Abstract
Optical pacing (OP) uses pulsed infrared light to initiate heartbeats in electrically-excitable cardiac tissues without employing exogenous agents. OP is an alternative approach to electrical pacing that may overcome some its disadvantages for some applications. In this review, we discuss the initial demonstrations, mechanisms, safety, advantages and applications of OP.
Keywords: Optical Pacing, Infrared Light, Infrared Control of Excitable Tissues
INTRODUCTION
Electrical cardiac pacing has been the gold standard for rate and rhythm control of the heart for decades. The experimental history of cardiac pacing reaches back to the 18th century, and the first pacemaker was implanted in a human in 1958.1 This technology became more understood as scientists in the 1960s made reproducible recordings of cardiac conduction.2 Comparing 12-lead ECGs and information from programmed electrical stimulation made it possible to classify arrhythmias based on their site of origin and mechanism.3 In research laboratories, electrical cardiac pacing has led to a better understanding behind the mechanisms of electrical propagation in both normal rhythm and arrhythmias.4,5 It has also allowed investigators to test antiarrhythmic compounds.5 In modern medicine, this has led to a variety of successful methods (e.g., pacemakers, antiarrhythmic drugs, radiofrequency ablation) to treat arrhythmias.
However, electric current has some limitations for cardiac pacing that constrain its use. First, electric current quickly spreads when applied to tissue which creates artifacts on nearby electrodes.6–8 This limits how close a stimulation electrode can be placed near a recording electrode in any setup. Second, current spread also restricts the spatial precision of the pacing site by stimulating an area greater than the electrode itself.9,10 Third, electrical pacing requires direct contact with the tissue which can increase the complexity of a setup. Fourth, devices, such as MRI and metal detectors can interfere with electrical signals.11 Fifth, there is a limited ability to selectively activate different types of cardiomyocytes. Finally, electrical pacemakers limit autonomic modulation of heart rate, such as occurs during exercise tolerance and emotional responses. There is a need to develop additional pacing strategies that overcome these limitations.
In this review we will examine a new experimental pacing modality, Optical Pacing (OP). OP uses pulses of infrared light directed at electrically-excitable cardiac tissues to initiate a heartbeat. No exogenous agents are required. OP is a subsection of infrared control – see Figure 1. Infrared control is defined as thermally-induced infrared light activation or inhibition of cardiac or neural tissue. OP shows promise in overcoming many of the limitations of electrical pacing and may develop into a complementary approach for rhythm control of cardiac tissue.
Figure 1. Infrared Control of Excitable Tissues (ICE-T).
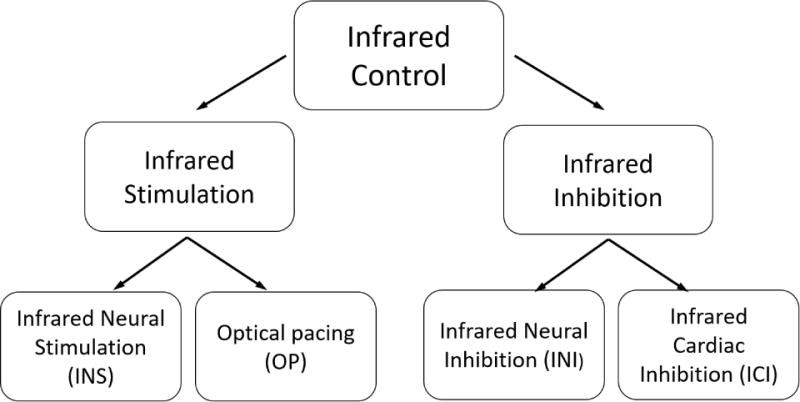
The diagram shows different categories of ICE-T. ICE-T is defined as thermally-induced infrared light activation or inhibition of cardiac or neural tissue. Infrared neural stimulation (INS) was first discovered in 200512,13, while infrared neural inhibition was discovered in 2012.14,15 Optical pacing was discovered in 2010,16 while infrared cardiac inhibition was first demonstrated in 2016.10
EARLY DEMONSTRATIONS
In the 60s and 70s, groups demonstrated that ultraviolet and visible light could modulate the excitability of myocardial tissue.17,18 In 2008, Smith et al. paced small groups of cardiomyocytes with limited success using a Ti:Sapphire laser (780 nm).19 By moving further in the infrared, Jenkins et al. paced the first intact heart.16 Briefly, a pulsed diode laser (λ = 1875 nm) coupled to a multimode fiber was positioned 500 μm from the heart’s inflow tract in a living embryonic quail. Quail embryos ranged from stage 12 to 18, all tubular heart stages. The optical fiber illuminated a spot size of 0.3 mm2 over the inflow tract of the heart tube, never making contact with the tissue. Reliable and repeatable pacing was achieved.
Figure 2 shows OP of an embryonic quail heart. The blue tracing represents the laser pulse, and the red tracing represents the heart rate. The heart was paced at 2Hz (Figure 2a), well above the intrinsic rate of the embryo, and the heart returned to its intrinsic rate when the laser stopped. The expansion of the tracings in Figure 2b clearly show the pulsed laser initiating the heartbeat. The pacing rate was changed from 2 Hz to 3 Hz and back to 2 Hz demonstrating the ability to quickly achieve capture at different frequencies. The radiant energy threshold at which 50% of embryos would pace was found to be 0.81 J/cm2/pulse.
Figure 2. Pacing of the embryonic quail heart from Jenkins et al16.
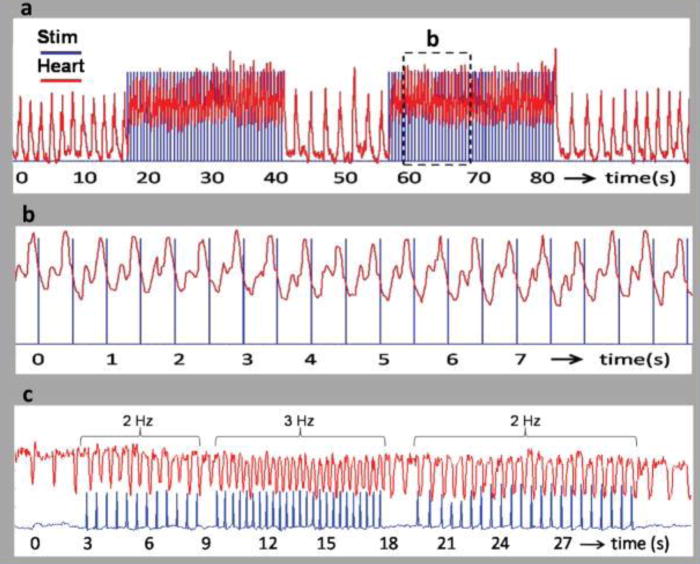
a,b, The trigger pulse (blue) from the pulsed laser is superimposed on the heart rate (red). (a) Recording from a stage 17 (59 hour) quail embryo paced at 2 Hz. The laser pulse duration was 1 ms and the radiant exposure was 0.92 J/cm2 per pulse. The laser pulses were turned on and off several times to demonstrate the robustness of optical pacing. The heart rate increased from 0.634 to 2 Hz when the stimulation laser pulses were started and decreased to 0.693 Hz by the end of the trace in 2a. (b) The dashed box in 2a is expanded for a close up view in 2b. Clearly the trigger pulse and LDV signal were synchronized with each laser pulse eliciting a heartbeat. (c) Recording from a stage 14 (53 hour) quail embryo. The laser pulse duration was 2 ms and the radiant exposure was 0.84 mJ/cm2 per pulse. The frequency of the laser pulses were varied from 2 Hz to 3 Hz and back to 2 Hz to demonstrate the ability of the embryo heart to follow the pulse frequency.
