Opinion Statement
Right ventricular (RV) structure and function is clinically important in a wide range of conditions. While conventional echocardiography (echo) methods are widely used, its limitations in RV assessment due its complex geometry are well recognized. New applications of traditional echo methods as well as emerging echo techniques including 3-dimensional (3D) echo and speckle tracking strain have the potential to overcome limitations of conventional echo though widespread clinical use remains to be seen. Volumetric methods using cardiac magnetic resonance (CMR) and computed tomography (CT) provide accurate assessment of RV function without geometric assumptions. In addition, tissue characterization imaging for myocardial scar and fat using CMR and CT provides important information regarding the RV beyond structure and function alone and has clinical applications for diagnosis and prognosis in a broad range of pathologies. Limitations also exist for these two advanced modalities including availability and patient suitability for CMR and need for contrast and radiation exposure for CT. The complementary role of each modality for the RV as well as emerging evidence for the use of each imaging method in diagnosis and management of RV pathologies are outlined in this study.
Introduction
Right ventricular (RV) assessment using complementary multi-modality imaging methods has drastically improved understanding of right ventricular anatomy and function. Echocardiography (echo) represents the most widely available tool to assess RV function, though its limitations in evaluation of the RV are well described. The emergence of advanced echo methods including 3D echo and strain imaging has the potential to overcome known limitations of traditional echo techniques. CMR and CT offer volumetric assessment of the right ventricle with high spatial resolution.
Right Ventricle Background
Anatomy and Function
The RV wraps around the left ventricle (LV) and is pyramidal in shape. Three distinct anatomic regions give rise to functionally distinct segments: the inflow tract, the body and the outflow tract. The RV is distinct from the LV in that it has a more apically displaced tricuspid valve (TV) septal leaflet relative to the mitral valve, a moderator band, three or more papillary muscles, a trileaflet tricuspid valve, and coarse trabeculations. In addition, the RV has a thinner wall and a mass one fifth of that of the LV.
The RV myocardium is made up of two layers. The first is a deep, subendocardial layer of longitudinal fibers that result in systolic contraction of the base toward the apex. The second is a superficial circumferential layer of fibers responsible for inward contraction. RV systolic function is characterized by RV free wall motion, the contraction of spiral muscles that enable basal-apical shortening characteristic of systole and interventricular sepal motion, which allows for biventricular interdependence.
The RV’s predominant motion involves longitudinal shortening. This includes systolic descent of the basal portion of the free wall toward the apex and peristaltic contraction from the inflow to the outflow portions of the RV. Unlike in the LV, twisting and rotational movements do not contribute significantly to RV contraction.
Cardiovascular Outcomes Are Predicted by RV Function
Increasing data support the importance of RV function with respect to clinical outcomes in a broad range of conditions. RV function quantified on echo and SPECT has been shown to be linked to increased morbidity and mortality, including prolonged hospital stays in a broad cohort of patients with ischemic and non-ischemic cardiomyopathy.1,2,3,4 RV function has also been shown to be linked to adverse cardiac outcomes and impaired functional status among patients with coronary artery disease5,6. Lastly prior studies have demonstrated RV size and function to be a critical prognostic indicator in pulmonary arterial hypertension (PAH) and congenital heart disease.7,8,9,10 Despite the role of the RV in stratification of prognosis, non-invasive evaluation of the RV remains challenging due to several key anatomic factors.
Challenges
The RV is crescentic in shape and, unlike the LV, cannot be characterized using simple geometric assumptions. The RV has three distinct anatomical components: the inlet, body and outlet – these three components of the RV cannot be simultaneously imaged in a single 2D plane. Furthermore, extensive trabeculations of the RV result in difficulties with image analysis leading to variability in reproducibility and accuracy. Marked load dependence can also lead to variation in shape and size, which can lead to day-to-day variation in volumes and ejection fraction. From a technical standpoint, the RV’s retrosternal position results in difficulties with ultrasound wave transmission11 and linear dimensions and area can differ markedly with minor rotations in the transducer12 during echo acquisition.
Multi-Modality Imaging
Using multiple imaging modalities to assess the RV enables evaluation of several key characteristics including volume, shape, mass, and tissue properties.13 The emergence of additional techniques, including strain using speckle tracking, and three-dimensional echo, as well as CMR and CT, allow for even better characterization of the RV. Strength and limitations of each modality are outlined in Table 1.
Table 1.
