Abstract
Purpose of Review
Current feeding advice to prevent pediatric obesity focusses on caregiver feeding behaviors. This review integrates newer data showing that child appetitive traits also have a genetic component.
Recent Findings
Caregiver feeding behaviors robustly correlate with child eating behaviors; however there is also a strong heritable component.
Summary
The satiety cascade delineates the biological drive underlying hunger, satiation and satiety. Innate individual differences exist for the components of the satiety cascade, which may explain the heritability of child eating behaviors. However, given the correlation of caregiver feeding behaviors with child eating behaviors any etiological model should include both genetic/biological components and environmental. Integrating the biological etiology of child eating behaviors into the current environmental model has implications for tailoring feeding advice which needs to move from a “one size fits all” approach, to one that is tailored to individual differences in children’s biological drives to appetite.
Keywords: appetitive traits, pediatric obesity, heritability, parenting, feeding style
Introduction
Children are thought to have a natural ability to self-regulate their eating behaviors and maintain energy balance which is achieved by responding to the internal cues the “satiety cascade”. The rise in pediatric obesity is, in part, attributed to caregiver feeding behaviors which cause children eat in response to external cues, rather than their internal biological signals of hunger, satiation and satiety. Hence, much advice for the prevention of pediatric obesity is targeted at developing responsive caregiver feeding behaviors considered to maintain the child’s tendency to eat and stop eating in response to their underlying appetitive drive. More recently, the role of biology in shaping child eating behaviors has been recognized, perhaps spurred on by the results of recent twin studies which suggest that child eating behaviors are moderately heritable. This raises the question of how much the feeding environment can really alter the child’s genetic predisposition to eat in a certain way, which has recently become the topic of high-profile debate (1). This review will describe those internal appetitive cues which are encapsulated by the “satiety cascade”, and those caregiver feeding behaviors thought to shape child eating behaviors. We will briefly make comments on why these caregiver feeding behaviors are falling out of favor as critical contributors to child obesity risk, with recourse to the evidence which suggests that child eating behaviors are partly genetic in origin. Our goal is to synthesize these two lines of evidence and suggest a new model of child eating behaviors which reflects the likelihood that gene-environment interplay underlies child eating. We will discuss the implications of this model for advice on child obesity prevention.
Child Appetitive Traits
Definition
Energy balance in childhood requires behaviors which match the amount of energy consumed via eating and drinking, with how much is expended via metabolism, direct waste or physical activity. In recognition of this first component (“consumption”), a growing body of research has focused not on what children eat, but how they eat. The “how” of child eating is measured across several well described eating phenotypes, collectively known as child appetitive traits. Most appetitive traits (Table 1) relate to a construct known as the “self-regulation of eating behaviors” which refers to “the ability, both inborn and socialized, to eat and stop eating in response to internal cues of hunger and fullness” (2). In other words, they measure one or more aspects of a child’s tendency to listen to the internal cues and signals from the satiety cascade which are mediated by energy intake and, in order to maintain energy balance, help the child to start eating when feeling hungry (“hunger”), stop eating when feeling full (“satiation”) and resist consuming further appetizing snack foods until hungry again (“satiety”).
Table 1.
Key Terms and Definitions
| Term | Definition |
|---|---|
| Appetite-related | |
| Hunger | Those internal cues and signals which bring about the initiation of eating |
| Satiation | Those internal cues and signals which bring about the cessation of eating |
| Satiety | Those internal cues and signals which prevent eating after the termination of a meal, until hunger returns |
| Appetitive Drive | Those internal cues and signals which collectively bring about hunger, satiation and satiety |
| Appetitive traits | Individual eating behaviors which may measure one of more of hunger, satiation and satiety |
| Appetite | A term collectively encompassing the tendency to eat when hungry (or not), stop eating when full (or not), and the extent to which eating during satiation occurs |
| Specific Eating Behaviors | |
| Enjoyment of Food | Responsivity to food as an external stimulus |
| Compensation | The ability to adjust eating behaviors in response to earlier energy intake and maintain energy balance |
| Eating in the absence of hunger (EAH) | Eating when having recently reported satiation |
| Eating rate | How quickly food is consumed, measured via metrics such as bites per second and the change in bites per second across a meal |
| Emotional over- and under-eating | Altering food intake in response to internal emotion cues |
| Food fussiness | Rejection of a wide number of foods |
| Food responsiveness | Eating in response to the presence of food, rather than internal signals of hunger |
| Satiety responsiveness | The extent to which appetizing snack foods are resisted in the period between satiation and hunger. |
| Slowness in eating | A reduction is eating speed across mealtimes |
| Delay of gratification (DOG) | The tendency to choose a larger, delayed reward over a small immediate reward |
| External Eating | Eating in response to the sight of smell of food (also called ‘food responsiveness’) |
Measurement
Appetitive traits are assessed using several methodologies. Compensation is usually measured through an experimental design known as a compensation trial, where individuals are given a “pre-load” of calories (usually in liquid form) before a meal, and the compensatory reduction in calories eaten at subsequent meal(s) is the outcome. The eating in the absence of hunger (EAH) and delay of gratification (DOG) protocols employ direct observation in a standardized setting. The EAH protocol measures the number of calories a child eats when not hungry, usually about 20 minutes after the child has been provided with a standardized meal and has reported satiation. In the DOG protocol, children are offered the choice of either a small food reward now or a larger food reward later, after sitting in a room in front of the smaller reward – the best known example of which is Walter Mischel’s “marshmallow test” (3). Other child appetitive traits are measured by caregiver reports (usually the mother), either using scales developed for children such as the Child Eating Behavior questionnaire (CEBQ; (4)), or using scales developed for adults but used on children such as the Dutch Eating Behavior Questionnaire (DEBQ; (5)).