This work was further developed by Jenkins et al20 in the adult rabbit heart. Adult New Zealand rabbit hearts were excised, cannulated, and perfused, allowing them to survive for 4-6 hours. Several electrodes were sutured to the epicardium to collect ECG data. Pulsed 1851 nm light from a diode was coupled to a multimode fiber placed on either the right or left atrium. With a larger beating heart, contact was not required for pacing, but was used to guarantee that the site of excitation did not change as the heart moved. Again, the laser signal and the ECG were recorded. In each recording, the laser pulse occurred at the beginning of each cardiac cycle. This with the timing between the pulse and QRS complex, showed that each pulse was initiating and not modulating the heartbeat, and that the conduction was propagated through the AV node. Hearts were successfully paced over a range of frequencies, each time returning to the intrinsic rate after conclusion of pacing. OP was accomplished from sites in both left and right atria, but exact locations differed among individual hearts. Consistent OP of the ventricles was not achieved. The stimulation threshold was found to be affected by the pacing frequency with higher frequencies requiring higher stimulation thresholds. Longer pulse widths (~10 ms) required lower radiant exposure levels to pace the heart as also observed in the embryonic heart.21 Pacing the adult heart was not as robust as embryo heart pacing with pacing times not extending past five minutes, although it is likely that further optimization of pacing protocols will lead to increased reliability and repeatability of adult heart OP in the future.
These studies demonstrated multiple concepts that are significant for future in vivo studies. First, non-contact pacing with infrared light is possible over a range of frequencies with living embryonic hearts. Second, each pulse of the laser initiates, not modulates, a heartbeat, and the heart is able to return to its intrinsic rate after pacing. Third, longer pulse widths (~10 ms) require less radiant exposure to achieve capture. Finally, the stimulation threshold increases as the pacing frequency increases.
MECHANISM
To improve the utility of OP, it is important to determine the mechanisms of action. Because light-tissue interactions are complex, it has been difficult to determine the underlying principles and several theories have arisen. All groups agree that OP works by producing a thermal gradient in the tissue, but how the thermal gradient is transduced by the tissue is still debated. Theories include induced capacitive currents,22,23 actions on mitochondrial calcium currents,24–26 actions on ion channels,27,28 and sarcomeric auto-oscillations.29,30 It is feasible that multiple effects combine to produce pacing or that an alternative process (e.g., photomechanical forces31) is responsible. Below we discuss details from some of the mechanism studies in cardiac tissue.
Soon after the first OP demonstration, Dittami et al26 found evidence that pulsed IR light (1862 nm) induced intracellular calcium transients. Using fluorescence confocal microscopy with rat ventricular cardiomyocytes, they compared evoked and spontaneous Ca2+ transients. Those transients evoked by IR were distinguished by spatial uniformity and temporal synchrony of Ca2+ release with the IR stimulus. They also had significantly lower amplitudes and faster recovery time constants than spontaneous transients. The response to IR pulses was dose dependent, as the probability of evoking a Ca2+ transient increased with greater IR energy per pulse. To further elucidate the mechanism behind IR-induced intracellular Ca2+ transients, the group tested 4 compounds that interfere with intracellular Ca2+ transport. Ryanodine, an alkaloid that binds strongly to ryanodine receptors (RyRs) in the sarcoplasmic reticulum, has a major role in calcium induced calcium release and locks the Ca2+/K+ channel in a slow-gating subconductive state.32 Ryanodine treatment did not abolish IR-evoked responses. However, it did significantly decrease the IR-evoked Ca2+ transient amplitude (see Figure 3A), which suggests that RyR release has an indirect contribution to the effect induced by infrared light. Three other compounds, all inhibitors of mitochondrial Ca2+ transporters with differing mechanism (CGP-37157 – inhibitor of mitochondrial Na+/Ca2+ exchanger; Ruthenium Red – inhibitor of the mitochondrial Ca2+ uniporter; and 2-APB – an IP3 channel antagonist), were strong inhibitors of the IR-evoked Ca2+ response (see figure 3B–D). Further work by Lumbreras et al. produced similar results in neonatal spiral and vestibular ganglion neurons.25 These findings strongly point to the mitochondrial Ca2+ exchanger and uniporter as likely sources of IR-evoked Ca2+ transients.
Figure 3. Infrared-induced calcium transients from Dittami et al26.
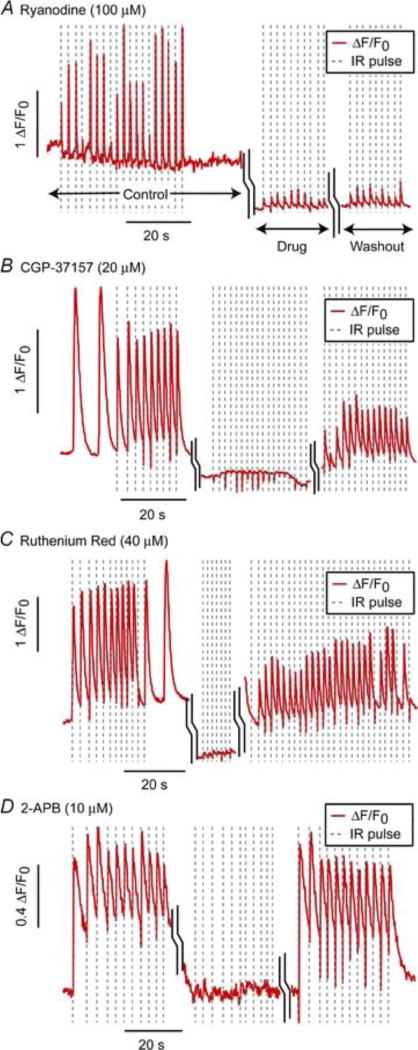
Pre-drug Ca2+ (control) fluorescence, followed by exposure to compounds that interfere with Ca2+transport and subsequent 4× washout (a) Ryanodine, an RyR channel antagonist, (b) CGP-37157, a known inhibitor of mNCX, (c) Ruthenium Red, an inhibitor of mCU, (d) 2-APB, an IP3 channel antagonist. Each was a strong inhibitor of the IR-evoked Ca2+ response, suggesting the mitochondrial uniporter and transporter as the source of IR-induced Ca2+ transients.
