SUMMARY
Setting
A high proportion of individuals with multi-drug-resistant tuberculosis (MDR-TB) develop permanent hearing loss due to ototoxicity caused by injectable aminoglycosides (AGs). The prevalence of AG-induced hearing loss is greatest in tuberculosis (TB) and human immunodeficiency virus (HIV) endemic countries in sub-Saharan Africa. However, whether HIV coinfection is associated with a higher incidence of AG-induced hearing loss during MDR-TB treatment is controversial.
Objective
To evaluate the impact of HIV coinfection on AG-induced hearing loss among individuals with MDR-TB in sub-Saharan Africa.
Design
This was a meta-analysis of articles published in PubMed, Embase, Scopus, Cumulative Index to Nursing and Allied Health Literature, Web of Science, Cochrane Review, and reference lists using search terms ‘hearing loss’, ‘aminoglycoside’, and ‘sub-Saharan Africa’.
Results
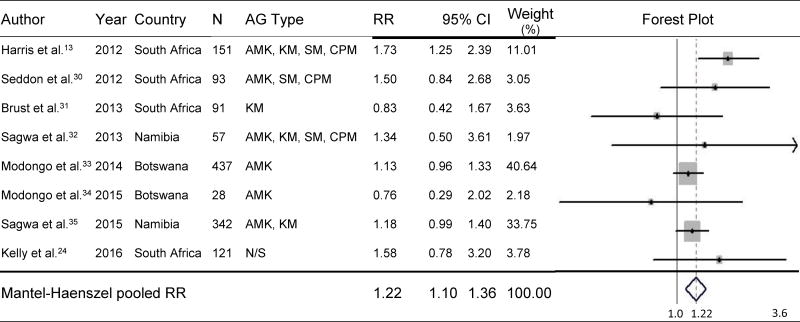
Eight studies conducted in South Africa, Botswana and Namibia and published between 2012 and 2016 were included. As the included studies were homogeneous (χ2=8.84, d.f.=7), a fixed-effects model was used. Individuals with MDR-TB and HIV coinfection had a 22% higher risk of developing AG-induced hearing loss than non-HIV-infected individuals (pooled relative risk=1.22; 95% CI=1.10–1.36) during MDR-TB treatment.
Conclusion
This finding is critical for TB programs with regard to the expansion of injectable-sparing regimens. Our findings lend credibility to using inject- able-sparing regimens and more frequent hearing monitoring, particularly in resource-limited settings for HIV-coinfected individuals.
Keywords: ototoxicity, sub-Saharan Africa, meta-analysis
INTRODUCTION
Multidrug-resistant Tuberculosis (MDR-TB), defined as TB resistant to at least isoniazid and rifampicin, is a global health emergency. MDR-TB treatment is prolonged (9–24 months), poorly efficacious (<50% treatment success), poorly tolerated and quite toxic.1,2 Despite advances in injectable-sparing regimens, the mainstay of MDR-TB treatment contains one second-line injectable, an aminoglycoside (AG), for at least 4 months in combination with four oral drugs.2 AGs include amikacin (AMK), kanamycin (KM), and streptomycin (SM), or the mechanistically similar cyclic peptide antibiotic, capreomycin (CPM).3 One of the main adverse reactions from AGs is sensorineural ototoxicity: SM is mainly vestibulotoxic, causing dizziness, ataxia, or nystagmus; AMK, KM, and CPM are predominantly cochleotoxic, resulting in tinnitus or hearing loss.4
AG-induced hearing loss begins at high frequencies, can progress even with AG discontinuation, and is permanent unless quickly identified.4 Hearing loss leads to social isolation, reduced quality of life, and threatens employment stability and family prosperity.5,6 The risk of AG-induced hearing loss may be impacted by human immunodeficiency virus (HIV) coinfection. Although the exact mechanism of AG ototoxicity is not known, it has been hypothesized that excessive AG accumulation in the inner ear catalyzes the formation of reactive oxygen species (ROS).7,8 When ROS formation overwhelms the capacity of the intrinsic protective and repair system, the sensory hair cells undergo apoptotic death, resulting in irreversible hearing loss.4,9 As chronic immune activation in HIV coinfection triggers massive ROS formation, people living with HIV (PLHIV), particularly those who are antiretroviral therapy (ART) naïve, may be more vulnerable to AG ototoxicity.10,11
Paradoxically, HIV treatment may also be associated with an increased risk of ototoxicity. Nucleoside reverse transcriptase inhibitors (NRTIs), a class of ART drugs, are mitochondrial-toxic, and cause mitochondrial damage in outer hair cells.12,13 Moreover, one NRTI, tenofovir disoproxil fumarate, is also nephrotoxic, and can compound AG-induced ototoxicity, as AGs are eliminated through the kidneys.12,13 Poly-pharmacy is common in MDR-TB and HIV treatment, with additional medications added to manage opportunistic infections or adverse drug reactions.14 This complexity may result in additional drug-drug interactions, pill fatigue and resultant non-adherence, or drug-induced renal impairment, any of which can affect the risk of ototoxicity.15
People in resource-limited settings are more likely to be at high risk for AG ototoxicity. Protein-energy malnutrition caused by insufficient intake of protein and calories is prominent in sub-Saharan Africa due to food insecurity.16,17 In the case of protein-energy malnutrition, albumin synthesis is impaired and changes in oncotic pressure lead to abnormal accumulation of fluid in the interstitium of hair cells,18,19 thereby worsening AG ototoxicity because AG is water-soluble.20 Furthermore, a dietary deficiency of protein and calories reduces the synthesis of antioxidant enzymes and antioxidant concentrations, leading to ROS overproduction.19,21 Due to the financial costs involved in frequent audiological assessment or therapeutic drug monitoring (i.e., daily blood tests for AG concentration), early detection of hearing loss is impractical in most sub-Saharan African countries, which leads to missed opportunities to prevent hearing loss.1,22