Association with weight status in children
Most child appetitive traits are considered to associate with weight status. On the one hand, there are those eating behaviors which help to maintain energy balance and tend to show an inverse association with BMI in childhood; these include compensation (6, 7), the DOG (8, 9), satiety responsiveness (10–13), slowness in eating (10, 11, 13), emotional undereating (10) and restrained eating (14–16). On the other hand, there are those behaviors which are theorized to positively associate with weight status and include EAH (17–22), eating rate (23), enjoyment of food (10, 11, 13), emotional overeating (10, 11, 13), desire to drink (10, 11), food fussiness (10, 11, 24), emotional (over)eating (14) and external eating (14, 15). This latter set of traits, which contribute to energy imbalance and so increase obesity risk, are thought to represent a failure to appropriately respond to internal appetitive cues. Much work has gone into mapping out the internal cues underlying eating behaviors, which are described by the satiety cascade, and proposed in the late 80s by Blundell, Rogers and Hill (Figure 1; (25)). The satiety cascade aims to describe the molecular milieu responsible for internals cues which should give rise to behaviors of meal initiation (hunger), cessation (satiation), and the ability to resist eating again until hunger returns (satiety).
Figure 1.
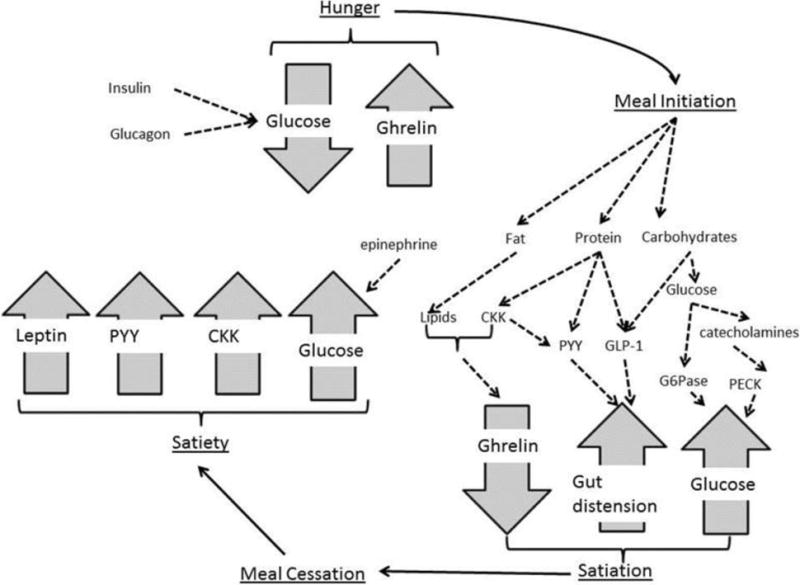
The role of the gut in appetitive drive
The Satiety Cascade
Mechanisms of meal initiation (Figure 1)
The internal factors involved in meal initiation are poorly understood compared to those which bring about the cessation of eating, and ghrelin and glucose are seen as the main protagonists driving when eating begins. Ghrelin is cleaved from preproghrelin which secreted by the gut and small intestine, and acetylated by the enzyme ghrelin O-acyltransferase into active (acyl ghrelin) and inactive (des-acyl ghrelin) isoforms. The active form finds receptors in hypothalamus, heart, lung, pancreas, adipose tissue and intestines. Recently research has suggested that the inactive form may also have receptors and play a role in eating behaviors although this potential role is not yet well delineated (26). Levels of ghrelin in the blood peak before meal onset and declines during the meal and after eating (27) leading it to be considered a orexigenic hormone which initiates food intake, even though mice lacking ghrelin do not exhibit reduced food intake (28).
In the case of glucose, rather than its presence as with the presence of ghrelin, it is the decline of glucose after a meal and its relative absence that experimental studies support as playing a role in meal initiation when registered by the hypothalamus (29–33). Although not often included as a core component of the satiety cascade insulin, glucagon and epinephrine must therefore play a role in meal initiation given their status as core components of the glycolysis.
Mechanisms of satiation (Figure 1)
The termination of a meal can be seen as a combination of the decline of meal initiation factors, and the rise of specific termination factors. The ingestion of glucose and protein are effective in reducing ghrelin levels, with lipids less effective at this and dependent on the presence of other hormones such as cholecystokinin (CKK; (34, 35). Similarly, the release of phosphoenolpyruvate carboxykinase (PECK) as induced by catecholamines such as adrenaline and noradrenaline, and glucose-6-phosphatase (G6Pase) after glucose ingestion releases glucose into bloodstream and contributes to satiation.
More specifically to satiation, administration of the CKK family of hormones results in a reduced meal size, and is most effective at achieving this when combined with gastric dissention (36). Delayed gastric emptying contributes to this process and is enabled by the release of Peptide YY (PYY; (37, 38)) either directly from food ingestion or from the release of CKK, and glucagon-like peptide 1 (GLP-1) which is released in a bi-phasic manner (first in response to ingesting carbohydrates, and then in response to ingesting lipids (39)) proportionately to the amount of calories consumed (40). Together, this highlights the interrelatedness of satiety factors in bringing about meal cessation.
Mechanisms of satiety (Figure 1)
Satiety represents the continuation of satiation, but also the failure of hunger to return, thus is dependent in part, on both those hunger and satiation factors outlined above, for example ghrelin should stay low and leptin high. Similarly, CKK, GLP-1 and PYY contribute to meal cessation - but this occurs before CKK fully elevates and CKK elevation may therefore be seen as a satiety hormone. Similarly, although administration of GLP-1 and PYY3-36 are recognized for their role in reducing intake at a meal (41), PYY3-36 administration leads to reduced food intake over 24h of up to 36% (41), again suggesting a role in satiety.
