Abstract
The advantage of adjuvant chemotherapy (ACT) for treating Stage III colon cancer patients is well established and widely accepted. However, many patients with Stage III colon cancer do not receive ACT. Moreover, there are controversies around the effectiveness of ACT for Stage II patients. We investigated the administration of ACT and its association with overall survival in resected Stage II (overall and stratified by low-/high-risk) and Stage III colon cancer patients in three European countries including The Netherlands (2009–2014), Belgium (2009–2013) and Sweden (2009–2014). Hazard ratios (HR) for death were obtained by Cox regression models adjusted for potential confounders. A total of 60244 resected colon cancer patients with pathological Stages II and III were analyzed. A small proportion (range 9–24%) of Stage II and over half (range 55–68%) of Stage III patients received ACT. Administration of ACT in Stages II and III tumors decreased with higher age of patients. Administration of ACT was significantly associated with higher overall survival in high-risk Stage II patients (in The Netherlands (HR; 95%CI = 0.82 (0.67–0.99), Belgium (0.73; 0.59–0.90) and Sweden (0.58; 0.44–0.75)), and in Stage III patients (in The Netherlands (0.47; 0.43–0.50), Belgium (0.46; 0.41–0.50) and Sweden (0.48; 0.43–0.54)). In Stage III, results were consistent across subgroups including elderly patients. Our results show an association of ACT with higher survival among Stage III and high-risk Stage II colon cancer patients. Further investigations are needed on the selection criteria of Stages II and III colon cancer patients for ACT.
Keywords: colon cancer, adjuvant chemotherapy, variations, survival
Complete surgical resection is the primary treatment of nonmetastatic colon cancer patients. Historically about 55% of patients experience recurrence after curative resection.1 However, more recent publications show a lower recurrence rate.2 Despite the vast improvement in the surgical techniques of colon cancer in recent years, many patients still recur with rates up to 29% in Stage II3–5 and 42% in Stage III,6 and adjuvant (postsurgical) chemotherapy (ACT) is the standard care for node-positive colon cancer patients (Stage III).7 The primary goal of ACT administration is to eliminate potential microscopic residual disease and thereby reduce the risk of recurrence. For Stage III, it has been estimated that ACT reduces the risk of recurrence by 14%.6 Although the advantage of ACT for treating Stage III colon cancer patients is well established and widely accepted, several studies have described potential underutilization of ACT in routine practice.8,9 Moreover, the completion rate of initiated ACT for Stage III colon cancer patients has been reported to be only 78%.10
Modest survival benefits of ACT administration have been reported for Stage II colon cancer patients.11,12 However, administration of ACT for Stage II patients remains a subject of ongoing debate and is recommended when specific features associated with poor prognosis are present.7,13 These include perforation or obstruction, histopathologic T4 tumor, suboptimal lymph node sampling, vascular or neural invasion, and poorly differentiated histology.14,15 There is little scientific evidence in the literature supporting the effectiveness of ACT in low-risk colon cancer patients.13,16 Nevertheless, guidelines published by the European Society for Medical Oncology (ESMO) state that ACT can also be considered in individual low-risk patients.17
This study is part of a EurocanPlatform project which is a consortium of major European cancer centers aiming at the enhanced translation of progress in oncological research into clinical practice and has been explained elsewhere.18,19 In this population-based study on Stages II and III colon cancer patients, we aimed to assess (1) variations in the administration of ACT across countries and over time, (2) the association of ACT administration with patient and tumor characteristics and (3) with overall survival.
Material and Methods
Study population
Population-based data were obtained from quality registries in three European countries, visually the Netherlands Cancer Registry (2009–2014), the Belgian Cancer Registry (2009–2013) and the Swedish ColoRectal Cancer Registry (2009–2014). Stage of the colon cancer (ICD-10: C18–C19) was defined according to the seventh edition of the pathological TNM classification.20 The analysis included cases with a first diagnosis of colon cancer at Stage II (pT3–4,N0,M0) or Stage III (pTany,N+,M0) who were surgically resected. Stage II patients were classified according to the presence of poor prognostic features which were defined by either of the following: poorly differentiated histology, histopathological T4, vascular/neural invasion (information available in Sweden only), and suboptimal lymph node sampling (information available in The Netherlands and Sweden). Suboptimal lymph node sampling was in Sweden defined as below 12 lymph nodes and in The Netherlands below 10 lymph nodes. We followed the national definitions for classification.21
Statistical methods
The distribution of basic patient and tumor characteristics across the three countries is presented. The annual age-standardized proportion of patients receiving ACT in each country was computed using the age distribution from The Netherlands as standard. The associations between the administration of ACT and gender, age group (<65, 65–69, 70–79, 80+), tumor location (right/left side, where the left side refers to location from distal to the splenic flexure), and type of surgery (open resection vs laparoscopy) were investigated by odds ratios (OR) using multiple logistic regression models, adjusting for the mentioned factors, and stratified by tumor stage (Stage II, low-/high-risk Stage II and Stage III).
The association between administration of ACT compared to surgery only with overall survival was investigated by hazard ratios (HR) using Cox regression models adjusted for gender, age group, tumor location, type of surgery, tumor size and lymph node counts. As patients receiving ACT must have survived until the start of ACT, no accounting for the immortal time of these patients in the Cox model might result in immortal time bias. While considering ACT as time-dependent covariate according to the Mantel–Byar method is the gold standard approach to reduce immortal time bias,22 it requires information on the date of ACT, which was not available in the Netherlands. To account at least partly for a potential immortal time bias without using information on date of ACT, the start of the follow-up was set to the date of surgery + 49 days (the median time from surgery to ACT in Sweden) for all patients. Moreover, as sensitivity analysis, we repeated the analysis for Belgium and Sweden using the Mantel–Byar method by estimating survival after surgery but counting patients as “untreated” before the start of ACT and “treated” afterward.
In further sensitivity analyses to evaluate the possible influence of classification differences of low- and high-risk Stage II groups in estimates of HRs between countries, the analyses were replicated by classifying low- and high-risk Stage II based on poorly differentiated histology and histopathological T4 only, the two factors available in all three countries. The overall survival was assessed up to five years after starting follow-up.
Patients with missing data in factors included in modelling were excluded from analyses (4% in The Netherlands, 5% in Belgium and 0.7% in Sweden). Statistical significance was defined by a two-sided p < 0.05 without correction for multiple testing.