In 2012, Shapiro et al22 found evidence that a rapid IR-induced temperature change excited cells by altering the electrical capacitance of the plasma membrane. These experiments were performed with untreated oocytes, cultured mammalian cells, and artificial lipid bilayers. Oocytes expressing voltage-gated Na+ or K+ channels and wild-type oocytes were exposed to IR pulses. All oocytes displayed inward currents under voltage-clamp conditions. Current magnitudes and reverse potentials were not altered by eliminating channel permeable ions in solution, replacing Na+ with K+ in buffer, or transporter inhibitors. Oocytes expressing voltage-gated channels also demonstrated action potentials when depolarized past threshold. In neuronal optical stimulation experiments, it had been hypothesized that water absorbed heat, which was then converted to energy for cell excitation. Therefore heavy water (D20) was used to replace normal water, as its absorption coefficient is much lower. With D20, there was a significant decrease in potentials compared to H20, which further endorsed the role of water in the IR-induced excitation mechanism. Similar results were found in the mammalian cells. Voltage-clamped artificial bilayers pulsed with IR light did elicit currents (Figure 4a and 4b). The shape of these currents were similar to the oocyte and mammalian cells. Furthermore, replacing H20 with D20 again significantly reduced the response (Fig 4c). Also, adding multivalent cations (Gd3+ ; MgCl2) to either the external or internal side of the membrane produced greater currents toward the other side. IR light induced depolarizations of up to 9 mV.
Fig. 4. IR light transiently alters membrane capacitance from Shapiro et al22.
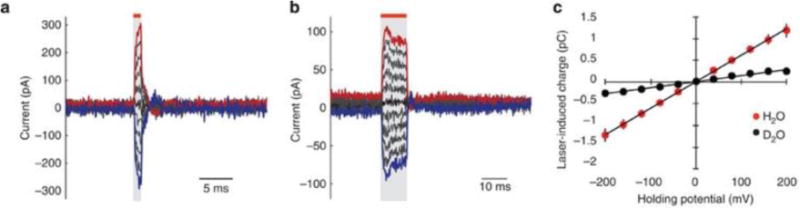
(a) As the energy of a 1 ms IR pulse is increased, higher capacitive currents are measured in response to the light in a voltage-clamped artificial bilayer. (b) As the energy of a 10ms IR pulse is increased, higher capacitive currents are measured in response to the light in an artificial bilayer. With each size pulse the current closely follows the timing of the IR pulse. Red bars and grey shading indicate the timing of laser stimulation. In both (a) and (b) the characteristic transient of a voltage-gated channel is not seen. (c) Q–V curves acquired in artificial bilayers with increasing IR stimulation in H2O (red) and D2O (blue). The different absorption of D2O results in a significantly decreased the IR-induced transient, further validating that H2O plays a significant role.
A couple years later, Liu et al23 also demonstrated that IR light can induce capacitive inward currents. They made voltage-clamp recordings in the neuromuscular junction of C. elegans. They showed that a temperature gradient of 500 °C/s (500 μs pulse) evoked ~35 pA of inward current, while increasing the temperature 0.25 °C. Currents were also recorded in the absence of extracellular Ca2+, Na+, and K+, again confirming that these currents are independent of major ionic conduction currents. Both Shapiro22 and Liu23 show convincing evidence that temperature gradients produced by IR light displace ions near the plasma membrane which leads to an inward capacitive current.
In 2012, Oyama et al29 demonstrated that IR pulses could induce contractions in rat cardiomyocytes kept in Ca2+ free solution. The temperature increase required for contraction was greater at room temperature than at 36 °C, but results also suggested that the rapid temperature increase, not the absolute temperature change, was important for contraction. An inhibitor of actin-myosin interaction (Blebbistatin) blocked contraction. Based on these findings, the group hypothesized that the IR-induced temperature change causes a partial dissociation of F-actin from tropomyosin allowing the myosin heads to attach and cause contraction. When the temperature returns to normal, the tropomyosin recovers its affinity for F-actin, and the muscle returns to its relaxed state.
Several years later, the Ishiwata group investigated how heating affects sarcomere states.29 Myocardial sarcomere function is based on an equilibrium of the binding or detachment of myosin heads and actin filaments. This equilibrium has a graded regulation, and so cardiac sarcomeres demonstrate spontaneous oscillations in a partially activated state. In myocytes, IR light induced high-frequency sarcomeric auto-oscillations (here termed as Hyperthermal Sarcomeric Oscillations, or HSOs) which stopped when the IR light was turned off. The HSOs were induced by IR pulses with and without the presence of ryanodine or thapsigargin, showing this occurs without changes in Ca2+. An actomyocin inhibitor (N-benzyl-ptoluenesulfonamide) significantly decreased the sarcomere length displacement during heating. At room temperature large increases (15-19 °C) were needed to induce HSOs, while much smaller increases (3.3 °C) were required at physiologic temperatures. They therefore concluded that the absolute intracellular temperature caused the HSOs, not the change in the temperature. The sum of the data show that HSOs can be induced by IR pulses and co-exist with slower Ca2+ dependent sarcomeric contractions. However, it has yet to be investigated as to whether these HSOs play a role in IR Pacing with in vivo hearts.
With each of these studies, there remains the common theme that the temperature increase is responsible for the physiologic changes seen with IR light. This evidence also suggests the IR-induced effects are multifactorial and can be related to Ca2+ transients, membrane capacitance changes, actin-myosin interactions or other factors. These factors are diagramed in Figure 5 for clarity. How these mechanisms may interact with each other to allow pacing of an in vivo heart is still under investigation.
Figure 5. Mechanisms of cardiomyocyte contraction caused by IR light.
