Two faces of herbivory
Herbivory occurs when animals consume plants; but the term hides two fundamentally different processes. One relates to the animal’s nutrition, the other to the plant’s survival and abundance. Both are central to the ecological process called “herbivory.” Evolutionary innovations in herbivore function has shaped shallow marine ecosystems from kelp forests to coral reefs.
Brief history of herbivory
Until the last ~66 million years, all herbivores were invertebrates and all autotrophs were either photosynthetic bacteria or algae. Fossil and molecular evidence suggests algae evolved before the first metazoans capable of feeding on them. However, soon after the Cambrian “explosion” when animals with hard parts first evolved, herbivory began. Chitons (a marine mollusk) were among the earliest suspected herbivores followed by snails. Some trilobites could have been herbivores but their primitive mouthparts likely limited their foraging capacity. The earliest herbivores were slow moving with limited range. It is likely that microcrustaceans such as amphipods and marine polychaetes fed on algae or algal detritus but we have no clear record of that. Small body size, limited mobility and mouthpart constraints best explain the likely weak interactions and modest impacts on algae through the Paleozoic Era. Accordingly, there are no reported grazing trace fossils from herbivores of that era. The early evolution of fishes likely included algal grazers, but larger rasping or deep grazing herbivores appear absent, as evidenced by Late Paleozoic reefs constructed of thin, leafy calcareous algae that had the fragility of potato chips.
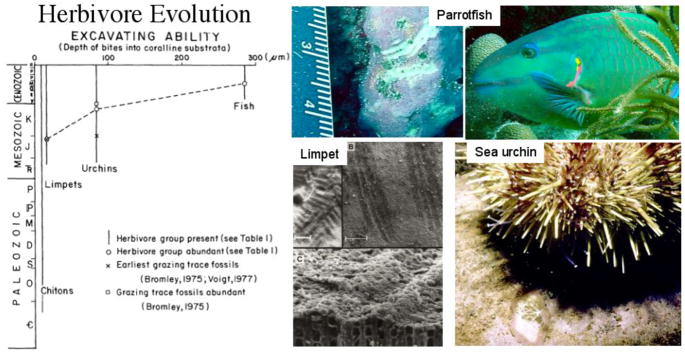
By mid-Jurassic, herbivory as an agent of disturbance, began escalating (Fig. 1). Sea urchins, had nearly disappeared during the end Permian mass extinction but surviving taxa radiated and functionally changed to become the first deep grazing marine herbivores. They were the first capable of biting deeply into calcium carbonate substrates and thus they could have been the first herbivore group capable of denuding the largest stands of macroalgae. Trace fossils of their distinctive star shaped bite marks first appeared in the fossil record during the Jurassic Period.
Fig. 1.
Evolution of deep-grazing herbivore groups since the Cambrian. The capacity to excavate calcareous coralline algae is taken as an indication of improved feeding capability. Left panel after Steneck 1983, Right photos include parrotfish bite marks, parrotfish, limpet bites (scale 0.5 mm) and sea urchin bites into calcareous algae (all photos by R. Steneck).
Sea urchins, although ubiquitous today, tend to thrive in shallow marine habitats devoid of urchin eating carnivores. Perhaps the best example of this comes from the Aleutian archipelago where urchins had been preferred food of sea otters until otters fell prey to humans in the 1700s to early 1900s. After a brief otter recovery, otter populations declined due to killer whale predation since the 1990s. Each time otters declined, sea urchin abundances increased and vast kelp forests were grazed to a barren state.
Sea urchin barrens are globally ubiquitous having been reported worldwide in areas where predator declines allow sea urchin population to escape predator control, resulting in urchin population explosions. It is possible that human fishing pressure on urchin predators has created conditions allowing a degree of sea urchin dominance not seen since the Mesozoic Era.
The rise of vertebrate herbivores: fishes, modern coral reefs, and Darwin’s Paradox
By far the greatest escalation of marine herbivores occurred with the diversification of advanced fishes during the early Cenozoic. The Eocene Epoch beginning 56 million years ago, contained several plant-eating fish families. During the early Cenozoic other vertebrate herbivores such as turtles, manatees and dugongs also evolved in the marine realm. Perhaps the pinnacle of dugong evolution was the giant (10 m) Steller’s sea cow that fed exclusively on kelp and algae along North Pacific shorelines. It went extinct less than 30 years after being discovered by Steller in the 1700s. Most other vertebrate herbivores feed on angiosperm sea grasses (which also evolved in the early Cenozoic). Most dugongs, manatees and herbivorous turtles were heavily hunted and persist today at population densities that are a fraction of their past densities.