Complementary Role of CMR, CT and Echo
| CMR | CT | 2D Echo | 3D Echo | |
|---|---|---|---|---|
| Imaging Variables | ||||
| RV Systolic Function | ||||
| Global Assessment | ✓ | ✓✓ | ✓✓ | ✓✓ |
| Regional Assessment | ✓✓ | ✓✓ | ✓ | ✓✓ |
| RV Volumes | ✓✓ | ✓✓ | - | ✓✓ |
| Tissue Characterization (e.g. fat) | ✓✓ | ✓ | - | - |
| RV Hemodynamics (e.g. PA pressure) | - | - | ✓✓ | - |
| Accessibility | ||||
| Availability/portability | - | - | ✓✓ | ✓✓ |
| Speed of Exam | - | ✓✓ | ✓✓ | ✓ |
| Contrast/Radiation Exposure | - | ✓✓ | - | - |
While CMR is currently considered the reference standard for evaluation of the RV, due to wider availability, two-dimensional (2D) echo is most frequently used in clinical practice. While 2D echo allows for calculation of RV wall thickness and size, three-dimensional (3D) echo provides en face planimetry of volumes and also provides full 3D dataset to assess for RV structural and regional abnormalities. Both 3D echo and CMR allow for absolute, accurate calculation of RV volume and RVEF. However, additional 2D echo methods, including fractional area shorting, TAPSE, the Tei index, Doppler tissue imaging and speckle tracing of the RV free wall, provides accurate assessment of the RV when used together thereby supporting 2D echo use as a screening tool. When echo assessment is inadequate and CMR is unavailable or unsuitable, CT is also used for quantitative RV assessment.14
Echocardiography
RV Linear Dimensions
Complex RV geometry and the lack of specific anatomic reference points make quantification of 2D linear measurements a challenge. Rather than using the conventional apical four-chamber view to calculate RV linear dimensions and areas, the RV-focused apical four-chamber view, accessible with a lateral or medial transducer orientation, allows for better estimation of dimensions.15 Guideline-driven recommendations emphasize that RV size should be measured from a four-chamber view, and that it be evaluated in the context of the left ventricle. A qualitative assessment of the RV using the LV as a reference in a standard apical four-chamber view provides a frame of reference. Without fixed reference points that ensure optimal RV linear measurements, the current recommendation is to make basal and mid cavity measurements followed by a longitudinal measurement.16
Further studies have validated the use of several linear measurements with 2D echo to improve accuracy. A study of 272 patients with CAD who underwent echocardiography and CMR within a narrow interval found that it was feasible to obtain a complete set of linear dimensions for all patients, and measurements made in parasternal long axis right ventricular outflow tract (RVOT) and four-chamber RV basal diameter were well-correlated with CMR volumes.17 In another study, linear fractional shortening on echo was used as a measure of RV dysfunction in a population of 168 patients with HFrEF who underwent echocardiography and CMR within a narrow interval. All fractional shortening indices were lower among patients with RV dysfunction, and acquisition of these indices was feasible in 93% of the patients within the population. When compared to CMR-quantified RVEF, fractional shortening measurements were independently associated with EF.18
RV Systolic Function: TAPSE, S′, FAC and RIMP
Several methods are used to calculate RV systolic function, including tricuspid annular plane systolic excursion (TAPSE), tissue Doppler-derived tricuspid lateral annular systolic velocity (S′), 2D fractional area change (FAC), and RV index of myocardial performance (RIMP).
TAPSE is obtained from a standard apical four-chamber view and measures systolic excursion of the RV annular segment along its longitudinal plane. The assumption is that the greater the RV systolic excursion measurement, the greater the function of the RV. Measurement of RV systolic function by way of TAPSE is simple, less dependent on optimal image quality, and fairly reproducible. However, the measurement is made in a single RV segment to represent a complex 3D structure. Therefore, it is both angle and load dependent.16 RV dysfunction as calculated by TAPSE has been more associated with the development of new onset heart failure in non-obese patients versus obese patients following acute myocardial infarction (MI).19
Systolic excursion velocity, known as RV S′, represents the longitudinal velocity of excursion of the tricuspid annulus and basal free wall segment of the RV by way of pulsed tissue Doppler and color-coded tissue Doppler.16 These are valuable regions for measurement within the RV given that they are among the most reliably and reproducibly imaged areas. An apical four-chamber view is used with tissue Doppler mode region of interest to highlight the RV free wall. S′ is read as the highest systolic velocity. Pulsed tissue Doppler derived S′ is considered a reproducible measure to assess basal RV function. Color-coded tissue Doppler has less evidence and a wider confidence interval, so it is currently used primarily for research. Limitations include angle dependence, technical issues such as alignment difficulties, and a lack of validation in non-sinus rhythm.