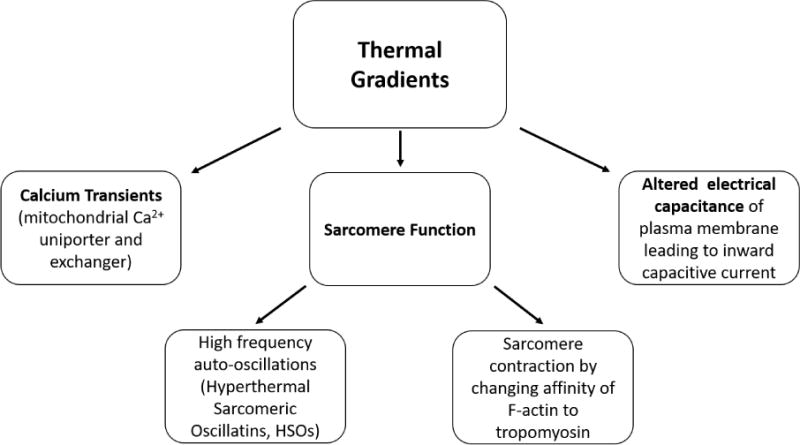
Calcium transients, altered electrical capacitance, and sarcomere function may each play a role.
SAFETY
To determine whether OP can be used in basic science studies and in the clinic, it is crucial to establish the safety of heating the tissue with IR light. Several INS studies suggest that IR light can be applied safely. Wells et al12,13,34 had previously found radiant exposures at which mammalian peripheral nerves could be repetitively stimulated without damage. The Richter group demonstrated that 4-8 hours of continuous infrared stimulation per day for a month for an optical cochlear implant in a cat model was safe.35 In a human feasibility study, Cayce et al. showed no histological evidence of tissue damage in resected dorsal roots harvested from patients undergoing dorsal rhizotomy after application of IR light in vivo.36 In the initial embryonic experiments by Jenkins et al.,16 transmission electron microscopy (TEM) was used to examine the cardiomyocytes targeted by the laser. Both control hearts and hearts paced slightly above threshold showed no ultrastructural abnormalities, while hearts paced far above threshold showed vacuolated mitochondria and slightly expanded nuclear envelopes and endoplasmic reticulum. Ford et al. showed that embryonic quail hearts paced slightly above the intrinsic rate developed similar to controls and showed no adverse effects.37 In the adult rabbit heart, propidium iodide (PI) was used to determine if OP caused cell membrane disruption.20 In some hearts, a small number of cells near the stimulation site were positively stained indicating some cell membrane disruption and a need to reduce stimulation thresholds. The radiant exposures required to pace the adult heart were approximately an order of magnitude higher than in the embryo. Although OP appears safe for some applications, further testing is needed.
ADVANTAGES
Using infrared light to control heart rate gives several advantages over electrical stimulation. Below we discuss some of the unique applications allowed by using light instead of current.
Studies of electrical activity in small tissues (< 2mm) have lacked a viable point stimulation technique. Point stimulation is needed to determine rate-dependent parameters such as conduction velocity and action potential duration. Without point stimulation one cannot quantify or compare electrophysiologic parameters in wild-type and mutant tissues. Unfortunately, electrical current delivery lacks spatial precision. Electrical stimulation also produces electrical artifacts on recording electrodes and at the stimulation site.6–9 Because light can be focused precisely and does not produce electrical artifacts, it can overcome the limitations of electrical stimulation. Wang et al. demonstrated point stimulation of embryonic quail, mouse and zebrafish hearts with infrared light.21,34 Briefly, a single-mode fiber delivered a 12 μm spot to pace the embryonic quail heart, while signals from voltage-sensitive dyes were recorded with an EMCCD camera (optical mapping – OM). Embryos were paced at a range of developmental stages. Figure 5 shows recording traces at specific locations in addition to activation and action potential duration maps for paced and un-paced, day 2 and day 5 quail embryos. The infrared light did induce a small artifact in the OM recording due to thermal lensing, but this artifact is significantly smaller than electrical pacing artifacts. More recently, Ma et al., utilized OP point stimulation in conjunction with volumetric optical mapping38. Gu et al. employed OP point stimulation to help demonstrate improved algorithms for quantify conduction velocity,39 and McPheeter et al., used OP point stimulation to demonstrate an all optical system for high-throughput screening of cardiac electrophysiology for human cardiomyocytes.40
Studies exploring modulation of heart rate in early embryos have not been practical because early embryos are so delicate and electrical stimulation requires contact which would cause damage. Since OP does not require contact, it can overcome this limitation. Ford et al used OP to study how changes in hemodynamics influences heart development and can lead to congenital heart defects (CHDs).37 Briefly, heart rate was increased with OP to increase regurgitant flow in the tubular heart (stage 14 – day 2). Optical coherence tomography41 simultaneously acquired pulsed and color Doppler images of blood flow in the inflow of the tubular heart. Embryos were then allowed to mature to stage 19 (day 3) or 34 (day 8) to assess for endocardial cushion defects and congenital heart disease in a four-chambered heart, respectively. Measured regurgitant blood flow on day 2 was strongly and inversely correlated with smaller endocardial cushions on day 3 (Figure 6). Those with the most regurgitant flow were more likely to die before stage 34, indicating that the most severe endocardial cushion alterations are lethal. Of the hearts that were paced and imaged at stage 34, all but one displayed a CHD. All defects were related to endocardial cushion defects. This type of non-invasive functional change in such small embryos is not currently inducible by other means. Other models of CHDs, such as those induced by ethanol exposure and cardiac neural crest cell ablation, also display increased regurgitant flow, abnormal endocardial cushions, and CHD at the 4-chambered stage (Figure 7). These data suggest that regurgitant flow may play a role in the development of CHD, making non-contact experimentation with live embryos crucial to exploring the pathology of CHDs. OP offers a unique ability to accomplish this.
Figure 6. Optical mapping of unpaced and optically paced hearts from Wang et al21.