Results
A total of 60244 surgically resected primary colon cancer patients with pathological Stage II (31200 patients) and Stage III (29044 patients) from the three European countries were included in this study (Table 1). Basic characteristics of patients and treatment details are summarized in Table 2. Distribution of patients by sex, age group and tumor location are similar between stages and across the three countries. Laparoscopic surgery was less applied in Sweden (11% in Stage II and 10% in Stage III) than in The Netherlands (44% in Stage II and 43% in Stage III). The curative surgical resection without ACT was applied to 76–91% of Stage II colon cancer patients. The highest frequency of ACT administration for Stage II colon cancer patients was observed in Belgium (24%), followed by Sweden (12%) and The Netherlands (9%). Within Stage II colon cancer, women were slightly more often classified in high-risk Stage II than men. Compared to left-sided tumors, right-sided tumors were associated with higher tumor grades, and hence right side tumors were more often observed in the high-risk Stage II group. In all countries, the high-risk Stage II patients received ACT (Netherlands, 17%; Belgium, 38%; Sweden, 18%) more frequently than low-risk patients (Netherlands and Sweden: 4% and Belgium: 19%). The frequencies of ACT administration for Stage III patients were 68% in Belgium, 61% in The Netherlands and 55% in Sweden. Higher frequency of ACT administration was observed in Belgium in all age subgroups of low-/high-risk Stage II, and in patients older than 70 years with Stage III disease compared to the other countries (Supporting Information, Fig. 1). Information about the time interval between surgery and ACT administration was available in Sweden and Belgium, and most of the patients received chemotherapy within eight weeks after surgery in these countries.
Table 1.
Participating countries, study period, follow-up time, and number of surgically resected stage II–III colon cancer patients
| Country1 | Period of study | Date of las follow-up |
Median follow-up (months) |
Number of patients | Available variables for risk stratification of stage II patients |
||
|---|---|---|---|---|---|---|---|
| Total (60244) |
Stage II (31200) |
Stage III (29044) |
|||||
| The Netherlands | 2009–2014 | April 2015 | 34 | 31879 | 16206 | 15673 | Grade of tumor, pT category, LN (≥10, <10) |
| Belgium | 2009–2013 | June 2016 | 59 | 14847 | 7921 | 6926 | Grade of tumor, pT category |
| Sweden | 2009–2014 | March 2016 | 51 | 13518 | 7073 | 6445 | Grade of tumor, pT category, LN (≥12, <12), vascular/neural invasion |
Data provided by: The Netherlands Cancer Registry, Belgian Cancer Registry, and Swedish Colorectal Cancer Registry.
Abbreviations: pT stage, pathological T-category (tumor size); LN, harvested lymph node count.
Table 2.
Basic characteristics of surgically resected colon cancer patients with stage II (total and low-/high-risk) and stage III disease
| Count (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| The Netherlands | Belgium | Sweden | |||||||||||||
|
|
|
|
|||||||||||||
| Stage II2 | Stage II2 | Stage II2 | |||||||||||||
|
|
|
|
|||||||||||||
| Basic characteristics1 | Total | Total | Low-risk | High-risk | Stage III | Total | Total | Low-risk | High-risk | Stage III | Total | Total | Low-risk | High-risk | Stage III |
| Total | 31879 | 16206 | 9733 | 5379 | 15673 | 14847 | 7921 | 5244 | 2231 | 6926 | 13518 | 7073 | 3085 | 3858 | 6445 |
|
| |||||||||||||||
| Sex | |||||||||||||||
|
| |||||||||||||||
| Men | 16527 (52) | 8260 (51) | 5174 (53) | 2515 (47) | 8267 (53) | 7618 (51) | 4099 (52) | 2871 (55) | 1014 (45) | 3519 (51) | 6600 (49) | 3441 (49) | 1590 (52) | 1786 (46) | 3159 (49) |
|
| |||||||||||||||
| Women | 15352 (48) | 7946 (49) | 4560 (47) | 2864 (53) | 7406 (47) | 7229 (49) | 3822 (48) | 2373 (45) | 1217 (55) | 3407 (49) | 6918 (51) | 3632 (51) | 1495 (48) | 2072 (54) | 3286 (51) |
|
| |||||||||||||||
| Mean age (±SD) | 71 (11) | 72 (11) | 71 (11) | 72 (11) | 69 (11) | 72 (11) | 73 (11) | 73 (11) | 74 (11) | 71 (12) | 73 (11) | 73 (11) | 73 (11) | 74 (11) | 72 (12) |
|
| |||||||||||||||
| Age at diagnosis | |||||||||||||||
|
| |||||||||||||||
| <60 | 4912 (15) | 2147 (13) | 1348 (14) | 638 (12) | 2765 (18) | 2062 (14) | 970 (12) | 655 (12) | 264 (12) | 1092 (16) | 1671 (12) | 755 (11) | 324 (11) | 415 (11) | 916 (14) |
|
| |||||||||||||||
| 60–69 | 8842 (28) | 4223 (26) | 2625 (27) | 1301 (24) | 4619 (29) | 3330 (22) | 1663 (21) | 1118 (21) | 448 (20) | 1667 (24) | 3063 (23) | 1494 (21) | 699 (23) | 763 (20) | 1569 (24) |
|
| |||||||||||||||
| 70–79 | 10869 (34) | 5728 (35) | 3459 (36) | 1891 (35) | 5141 (33) | 4810 (32) | 2606 (33) | 1755 (33) | 707 (32) | 2204 (32) | 4740 (35) | 2538 (36) | 1132 (37) | 1365 (35) | 2202 (34) |
|
| |||||||||||||||
| 80+ | 7256 (23) | 4108 (25) | 2302 (24) | 1549 (29) | 3148 (20) | 4645 (31) | 2682 (34) | 1716 (33) | 812 (36) | 1963 (28) | 4044 (30) | 2286 (32) | 930 (30) | 1315 (34) | 1758 (27) |
|
| |||||||||||||||
| Tumor location | |||||||||||||||
|
| |||||||||||||||
| Right | 15846 (51) | 8381 (53) | 4882 (51) | 2898 (56) | 7465 (49) | 7017 (50) | 3876 (52) | 2399 (48) | 1240 (59) | 3141 (48) | 7877 (58) | 4252 (60) | 1780 (58) | 2387 (62) | 3625 (56) |
|
| |||||||||||||||
| Left | 15138 (49) | 7319 (47) | 4620 (49) | 2252 (44) | 7819 (51) | 7084 (50) | 3639 (48) | 2612 (52) | 853 (41) | 3445 (52) | 5632 (42) | 2819 (40) | 1305 (42) | 1469 (38) | 2813 (44) |
|
| |||||||||||||||
| Type of surgery | |||||||||||||||
|
| |||||||||||||||
| Open resection | 17872 (56) | 9025 (56) | 4904 (50) | 3555 (66) | 8847 (57) | – | – | – | – | – | 12055 (90) | 6282 (89) | 2658 (87) | 3513 (92) | 5773 (90) |
|
| |||||||||||||||
| Laparoscopy | 13966 (44) | 7162 (44) | 4820 (50) | 1816 (34) | 6804 (43) | – | – | – | – | – | 1377 (10) | 751 (11) | 407 (13) | 325 (8) | 626 (10) |
|
| |||||||||||||||
| Treatment | |||||||||||||||
|
| |||||||||||||||
| Surgery alone | 20964 (66) | 14788 (91) | 9304 (96) | 4443 (83) | 6176 (39) | 8182 (55) | 5992 (76) | 4258 (81) | 1374 (62) | 2190 (32) | 9130 (68) | 6239 (88) | 2961 (96) | 3153 (82) | 2891 (45) |
|
| |||||||||||||||
| Surgery+ACT3 | 10915 (34) | 1418 (9) | 430 (4) | 936 (17) | 9497 (61) | 6665 (45) | 1929 (24) | 986 (19) | 857 (38) | 4736 (68) | 4388 (32) | 834 (12) | 124 (4) | 705 (18) | 3554 (55) |
Unknown tumor location: 895(3%) in The Netherlands; 746 (5%) in Belgium; 9 (<1%) in Sweden; Unknown type of surgery: 41 (<1%) in The Netherlands; 86 (<1%) in Sweden. These unknown data were similarly distributed by categories of patients’ characteristics.
