Abstract
Glioblastoma (GBM) remains incurable, and recurrent tumors that rarely respond to standard-of-care radiation and chemo therapies. Therefore, strategies that enhance the effects of these therapies should provide significant benefits to GBM patients. We have developed a nanoparticle delivery vehicle that can stably bind and protect nucleic acids for specific delivery into brain tumor cells. These nanoparticles can deliver therapeutic siRNAs to sensitize GBM cells to radiotherapy and improve GBM treatment via systemic administration. We show that nanoparticle-mediated knockdown of the DNA repair protein apurinic endonuclease 1 (Ape1) sensitizes GBM cells to radiotherapy and extend survival in a genetic mouse model of GBM. Specific knockdown of Ape1 activity by 30% in brain tumor tissue doubled the extended survival achieved with radiotherapy alone. Ape1 is a promising target for increasing the effectiveness of radiotherapy, and nanoparticle-mediated delivery of siRNA is a promising strategy for tumor specific knockdown of Ape1.
Keywords: Ape1/APEX1, siRNA, nanoparticle, glioblastoma, radiosensitization
Introduction
The widespread study of nanotechnology has produced many different nanoparticle (NP)-based delivery vehicles that have shown promise in improving cancer therapy.1 However, the successful translation of these nanotechnologies into clinical use, and even into Phase I clinical trials, has been severely limited.2 The systems are either too complicated for scale-up in production, or are too restrictive in the patient populations that could potentially benefit. The first nanotechnologies to enter into clinical trials will be those that directly improve upon standard of care by 1) improving delivery of chemotherapy payloads, 2) enhancing the surgeons ability to visualize and remove tumor cells, and 3) sensitizing tumor cells to radiation and chemo therapies by inhibiting resistance pathways.3
Radiotherapy is an integral component in the treatment of glioblastoma (GBM), but is not always effective especially in recurrent tumors. The cytotoxicity of radiotherapy is caused by DNA double-strand breaks (DSBs) generated directly by the radiation or indirectly through the generation of reactive oxygen species. However, only approximately 2% of the damage caused by radiotherapy generates these cytotoxic DSBs directly.4 The majority of radiation-induced DNA damage are abasic sites or single strand breaks with defragmented deoxyribose. These lesions (herein collectively called abasic lesions), which account for 60% of the damage caused by radiotherapy, are repaired through the base excision repair (BER) pathway. Critical to the function of BER is apurinic endonuclease 1 (Ape1), which cleaves the abasic lesion for repair. In the absence of BER repair, these abasic lesions will evolve into cytotoxic DSBs, and thus Ape1 represents an ideal target for enhancing the effects of radiotherapy. Indeed, Ape1 activity in tumor tissue is inversely correlated with survival strongly suggesting its clinical relevancy.5–8 Additionally, Ape1 expression in GBM cell lines is predictive of their radiosensitivity.9 However, no Ape1 inhibitors are approved for clinical use,10–15 with only one, methylamine, making it to Phase I and II clinical trials.16 As opposed to these small molecule Ape1 inhibitors, delivery of antisense oligonucleotides such as short interfering RNAs (siRNA) is advantageous because of the potency and efficiency of the RNA interference (RNAi) pathway.17 Nevertheless, translating RNAi therapies into clinical use has been fraught with challenges.18
We have developed an image-guided, multifunctional, tumor targeted NP capable of delivering oligonucleotide payloads into brain cancer cells and tumors. These NPs comprise a superparamagnetic iron oxide core that provides T2 contrast in MRI, which is coated with a copolymer of chitosan, PEG, and polyethyleneimine (PEI).19 Combined, this system is able to overcome the biological barriers that would otherwise prevent transport of small oligonucleotides such as siRNA into tumors to enter the RNAi pathway to inhibit gene expression.19–24 The siRNA bound NP (NP:siRNA) is then surface functionalized with the tumor targeting peptide, chlorotoxin, which improves NP uptake by tumor cells.21, 22, 25 We show that these nanoparticles are able to deliver anti-Ape1 siRNA to pediatric ependymoma and medulloblastoma cells and sensitize them to radiotherapy in vitro.20 Here, we test the ability of the nanoparticles to delivery anti-Ape1 siRNA to GBM cells and tumors using a genetic mouse model of GBM to enhance the effects of radiotherapy. We utilized a genetic mouse model of GBM because it more accurately reflects the etiology and growth behavior of human brain tumors than human orthotopic xenografts. More importantly, these genetic models require the use of siRNA against mouse sequences, which allows for a more accurate assessment of off-target effects since siRNA against human sequences may not knockdown expression in off target tissues and thus lead to inaccurate conclusions about potential off-target effects.
Methods
Materials
All cell culture reagents were purchased from Invitrogen unless otherwise noted. All chemicals were purchased from Sigma unless otherwise noted. The GBM-derived SF763 cell line was maintained in DMEM supplemented with 10% FBS and 1% antibiotic-antimycotic in a 37°C humidified 95/5% air/CO2 incubator. siApe1 was purchased as a SMARTpool consisting of four validated siRNA sequences against human APEX1 for in vitro trials and mouse APEX1 for in vivo trials (Thermo Scientific). The anti-green fluorescent protein siRNA (siGFP) was used as a non-specific sequence control as previously described.20, 22, 23, 26 Fluorophore labeled siRNA (Dy677 modified 5′ end of the antisense strand) was purchased from Dharmacon.