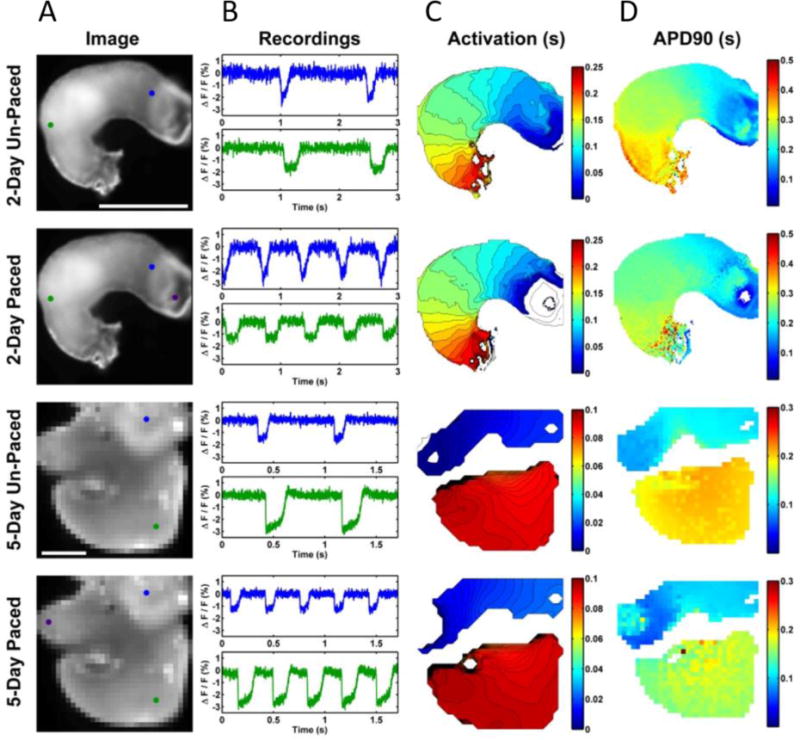
2 and 5 day excised quail hearts (a) have markers for the AV junction (2 day, blue), atrium (5 day, blue), ventricle (green). The purple markers indicate the position of the pacing laser. Scale bars are 500 m. (b) shows representative recordings at blue and green location for paced and un-paced hearts. (c) shows the resulting activation maps and (d) the action potential durations.
Figure 7. Influence of regurgitant flow on cardiac cushion volume from Ford et al37.
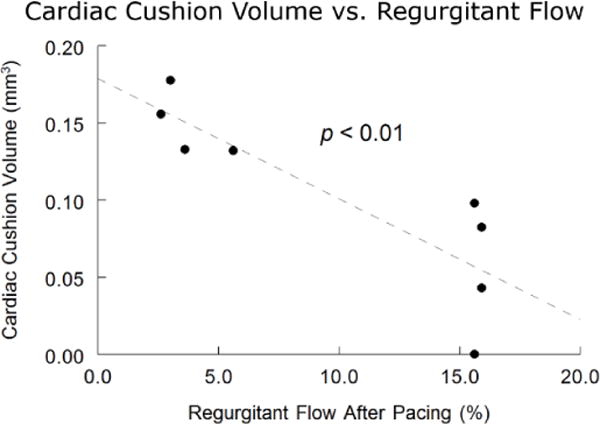
The amount of regurgitant flow experienced by quail embryos optically paced on day 2 was inversely correlated to endocardial cushion size 24 hours later.
Recently, Greenberg et al., showed that neural crest cells can be differentiated to a cardiomyogenic lineage.42 They used OP to stimulate cell aggregates to study their contractile ability. The aggregates could be paced for 10 minutes at frequencies ranging from 0.25-2 Hz. OP can be a useful technique to study cardiac differentiation in the future.
Another advantage of OP is the ability to rapidly and reversibly inhibit action potentials with high spatial precision. Wang et al10 found that IR light directed at the atrium of a beating heart could block the initiation of contraction (figure 8b). Focusing on the ventricle blocked propagation of the calcium transients in that area, which led to blockage of calcium transients downstream of that point, but not upstream (figure 8a). OM with voltage-sensitive dyes showed that depolarization stopped at the site of inhibition, indicating that IR inhibition blocks membrane depolarization which leads to no Ca2+ transients and contraction. IR suppression has also been described in neurons,14,43 indicating a broader physiological importance for this technology. In physiologic experiments this shows great potential for investigation of disease processes by allowing temporary disruption of normal function without physical contact with the tissue. It may also allow for tissue collection or manipulation in a temporarily motionless heart.
Figure 8. Comparison of pacing-induced cardiac defects with fetal alcohol syndrome (ethanol exposed) and velo-cardio-facial syndrome/Digeorge (cardiac neural crest cell (CNCC) ablation) models from Ford et al37.
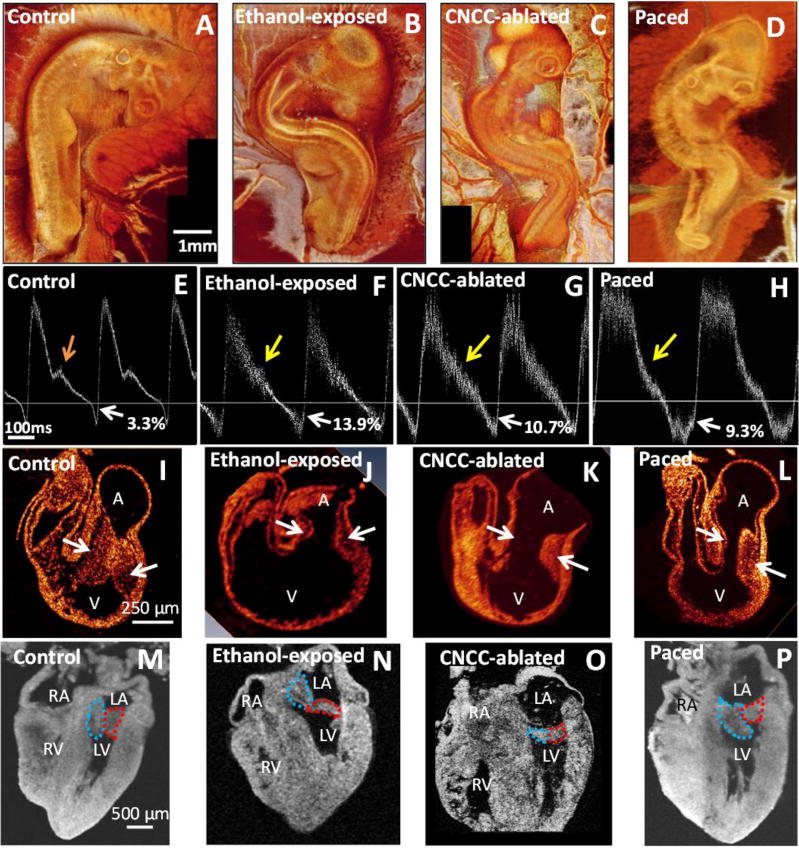
(a–d) are representative day 3 3-D OCT reconstructions. The experimental models show a spinal curvature similar to scoliosis, which is a common comorbidity of CHDs. (e–h) are representative day 3 pulsed-Doppler traces. The horizontal line delineates forward (above) and regurgitant (below) flows. Control embryos (e) always have a notch indicated by the orange arrow and mild regurgitation (white arrow, 1-3%). Pulsed-Doppler waveforms from each of the experimental models often have reduced or absent shoulders (yellow arrows) and significantly increased retrograde flow (white arrows). (i–l) show day 3 cross sections of the heart, and the experimental models have distorted and smaller endocardial cushions (arrows) than the control. (m–p) show the resultant heart morphology at day 8, with mitral leaflets outlined in red and blue. Valves are thicker at this stage of development than at maturity. The ethanol-exposed (m), CNCC-ablated (n), and paced (o) hearts show unusual morphology and coaptation of the leaflets. The paced heart (p) demonstrates severe right ventricular hypoplasia, tricuspid atresia, and a malformed mitral valve. We believe altered hemodynamics is a contributing factor in all three models and explains the similar phenotypes. Altered hemodynamics leads to smaller cardiac cushions, which develop into abnormally shaped valve leaflets. A – atrial region; V – ventricular region; LA – left atrium; RA – right atrium; LV left ventricle; RV – right ventricle
An increase in baseline temperature may cause infrared Inhibition, in contrast to a spatiotemporal thermal gradient (dT/dt, dT/dz) which is believed to cause excitation. One can either set laser parameters to achieve brief temperature transients for stimulation or baseline temperature increases for inhibition. The mechanism of IR inhibition may result from non-uniform rate increases in temperature-dependent Hodgkin-Huxley gating mechanisms. As the temperature increases, the Na2+ channel inactivation rate and K+ channel activation rate overwhelm the Na2+channel re-activation rate.44–46 Further studies are needed to ascertain the mechanism for IR inhibition.