FAC is a percentage derived from the difference between end-diastolic area and end-systolic area of the RV, divided by end-diastolic area and multiplied by 100.16 RV FAC correlates well with RVEF determined by CMR.20 RV dysfunction, defined by percent of FAC decrease, was identified as an independent predictor of death and the development of HFrEF following MI.21 The VALIANT ECHO study then demonstrated that decreases in FAC after MI were associated with an increased risk of non-fatal and fatal cardiovascular outcomes, including heart failure and stroke.22 Limitations to widespread use of FAC center on poor reproducibility, challenges in image quality and discernment of endocardial trabeculations from the free wall borders, and RV apical foreshortening. Furthermore, the RV outflow tract is not part of the calculation, so its contribution is left out.
RIMP allows for an estimate of RV systolic and diastolic function. It is defined as the ratio of isovolumic time to ejection time and is obtained by way of pulsed Doppler and tissue Doppler methods.16 It depends on a constant R-R interval in order to minimize error. Furthermore, it is less reliable with elevated right atrial (RA) pressures. RIMP continues to have utility in the determination of RV function across a range of presentations. It is highly reproducible and enables calculation of RV function. In a recent study of 268 patients post-TAVR, measurements of RV dysfunction by way of RIMP were associated with worse survival following the procedure.23 However, it is important to avoid RIMP if patients have varying R-R intervals and elevated RA pressures.
3D Echocardiography
There are numerous advantages to 3D echocardiography. First, it allows visualization of inflow, outflow and apical regions of the RV without geometric assumptions. In addition, the technique is well validated against CMR derived volume (though typically underestimated in CMR).24 The validation of 3D echo against CMR has also been demonstrated in a population of patients with LV dysfunction.25 3D echo measurements also correlate well with data obtained by 2D and Doppler methods.26 Calculation of RV volumes by way of 3D imaging requires specific equipment and training and depends heavily on adequate 2D image quality, a regular rhythm, and patient cooperation. In addition to RV and stroke volumes, ejection fraction can also be calculated.15 The absence of sufficient established reference values was cited as a limitation in the past; however, reference values are now available (Table 2).15
Table 2.
Right Ventricular Normative Values
| Cardiac Magnetic Resonance1 | 3D Echocardiography2 | Computed Tomography3 | |||
|---|---|---|---|---|---|
|
|
|
|
|||
| Men | Women | Men | Women | Both genders | |
|
|
|
|
|
|
|
| Mean ± SD (95% CI) |
Mean ± SD (95% CI) |
Mean ± SD (95%CI) |
Mean ± SD (95% CI) |
Mean ± SD (95% CI) |
|
| RV end-diastolic volume | |||||
| mL | 142 ± 31 (96–201) |
110 ± 24 (77–155) |
- | - | 175 ± 48 (81–269) |
| mL/m2 | 82 ± 16 (57–101) |
69 ± 14 (49–95) |
61 ± 13 (35–87) |
53 ± 11 (32–74) |
93 ± 20 (54–133) |
| RV end-systolic volume | |||||
| mL | 54 ± 17 (28–85) |
35 ± 13 (18–57) |
- | - | 82 ± 29 (25–139) |
| mL/m2 | 31 ± 9 (17–48) |
22 ± 8 (10–34) |
27 ± 9 (10–44) |
22 ± 7 (8–36) |
- |
| RVEF (%) | 62 ± 1 (50–75) |
69 ± 1 (58–81) |
- | - | 58 ± 8 (42–74) |
Recent analyses demonstrate the prognostic value of RVEF calculated by 3D echo with a significant association between 3D RVEF and future cardiac death and major adverse cardiac events.24 3D echo will also play a key role prior to and following percutaneous valve replacement.27, 28 In a study that compared 2D versus 3D echo methods in a population of patients with congenital heart disease, it was found that 3D echo improved RV size and function assessment.29
Strain
Automated functional imaging allows for the assessment of global and regional RV function. Myocardial strain can be evaluated by tracking frame-to-frame movement of the speckles, or natural acoustic markers, within the myocardium on two-dimensional gray scale images. This method does not rely on geometric assumptions. It provides a sensitive and rapid measure of segmental, mechanical changes within the RV as well as global longitudinal RV function.30 Evaluation of the RV is performed in a two-chamber view. Speckle tracking is an angle-independent method, which is based upon routine 2D images.