The most famous satiety hormone may be leptin which is mostly secreted by adipocytes after a meal (with higher levels after fat based meals, as compared to carbohydrate based meals (42, 43)), and known to cross the blood-brain barrier where it binds to receptors in several regions known to control feeding, energy expenditure and hormones. Leptin plays a complex role in many obesity-related phenotypes, such as glucose turnover (44) and blood pressure,(45) and its role in satiety is most strongly indicated by leptin-null mice (Lepob/ob) who show extreme hyperphagia, which can be reversed with an infusion of leptin in a dose-repose manner.(46) Leptin, is therefore, an orthorexic hormone thought to prevent the initiation of eating in the absence of hunger and therefore although it may play a role in satiation is one of the key satiety hormones. Leptin is an agonist of ghrelin, highlighting the difficulties in distinguishing separable biological components representing hunger, satiation and satiety.
Brain mechanisms and eating behaviors
Many components of the satiety cascade described above originate in the gut and intestine abut have principal receptor sites within the brain region of the hypothalamus, enabling the biology of the satiety cascade to drive behavior. Upon coupling with the molecular milieu of the satiety cascade, the hypothalamus secretes neurotransmitters and further hormones contributing to energy homeostasis. The arcuate nucleus (ARC) secretes neuropeptides which are both anorexigenic (e.g. proopiomelanocortin [POMC], cocaine- and amphetamine- related transcript [CART], and neurotensin), and orexigenic (e.g. neuropeptide Y [NPY], agouti-related protein (AgRP), ghrelin, and galanin). This secretion of neuropeptides and neurotransmitters is regulated, in part, by energy intake and circulating hormones, including some of those by the satiety cascade (see (47) for a review), but also by more psychological factors such as reward sensitivity which illustrates the how eating behaviors are at the intersection of biology and behavior.
The interaction of biology and Parenting
Early work in the 1980s showed that the majority of preschool children (ages 3-5 years) would reduce the number of calories consumed immediately after a preload (48), a finding largely replicated (7, 49). This same compensatory mechanism is thought to be seen in very young infants who will adjust their volume of intake based on the caloric density of formula (50), and is thought to decline across childhood (49). The compensation of a preload in such young children was taken as evidence that children are born with the ability to self-regulate their eating behaviors via the satiety cascade, but this ability becomes dysregulated as children stop responding appropriately to the cue and signals of the satiety cascade, and start responding more to external cues for eating initiation and cessation. Perhaps because we are in the tail end of an era where parenting, specifically warm and responsive parenting, is considered critical for healthy child development (see (1)) the dysregulation of eating behaviors which poses such a risk for pediatric obesity, was seen as arising from caregiver (often mother) feeding behaviors, rather than the dysregulation of biology.
Parenting and Child Appetitive Traits
Defining Caregiver Feeding Behaviors
Contemporary literature makes a distinction between caregiver feeding practices and caregiver feeding styles. Practices represent one or more goal-oriented feeding behaviors, while styles reflect the recognition that parenting practices arise from underlying dimensions, which produce stable patterns of behavior (51). Mirroring the general parenting literature, the two dimensions of demandingness (represented by feeding behaviors associated with parental control and supervision) and responsiveness (represented by feeding behaviors associated with parental warmth, acceptance, and involvement), have been used to describe four caregiver feeding styles: authoritative (high demandingness/high responsiveness); authoritarian (high demandingness/low responsiveness), indulgent (low demandingness/high responsiveness) and uninvolved (low demandingness/low responsiveness). Each style thus represents general attitudes towards feeding with certain feeding practices embedded within each feeding style (52).
Parent Feeding Practices and Child Appetitive Traits
The association of caregiver feeding behaviors with the development of child appetitive traits begins early, with the notion that bottle feeding, over that of the breast, may be associated with poorer self-regulation of eating behaviors later on (53). Upon the introduction of solid foods, controlling feeding practices (pressure to eat and restriction) are the caregiver feeding behaviors thought to encourage children to respond more to external cues in the initiation and cessation of eating, rather than the internal cues of the satiety cascade. Parental pressure to eat shows associations with disinhibited eating, emotional eating (54), food avoidance behaviors (55) and food fussiness (56), alongside less slowness in eating (56) and enjoyment of food (57). However, some studies have suggested pressure to eat may also be associated with higher satiety sensitivity (56, 58), which would suggest better responsivity to internal cues. Like control, restriction is generally adopted to promote a healthy diet; however, it is now thought to increase the child’s tendency to listen to external, rather than internal, cues of hunger from the satiety cascade. Restriction has been associated with increased parent reported food responsiveness (56, 58), higher self-reported disinhibited eating (54), increased parent-reported food responsiveness (56) and more calories consumed during the EAH protocol (59). Restriction has also been associated with emotional over- and under- eating (60) which again, represents appetitive behaviors in response to cues other than those of the satiety cascade. Highly controlling feeding (a combination of restriction and pressure to eat) is associated with poorer compensation (6).
Autonomy promoting feeding practices such as modeling and monitoring are not thought to dysregulate eating behaviors. Modelling and monitoring have been associated with better child appetite regulation (56, 57, 60, 61), and lower food responsivity and emotional overeating (62), although monitoring may be associated with controlling or restrictive feeding behaviors and hence the association with child BMI is less clear.
Less research has focused on the association of styles over practices with child appetitive traits; however, research does show that an indulgent feeding style is associated with poorer satiety responsiveness and enjoyment of food (63), as well as increased weight status (suggesting energy dysregulation; (64)), especially compared to an authoritarian style (65, 66).
Implications and problems with the parenting etiology of child appetitive traits
Current theory and practice has clearly adopted the “biological regulation of hunger satiation and satiety, which is dysregulated by caregiver feeding behavior” models, something we term the “parenting trumps biology” approach. Thus feeding advice is tailored around effecting caregiver behaviors which represent a “hands-off” approach to child energy balance, and are theorized to allow a child to maintain his or her innate tendency to self-regulate their eating behaviors by responding to their underlying appetitive drive, driven by cues from the satiety cascade. This advice may be typified by Ellen Satter’s “division of responsibility” model where caregiver decide when and what a child should eat, but the child is left to decide if and how much to eat (67). Several problems have been raised with this approach; most controversial are those that claim that it is simply not working (see (1)), although it is not clear whether this is because the model itself is inaccurate, or because changing parent feeding behavior is a complex and difficult process. More problematic seems to be that this model fails to integrate findings from twin and sibling studies, as well as those from molecular genetics, which suggest that there is a heritable component to child appetitive traits, and thus a core component of individual differences in these, which do not reflect the feeding environment.