Nanoparticle synthesis
Nanoparticles were synthesized as described previously,19–22 with modification. Synthesis of siloxane poly(ethylene glycol) (PEG) monolayer coated iron oxide nanoparticles was performed as described previously.27 Amine terminated PEG on the nanoparticles were then conjugated to a copolymer of chitosan, PEG, and PEI prepared by attaching PEI to PEG-grafted-chitosan.28 Amine groups on 25,000 Da polyethylenimine (PEI, Sigma-Aldrich) were thiolated with 2-iminothiolane (Traut’s reagent, Molecular Biosciences) for 1 hr in thiolation buffer (100 mM sodium bicarbonate, pH 8.0). Concurrently, chitosan-grafted-PEG (CP) was made thiol reactive with N-succinimidyl iodoacetate (SIA, Molecular Biosciences) at a 1:10 molar ratio in thiolation buffer before removing unreacted SIA reagent using a Zeba spin column (Thermo Fisher Scientific) equilibrated with thiolation buffer. The functionalized CP was then added to thiolated PEI for attachment through the formation of a thioether bond. After reaction at room temperature for 4 hr, the resultant mixture was dialyzed with a dialysis membrane (MW 50,000 cutoff, Spectrum Labs) against distilled water, and the solution was subsequently lyophilized. The resultant polymer was conjugated to nanoparticles (NPs) by first functionalizing with iodoacetyl groups using SIA at a 1:10 molar ratio. The excess SIA was purified using a Zeba spin column equilibrated with thiolation buffer. NPs were simultaneously reacted with Traut’s reagent at room temperature for 1 hr, then combined with the activated polymer overnight before purifying through a Zeba spin column equilibrated with 20 mM HEPES buffer (pH 7.4). To attach siRNA, NPs and siRNA were mixed in 20 mM HEPES buffer (pH 7.4) for 30 min to allow formation of NP:siRNA complexes. Afterwards, a 1 mg/mL solution of CTX was thiolated through reaction with Traut’s reagent at a 1.2:1 molar ratio for 1 hr in the dark at room temperature. Concurrently, SIA was conjugated to amine functional groups on NP at 1 mg of SIA/mg iron in the dark with gentle rocking for 1 hr. Subsequently the thiolated CTX was reacted with the iodoacetyl groups on the SIA at 1 mg CTX per 0.9 mg Fe. The size and zeta potential of the resulting NPs were characterized in 20mM HEPES buffer (pH 7.4) using a DTS Zetasizer Nano (Malvern Instruments).
In vitro cell transfections
SF767 cells were plated at 30,000 cells per well in 24 well plates and transfected with NP-siRNA at 100 nM of siRNA in 500 μL fully supplemented culture medium. After incubation for 24 hr, the transfection medium was replaced with fresh medium, and cells were incubated for an additional 48 hr before analysis.
Quantitative RT-PCR
RNA was extracted from cells using the Qiagen RNeasy kit, and cDNA was prepared using the iScript cDNA Synthesis Kit (BioRad) following the manufacturer’s protocol. Quantitative RT-PCR (qRT-PCR) was used to evaluate the relative expression levels of Ape1 utilizing human β-actin as a reference gene. SYBR Green PCR Master mix (Bio-Rad) was used for template amplification with a primer for each of the transcripts in a Bio-Rad CFX96 real-time PCR detection system. Thermocycling for all targets was carried out in a solution of 20 μl containing 0.5 μM primers and 4 pg of cDNA from the reverse transcription reaction at 95°C for 2 min, 40 cycles of denaturation (15 sec, 95°C), annealing (30 sec, 58°C), and extension (30 sec, 72°C). Primers used for Ape1 were forward: CAACACACCCTATGCCTACA, reverse: GTAACAGAGAGTGGGACAA, and for β-actin were forward: AGCGAGCATCCCCCAAAGTT, reverse: GGGCACGAAGGCTCATCATT.
Cell survival assays
The clonogenic assay was used for assessing radiation sensitivity as it provides the best indication of replicative cell death, as opposed to measuring changes in cell density or metabolism (e.g., XTT, Alamar blue, CellTiter Glo). NP-treated cells were harvested by trypsinization, resuspended in medium and were immediately irradiated at 4 gray (Gy)/min with 137Cs-γ-rays. Six well trays were then inoculated with 250, 500 or 1,000 irradiated cells in 2 mL supplemented medium. Plates were incubated until colonies of ≥ 50 cells were formed after 10–14 days. Colonies were stained with 0.5% methylene blue in methanol/water (1:1 v/v) and counted using a dissecting microscope. Survival is the fraction of colonies formed by treated cells compared to untreated controls.
Genetic mouse model
The animal studies conducted in these experiments were done in accordance with a protocol approved by the Institutional Animal Care and Use Committee at the University of Washington. Veterinary care of the animals involved in this work is available at all times at the University of Washington Office of Veterinary Services, which is dedicated to the improvement of health and medicine of both humans and animals through the humane and ethical use of animals in biomedical research. Genetic mouse models of GBM were made using the RCAS/tv-a system to drive PDGF-B expression in N/tv-a; Ink4a-arf−/−; Ptenfl/fl background mice as described previously.29, 30 Mice entered the treatment studies 21 days post-inoculation when tumors begin to form. Mice receiving NP treatment were injected intravenously through the tail vein with 20 μg siRNA loaded in 10 μg NPs (Fe equivalent) suspended in 100 μL HEPES buffer daily for five days. Each injection of NPs was followed 24 hrs later by whole brain exposure to 2 Gy 137Cs-γ-rays at 1 Gy/min under isoflurane anesthesia. Animal weights were monitored and death defined as weight loss greater than 15% caused by tumor formation. Progression was defined as the first day of weight loss prior to reaching 15%. Tissues were collected from CO2 euthanized animals and placed into formalin for histology or flash frozen and stored at −80°C for Ape1 activity biochemical assays.
