Abstract
A concise synthesis of protected 5-cyano-L-tryptophan (Trp5CN) has been developed for 2D IR spectroscopic investigations within peptides or proteins. To assess the potential of differently substituted cyano-tryptophans, several model cyanoindole systems were characterized using IR spectroscopy. Upon assessment of their spectroscopic properties, Trp5CN was integrated into a model peptide sequence, Trp5CN–Gly–Phe4CN, to elucidate its structure. This peptide demonstrates the capability of this probe, Trp5CN and Phe4CN, to capture structural information via 2D IR spectroscopy. The 2D IR spectrum of the peptide in water was simulated to reveal a unique spectral signature resulting from the presence of dipolar coupling. The coupling strength between cyano-labels was determined to be 1.4 cm−1 by matching the slopes along the max contour between the simulated and experimental spectrum. Using transition dipole coupling, a distance between the two probes of 13 Å was calculated.
Keywords: Cyano-Tryptophan, 2D IR, Vibrational Coupling, Spectroscopic Reporters, Probe Pairs
COMMUNICATION
A reliable synthesis of protected 5-cyano-L-tryptophan has been developed for two-dimensional infrared (2D IR) spectroscopic investigations within a model peptide, Trp5CN–Gly–Phe4CN. In this short peptide, vibrational coupling between a cyano-labeled probe pair was captured. The coupling strength between cyano-labels was determined to be 1.4 cm−1 by matching the slopes along the max contour between the simulated and experimental spectrum. Using transition dipole coupling, a distance between the two probes of 13 Å was calculated.

Introduction
Many interesting questions in structural dynamics of peptides and proteins can be addressed by incorporation of unnatural amino acid-based vibrational probes. The structure evolution, even when it is ultrafast, can be followed by infrared spectroscopy or two-dimensional infrared (2D IR) spectroscopy. Several non-native amino acid side chain probes, such as azido-, cyano-, thiocyanate, selenocyanate and isonitrile have been developed to monitor hydration, changes in electric field strength, chemical exchange, drug binding and drug-enzyme interactions.[1] For example, Herschlag et al. utilized the SCN probe to capture the local environment changes in the active site of the bacterial enzyme ketosteroid isomerase.[1d] For studying folded and unfolded states of protein backbones, the N3 asymmetric stretching region of azidohomoalanine shows a significant spectral shift upon folding.[2]
Although all of the aforementioned vibrational probes offer varying capabilities of measuring structure and dynamics, they have certain limitations that require the development of infrared probes that mitigate these issues. In particular, the sensitivity of the cyano-stretching frequency as a local environment reporter has been established through the use of 4-cyano-phenylalanine (Phe4CN) in both infrared and two-dimensional infrared studies. The CN vibrational transition is located in an isolated region, ranging from 2200 to 2300 cm−1, far from other vibrational modes found in biomolecules. The multifaceted nature of nitriles has enabled the investigation of peptide side chain orientation and hydration status upon binding to lipid membranes, amyloid fiber structure and conformation, dynamics of an HIV-1 reverse transcriptase inhibitor, local environments of DNA fragments, ligand-protein interactions, and many more applications.[1n, 3]
Although analogs of phenylalanine (Phe) and alanine are commercially available, other cyano- derivatized amino acids are not and have remained difficult to obtain. The utility of 5-cyano-tryptophan (Trp5CN) as a potential infrared probe with a larger sensitivity for the local environment, moreover, has been demonstrated through analysis of the infrared spectrum by Gai and coworkers.[3e, 4] These studies show Trp5CN has a larger molar absorption cross section than Phe4CN and a higher sensitivity to the degree of hydration due to the larger ‘antenna effect’ of the indole ring.[5] Furthermore, this spectroscopic reporter is capable of exposing the solvent dynamics around side chains within indolicidin upon binding to a membrane. By comparing two Trp5CN labelled peptides, a narrowing of the spectral linewidth was observed upon binding to dodecylphosphocholine (DPC) micelles for one labelled side chain that becomes sequestered in the membrane mimic, while the other labelled side chain showed no significant change in bandwidth compared to the peptide in bulk water, revealing exposure to solvent.[3e] Overall, these prior results show that the cyanoindole moiety is highly sensitive to the degree of hydration around the sidechain.