Genetics and Individual Differences in Child Appetitive Traits
Heritability
Based upon the pattern of twin correlations between groups of MZ and DZ twin pairs for an appetitive trait, twin studies have decomposed the variance in child appetitive traits into separate underlying genetic and environmental influences, and provided some of the strongest evidence that these traits are heritable. While range of heritability estimates for child appetitive traits is large, with genetic influences estimated to account for 0-75% most estimates in the upper moderate range of heritability with genetic influences shown to account for 50% or more of individual differences in slowness in eating (68), satiety responsiveness (68, 69), calories consumed during the EAH protocol (21), enjoyment of food (33), and eating rate(69). These moderate to high heritabilites have been reported across childhood (21), in infants aged three months (68), and in middle childhood at 8-11 (69), and 10-12 years of age (23). Only one study reported a heritability lower than 50%, for compensation in 4-7 years olds (70). There is not a clear suggestion why this study found a lower heritability, although it is notable for being the only study to employ an experimental protocol to assess the eating behavior.
Genetic Associations
Candidate gene studies of child appetitive traits have focused on examining the potential role of the fat mass and obesity associated (FTO) gene, which has shown the most consistent associations with BMI (71, 72). FTO is largely expressed in the brain, in particular, in the hypothalamus (73) which is known for its role in behaviors that affect energy homeostasis, as well as being expressed in the central and peripheral nervous tissues(74). FTO is also expressed in the cortex, hippocampus, and cerebellum(74), which drive reward-driven and energy-driven food intake in rats (74). The A allele of rs9939609 was associated with greater food intake during a test meal, and lower compensation during a compensation trial (75), as well as grams of palatable food consumed during the EAH protocol (76). These experimental and observational data have been supported by parent reports of child eating behaviors (which due to the lower demands on data collection tend to have larger sample sizes), where the same A allele has been associated with higher food responsiveness (4, 77) and lower satiety responsiveness (4), with a suggestion the A allele may also be associated with enjoyment of food (78). One study examined both eating behaviors and BMI, suggesting that FTO acts on eating behavior directly (in this case satiety responsiveness), and through this association is seen to predict BMI, although this single study needs replication (78).
As yet, the literature lacks other robust associations between loci of genetic variation and child appetitive traits, and the small effect size estimated for associations between FTO and child eating behaviors indicate that much of the heritable variance in child appetitive traits remains unaccounted for. This problem is known as the problem of the “missing heritability” (79) and is common for all traits – even for adult BMI, subject to some of the largest genetic investigations to date, less than 10% of the heritable variance has been accounted for by genetic studies with replicated loci (71) – but may be particularly problematic for behavioral traits (80). Genome-wide approaches are generally considered the preferred approach to finding the missing heritability (79), but these have their own statistical problems thought to contribute to the problem of the missing heritability with inadequate statistical approaches to account for the large number of variants surveyed combined with small anticipated effect sizes for each individual variants leading to both false positives and false negatives. To reduce the rate of type I and type II errors in genome-wide association studies (GWAS), it is now recognized that very large sample sizes are needed which may be particularly prohibitive in the case of behavioral traits such as child eating behaviors, where observational and even questionnaire data can be costly and time consuming to collect. Thus, for child eating behaviors, a candidate gene approach may be necessary, but as yet, there are few suggestions for which genetic loci to select a priori for analysis.
Genetic basis of the Satiety Cascade
The satiety cascade underlies appetitive drive, and the majority of the molecular components of the satiety cascade are produced and encoded by genes, despite their stimulation from other factors such as food intake and the mediation of their release from both internal and external factors such as adiposity and meal timing. In addition, the genes involved in the synthesis or regulation of factors of the satiety cascade are polymorphic, with evidence to suggest that genetic variation in these genes contributes to variation in plasma levels and/or associated behaviors. For example, leptin is encoded by the leptin gene (LEP) and regulated by a genes which include the leptin receptor gene (LEPR) and the peroxisome proliferator-activated receptor-gamma gene (PPARG) (81). LEP knockout (ob/ob) mice show extreme hyperphagia as do children missing a single nucleotide in LEP (guanine) and who produce small quantities of leptin (82). Ghrelin is cleaved from ghrelin-obestatin preproprotein, with this latter being encoded by the ghrelin (GHRELIN) gene. GHRELIN is also polymorphic and variation has been associated with anorexia and bulimia which is associated with an altered satiety cascade (83). The elevated ghrelin (and ensuing hyperphagia) see in Prada-Willi syndrome which arises from the silencing of the paternally-inherited 15q11-q13 region suggests that, like with leptin, trans-genes regulate ghrelin levels. Thus it seems likely that genetic variation will contribute to individual differences in the satiety cascade and may therefore be, in part, responsible for obesity-related appetitive traits alongside aspects of the feeding environment.
Conclusions
Using Biological Understanding to Improve Behavioral Advice
The prevalent notion is that children can maintain energy balance through responding to the internal cues of hunger, satiation and satiety provided by the components of the satiety cascade. This ability to modulate eating behaviors based on energy balance needs is termed the self-regulation of eating behaviors, and the dysregulation of eating behaviors and any ensuing obesity risk is seen to arise from the environment – in the case of very young children mostly caregiver feeding behaviors are considered responsible. Evidence from twin and molecular studies point towards the likelihood that children will have individual differences in their satiety cascade. Thus, we might expect that children also have innate differences in their appetitive drive. At one end of the spectrum, some children will have strong, appropriate internals cues of hunger, satiation and/or satiety, while others will have weaker, or shorter, ones. Thus, it is reasonable to deduce that some children may have a strong underlying biological drive to self-regulate their eating behaviors, while some will have a less strong drive. Support for the notion that children have innate, and early, differences in the their appetitive traits can be found not only in the heritability seen for eating behaviors of very young infants (84), but also in close examination of the early data from the compensation trials which shows individual differences in young children’s ability to compensate for a caloric preload, including from infancy.
