The structures of two new chalcone derivatives have been determined and are investigated using Hirshfeld surface analysis and molecular electrostatic potential techniques.
Keywords: chalcone, crystal Structure, DFT, molecular electrostatic potential, Hirshfeld surface
Abstract
The molecular and crystal structure of two new chalcone derivatives, (E)-1-(anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one, C28H25NO, (I), and (E)-1-(anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one, C35H25NO, (II), with the fused-ring system at the same position are described. In the crystals of (I) and (II), the molecules are linked via C—H⋯O hydrogen bonds into inversion dimers, forming R 2 2(22) and R 2 2(14) ring motifs, respectively. Weak intermolecular C—H⋯π interactions further help to stabilize the crystal structure, forming a two-dimensional architecture. The molecular structures are optimized using density functional theory (DFT) at B3LYP/6–311 G++(d,p) level and compared with the experimental results. The smallest HOMO–LUMO energy gaps of (I) (exp . 2.76 eV and DFT 3.40 eV) and (II) (exp . 2.70 eV and DFT 3.28 eV) indicates the suitability of these crystals in optoelectronic applications. All intermolecular contacts and weaker contributions involved in the supramolecular stabilization are investigated using Hirshfeld surface analysis. The molecular electrostatic potential (MEP) further identifies the positive, negative and neutral electrostatic potential regions of the molecules.
Chemical context
Chalcone derivatives have attracted significant attention in the past few decades mainly because of their availability of high optical non-linearities resulting from the significant delocalization of the electron clouds throughout the chalcone system (D’silva et al., 2011 ▸). A chalcone molecule with a π-conjugated system provides a large charge-transfer axis with appropriate substituent groups on the two aromatic terminal rings. Furthermore, π-conjugated molecular materials with fused rings are the focus of considerable interest in the emerging area of organic electronics, since the combination of excellent charge-carrier mobility and a high stability structure leads to potential optoelectronic applications (Wu et al., 2010 ▸). As part of our studies in this area, the chalcone compounds (E)-1-(anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one, (I), and (E)-1-(anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one, (II), were successfully synthesized and their crystal structures are reported herein.
Structural commentary
The title compounds (I) and (II) (Fig. 1 ▸) crystallize in he triclinic and monoclinic space groups P
 and C2/c, respectively. The bond lengths and angles are in normal ranges. The calculated values of compounds (I) and (II) determined from B3LYP/6-311G(d,p) calculations (given in the Supporting information) may provide information about the geometry of the molecules. From the results, it can be concluded that this basis set is comparable in its approach to the experimental data. The slight deviations from the experimental values are due to the fact that the optimization is performed in an isolated condition, whereas the crystal environment and hydrogen-bonding interactions affect the results of the X-ray structure (Zainuri et al., 2017 ▸).
and C2/c, respectively. The bond lengths and angles are in normal ranges. The calculated values of compounds (I) and (II) determined from B3LYP/6-311G(d,p) calculations (given in the Supporting information) may provide information about the geometry of the molecules. From the results, it can be concluded that this basis set is comparable in its approach to the experimental data. The slight deviations from the experimental values are due to the fact that the optimization is performed in an isolated condition, whereas the crystal environment and hydrogen-bonding interactions affect the results of the X-ray structure (Zainuri et al., 2017 ▸).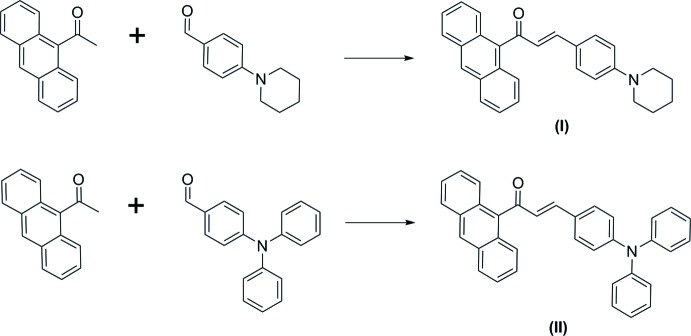
Figure 1.
(a) The molecular structure of compounds (I) and (II) with 50% probability displacement ellipsoids. (b) The optimized structures of compounds (I) and (II) at the DFT/B3LYP 6–311++G(d,p) level.
Compounds (I) and (II) contain an anthracene fused ring system and a 1-phenylpiperidine or triphenylamine substituent, representing a D–π–D intermolecular charge-transfer system. The piperidine ring (N1/C24–C28) in (I) adopts a chair conformation with puckering parameters Q = 0.521 (4), Θ = 3.1 (3)° and φ = 221 (6)°. The enone moiety (O1/C15–C17) in compounds (I) and (II) adopts an s-trans configuration with respect to the C15=O1 and C16=C17 bonds. Both compounds (I) and (II) are twisted at the C14—C15 bonds with C1—C14—C15—C16 torsion angles of 101.5 (3) and 93.66 (18)°, respectively. The corresponding torsion angles from the DFT study are 88.68 and 90.29°. In addition, the C17—C18 bond is also twisted slightly in (I) and (II) with the C16—C17—C18—C19 torsion angles being 171.5 (3)° (Exp) and 179.22° (DFT) in (I) and −164.77 (16)° (Exp) and 175.94° (DFT) in (II). The torsion angle difference between the experimental and DFT studies are due to the formation of intermolecular interactions involving the anthracene fused-ring system and the terminal substituent of the 1-phenylpiperidine and triphenylamine units. The observed intermolecular interactions in the crystal packing are the main cause of the angle deviation between the experimental and the theoretical results.
The enone moiety for (I) [O1/C15–C17, maximum deviation of 0.052 (3) Å at C16] forms dihedral angles of 82.9 (3), 12.0 (3) and 8.1 (3)° with the anthracene ring system (C1–C14), the benzene ring (C18-C23) and the piperidine ring (N1/C24–C28), respectively. The anthracene ring system forms dihedral angles of 86.74 (10) and 85.55 (12)° with the 1-phenylpiperidine rings C18–C23 and N1/C24–C28, respectively. Meanwhile, in compound (II), the enone moiety [O1/ C15–C17, maximum deviation of 0.0287 (15) Å at C16] forms dihedral angles of 87.30 (16), 17.13 (16), 72.55 (17) and 79.16 (16)° with the anthracene ring system (C1–C14) and the benzene rings C18–C23, C24–C29 and C30–C35, respectively. The dihedral angle between the anthracene ring system and the triphenylamine benzene rings C18–C23, C24–C29 and C30–C35 are 75.86 (6), 79.81 (8) and 12.84 (8)°, respectively. The large dihedral-angle deviation indicates that the possibility for electronic effects between the anthracene units through the enone moiety has decreased (Jung et al., 2008 ▸). Furthermore, the bulkiness of the anthracene ring system gives rise to a highly twisted structure for both compounds (Zainuri et al., 2018a ▸,b ▸).
Supramolecular features
In the crystal packing of compound (I), the molecules are connected via intermolecular C28—H28B⋯O1i interactions (Table 1 ▸), forming inversion dimers with  (22) ring motifs. These ring motifs further link into one-dimensional columns along the b-axis direction (Fig. 2 ▸). The crystal packing is stabilized by weak C28—H28A⋯ Cg1ii interactions (Table 1 ▸). Together, these interactions connect the molecules into sheets parallel to the ac plane.
(22) ring motifs. These ring motifs further link into one-dimensional columns along the b-axis direction (Fig. 2 ▸). The crystal packing is stabilized by weak C28—H28A⋯ Cg1ii interactions (Table 1 ▸). Together, these interactions connect the molecules into sheets parallel to the ac plane.
Table 1. Hydrogen-bond geometry (Å, °) for (I) .
Cg1 is the centroid of the C18–C23 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C28—H28B⋯O1i | 0.97 | 2.36 | 3.262 (4) | 154 |
| C28—H28A⋯Cg1ii | 0.97 | 2.95 | 3.861 (4) | 157 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
Figure 2.
The crystal packing of (I) showing weak C—H⋯O and C—H⋯π interactions.
Similary, in compound (II), C23—H23A⋯ O1i (Table 1 ▸ and Fig. 3 ▸) hydrogen bonds connect the molecules into centrosymmetric dimers, forming  (14) ring motifs. These dimers are further linked into infinite columns along the c-axis direction. C29—H29A⋯ Cg1ii interactions (Table 2 ▸) are also observed. As in (I), the crystal structure comprises sheets parallel to the ac plane.
(14) ring motifs. These dimers are further linked into infinite columns along the c-axis direction. C29—H29A⋯ Cg1ii interactions (Table 2 ▸) are also observed. As in (I), the crystal structure comprises sheets parallel to the ac plane.
Figure 3.
The weak C—H⋯ O and C—H⋯π interactions in compound (II).
Table 2. Hydrogen-bond geometry (Å, °) for (II) .
Cg1 is the centroid of the C18–C23 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C23—H23A⋯O1i | 0.93 | 2.40 | 3.221 (2) | 147 |
| C29—H29A⋯Cg1ii | 0.93 | 2.96 | 3.739 (19) | 142 |
Symmetry codes: (i)  ; (ii)
; (ii)  .