With the great promise of cyano-labeled tryptophan as a spectroscopic reporter, we developed a scalable, modular synthesis to further investigate this potential.[6] Furthermore, we then incorporated a cyano-labeled tryptophan and another cyano-probe within the same short peptide to develop a novel cyano- label probe pair that allowed for the detection of structural information through 2D IR spectroscopy. These novel side chain probes coupled with the structural methods will usher in the ability to directly observe the dynamics in side chain gating mechanisms such as found in the M2 Influenza virus [4] as well as localized detection of aggregates found in Alzheimer’s and other protein misfolding diseases.
Results and Discussion
Cyanoindole Linear Infrared Study
In order to assess the potential of differently substituted cyano-tryptophans, several model indole systems were analyzed in the infrared. The cyano- label was positioned at the 3, 4, 5, and 6 carbons on the indole ring to determine the variations in peak position and extinction coefficients in THF. Figure 1a demonstrates that indole3CN exhibited the largest extinction coefficient followed by indole6CN and indole5CN, while indole4CN showed the lowest peak extinction coefficient. Although indole3CN exhbited the largest extinction coefficient, it is rendered unusable as a cyano- label in tryptophan because the 3-position links to the alpha carbon of the amino acid. Thus, the most promising cyano-positions of the indole are positions 5 and 6, with indole6CN showing ~1.2 fold increase in dipole strength compared to indole5CN.
Figure 1.
a) FTIR spectra of equimolar indole3CN (blue), indole4CN (red), indole5CN (green), and indole6CN (purple) in THF b) Linear infrared spectra of Trp5CN in water (blue) and Fmoc-Trp5CN in THF (red).
In addition to differences in dipole strength, there are small, but distinguishable, differences in the vibrational frequencies (~1–2 cm−1) of the transitions of cyanoindole derivatives, making it unlikely that two cyano-tryptophan derivations can be observed in a single peptide without significant spectral overlap. Three of the four cyano- derivatives exhibit only one prominent transition. Indole4CN exhibits two distinct transitions at 2214 and 2230 cm−1. Prior studies have indicated the origin of the second peak is due to a Fermi resonance between the fundamental and the ring breathing modes.[7] The Fermi resonance combined with the lower extinction coefficients render the 4-cyano analog less desirable as an infrared probe. The 5-cyano-L-tryptophan derivative was then synthesized as a potential infrared probe for further investigation.
Synthesis of (S)-Nα-Boc/Fmoc-N(in)-Boc-5-cyano-tryptophan
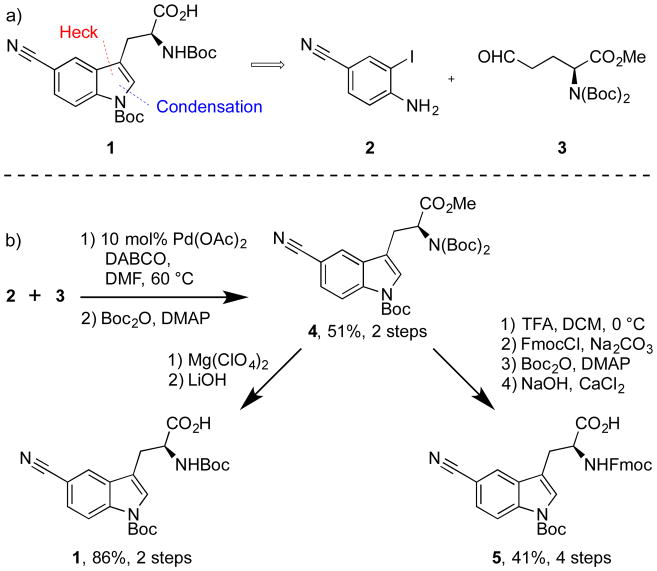
Although 5-cyano-L-tryptophan has been previously synthesized starting from L-tryptophan,[8] the route’s reliance on an electrophilic aromatic bromination/high-temperature cyanation sequence to set the regiochemistry precludes the synthesis of other regioisomers.[9] Therefore, a route (Figure 2a) applicable to multiple protected cyano-tryptophan regioisomers was envisioned.[10] The key step in this route utilizes a modification of Zhu’s indole synthesis[11] to construct the tryptophan core (4) via the condensation-cyclization[12] of aldehyde 3[13] and 4-cyano-2-iodoaniline (2) [14] followed by treatment of the crude material with Boc2O (Figure 2b).[11] This reaction sequence has been performed on a scale to provide greater than 5 g of 4 (See SI). Selective removal of one Boc-group from the bis-Boc-carbonate with Mg(ClO4)2[15] and hydrolysis of the methyl ester with LiOH, afforded (S)-Nα-Boc-N(in)-Boc-5-cyano-tryptophan (1) suitable for peptide synthesis. Exchange of the Boc-protecting group on the N-terminus for an Fmoc-group[16], was achieved through a 4-step route starting with 4 (41% over 4 steps).