Ape1 activity assay
Abasic site endonuclease activity was measured in cleared supernatants of tissue extracts. Total protein content in tissue extracts was quantified using Bradford’s reagent for normalization between samples and estimation of cell number. The assay measures conversion of acid-treated, supercoiled plasmid DNA to relaxed form caused by incision at abasic sites as detailed elsewhere.5–8 In the presence of Ape1 activity, the cleaved abasic site relaxes the supercoiled plasmid to its open circular form. Relaxed plasmid was quantitated by comparison with known amounts of linearized substrate allowing estimation of activity expressed as fmoles lesions incised per minute per cell (fmol/min/cell).
Statistics
Data shown are mean ± standard deviation. Statistical significance was determined using Student’s t-test or the log-rank (Mantel-Cox) test for survival data using the Prism software package. We considered p-values of 0.05 or less significant.
Results
Proper NP physicochemical properties will ensure ideal trafficking within the human body and target cells.3 The composition of our siRNA-bound NP is shown in Figure 1A. The NP has a hydrodynamic size of 48.5 ± 4.0 nm as determined by dynamic light scattering, and a positive zeta potential, a measure of NP surface charge, of 13 ± 3.4 mV. The physicochemical properties of these NPs are ideal for enhancing nucleic acid protection, promoting cellular uptake, and maximizing tumor delivery.
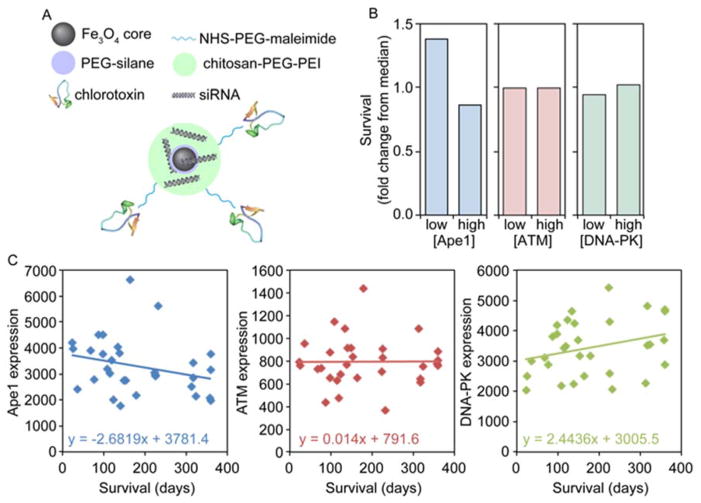
Figure 1.
NP design and clinical relevance of Ape1 target. A) Schematic of NP components. B) Fold change in survival as compared to median with low and high expression of the DNA repair enzymes Ape1, ATM, and DNA-PK as determined from TCGA portal data. Survival is significantly higher (~50% over median) only in patients with lower than median Ape1 expression. C) Ape1 expression is inversely correlated with survival in patients that received at least 40 Gy as determined from TCGA portal data (RNA seq V2).
To test the clinical relevancy of Ape1 as a target for improving GBM therapy, we utilized The Cancer Genome Atlas (TCGA) RNA sequencing data31, 32 to assess the effect of Ape1 expression on patient survival. Patients with lower than median Ape1 expression levels had nearly 50% longer survival times than median, and nearly double the survival time as those with higher than median Ape1 expression (Figure 1B). As a comparison, the DNA enzymes critical for repair in the DSB repair pathways including ATM (ataxia telangiectasia mutated; homologous recombination) and DNA-PK (DNA-dependent protein kinase; non-homologous end joining) showed no difference in survival based on expression. More importantly, in patients that received a radiation dose of at least 40 gray (Gy) out of the 60 Gy standard, time of survival decreased as Ape1 expression increased (Figure 1C) further suggesting Ape1 expression as a predictive marker.5–8 This was not observed with ATM, but DNA-PK expression level was directly correlated with survival suggesting a role for active non-homologous end joining DSB repair in enhancing radiosensitivity, as seen in previous studies.33, 34 This strongly suggests Ape1 as a key repair enzyme that greatly diminishes patient response to standard of care therapy, and should be a good therapeutic target.
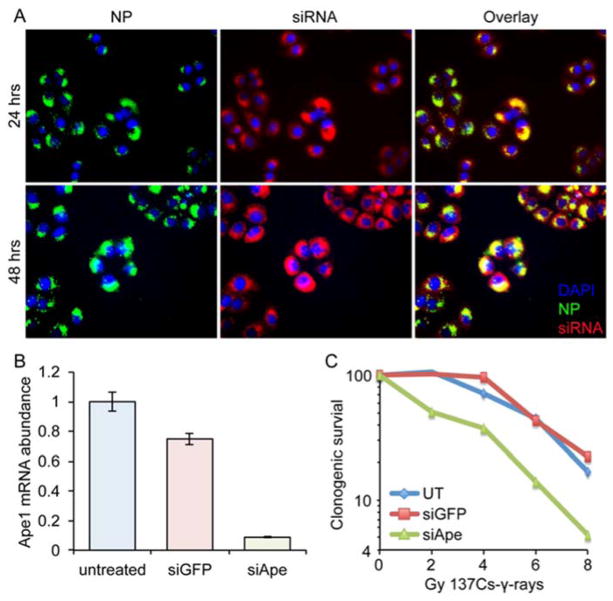
NPs were efficiently taken up into GBM cells and became well distributed in the perinuclear region (Figure 2A), the intracellular site of action for siRNA, within 24 hrs after treatment. Large amounts of siRNA was still bound to NPs as seen from significant yellow color around the nucleus indicating overlap of NP and siRNA signals. After 48 hrs, siRNA was largely released from NPs as seen from more red visible from siRNA alone. There was still a significant amount of siRNA still bound to the NP after 48 hrs (yellow in the overlay), which suggests limited efficiency of siRNA release from the NP. Release of siRNA from the NP may not be required to achieve knockdown, as we and others have observed previously,35, 36 or it is possible that only minimal release of siRNA from the NP is needed to achieve high target gene knockdown.37 Combined, these data indicate the NPs should function well to deliver anti-Ape1 siRNA into target GBM cells. To test the ability of the NPs to knockdown Ape1 expression in GBM cells, we incubated SF767 human GBM cells with NPs loaded with siApe1 (NP:siApe1) or with siRNA against GFP as a control (NP:siGFP). PCR analysis showed a significant reduction in Ape1 mRNA abundance in cells treated with NP:siApe1, whereas control NP:siGFP had little effect on expression (Figure 2B). These data show that NP:siApe1 is able to protect siApe1 against lysosomal degradation after endocytosis and facilitates siRNA entry into the RNAi pathway.