Low-/high-risk could not be classified due to missing information of prognostic factors: The Netherlands 3%, Belgium 3% and Sweden 1%.
Time from surgery to ACT was: Median (interquartile range) = 6 (5–8) weeks in Belgium and 7 (6–8) weeks in Sweden.
Abbreviations: ACT, Adjuvant chemotherapy; –, data no available.
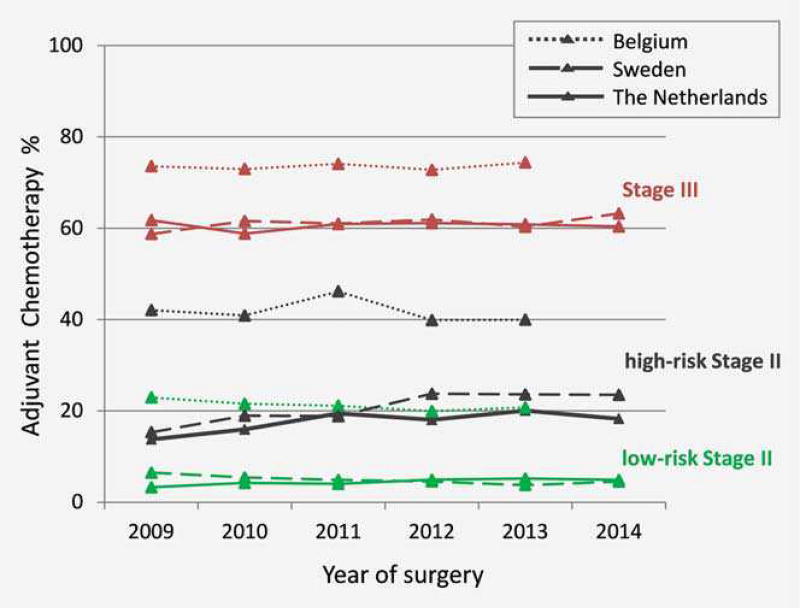
Figure 1 shows the age-standardized frequencies of ACT administration for patients in each country during the study period. For the low-risk Stage II and Stage III patients, the proportion of patients receiving ACT was stable over time in all countries. For high-risk Stage II, the proportion of patients who received ACT slightly increased initially in The Netherlands (between 2009 and 2012) and Sweden (between 2009 and 2011) but was stable afterward. For Belgium, no trend change was observed.
Figure 1.
Age-standardized trend of administration of adjuvant chemotherapy in low-/high-risk Stage II and Stage III patients resected between 2009 and 2014.
Table 3 shows the odds ratios for the association of gender, age group, tumor location and type of surgery with ACT administration after mutual adjustment for each of these factors. The frequency of ACT administration decreased strongly with age, at all stages and stage subgroups, and in all countries. In Sweden, women with Stages II and III disease received ACT significantly more often than men (OR; 95%CI for Stage II: 1.20; 1.03–1.40, for Stage III: 1.16; 1.02–1.32). In Belgium, for patients with Stage III disease, ACT was less often used for women compared to men (0.87; 0.77–0.99).
Table 3.
Odds ratios of administration of adjuvant chemotherapy versus surgery only, adjusted for confounding factors, and stratified by tumor stage.