.
UV–Vis absorption analysis
The strongest absorption and smaller energy gap, particularly in the visible region, is important feature in the suitability for optoelectronic application. The electronic absorption and excitation properties of (I) and (II) were estimated theoretically by applying the time-dependent DFT approach at the B3LYP level of theory with the 6-311++G(d,p) basis set. The experimental absorptions (Fig. 4 ▸) of (I) and (II) are reported at 396 and 406 nm, while simulated values are observed at 397 and 415 nm, respectively. The theoretical wavelengths are shifted to higher wavelengths because the calculations are confined to the gaseous equivalent whereas the observations are from the solution state.
Figure 4.
UV–Vis absorption spectra for compounds (I) and (II).
The experimental energy band gaps for (I) and (II) are 2.76 and 2.70 eV, respectively, through an extrapolation of the linear trend. The calculations of the molecular orbital geometry show that the absorption maxima of the molecules correspond to the electron transition between the frontier orbitals highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO) (Fig. 5 ▸). The predicted energy gaps for compounds (I) and (II) are 3.40 and 3.28 eV, respectively. The small HOMO–LUMO energy gap in these compounds shows the chemical reactivity is stronger and the kinetic stability is weaker, which in turn increase the polarizability and NLO activity (Maidur et al., 2018 ▸).
Figure 5.
The electron distribution of the HOMO and LUMO energy levels of (I) and (II).
Hirshfeld surface analysis
Hirshfeld surface analysis assigns intermolecular interactions inside the unit-cell packing. The dnorm, shape-index and de (Wolff et al., 2012 ▸) surfaces are presented in Fig. 6 ▸ a, b and c, respectively. All C—H⋯ O and C—H⋯π contacts are recognized in the dnorm mapped surface as deep-red depression areas in Fig. 6 ▸ a. The C—H⋯ O contacts are observed in both compounds (I) and (II). The presence of C—H⋯π interactions is indicated through the combination of pale-orange and bright-red spots, which are present on the shape-index surface, identified with black arrows (Fig. 6 ▸ b).
Figure 6.
View of the Hirshfeld Surfaces, showing (a) d norm with the red spot showing the involvement of the C—H⋯O interactions, (b) mapped over shape-index and (c) mapped over d e with the pale-orange spot inside the black arrows indicating the C—H⋯π interactions.
Two-dimensional fingerprint plots as shown in Fig. 7 ▸. These illustrate the difference between the intermolecular interaction patterns and the major intermolecular contacts associated in both compounds. The H⋯H contacts appear to be the major contributor to the Hirshfeld surface; these are shown in Fig. 7 ▸ b as one distinct spike with a minimum value de + di that is approximately less than the sum of van der Waals radii (2.4 Å). Furthermore, the intermolecular C—H⋯π interactions for compounds (I) and (II) are characterized by the short interatomic C⋯H/H⋯C contacts with percentage contributions of 21.7% (I) and 30.6% (II), showing two distinct spikes with de + di ∼2.8 Å (I) and 2.7 Å (II). Additionally, the O⋯H/H⋯O contacts indicate the presence of intermolecular C—H⋯ O interactions with percentage contributions of 8.0% (I) and 6.5% (II) and are indicated by a pair of wings at de + di ∼2.3 Å (Fig. 7 ▸ c).
Figure 7.
Fingerprint plots of interactions, listing the percentage of contacts (a) full two-dimensional fingerprint plots; (b) H⋯H (c) O⋯H/H⋯O and (d) C⋯H/H⋯C contributions to the total Hirshfeld surface. The outline of the full fingerprint plots is shown in grey.
Molecular Electrostatic Potential
The molecular electrostatic potential (MEP) has become firmly established as an effective guide to molecular interactions. The importance of MEPs lies in the fact that it simultaneously displays molecular size and shape, as well as positive, negative and neutral electrostatic potential regions, in terms of colour grading and is useful in suties of the molecular structure and its physicochemical property relationship (Murray & Sen, 1996 ▸; Scrocco & Tomasi, 1978 ▸). The MEP maps of (I) and (II) molecules were calculated theoretically at the B3LYP/6-311G++(d,p) level of theory and the obtained plots are shown in Fig. 8 ▸. The red-coloured region is nucleophile and electron rich, whereas the blue colour indicates the electrophile region with poor electrons in the vicinity, and the remaining white region shows the neutrality of atoms. These sites given information about the region from where the molecule can have intermolecular interactions (Gunasekaran & Srinivasan, 2008 ▸).
Figure 8.
The total electron density three-dimensional surface mapped for (a) compound (I) and (b) compound (II) with the electrostatic potential calculated at the B3LYP/6–311 G++ (d,p) level.
In (I) and (II), the reactive sites are near the C=O group; this is the region having the most negative potential spots (red colour), all over the oxygen atom due to the C—H⋯ O interactions in the crystal structure. The negative potential values of compounds (I) and (II) of −0.06268 and −0.06453 a.u. indicate the strongest repulsion (electrophilic attack). Meanwhile, the most positive regions for (I) and (II) are localized on the hydrogen atoms and show the strongest attraction (nucleophilic attack) sites involving the anthrancene group and its subtsituent groups of the 1-phenylpiperidine (I) and triphenylamine (II) moieties.
Database survey
A survey of Cambridge Structural Database (CSD, Version 5.38, last update Nov 2016; Groom et al., 2016 ▸) revealed fused-ring substituted chalcones similar to (I) and (II). There are four compounds that have ananthracene–ketone substituent on the chalcone: 9-anthryl styryl ketone and 9,10-anthryl bis(styryl ketone) (Harlow et al., 1975 ▸), (2E)-1-(anthracen-9-yl)-3-[4-(propan-2-yl)phenyl]prop-2-en-1-one (Girisha et al., 2016 ▸) and (E)-1-(anthracen-9-yl)-3-(2-chloro-6-fluorophenyl)prop-2-en- 1-one (Abdullah et al., 2016 ▸). Zainuri et al., 20182018a ▸,b ▸) reported two anthracene substituents on the chalcone (E)-1,3-bis(anthracen-9-yl)prop-2-en-1-one. Other related compounds include 1-(anthracen-9-yl)-2-methylprop-2-en-1-one (Agrahari et al., 2015 ▸) and 9-anthroylacetone (Cicogna et al., 2004 ▸).
Synthesis and crystallization
A mixture of 9-acetylanthrancene (0.5 mmol) and 4-(piperidin-1-yl)benzaldehyde (0.5 mmol) and 4-(diphenylamino)benzaldehyde (0.5 mmol) for compound (I) and (II), respectively, was dissolved in methanol (20 ml). A catalytic amount of NaOH (5 ml, 20%) was added to the solution dropwise with vigorous stirring. The reaction mixture was stirred for about 5–6 h at room temperature. After stirring, the contents of the flask were poured into ice-cold water (50 ml). The resultant crude products were filtered, washed successively with distilled water and recrystallized to get the corresponding chalcones. Crystals suitable for X-ray diffraction were obtained by the slow evaporation technique from acetone.
Refinement
Crystal data collection and structure refinement details are summarized in Table 3 ▸. All H atoms were positioned geometrically [C—H = 0.93 and 0.97 Å (in (I)) and 0.93 Å (in (II))] and refined using riding model with U iso(H) = 1.2U eq(C).
Table 3. Experimental details.