Figure 2.
a) Retrosynthesis of (S)-Nα-Boc-N(in)-Boc-5-cyano-tryptophan.
b) Synthesis of (S)-Nα-Boc/Fmoc-N(in)-Boc-5-cyano-tryptophan
Infrared Studies of 5-Cyano-Tryptophan and Transition Dipole Strengths
The linear IR spectra of Trp5CN in water and Fmoc-Trp5CN in THF both exhibit a single transition associated with the -CN stretch. These transitions occur at 2227 and 2225 cm−1 in water and THF, respectively (Figure 1b). The bandwidth of Trp5CN in water (14.4 ± 1.3 cm−1) is approximately twice as large as observed in THF (7.6 ± 0.2 cm−1). Although there is not a significant solvachromatic shift of the vibrational frequency, the two fold increase in bandwidth suggests sensitivity to the number of microstructures available upon interaction of water and the cyanoindole moiety[12] detectable via 2D IR spectroscopy. In the spectral profile of Fmoc-Trp5CN in THF, a slight asymmetry of the band (left shoulder) is observed. This asymmetry has been detected within other cyano- substituted aromatic side chains, and it is likely due to a slight Fermi resonance.[7] Another possibility is that two conformers of the molecule are present in THF whereas only one is seen in water, as seen in other ring systems.[1i] In our current work, the transition dipole strength (μ) of Trp5CN in water was measured to be 0.49 ± 0.02 D using methods reported by Zanni and coworkers.[17] This value is larger than the transition dipole strength of the commonly used cyano- probe, Phe4CN (0.38 ± 0.02 D). With this increase in transition dipole strength, the peptide concentrations required for experiments can be reduced and the signals become easier to observe, especially for 2D IR where the signal is dependent on |μ|4.
The synthesized Trp5CN was incorporated into a short model peptide in conjunction with Phe4CN, Trp5CN–Gly–Phe4CN, to address whether this probe pair has the potential to vibrationally couple over short distances for structural determination. The infrared spectrum of this peptide in water, shown in Figure 4, has two distinct transitions at 2227 and 2233 cm−1. The presence of these two probes within a close spatial proximity in the molecule results in significant dipolar coupling as indicated by the spectral intensities. As a result, the ratio of the relative intensities of the infrared transitions, 1.15:1 (Trp5CN:Phe4CN) is sufficiently less than expected for isolated transitions based on prior literature [18] as well as the measured transition dipole of 5-cyanotryptophan (1.7:1).
Figure 4.
Simulated 2D IR spectra of Trp5CN–Gly–Phe4CN in water, zero coupling (top) and 1.4 cm−1 coupling (bottom).
Typically, a splliting in the vibrational frequencies is expected when coupling is present. No changes in the vibrational frequency were observed for these coupled transitions in the infrared spectrum. Assuming a simple bilinear coupling model, the vibrational frequencies were determined to vary by less than 1 cm−1 and within the limits of the FTIR detection. On the other hand, the relative intensities of the two transitions still vary significantly with the magnitude of the vibrational coupling, as observed in our experimental results similar to Krummel et al.[19] Although vibrational coupling of this nature is often difficult to assess via linear infrared spectroscopy, a much clearer picture of the vibrational coupling can be detected via 2D IR spectroscopy by careful analysis of the spectral shape or oftentimes by the presence cross peaks.[20] Therefore, 2D IR was invoked to investigate the coupling for these two vibrational probes.