Thus, it seems that the developmental psychologists and the behavioral geneticists are at an impasse: the developmental psychologists point to robust associations between the caregiver feeding environment and child appetitive traits, and by turn the behavior geneticists point to the heritable components of child eating behaviors. Of course, because a trait is heritable does not mean the environment cannot modify it – the most famous example being of course phenylketonuria (PKU) a Medelian disorder (i.e. caused by a single gene) which causes extreme mental disabilities, which are entirely prevented by the dietary avoidance of phenylalanine. And so, the evidence from genetics is not designed to indicate that the feeding environment does not matter, or does not matter for some children. Rather, the study of genetics raises the question about whether all caregivers should be subjected to the same feeding advice. Surely, children with an appropriate appetitive drive where strong signals of hunger and satiety accurately reflect energy balance needs should need a different feeding environment that those children with a more dysregulated biology. If some children have an appetitive drive associated with increased hunger, or reduced satiety, is the advice to allow these children to self-regulate the amount they eat wise?
The future
The etiology of child appetitive traits (and so feeding advice) must integrate knowledge on the influence of genetics and the satiety cascade on child appetitive traits, with knowledge on the influence of caregiver feeding behaviors and other environmental factors. Towards this goal, several research questions must be addressed, which include (1) what is the full the underlying biology of hunger, satiation and satiety? (2 What is the correlation between individual differences in the satiety cascade and child appetitive traits? (3) To what extent do caregiver feeding behaviors which associate with children’s tendency tailor their eating behaviors to external and not internal cues mean that appetitive traits as observed may not reflect the underlying biology of the satiety cascade? And (4) To what extent does the environment alter the underlying biology of the satiety cascade (through, for example, changes in gene expression)?
Building a biobehavioral model of gene-environment interplay to better understand child appetitive traits will require intensive research which draws upon the genomics, psychometrics, developmental psychology and molecular biology as disciplines. The investment will be worth it: child overweight and obesity now affects up to one-third of US preschoolers (85), and is a leading cause of racial inequalities in health (85). Developing more effective obesity prevention and intervention strategies is a vital public health goal and will require careful consideration of all the evidence present, as well as the generation of new molecular and sociobehavioral data. Only by bringing these fields together, however, do we stand the best chance at reducing child obesity risk.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest: Alexis C. Wood, Shabnam Momin, Mackenzie Senn, and Sheryl O. Hughes declare they have no conflict of interest.
Human and Animal Rights and Informed Consent: This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Lumeng J. Too indulgent or not sensitive enough: mothering in the current historical era and its relevance to childhood obesity. Nature Publishing Group; 2017. [DOI] [PubMed] [Google Scholar]
- 2.Baumeister R, Vohs K. Handbook of self-regulation: Research, theory and applications. 1st. New York, NY: The Guilford Press; 2004. [Google Scholar]
- 3.Mischel W, Shoda Y, Rodriguez ML. Delay of gratification in children. Science. 1989;244(4907):933–8. doi: 10.1126/science.2658056. [DOI] [PubMed] [Google Scholar]
- 4.Wardle J, Guthrie CA, Sanderson S, Rapoport L. Development of the Children’s Eating Behaviour Questionnaire. J Child Psychol Psychiatry. 2001;42(7):963–70. doi: 10.1111/1469-7610.00792. Epub 2001/11/06. [DOI] [PubMed] [Google Scholar]
- 5.Van Strien T, Frijters JE, Bergers G, Defares PB. The Dutch Eating Behavior Questionnaire (DEBQ) for assessment of restrained, emotional, and external eating behavior. Int J Eat Disord. 1986;5(2):295–315. [Google Scholar]
- 6.Johnson SL, Birch LL. Parents’ and children’s adiposity and eating style. Pediatrics. 1994;94(5):653–61. [PubMed] [Google Scholar]
- 7.Kral TV, Allison DB, Birch LL, Stallings VA, Moore RH, Faith MS. Caloric compensation and eating in the absence of hunger in 5-to 12-y-old weight-discordant siblings. The American journal of clinical nutrition. 2012;96(3):574–83. doi: 10.3945/ajcn.112.037952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schlam TR, Wilson NL, Shoda Y, Mischel W, Ayduk O. Preschoolers’ delay of gratification predicts their body mass 30 years later. The Journal of pediatrics. 2013;162(1):90–3. doi: 10.1016/j.jpeds.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeyave DM, Coleman S, Appugliese D, Corwyn RF, Bradley RH, Davidson NS, et al. Ability to delay gratification at age 4 years and risk of overweight at age 11 years. Arch Pediatr Adolesc Med. 2009;163(4):303–8. doi: 10.1001/archpediatrics.2009.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Viana V, Sinde S, Saxton JC. Children’s Eating Behaviour Questionnaire: associations with BMI in Portuguese children. Br J Nutr. 2008;100(2):445–50. doi: 10.1017/S0007114508894391. Epub 2008/02/16. [DOI] [PubMed] [Google Scholar]
- 11.Webber L, Hill C, Saxton J, Van Jaarsveld CH, Wardle J. Eating behaviour and weight in children. Int J Obes (Lond) 2009;33(1):21–8. doi: 10.1038/ijo.2008.219. Epub 2008/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carnell S, Wardle J. Appetite and adiposity in children: evidence for a behavioral susceptibility theory of obesity. Am J Clin Nutr. 2008;88(1):22–9. doi: 10.1093/ajcn/88.1.22. Epub 2008/07/11. [DOI] [PubMed] [Google Scholar]