LIGHT SOURCES
As described above, a variety of light sources and protocols have been used to stimulate or inhibit cardiomyocytes. These have been summarized in Table 1. For neural applications, groups have used various laser choices including holmium:YAG,12,13,36,47 free-electron laser,13 thulium fiber (IPG Photonics)48,49 and various diode lasers. 47,50–55
Table 1.
| Application | Light Source | Wavelength | Frequencies | Pulse Widths | Radiant Exposure Thresholds |
|---|---|---|---|---|---|
| Embryonic heart stimulation | Capella | 1875nm,16 1860nm37 | 2-3Hz | 2ms,16 10ms37 | 0.81 J/cm2 16 0.63-1.38 J/cm2 37 |
| QPhotonics21 | 1440nm, 1465nm | 1-3Hz | 20ms | 0.31 J/mm2 0.60 J/mm2 | |
| Adult heart stimulation20 | Capella | 1851nm | 2.5-3.3 Hz | 2.5 - 12ms | 7.8-11.8 J/cm2 |
| Rat neonatal ventricular cardiomyocytes26 | Capella | 1862nm | 0.75-1Hz | 1.75 - 4.0ms | 5.1-11.6 J/cm2 |
| Rat neonatal cardiomyocytes29 | KPS-STD-BT-RFL-1455-02-CO | 1455nm | 2.5 Hz | 200ms | NA |
| Neural crest cell-derived cardiomyogenic lineage42 | Capella | 1862nm | 0.25-2Hz | 2ms | 668mJ/cm2 |
| Embryonic heart inhibition10 | QPhotonics (PUMA) | 1463nm | 200Hz | 200μs | 66 mJ/cm2/pulse |
Conclusion
OP is a potential alternative or complementary approach to electrical pacing to increase its utility. OP overcomes several disadvantages of electrical pacing including the ability to be noncontact, superior spatial precision, lack of electrical artifacts, and no interference with magnetic or electric fields. Several applications that benefit from OP have already been identified and demonstrated. Future studies are needed to further optimize OP protocols, determine the mechanism of OP, increase safety factors for some applications, and improve infrared light delivery methods.
Figure 9. Demonstration of IR Inhibition from Wang et al10.
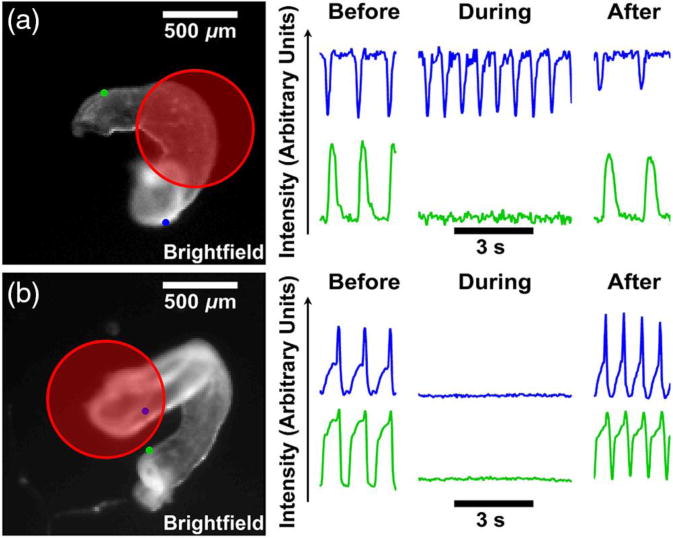
(a) The inhibition laser focused on the ventricle (red circle) stops contractions in the outflow tract (green dot and traces). However, contractions in the atrium are unaffected (blue). (b) When positioned on the atrium, the inhibition laser stops all contractions. Contractions resume after the inhibition laser stops.
Acknowledgments
This work was partially funded by the National Institutes of Health (NIH) through the National Heart, Lung, Blood Institute grant R01HL126747.