2D IR Spectroscopy and Analysis
The 2D IR spectrum of Trp5CN–Gly–Phe4CN in water measured at 150 fs waiting time reveals two transitions with a distortion/change in the lineshape (Figure 3). For Trp5CN, the 0→1 positive going transition is observed at {ωt,ωτ} = 2225,2227 cm−1 along with an anharmonically shifted negative transition located 21 ± 1 cm−1 along ωt. The second position-going transition resulting from the cyano- mode of the Phe4CN is observed at {ωt,ωτ} = 2231,2233 cm−1. Significant inhomogenous broadening is present in both trasnitions as expected from the different microstructures available in bulk water. The population relaxations, T10, of Trp5CN and Phe4CN in water were measured to be 1.7 ± 0.1 ps and 2.0 ± 0.1 ps, respectively. By comparing the 2D spectral line shapes of the peptide to a 50/50 mixture of the amino acids as a control (See Figure S8), it is evident that the spectral distortions in the peptide spectrum are due to the presence of vibrational coupling. Also, the intensities of the cross peaks become more prominent at later times as seen in Figure S4-a. The decay of the cross peaks intensity as a function of waiting time suggests the presence of vibrational coupling between transitions. An average of 1D slices of the 2D IR spectrum along ωτ indicates the presence of cross peak within the lineshape that become more apparent upon first derivative analysis shown in Figure S9. Quantitively, it can be shown that the significant distortion in the shape of the center line contours of both the positive and negative bands is indicative of the vibrational coupling (see SI for more details). These observed spectral characteristics were quantified by the slope of the line passing through the max contour of the positive bands accounting for 12% of the amplitude. The values of the slopes were 0.77 and 0.47 for Trp5CN and Phe4CN, respectively. Then, the magnitude of the vibrational coupling was determined by simulating the 2D IR spectrum of this peptide in water to best match the observed experimental slopes through the max contour. In order to reasonably reproduce the observed 2D IR spectrum, a vibrational coupling of 1.4 cm−1 was required between the two modes (Figure 4).
Figure 3.
FTIR and 2D IR spectrum of Trp5CN–Gly–Phe4CN in water at 150 fs waiting time.
Briefly, the 2D IR simulation was performed for the standard response functions within the weak coupling limit, utilizing the measured experimental parameters and assuming FFCF parameters for the modes were exposed to water. To evaluate the coupling parameter, a range of values between 0 and 2 were simulated and the resulting spectra were analyzed by measuring the slope along the max contour (see SI for complete analysis). By comparing the simulated spectrum for zero coupling and a coupling strength of 1.4 cm−1 (Figure 4), there are very distinct differences. Assuming zero coupling, two distinguishable bands were observed on the diagonal at {ωt,ωτ} = 2227 cm−1 and {ωt,ωτ} = 2233 cm−1 with the slope of max contour having values of 0.66 and 0.70. Also, no apparent distortion was present in the anharmonically shifted transitions. However, as the coupling strength was increased, the slopes along the max contour changed as a result of distortions caused by the addition of weak cross peak (see SI). Once a value of 1.4 cm−1 is reached for the coupling, the slopes along the max contours of the positive bands, 0.78 and 0.50, are in reasonable agreement with the experimental 2D IR spectrum and the distortion in the negative 1→2 transition distortion is also reproduced. To further assess the overall change in spectral shape upon coupling and its effects on the max contour slope of CN symmetric stretch, the linear infrared and 2D IR spectra of the Phe4CN amino acid in water were collected and compared. The slope along the max contour of the positive band of the 2D IR spectrum of the single labelled amino acid at 150 fs waiting time is 0.71 matching the slope of the simulated spectrum with zero coupling and 1.5x less than the transition in the model peptide (see SI). Together, these results establish the utility of quantifying the vibrational coupling magnitude of overlapping nitrile transitions via the slope along the max contour.
