AE | $12,952 | $12,522 | $13,381 | HCUP* |
| Surgery with Grade 3 or 4 AE | $39,651 | $37,701 | $41,601 | HCUP* |
| Admission for Grade 3 or 4 Chemotherapy-related AE | $9,089 | $8,776 | $9,402 | HCUP* |
| Total Surgical Procedure Cost | ||||
| PCS | $2,829 | $2,622 | $3,036 | CMS † |
| NACT | $1,709 | $1,583 | $1,834 | CMS † |
| Base Surgery Cost | ||||
| PCS | $2,199 | $2,040 | $2,358 | CMS† |
| NACT | $1,501 | $1,392 | $1,611 | CMS † |
| Chemotherapy Administration‡ | ||||
| Infusion Costs Per Cycle | $221 | CMS † | ||
| Chemotherapy Cost Per Cycle | $112 | CMS † | ||
| Total Chemotherapy Cost Per 3 Cycles | $999 | $899 | $1,099 | CMS † |
Data for inpatient stays in 2013;
Medicare reimbursement database maintained by the American Medical Association;
Chemotherapy administration costs do not include supportive care medications (e.g. steroids, diphenhydramine, antiemetics).
Abbreviations: AE: adverse event; PCS: primary cytoreductive surgery; NACT: neoadjuvant chemotherapy; HCUP: Agency for Healthcare Quality and Research’s Healthcare Cost and Utilization Project; CMS: Centers for Medicare and Medicaid Services.
Table 3.
Estimated rates of additional procedures during cytoreductive surgery – either primary or interval cytoreduction.
| Rates of Additional Procedure | ||||||||
|---|---|---|---|---|---|---|---|---|
| Procedure | PCS Base Case | Min | Max | ICS Base Case | Min | Max | Cost* | Source |
| Small bowel resection †, ‡, §, ‖ | 0.141 | 0.067 | 0.215 | 0.041 | 0.006 | 0.077 | $1358 | CMS¶ |
| Large bowel resection †, ‡, §, ‖ | 0.218 | 0.104 | 0.331 | 0.071 | 0.005 | 0.138 | $1491 | CMS¶ |
| Upper abdominal surgery (splenectomy) †, §, ‖ | 0.391 | 0 | 0.978 | 0.158 | 0.051 | 0.265 | $288 | CMS¶ |
In 2015 US dollars. Rates estimated from aggregate data from
EORTC 55971,
CHORUS,
SCORPION, and
Medicare reimbursement database maintained by the American Medical Association.
Abbreviations: PCS: primary cytoreductive surgery; ICS: interval cytoreductive surgery; CMS: Centers for Medicare and Medicaid Services.
Chemotherapy costs were informed by CMS Physician Fee Schedule (using CPT code 96413 and 96415 to account for four total hours of chemotherapy infusion), and CMS Medicare Part B drug average sales prices for carboplatin and paclitaxel. Cost of care for patients with adverse reactions to chemotherapy were obtained from HCUP using the International Classification of Diseases, ninth revision (ICD-9) codes (infection – septicemia 038.9; metabolic – hypomagnesemia 275.2 and hypokalemia 276.8; thrombocytopenia 287.5; neurologic – neuropathy due to drugs 357.6; gastrointestinal – nausea and vomiting 787.01 and intestinal obstruction 560.9; genitourinary – renal failure 586.0; fever 780.6; pain – unspecified abdominal pain 789.0; and hematologic – neutropenia 288.00 and anemia 285.3). All cost estimates were calculated in 2015 US dollars; those data from earlier years were adjusted for inflation using the appropriate Consumer Prices Indices Medical Care Component factor from the Bureau of Labor Statistics (BLS) website.[18] We then used the mean value of all the costs obtained from our ICD-9 codes. This calculated mean value was the value used to estimate the adverse reactions to chemotherapy in our model.
Utility inputs were obtained from previous studies (see table 4).[19,20] In order to account for the 7-month time horizon, utilities were multiplied by the number of months spent in each health state in order to determine the quality-adjusted life-months (ex. 1 month for patients undergoing surgery in either the NACT or PCS arm). To ease comparability with commonly used willingness-to-pay thresholds, quality-adjusted life-months were converted to QALYs prior to generating ICERs.[21]
Table 4.