Figure 2.
In vitro NP-mediated delivery of siRNA to SF767 human GBM cells. A) In vitro uptake and cellular distribution of NPs (green) and fluorophore labeled siRNA (red) in SF767 cells after 24 and 48 hrs. Yellow indicates siRNA still bound to NPs. B) Knockdown of Ape1 as determined by PCR. C) Clonogenic survival after exposure to various doses of RT from γ-rays generated by 137Cs.
The effect of NP:siApe1 on the radiosensitivity of SF767 cells was evaluated using clonogenic survival assays. Cells treated with NP:siApe1, NP:siGFP, or left untreated (UT) were exposed to various doses of 137Cs-γ-rays and clonogenic survival was assessed after 10 days. Cells treated with NP:siApe1 displayed greater sensitivity to various doses of 137Cs-γ-rays compared to UT and NP:siGFP treated cells (Figure 2C). The significance of NP:siApe1 treatment was most notable at doses of 2 Gy 137Cs-γ-rays or less, the standard fractionated dose in most RT treatment regimens.38, 39 The detectible shoulder of resistance displayed by UT and NP:siGFP treated cells was eliminated with NP:siApe1 treatment, indicating that repair of radiation damage by Ape1 was the predominant repair mechanism of SF767 cells at low doses.
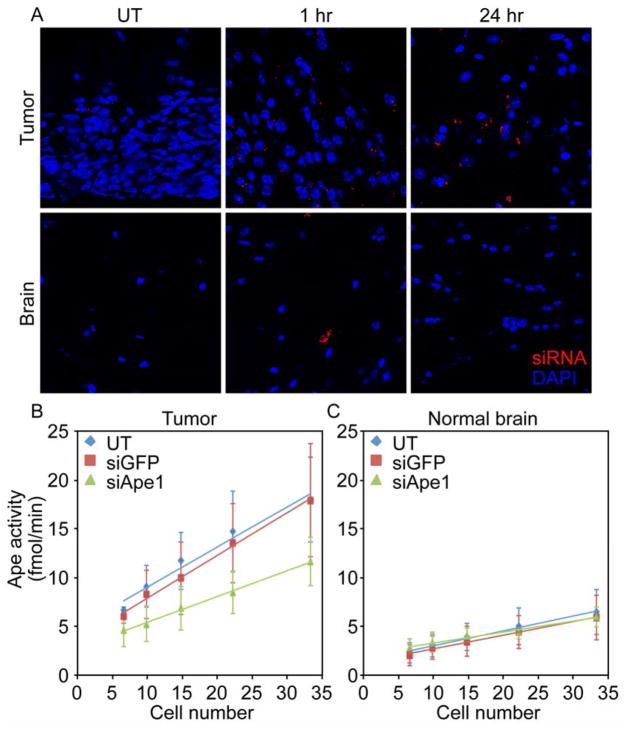
We next tested the ability of the NPs for in vivo delivery to GBM tumors in genetic mouse models of GBM that reflect the etiology, progression, and penetrance of human disease better than xenograft models. NPs were first loaded with fluorophore labeled siRNA for observation of NP-mediated delivery and retention of siRNA specifically in tumor tissue. Figure 3A shows red fluorescence from siRNA distributed in the tumor and a few fluorescence spots in contralateral normal brain, likely in brain vessels, at 1 hr post-injection through the tail vein. After 24 hrs, siRNA is only observed in the tumor tissue showing retention in the tumor for over a day and rapid clearance from normal brain suggesting uptake of nanoparticles and subsequent knockdown of Ape1 would only occur in target tumor tissue.
Figure 3.
In vivo NP delivery and knockdown efficiency. A) Uptake and retention of NP-mediated delivery of siRNA (red) into brain tumors and normal brain. B–C) Ape1 activity as determined by the Ape1 activity biochemical assay in B) tumor, C) normal brain.
The suppression of Ape1 activity was assayed by measuring abasic endonuclease activity determined using a biochemical assay (Figure 3B, C). Significant knockdown of Ape1 activity was observed in tumor tissue from mice injected with NP:siApe1 (0.26 fmol/min/cell), whereas mice injected with NP:siGFP (0.41 fmol/min/cell) and UT (0.44 fmol/min/cell, without NP) control animals showed similar higher Ape1 activities. Importantly, the reduction in Ape1 activity was only observed in tumor tissue and did not extend into normal brain. Ape1 activity in normal brain tissue contralateral to the tumor were similar for mice that were left untreated (0.15 fmol/min/cell), treated with siGFP (0.14 fmol/min/cell), or treated with siApe1 (0.11 fmol/min/cell). This shows the specificity of delivery and the potential safety of this treatment strategy since normal brain would not also be sensitized to radiotherapy.