| (I) | (II) | |
|---|---|---|
| Crystal data | ||
| Chemical formula | C28H25NO | C35H25NO |
| M r | 391.49 | 475.56 |
| Crystal system, space group | Triclinic, P

|
Monoclinic, C2/c |
| Temperature (K) | 296 | 296 |
| a, b, c (Å) | 8.0535 (15), 9.0457 (17), 15.352 (3) | 31.2875 (16), 9.0470 (4), 18.3643 (8) |
| α, β, γ (°) | 106.553 (4), 101.572 (4), 94.385 (4) | 90, 99.388 (3), 90 |
| V (Å3) | 1039.6 (3) | 5128.5 (4) |
| Z | 2 | 8 |
| Radiation type | Mo Kα | Mo Kα |
| μ (mm−1) | 0.08 | 0.07 |
| Crystal size (mm) | 0.64 × 0.23 × 0.10 | 0.96 × 0.23 × 0.17 |
| Data collection | ||
| Diffractometer | Bruker SMART APEXII DUO CCD area-detector | Bruker SMART APEXII DUO CCD area-detector |
| Absorption correction | Multi-scan (SADABS; Bruker, 2009 ▸) | Multi-scan (SADABS; Bruker, 2009 ▸) |
| T min, T max | 0.724, 0.972 | 0.645, 0.957 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 27976, 4812, 2122 | 98729, 7726, 4183 |
| R int | 0.079 | 0.076 |
| (sin θ/λ)max (Å−1) | 0.652 | 0.712 |
| Refinement | ||
| R[F 2 > 2σ(F 2)], wR(F 2), S | 0.068, 0.246, 1.01 | 0.057, 0.144, 1.02 |
| No. of reflections | 4812 | 7726 |
| No. of parameters | 271 | 334 |
| H-atom treatment | H-atom parameters constrained | H-atom parameters constrained |
| Δρmax, Δρmin (e Å−3) | 0.26, −0.19 | 0.13, −0.14 |
Supplementary Material
Crystal structure: contains datablock(s) I, II. DOI: 10.1107/S2056989018006527/lh5873sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018006527/lh5873Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989018006527/lh5873IIsup3.hkl
Comparison between selected calculated (DFT) and experimental geometrical data. DOI: 10.1107/S2056989018006527/lh5873sup4.pdf
Additional supporting information: crystallographic information; 3D view; checkCIF report
supplementary crystallographic information
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Crystal data
| C28H25NO | Z = 2 |
| Mr = 391.49 | F(000) = 416 |
| Triclinic, P1 | Dx = 1.251 Mg m−3 |
| a = 8.0535 (15) Å | Mo Kα radiation, λ = 0.71073 Å |
| b = 9.0457 (17) Å | Cell parameters from 1764 reflections |
| c = 15.352 (3) Å | θ = 2.4–19.6° |
| α = 106.553 (4)° | µ = 0.08 mm−1 |
| β = 101.572 (4)° | T = 296 K |
| γ = 94.385 (4)° | Plate, yellow |
| V = 1039.6 (3) Å3 | 0.64 × 0.23 × 0.10 mm |
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Data collection
| Bruker SMART APEXII DUO CCD area-detector diffractometer | 2122 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.079 |
| φ and ω scans | θmax = 27.6°, θmin = 1.4° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −10→10 |
| Tmin = 0.724, Tmax = 0.972 | k = −11→11 |
| 27976 measured reflections | l = −19→19 |
| 4812 independent reflections |
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.068 | H-atom parameters constrained |
| wR(F2) = 0.246 | w = 1/[σ2(Fo2) + (0.115P)2 + 0.0669P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 4812 reflections | Δρmax = 0.26 e Å−3 |
| 271 parameters | Δρmin = −0.19 e Å−3 |
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Special details
| Experimental. The following wavelength and cell were deduced by SADABS from the direction cosines etc. They are given here for emergency use only: CELL 0.71134 9.070 15.379 16.135 101.576 94.356 106.571 |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | −0.3955 (3) | 0.4115 (3) | 0.73584 (14) | 0.0949 (8) | |
| N1 | 0.6347 (3) | 0.8072 (2) | 1.09764 (14) | 0.0575 (6) | |
| C1 | −0.2379 (3) | 0.4582 (3) | 0.57411 (17) | 0.0542 (6) | |
| C2 | −0.1842 (3) | 0.3116 (3) | 0.5702 (2) | 0.0694 (8) | |
| H1 | −0.1747 | 0.2771 | 0.6224 | 0.083* | |
| C3 | −0.1466 (4) | 0.2206 (4) | 0.4920 (2) | 0.0848 (9) | |
| H2 | −0.1098 | 0.1253 | 0.4912 | 0.102* | |
| C4 | −0.1627 (4) | 0.2695 (4) | 0.4119 (2) | 0.0884 (10) | |
| H3 | −0.1375 | 0.2059 | 0.3583 | 0.106* | |
| C5 | −0.2139 (4) | 0.4060 (4) | 0.4123 (2) | 0.0788 (9) | |
| H4 | −0.2249 | 0.4360 | 0.3585 | 0.095* | |
| C6 | −0.2517 (3) | 0.5066 (3) | 0.49269 (16) | 0.0575 (7) | |
| C7 | −0.3004 (3) | 0.6497 (3) | 0.49477 (18) | 0.0632 (7) | |
| H5 | −0.3109 | 0.6799 | 0.4411 | 0.076* | |
| C8 | −0.3343 (3) | 0.7503 (3) | 0.57345 (18) | 0.0579 (7) | |
| C9 | −0.3802 (3) | 0.8996 (4) | 0.5773 (2) | 0.0726 (8) | |
| H6 | −0.3851 | 0.9339 | 0.5253 | 0.087* | |
| C10 | −0.4167 (4) | 0.9924 (4) | 0.6537 (3) | 0.0812 (9) | |
| H10 | −0.4481 | 1.0895 | 0.6542 | 0.097* | |
| C11 | −0.4080 (4) | 0.9442 (4) | 0.7330 (2) | 0.0791 (9) | |
| H11 | −0.4349 | 1.0089 | 0.7857 | 0.095* | |
| C12 | −0.3612 (3) | 0.8049 (3) | 0.73383 (19) | 0.0657 (7) | |
| H12 | −0.3546 | 0.7758 | 0.7877 | 0.079* | |
| C13 | −0.3216 (3) | 0.7014 (3) | 0.65471 (16) | 0.0533 (6) | |
| C14 | −0.2753 (3) | 0.5560 (3) | 0.65304 (16) | 0.0529 (6) | |
| C15 | −0.2738 (3) | 0.5015 (3) | 0.73695 (17) | 0.0604 (7) | |
| C16 | −0.1294 (3) | 0.5534 (3) | 0.81633 (17) | 0.0610 (7) | |
| H16 | −0.1401 | 0.5308 | 0.8705 | 0.073* | |
| C17 | 0.0172 (3) | 0.6311 (3) | 0.81710 (17) | 0.0550 (6) | |
| H17 | 0.0211 | 0.6584 | 0.7635 | 0.066* | |
| C18 | 0.1718 (3) | 0.6790 (3) | 0.89032 (16) | 0.0519 (6) | |
| C19 | 0.3209 (3) | 0.7416 (3) | 0.87506 (18) | 0.0667 (8) | |
| H19 | 0.3190 | 0.7560 | 0.8173 | 0.080* | |
| C20 | 0.4712 (3) | 0.7833 (3) | 0.94162 (18) | 0.0680 (8) | |
| H20 | 0.5679 | 0.8259 | 0.9279 | 0.082* | |
| C21 | 0.4836 (3) | 0.7640 (3) | 1.02901 (16) | 0.0516 (6) | |
| C22 | 0.3322 (3) | 0.7030 (3) | 1.04536 (17) | 0.0610 (7) | |
| H22 | 0.3334 | 0.6892 | 1.1032 | 0.073* | |
| C23 | 0.1831 (3) | 0.6632 (3) | 0.97861 (17) | 0.0611 (7) | |
| H23 | 0.0850 | 0.6240 | 0.9927 | 0.073* | |