Transition Dipole Coupling Model
Transition dipole coupling (TDC) is a simple model that relates the observed experimental coupling to the spatial distance between the two dipoles of the vibrational probes within the molecule. According to TDC (Eq. 1),
| (1) |
where μi and μj are the transition dipole strengths of the probes, rij is the distance between the probes, and βij is the coupling constant.[20]
By applying the above mentioned transition dipole strengths and the experimental coupling along with the angles between dipoles detemined from the linear IR intensities, the distance between cyano probes was calculated to be ~ 13 Å. An average value for the distance was then determined by running Density Functional Theory (DFT) energy minimizations (Gaussian09) on an ensemble of starting conformations of the short peptide (more details in the SI). A distance of 13.3 ± 1.04 Å was calculated for the ensemble average of the conformations of the Trp5CN–Gly–Phe4CN peptide, which closely matches the experimental value. Any slight differences in the distances can be due to the lack of sufficient sampling of the peptide structures for the theoretical ensemble. Overall, the methodology put forth above provides a spectral tool to access structural information in peptides for cyano-cyano probe pairs.
Conclusions
In this work, Nα-Boc-N(in)-Boc-5-cyano-tryptophan and Nα-Fmoc-N(in)-Boc-5-cyano-tryptophan were synthesized in 44% and 21% respectively. The motivation for this synthesis is provided by the potential of cyano-labeled indoles as non-native labels in peptides and proteins via tryptophan. The synthesized 5-cyano-L-tryptophan was incorporated into a short peptide system as second cyano- label, Trp5CN–Gly–Phe4CN, to validate the efficacy of measuring spatial distance using this probe pair via 2D IR spectroscopy. The vibrational coupling, 1.4 cm−1 between the cyano- probes was quantified via simulation of the 2D IR spectrum and analysis of the slope along the max contours of the positive transitions. The spatial distance, ~13 Å, between the two labels was found experimentally using transition dipole coupling theory and confirmed theoretically. Other cyano-tryptophan regioisomers are currently being synthesized, and the spectroscopic studies of such compounds will be presented in due course.
Experimental Section
5-Cyano-L-tryptophan (Trp5CN) was synthesized as described above and characterized via Fourier transform infrared (FTIR) spectroscopy. Additionally, a short peptide system, Trp5CN–Gly–Phe4CN, was synthesized via standard fluorenylmethyloxycarbonyl chloride (FMOC) solid-state peptide synthesis to include the Trp5CN. FTIR spectra (Nicolet 6700, Thermo Scientific) of Trp5CN, indole3CN, indole4CN, indole5CN, indole6CN, and the short peptide Trp5CN–Gly–Phe4CN were collected with 1 cm−1 resolution and a nitrogen-cooled MCT detector. 2D IR spectra were measured using the box-CARS configuration as described in prior literature.[20e] The optical densities for both methods were between 0.01–0.03. See SI for more details.
Supplementary Material
Acknowledgments
The research was supported by NIH (R15GM1224597) to MJT. PHG and MWF acknowledge the University of Scranton for support for this research.
Footnotes