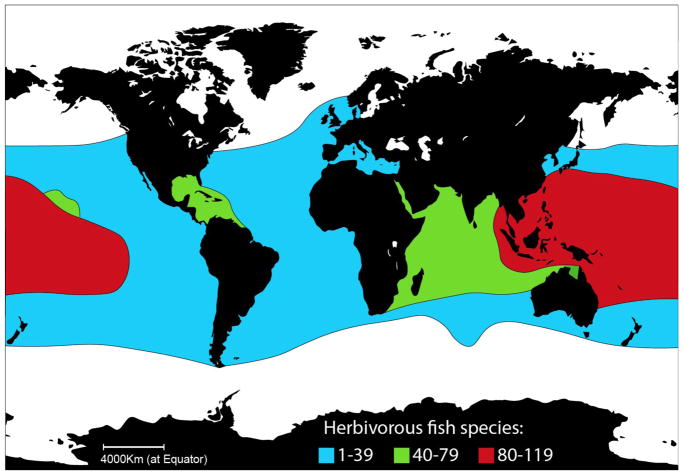
The Eocene ushered in the era of fish-based herbivory in marine systems. When we think of this herbivory, the immediate picture is one of colorful parrotfishes or surgeonfishes grazing on coral reefs. However, herbivory by fishes is not just a tropical phenomenon. Fishes eat plant material from the temperate Atlantic coasts in the north to the Sub-Antarctic islands in the south (Fig. 2). However, it is the diversity, intensity and frequency of fish grazing that sets the tropics apart. Nowhere on earth is fish-based grazing as diverse or intense as on coral reefs, where parrotfishes, surgeonfishes, rabbitfishes, and others graze algae down to a highly productive but diminutive 3mm high carpet of filamentous algae called “turfs”. Algal turfs are the basis of most primary productivity in tropical reef systems.
Fig 2.
Global distribution of species richness among modern herbivorous fishes.
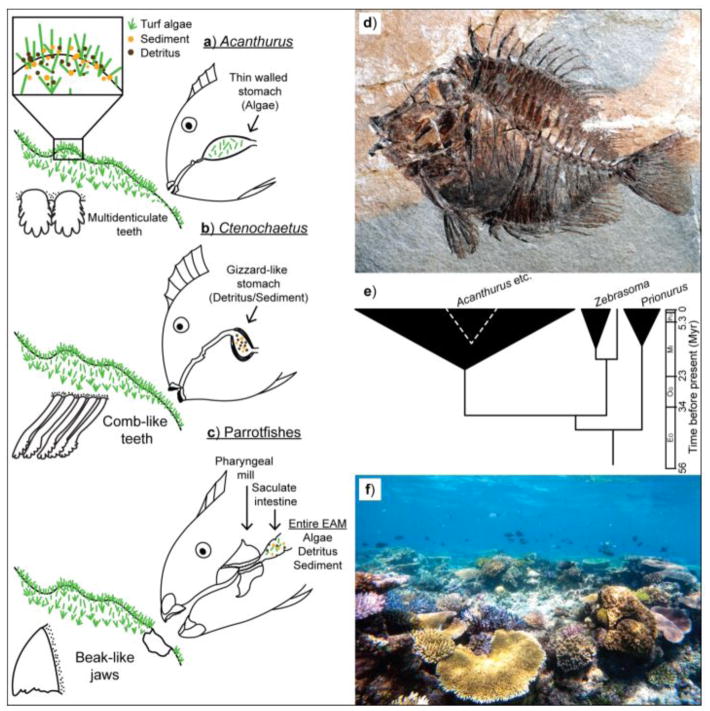
In the early Eocene, surgeonfishes and rabbitfishes were the first herbivorous fishes capable of rapidly denuding algae; including both larger fleshy algae and short algal turfs (Fig. 3). Because of the mobility and agility of modern fishes (for the first time they can hover, bite, and suck benthic habitats), algae lost their traditional refuges. Unlike sea urchins, fishes can move rapidly, graze three-dimensional surfaces, and consume algae with great efficiency. This efficiency is due in large part to the evolutionary versatility of the mouthparts of grazing fishes. Parrotfishes evolved fused parrot-like “beaks” that excavate calcium carbonate coral substrates and they have a secondary grinding structure in their “throat” to pulverize the reef rock they ingest (Figs. 1,3).