| C24 | 0.7882 (3) | 0.8619 (4) | 1.0742 (2) | 0.0757 (9) | |
| H24A | 0.7613 | 0.9339 | 1.0389 | 0.091* | |
| H24B | 0.8275 | 0.7740 | 1.0346 | 0.091* | |
| C25 | 0.9284 (4) | 0.9408 (4) | 1.1579 (2) | 0.0930 (11) | |
| H25A | 0.8976 | 1.0392 | 1.1913 | 0.112* | |
| H25B | 1.0317 | 0.9634 | 1.1378 | 0.112* | |
| C26 | 0.9649 (4) | 0.8453 (4) | 1.2232 (2) | 0.0958 (11) | |
| H26A | 1.0141 | 0.7547 | 1.1941 | 0.115* | |
| H26B | 1.0468 | 0.9066 | 1.2800 | 0.115* | |
| C27 | 0.8026 (4) | 0.7947 (4) | 1.2463 (2) | 0.0897 (10) | |
| H27A | 0.8248 | 0.7249 | 1.2836 | 0.108* | |
| H27B | 0.7629 | 0.8850 | 1.2832 | 0.108* | |
| C28 | 0.6666 (4) | 0.7140 (4) | 1.1604 (2) | 0.0755 (9) | |
| H28A | 0.7004 | 0.6167 | 1.1277 | 0.091* | |
| H28B | 0.5614 | 0.6895 | 1.1782 | 0.091* |
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0856 (14) | 0.1238 (18) | 0.0697 (13) | −0.0401 (13) | 0.0025 (11) | 0.0461 (13) |
| N1 | 0.0538 (12) | 0.0678 (14) | 0.0501 (12) | 0.0009 (10) | 0.0081 (10) | 0.0215 (10) |
| C1 | 0.0478 (14) | 0.0621 (17) | 0.0490 (15) | −0.0043 (12) | 0.0073 (11) | 0.0170 (13) |
| C2 | 0.0658 (18) | 0.0722 (19) | 0.0683 (19) | −0.0012 (15) | 0.0129 (15) | 0.0236 (15) |
| C3 | 0.085 (2) | 0.069 (2) | 0.090 (2) | 0.0049 (16) | 0.0221 (19) | 0.0092 (19) |
| C4 | 0.091 (2) | 0.093 (3) | 0.066 (2) | −0.003 (2) | 0.0264 (18) | −0.0005 (19) |
| C5 | 0.080 (2) | 0.094 (2) | 0.0528 (17) | −0.0077 (19) | 0.0148 (15) | 0.0124 (17) |
| C6 | 0.0506 (14) | 0.0739 (18) | 0.0416 (14) | −0.0052 (13) | 0.0062 (11) | 0.0145 (13) |
| C7 | 0.0575 (16) | 0.088 (2) | 0.0448 (15) | −0.0011 (15) | 0.0026 (12) | 0.0307 (15) |
| C8 | 0.0485 (14) | 0.0717 (18) | 0.0531 (16) | −0.0001 (13) | 0.0063 (12) | 0.0249 (14) |
| C9 | 0.0624 (17) | 0.084 (2) | 0.078 (2) | 0.0066 (16) | 0.0074 (15) | 0.0418 (18) |
| C10 | 0.0686 (19) | 0.077 (2) | 0.096 (3) | 0.0075 (16) | 0.0124 (18) | 0.027 (2) |
| C11 | 0.0720 (19) | 0.079 (2) | 0.075 (2) | 0.0070 (17) | 0.0159 (16) | 0.0079 (17) |
| C12 | 0.0608 (17) | 0.0736 (19) | 0.0567 (17) | −0.0009 (14) | 0.0121 (13) | 0.0146 (15) |
| C13 | 0.0425 (13) | 0.0679 (17) | 0.0446 (14) | −0.0048 (12) | 0.0042 (11) | 0.0169 (13) |
| C14 | 0.0476 (14) | 0.0642 (16) | 0.0433 (14) | −0.0082 (12) | 0.0044 (11) | 0.0196 (12) |
| C15 | 0.0612 (16) | 0.0677 (17) | 0.0519 (15) | −0.0027 (14) | 0.0111 (13) | 0.0223 (13) |
| C16 | 0.0687 (17) | 0.0704 (17) | 0.0447 (14) | −0.0022 (14) | 0.0075 (13) | 0.0257 (13) |
| C17 | 0.0633 (16) | 0.0592 (15) | 0.0445 (14) | 0.0049 (13) | 0.0091 (12) | 0.0221 (12) |
| C18 | 0.0569 (15) | 0.0563 (15) | 0.0434 (14) | 0.0056 (12) | 0.0109 (12) | 0.0178 (11) |
| C19 | 0.0674 (17) | 0.088 (2) | 0.0476 (15) | −0.0017 (15) | 0.0129 (14) | 0.0283 (14) |
| C20 | 0.0586 (16) | 0.092 (2) | 0.0555 (17) | −0.0045 (15) | 0.0151 (14) | 0.0292 (15) |
| C21 | 0.0545 (15) | 0.0567 (15) | 0.0450 (14) | 0.0055 (12) | 0.0129 (12) | 0.0178 (11) |
| C22 | 0.0630 (17) | 0.0770 (18) | 0.0419 (14) | −0.0010 (14) | 0.0112 (13) | 0.0205 (13) |
| C23 | 0.0563 (15) | 0.0764 (18) | 0.0519 (15) | −0.0021 (13) | 0.0145 (13) | 0.0232 (13) |
| C24 | 0.0600 (17) | 0.095 (2) | 0.073 (2) | −0.0034 (16) | 0.0141 (15) | 0.0316 (17) |
| C25 | 0.0672 (19) | 0.103 (2) | 0.105 (3) | −0.0109 (18) | −0.0082 (19) | 0.052 (2) |
| C26 | 0.065 (2) | 0.109 (3) | 0.108 (3) | −0.0009 (18) | −0.0116 (18) | 0.049 (2) |
| C27 | 0.074 (2) | 0.122 (3) | 0.076 (2) | 0.0079 (19) | −0.0034 (17) | 0.051 (2) |
| C28 | 0.0664 (18) | 0.087 (2) | 0.078 (2) | 0.0026 (15) | 0.0066 (15) | 0.0422 (17) |
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Geometric parameters (Å, º)
| O1—C15 | 1.219 (3) | C15—C16 | 1.442 (3) |
| N1—C21 | 1.386 (3) | C16—C17 | 1.323 (3) |
| N1—C24 | 1.445 (3) | C16—H16 | 0.9300 |
| N1—C28 | 1.449 (3) | C17—C18 | 1.442 (3) |
| C1—C14 | 1.385 (3) | C17—H17 | 0.9300 |
| C1—C2 | 1.415 (4) | C18—C19 | 1.379 (3) |
| C1—C6 | 1.425 (3) | C18—C23 | 1.389 (3) |
| C2—C3 | 1.354 (4) | C19—C20 | 1.366 (3) |
| C2—H1 | 0.9300 | C19—H19 | 0.9300 |
| C3—C4 | 1.406 (4) | C20—C21 | 1.387 (3) |
| C3—H2 | 0.9300 | C20—H20 | 0.9300 |
| C4—C5 | 1.331 (4) | C21—C22 | 1.398 (3) |
| C4—H3 | 0.9300 | C22—C23 | 1.361 (3) |
| C5—C6 | 1.413 (4) | C22—H22 | 0.9300 |
| C5—H4 | 0.9300 | C23—H23 | 0.9300 |
| C6—C7 | 1.375 (4) | C24—C25 | 1.486 (4) |
| C7—C8 | 1.380 (4) | C24—H24A | 0.9700 |
| C7—H5 | 0.9300 | C24—H24B | 0.9700 |
| C8—C9 | 1.415 (4) | C25—C26 | 1.500 (4) |
| C8—C13 | 1.426 (3) | C25—H25A | 0.9700 |
| C9—C10 | 1.336 (4) | C25—H25B | 0.9700 |
| C9—H6 | 0.9300 | C26—C27 | 1.493 (4) |
| C10—C11 | 1.396 (4) | C26—H26A | 0.9700 |
| C10—H10 | 0.9300 | C26—H26B | 0.9700 |
| C11—C12 | 1.345 (4) | C27—C28 | 1.491 (4) |
| C11—H11 | 0.9300 | C27—H27A | 0.9700 |
| C12—C13 | 1.417 (3) | C27—H27B | 0.9700 |
| C12—H12 | 0.9300 | C28—H28A | 0.9700 |
| C13—C14 | 1.389 (3) | C28—H28B | 0.9700 |
| C14—C15 | 1.503 (3) | ||
| C21—N1—C24 | 118.4 (2) | C16—C17—C18 | 128.4 (2) |
| C21—N1—C28 | 117.4 (2) | C16—C17—H17 | 115.8 |
| C24—N1—C28 | 113.0 (2) | C18—C17—H17 | 115.8 |
| C14—C1—C2 | 123.1 (2) | C19—C18—C23 | 115.7 (2) |
| C14—C1—C6 | 119.1 (2) | C19—C18—C17 | 120.8 (2) |
| C2—C1—C6 | 117.7 (2) | C23—C18—C17 | 123.5 (2) |
| C3—C2—C1 | 121.2 (3) | C20—C19—C18 | 122.5 (2) |
| C3—C2—H1 | 119.4 | C20—C19—H19 | 118.8 |
| C1—C2—H1 | 119.4 | C18—C19—H19 | 118.8 |
| C2—C3—C4 | 120.3 (3) | C19—C20—C21 | 121.9 (2) |
| C2—C3—H2 | 119.9 | C19—C20—H20 | 119.1 |
| C4—C3—H2 | 119.9 | C21—C20—H20 | 119.1 |
| C5—C4—C3 | 120.5 (3) | N1—C21—C20 | 122.6 (2) |
| C5—C4—H3 | 119.7 | N1—C21—C22 | 121.5 (2) |
| C3—C4—H3 | 119.7 | C20—C21—C22 | 115.9 (2) |
| C4—C5—C6 | 121.4 (3) | C23—C22—C21 | 121.6 (2) |