Supporting information for this article is given via a link at the end of the document.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.a) Getahun Z, Huang CY, Wang T, De Leon B, DeGrado WF, Gai F. J Am Chem Soc. 2003;125:405–11. doi: 10.1021/ja0285262. [DOI] [PubMed] [Google Scholar]; b) Zheng J, Kwak K, Asbury J, Chen X, Piletic IR, Fayer MD. Science. 2005;309:1338–43. doi: 10.1126/science.1116213. [DOI] [PubMed] [Google Scholar]; c) Suydam IT, Snow CD, Pande VS, Boxer SG. Science. 2006;313:200–4. doi: 10.1126/science.1127159. [DOI] [PubMed] [Google Scholar]; d) Sigala PA, Fafarman AT, Bogard PE, Boxer SG, Herschlag D. J Am Chem Soc. 2007;129:12104–5. doi: 10.1021/ja075605a. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Fafarman AT, Sigala PA, Herschlag D, Boxer SG. J Am Chem Soc. 2010;132:12811–3. doi: 10.1021/ja104573b. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Taskent-Sezgin H, Chung J, Banerjee PS, Nagarajan S, Dyer RB, Carrico I, Raleigh DP. Angew Chem Int Ed. 2010;49:7473–5. doi: 10.1002/anie.201003325. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Bischak CG, Longhi S, Snead DM, Costanzo S, Terrer E, Londergan CH. Biophys J. 2010;99:1676–83. doi: 10.1016/j.bpj.2010.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Urbanek DC, Vorobyev DY, Serrano AL, Gai F, Hochstrasser RM. J Phys Chem Lett. 2010;1:3311–5. doi: 10.1021/jz101367d. [DOI] [PMC free article] [PubMed] [Google Scholar]; i) Tucker MJ, Gai XS, Fenlon EE, Brewer SH, Hochstrasser RM. Phys Chem Chem Phys. 2011;13:2237–41. doi: 10.1039/c0cp01625j. [DOI] [PMC free article] [PubMed] [Google Scholar]; j) Chung JK, Thielges MC, Fayer MD. Proc Natl Acad Sci U S A. 2011;108:3578–83. doi: 10.1073/pnas.1100587108. [DOI] [PMC free article] [PubMed] [Google Scholar]; k) Bagchi S, Fried SD, Boxer SG. J Am Chem Soc. 2012;134:10373–6. doi: 10.1021/ja303895k. [DOI] [PMC free article] [PubMed] [Google Scholar]; l) Rock W, Li YL, Pagano P, Cheatum CM. J Phys Chem A. 2013;117:6073–83. doi: 10.1021/jp312817t. [DOI] [PMC free article] [PubMed] [Google Scholar]; m) Maj M, Ahn C, Kossowska D, Park K, Kwak K, Han H, Cho M. Phys Chem Chem Phys. 2015;17:11770–8. doi: 10.1039/c5cp00454c. [DOI] [PubMed] [Google Scholar]; n) Levin DE, Schmitz AJ, Hines SM, Hines KJ, Tucker MJ, Brewer SH, Fenlon EE. RSC Adv. 2016;6:36231–7. doi: 10.1039/C5RA27363C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taskent-Sezgin H, Chung J, Banerjee PS, Nagarajan S, Dyer RB, Carrico I, Raleigh DP. Angew Chem Int Ed. 2010;49:7473–5. doi: 10.1002/anie.201003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) Tucker MJ, Getahun Z, Nanda V, DeGrado WF, Gai F. J Am Chem Soc. 2004;126:5078–9. doi: 10.1021/ja032015d. [DOI] [PubMed] [Google Scholar]; b) Schultz KC, Supekova L, Ryu Y, Xie J, Perera R, Schultz PG. J Am Chem Soc. 2006;128:13984–5. doi: 10.1021/ja0636690. [DOI] [PubMed] [Google Scholar]; c) Aprilakis KN, Taskent H, Raleigh DP. Biochemistry. 2007;46:12308. doi: 10.1021/bi7010674. [DOI] [PubMed] [Google Scholar]; d) Fang C, Bauman JD, Das K, Remorino A, Arnold E, Hochstrasser RM. Proc Natl Acad Sci U S A. 2008;105:1472–7. doi: 10.1073/pnas.0709320104. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Waegele MM, Tucker MJ, Gai F. Chem Phys Lett. 2009;478:249–53. doi: 10.1016/j.cplett.2009.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; f) Zimmermann J, Thielges MC, Seo YJ, Dawson PE, Romesberg FE. Angew Chem Int Ed. 2011;50:8333–7. doi: 10.1002/anie.201101016. [DOI] [PubMed] [Google Scholar]; g) Ma J, Pazos IM, Zhang W, Culik RM, Gai F. Annu Rev Phys Chem. 2015;66:357–77. doi: 10.1146/annurev-physchem-040214-121802. [DOI] [PMC free article] [PubMed] [Google Scholar]; h) Maj M, Ahn C, Blasiak B, Kwak K, Han H, Cho M. J Phys Chem B. 2016;120:10167–80. doi: 10.1021/acs.jpcb.6b04319. [DOI] [PubMed] [Google Scholar]