Utility weight estimates.
| Utility Weights | Base Case |
|---|---|
| Before Treatment | 0.72 |
| Neoadjuvant Chemotherapy Start | 0.67 |
| Neoadjuvant ICS Surgery | 0.74 |
| Neoadjuvant Chemotherapy End | 0.74 |
| PCS Surgery | 0.67 |
| PCS Chemotherapy | 0.67 |
| Chemotherapy to G3/G4 event | 0.5 |
| No Further Therapy | 0.5 |
Assumptions and Uncertainty Analyses
All patients were assumed to have been evaluated by a gynecologic oncologist. For ease of comparison, we assumed that surgery and its associated recovery required one month, and that each chemotherapy cycle (of which there are 6) also lasts for one month, giving a 7-month analytic time horizon. We specified uncertainty in utility inputs by a standard error of 0.05, as stated in the previous studies.[19,20] Based on CTCAE v 4.0, we assumed that a hospital stay would be required for all grade 3 or 4 AE related to chemotherapy. Grade 3 and 4 surgical AE were considered major complications when analyzing cost parameters. All patients who recovered from a grade 3 or 4 AE (either surgical or chemotherapy related) were assumed to proceed through the model without changes in chemotherapy dosing or surgical planning.
To gain greater insight into the impact of uncertainty in our model, we chose to conduct probabilistic sensitivity analyses (PSA), where we varied all input parameters within specified bounds of uncertainty (95% confidence intervals from the aggregate probabilities of the random-effects meta-analytic model where available, or other assumed bounds specified above). PSA was performed with Monte Carlo simulation for 10,000 trials. Similar PSAs were performed with the removal of non-randomized data and with a 3-week chemotherapy cycle instead of 4.
Results
Base Case
Using base case probabilities and costs, NACT cost $20,762 per patient, whereas PCS cost $27,796 per patient. Thus NACT saved $7,034 per patient over the 7-month time horizon compared to PCS. Additionally, our model estimated that the NACT alternative was associated with 0.973 life-years per patient versus 0.892 life-years per patient in the PCS arm. When comparing NACT to PCS in our model, we find a difference of 0.081 life-years per person in favor of NACT. Additionally, hypothetical individuals in the NACT alternative had 0.383 QALYs, which was 0.035 QALYs higher than the PCS arm (0.348 QALYs). Thus, when using our cost savings and differences in QALY to calculate our ICER, we find that the NACT alternative dominated the PCS alternative.
Sensitivity Analyses
Results from our PSA are presented as an ICER scatterplot (see figure 2). We find that 99.4% of our results from our PSA fall in the lower right quadrant (i.e. indicating a “dominant” treatment strategy). The remainder were located in the lower left quadrant and below the $50,000 per QALY and $100,000 per QALY willingness-to-pay thresholds. The results were similar with the removal of non-randomized data and when varying chemotherapy cycles from 4 to 3 weeks.
Figure 2.
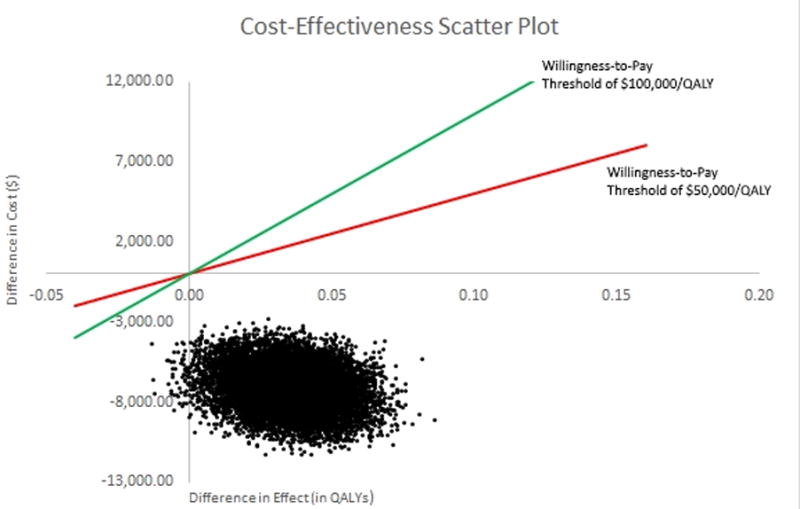
Cost-effectiveness scatter plot. Comparison between primary cytoreductive surgery and neoadjuvant chemotherapy for treating women with advanced stage epithelial ovarian carcinoma.