In a study of 125 patients, global longitudinal RV strain measurements were obtained with manual tracing. These strain measurements were found to correlate well with standard measures of RV chamber and RVOT performance. TAPSE and RVOT systolic excursion were noted to correlate most closely with strain measures.31 Global longitudinal strain measures were also found to be predictive of outcomes in a population of 98 patients with HFrEF, and in another study of 60 patients with HFrEF, global longitudinal strain was noted to be the only variable associated with cardiac death or heart failure hospitalization.32, 33 Strain in this population of 60 patients was a stronger predictor of outcome than TAPSE, RV fractional area change, RV myocardial performance index and tissue Doppler peak myocardial velocity
Cardiac MRI
CMR represents the reference standard to accurately assess the dimensions, mass, and function of the RV. CMR provides high-resolution 3D images of the RV, which enable accurate assessments of RV systolic and diastolic volumes, which can then be used to calculate ejection fraction. Steady-state free precession (SSFP) is the pulse sequence typically used for cine-CMR for volumetric quantification and provides outstanding endocardial definition. CMR phase velocity encoded imaging can also be used to calculate flow through semilunar and AV valves, allowing for quantification of regurgitant fractions, cardiac output and shunt fraction.12 CMR methods correlate closely with in vivo pathology; it is accurate and highly reproducible leading to minimal operator variation. Fully automated segmentation framework now exists for the RV34 and further work is needed to validate these methods to increase efficiency. Limitations of CMR includes breath hold requirement for image acquisition, patient contraindication including pacemaker/defibrillator as well as cost and availability issues. Methods including parallel imaging and navigator based techniques can be used to truncate or eliminate breath hold times.
CMR is known to provide valuable prognostic information for the RV. In addition to accurate quantification of RV size and function, CMR provides information regarding RV tissue properties, which cannot be done with existing echo techniques. For example, in pulmonary hypertension (PH), late gadolinium enhancement (LGE) at the RV insertion point (RVIP) has been shown to provide risk stratification for morbidity and mortality incremental to traditional CMR indices including EF. LGE at RVIP has been associated with more advanced disease and poor prognosis PH.35 Non-ischemic fibrosis identified on CMR has also been linked to greater degree of RV dilation and afterload and is independently associated with increased mortality in patients with cardiomyopathy.36, 37 In patients with arrhythmogenic RV Cardiomyopathy (ARVC), CMR indices are critical components of the diagnostic criteria, which includes CMR derived RVEF and end diastolic volume.38 RV volumetric and functional assessment is important in a wide range of congenital heart disease conditions. For example, RV functional indices are used as for assessment of pulmonary valve replacement in patients with repaired tetralogy of Fallot.
CT
CT functional assessment of the RV is performed using retrospective ECG gating. While this method requires increased radiation exposure, it provides dynamic images throughout the cardiac cycle allowing for precise and reproducible quantification of RV volume and function.39 While CMR is traditionally used more frequently for quantification of volumes and ejection fraction, CT can be used as an alternative modality when CMR is contraindicated or unavailable. 14 CT like CMR provides multiplanar imaging of the RV with excellent endocardial definition and high spatial resolution.38 CT can also provide assessment of tissue properties including identification of myocardial replacement with fat as seen in ARVC.
Setting CT apart from echo and CMR is its ability to comprehensively assess the pulmonary arterial vasculature as well as lung parenchyma and thereby provide information regarding pathophysiology of RV dysfunction and its sequalae. Concurrent chest CT and cardiac CT can be employed as a single exam in select cohorts for this purpose. Limitations to widespread use of CT are due to use of nephrotoxic contrast and ionizing radiation, and limited accessibility as compared to echo. It is also important to note that unlike echo and CMR, CT is unable to assess right-sided valvular and hemodynamic parameters. Lastly, non-gated CT has also been shown to be useful in the evaluation of the RV. For example, in acute pulmonary embolism, ventricular diameter ratio has been noted as an independent predictor of 30-day mortality.40 Septal displacement and contrast reflux within the inferior vena cava are also implicated in RV failure and worse outcomes.41
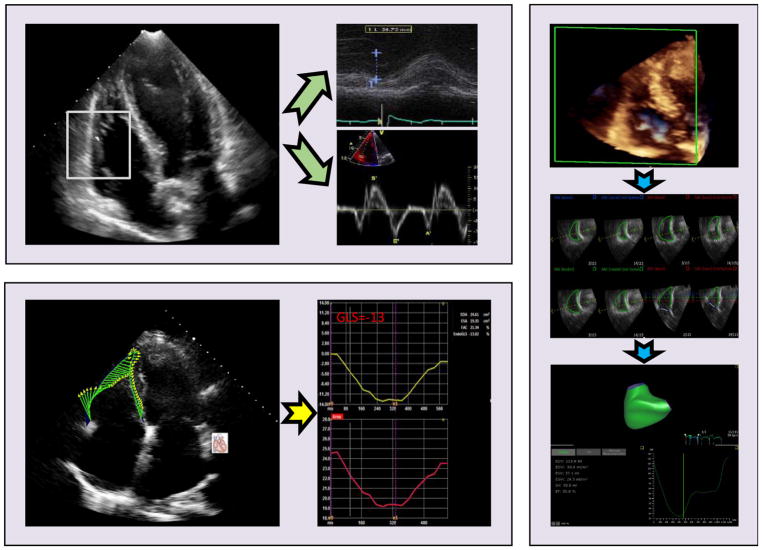
Figure.