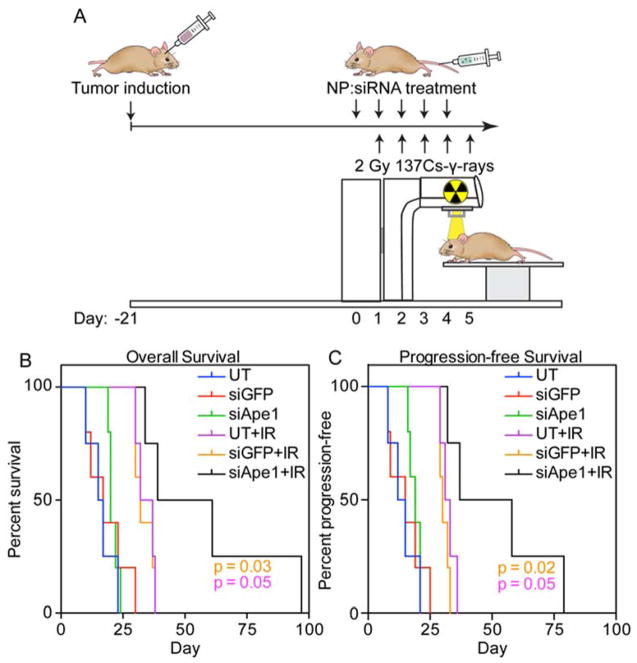
The ability of NP-mediated Ape1 suppression to extend survival in mice treated with 10 Gy 137Cs-γ-rays fractionated radiotherapy was tested in these genetic mouse models of GBM. Mice were injected intravenously through the tail vein with NP:siApe1, NP:siGFP as a control, or left uninjected (untreated, UT) for five subsequent days, with each injection followed up 24 hrs later with 2 Gy 137Cs-γ-rays whole brain irradiation (Figure 4A). NP:siGFP treatment had no effect on overall survival (Figure 4B) suggesting the lack of off-target effects from the NP or siRNA. Radiotherapy extended overall survival in these animals (UT and NP:siGFP) from 16 and 17 days, respectively, to 35 and 32 days, respectively, a response expected for this animal model.40 Treatment with NP:siApe1 alone slightly increased overall survival to 20 days, but was not significant as compared to UT and NP:siGFP. However, 10 Gy 137Cs-γ-rays fractionated radiotherapy with NP:siApe1 treatment showed a significant overall survival advantage to 50 days, doubling the extension in survival of these animals as compared to the addition of RT only. A similar trend was observed with progression-free survival, where progression was defined as the first day of a downward trend in weight loss towards the final 15%. NP:siApe1 treatment combined with radiotherapy significantly extended progression-free survival from 32 and 30 days (UT and NP:siGFP) to 48 days.
Figure 4.
Combined NP treatment and radiotherapy extends survival in a genetic mouse model of GBM. A) Treatment timeline for tumor induction, NP injections, and gamma irradiation. B) Overall survival following treatments. C) Progression-free survival following treatments.
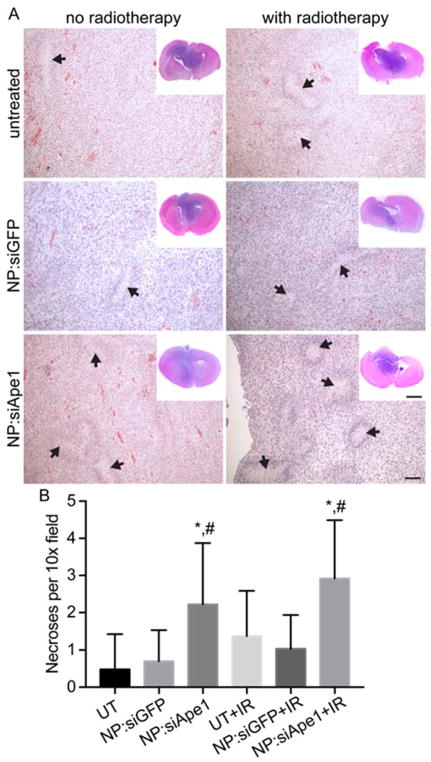
Brains were collected from animals at the time of euthanasia for histological analysis of brain tumors at their end-point. Brain tumors from animals that received NP:siApe1 treatment showed a higher density of necrotic regions (Figure 5A), with quantification revealing the highest density being in tumors from animals that received the combination NP:siApe1 and radiotherapy (Figure 5B). This may suggest these tumors were more responsive to radiotherapy with areas of necrosis indicative of treatment-mediated cell kill. However, an increase in pseudopalisading necrosis is indicative of an increase in tumor aggressiveness, suggesting the recurrent tumor might be more aggressive than the primary tumor, a common finding with recurrent GBM. Therefore, these mice that received both NP:siApe1 and RT survived longer but died with tumors having histopathological indicators of higher aggression. Data suggest that this treatment, although more effective at debulking the tumor, left behind aggressive, resistant cells.
Figure 5.
Histological analysis of brain tumors. A) Hematoxylin and Eosin stained brain sections showing necrotic regions (black arrows). Scale bars are 100 μm for 10x images and 250 μm for whole brain insets. B) Quantification of necrotic regions from 3 10x field views per slice, 3 slices per mouse, 3 mice per treatment condition. * indicates statistical difference (p < 0.01) as compared to UT or UT+IR within the same group, # indicates statistical difference (p < 0.01) as compared to NP:siGFP or NP:siGFP+IR within the same group.