- 13.Sleddens EF, Kremers SP, Thijs C. The children’s eating behaviour questionnaire: factorial validity and association with Body Mass Index in Dutch children aged 6–7. Int J Behav Nutr Phys Act. 2008;5:49. doi: 10.1186/1479-5868-5-49. Epub 2008/10/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braet C, Van Strien T. Assessment of emotional, externally induced and restrained eating behaviour in nine to twelve-year-old obese and non-obese children. Behav Res Ther. 1997;35(9):863–73. doi: 10.1016/s0005-7967(97)00045-4. [DOI] [PubMed] [Google Scholar]
- 15.Baños R, Cebolla A, Etchemendy E, Felipe S, Rasal P, Botella C. Validation of the Dutch Eating Behavior Questionnaire for Children (DEBQ-C) for use with Spanish children. Nutr Hosp. 2011;26(4) doi: 10.1590/S0212-16112011000400032. [DOI] [PubMed] [Google Scholar]
- 16.van Strien T, Oosterveld P. The children’s DEBQ for assessment of restrained, emotional, and external eating in 7‐to 12‐year‐old children. Int J Eat Disord. 2008;41(1):72–81. doi: 10.1002/eat.20424. [DOI] [PubMed] [Google Scholar]
- 17.Butte NF, Cai G, Cole SA, Wilson TA, Fisher JO, Zakeri IF, et al. Metabolic and behavioral predictors of weight gain in Hispanic children: the Viva la Familia Study. Am J Clin Nutr. 2007;85(6):1478–85. doi: 10.1093/ajcn/85.6.1478. Epub 2007/06/09. [DOI] [PubMed] [Google Scholar]
- 18.Faith MS, Berkowitz RI, Stallings VA, Kerns J, Storey M, Stunkard AJ. Eating in the absence of hunger: a genetic marker for childhood obesity in prepubertal boys? Obesity (Silver Spring) 2006;14(1):131–8. doi: 10.1038/oby.2006.16. Epub 2006/02/24. [DOI] [PubMed] [Google Scholar]
- 19.Faith MS, Carnell S, Kral TV. Genetics of food intake self-regulation in childhood: literature review and research opportunities. Hum Hered. 2013;75(2–4):80–9. doi: 10.1159/000353879. Epub 2013/10/02. [DOI] [PubMed] [Google Scholar]
- 20.Fisher JO, Birch LL. Eating in the absence of hunger and overweight in girls from 5 to 7 y of age. Am J Clin Nutr. 2002;76(1):226–31. doi: 10.1093/ajcn/76.1.226. Epub 2002/06/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher JO, Cai G, Jaramillo SJ, Cole SA, Comuzzie AG, Butte NF. Heritability of hyperphagic eating behavior and appetite-related hormones among Hispanic children. Obesity (Silver Spring) 2007;15(6):1484–95. doi: 10.1038/oby.2007.177. Epub 2007/06/15. [DOI] [PubMed] [Google Scholar]
- 22.Hill C, Llewellyn CH, Saxton J, Webber L, Semmler C, Carnell S, et al. Adiposity and ‘eating in the absence of hunger’ in children. Int J Obes (Lond) 2008;32(10):1499–505. doi: 10.1038/ijo.2008.113. Epub 2008/07/23. [DOI] [PubMed] [Google Scholar]
- 23.Llewellyn CH, Van Jaarsveld CH, Boniface D, Carnell S, Wardle J. Eating rate is a heritable phenotype related to weight in children. The American journal of clinical nutrition. 2008;88(6):1560–6. doi: 10.3945/ajcn.2008.26175. [DOI] [PubMed] [Google Scholar]
- 24.Dubois L, Farmer A, Girard M, Peterson K, Tatone-Tokuda F. Problem eating behaviors related to social factors and body weight in preschool children: A longitudinal study. International Journal of Behavioral Nutrition and Physical Activity. 2007;4(1):9. doi: 10.1186/1479-5868-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blundell J, Rogers P, Hill A. Evaluating the satiating power of foods: implications for acceptance and consumption. In: Solms J, et al., editors. Food acceptance and nutrition. 1987. [Google Scholar]
- 26.Seim I, Josh P, Cunningham P, Herington A, Chopin L. Ghrelin axis genes, peptides and receptors: recent findings and future challenges. Mol Cell Endocrinol. 2011;340(1):3–9. doi: 10.1016/j.mce.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 28.Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, et al. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A. 2004;101(21):8227–32. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mayer J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci. 1955;63(1):15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- 30.Campfield LA, Brandon P, Smith FJ. On-line continuous measurement of blood glucose and meal pattern in free-feeding rats: the role of glucose in meal initiation. Brain Res Bull. 1985;14(6):605–16. doi: 10.1016/0361-9230(85)90110-8. [DOI] [PubMed] [Google Scholar]
- 31.Campfield L, Smith F. Transient declines in blood glucose signal meal initiation. Int J Obes. 1990;14:15–31. discussion -4. [PubMed] [Google Scholar]
- 32.Smith FJ, Campfield LA. Meal initiation occurs after experimental induction of transient declines in blood glucose. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1993;265(6):R1423–R9. doi: 10.1152/ajpregu.1993.265.6.R1423. [DOI] [PubMed] [Google Scholar]
- 33.Van Itallie TB, Beaudoin R, Mayer J. Arleriovenous Glucose Differences, Metabolic Hypoglycemia and Food Intake in Man. The American Journal of Clinical Nutrition. 1953;1(3):208–17. doi: 10.1093/ajcn/1.3.208. [DOI] [PubMed] [Google Scholar]
- 34.Overduin J, Frayo RS, Grill HJ, Kaplan JM, Cummings DE. Role of the duodenum and macronutrient type in ghrelin regulation. Endocrinology. 2005;146(2):845–50. doi: 10.1210/en.2004-0609. [DOI] [PubMed] [Google Scholar]
- 35.Degen L, Drewe J, Piccoli F, Grani K, Oesch S, Bunea R, et al. Effect of CCK-1 receptor blockade on ghrelin and PYY secretion in men. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2007;292(4):R1391–R9. doi: 10.1152/ajpregu.00734.2006. [DOI] [PubMed] [Google Scholar]
- 36.Little T, Horowitz M, Feinle‐Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obesity reviews. 2005;6(4):297–306. doi: 10.1111/j.1467-789X.2005.00212.x. [DOI] [PubMed] [Google Scholar]