M-mode derived TAPSE and tissue Doppler measured S′ obtained in apical four-chamber view (normal >1.6 cm and >10 cm/s, respectively) [top left]. Speckle tracking echo for RV global longitudinal strain assessment (normal >20%) [bottom left]. 3D full volume acquisition in RV focused view for RV volumes and ejection fraction (normal >44%) [right].
Acknowledgments
Sources of Funding: 1R01HL128278-01 (JWW)
References
- 1*.Yamin PP, Raharjo SB, Putri VK, Hersunarti N. Right ventricular dysfunction as predictor of longer hospital stay in patients with acute decompensated heart failure: a prospective study in Indonesian population. Cardiovasc Ultrasound. 2016;14:25. doi: 10.1186/s12947-016-0069-0. A prospective cohort study that investigated the prognostic value of RV dysfunction in predicting longer length of stay in acute decompensated heart failure patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polak JF, Holman BL, Wynne J, Colucci WS. Right ventricular ejection fraction: an indicator of increased mortality in patients with congestive heart failure associated with coronary artery disease. J Am Coll Cardiol. 1983;2:217–24. doi: 10.1016/s0735-1097(83)80156-9. [DOI] [PubMed] [Google Scholar]
- 3.Di Salvo TG, Mathier M, Semigran MJ, Dec GW. Preserved right ventricular ejection fraction predicts exercise capacity and survival in advanced heart failure. J Am Coll Cardiol. 1995;25:1143–53. doi: 10.1016/0735-1097(94)00511-n. [DOI] [PubMed] [Google Scholar]
- 4.Gavazzi A, Berzuini C, Campana C, Inserra C, Ponzetta M, Sebastiani R, Ghio S, Recusani F. Value of right ventricular ejection fraction in predicting short-term prognosis of patients with severe chronic heart failure. J Heart Lung Transplant. 1997;16:774–85. [PubMed] [Google Scholar]
- 5**.Kim J, Di Franco A, Seoane T, Srinivasan A, Kampaktsis PN, Geevarghese A, Goldburg SR, Khan SA, Szulc M, Ratcliffe MB, Levine RA, Morgan AE, Maddula P, Rozenstrauch M, Shah T, Devereux RB, Weinsaft JW. Right Ventricular Dysfunction Impairs Effort Tolerance Independent of Left Ventricular Function Among Patients Undergoing Exercise Stress Myocardial Perfusion Imaging. Circ Cardiovasc Imaging. 2016:9. doi: 10.1161/CIRCIMAGING.116.005115. Regional LV ischemia involving the inferior and lateral walls led to an increased likelihood of RV dysfunction in a population of over 2000 patients who underwent exercise stress myocardial perfusion imaging and echo. RV dysfunction was noted to impair exercise tolerance independent of LV dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Chang WT, Liu YW, Liu PY, Chen JY, Lee CH, Li YH, Tsai LM, Tsai WC. Association of Decreased Right Ventricular Strain with Worse Survival in Non-Acute Coronary Syndrome Angina. J Am Soc Echocardiogr. 2016;29:350–358 e4. doi: 10.1016/j.echo.2015.11.015. A study that validated the prognostic value of RV strain in patients with non- acute coronary syndrome angina. RV free wall longitudinal strain was significantly related to cardiovascular outcomes and hemodynamically unstable ventricular arrhythmia in patients with non–ACS angina. [DOI] [PubMed] [Google Scholar]
- 7*.Amsallem M, Sweatt AJ, Aymami MC, Kuznetsova T, Selej M, Lu H, Mercier O, Fadel E, Schnittger I, McConnell MV, Rabinovitch M, Zamanian RT, Haddad F. Right Heart End-Systolic Remodeling Index Strongly Predicts Outcomes in Pulmonary Arterial Hypertension: Comparison With Validated Models. Circ Cardiovasc Imaging. 2017;10 doi: 10.1161/CIRCIMAGING.116.005771. A prospective study that demonstrated RV end-systolic remodeling index. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8**.Guazzi M, Dixon D, Labate V, Beussink-Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure With Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc Imaging. 2017 doi: 10.1016/j.jcmg.2016.12.024. A study of over 300 patients with HFpEF in which TAPSE/PASP ratio was discovered as an independent predictor of worse outcomes in the population. [DOI] [PubMed] [Google Scholar]