Discussion
Radiotherapy (RT) is an integral component in the treatment of GBM. While there has been much improvement in radiotherapy methods to reduce off-target effects such as gamma knife and protons, there is still opportunity to increase the efficiency of RT to increase cell kill. A 30-fold increase in effectiveness by inhibiting the BER pathway can be achieved theoretically by increasing the number of RT-induced lesions that lead to cell death.4
There have been many small molecule inhibitors of Ape1 developed, but the likelihood of off-target effects and difficulty of getting small molecule drugs across the blood-brain barrier (BBB) greatly limits their clinical utility. RNA interference offers the possibility of gene-specific therapies through delivery of synthetic siRNAs into tumor cells. However, there are numerous anatomical and physiological barriers that must be bypassed for targeted intracellular delivery of siRNA which alone is highly unstable.
To evaluate the ability of these NPs to knock down Ape1 expression and increase radiosensitivity in GBM, we treated human SF767 GBM cells with NPs carrying siRNA against Ape1 (siApe1). Treated cells showed significantly decreased Ape1 expression as determined by PCR, which led to more cell kill after exposure to various doses of 137Cs-γ-rays as determined by clonogenic assays. These results, combined with our previous findings,5–8, 20 strongly indicate Ape1 as an ideal targeted for radiosensitization.
In vivo, we observed significant knockdown of Ape1 activity only in brain tumors from animalstreated with nanoparticles carrying siApe1. This 40% reduction in Ape1 activity would be expected to be clinically relevant since the hazard ratio for death has been shown to increase by 1.89 for every 0.01 unit increase in Ape1 activity in ependymoma patients.7 In other words, a 40% reduction in Ape1 activity would decrease the chance of death five-fold. Indeed, animals receiving both fractionated radiotherapy and nanoparticle-mediated siApe1 treatment lived significantly longer than those that received radiotherapy alone and radiotherapy and control nanoparticle treatment. The inclusion of nanoparticle-bound siApe1 treatment with radiotherapy doubled the extension in survival gained by radiotherapy alone suggesting the utility of this treatment.
Brains taken at the time of death (euthanized at 15% weight loss) from animals receiving both siApe1 treatment and radiotherapy showed larger areas of necrosis than control animals and those receiving only radiotherapy. This apparent increase in tumor cell kill suggests a higher proportion of cells became sensitive to radiotherapy and allowed the animals to live longer. The lack of significant necrosis apparent in control nanoparticle treatments indicates the increased radiosensitivity wasn’t solely caused by the presence of the nanoparticle cores, which has been proposed and shown to enhance the effects of radiotherapy through an Auger effect.41–43 Instead, increased necrosis was likely caused by a greater proportion of cells being sensitized to radiotherapy because of inhibited abasic site repair activity. However, an increase in pseudopalisading necrosis is also a pathological finding in more aggressive GBMs. Therefore, the tumors recurring in mice receiving combined siApe1 treatment and radiotherapy may be more aggressive than others. However, these mice survived longer indicating a more effective treatment that killed off a higher proportion of cells but left a smaller subpopulations of more aggressive, resistant cells. This would suggest that this smaller surviving cell population in these tumors relied on additional DNA repair pathways to promote survival and increased aggression, or that there were alterations to remaining Ape1 acetylation that promoted enhanced AP-endonuclease activity.44 Future studies should identify multiple DNA repair pathways to inhibit simultaneously to increase initial cell kill and reduce the proportion of recurrent cell survival.
In conclusion, we have shown that systemic delivery of nanoparticle-mediated siRNA targeting Ape1 can knock down expression and activity of Ape1 in GBM cells and tumors. The observed knockdown is expected to be clinically relevant as patient survival is inversely correlated with Ape1 expression and activity. Indeed, in a genetic mouse model of GBM we observed doubled extension in survival in animals that received NP:siApe1 treatment combined with fractionated radiotherapy as compared to fractionated radiotherapy alone. This strongly indicates Ape1 as a promising target to sensitize brain tumor cells to radiotherapy and NPs as an ideal method for Ape1 inhibition specifically in brain tumors to improve treatment and extend survival.
Acknowledgments
This work was supported in part by the grants of National Institutes of Health R01CA161953 and Seattle Children’s Hospital (M.Z and R.E) and the National Institutes of Health (R01CA195718, U0 1CA160882, U54CA193461) (E.H). F.K. acknowledges support from the American Brain Tumor Association Basic Research Fellowship in Honor of Susan Kramer. We thank Prof. Jeffery Schwartz for use of the gamma irradiator, and the University of Washington Department of Pathology service core for preparing and staining tissue sections.