Discussion
AEOC remains a highly fatal and aggressive disease. When evaluating treatment options for women with AEOC, the balance of survival and quality of life is of utmost importance. However, health care spending has reached roughly 18% of the gross domestic product and estimates suggest a continual increase in health care expenditure; thus, consideration for overall cost-effective care becomes increasingly important.[11,22]
The RCT data thus far reflect that NACT is non-inferior to PCS, this conclusion remains a point of contention. NACT usage has continued to increase, but the utilization of NACT remains much lower in comparison to PCS.[23–26] The clinical trials which have survival data available have been largely criticized for low optimal debulking rates.[7,9] Trials with higher rates of optimal debulking rates have yet to mature in regards to overall survival and non-inferiority data.[6,8]
We sought to contribute to the conversation on deciding between NACT and PCS when treating patients with AEOC using a cost-effectiveness approach. We built a Markov model with a structure that accommodates health states associated with either treatment strategies, and integrated inputs from multiple sources, including the four studies that informed the SGO and ASCO practice guidelines. Our findings suggest that when compared to PCS, NACT is the dominant treatment strategy within our 7-month time horizon.
To our knowledge, this is the first cost effectiveness study for NACT in ovarian cancer that takes into account both surgical AEs and chemotherapy-related AEs. Furthermore, this is the only model that takes into account all RCT data utilized by the SGO and ASCO practice guidelines. Previous data either only presented surgical complications [16] or relied on Surveillance, Epidemiology and End Results (SEER)-Medicare data to examine costs without presenting a probabilistic decision model.[27,28] Rowland, et al. examined a longer time horizon, did not utilize chemotherapy related AEs, and incorporated data from EORTC 55971. Their results suggest that at a willingness-to-pay threshold of $100,000 per QALY, NACT was more cost-effective 60% of the time in women older than 65 with AEOC.[16] Forde, et al. were able to show that NACT was only more cost-effective for women with stage IV AEOC; however, they assumed that PCS offered a survival benefit to NACT. This assumption has not been reflected in any RCT data. Moreover, their model is only informed by the SEER-Medicare database and National Cancer Institute data for survival estimates.[27] Furthermore, their cohort spans a timeframe in which chemotherapy used for AEOC changed to include platinum based chemotherapy; thus, the standard of treatment of the disease drastically changed during their evaluation period.[29,30] Similarly, Poonawalla, et al. used SEER-Medicare database and showed that for patients who were deemed “high risk” (e.g. older age, higher stage disease, or more co-morbidities), NACT was cost-effective compared to PCS.[28] Unfortunately, no decision analysis model was utilized to calculate cost-effectiveness. Therefore, we feel that our model is more applicable in regards to the real-world administration of NACT for women with AEOC.
One of the strengths of our study is that we chose to improve the precision and robustness of our aggregated parameter input estimates by combining data from four RCTs (viz. EORTC55971, CHORUS, JCOG0602, and SCORPION) and an observational study that was done across six National Cancer Institute-designated cancer centers (Meyer, et al).[6–9,13] Although the latter is prone to selection bias, authors used propensity-score matching to minimize the effect of treatment selection on their effect estimates. The addition of non-randomized data does introduce bias compared to using solely RCT data; however, we felt that the real-world evidence on NACT compared to PCS, specifically in the US, would improve the robustness of the clinical variables incorporated into our model. Though there may be heterogeneity in all the studies used, aggregating the data in a random-effects meta-analytic model accommodates for these differences. Further, our findings were robust to exclusion of the observational study data from the aggregated estimate. In other words, the results when we excluded the non-randomized data are similar to when these data are included. Due to the non-inferiority found in the RCTs, no single characteristic predicts outcomes for NACT nor PCS. Thus, regardless of first or second order uncertainty, our model would yield similar results.
In addition, we utilized a short time horizon with the understanding that available data to date reveal no differences in survival between NACT and PCS. Thus, we felt the short-term morbidity represented the most-important consideration for analysis. In order to allow comparability across other cost-effectiveness studies, we converted quality-adjusted life-months to QALYs prior to calculation of ICERs, but it is important to note that our QALY estimates are driven by the available data and not directly extendable beyond our 7-month time horizon.