Fig. 3.
Feeding capabilities and diversification of selected taxa of herbivorous fishes. Anatomically different feeding apparatus of Acanthurus surgeonfishes (a) which typically remove algae to be processed in an acidic thin-walled stomach, to the closely-related Ctenochaetus (b) which selectively removes fine particles that are ground in a gizzard-like stomach and parrotfishes (c) which excavate the reef rock along with epilithic and endolithic algal turf (with all its constituents) to be triturated in a pharyngeal mill. Surgeonfishes (Acanthurus spp) (d) evolved in the Eocene 50Ma but diversified explosively in the Miocene revolutionizing herbivory and benthic interactions (e) leading to the heavily grazed reefs we see today (f). (d) and 9e) modified after Bellwood et al 2016.
Modern reef fishes have had two major evolutionary phases since the end of the Mesozoic. In the early Cenozoic, carnivorous reef fishes were relatively large, feeding on large prey, including herbivores. In the Miocene (23 MYBP) most of the world was relatively warm and reef ecosystems underwent a dramatic transformation. Shallow marine habitats became dominated by smaller fishes and herbivory by fishes changed fundamentally. With the explosive evolution of parrotfishes and surgeonfishes that remove algae from reef surfaces, there also came a new but inconspicuous source of organic and nitrogen-rich material from a matrix of fine filamentous algae, detritus and bacteria. Thus, these “herbivorous” fishes are microphages or detritivores from a nutritional perspective but herbivores from an ecological perspective as biogenic agents of disturbance that crop algae. This distinction is important because by consuming this algal matrix, including particulate material such as tiny copepods, these post-Miocene fishes are eating food that was traditionally consumed by invertebrates. Rather than fishes eating invertebrates that eat this matrix, fishes are eating the energy rich benthic particulates themselves. Cutting out the middle-man and opening up a new world for high intensity, high-energy herbivory.
This explosion of new herbivores in the Miocene (Fig. 3) appears to have coincided with the formation of modern coral reefs, and the evidence suggests that this intense grazing may have permitted fishes to move into shallow waters (following productive algal turfs), with intense grazing opening up new areas of shallow water for colonization by grazing resistant organisms such as calcareous algae (Fig. 1) and corals. Thus, the proliferation of shallow-water corals and the formation and growth of coral reefs may be a direct consequence of escalation in herbivore feeding capabilities.
Charles Darwin was struck by how many fish inhabit coral reefs given the apparent absence of food. This has been called “Darwin’s Paradox”. Research found that increasing grazing frequency selected for diminutive algae with high mass-specific productivity. Thus, as herbivores improved their efficiency, algal biomass declined, but mass-specific productivity increased (i.e., higher productivity to biomass P/B ratios). The turf algal matrix including nitrogen-fixing cyanobacteria creates a microecosystem within its 2 – 3 mm canopy. Extremely high rates of consumption are met with extremely high rates of productivity. Darwin was looking for biomass sufficient to support all the reef fish but he failed to realize it was the high productivity of this ecosystem that supported the fish he described while sailing on the Beagle.
Living with the enemy: diffuse coevolution that allows algae to coexist with herbivores
The evolution of rapidly moving fishes with powerful mouthparts and high metabolic rates produced tremendous selection for seaweeds to tolerate, escape, or deter herbivores. Succeeding at any one of these strategies lowers herbivore impact tremendously and prevents seaweeds from being driven to local extinction. Tolerance involves persisting despite heavy herbivory by minimizing the suppression of seaweed fitness that occurs when parts are consumed. As an example, small turf algae persist via basal sections in cracks and crevices that regenerate following herbivory, and by having spores or vegetative parts that survive gut passage, with herbivores acting as “seed” dispersers for these species.