- 9*.Sano H, Tanaka H, Motoji Y, Fukuda Y, Mochizuki Y, Hatani Y, Matsuzoe H, Hatazawa K, Shimoura H, Ooka J, Ryo-Koriyama K, Nakayama K, Matsumoto K, Emoto N, Hirata KI. Right ventricular relative wall thickness as a predictor of outcomes and of right ventricular reverse remodeling for patients with pulmonary hypertension. Int J Cardiovasc Imaging. 2017;33:313–321. doi: 10.1007/s10554-016-1004-z. In a population over 50 patients with pulmonary hypertension followed over 5 years, RV- relative wall thickness was noted to predict RV reverse remodeling in patients with HF who had received treatment. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler M, Leipsic J, Trinh P, Raju R, Alaamri S, Thompson CR, Moss R, Munt B, Kiess M, Grewal J. Right Ventricular Assessment in Adult Congenital Heart Disease Patients with Right Ventricle-to-Pulmonary Artery Conduits. J Am Soc Echocardiogr. 2015;28:522–32. doi: 10.1016/j.echo.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Vitarelli A, Terzano C. Do we have two hearts? New insights in right ventricular function supported by myocardial imaging echocardiography. Heart Fail Rev. 2010;15:39–61. doi: 10.1007/s10741-009-9154-x. [DOI] [PubMed] [Google Scholar]
- 12.Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–48. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999;1:7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 14**.Sanz J, Conroy J, Narula J. Imaging of the right ventricle. Cardiol Clin. 2012;30:189–203. doi: 10.1016/j.ccl.2012.03.001. A fundamental document with guidelines for chamber quantification. [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16**.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK, Schiller NB. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 786–8. A fundamental document with guidelines for chamber quantifiaction. [DOI] [PubMed] [Google Scholar]
- 17**.Kim J, Srinivasan A, Seoane T, Di Franco A, Peskin CS, McQueen DM, Paul TK, Feher A, Geevarghese A, Rozenstrauch M, Devereux RB, Weinsaft JW. Echocardiographic Linear Dimensions for Assessment of Right Ventricular Chamber Volume as Demonstrated by Cardiac Magnetic Resonance. J Am Soc Echocardiogr. 2016;29:861–70. doi: 10.1016/j.echo.2016.05.002. RV linear fractional shortening helps appreciate RV ejection fraction as quantified by Cardiac MR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18**.Srinivasan A, Kim J, Khalique O, Geevarghese A, Rusli M, Shah T, Di Franco A, Alakbarli J, Goldburg S, Rozenstrauch M, Devereux RB, Weinsaft JW. Echocardiographic linear fractional shortening for quantification of right ventricular systolic function-A cardiac magnetic resonance validation study. Echocardiography. 2017;34:348–358. doi: 10.1111/echo.13438. A study of over 100 patients which demonstrated that obese patients had better RV function measured by TAPSE at the time of MRI when compared non-obese patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alhamshari YS, Alnabelsi T, Mulki R, Cepeda-Valery B, Figueredo VM, Romero-Corral A. Right ventricular function measured by TAPSE in obese subjects at the time of acute myocardial infarction and 2year outcomes. Int J Cardiol. 2017;232:181–185. doi: 10.1016/j.ijcard.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Anavekar NS, Gerson D, Skali H, Kwong RY, Yucel EK, Solomon SD. Two-dimensional assessment of right ventricular function: an echocardiographic-MRI correlative study. Echocardiography. 2007;24:452–6. doi: 10.1111/j.1540-8175.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- 21.Zornoff LA, Skali H, Pfeffer MA, St John Sutton M, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moye LA, Lewis SJ, Braunwald E, Solomon SD Investigators S. Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction. J Am Coll Cardiol. 2002;39:1450–5. doi: 10.1016/s0735-1097(02)01804-1. [DOI] [PubMed] [Google Scholar]