- 37.Rehfeld J. Incretin physiology beyond glucagon‐like peptide 1 and glucose‐dependent insulinotropic polypeptide: cholecystokinin and gastrin peptides. Acta physiologica. 2011;201(4):405–11. doi: 10.1111/j.1748-1716.2010.02235.x. [DOI] [PubMed] [Google Scholar]
- 38••.Dockray GJ. Cholecystokinin. Current Opinion in Endocrinology, Diabetes and Obesity. 2012;19(1):8–12. doi: 10.1097/MED.0b013e32834eb77d. This publication provides an up-to-date summary of how the gut hormones in the statiety cascade can inform behaviors via recpetors on the brain. [DOI] [PubMed] [Google Scholar]
- 39.Elliott R, Morgan L, Tredger J, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7–36) amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138(1):159–66. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 40.Ørskov C, Rabenhøj L, Wettergren A, Kofod H, Holst JJ. Tissue and plasma concentrations of amidated and glycine-extended glucagon-like peptide I in humans. Diabetes. 1994;43(4):535–9. doi: 10.2337/diab.43.4.535. [DOI] [PubMed] [Google Scholar]
- 41.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY3-36 physiologically inhibits food intake. Nature. 2002;418(6898):650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 42.Kenward MG, Molenberghs G. Likelihood based frequentist inference when data are missing at random. Statistical Science. 1998:236–47. [Google Scholar]
- 43.Polderman TJ, Posthuma D, De Sonneville LM, Stins JF, Verhulst FC, Boomsma DI. Genetic analyses of the stability of executive functioning during childhood. Biol Psychol. 2007;76(1):11–20. doi: 10.1016/j.biopsycho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann W, Friese M, Roefs A. Three ways to resist temptation: The independent contributions of executive attention, inhibitory control, and affect regulation to the impulse control of eating behavior. J Exp Soc Psychol. 2009;45(2):431–5. [Google Scholar]
- 45.Mark AL, Shaffer RA, Correia ML, Morgan DA, Sigmund CD, Haynes WG. Contrasting blood pressure effects of obesity in leptin‐deficient ob/ob mice and agouti yellow obese mice. J Hypertens. 1999;17(12):1949–53. doi: 10.1097/00004872-199917121-00026. [DOI] [PubMed] [Google Scholar]
- 46.Harris RB, Zhou J, Redmann SM, Jr, Smagin GN, Smith SR, Rodgers E, et al. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139(1):8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 47.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behav Brain Res. 2010;209(1):1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Birch LL, Deysher M. Conditioned and unconditioned caloric compensation: evidence for self-regulation of food intake in young children. Learn Motiv. 1985;16(3):341–55. [Google Scholar]
- 49.Cecil JE, Palmer CN, Wrieden W, Murrie I, Bolton-Smith C, Watt P, et al. Energy intakes of children after preloads: adjustment, not compensation. The American journal of clinical nutrition. 2005;82(2):302–8. doi: 10.1093/ajcn.82.2.302. [DOI] [PubMed] [Google Scholar]
- 50.Samuel J, Anderson TA, Nelson SE. Influence of formula concentration on caloric intake and growth of normal infants. Acta Paediatr. 1975;64(2):172–81. doi: 10.1111/j.1651-2227.1975.tb03818.x. [DOI] [PubMed] [Google Scholar]
- 51.Darling N, Steinberg L. Parenting style as context: An integrative model. Psychol Bull. 1993;113(3):487. [Google Scholar]
- 52••.Hughes SO, Power TG, Papaioannou MA, Cross MB, Nicklas TA, Hall SK, et al. Emotional climate, feeding practices, and feeding styles: an observational analysis of the dinner meal in Head Start families. Int J Behav Nutr Phys Act. 2011;8:60. doi: 10.1186/1479-5868-8-60. Epub 2011/06/15. The first paper to coalesce parent feeding practices into specific styles mirroring the general parenting literature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DiSantis KI, Collins BN, Fisher JO, Davey A. Do infants fed directly from the breast have improved appetite regulation and slower growth during early childhood compared with infants fed from a bottle? International Journal of Behavioral Nutrition and Physical Activity. 2011;8(1):89. doi: 10.1186/1479-5868-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carper J, Fisher JO, Birch LL. Young girls’ emerging dietary restraint and disinhibition are related to parental control in child feeding. Appetite. 2000;35(2):121–9. doi: 10.1006/appe.2000.0343. [DOI] [PubMed] [Google Scholar]
- 55.Powell FC, Farrow CV, Meyer C. Food avoidance in children. The influence of maternal feeding practices and behaviours. Appetite. 2011;57(3):683–92. doi: 10.1016/j.appet.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Webber L, Cooke L, Hill C, Wardle J. Associations between children’s appetitive traits and maternal feeding practices. J Am Diet Assoc. 2010;110(11):1718–22. doi: 10.1016/j.jada.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 57.Gregory JE, Paxton SJ, Brozovic AM. Maternal feeding practices, child eating behaviour and body mass index in preschool-aged children: a prospective analysis. International Journal of Behavioral Nutrition and Physical Activity. 2010;7(1):55. doi: 10.1186/1479-5868-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58•.Carnell S, Benson L, Driggin E, Kolbe L. Parent feeding behavior and child appetite: associations depend on feeding style. Int J Eat Disord. 2014;47(7):705–9. doi: 10.1002/eat.22324. Cross-sectional analysis of parent feeding nbehaviors and child appetitive traits, noted for its comprehensive assessment of both. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jansen E, Mulkens S, Jansen A. Do not eat the red food!: prohibition of snacks leads to their relatively higher consumption in children. Appetite. 2007;49(3):572–7. doi: 10.1016/j.appet.2007.03.229. [DOI] [PubMed] [Google Scholar]