| C4—C5—H4 | 119.3 | C23—C22—H22 | 119.2 |
| C6—C5—H4 | 119.3 | C21—C22—H22 | 119.2 |
| C7—C6—C5 | 122.0 (3) | C22—C23—C18 | 122.5 (2) |
| C7—C6—C1 | 119.2 (2) | C22—C23—H23 | 118.8 |
| C5—C6—C1 | 118.8 (3) | C18—C23—H23 | 118.8 |
| C6—C7—C8 | 122.5 (2) | N1—C24—C25 | 112.8 (2) |
| C6—C7—H5 | 118.7 | N1—C24—H24A | 109.0 |
| C8—C7—H5 | 118.7 | C25—C24—H24A | 109.0 |
| C7—C8—C9 | 123.1 (3) | N1—C24—H24B | 109.0 |
| C7—C8—C13 | 118.3 (3) | C25—C24—H24B | 109.0 |
| C9—C8—C13 | 118.6 (3) | H24A—C24—H24B | 107.8 |
| C10—C9—C8 | 121.6 (3) | C24—C25—C26 | 113.0 (3) |
| C10—C9—H6 | 119.2 | C24—C25—H25A | 109.0 |
| C8—C9—H6 | 119.2 | C26—C25—H25A | 109.0 |
| C9—C10—C11 | 120.1 (3) | C24—C25—H25B | 109.0 |
| C9—C10—H10 | 119.9 | C26—C25—H25B | 109.0 |
| C11—C10—H10 | 119.9 | H25A—C25—H25B | 107.8 |
| C12—C11—C10 | 120.7 (3) | C27—C26—C25 | 109.6 (2) |
| C12—C11—H11 | 119.7 | C27—C26—H26A | 109.8 |
| C10—C11—H11 | 119.7 | C25—C26—H26A | 109.8 |
| C11—C12—C13 | 121.7 (3) | C27—C26—H26B | 109.8 |
| C11—C12—H12 | 119.2 | C25—C26—H26B | 109.8 |
| C13—C12—H12 | 119.2 | H26A—C26—H26B | 108.2 |
| C14—C13—C12 | 123.0 (2) | C28—C27—C26 | 111.7 (3) |
| C14—C13—C8 | 119.8 (2) | C28—C27—H27A | 109.3 |
| C12—C13—C8 | 117.3 (3) | C26—C27—H27A | 109.3 |
| C1—C14—C13 | 121.1 (2) | C28—C27—H27B | 109.3 |
| C1—C14—C15 | 119.6 (2) | C26—C27—H27B | 109.3 |
| C13—C14—C15 | 119.2 (2) | H27A—C27—H27B | 107.9 |
| O1—C15—C16 | 121.0 (2) | N1—C28—C27 | 112.6 (2) |
| O1—C15—C14 | 118.7 (2) | N1—C28—H28A | 109.1 |
| C16—C15—C14 | 120.2 (2) | C27—C28—H28A | 109.1 |
| C17—C16—C15 | 124.3 (2) | N1—C28—H28B | 109.1 |
| C17—C16—H16 | 117.9 | C27—C28—H28B | 109.1 |
| C15—C16—H16 | 117.9 | H28A—C28—H28B | 107.8 |
| C14—C1—C2—C3 | −179.0 (2) | C8—C13—C14—C15 | −175.9 (2) |
| C6—C1—C2—C3 | 0.3 (4) | C1—C14—C15—O1 | −77.6 (3) |
| C1—C2—C3—C4 | −1.0 (5) | C13—C14—C15—O1 | 99.6 (3) |
| C2—C3—C4—C5 | 0.5 (5) | C1—C14—C15—C16 | 101.5 (3) |
| C3—C4—C5—C6 | 0.6 (5) | C13—C14—C15—C16 | −81.3 (3) |
| C4—C5—C6—C7 | 178.3 (3) | O1—C15—C16—C17 | 169.2 (3) |
| C4—C5—C6—C1 | −1.3 (4) | C14—C15—C16—C17 | −9.8 (4) |
| C14—C1—C6—C7 | 0.6 (4) | C15—C16—C17—C18 | −175.6 (2) |
| C2—C1—C6—C7 | −178.8 (2) | C16—C17—C18—C19 | 171.5 (3) |
| C14—C1—C6—C5 | −179.8 (2) | C16—C17—C18—C23 | −7.2 (4) |
| C2—C1—C6—C5 | 0.8 (3) | C23—C18—C19—C20 | 1.1 (4) |
| C5—C6—C7—C8 | −178.6 (2) | C17—C18—C19—C20 | −177.7 (3) |
| C1—C6—C7—C8 | 1.0 (4) | C18—C19—C20—C21 | 0.5 (5) |
| C6—C7—C8—C9 | 178.3 (2) | C24—N1—C21—C20 | −5.5 (4) |
| C6—C7—C8—C13 | −1.4 (4) | C28—N1—C21—C20 | −146.8 (3) |
| C7—C8—C9—C10 | 178.1 (3) | C24—N1—C21—C22 | 176.8 (2) |
| C13—C8—C9—C10 | −2.1 (4) | C28—N1—C21—C22 | 35.5 (3) |
| C8—C9—C10—C11 | 0.9 (4) | C19—C20—C21—N1 | −179.3 (2) |
| C9—C10—C11—C12 | 0.7 (5) | C19—C20—C21—C22 | −1.5 (4) |
| C10—C11—C12—C13 | −0.9 (4) | N1—C21—C22—C23 | 178.8 (2) |
| C11—C12—C13—C14 | −179.0 (2) | C20—C21—C22—C23 | 0.9 (4) |
| C11—C12—C13—C8 | −0.4 (4) | C21—C22—C23—C18 | 0.7 (4) |
| C7—C8—C13—C14 | 0.3 (3) | C19—C18—C23—C22 | −1.7 (4) |
| C9—C8—C13—C14 | −179.4 (2) | C17—C18—C23—C22 | 177.1 (2) |
| C7—C8—C13—C12 | −178.4 (2) | C21—N1—C24—C25 | 165.5 (2) |
| C9—C8—C13—C12 | 1.8 (3) | C28—N1—C24—C25 | −51.5 (3) |
| C2—C1—C14—C13 | 177.7 (2) | N1—C24—C25—C26 | 51.8 (4) |
| C6—C1—C14—C13 | −1.7 (3) | C24—C25—C26—C27 | −52.4 (4) |
| C2—C1—C14—C15 | −5.2 (4) | C25—C26—C27—C28 | 53.5 (4) |
| C6—C1—C14—C15 | 175.5 (2) | C21—N1—C28—C27 | −163.3 (2) |
| C12—C13—C14—C1 | 179.9 (2) | C24—N1—C28—C27 | 53.3 (3) |
| C8—C13—C14—C1 | 1.2 (4) | C26—C27—C28—N1 | −54.9 (4) |
| C12—C13—C14—C15 | 2.7 (4) |
(E)-1-(Anthracen-9-yl)-3-[4-(piperidin-1-yl)phenyl]prop-2-en-1-one (I) . Hydrogen-bond geometry (Å, º)
Cg1 is the centroid of the C18–C23 ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C28—H28B···O1i | 0.97 | 2.36 | 3.262 (4) | 154 |
| C28—H28A···Cg1ii | 0.97 | 2.95 | 3.861 (4) | 157 |
Symmetry codes: (i) −x, −y+1, −z+2; (ii) −x+1, −y+1, −z+2.
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Crystal data
| C35H25NO | F(000) = 2000 |
| Mr = 475.56 | Dx = 1.232 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| a = 31.2875 (16) Å | Cell parameters from 9835 reflections |
| b = 9.0470 (4) Å | θ = 2.3–21.9° |
| c = 18.3643 (8) Å | µ = 0.07 mm−1 |
| β = 99.388 (3)° | T = 296 K |
| V = 5128.5 (4) Å3 | Block, yellow |
| Z = 8 | 0.96 × 0.23 × 0.17 mm |
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Data collection
| Bruker SMART APEXII DUO CCD area-detector diffractometer | 4183 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.076 |
| φ and ω scans | θmax = 30.4°, θmin = 1.3° |
| Absorption correction: multi-scan (SADABS; Bruker, 2009) | h = −44→44 |
| Tmin = 0.645, Tmax = 0.957 | k = −12→12 |
| 98729 measured reflections | l = −26→26 |
| 7726 independent reflections |
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Refinement
| Refinement on F2 | 0 restraints |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.057 | H-atom parameters constrained |
| wR(F2) = 0.144 | w = 1/[σ2(Fo2) + (0.0415P)2 + 2.2393P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max = 0.001 |
| 7726 reflections | Δρmax = 0.13 e Å−3 |
| 334 parameters | Δρmin = −0.14 e Å−3 |
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Special details
| Experimental. The following wavelength and cell were deduced by SADABS from the direction cosines etc. They are given here for emergency use only: CELL 0.71163 9.142 16.459 18.559 99.001 89.988 106.089 |
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.54087 (4) | 0.92358 (14) | 0.60195 (7) | 0.0596 (3) | |
| O1 | 0.76241 (4) | 0.60494 (16) | 0.40875 (8) | 0.0900 (4) | |