| OR1 (95%CI) | ||||||||
|
|
||||||||
| The Netherlands | Belgium | |||||||
|
|
|
|||||||
| Stage II | Stage II | |||||||
|
|
|
|||||||
| Basic characteristics | Total | Low-risk | High-risk | Stage III | Total | Low-risk | High-risk | Stage III |
|
| ||||||||
| Sex | ||||||||
|
| ||||||||
| Men | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
|
| ||||||||
| Women | 1.08 (0.96–1.21) | 0.81 (0.66–1.00) | 1.16 (0.99–1.36) | 1.01 (0.93–1.10) | 0.99 (0.88–1.11) | 0.89 (0.77–1.05) | 0.94 (0.76–1.17) | 0.87 (0.77–0.99) |
|
| ||||||||
| Age group | ||||||||
|
| ||||||||
| <60 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
|
| ||||||||
| 60–69 | 0.65 (0.56–0.74) | 0.54 (0.43–0.69) | 0.63 (0.51–0.77) | 0.59 (0.51–0.68) | 0.66 (0.56–0.78) | 0.67 (0.55–0.82) | 0.53 (0.37–0.77) | 0.58 (0.42–0.79) |
|
| ||||||||
| 70–79 | 0.22 (0.19–0.26) | 0.24 (0.18–0.32) | 0.16 (0.13–0.20) | 0.18 (0.16–0.21) | 0.30 (0.25–0.35) | 0.27 (0.22–0.33) | 0.24 (0.17–0.33) | 0.19 (0.14–0.26) |
|
| ||||||||
| 80+ | 0.02 (0.02–0.04) | 0.04 (0.02–0.07) | 0.01 (0.01–0.02) | 0.02 (0.01–0.02) | 0.05 (0.04–0.06) | 0.05 (0.04–0.07) | 0.03 (0.02–0.04) | 0.02 (0.02–0.03) |
|
| ||||||||
| Tumor location | ||||||||
|
| ||||||||
| Right | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) |
|
| ||||||||
| Left | 1.35 (1.20–1.52) | 1.61 (1.31–1.99) | 1.40 (1.20–1.65) | 0.98 (0.90–1.06) | 1.35 (1.20–1.52) | 1.58 (1.35–1.85) | 1.51 (1.22–1.86) | 1.15 (1.01–1.31) |
|
| ||||||||
| Type of surgery | ||||||||
|
| ||||||||
| Open resection | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | – | – | – | – |
|
| ||||||||
| Laparoscopy | 0.53 (0.47–0.60) | 0.58 (0.47–0.71) | 0.79 (0.67–0.93) | 1.64 (1.51–1.78) | – | – | – | – |
|
| ||||||||
| OR1 (95%CI) | ||||||||
|
| ||||||||
| Sweden | ||||||||
|
| ||||||||
| Stage II | ||||||||
|
|
||||||||
| Basic characteristics | Total | Low-risk | High-risk | Stage III | ||||
|
| ||||||||
| Sex | ||||||||
|
| ||||||||
| Men | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||
|
| ||||||||
| Women | 1.20 (1.03–1.40) | 1.01 (0.69–1.47) | 1.14 (0.95–1.37) | 1.16 (1.02–1.32) | ||||
|
| ||||||||
| Age group | ||||||||
|
| ||||||||
| <60 | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||
|
| ||||||||
| 60–69 | 0.50 (0.41–0.61) | 0.39 (0.26–0.59) | 0.51 (0.40–0.66) | 0.68 (0.53–0.86) | ||||
|
| ||||||||
| 70–79 | 0.19 (0.16–0.24) | 0.10 (0.06–0.16) | 0.19 (0.15–0.24) | 0.19 (0.16–0.24) | ||||
|
| ||||||||
| 80+ | 0.01 (0.01–0.02) | 0.02 (0.01–0.06) | 0.01 (0.00–0.02) | 0.01 (0.01–0.01) | ||||
|
| ||||||||
| Tumor location | ||||||||
|
| ||||||||
| Right | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||
|
| ||||||||
| Left | 1.28 (1.09–1.49) | 1.61 (1.10–2.37) | 1.37 (1.14–1.64) | 1.05 (0.92–1.20) | ||||
|
| ||||||||
| Type of surgery | ||||||||
|
| ||||||||
| Open resection | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | 1.00 (ref.) | ||||
|
| ||||||||
| Laparoscopy | 0.56 (0.42–0.73) | 0.52 (0.28–0.95) | 0.75 (0.54–1.04) | 1.28 (1.03–1.59) | ||||
Odds ratio (OR) and 95% confidence interval of patient characteristics associated with the administration of adjuvant therapy with respect to a reference subgroup (ref.). The OR of each factor was obtained from multiple logistic regression models adjusted for all factors listed in the table. ORs displayed in bold are statistically significant, p values <0.05. Underlined ORs (ORs > 1) indicate more often administration of the respective adjuvant chemotherapy.
– Data no available.
Administration of ACT was significantly more frequent in patients with left-sided tumors in Stage II disease (in total and in low-/high-risk subgroups) in all countries. For Stage III patients, a significant association of ACT with left-sided tumors was observed in Belgium (OR; 95%CI: 1.15; 1.01–1.31) only. Utilization of laparoscopy was associated with a significantly lower frequency of ACT administration in low- and high-risk Stage II disease, but with a significantly higher frequency of ACT administration in Stage III disease in the Netherlands and Sweden (Belgium had no information on the type of surgery).
Survival of patients
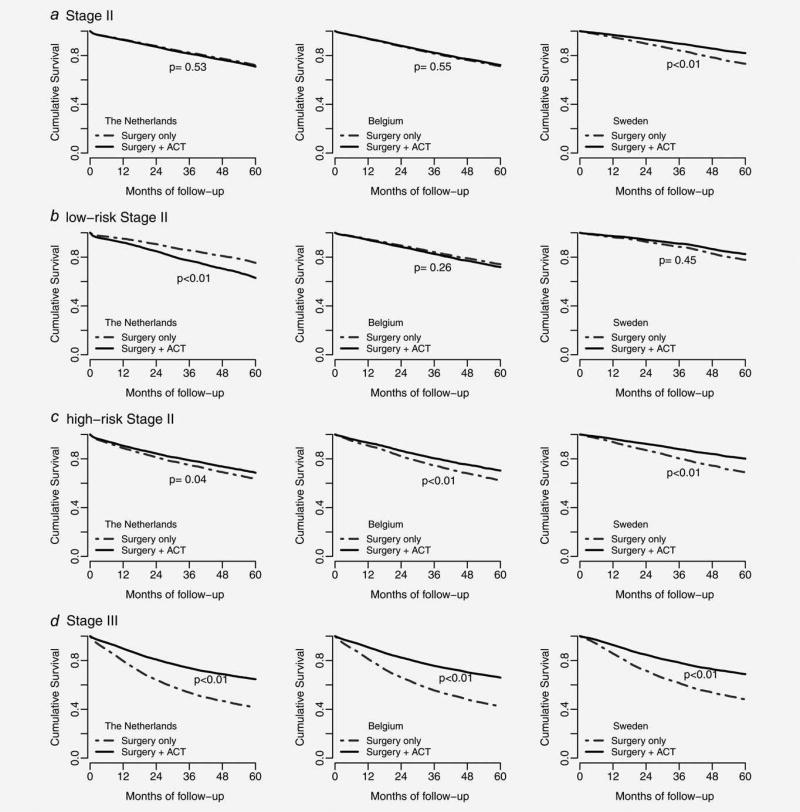
Median follow-up time was 34, 59 and 51 months for patients in The Netherlands, Belgium and Sweden, respectively. The HR for death after adjustment for prognostic factors (Table 4) showed a significant association between ACT and better survival of Stage II colon cancer patients in Sweden (HR; 95% CI: 0.62; 0.49–0.79). Administration of ACT was significantly associated with better overall survival in high-risk Stage II patients in all countries (HR (95%CI) in The Netherlands: 0.82 (0.67–0.99), Belgium: 0.73 (0.59–0.90) and Sweden: 0.58 (0.44–0.75)). In The Netherlands administration of ACT was significantly associated with higher mortality in low-risk Stage II patients overall, and particularly in patients with left-sided tumors, and patients undergoing laparoscopic surgery. Significantly higher survival was observed in Stage III colon cancer patients treated with ACT compared with those who underwent surgery only, in all countries. This finding was also observed in all subgroups of Stage III colon cancer patients defined by gender, age, tumor location and type of surgery. Adjusted survival curves are shown in Figure 2. The unadjusted 1-, 3- and 5-year probabilities of survival estimates stratified by stage and treatment type in each country are shown in Supporting Information, Table 1.