Limitations to our study include the unequally presented QOL data within the RCTs. Therefore, we relied on previously published literature on utility weights.[19,20] One study explored chemotherapy related toxicities, but did not include all the grade 3 or 4 AE found in our model.[19] Their utility estimated included fatigue, but our model did not. The missing variable would underestimate the changes in QALYs found in our results; however, the incidence is rare, ranging from 1% to 10%. Thus, the intervals in our PSA would account for this variability. The other study examined NACT in an alternative setting (allowing for initial exploratory laparotomy in patients undergoing NACT), but we felt those utility weights were similar enough to include in our model.[20] Although utility weights were varied in our sensitivity analysis to account for potential differences, they may not reflect all real-world experiences of patients with AEOC.
Moreover, HCUP data is a commonly used to estimate costs[16]; however, they serve merely as proxies. The CPT codes associated in our study for ICS and PCS were not designed to differentiate between patients undergoing NACT nor patients undergoing PCS. HCUP data cannot to differentiate stage or histology when construction cost estimates; however, our costs estimates are similar to another studying using HCUP to explore NACT.[16] In order to investigate if changes in our costs estimates for surgery would largely change our findings, we decided to make the base case cost and cost range equal for PCS and ICS in our PSA. Even when these costs were kept equal, our original findings remained robust. When costs were made equal, NACT dominated PCS in the base case. In addition, NACT was over 99% cost-effective at willingness-to-pay thresholds of $50,000 per QALY and $100,000 per QALY.
We acknowledge that within current practice patterns, all patients with grade 3 or 4 chemotherapy AE may not require hospital admission; however, this is the strict definition presented in both the CTCAE and the only data we have based on RCTs. We focused specifically on thrombocytopenia and neuropathy; both these AE are unlikely to be admitted outside of RCTs. These AE only account for 8% of all AE reported in the RCTs used to inform our data; thus, the percentage added to overall cost is likely to be small. We removed the cost of thrombocytopenia and neuropathy from our overall cost of chemotherapeutic AEs. The new cost range still fell within the range performed in our PSA. Thus, the findings are robust even with the removal of costs from neuropathy and thrombocytopenia.
We also acknowledge that our model may be missing some nuances in the diagnosis and treatment of AEOC. Some patients may undergo a diagnostic laparoscopy or a computed tomography (CT) guided biopsy prior to initiation of NACT. Additionally, patients in either arm may receive a CT scan of their abdomen and pelvis prior to treatment or prior to ICS. Thus, we ran an iteration of our model where women receive a CT scan at the time of diagnosis and women in the NACT arm underwent a CT scan prior to ICS. Women undergoing NACT also underwent CT biopsy or diagnostic laparoscopy prior to initiation of chemotherapy. Our results remain robust even with these additional costs and changes in QOL. Base case analysis shows that NACT remains dominant over PCS; PSA shows that NACT is more cost-effective in over 99% of iterations at willingness-to-pay thresholds of $50,000 per QALY or $100,000 per QALY.
As reflected in the RCTs, a subset of patients from the model in the NACT arm fail to undergo ICS after initial chemotherapy. These patients represent a small proportion of patients that have a poor prognosis. ICS may be excluded for a multitude of reasons (e.g. progression of disease); however, treatment is individualized and difficult to account for in our model. Likewise, a similar subset of patients in the PCS arm experience a similarly poor prognosis; however, the outcomes of both groups are beyond the time-horizon of our model.
Using data from RCTs offers a unique set of biases. The women enrolled into RCTs for NACT may have been patients who are more chronically ill. Moreover, women not enrolled may have been felt to be better surgical candidates or have disease that may be more likely to be optimally cytoreduced. Although results from RCTs may not be generalizable, the data from RCTs offers the least bias to inform a cost-effective model.