Abbreviations
- GBM
glioblastoma
- Ape1
apurinic endonuclease 1
- DSB
double strand break
- BER
base excision repair
- siGFP
siRNA against green fluorescent protein
- siApe1
siRNA against Ape1
- PEI
polyethyleneimine
- CTX
chlorotoxin
- CP
chitosan-grafted PEG
- NP
nanoparticle
- TCGA
The Cancer Genome Atlas
- RT
radiotherapy
- ATM
ataxia telangiectasia mutated
- DNA-PK
DNA-dependent protein kinase
- Gy
gray
References
- 1.Bertrand N, Wu J, Xu X, Kamaly N, Farokhzad OC. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Advanced drug delivery reviews. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wicki A, Witzigmann D, Balasubramanian V, Huwyler J. Nanomedicine in cancer therapy: challenges, opportunities, and clinical applications. J Control Release. 2015;200:138–57. doi: 10.1016/j.jconrel.2014.12.030. [DOI] [PubMed] [Google Scholar]
- 3.Kievit FM, Zhang M. Cancer nanotheranostics: improving imaging and therapy by targeted delivery across biological barriers. Adv Mater. 2011;23:H217–47. doi: 10.1002/adma.201102313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung H, Demple B. Distinct roles of Ape1 protein in the repair of DNA damage induced by ionizing radiation or bleomycin. The Journal of biological chemistry. 2011;286:4968–77. doi: 10.1074/jbc.M110.146498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bobola MS, Blank A, Berger MS, Stevens BA, Silber JR. Apurinic/apyrimidinic endonuclease activity is elevated in human adult gliomas. Clin Cancer Res. 2001;7:3510–8. [PubMed] [Google Scholar]
- 6.Bobola MS, Finn LS, Ellenbogen RG, Geyer JR, Berger MS, Braga JM, et al. Apurinic/apyrimidinic endonuclease activity is associated with response to radiation and chemotherapy in medulloblastoma and primitive neuroectodermal tumors. Clin Cancer Res. 2005;11:7405–14. doi: 10.1158/1078-0432.CCR-05-1068. [DOI] [PubMed] [Google Scholar]
- 7.Bobola MS, Jankowski PP, Gross ME, Schwartz J, Finn LS, Blank A, et al. Apurinic/apyrimidinic endonuclease is inversely associated with response to radiotherapy in pediatric ependymoma. Int J Cancer. 2011;129:2370–9. doi: 10.1002/ijc.25900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silber JR, Bobola MS, Blank A, Schoeler KD, Haroldson PD, Huynh MB, et al. The apurinic/apyrimidinic endonuclease activity of Ape1/Ref-1 contributes to human glioma cell resistance to alkylating agents and is elevated by oxidative stress. Clin Cancer Res. 2002;8:3008–18. [PubMed] [Google Scholar]
- 9.Naidu MD, Mason JM, Pica RV, Fung H, Pena LA. Radiation resistance in glioma cells determined by DNA damage repair activity of Ape1/Ref-1. Journal of radiation research. 2010;51:393–404. doi: 10.1269/jrr.09077. [DOI] [PubMed] [Google Scholar]
- 10.Al-Safi RI, Odde S, Shabaik Y, Neamati N. Small-molecule inhibitors of APE1 DNA repair function: an overview. Current molecular pharmacology. 2012;5:14–35. [PubMed] [Google Scholar]
- 11.Dorjsuren D, Kim D, Vyjayanti VN, Maloney DJ, Jadhav A, Wilson DM, 3rd, et al. Diverse small molecule inhibitors of human apurinic/apyrimidinic endonuclease APE1 identified from a screen of a large public collection. PloS one. 2012;7:e47974. doi: 10.1371/journal.pone.0047974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Gerson SL. Therapeutic impact of methoxyamine: blocking repair of abasic sites in the base excision repair pathway. Current opinion in investigational drugs. 2004;5:623–7. [PubMed] [Google Scholar]
- 13.Sultana R, McNeill DR, Abbotts R, Mohammed MZ, Zdzienicka MZ, Qutob H, et al. Synthetic lethal targeting of DNA double-strand break repair deficient cells by human apurinic/apyrimidinic endonuclease inhibitors. Int J Cancer. 2012;131:2433–44. doi: 10.1002/ijc.27512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Srinivasan A, Wang L, Cline CJ, Xie Z, Sobol RW, Xie XQ, et al. Identification and characterization of human apurinic/apyrimidinic endonuclease-1 inhibitors. Biochemistry. 2012;51:6246–59. doi: 10.1021/bi300490r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbotts R, Jewell R, Nsengimana J, Maloney DJ, Simeonov A, Seedhouse C, et al. Targeting human apurinic/apyrimidinic endonuclease 1 (APE1) in phosphatase and tensin homolog (PTEN) deficient melanoma cells for personalized therapy. Oncotarget. 2014;5:3273–86. doi: 10.18632/oncotarget.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DM, 3rd, Simeonov A. Small molecule inhibitors of DNA repair nuclease activities of APE1. Cellular and molecular life sciences: CMLS. 2010;67:3621–31. doi: 10.1007/s00018-010-0488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–38. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nature reviews Cancer. 2011;11:59–67. doi: 10.1038/nrc2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kievit FM, Veiseh O, Bhattarai N, Fang C, Gunn JW, Lee D, et al. PEI-PEG-Chitosan Copolymer Coated Iron Oxide Nanoparticles for Safe Gene Delivery: synthesis, complexation, and transfection. Adv Funct Mater. 2009;19:2244–2251. doi: 10.1002/adfm.200801844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kievit FM, Stephen ZR, Wang K, Dayringer CJ, Sham JG, Ellenbogen RG, et al. Nanoparticle mediated silencing of DNA repair sensitizes pediatric brain tumor cells to gamma-irradiation. Molecular oncology. 2015;9:1071–80. doi: 10.1016/j.molonc.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kievit FM, Veiseh O, Fang C, Bhattarai N, Lee D, Ellenbogen RG, et al. Chlorotoxin labeled magnetic nanovectors for targeted gene delivery to glioma. ACS Nano. 2010;4:4587–94. doi: 10.1021/nn1008512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veiseh O, Kievit FM, Fang C, Mu N, Jana S, Leung MC, et al. Chlorotoxin bound magnetic nanovector tailored for cancer cell targeting, imaging, and siRNA delivery. Biomaterials. 