Rather than tolerating herbivory, some seaweeds escape in space or time by growing in seasons or areas where herbivores are rare due to physical stresses or fear of attack by predators. Unstructured sand-plains or shallow reef flats without topographic complexity serve as spatial escapes for seaweeds because herbivores rarely forage there due to exposure to their own predators in these unstructured habitats. These spatial escapes can occur due to complex interactions among physical processes (tidal height), predator behavior, and herbivore fear. On Pacific reef-flats subject to appreciable tides, palatable seaweeds commonly occur on the upper portions of patch reefs. Herbivorous fishes could browse these seaweeds during high tide but do not. These fishes feed primarily at low tide when the reefs are too shallow for large predators like sharks; when large predators invade the reef at high tide, herbivorous fishes suppress feeding in favor of increased vigilance. Thus, fear of consumers produces an escape for palatable seaweeds.
To co-occur with herbivores, most seaweeds must be defended. Morphological and structural defenses may work against some herbivores (e.g. Fig. 1), but once fishes evolved the strong feeding abilities discussed above, seaweeds were selected to evolve chemical defenses, such as the great variety of bioactive terpenoids and phlorotannins that defend seaweeds from numerous consumers. That herbivorous fishes in the tropics are important agents selecting for seaweed chemical defenses is consistent with the more potent chemical defenses there compared to temperate seaweeds. Similarly, because more chemically defended seaweeds surround tropical herbivores, tropical herbivores are more resistant to seaweed defenses than are temperate herbivores.
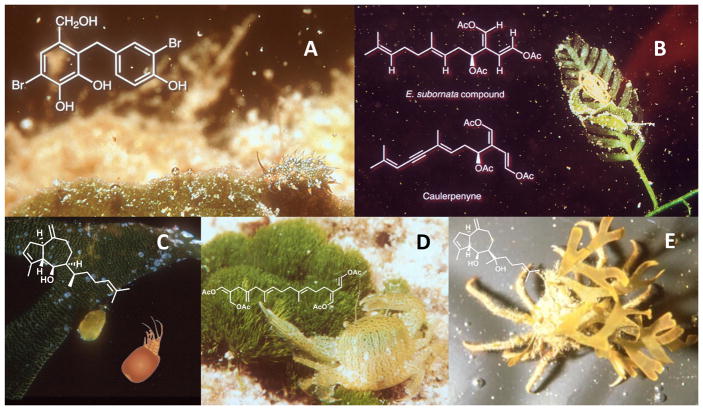
Larger generalist herbivores like fishes, view seaweeds primarily as food and are mobile enough to scour a reef in search of the best seaweeds. In contrast, small, less mobile herbivores such as tube-building amphipods or polychaetes, and many small crabs and gastropods (mesograzers) must view seaweeds as both foods and habitats because they commonly live on the alga they consume. Living on seaweeds palatable to herbivorous fishes is not a wise survival strategy. Thus, once seaweeds are well defended from fishes, they become an “evolutionary opportunity” for mesograzers because they represent both safe living spaces and food, if the mesograzers can evolve tolerance to the defense – many have. Numerous mesograzers (amphipods, isopods, polychaetes, crabs, sea slugs and other gastropods) selectively consume toxic seaweeds (Fig 4). Mesograzers evolve onto toxic seaweeds to escape predation, not due to the nutritional value of these foods.
Fig. 4.
Small herbivores that deter enemies using toxic hosts. A) The sea slug Costasiella ocellifera eats only the green alga Avrainvillea longicaulis, sequesters the compound shown, and is therefore rejected by fish predators. B) A similar interaction for the sea slug Elysia sp. It consumes Caulerpa sp., sequesters and converts its compound for storage and protection of itself and its eggs (the yellow mass on the alga). C) The amphipod Pseudamphithoides incurvaria creates a domicile from its algal host (Dictyota). Pachydictyol-A cues this behavior and deters fishes from consuming the amphipod. The drawing shows the amphipod more clearly. D) The crab Caphyra rotundifrons lives in and consumes only the green alga Chlorodesmis fastigiata, which is protected from herbivorous fishes by the cytotoxic compound chlorodesmin. Chlorodesmin, deters fish feeding but stimulates feeding by the crab. E) The crab Libinia dubia decorates with the chemically-defended alga Dictyota. Dictyot-E from the alga cues decoration and deters fishes from consuming the crab.