- 22**.Anavekar NS, Skali H, Bourgoun M, Ghali JK, Kober L, Maggioni AP, McMurray JJ, Velazquez E, Califf R, Pfeffer MA, Solomon SD. Usefulness of right ventricular fractional area change to predict death, heart failure, and stroke following myocardial infarction (from the VALIANT ECHO Study) Am J Cardiol. 2008;101:607–12. doi: 10.1016/j.amjcard.2007.09.115. A study of over 250 patients undergoing TAVR demonstrated that those with RV dilation had worse survival. [DOI] [PubMed] [Google Scholar]
- 23*.Ito S, Pislaru SV, Soo WM, Huang R, Greason KL, Mathew V, Sandhu GS, Eleid MF, Suri RM, Oh JK, Nkomo VT. Impact of right ventricular size and function on survival following transcatheter aortic valve replacement. Int J Cardiol. 2016;221:269–74. doi: 10.1016/j.ijcard.2016.07.085. 3DTTE-determined RV ejection fraction was independently associated with cardiac outcomes in 60 patients followed over 4 years. [DOI] [PubMed] [Google Scholar]
- 24**.Nagata Y, Wu VC, Kado Y, Otani K, Lin FC, Otsuji Y, Negishi K, Takeuchi M. Prognostic Value of Right Ventricular Ejection Fraction Assessed by Transthoracic 3D Echocardiography. Circ Cardiovasc Imaging. 2017:10. doi: 10.1161/CIRCIMAGING.116.005384. Real time three-dimensional echocardiography derived RV volumes and EF correlated well with CMR measures in a group of patients with established LV dysfunction. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Cohen SB, Atalay MK, Maslow AD, Poppas A. Quantitative Assessment of Right Ventricular Volumes and Ejection Fraction in Patients with Left Ventricular Systolic Dysfunction by Real Time Three-Dimensional Echocardiography versus Cardiac Magnetic Resonance Imaging. Echocardiography. 2015;32:805–12. doi: 10.1111/echo.12715. [DOI] [PubMed] [Google Scholar]
- 26*.Tamborini G, Brusoni D, Torres Molina JE, Galli CA, Maltagliati A, Muratori M, Susini F, Colombo C, Maffessanti F, Pepi M. Feasibility of a new generation three-dimensional echocardiography for right ventricular volumetric and functional measurements. Am J Cardiol. 2008;102:499–505. doi: 10.1016/j.amjcard.2008.03.084. Structural valve deterioration was not associated with severe stenosis in most of the patients who underwent TAVI and had no significant impact on and clinical outcome. [DOI] [PubMed] [Google Scholar]
- 27*.Muratori M, Fusini L, Tamborini G, Gripari P, Ghulam Ali S, Mapelli M, Fabbiocchi F, Trabattoni P, Roberto M, Agrifoglio M, Alamanni F, Bartorelli AL, Pepi M. Five-year echocardiographic follow-up after TAVI: structural and functional changes of a balloon-expandable prosthetic aortic valve. Eur Heart J Cardiovasc Imaging. 2017 doi: 10.1093/ehjci/jex046. A prospective study that demonstrated RV volumes and systolic function were preserved after percutaneous mitral valve repair. [DOI] [PubMed] [Google Scholar]
- 28.Gripari P, Tamborini G, Bottari V, Maffessanti F, Carminati MC, Muratori M, Vignati C, Bartorelli AL, Alamanni F, Pepi M. Three-Dimensional Transthoracic Echocardiography in the Comprehensive Evaluation of Right and Left Heart Chamber Remodeling Following Percutaneous Mitral Valve Repair. J Am Soc Echocardiogr. 2016;29:946–954. doi: 10.1016/j.echo.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 29*.van der Zwaan HB, Geleijnse ML, McGhie JS, Boersma E, Helbing WA, Meijboom FJ, Roos-Hesselink JW. Right ventricular quantification in clinical practice: two-dimensional vs. three-dimensional echocardiography compared with cardiac magnetic resonance imaging. Eur J Echocardiogr. 2011;12:656–64. doi: 10.1093/ejechocard/jer107. A review that demonstrates an association between RV dysfunction and higher cardiovascular and overall mortality in patients with heart failure, irrespective of ejection fraction. [DOI] [PubMed] [Google Scholar]
- 30*.Tadic M, Pieske-Kraigher E, Cuspidi C, Morris DA, Burkhardt F, Baudisch A, Hassfeld S, Tschope C, Pieske B. Right ventricular strain in heart failure: Clinical perspective. Arch Cardiovasc Dis. 2017 doi: 10.1016/j.acvd.2017.05.002. Automated functional imaging allows for assessment of RV global systolic function without traditional echocardiographic measures. [DOI] [PubMed] [Google Scholar]