2010;31:8032–42. doi: 10.1016/j.biomaterials.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang K, Kievit FM, Sham JG, Jeon M, Stephen ZR, Bakthavatsalam A, et al. Iron-Oxide-Based Nanovector for Tumor Targeted siRNA Delivery in an Orthotopic Hepatocellular Carcinoma Xenograft Mouse Model. Small. 2016;12:477–87. doi: 10.1002/smll.201501985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K, Kievit FM, Jeon M, Silber JR, Ellenbogen RG, Zhang M. Nanoparticle-Mediated Target Delivery of TRAIL as Gene Therapy for Glioblastoma. Advanced healthcare materials. 2015;4:2719–26. doi: 10.1002/adhm.201500563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang K, Kievit FM, Florczyk SJ, Stephen ZR, Zhang M. 3D Porous Chitosan-Alginate Scaffolds as an In Vitro Model for Evaluating Nanoparticle-Mediated Tumor Targeting and Gene Delivery to Prostate Cancer. Biomacromolecules. 2015;16:3362–72. doi: 10.1021/acs.biomac.5b01032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok H, Veiseh O, Fang C, Kievit FM, Wang FY, Park JO, et al. pH-Sensitive siRNA nanovector for targeted gene silencing and cytotoxic effect in cancer cells. Mol Pharm. 2010;7:1930–9. doi: 10.1021/mp100221h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang C, Bhattarai N, Sun C, Zhang M. Functionalized nanoparticles with long-term stability in biological media. Small. 2009;5:1637–41. doi: 10.1002/smll.200801647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhattarai N, Ramay HR, Gunn J, Matsen FA, Zhang M. PEG-grafted chitosan as an injectable thermosensitive hydrogel for sustained protein release. Journal of controlled release: official journal of the Controlled Release Society. 2005;103:609–24. doi: 10.1016/j.jconrel.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 29.Ozawa T, Riester M, Cheng YK, Huse JT, Squatrito M, Helmy K, et al. Most human non-GCIMP glioblastoma subtypes evolve from a common proneural-like precursor glioma. Cancer Cell. 2014;26:288–300. doi: 10.1016/j.ccr.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling Adult Gliomas Using RCAS/t-va Technology. Transl Oncol. 2009;2:89–U45. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao JJ, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci Signal. 2013:6. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cerami E, Gao JJ, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burma S, Chen DJ. Role of DNA-PK in the cellular response to DNA double-strand breaks. DNA repair. 2004;3:909–18. doi: 10.1016/j.dnarep.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Santivasi WL, Xia F. The role and clinical significance of DNA damage response and repair pathways in primary brain tumors. Cell & bioscience. 2013;3:10. doi: 10.1186/2045-3701-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Veiseh O, Kievit FM, Mok H, Ayesh J, Clark C, Fang C, et al. Cell transcytosing poly-arginine coated magnetic nanovector for safe and effective siRNA delivery. Biomaterials. 2011;32:5717–25. doi: 10.1016/j.biomaterials.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh N, Agrawal A, Leung AK, Sharp PA, Bhatia SN. Effect of nanoparticle conjugation on gene silencing by RNA interference. J Am Chem Soc. 2010;132:8241–3. doi: 10.1021/ja102132e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei J, Jones J, Kang J, Card A, Krimm M, Hancock P, et al. RNA-Induced Silencing Complex-Bound Small Interfering RNA Is a Determinant of RNA Interference-Mediated Gene Silencing in Mice. Mol Pharmacol. 2011;79:953–963. doi: 10.1124/mol.110.070409. [DOI] [PubMed] [Google Scholar]
- 38.Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard-risk medulloblastoma: results from the randomized multicenter HIT-SIOP PNET 4 trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30:3187–93. doi: 10.1200/JCO.2011.39.8719. [DOI] [PubMed] [Google Scholar]
- 39.Stuben G, Stuschke M, Kroll M, Havers W, Sack H. Postoperative radiotherapy of spinal and intracranial ependymomas: analysis of prognostic factors. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 1997;45:3–10. doi: 10.1016/s0167-8140(97)00138-2. [DOI] [PubMed] [Google Scholar]
- 40.Leder K, Pitter K, Laplant Q, Hambardzumyan D, Ross BD, Chan TA, et al. Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell. 2014;156:603–16. doi: 10.1016/j.cell.2013.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pradhan AK, Nahar SN, Montenegro M, Yu Y, Zhang HL, Sur C, et al. Resonant X-ray enhancement of the Auger effect in high-Z atoms, molecules, and nanoparticles: potential biomedical applications. J Phys Chem A. 2009;113:12356–63. doi: 10.1021/jp904977z. [DOI] [PubMed] [Google Scholar]
- 42.Choi GH, Seo SJ, Kim KH, Kim HT, Park SH, Lim JH, et al. Photon activated therapy (PAT) using monochromatic Synchrotron x-rays and iron oxide nanoparticles in a mouse tumor model: feasibility study of PAT for the treatment of superficial malignancy. Radiat Oncol. 2012:7. doi: 10.1186/1748-717X-7-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antosh MP, Wijesinghe DD, Shrestha S, Lanou R, Huang YH, Hasselbacher T, et al. Enhancement of radiation effect on cancer cells by gold-pHLIP. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:5372–5376. doi: 10.1073/pnas.1501628112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sengupta S, Mantha AK, Song H, Roychoudhury S, Nath S, Ray S, et al. Elevated level of acetylation of APE1 in tumor cells modulates DNA damage repair. Oncotarget. 2016;7:75197–75209. doi: 10.18632/oncotarget.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]