Seaweed defenses were initially interpreted as arising due to selection from large generalist herbivores, with mesograzers representing minimal selection pressure. However, there is accumulating evidence that seaweed traits are shaped by mesograzers as well. As examples, several seaweeds induce increased chemical defenses in response to mesograzer feeding, and some can sense the branches on which mesograzers are feeding and drop those branches to rid themselves of mesograzers.
Some seaweeds employ multiple defensive strategies and may vary these with the identity of the herbivore. Green seaweeds in the genus Halimeda are heavily calcified. They produce their new, and most vulnerable tissues at night when herbivorous fishes are sleeping. These segments contain a suite of noxious chemicals that convert to even more potent forms once the plant is bitten. Other seaweeds, use chemical cues to sense attacks on nearby conspecifics; they can identify the attacking herbivore, and upregulate the appropriate chemical defense effective against that particular consumer. Thus, like nations, seaweeds have developed early warning systems that detect impending attack.
The variance in effectiveness of seaweed chemical defense against generalist fishes versus mesograzers is less consistent among herbivorous fishes, but does occur. Chemically noxious seaweeds are avoided by many herbivorous fishes but eaten selectively by others. The green alga Chlorodesmis fastigiata produces cytotoxic terpenoids that deter most fishes, but the rabbitfish Siganus argenteus targets and selectively consumes this seaweed. Several chemically noxious seaweeds appear to be eaten by only one, or a few, fishes. Mechanisms generating this feeding variance are unknown, but could involve evolved tolerance, unique microbial symbionts that degrade these algal toxins, or other mechanisms. This variance in feeding generates a critical function for herbivore biodiversity. Without a biodiverse mix of herbivores on reefs, some defended seaweeds might escape herbivore control, dominate the reef, suppress corals, and make the reef less habitable for the many herbivores that cannot consume this seaweed. Thus, herbivores that compete for some, but not all, foods may be dependent on their competitors to keep reefs devoid of chemically defended seaweeds thereby facilitating corals and indirectly fishes that depend on the topographic complexity that these foundation species create.
Herbivory collides with the Anthropocene
Evolved diversification and improvements in the feeding capabilities of marine herbivores over millions of years functionally increased their capacity to denude shallow coastal zones of marine algae. The bigger picture of course involves entire food webs. Fishing pressure in the tropics has shifted from preferred carnivores such as snappers and groupers to parrotfishes and surgeonfishes, thus releasing reef algae from herbivore control. As a result, most coral reefs in the Caribbean are now more accurately algal reefs.
A few herbivore groups such as tropical fishes and ubiquitous sea urchins can be strong interactors that drive inverse relationships between herbivore abundance and their algal prey. The collateral impacts include less conspicuous organisms that require algae for food or habitat (Fig. 4).
While herbivorous fishes are most diverse and important in the tropics, they decline in impact towards colder water environments nearer the poles (Fig. 2). However, as our planet warms, tropical herbivorous fishes are invading areas farther from the equator than ever reported before. Today there are a few examples of “tropicalization” and denuding of kelp forests by newly arrived herbivorous fishes. Clearly, marine herbivory today is shaped by human activities like never before.
Human hunting and fishing pressure is disrupting food webs and plant-herbivore interactions. In relatively predator free environments where herbivorous fishes are rare or absent (Fig. 2), sea urchin populations rise to dominance (e.g. the sea otter example above). Often such locations became targeted for sea urchin harvesting, resulting in the loss of urchins and a return to algal dominance.
In Maine, USA, extirpation of almost all predatory fishes such as cod allowed sea urchins to proliferate and deforest kelp and other bushy seaweeds. However, the sea urchin fishery removed enough urchins to allow kelp forests and seaweeds to return. Prolific bushy algae then became a nursery habitat for juvenile crabs. In this predator free environment abundant adult crabs ate virtually all the sea urchins. As a result, Maine’s coastal ecosystem quickly flipped and locked into an alternative herbivore-free state for the past several decades.
All of this illustrates how herbivores shaped shallow marine ecosystems. It also suggests how humans in the Anthropocene may be pushing reefs back to conditions typical of the mid-Jurassic and in the last example, possibly back to the macroherbivore-free Paleozoic.