| C1 | 0.69686 (5) | 0.56889 (18) | 0.25826 (8) | 0.0548 (4) | |
| C2 | 0.70542 (5) | 0.7199 (2) | 0.24498 (10) | 0.0667 (4) | |
| H2A | 0.7163 | 0.7808 | 0.2845 | 0.080* | |
| C3 | 0.69796 (6) | 0.7766 (3) | 0.17592 (12) | 0.0841 (6) | |
| H3A | 0.7036 | 0.8759 | 0.1684 | 0.101* | |
| C4 | 0.68185 (8) | 0.6870 (3) | 0.11597 (12) | 0.0993 (7) | |
| H4A | 0.6766 | 0.7273 | 0.0688 | 0.119* | |
| C5 | 0.67379 (8) | 0.5439 (3) | 0.12545 (11) | 0.0930 (7) | |
| H5A | 0.6634 | 0.4860 | 0.0845 | 0.112* | |
| C6 | 0.68065 (6) | 0.4783 (2) | 0.19662 (9) | 0.0677 (5) | |
| C7 | 0.67269 (6) | 0.3305 (2) | 0.20807 (11) | 0.0783 (5) | |
| H7A | 0.6621 | 0.2718 | 0.1675 | 0.094* | |
| C8 | 0.67976 (6) | 0.26679 (19) | 0.27691 (10) | 0.0662 (5) | |
| C9 | 0.67272 (7) | 0.1140 (2) | 0.28858 (15) | 0.0909 (7) | |
| H9A | 0.6619 | 0.0544 | 0.2485 | 0.109* | |
| C10 | 0.68128 (8) | 0.0541 (2) | 0.35592 (16) | 0.0987 (7) | |
| H10A | 0.6765 | −0.0462 | 0.3622 | 0.118* | |
| C11 | 0.69737 (7) | 0.1414 (2) | 0.41678 (13) | 0.0858 (6) | |
| H11A | 0.7036 | 0.0983 | 0.4633 | 0.103* | |
| C12 | 0.70399 (6) | 0.2875 (2) | 0.40906 (10) | 0.0681 (5) | |
| H12A | 0.7141 | 0.3442 | 0.4505 | 0.082* | |
| C13 | 0.69584 (5) | 0.35568 (17) | 0.33898 (9) | 0.0545 (4) | |
| C14 | 0.70393 (4) | 0.50520 (17) | 0.32830 (8) | 0.0504 (3) | |
| C15 | 0.72316 (5) | 0.59679 (18) | 0.39314 (8) | 0.0554 (4) | |
| C16 | 0.69564 (5) | 0.67286 (17) | 0.43679 (8) | 0.0553 (4) | |
| H16A | 0.7088 | 0.7352 | 0.4741 | 0.066* | |
| C17 | 0.65301 (5) | 0.66021 (16) | 0.42750 (8) | 0.0506 (3) | |
| H17A | 0.6402 | 0.5991 | 0.3894 | 0.061* | |
| C18 | 0.62422 (5) | 0.73214 (16) | 0.47080 (7) | 0.0484 (3) | |
| C19 | 0.58198 (5) | 0.68422 (18) | 0.46718 (8) | 0.0568 (4) | |
| H19A | 0.5719 | 0.6082 | 0.4348 | 0.068* | |
| C20 | 0.55459 (5) | 0.74561 (18) | 0.50998 (9) | 0.0585 (4) | |
| H20A | 0.5264 | 0.7100 | 0.5065 | 0.070* | |
| C21 | 0.56838 (5) | 0.86064 (16) | 0.55852 (8) | 0.0498 (3) | |
| C22 | 0.61055 (5) | 0.91102 (16) | 0.56133 (8) | 0.0511 (3) | |
| H22A | 0.6205 | 0.9887 | 0.5927 | 0.061* | |
| C23 | 0.63763 (5) | 0.84824 (16) | 0.51872 (8) | 0.0508 (3) | |
| H23A | 0.6657 | 0.8841 | 0.5219 | 0.061* | |
| C24 | 0.50401 (5) | 0.84596 (19) | 0.61751 (8) | 0.0561 (4) | |
| C25 | 0.50740 (6) | 0.7044 (2) | 0.64455 (10) | 0.0742 (5) | |
| H25A | 0.5343 | 0.6582 | 0.6533 | 0.089* | |
| C26 | 0.47145 (8) | 0.6306 (3) | 0.65871 (12) | 0.0934 (7) | |
| H26A | 0.4740 | 0.5341 | 0.6764 | 0.112* | |
| C27 | 0.43226 (8) | 0.6972 (3) | 0.64707 (13) | 0.1015 (8) | |
| H27A | 0.4079 | 0.6470 | 0.6568 | 0.122* | |
| C28 | 0.42867 (7) | 0.8379 (3) | 0.62104 (12) | 0.0937 (7) | |
| H28A | 0.4017 | 0.8837 | 0.6132 | 0.112* | |
| C29 | 0.46430 (6) | 0.9135 (2) | 0.60614 (9) | 0.0714 (5) | |
| H29A | 0.4615 | 1.0099 | 0.5885 | 0.086* | |
| C30 | 0.55076 (5) | 1.06241 (18) | 0.63734 (9) | 0.0572 (4) | |
| C31 | 0.55374 (7) | 1.0733 (2) | 0.71230 (10) | 0.0883 (6) | |
| H31A | 0.5491 | 0.9905 | 0.7400 | 0.106* | |
| C32 | 0.56357 (9) | 1.2064 (3) | 0.74657 (13) | 0.1146 (9) | |
| H32A | 0.5654 | 1.2134 | 0.7975 | 0.137* | |
| C33 | 0.57072 (8) | 1.3275 (3) | 0.70722 (16) | 0.1042 (8) | |
| H33A | 0.5779 | 1.4168 | 0.7311 | 0.125* | |
| C34 | 0.56734 (7) | 1.3186 (2) | 0.63278 (13) | 0.0865 (6) | |
| H34A | 0.5718 | 1.4023 | 0.6056 | 0.104* | |
| C35 | 0.55728 (6) | 1.18625 (19) | 0.59741 (10) | 0.0695 (5) | |
| H35A | 0.5549 | 1.1806 | 0.5463 | 0.083* |
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0647 (8) | 0.0574 (8) | 0.0621 (8) | −0.0005 (6) | 0.0262 (6) | −0.0097 (6) |
| O1 | 0.0500 (7) | 0.1216 (11) | 0.0971 (10) | −0.0044 (7) | 0.0079 (6) | −0.0522 (9) |
| C1 | 0.0488 (8) | 0.0628 (9) | 0.0562 (9) | −0.0018 (7) | 0.0182 (7) | −0.0101 (8) |
| C2 | 0.0596 (10) | 0.0703 (11) | 0.0742 (11) | −0.0030 (8) | 0.0227 (8) | −0.0012 (9) |
| C3 | 0.0780 (13) | 0.0912 (15) | 0.0907 (15) | 0.0032 (11) | 0.0360 (11) | 0.0192 (12) |
| C4 | 0.1056 (17) | 0.131 (2) | 0.0662 (13) | 0.0071 (16) | 0.0295 (12) | 0.0171 (14) |
| C5 | 0.1064 (17) | 0.1208 (19) | 0.0534 (11) | −0.0031 (15) | 0.0174 (10) | −0.0125 (12) |
| C6 | 0.0677 (11) | 0.0839 (13) | 0.0533 (10) | −0.0038 (9) | 0.0155 (8) | −0.0155 (9) |
| C7 | 0.0864 (13) | 0.0825 (13) | 0.0673 (12) | −0.0145 (10) | 0.0162 (10) | −0.0346 (10) |
| C8 | 0.0661 (10) | 0.0614 (10) | 0.0748 (12) | −0.0072 (8) | 0.0226 (9) | −0.0239 (9) |
| C9 | 0.1045 (16) | 0.0618 (12) | 0.1124 (18) | −0.0143 (11) | 0.0356 (14) | −0.0324 (12) |
| C10 | 0.1129 (18) | 0.0577 (12) | 0.136 (2) | −0.0023 (12) | 0.0523 (16) | −0.0029 (14) |
| C11 | 0.0895 (14) | 0.0722 (13) | 0.1014 (16) | 0.0072 (11) | 0.0322 (12) | 0.0109 (12) |
| C12 | 0.0664 (11) | 0.0692 (11) | 0.0717 (11) | 0.0008 (9) | 0.0200 (9) | −0.0027 (9) |
| C13 | 0.0498 (8) | 0.0561 (9) | 0.0607 (9) | −0.0017 (7) | 0.0187 (7) | −0.0112 (8) |
| C14 | 0.0435 (7) | 0.0578 (9) | 0.0522 (8) | −0.0015 (6) | 0.0145 (6) | −0.0144 (7) |
| C15 | 0.0483 (8) | 0.0612 (9) | 0.0571 (9) | −0.0016 (7) | 0.0097 (7) | −0.0127 (7) |
| C16 | 0.0543 (9) | 0.0608 (9) | 0.0505 (8) | −0.0010 (7) | 0.0077 (7) | −0.0166 (7) |
| C17 | 0.0549 (9) | 0.0534 (8) | 0.0434 (8) | −0.0002 (7) | 0.0080 (6) | −0.0074 (6) |
| C18 | 0.0504 (8) | 0.0520 (8) | 0.0429 (7) | 0.0036 (6) | 0.0077 (6) | −0.0048 (6) |
| C19 | 0.0553 (9) | 0.0608 (9) | 0.0545 (9) | −0.0031 (7) | 0.0090 (7) | −0.0154 (7) |
| C20 | 0.0509 (8) | 0.0650 (10) | 0.0611 (9) | −0.0042 (7) | 0.0133 (7) | −0.0117 (8) |
| C21 | 0.0547 (8) | 0.0525 (8) | 0.0438 (8) | 0.0059 (7) | 0.0128 (6) | −0.0022 (6) |
| C22 | 0.0566 (9) | 0.0511 (8) | 0.0448 (8) | 0.0026 (7) | 0.0058 (6) | −0.0077 (6) |