Table 4.
Adjusted Hazard Ratio of association of administration of adjuvant therapy with overall survival, after adjustment for confounding factors, stratified by stage and patients’ characteristics
| HR1 (95%CI) | ||||||||
|
|
||||||||
| The Netherlands | Belgium | |||||||
|
|
|
|||||||
| Stage II | Stage II | |||||||
|
|
|
|||||||
| Basic characteristics | Total2,3 | Low-risk | High-risk2,3 | Stage III2,3 | Total2 | Low-risk | High-risk2 | Stage III2 |
|
| ||||||||
| Overall | 1.05 (0.90–1.23) | 1.70 (1.29–2.25) | 0.82 (0.67–0.99) | 0.47 (0.43–0.50) | 0.96 (0.84–1.10) | 1.12 (0.92–1.35) | 0.73 (0.59–0.90) | 0.46 (0.41–0.50) |
|
| ||||||||
| Gender | ||||||||
|
| ||||||||
| Men | 1.02 (0.82–1.27) | 1.60 (1.12–2.29) | 0.76 (0.57–1.00) | 0.48 (0.43–0.53) | 0.86 (0.72–1.04) | 1.04 (0.82–1.31) | 0.62 (0.46–0.84) | 0.44 (0.38–0.50) |
|
| ||||||||
| Women | 1.08 (0.85–1.37) | 1.91 (1.23–2.97) | 0.89 (0.67–1.18) | 0.45 (0.40–0.50) | 1.11 (0.90–1.36) | 1.27 (0.92–1.76) | 0.87 (0.64–1.17) | 0.48 (0.41–0.56) |
|
| ||||||||
| Age group | ||||||||
|
| ||||||||
| <60 | 0.88 (0.59–1.34) | 2.54 (1.48–4.36) | 0.47 (0.27–0.81) | 0.37 (0.30–0.46) | 1.93 (1.11–3.35) | 1.67 (0.86–3.23) | NE | 0.33 (0.21–0.51) |
|
| ||||||||
| 60–69 | 0.95 (0.72–1.26) | 1.19 (0.68–2.10) | 0.89 (0.65–1.23) | 0.38 (0.33–0.43) | 0.96 (0.71–1.30) | 1.15 (0.79–1.70) | 0.63 (0.39–1.02) | 0.36 (0.27–0.48) |
|
| ||||||||
| 70–79 | 0.99 (0.77–1.28) | 1.63 (1.03–2.58) | 0.85 (0.63–1.15) | 0.49 (0.45–0.55) | 0.70 (0.56–0.87) | 0.78 (0.58–1.06) | 0.60 (0.44–0.83) | 0.39 (0.34–0.46) |
|
| ||||||||
| 80+ | 1.15 (0.62–2.16) | 2.01 (0.83–4.85) | 0.67 (0.25–1.81) | 0.70 (0.58–0.84) | 1.22 (0.94–1.59) | 1.85 (1.29–2.65) | 0.84 (0.57–1.26) | 0.58 (0.50–0.67) |
|
| ||||||||
| Tumor location | ||||||||
|
| ||||||||
| Right | 0.94 (0.74–1.20) | 1.33 (0.83–2.14) | 0.77 (0.58–1.03) | 0.47 (0.42–0.52) | 1.11 (0.91–1.35) | 1.45 (1.08–1.95) | 0.89 (0.67–1.18) | 0.51 (0.45–0.59) |
|
| ||||||||
| Left | 1.17 (0.95–1.45) | 1.98 (1.40–2.79) | 0.86 (0.66–1.14) | 0.46 (0.42–0.52) | 0.84 (0.70–1.02) | 0.95 (0.74–1.22) | 0.56 (0.41–0.78) | 0.40 (0.34–0.46) |
|
| ||||||||
| Type of surgery | ||||||||
|
| ||||||||
| Open resection | 0.93 (0.77–1.12) | 1.30 (0.90–1.89) | 0.78 (0.62–0.98) | 0.47 (0.43–0.51) | – | – | – | – |
|
| ||||||||
| Laparoscopy | 1.47 (1.10–1.96) | 2.56 (1.68–3.90) | 0.97 (0.65–1.45) | 0.46 (0.40–0.52) | – | – | – | – |
|
| ||||||||
| HR1 (95%CI) | ||||||||
|
|
||||||||
| Sweden | ||||||||
|
|
||||||||
| Stage II | ||||||||
|
|
||||||||
| Basic characteristics | Total2,3 | Low-risk | High-risk2,3 | Stage III2,3 | ||||
|
| ||||||||
| Overall | 0.62 (0.49–0.79) | 0.76 (0.37–1.57) | 0.58 (0.44–0.75) | 0.48 (0.43–0.54) | ||||
|
| ||||||||
| Gender | ||||||||
|
| ||||||||
| Men | 0.59 (0.42–0.83) | 0.85 (0.37–1.97) | 0.52 (0.36–0.75) | 0.45 (0.39–0.52) | ||||
|
| ||||||||
| Women | 0.66 (0.46–0.95) | 0.55 (0.13–2.28) | 0.64 (0.44–0.94) | 0.53 (0.44–0.62) | ||||
|
| ||||||||
| Age group | ||||||||
|
| ||||||||
| <60 | 1.12 (0.60–2.09) | 1.55 (0.55–4.40) | 1.07 (0.49–2.34) | 0.43 (0.29–0.65) | ||||
|
| ||||||||
| 60–69 | 0.66 (0.42–1.03) | 0.63 (0.15–2.63) | 0.63 (0.39–1.01) | 0.44 (0.34–0.56) | ||||
|
| ||||||||
| 70–79 | 0.46 (0.31–0.69) | 0.40 (0.06–2.88) | 0.46 (0.30–0.68) | 0.50 (0.43–0.58) | ||||
|
| ||||||||
| 80+ | NE | NE | NE | 0.49 (0.36–0.67) | ||||
|
| ||||||||
| Tumor location | ||||||||
|
| ||||||||
| Right | 0.49 (0.34–0.71) | 0.92 (0.33–2.52) | 0.45 (0.30–0.67) | 0.55 (0.48–0.64) | ||||
|
| ||||||||
| Left | 0.79 (0.56–1.11) | 0.71 (0.26–2.00) | 0.72 (0.50–1.04) | 0.40 (0.33–0.48) | ||||
|
| ||||||||
| Type of surgery | ||||||||
|
| ||||||||
| Open resection | 0.61 (0.47–0.78) | 0.79 (0.38–1.62) | 0.56 (0.43–0.73) | 0.49 (0.43–0.55) | ||||
|
| ||||||||
| Laparoscopy | 0.89 (0.29–2.74) | NE | 1.04 (0.30–3.65) | 0.42 (0.25–0.70) | ||||
Hazard ratio (HR) and 95% confidence interval estimate of adjuvant administration by subgroup of patients. HR was obtained from multiple Cox regression model adjusted for all other factor listed in the table. HRs displayed in bold are statistically significant, p values <0·05. HRs displayed with underline bold are statistically significant increased mortality rates.