References
- 1.Aquilina O. A brief history of cardiac pacing. Images Paediatr Cardiol. 2006;8(2):17–81. http://www.ncbi.nlm.nih.gov/pubmed/22368662. [PMC free article] [PubMed] [Google Scholar]
- 2.Scherlag BJ, Lau SH, Helfant RH, Berkowitz WD, Stein E, Damato AN. Catheter technique for recording His bundle activity in man. Circulation. 1969;39(1):13–18. doi: 10.1161/01.cir.39.1.13. [DOI] [PubMed] [Google Scholar]
- 3.WELLENS HJJ. Twenty-Five Years of Insights into the Mechanisms of Supraventricular Arrhythmias. J Cardiovasc Electrophysiol. 2003;14(9):1020–1025. doi: 10.1046/j.1540-8167.2003.03282.x. [DOI] [PubMed] [Google Scholar]
- 4.Josephson ME, Horowitz LN, Farshidi A, Spear JF, Kastor JA, Moore EN. Recurrent sustained ventricular tachycardia. 2. Endocardial mapping. Circulation. 1978;57(3) doi: 10.1161/01.cir.57.3.440. [DOI] [PubMed] [Google Scholar]
- 5.Efimov IR, Nikolski VP, Salama G. Optical Imaging of the Heart. Circ Res. 2004;95(1) doi: 10.1161/01.RES.0000130529.18016.35. [DOI] [PubMed] [Google Scholar]
- 6.Weidmann S. The electrical constants of Purkinje fibres. J Physiol. 1952;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weidmann S. Electrical constants of trabecular muscle from mammalian heart. J Physiol. 1970;210(4):1041–1054. doi: 10.1113/jphysiol.1970.sp009256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akar FG, Roth BJ, Rosenbaum DS. Optical measurement of cell-to-cell coupling in intact heart using subthreshold electrical stimulation. Am J Physiol Hear Circ Physiol. 2001;281(2):H533–42. doi: 10.1152/ajpheart.2001.281.2.H533. [DOI] [PubMed] [Google Scholar]
- 9.Butson CR, McIntyre CC. Role of Electrode Design on the Volume of Tissue Activated During Deep Brain Stimulation. J Neural Eng. 2011;4(164):1–8. doi: 10.1126/scisignal.2001449.Engineering. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YT, Rollins AM, Jenkins MW. Infrared inhibition of embryonic hearts. J Biomed Opt. 2016;21(6):60505. doi: 10.1117/1.JBO.21.6.060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosen MR, Brink PR, Cohen IS, Robinson RB. Genes, stem cells and biological pacemakers. Cardiovasc Res. 2004;64(1):12–23. doi: 10.1016/j.cardiores.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Wells J, Kao C, Jansen ED, Konrad P, Mahadevan-jansen A. Application of infrared light for in vivo neural stimulation. J Biomed Opitics. 2005;10(6) doi: 10.1117/1.2121772. December 2005: 64003. [DOI] [PubMed] [Google Scholar]
- 13.Wells J, Kao C, Mariappan K, et al. Optical stimulation of neural tissue in vivo. Opt Lett. 2005;30(5):504. doi: 10.1364/OL.30.000504. [DOI] [PubMed] [Google Scholar]
- 14.Duke AR, Jenkins MW, Lu H, McManus JM, Chiel HJ, Jansen ED. Transient and selective suppression of neural activity with infrared light. Sci Rep. 2013;3:2600. doi: 10.1038/srep02600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duke AR, Lu H, Jenkins MW, et al. Spatial and temporal variability in response to hybrid electro-optical stimulation. J Neural Eng. 2012;9(3):36003. doi: 10.1088/1741-2560/9/3/036003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins MW, Duke AR, Gu S, et al. Optical pacing of the embryonic heart. Nat Photonics. 2010;4:623–626. doi: 10.1038/nphoton.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fork RL. Laser stimulation of nerve cells in Aplysia. Science. 1971;171(3974):907–908. doi: 10.1126/science.171.3974.907. [DOI] [PubMed] [Google Scholar]
- 18.Gimeno MA, Roberts CM, Webb JL. Acceleration of Rate of the Early Chick Embryo Heart by Visible Light. Nature. 1967;214(5092):1014–1016. doi: 10.1038/2141014a0. [DOI] [PubMed] [Google Scholar]
- 19.Smith NI, Kumamoto Y, Iwanaga S, Ando J, Fujita K, Kawata S. A femtosecond laser pacemaker for heart muscle cells. Opt Express. 2008;16(12):8604. doi: 10.1364/OE.16.008604. [DOI] [PubMed] [Google Scholar]
- 20.Jenkins MW, Wang YT, Doughman YQ, Watanabe M, Cheng Y, Rollins AM. Optical pacing of the adult rabbit heart. Biomed Opt Express. 2013;4(9):1626–1635. doi: 10.1364/BOE.4.001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YT, Gu S, Ma P, Watanabe M, Rollins AM, Jenkins MW. Optical stimulation enables paced electrophysiological studies in embryonic hearts. Biomed Opt Express. 2014;5(4):1000–1013. doi: 10.1364/BOE.5.001000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shapiro MG, Homma K, Villarreal S, Richter C-P, Bezanilla F. Infrared light excites cells by changing their electrical capacitance. Nat Commun. 2012;3:736. doi: 10.1038/ncomms1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Frerck MJ, Holman HA, Jorgensen EM, Rabbitt RD. Exciting cell membranes with a blustering heat shock. Biophys J. 2014;106(8):1570–1577. doi: 10.1016/j.bpj.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cayce JM, Bouchard MB, Chernov MM, et al. Calcium imaging of infrared-stimulated activity in rodent brain. Cell Calcium. 2014;55(4):183–190. doi: 10.1016/j.ceca.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lumbreras V, Bas E, Gupta C, Rajguru SM. Pulsed infrared radiation excites cultured neonatal spiral and vestibular ganglion neurons by modulating mitochondrial calcium cycling. J Neurophysiol. 2014;112(6):1246–1255. doi: 10.1152/jn.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dittami GM, Rajguru SM, Lasher RA, Hitchcock RW, Rabbitt RD. Intracellular calcium transients evoked by pulsed infrared radiation in neonatal cardiomyocytes. J Physiol. 2011;589(6):1295–1306. doi: 10.1113/jphysiol.2010.198804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao J, Liu B, Qin F. Rapid temperature jump by infrared diode laser irradiation for patch-clamp studies. Biophys J. 2009;96(9):3611–3619. doi: 10.1016/j.bpj.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Albert ES, Bec JM, Desmadryl G, et al. TRPV4 channels mediate the infrared laser-evoked response in sensory neurons. J Neurophysiol. 2012;107:3227–3234. doi: 10.1152/jn.00424.2011. [DOI] [PubMed] [Google Scholar]
- 29.Oyama K, Mizuno A, Shintani SA, et al. Microscopic Heat Pulses Induce Contraction of Cardiomyocytes without Calcium Transients. Biochem Biophys Res Commun. 2012;417 doi: 10.1016/j.bbrc.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Shintani SA, Oyama K, Fukuda N, Ishiwata S. High-Frequency Sarcomeric Auto-Oscillations Induced by Heating in Living Neonatal Cardiomyocytes of the Rat. Biochem Biophys Res Commun. 2015;457 doi: 10.1016/j.bbrc.2014.12.077. [DOI] [PubMed] [Google Scholar]