- 60.Haycraft E, Blissett J. Predictors of paternal and maternal controlling feeding practices with 2-to 5-year-old children. Journal of nutrition education and behavior. 2012;44(5):390–7. doi: 10.1016/j.jneb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 61.Farrow C, Galloway A, Fraser K. Sibling eating behaviours and differential child feeding practices reported by parents. Appetite. 2009;52(2):307–12. doi: 10.1016/j.appet.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 62.Garcia KS, Power TG, Fisher JO, O’Connor TM, Hughes SO. Latina mothers’ influences on child appetite regulation. Appetite. 2016;103:200–7. doi: 10.1016/j.appet.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frankel LA, O’Connor TM, Chen T-A, Nicklas T, Power TG, Hughes SO. Parents’ perceptions of preschool children’s ability to regulate eating. Feeding style differences. Appetite. 2014;76:166–74. doi: 10.1016/j.appet.2014.01.077. [DOI] [PubMed] [Google Scholar]
- 64.Hughes SO, Shewchuk RM, Baskin ML, Nicklas TA, Qu H. Indulgent feeding style and children’s weight status in preschool. Journal of developmental and behavioral pediatrics: JDBP. 2008;29(5):403. doi: 10.1097/DBP.0b013e318182a976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hughes SO, Power TG, Fisher JO, Mueller S, Nicklas TA. Revisiting a neglected construct: parenting styles in a child-feeding context. Appetite. 2005;44(1):83–92. doi: 10.1016/j.appet.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 66.Tovar A, Hennessy E, Pirie A, Must A, Gute DM, Hyatt RR, et al. Feeding styles and child weight status among recent immigrant mother-child dyads. International Journal of Behavioral Nutrition and Physical Activity. 2012;9(1):62. doi: 10.1186/1479-5868-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Satter E. How to get your kid to eat: But not too much. Bull Publishing Company; 2012. [Google Scholar]
- 68.Llewellyn CH, van Jaarsveld CH, Plomin R, Fisher A, Wardle J. Inherited behavioral susceptibility to adiposity in infancy: a multivariate genetic analysis of appetite and weight in the Gemini birth cohort. Am J Clin Nutr. 2012;95(3):633–9. doi: 10.3945/ajcn.111.023671. Epub 2012/01/27. [DOI] [PubMed] [Google Scholar]
- 69.Carnell S, Haworth CM, Plomin R, Wardle J. Genetic influence on appetite in children. Int J Obes (Lond) 2008;32(10):1468–73. doi: 10.1038/ijo.2008.127. Epub 2008/08/06. [DOI] [PubMed] [Google Scholar]
- 70.Faith MS, Pietrobelli A, Heo M, Johnson SL, Keller KL, Heymsfield SB, et al. A twin study of self-regulatory eating in early childhood: Estimates of genetic and environmental influence, and measurement considerations. International journal of obesity (2005) 2012;36(7):931. doi: 10.1038/ijo.2011.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. doi: 10.1038/nature14177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42(11):937–48. doi: 10.1038/ng.686. Epub 2010/10/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olszewski PK, Fredriksson R, Olszewska AM, Stephansson O, Alsiö J, Radomska KJ, et al. Hypothalamic FTO is associated with the regulation of energy intake not feeding reward. BMC neuroscience. 2009;10(1):129. doi: 10.1186/1471-2202-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fredriksson R, Hägglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149(5):2062–71. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- 75.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359(24):2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- 76.Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. The American journal of clinical nutrition. 2009;90(6):1483–8. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Velders FP, De Wit JE, Jansen PW, Jaddoe VW, Hofman A, Verhulst FC, et al. FTO at rs9939609, food responsiveness, emotional control and symptoms of ADHD in preschool children. PLoS One. 2012;7(11):e49131. doi: 10.1371/journal.pone.0049131. Epub 2012/11/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wardle J, Carnell S, Haworth CM, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. The Journal of Clinical Endocrinology & Metabolism. 2008;93(9):3640–3. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- 79.Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461(7265):747–53. doi: 10.1038/nature08494. Epub 2009/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wood AC, Neale MC. Twin studies and their implications for molecular genetic studies: endophenotypes integrate quantitative and molecular genetics in ADHD research. J Am Acad Child Adolesc Psychiatry. 2010;49(9):874–83. doi: 10.1016/j.jaac.2010.06.006. Epub 2010/08/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paracchini V, Pedotti P, Taioli E. Genetics of leptin and obesity: a HuGE review. Am J Epidemiol. 2005;162(2):101–14. doi: 10.1093/aje/kwi174. [DOI] [PubMed] [Google Scholar]
- 82.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- 83.Cellini E, Nacmias B, Brecelj-Anderluh M, Badía-Casanovas A, Bellodi L, Boni C, et al. Case– control and combined family trios analysis of three polymorphisms in the ghrelin gene in European patients with anorexia and bulimia nervosa. Psychiatr Genet. 2006;16(2):51–2. doi: 10.1097/01.ypg.0000194444.89436.e9. [DOI] [PubMed] [Google Scholar]
- 84.Llewellyn CH, van Jaarsveld CH, Johnson L, Carnell S, Wardle J. Nature and nurture in infant appetite: analysis of the Gemini twin birth cohort. Am J Clin Nutr. 2010;91(5):1172–9. doi: 10.3945/ajcn.2009.28868. Epub 2010/03/26. [DOI] [PubMed] [Google Scholar]
- 85.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–14. doi: 10.1001/jama.2014.732. Epub 2014/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]