HR was additionally adjusted by tumor size.
HR was additionally adjusted by lymph node counts: <10, ≥10 for The Netherlands, and <12, ≥12 for Sweden.
Abbreviation: NE, no enough data for HR calculation, –, data no available.
Figure 2.
Adjusted survival curves stratified by total Stage II (a), low-risk Stage II (b), high-risk Stage II (c) and Stage III (d), for patients receiving postsurgical adjuvant chemotherapy (ACT) and surgery only. Classification of low- and high-risk Stage II was done according to available high-risk prognostic factors for each country shown in Table 1. The survival estimates were obtained from Cox regression models with adjustment for gender, age group, tumor location, tumor size, type of surgery and lymph node count (the two latter factors were not available in Belgium).
No substantial changes in the overall hazard ratios for death were observed when ACT was added as a time-varying factor in the survival Cox models in Belgium and Sweden (Supporting Information, Table 2). Results were also consistent when Stage II patients were classified into low- and high-risk based on two factors of tumor size and histological grade, in the overall and subgroup analyses. Adjusted survival curves are displayed in Supporting Information, Figure 2.
Discussion
In this population-based retrospective cohort study, we investigated the administration of ACT for Stages II and III colon cancer patients in three different European countries. This study showed that a small proportion (varying from 9% to 24%) of Stage II and over half of Stage III (varying from 55% to 68%) colon cancer patients received ACT in the studied countries. ACT was more commonly used in all stage and age subgroups of patients in Belgium compared with The Netherlands and Sweden. Application of ACT compared to surgery only was associated with significantly higher survival in high-risk Stage II and Stage III, but not in low-risk Stage II colon cancer patients. The advantage in survival in Stage III patients receiving ACT was observed in all age subgroups of patients, though older patients were significantly less likely to receive ACT compared to their younger counterparts. We observed a similar inverse association between ACT administration and age of patients with Stage II (in total and low-/high-risk subgroups) colon cancer across studied countries.
Our finding of considerable differences across studied populations in the proportion of Stage II colon cancer patients who received ACT is consistent with the results of a European comparison pertaining to earlier years (between 2007 and 2009), showing the highest proportion of ACT administration in Belgium among other countries.23 The observed higher ACT utilization in Belgium also in this study is likely related to country-specific risk interpretation and considerations of starting treatment.24 It has been suggested that many of the European countries produce their own risk assessment rules which are adapted to their domestic resources and reimbursement system, availability of therapy facilities and interpretation of the present knowledge.25 For example, the ESMO recommends ACT administration for Stage II colon cancer patients with less than twelve examined nodes as a high-risk group.7 This recommendation is followed in most other countries except the Netherlands where cases with <10 examined nodes are classified in the poor prognostic group.21 In this study, we cannot explain the reason for higher ACT administration in Belgium. Further studies on selection criteria of colon cancer patients for ACT administration in European countries are needed to explore the reasons underlying major differences of care and their implications on patient outcomes. There are increasing efforts towards more personalized selection of patients for ACT in routine practice, such as detecting mutations of oncogenes (e.g., KRAS, BRAF and PIK3CA) and assessing DNA microsatellite instability.26,27
Several investigators have reported potential underutilization of ACT in Stage III colon cancer patients, despite the unequivocal recommendation of guidelines for ACT administration.8,9 For obvious reasons, all guidelines are based upon results from randomized clinical trials (RCTs), revealing gains from ACT. However, the RCTs reporting gains were conducted decades ago, and development in staging, surgery and pathology has decreased the risks of recurrence.2 The extent of this risk decrease is not clearly known,6 but the benefit of adjuvant therapy has in absolute terms decreased in recent years.2 Our finding that more than one-third of the studied Stage III patients (range 32–45%) did not receive ACT is consistent with results of a recent study from the US.9 In both the US study and this study, administration of ACT was inversely associated with age of patients. Elderly patients less often receive reference treatments and are underrepresented in the adjuvant RCTs, mainly because of their greater numbers of comorbidities, intolerance to the treatment-related toxicity, shorter natural life expectancies and reluctance for treatment.27–29
Compared to Stage III colon cancer, the survival differences according to the use of ACT were less pronounced for Stage II patients. Most RCTs on ACT addressed a mixed group of Stages II and III colon cancer patients and evidence regarding the Stage II patients therefore mostly come from post hoc subgroup analyses which were often unpowered to show a potential survival benefit.30,31 A recent Cochrane meta-analysis of 25 adjuvant RCTs in Stage II colon cancer patients demonstrated a modest but statistically significant enhancement of survival for patients receiving ACT compared to surgery only.32 However, a large observational study from the US did not show survival advantages of Stage II patients regardless of good or poor prognostic features.13 Similar results were reported in a study from Ontario, Canada,33 while another study showed improved survival associated with ACT regardless of high-risk features.34 Moreover, a recent large cohort study from England reported an increased risk of colorectal cancer death and no significant risk of death from other causes for Stage II patients receiving ACT.35 We observed ACT administration to be associated with higher survival in total Stage II patients in Sweden, and in high-risk Stage II patients in all countries. These patterns may support suggestions that the association of ACT with higher survival of high-risk Stage II patients seen in observational studies are primarily related to patient selection rather than reflect the direct benefit from ACT.16 For instance, the observed overall survival benefit of ACT in elderly patients in this study might be related to the selection of patients who were medically more fit for treatment.