- 4.Markiewicz BN, Lemmin T, Zhang W, Ahmed IA, Jo H, Fiorin G, Troxler T, DeGrado WF, Gai F. Phys Chem Chem Phys. 2016;18:28939–50. doi: 10.1039/c6cp03426h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Lindquist BA, Corcelli SA. J Phys Chem B. 2008;112:6301–3. doi: 10.1021/jp802039e. [DOI] [PubMed] [Google Scholar]; b) Waegele MM, Gai F. J Phys Chem Lett. 2010;1:781–6. doi: 10.1021/jz900429z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Goldberg JM, Batjargal S, Petersson EJ. J Am Chem Soc. 2010;132:14718–20. doi: 10.1021/ja1044924. [DOI] [PubMed] [Google Scholar]; b) Tucker MJ, Courter JR, Chen J, Atasoylu O, Smith AB, 3rd, Hochstrasser RM. Angew Chem Int Ed. 2010;49:3612–6. doi: 10.1002/anie.201000500. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Speight LC, Muthusamy AK, Goldberg JM, Warner JB, Wissner RF, Willi TS, Woodman BF, Mehl RA, Petersson EJ. J Am Chem Soc. 2013;135:18806–14. doi: 10.1021/ja403247j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipkin JS, Song R, Fenlon EE, Brewer SH. J Phys Chem Lett. 2011;2:1672–6. doi: 10.1021/jz2006447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dua RK, Phillips RS. Tetrahedron Lett. 1992;33:29–33. [Google Scholar]
- 9.Bartoccini F, Bartolucci S, Mari M, Piersanti G. Org Biomol Chem. 2016;14:10095–100. doi: 10.1039/c6ob01791f. [DOI] [PubMed] [Google Scholar]
- 10.a) Humphrey GR, Kuethe JT. Chem Rev. 2006;106:2875–911. doi: 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]; b) Cacchi S, Fabrizi G. Chem Rev. 2011;111:215–83. doi: 10.1021/cr100403z. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y, Zhu J. J Org Chem. 2006;71:7826–34. doi: 10.1021/jo061471s. [DOI] [PubMed] [Google Scholar]
- 12.Chuang KV, Kieffer ME, Reisman SE. Org Lett. 2016;18:4750–3. doi: 10.1021/acs.orglett.6b02477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokotos G, Padro JM, Martín T, Gibbons WA, Martín VS. J Org Chem. 1998;63:3741–4. [Google Scholar]
- 14.Liu CY, Knochel P. Org Lett. 2005;7:2543–6. doi: 10.1021/ol0505454. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez JN, Ramirez MA, Martin VS. J Org Chem. 2003;68:743–6. doi: 10.1021/jo026300b. [DOI] [PubMed] [Google Scholar]
- 16.a) Richard DJ, Schiavi B, Joullie MM. Proc Natl Acad Sci U S A. 2004;101:11971–6. doi: 10.1073/pnas.0401407101. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Barbie P, Kazmaier U. Org Biomol Chem. 2016;14:6055–64. doi: 10.1039/c6ob00801a. [DOI] [PubMed] [Google Scholar]
- 17.Grechko M, Zanni MT. J Chem Phys. 2012;137:1–9. doi: 10.1063/1.4764861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.a) Waegele MM, Culik RM, Gai F. J Phys Chem Lett. 2011;2:2598–609. doi: 10.1021/jz201161b. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Urbanek DC, Vorobyev DY, Serrano AL, Gai F, Hochstrasser RM. J Phys Chem Lett. 2010;1:3311–5. doi: 10.1021/jz101367d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krummel AT, Zanni MT. J Phys Chem B. 2008;112:1336–8. doi: 10.1021/jp711558a. [DOI] [PubMed] [Google Scholar]
- 20.a) DeFlores LP, Ganim Z, Ackley SF, Chung HS, Tokmakoff A. J Phys Chem B. 2006;110:18973–80. doi: 10.1021/jp0603334. [DOI] [PubMed] [Google Scholar]; b) Bagchi S, Falvo C, Mukamel S, Hochstrasser RM. J Phys Chem B. 2009;113:11260–73. doi: 10.1021/jp900245s. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Remorino A, Korendovych IV, Wu Y, DeGrado WF, Hochstrasser RM. Science. 2011;332:1206–9. doi: 10.1126/science.1202997. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Woys AM, Almeida AM, Wang L, Chiu CC, McGovern M, de Pablo JJ, Skinner JL, Gellman SH, Zanni MT. J Am Chem Soc. 2012;134:19118–28. doi: 10.1021/ja3074962. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Schmitz AJ, Hogle DG, Gai XS, Fenlon EE, Brewer SH, Tucker MJ. J Phys Chem B. 2016;120:9387–94. doi: 10.1021/acs.jpcb.6b07212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.