| C23 | 0.0495 (8) | 0.0543 (8) | 0.0486 (8) | 0.0001 (7) | 0.0080 (6) | −0.0050 (7) |
| C24 | 0.0606 (9) | 0.0653 (10) | 0.0448 (8) | −0.0003 (8) | 0.0161 (7) | −0.0058 (7) |
| C25 | 0.0765 (12) | 0.0699 (12) | 0.0784 (12) | −0.0023 (9) | 0.0189 (9) | 0.0062 (10) |
| C26 | 0.1081 (18) | 0.0881 (15) | 0.0889 (15) | −0.0294 (14) | 0.0308 (13) | −0.0025 (12) |
| C27 | 0.0915 (17) | 0.130 (2) | 0.0908 (16) | −0.0422 (16) | 0.0377 (13) | −0.0292 (15) |
| C28 | 0.0591 (12) | 0.134 (2) | 0.0910 (15) | −0.0019 (13) | 0.0198 (10) | −0.0251 (15) |
| C29 | 0.0676 (11) | 0.0879 (13) | 0.0604 (10) | 0.0107 (10) | 0.0152 (8) | −0.0034 (9) |
| C30 | 0.0609 (9) | 0.0586 (9) | 0.0549 (9) | 0.0057 (7) | 0.0179 (7) | −0.0100 (8) |
| C31 | 0.1208 (17) | 0.0893 (14) | 0.0589 (11) | −0.0092 (13) | 0.0272 (11) | −0.0114 (10) |
| C32 | 0.155 (2) | 0.119 (2) | 0.0749 (15) | −0.0213 (18) | 0.0321 (15) | −0.0418 (15) |
| C33 | 0.1124 (18) | 0.0869 (16) | 0.118 (2) | −0.0103 (14) | 0.0336 (15) | −0.0477 (15) |
| C34 | 0.0954 (15) | 0.0605 (11) | 0.1077 (17) | 0.0056 (10) | 0.0284 (12) | −0.0109 (11) |
| C35 | 0.0818 (12) | 0.0621 (11) | 0.0662 (11) | 0.0100 (9) | 0.0166 (9) | −0.0025 (9) |
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Geometric parameters (Å, º)
| N1—C21 | 1.3876 (18) | C17—H17A | 0.9300 |
| N1—C24 | 1.419 (2) | C18—C19 | 1.382 (2) |
| N1—C30 | 1.425 (2) | C18—C23 | 1.390 (2) |
| O1—C15 | 1.2170 (18) | C19—C20 | 1.371 (2) |
| C1—C14 | 1.394 (2) | C19—H19A | 0.9300 |
| C1—C2 | 1.421 (2) | C20—C21 | 1.392 (2) |
| C1—C6 | 1.422 (2) | C20—H20A | 0.9300 |
| C2—C3 | 1.353 (3) | C21—C22 | 1.389 (2) |
| C2—H2A | 0.9300 | C22—C23 | 1.3672 (19) |
| C3—C4 | 1.394 (3) | C22—H22A | 0.9300 |
| C3—H3A | 0.9300 | C23—H23A | 0.9300 |
| C4—C5 | 1.336 (3) | C24—C29 | 1.370 (2) |
| C4—H4A | 0.9300 | C24—C25 | 1.372 (2) |
| C5—C6 | 1.420 (3) | C25—C26 | 1.369 (3) |
| C5—H5A | 0.9300 | C25—H25A | 0.9300 |
| C6—C7 | 1.382 (3) | C26—C27 | 1.352 (3) |
| C7—C8 | 1.374 (3) | C26—H26A | 0.9300 |
| C7—H7A | 0.9300 | C27—C28 | 1.358 (3) |
| C8—C13 | 1.419 (2) | C27—H27A | 0.9300 |
| C8—C9 | 1.422 (3) | C28—C29 | 1.373 (3) |
| C9—C10 | 1.337 (3) | C28—H28A | 0.9300 |
| C9—H9A | 0.9300 | C29—H29A | 0.9300 |
| C10—C11 | 1.394 (3) | C30—C31 | 1.368 (2) |
| C10—H10A | 0.9300 | C30—C35 | 1.372 (2) |
| C11—C12 | 1.349 (3) | C31—C32 | 1.370 (3) |
| C11—H11A | 0.9300 | C31—H31A | 0.9300 |
| C12—C13 | 1.412 (2) | C32—C33 | 1.351 (3) |
| C12—H12A | 0.9300 | C32—H32A | 0.9300 |
| C13—C14 | 1.396 (2) | C33—C34 | 1.356 (3) |
| C14—C15 | 1.494 (2) | C33—H33A | 0.9300 |
| C15—C16 | 1.443 (2) | C34—C35 | 1.374 (3) |
| C16—C17 | 1.322 (2) | C34—H34A | 0.9300 |
| C16—H16A | 0.9300 | C35—H35A | 0.9300 |
| C17—C18 | 1.4494 (19) | ||
| C21—N1—C24 | 120.91 (13) | C19—C18—C23 | 117.05 (13) |
| C21—N1—C30 | 121.00 (13) | C19—C18—C17 | 120.52 (13) |
| C24—N1—C30 | 117.88 (12) | C23—C18—C17 | 122.41 (13) |
| C14—C1—C2 | 123.36 (15) | C20—C19—C18 | 121.84 (14) |
| C14—C1—C6 | 118.58 (15) | C20—C19—H19A | 119.1 |
| C2—C1—C6 | 118.06 (16) | C18—C19—H19A | 119.1 |
| C3—C2—C1 | 121.13 (18) | C19—C20—C21 | 120.81 (14) |
| C3—C2—H2A | 119.4 | C19—C20—H20A | 119.6 |
| C1—C2—H2A | 119.4 | C21—C20—H20A | 119.6 |
| C2—C3—C4 | 120.3 (2) | N1—C21—C22 | 121.24 (13) |
| C2—C3—H3A | 119.8 | N1—C21—C20 | 121.20 (14) |
| C4—C3—H3A | 119.8 | C22—C21—C20 | 117.55 (13) |
| C5—C4—C3 | 120.8 (2) | C23—C22—C21 | 121.07 (14) |
| C5—C4—H4A | 119.6 | C23—C22—H22A | 119.5 |
| C3—C4—H4A | 119.6 | C21—C22—H22A | 119.5 |
| C4—C5—C6 | 121.5 (2) | C22—C23—C18 | 121.66 (14) |
| C4—C5—H5A | 119.3 | C22—C23—H23A | 119.2 |
| C6—C5—H5A | 119.3 | C18—C23—H23A | 119.2 |
| C7—C6—C5 | 122.72 (18) | C29—C24—C25 | 119.11 (17) |
| C7—C6—C1 | 119.09 (17) | C29—C24—N1 | 119.71 (16) |
| C5—C6—C1 | 118.19 (18) | C25—C24—N1 | 121.19 (15) |
| C8—C7—C6 | 122.66 (16) | C26—C25—C24 | 120.5 (2) |
| C8—C7—H7A | 118.7 | C26—C25—H25A | 119.8 |
| C6—C7—H7A | 118.7 | C24—C25—H25A | 119.8 |
| C7—C8—C13 | 118.97 (16) | C27—C26—C25 | 120.3 (2) |
| C7—C8—C9 | 122.75 (18) | C27—C26—H26A | 119.9 |
| C13—C8—C9 | 118.26 (19) | C25—C26—H26A | 119.9 |
| C10—C9—C8 | 121.4 (2) | C26—C27—C28 | 119.7 (2) |
| C10—C9—H9A | 119.3 | C26—C27—H27A | 120.2 |
| C8—C9—H9A | 119.3 | C28—C27—H27A | 120.2 |
| C9—C10—C11 | 120.3 (2) | C27—C28—C29 | 120.9 (2) |
| C9—C10—H10A | 119.9 | C27—C28—H28A | 119.5 |
| C11—C10—H10A | 119.9 | C29—C28—H28A | 119.5 |
| C12—C11—C10 | 120.8 (2) | C24—C29—C28 | 119.6 (2) |
| C12—C11—H11A | 119.6 | C24—C29—H29A | 120.2 |
| C10—C11—H11A | 119.6 | C28—C29—H29A | 120.2 |
| C11—C12—C13 | 121.06 (18) | C31—C30—C35 | 119.13 (17) |
| C11—C12—H12A | 119.5 | C31—C30—N1 | 119.80 (16) |
| C13—C12—H12A | 119.5 | C35—C30—N1 | 121.07 (14) |
| C14—C13—C12 | 122.79 (15) | C30—C31—C32 | 120.0 (2) |
| C14—C13—C8 | 119.02 (15) | C30—C31—H31A | 120.0 |
| C12—C13—C8 | 118.17 (15) | C32—C31—H31A | 120.0 |
| C1—C14—C13 | 121.68 (14) | C33—C32—C31 | 120.7 (2) |
| C1—C14—C15 | 119.26 (14) | C33—C32—H32A | 119.6 |
| C13—C14—C15 | 118.95 (14) | C31—C32—H32A | 119.6 |
| O1—C15—C16 | 120.72 (14) | C32—C33—C34 | 119.9 (2) |
| O1—C15—C14 | 118.75 (13) | C32—C33—H33A | 120.1 |
| C16—C15—C14 | 120.53 (13) | C34—C33—H33A | 120.1 |
| C17—C16—C15 | 124.60 (14) | C33—C34—C35 | 120.2 (2) |
| C17—C16—H16A | 117.7 | C33—C34—H34A | 119.9 |
| C15—C16—H16A | 117.7 | C35—C34—H34A | 119.9 |
| C16—C17—C18 | 126.49 (14) | C30—C35—C34 | 120.09 (18) |
| C16—C17—H17A | 116.8 | C30—C35—H35A | 120.0 |
| C18—C17—H17A | 116.8 | C34—C35—H35A | 120.0 |
| C14—C1—C2—C3 | −179.93 (15) | C16—C17—C18—C19 | −164.77 (16) |