In contrast to the observations in high-risk Stage II patients, we did not observe an association between survival and ACT in low-risk Stage II patients in Belgium and Sweden, and low-risk Stage II patients receiving ACT in The Netherlands showed lower survival compared with patients who did not receive ACT. This observation might possibly be explained by side effects of chemotherapy exceeding beneficial effects in the low-risk group.16 However, patient selection is also a reasonable explanation. Our findings support suggestions that the ACT administration for patients with Stage II colon cancer could often be spared with proper risk stratification based on clinicopathologic and molecular markers.27
A limitation of this study is that not all factors for classification of Stage II patients in low- and high-risk were available in all countries. Thus, some high-risk patients might have been wrongly classified as low-risk patients and the proportion of misclassification might be different across countries, depending on the availability of information on the risk factors. This may partly explain observed differences across countries. However, classification of Stage II patients was based only on the factors that were available in all countries, pT category and tumor grade did not materially change the results. Moreover, pT4 stage is also the most powerful factor for recurrence in many studies.16,36 Another limitation is the lack of data on patients’ comorbidities, which has been shown to be associated with poorer prognosis37,38 and has, therefore, strong influence on decisions to apply ACT. The lack of data on chemotherapy regimen administered to patients, which has shown to influence survival for Stage III39 but not for Stage II,34 further limits our study. As details of recurrence of the tumor and cause of death were no available, we could not perform recurrence-free survival analysis. A major strength of the study includes the presence of most recent, high-quality, long-term population-based data with large sample size and very good completeness of follow-up information. Another strength is the full spectrum of age distribution including elderly patients who are often excluded from RCTs. Therefore, this study may help fill a knowledge gap left by RCTs and provide real-world results regarding ACT use and associated outcomes in different healthcare systems.
Summing up, we observed large differences in the proportion of Stages II and III colon cancer patients receiving ACT between Belgium and the other studied populations (Sweden and The Netherlands). The reason for the large variation in the administration of ACT for Stages II and III colon cancer patients across studied populations needs further investigation. The results of this study are consistent with an overall survival advantage of ACT for Stage III and high-risk Stage II but not for low-risk Stage II colon cancer patients. Further investigations are needed to elucidate the reasons for the differences in selection of Stages II and III colon cancer patients for ACT and their implications for prognosis in different European countries.
Supplementary Material
What’s new?
Adjuvant chemotherapy (ACT) is recommended for Stage III and high-risk Stage II colon cancer, to eliminate any microscopic residual disease. However, it is not clear how much benefit ACT actually provides. One reason is that there are wide variations in whether these patients receive ACT or not. In this European study, the authors found that ACT was consistently associated with improved overall survival. The reasons for differences in administration of ACT and their implications for prognosis should therefore be investigated.
Acknowledgments
Other Information
The study was funded by the FP7 program of the European Commission as part of an EUROCANPlatform project (project number: 260791). Additional support was obtained from the German Cancer Aid (Deutsche Krebshilfe, Reference Number 111365). The sponsors had no role in data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data and had the final responsibility to submit for publication. This study was carried out in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) and was approved by the ethics committee of the Medical Faculty of the University of Heidelberg, Germany.
Abbreviations
- ACT
adjuvant chemotherapy
- CI
confidence interval
- HR
hazard ratio
- OR
odds ratio
Footnotes
Additional Supporting Information may be found in the online version of this article.
Conflict of Interest Statement: We declare that we have no conflict of interest.
Contributors
MB coordinated data acquisition from the countries and participated in data harmonization and interpretation of results and wrote the manuscript. YB participated in data harmonization, performed the statistical analysis and contributed to the interpretation of the results. LJ contributed to interpretation of the results and writing of the manuscript. VL, FvE, LvE, EV, AS and BG provided valuable data from their respective participating registries, reviewed the manuscript and contributed to the discussion of results. CMU and PSK participated in protocol development, reviewed the manuscript and contributed to discussion and interpretation of results. HB designed and supervised the study, interpreted results and contributed to manuscript writing. All authors critically revised the manuscript and approved the final version.
References
- 1.Galandiuk S, Wieand HS, Moertel CG, et al. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27–32. [PubMed] [Google Scholar]
- 2.Pahlman LA, Hohenberger WM, Matzel K, et al. Should the benefit of adjuvant chemotherapy in colon cancer be re-evaluated? J Clin Oncol. 2016;34:1297–9. doi: 10.1200/JCO.2015.65.3048. [DOI] [PubMed] [Google Scholar]
- 3.Bernhoff R, Martling A, Sjovall A, et al. Improved survival after an educational project on colon cancer management in the county of Stockholm–A population based cohort study. Eur J Surg Oncol. 2015;41:1479–84. doi: 10.1016/j.ejso.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol. 2015;16:161–8. doi: 10.1016/S1470-2045(14)71168-4. [DOI] [PubMed] [Google Scholar]
- 5.Deijen CL, Vasmel JE, de Lange-de Klerk ESM, et al. Ten-year outcomes of a randomised trial of laparoscopic versus open surgery for colon cancer. Surg Endosc. 2017;31:2607–15. doi: 10.1007/s00464-016-5270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bockelman C, Engelmann BE, Kaprio T, et al. Risk of recurrence in patients with colon cancer stage II and III: a systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16. doi: 10.3109/0284186X.2014.975839. [DOI] [PubMed] [Google Scholar]
- 7.Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol. 2012;23:2479–516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 8.Winget M, Hossain S, Yasui Y, et al. Characteristics of patients with stage iii colon adenocarcinoma who fail to receive guideline-recommended treatment. Cancer Am Cancer Soc. 2010;116:4849–56. doi: 10.1002/cncr.25250. [DOI] [PubMed] [Google Scholar]