Contributor Information
Robert S. Steneck, Professor of Marine Biology, Oceanography and Marine Policy, School of Marine Sciences, University of Maine, Darling Marine Center, Walpole, Maine
David R. Bellwood, Distinguished Professor of Marine Biology, College of Science and Engineering & ARC Centre of Excellence for Coral Reef Studies, James Cook University, Townsville, Qld. 4811, AUSTRALIA.
Mark E. Hay, Teasley and Regents’ Professor, School of Biology, Georgia Institute of Technology, Atlanta, GA 30332-0230
Additional Readings
- Andrew N, Agatsuma Y, Ballesteros E, Bazhin A, Creaser E, Barnes D, Botsford L, Bradbury A, Campbell A, Dixon J, et al. Status and management of world sea urchin fisheries. Oceanography and Marine Biology-An Annual Review. 2003;40:343–425. [Google Scholar]
- Bellwood DR, Goatley CHR, Bellwood O. The evolution of fishes and corals on reefs: form, function and interdependence. Biol Rev. 2016 doi: 10.1111/brv.12259. [DOI] [PubMed] [Google Scholar]
- Bellwood DR, Goatley CHR, Brandl SJ, Bellwood O. Fifty million years of herbivory on coral reefs: fossils, fish and functional innovations. Proc R Soc B Biol Sci. 2014;281:20133046. doi: 10.1098/rspb.2013.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkepile DE, Hay ME. Herbivore species richness and feeding complementarity affect community structure and function on a coral reef. Proceedings of the National Academy of Sciences, USA. 2008;105:16201–16206. doi: 10.1073/pnas.0801946105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements KD, German DP, Piché J, Tribollet AD, Howard Choat J. Integrating ecological roles and trophic resources on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc. 2016 doi: 10.1111/bij.12914. [DOI] [Google Scholar]
- Filbee-Dexter K, Scheibling RE. Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Mar Ecol Prog Ser. 2014;49 doi: 10.3354/meps10573. [DOI] [Google Scholar]
- Harborne AR, Renaud PG, Tyler EH, Mumby PJ. Reduced density of the herbivorous urchin Diadema antillarum inside a Caribbean marine reserve linked to increased predation pressure by fishes. Coral Reefs. 2009;28:783–791. [Google Scholar]
- Hay ME. Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Annual Review of Marine Science. 2009;1:193–212. doi: 10.1146/annurev.marine.010908.163708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore AGB, Campbell AH, Coleman RA, Edgar GJ, Jormalainen V, Reynolds PL, Sotka EE, Stachowicz JJ, Taylor RB, Vanderklift MA, Duffy JE. Global patterns in the impact of marine herbivores on benthic primary producers. Ecology Letters 15: 912–922.populations, communities, and ecosystems. Annual Review of Marine Sciences. 2012;1:193–212. doi: 10.1111/j.1461-0248.2012.01804.x. [DOI] [PubMed] [Google Scholar]
- Rasher DB, Stout EP, Engel S, Shearer TL, Kubanek J, Hay ME. Marine and terrestrial herbivores display convergent chemical ecology despite 400 million years of independent evolution. Proceedings of the National Academy of Sciences. 2015;112:12110–12115. doi: 10.1073/pnas.1508133112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steneck RS. Escalating herbivory and resulting adaptive trends in calcareous algae. Paleobiology. 1983;9:45–63. [Google Scholar]
- Steneck RS. Sea urchins as drivers of shallow benthic marine community structure. In: Lawrence JM, editor. Sea Urchins: Biology and Ecology. Ch. 14. Elsevier B. V; 2013. [Google Scholar]
- Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ. Kelp forest ecosystem: biodiversity, stability, resilience and their future. Environmental Conservation. 2002;29(4):436–459. [Google Scholar]
- Steneck RS, Leland A, McNaught DC, Vavrinec J. Ecosystem flips, locks, and feedbacks: the lasting effects of fisheries on Maine’s kelp forest ecosystem. Bulletin of Marine Science. 2013;89(1):31–55. [Google Scholar]
- Verges A, Doropoulos C, Malcolm HA, Skye M, Garcia-Piza M, Marzinelli EM, Campbell A, Ballesteros HE, Hoey AS, Vila-Concejo A, Bozec Y, Steinberg PD. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. 2016 doi: 10.1073/pnas.1610725113. PNAS.org/cgi/doi/10.1073/pnas.1610725113. [DOI] [PMC free article] [PubMed]