- 31.Teudt IU, Maier H, Richter C-P, Kral A. Acoustic Events and ‘Optophonic’ Cochlear Responses Induced by Pulsed Near-Infrared LASER. IEEE Trans Biomed Eng. 2011;58(6) doi: 10.1109/TBME.2011.2108297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fill M, Copello JA, Adams B, et al. Ryanodine receptor calcium release channels. Physiol Rev. 2002;82(4):893–922. doi: 10.1152/physrev.00013.2002. [DOI] [PubMed] [Google Scholar]
- 33.Wells J, Kao C, Konrad P, et al. Biophysical Mechanisms of Transient Optical Stimulation of Peripheral Nerve. Biophys J. 2007;93(7):2567–2580. doi: 10.1529/biophysj.107.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wells JD, Thomsen S, Whitaker P, et al. Optically mediated nerve stimulation: Identification of injury thresholds. Lasers Surg Med. 2007;39(6):513–526. doi: 10.1002/lsm.20522. [DOI] [PubMed] [Google Scholar]
- 35.Matic AI, Robinson AM, Young HK, et al. Behavioral and Electrophysiological Responses Evoked by Chronic Infrared Neural Stimulation of the Cochlea. In: Sokolowski B, editor. PLoS One. 3. Vol. 8. 2013. p. e58189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cayce JM, Wells JD, Malphrus JD, et al. Infrared neural stimulation of human spinal nerve roots in vivo. Neurophotonics. 2015;2(1):15007. doi: 10.1117/1.NPh.2.1.015007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford SM, McPheeters MT, Wang YT, et al. Increased regurgitant flow causes endocardial cushion defects in an avian embryonic model of congenital heart disease. Congenit Heart Dis. 2017 Feb; doi: 10.1111/chd.12443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ma P, Chan DC, Gu S, Watanabe M, Jenkins MW, Rollins AM. Volumetric optical mapping in early embryonic hearts using light-sheet microscopy. Biomed Opt Express. 2016;7(12):5120. doi: 10.1364/BOE.7.005120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gu S, Wang YT, Ma P, Werdich AA, Rollins AM, Jenkins MW. Mapping conduction velocity of early embryonic hearts with a robust fitting algorithm. Biomed Opt Express. 2015;6(6):2138–2157. doi: 10.1364/BOE.6.002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McPheeters MT, Wang YT, Laurita KR, Jenkins M. An optical system for high-throughput screening of cardiac electrophysiology for human cardiomyocytes. SPIE Photonics West/BiOS. 2017 doi: 10.1371/journal.pone.0183761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenkins MW, Watanabe M, Rollins AM. Longitudinal Imaging of Heart Development With Optical Coherence Tomography. IEEE J Sel Top Quantum Electron. 2012;18(3):1166–1175. doi: 10.1109/JSTQE.2011.2166060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenberg JM, Lumbreras V, Pelaez D, Rajguru SM, Cheung HS. Neural Crest Stem Cells Can Differentiate to a Cardiomyogenic Lineage with an Ability to Contract in Response to Pulsed Infrared Stimulation. Tissue Eng Part C Methods. 2016;22(10):982–990. doi: 10.1089/ten.tec.2016.0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lothet EH, Kilgore KL, Bhadra N, et al. Alternating current and infrared produce an onset-free reversible nerve block. Neurophotonics. 2014;1(1):11010. doi: 10.1117/1.NPh.1.1.011010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodgkin AL, Katz B. The effect of temperature on the electrical activity of the giant axon of the squid. J Physiol. 1949;109(1–2):240–249. doi: 10.1113/jphysiol.1949.sp004388. http://www.ncbi.nlm.nih.gov/pubmed/15394322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rattay F, Aberham M. Modeling axon membranes for functional electrical stimulation. IEEE Trans Biomed Eng. 1993;40(12):1201–1209. doi: 10.1109/10.250575. [DOI] [PubMed] [Google Scholar]
- 46.Zongxia Mou, Triantis IF, Woods VM, Toumazou C, Nikolic K. A Simulation Study of the Combined Thermoelectric Extracellular Stimulation of the Sciatic Nerve of the Xenopus Laevis: The Localized Transient Heat Block. IEEE Trans Biomed Eng. 2012;59(6):1758–1769. doi: 10.1109/TBME.2012.2194146. [DOI] [PubMed] [Google Scholar]
- 47.Stahl CSD, Tozburun S, Hutchens TC, et al. Comparison of three pulsed infrared lasers for optical stimulation of the rat prostate cavernous nerves. In: Kollias N, Choi B, Zeng H, et al., editors. International Society for Optics and Photonics. 2013. p. 85655N. [DOI] [Google Scholar]
- 48.Fried NM, Lagoda GA, Scott NJ, Su L-M, Burnett AL. Noncontact Stimulation of the Cavernous Nerves in the Rat Prostate Using a Tunable-Wavelength Thulium Fiber Laser. J Endourol. 2008;22(3):409–414. doi: 10.1089/end.2008.9996. [DOI] [PubMed] [Google Scholar]
- 49.Fried NM, Rais-Bahrami S, Lagoda GA, Chuang A-Y, Su L-M, Burnett AL., III Identification and Imaging of the Nerves Responsible for Erectile Function in Rat Prostate, In Vivo, Using Optical Nerve Stimulation and Optical Coherence Tomography. IEEE J Sel Top Quantum Electron. 2007;13(6):1641–1645. doi: 10.1109/JSTQE.2007.910119. [DOI] [Google Scholar]
- 50.Tozburun S, Lagoda GA, Burnett AL, Fried NM. Infrared Laser Nerve Stimulation as a Potential Diagnostic Method for Intra-Operative Identification and Preservation of the Prostate Cavernous Nerves. IEEE J Sel Top Quantum Electron. 2014;20(2):299–306. doi: 10.1109/JSTQE.2013.2293273. [DOI] [Google Scholar]
- 51.Tozburun S, Stahl CD, Hutchens TC, Lagoda GA, Burnett AL, Fried NM. Continuous-wave Infrared Subsurface Optical Stimulation of the Rat Prostate Cavernous Nerves Using a 1490-nm Diode Laser. Urology. 2013;82(4):969–973. doi: 10.1016/j.urology.2013.06.031. [DOI] [PubMed] [Google Scholar]
- 52.Tozburun S, Lagoda GA, Burnett AL, Fried NM. Continuous-Wave Laser Stimulation of the Rat Prostate Cavernous Nerves Using a Compact and Inexpensive All Single Mode Optical Fiber System. J Endourol. 2011;25(11):1727–1731. doi: 10.1089/end.2011.0172. [DOI] [PubMed] [Google Scholar]
- 53.Young HK, Tan X, Xia N, Richter C-P. Target structures for cochlear infrared neural stimulation. Neurophotonics. 2015;2(2):25002. doi: 10.1117/1.NPh.2.2.025002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richter C-P, Rajguru S, Stafford R, Stock SR. Radiant energy during infrared neural stimulation at the target structure. Proc SPIE–the Int Soc Opt Eng. 2013;8565:85655P. doi: 10.1117/12.2013849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Richter C-P, Young H. Responses to amplitude modulated infrared stimuli in the guinea pig inferior colliculus. Proc SPIE–the Int Soc Opt Eng. 2013;8656:85655U. doi: 10.1117/12.2013847. [DOI] [PMC free article] [PubMed] [Google Scholar]