The decision to treat patients with AEOC should be made in conjunction with a patient’s preferences; however, the data surrounding NACT and PCS remain controversial and complex. As more data mature from RCTs, we will gain additional insight regarding the outcomes of both NACT and PCS. Survival data, QOL metrics, and short term morbidity will all help inform future models of cost-effectiveness. Given the available literature, our robust model suggests that NACT is the dominant approach to treatment of AEOC. Once more data matures from RCTs regarding long-term survival, we hope to construct cost-effective models that can account for possible differences in survival and QOL. It is imperative that more of these models be constructed in order to compare outcomes of both treatment modalities and reduce health care spending.
Supplementary Material
Footnotes
DISCLOSURE STATEMENT: The authors report no conflict of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Van der Burg ME, Van Lent M, Buyse M, Kobierska A, Colombo N, Favalli G, Lacave AJ, Nardi M, Renard J, Pecorelli S. The Effect of Debulking Surgery After Induction Chemotherapy on the Prognosis in Advanced Epithelial Ovarian Cancer. N Engl J Med. 1995;332(10):629–634. doi: 10.1056/NEJM199503093321002. [DOI] [PubMed] [Google Scholar]
- 3.Vergote I, van Gorp T, Amant F, Leunen K, Neven P, Berteloot P. Timing of debulking surgery in advanced ovarian cancer. Int J Gynecol Cancer. 2008;18(Suppl 1):11–9. doi: 10.1111/j.1525-1438.2007.01098.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Greasman WT, Berman M, Ball H, Berek JS. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. Am J Obstet Gynecol. 1994;170(4) doi: 10.1016/s0002-9378(94)70090-7. 974-9-80. [DOI] [PubMed] [Google Scholar]
- 5.Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence-free interval and survival in small-volume stage III epithelial ovarian cancer: A gynecologic oncology group study. Gynecol Oncol. 1992;47(2):159–66. doi: 10.1016/0090-8258(92)90100-w. [DOI] [PubMed] [Google Scholar]
- 6.Fagotti A, Ferrandina G, Vizzielli G, Fanfani F, Gallotta V, Chiantera V, Costantini B, Margariti PA, Alletti SG, Cosentino F, Tortorella L. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. Eur J Cancer. 2016;59:22–33. doi: 10.1016/j.ejca.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M, Dobbs S. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): An open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386(9990):249–57. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 8.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, Wakabayashi M, Takehara K, Saito M, Ushijima K, Kobayashi H. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Gr. Eur J Cancer. 2016;64:22–31. doi: 10.1016/j.ejca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, Van Der Burg ME, Lacave AJ, Panici PB, Kenter GG. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943–53. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 10.Wright AA, Bohlke K, Armstrong DK, Bookman A, Cliby WA, Coleman RL, Dizon DS, Kash JJ, Meyer LA, Moore KN, Olawaiye AB. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer : Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(28):3460–73. doi: 10.1200/JCO.2016.68.6907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keehan SP, Cuckler GA, Sisko AM, Madison AJ, Smith SD, Stone DA, Poisal JA, Wolfe CJ, Lizonitz JM. National Health Expenditure Projections, 2014-24: Spending Growth Faster Than Recent Trends. Health Aff. 2015;34(8):1407–17. doi: 10.1377/hlthaff.2015.0600. [DOI] [PubMed] [Google Scholar]
- 12.Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, Dicker AP, Ganz PA, Hoverman JR, Langdon R, Lyman GH. American Society of Clinical Oncology statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015;33(23):2563–77. doi: 10.1200/JCO.2015.61.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer LA, Cronin AM, Sun CC, Bixel K, Bookman MA, Cristea MC, Griggs JJ, Levenback CF, Burger RA, Mantia-Smaldone G, Matulonis UA. Use and Effectiveness of Neoadjuvant Chemotherapy for Treatment of Ovarian Cancer. J Clin Oncol. 2016;32(32):3854–3863. doi: 10.1200/JCO.2016.68.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fryar CD, Gu Q, Ogden CL. Anthropometric reference data for children and adults: United States, 2007-2010. Vital Health Stat. 2012;11(252):1–48. [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network: Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer (version 1.2017) 2017 update. Available at: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf. Retrieved June 1, 2017.