- 31*.Lopez-Candales A. Applicability of automated functional imaging for assessing right ventricular function. Echocardiography. 2013;30:919–28. doi: 10.1111/echo.12174. In a population of patient referred for heart transplant, RV longitudinal strain is a stronger predictor of outcome than LV longitudinal strain. [DOI] [PubMed] [Google Scholar]
- 32*.Cameli M, Righini FM, Lisi M, Bennati E, Navarri R, Lunghetti S, Padeletti M, Cameli P, Tsioulpas C, Bernazzali S, Maccherini M, Sani G, Henein M, Mondillo S. Comparison of right versus left ventricular strain analysis as a predictor of outcome in patients with systolic heart failure referred for heart transplantation. Am J Cardiol. 2013;112:1778–84. doi: 10.1016/j.amjcard.2013.07.046. In a population of patients with heart failure and depressed ejection fraction, RV longitudinal strain was a valuable prognostic variable. [DOI] [PubMed] [Google Scholar]
- 33*.Vizzardi E, D’Aloia A, Caretta G, Bordonali T, Bonadei I, Rovetta R, Quinzani F, Bugatti S, Curnis A, Metra M. Long-term prognostic value of longitudinal strain of right ventricle in patients with moderate heart failure. Hellenic J Cardiol. 2014;55:150–5. Deep learning algorithms can be used for automatic segmentation of the RV. [PubMed] [Google Scholar]
- 34.Avendi MR, Kheradvar A, Jafarkhani H. Automatic segmentation of the right ventricle from cardiac MRI using a learning-based approach. Magn Reson Med. 2017 doi: 10.1002/mrm.26631. [DOI] [PubMed] [Google Scholar]
- 35**.Freed BH, Gomberg-Maitland M, Chandra S, Mor-Avi V, Rich S, Archer SL, Jamison EB, Jr, Lang RM, Patel AR. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012;14:11. doi: 10.1186/1532-429X-14-11. In a study of over 100 patients with RV dysfunction, Non-Ischemic Septal Fibrosis was independently associated with RV chamber dilation and afterload. Therefore, NIF is linked to adverse RV chamber remodeling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36**.Kim J, Medicherla CB, Ma CL, Feher A, Kukar N, Geevarghese A, Goyal P, Horn E, Devereux RB, Weinsaft JW. Association of Right Ventricular Pressure and Volume Overload with Non-Ischemic Septal Fibrosis on Cardiac Magnetic Resonance. PLoS One. 2016;11:e0147349. doi: 10.1371/journal.pone.0147349. A review that allows for an overview of RV imaging by CMR and CT. An emphasis is placed on RV structure and function, as well as flow and tissue character. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 38.Kochav J, Simprini L, Weinsaft JW. Imaging of the right heart--CT and CMR. Echocardiography. 2015;32(Suppl 1):S53–68. doi: 10.1111/echo.12212. [DOI] [PubMed] [Google Scholar]
- 39**.Tadic M. Multimodality Evaluation of the Right Ventricle: An Updated Review. Clin Cardiol. 2015;38:770–6. doi: 10.1002/clc.22443. A reivew that summarizes available modailties to image the RV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schoepf UJ, Kucher N, Kipfmueller F, Quiroz R, Costello P, Goldhaber SZ. Right ventricular enlargement on chest computed tomography: a predictor of early death in acute pulmonary embolism. Circulation. 2004;110:3276–80. doi: 10.1161/01.CIR.0000147612.59751.4C. [DOI] [PubMed] [Google Scholar]
- 41.Kang DK, Thilo C, Schoepf UJ, Barraza JM, Jr, Nance JW, Jr, Bastarrika G, Abro JA, Ravenel JG, Costello P, Goldhaber SZ. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011;4:841–9. doi: 10.1016/j.jcmg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Tandri H, Daya SK, Nasir K, Bomma C, Lima JA, Calkins H, Bluemke DA. Normal reference values for the adult right ventricle by magnetic resonance imaging. Am J Cardiol. 2006;98:1660–4. doi: 10.1016/j.amjcard.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 43.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, Weinsaft JW, Shaw LJ, Berman DS, Callister TQ, Min JK. Cardiac chamber volumes, function, and mass as determined by 64-multidetector row computed tomography: mean values among healthy adults free of hypertension and obesity. JACC Cardiovasc Imaging. 2008;1:782–6. doi: 10.1016/j.jcmg.2008.04.015. [DOI] [PubMed] [Google Scholar]