- 9.Hines RB, Bimali M, Johnson AM, et al. Prevalence and survival benefit of adjuvant chemotherapy in stage III colon cancer patients: comparison of overall and age-stratified results by multivariable modeling and propensity score methodology in a population-based cohort. Cancer Epidemiol. 2016;44:77–83. doi: 10.1016/j.canep.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Dobie SA, Baldwin LM, Dominitz JA, et al. Completion of therapy by Medicare patients with stage III colon cancer. J Natl Cancer Inst. 2006;98:610–9. doi: 10.1093/jnci/djj159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris M, Platell C, McCaul K, et al. Survival rates for stage II colon cancer patients treated with or without chemotherapy in a population-based setting. Int J Colorectal Dis. 2007;22:887–95. doi: 10.1007/s00384-006-0262-y. [DOI] [PubMed] [Google Scholar]
- 12.Benson AB., 3rd New approaches to the adjuvant therapy of colon cancer. Oncologist. 2006;11:973–80. doi: 10.1634/theoncologist.11-9-973. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol. 2011;29:3381–8. doi: 10.1200/JCO.2010.34.3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. JCO. 2004;22:3408–19. doi: 10.1200/JCO.2004.05.063. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava G, Renfro LA, Behrens RJ, et al. Prospective multicenter study of the impact of oncotype DX colon cancer assay results on treatment recommendations in stage II colon cancer patients. Oncologist. 2014;19:492–7. doi: 10.1634/theoncologist.2013-0401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Kennecke HF, Renouf DJ, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer Am Cancer Soc. 2015;121:527–34. doi: 10.1002/cncr.29072. [DOI] [PubMed] [Google Scholar]
- 17.Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24:vi64–72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 18.McVie G, Ringborg U. EurocanPlatform, an FP7 project of the European Commission-first year commentary. Ecancermedicalscience. 2012;6:ed13. doi: 10.3332/ecancer.2012.ed13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Babaei M, Balavarca Y, Jansen L, et al. Minimally invasive colorectal cancer surgery in Europe implementation and outcomes. Medicine. 2016;95:e3812. doi: 10.1097/MD.0000000000003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratto C, Ricci R. Potential pitfalls concerning colorectal cancer classification in the seventh edition of the AJCC Cancer Staging Manual. Dis Colon Rectum. 2011;54:E232–E. doi: 10.1097/DCR.0b013e31821def52. [DOI] [PubMed] [Google Scholar]
- 21.Netherlands Comprehensive Cancer Centre. National Evidence Based Guideline for Colon Cancer. 2014 Available from < http://www.oncoline.nl/coloncarcinoom>.
- 22.Mi XJ, Hammill BG, Curtis LH, et al. Use of the landmark method to address immortal person-time bias in comparative effectiveness research: a simulation study. Statist Med. 2016;35:4824–36. doi: 10.1002/sim.7019. [DOI] [PubMed] [Google Scholar]
- 23.Breugom AJ, Bastiaannet E, Boelens PG, et al. Adjuvant chemotherapy and relative survival of patients with stage II colon cancer - A EURECCA international comparison between the Netherlands, Denmark, Sweden, England, Ireland, Belgium, and Lithuania. Eur J Cancer. 2016;63:110–7. doi: 10.1016/j.ejca.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Peeters M, Zlotta A, Roucoux F, et al. Nationale Richtlijnen van het College voor oncologie Reports. 29A. Brussel: FederaalKenniscentrum voor de gezondheidszorg (KCE); Apr, 2006. KCERef. D/2006/10.273/12. [Google Scholar]
- 25.Kanavos P, Schurer W. The dynamics of colorectal cancer management in 17 countries. Eur J Health Econ. 2010;10:S115–29. doi: 10.1007/s10198-009-0201-2. [DOI] [PubMed] [Google Scholar]
- 26.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 27.Dienstmann R, Salazar R, Tabernero J. Personalizing colon cancer adjuvant therapy: selecting optimal treatments for individual patients. JCO. 2015;33:1787–96. doi: 10.1200/JCO.2014.60.0213. [DOI] [PubMed] [Google Scholar]
- 28.Pasetto LM, Rossi E, Jirillo A, et al. Colorectal cancer adjuvant treatment in elderly patients. Crit Rev Oncol Hematol. 2005;55:201–6. doi: 10.1016/j.critrevonc.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 29.El Shayeb M, Scarfe A, Yasui Y, et al. Reasons physicians do not recommend and patients refuse adjuvant chemotherapy for stage III colon cancer: a population based chart review. BMC Res Notes. 2012;5:269. doi: 10.1186/1756-0500-5-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. JCO. 2012;30:3353–60. doi: 10.1200/JCO.2012.42.5645. [DOI] [PubMed] [Google Scholar]
- 31.Hendlisz A, Puleo F, Deleporte A, et al. In deciphering the future of adjuvant treatment in colon cancer, the journey matters more than the achievements. Curr Colorectal Cancer Rep. 2016;12:57–66. [Google Scholar]
- 32.Meyers BM, Al-Shamsi HO, Figueredo AT. Cochrane systematic review and meta-analysis of adjuvant therapy for stage II colon cancer. ASCO Annual Meeting Proceedings. 2015;33:3513. [Google Scholar]
- 33.Booth CM, Nanji S, Wei X, et al. Adjuvant chemotherapy for stage II colon cancer: practice patterns and effectiveness in the general population. Clin Oncol. 2017;29:e29–38. doi: 10.1016/j.clon.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Casadaban L, Rauscher G, Aklilu M, et al. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer Am Cancer Soc. 2016;122:3277–87. doi: 10.1002/cncr.30181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hippisley-Cox J, Coupland C. Development and validation of risk prediction equations to estimate survival in patients with colorectal cancer: cohort study. BMJ. 2017;357:j2497. doi: 10.1136/bmj.j2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verhoeff SR, van Erning FN, Lemmens VE, et al. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer. 2016;139:187–93. doi: 10.1002/ijc.30053. [DOI] [PubMed] [Google Scholar]
- 37.Ostenfeld EB, Norgaard M, Thomsen RW, et al. Comorbidity and survival of Danish patients with colon and rectal cancer from 2000–2011: a population-based cohort study. Clin Epidemiol. 2013;5:65–74. doi: 10.2147/CLEP.S47154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sarfati D, Hill S, Blakely T, et al. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: a retrospective cohort study. BMC Cancer. 2009;9:116. doi: 10.1186/1471-2407-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hsiao FY, Mullins CD, Onukwugha E, et al. Comparative effectiveness of different chemotherapeutic regimens on survival of people aged 66 and older with stage III colon cancer: a “real world” analysis using Surveillance, Epidemiology, and End Results-Medicare data. J Am Geriatr Soc. 2011;59:1717–23. doi: 10.1111/j.1532-5415.2011.03501.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.