- 16.Rowland MR, Lesnock JL, Farris C, Kelley JL, Krivak TC. Cost-utility comparison of neoadjuvant chemotherapy versus primary debulking surgery for treatment of advanced-stage ovarian cancer in patients 65 years old or older. Am J Obstet Gynecol. 2015;212(6):763-e1. doi: 10.1016/j.ajog.2015.01.053. [DOI] [PubMed] [Google Scholar]
- 17.Havrilesky LJ, Alvarez Secord A, Darcy KM, Darcy KM, Armstrong DK, Kulasingam S. Cost effectiveness of intraperitoneal compared with intravenous chemotherapy for women with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2008;26(25):4144–4150. doi: 10.1200/JCO.2007.13.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bureau of Labor Statistics: Consumer Price Index Detailed Report, 2016 update. https://www.bls.gov/cpi/cpid1512.pdf. Retreived June 1, 2017.
- 19.Havrilesky LJ, Broadwater G, Davis DM, Nolte KC, Barnett JC, Myers ER, Kulasingam S. Determination of quality of life-related utilities for health states relevant to ovarian cancer diagnosis and treatment. Gynecol Oncol. 2009;113(2):216–20. doi: 10.1016/j.ygyno.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoyer T, Bekkers R, Gooszen H, Massuger L, Rovers M, Grutters JP. Cost-effectiveness of early-initiated treatment for advanced-stage epithelial ovarian cancer patients: a modeling study. Int J Gynecol Cancer. 2014;24(1):75–84. doi: 10.1097/IGC.0000000000000025. [DOI] [PubMed] [Google Scholar]
- 21.Erim DO, Mahendraratnam N, Okafor PN, Wheeler SB. The value of vedolizumab as rescue therapy in moderate-severe Crohn’s disease patients with adalimumab non-response in the USA. J Crohns Colitis. 2015;9(8):669–75. doi: 10.1093/ecco-jcc/jjv090. [DOI] [PubMed] [Google Scholar]
- 22.Martin AB, Hartman M, Washington B, Catlin A. National Health Spending: Faster Growth In 2015 As Coverage Expands And Utilization Increases. Health Aff. 2016;36(1):1–11. doi: 10.1377/hlthaff.2016.1330. [DOI] [PubMed] [Google Scholar]
- 23.Dewdney SB, Rimel BJ, Reinhart AJ, Kizner NT, Brooks RA, Massad LS, Zighelboim I. The role of neoadjuvant chemotherapy in the management of patients with advanced stage ovarian cancer: survey results from members of the Society of Gynecologic Oncologists. Gynecol Oncol. 2010;119(1):18–21. doi: 10.1016/j.ygyno.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Mueller JJ, Zhou QC, Iasonos A, O’Cearbhaill RE, Alvi FA, El Haraki A, Eriksson AG, Gardner GJ, Sonoda Y, Levine DA, Aghajanian C. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecol Oncol. 2016;140(3):436–42. doi: 10.1016/j.ygyno.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauh-Hain JA, Melamed A, Wright A, Gockley A, Clemmer JT, Schorge JO, Del Carmen MG, Keating NL. Overall Survival Following Neoadjuvant Chemotherapy vs Primary Cytoreductive Surgery in Women With Epithelial Ovarian Cancer: Analysis of the National Cancer Database. JAMA Oncol. 2017;3(1):76–82. doi: 10.1001/jamaoncol.2016.4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright JD, Ananth CV, Tsui J, Glied SA, Burke WM, Lu YS, Neugut AI, Herzog TJ, Hershman DL. Comparative effectiveness of upfront treatment strategies in elderly women with ovarian cancer. Cancer. 2014;120(8):1246–54. doi: 10.1002/cncr.28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forde GK, Chang J, Ziogas A. Cost effectiveness of primary debulking surgery when compared to neoadjuvant chemotherapy in the management of stages IIIC and IV epithelial ovarian cancer. ClincoEconomics Outcomes. 2016;8:397–406. doi: 10.2147/CEOR.S91844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poonawalla IB, Lairson DR, Chan W, Piller LB, Du XL. Cost-Effectiveness of Neoadjuvant Chemotherapy versus Primary Surgery in Elderly Patients with Advanced Ovarian Cancer. Value Health. 2015;18(4):387–95. doi: 10.1016/j.jval.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 29.McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients With Stage III and Stage IV Ovarian Cancer. N Engl J Med. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 30.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, Mannel RS, DeGeest K, Hartenbach EM, Baergen R. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2003;21(17):3194–200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