| C6—C1—C2—C3 | 0.7 (2) | C16—C17—C18—C23 | 13.6 (2) |
| C1—C2—C3—C4 | −0.4 (3) | C23—C18—C19—C20 | −1.4 (2) |
| C2—C3—C4—C5 | −0.5 (3) | C17—C18—C19—C20 | 176.98 (15) |
| C3—C4—C5—C6 | 0.9 (4) | C18—C19—C20—C21 | 0.7 (3) |
| C4—C5—C6—C7 | −179.8 (2) | C24—N1—C21—C22 | −159.50 (14) |
| C4—C5—C6—C1 | −0.6 (3) | C30—N1—C21—C22 | 15.1 (2) |
| C14—C1—C6—C7 | −0.3 (2) | C24—N1—C21—C20 | 21.1 (2) |
| C2—C1—C6—C7 | 179.07 (16) | C30—N1—C21—C20 | −164.28 (15) |
| C14—C1—C6—C5 | −179.64 (16) | C19—C20—C21—N1 | 179.90 (15) |
| C2—C1—C6—C5 | −0.2 (2) | C19—C20—C21—C22 | 0.5 (2) |
| C5—C6—C7—C8 | 179.33 (18) | N1—C21—C22—C23 | 179.68 (14) |
| C1—C6—C7—C8 | 0.1 (3) | C20—C21—C22—C23 | −0.9 (2) |
| C6—C7—C8—C13 | 0.1 (3) | C21—C22—C23—C18 | 0.2 (2) |
| C6—C7—C8—C9 | −178.22 (18) | C19—C18—C23—C22 | 1.0 (2) |
| C7—C8—C9—C10 | 177.7 (2) | C17—C18—C23—C22 | −177.38 (14) |
| C13—C8—C9—C10 | −0.6 (3) | C21—N1—C24—C29 | −129.43 (16) |
| C8—C9—C10—C11 | 0.2 (3) | C30—N1—C24—C29 | 55.8 (2) |
| C9—C10—C11—C12 | 0.9 (3) | C21—N1—C24—C25 | 51.2 (2) |
| C10—C11—C12—C13 | −1.6 (3) | C30—N1—C24—C25 | −123.52 (17) |
| C11—C12—C13—C14 | −177.34 (16) | C29—C24—C25—C26 | 1.2 (3) |
| C11—C12—C13—C8 | 1.1 (2) | N1—C24—C25—C26 | −179.45 (16) |
| C7—C8—C13—C14 | 0.1 (2) | C24—C25—C26—C27 | −0.9 (3) |
| C9—C8—C13—C14 | 178.47 (16) | C25—C26—C27—C28 | 0.2 (3) |
| C7—C8—C13—C12 | −178.37 (16) | C26—C27—C28—C29 | 0.2 (3) |
| C9—C8—C13—C12 | 0.0 (2) | C25—C24—C29—C28 | −0.8 (3) |
| C2—C1—C14—C13 | −178.85 (14) | N1—C24—C29—C28 | 179.80 (16) |
| C6—C1—C14—C13 | 0.5 (2) | C27—C28—C29—C24 | 0.2 (3) |
| C2—C1—C14—C15 | −2.6 (2) | C21—N1—C30—C31 | −123.00 (18) |
| C6—C1—C14—C15 | 176.76 (14) | C24—N1—C30—C31 | 51.7 (2) |
| C12—C13—C14—C1 | 178.00 (14) | C21—N1—C30—C35 | 57.2 (2) |
| C8—C13—C14—C1 | −0.4 (2) | C24—N1—C30—C35 | −128.10 (17) |
| C12—C13—C14—C15 | 1.7 (2) | C35—C30—C31—C32 | −0.7 (3) |
| C8—C13—C14—C15 | −176.67 (13) | N1—C30—C31—C32 | 179.5 (2) |
| C1—C14—C15—O1 | −87.0 (2) | C30—C31—C32—C33 | −0.5 (4) |
| C13—C14—C15—O1 | 89.39 (19) | C31—C32—C33—C34 | 1.3 (4) |
| C1—C14—C15—C16 | 93.66 (18) | C32—C33—C34—C35 | −0.9 (4) |
| C13—C14—C15—C16 | −89.99 (18) | C31—C30—C35—C34 | 1.0 (3) |
| O1—C15—C16—C17 | −174.13 (17) | N1—C30—C35—C34 | −179.15 (16) |
| C14—C15—C16—C17 | 5.2 (3) | C33—C34—C35—C30 | −0.2 (3) |
| C15—C16—C17—C18 | 178.77 (15) |
(E)-1-(Anthracen-9-yl)-3-[4-(diphenylamino)phenyl]prop-2-en-1-one (II) . Hydrogen-bond geometry (Å, º)
Cg1 is the centroid of the C18–C23 ring.
| D—H···A | D—H | H···A | D···A | D—H···A |
| C23—H23A···O1i | 0.93 | 2.40 | 3.221 (2) | 147 |
| C29—H29A···Cg1ii | 0.93 | 2.96 | 3.739 (19) | 142 |
Symmetry codes: (i) −x+3/2, −y+3/2, −z+1; (ii) x+3/2, y+5/2, z+1.
Funding Statement
This work was funded by Malaysian Government grant My Brain15 to D.A. Zainuri. Universiti Sains Malaysia grants 203/PFIZIK/6711572 and 304/PFIZIK/6313336.
References
- Abdullah, A. A., Hassan, N. H. H., Arshad, S., Khalib, N. C. & Razak, I. A. (2016). Acta Cryst. E72, 648–651. [DOI] [PMC free article] [PubMed]
- Agrahari, A., Wagers, P. O., Schildcrout, S. M., Masnovi, J. & Youngs, W. J. (2015). Acta Cryst. E71, 357–359. [DOI] [PMC free article] [PubMed]
- Bruker (2009). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Cicogna, F., Ingrosso, G., Lodato, F., Marchetti, F. & Zandomeneghi, M. (2004). Tetrahedron, 60, 11959–11968.
- D’silva, E. D., Podagatlapalli, G. K., Rao, S. V., Rao, D. N. & Dharmaprakash, S. M. (2011). Cryst. Growth Des. 11, 5326–5369.
- Girisha, M., Yathirajan, H. S., Jasinski, J. P. & Glidewell, C. (2016). Acta Cryst. E72, 1153–1158. [DOI] [PMC free article] [PubMed]
- Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. (2016). Acta Cryst. B72, 171–179. [DOI] [PMC free article] [PubMed]
- Gunasekaran, S., Kumaresan, S., Arunbalaji, R., Anand, G. & Srinivasan, S. (2008). J. Chem. Sci. 120, 315–324.
- Harlow, R. L., Loghry, R. A., Williams, H. J. & Simonsen, S. H. (1975). Acta Cryst. B31, 1344–1350.
- Jung, Y., Son, K., Oh, Y. E. & Noh, D. (2008). Polyhedron, 27, 861–867.
- Maidur, S. R., Jahagirdar, J. R., Patil, P. S., Chia, T. S. & Quah, C. K. (2018). Opt. Mater. 75, 580–594.
- Murray, J. S. & Sen, K. (1996). Molecular Electrostatic Potentials, Concepts and Applications. Amsterdam: Elsevier.
- Scrocco, E. & Tomasi, J. (1978). Advances in Quantum Chemistry. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sheldrick, G. M. (2015). Acta Cryst. C71, 3–8.
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Wolff, S. K., Grimwood, D. J., McKinnon, J. J., Turner, M. J., Jayatilaka, D. & Spackman, M. A. (2012). CrystalExplorer. University of Western Australia, Perth.
- Wu, W., Liu, Y. & Zhu, D. (2010). Chem. Soc. Rev. 39, 1489–1502. [DOI] [PubMed]
- Zainuri, D. A., Arshad, S., Khalib, N. C., Razak, A. I., Pillai, R. R., Sulaiman, F., Hashim, N. S., Ooi, K. L., Armaković, S., Armaković, S. J., Panicker, Y. & Van Alsenoy, C. (2017). J. Mol. Struct. 1128, 520–533.
- Zainuri, D. A., Razak, I. A. & Arshad, S. (2018a). Acta Cryst. E74, 492–496. [DOI] [PMC free article] [PubMed]
- Zainuri, D. A., Razak, I. A. & Arshad, S. (2018b). Acta Cryst. E74, 650–655. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, II. DOI: 10.1107/S2056989018006527/lh5873sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S2056989018006527/lh5873Isup2.hkl
Structure factors: contains datablock(s) II. DOI: 10.1107/S2056989018006527/lh5873IIsup3.hkl
Comparison between selected calculated (DFT) and experimental geometrical data. DOI: 10.1107/S2056989018006527/lh5873sup4.pdf
Additional supporting information: crystallographic information; 3D view; checkCIF report