Despite these known risks, whether HIV coinfection leads to a higher incidence of AG-induced hearing loss during MDR-TB treatment is controversial. The objective of the present study was to systematically review the literature and estimate the effect size of the association between HIV coinfection and AG-induced hearing loss among MDR-TB-infected individuals in sub-Saharan Africa.
METHODS
The review process was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards.23 Institutional review board approval was not required for this meta-analysis.
Inclusion/exclusion criteria
The inclusion criteria for participants were: 1) known or presumptive TB with isoniazid resistance, rifampicin resistance, or MDR-TB on microbiologic tests (determined either on culture with drug susceptibility testing or using cartridge-based Xpert® MTB/RIF; Cepheid, Sunnyvale, CA, USA), and 2) use of second-line injectable anti-tuberculosis drugs (AMK, KM, SM, or CPM). Hearing loss in study participants should have been observed either prospectively or retrospectively during and/or after treatment with injectables. All ages and both sexes were included in our analyses.
The following diagnoses of AG-induced hearing loss were accepted: 1) audiometric hearing loss, defined as worsening of hearing threshold confirmed using audiometry; 2) self-reported hearing loss, defined as symptomatic hearing loss reported by patients after AG initiation; and 3) clinician-identified hearing loss, diagnosed by clinicians in the absence of audiometry. In our analysis, a broader definition of AG-induced hearing loss was accepted because regular audiological assessments are rarely conducted in many sub-Saharan African countries due to the shortage of trained audiologists or testing equipment. This definition of hearing loss was supported by a recent study that concluded that patient self-report of hearing loss was highly concordant with clinician-identified hearing loss in the setting of monthly audiological testing.24 Only studies written in English were included.
Studies were excluded if they did not include participants’ HIV status as a study variable. We also excluded studies if full-text versions were not available (e.g., conference abstracts), if the study did not have a quantitative design, or if studies reported the protocol only with no measured outcomes.
Search and selection process
PubMed, Embase, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Scopus, Web of Science, and Cochrane Review were searched using the following MeSH terms: ‘hearing loss’, ‘aminoglycosides’ and ‘Africa South of the Sahara’. Our initial search was not limited by the year of publication. Electronic searches were supplemented by manual searches of references found in identified articles and bibliographies.
Our initial database search, conducted on 19 December 2016, resulted in 367 citations. After removing duplicates, 79 titles with abstracts were reviewed for relevance by HH. Twenty-one articles were passed onto the next full-text review process. Of the 12 full-text articles that were selected by HH and confirmed by CB, six studies reporting the number of participants who developed AG-induced hearing loss and their baseline HIV coinfection status provided useful data for a meta-analysis. We contacted the six corresponding authors of the eligible studies to request unpublished descriptive statistical data to calculate the cumulative incidence of hearing loss and prevalence of HIV coinfection; of these, two authors provided the requested data, which were finally added to the study data set on 10 July 2017. Eight studies were included in our analysis; four studies were excluded due to lack of useful data required for a meta-analysis (Appendix Figure A.1).
Data quality assessment
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used to evaluate the quality of the original studies.25 Three main themes were evaluated: selection of samples (four items), comparability of cohorts (one item), and ascertainment of outcome (three items).
In this meta-analysis, comparability was assessed as to whether the original studies isolated conductive hearing loss (e.g., cerumen impaction or middle ear infection) using otoscopy or tympanometry, because AG mainly causes cochlear-toxic sensorineural hearing loss. One point was awarded for each quality item; a total of eight points thus indicated the highest quality. In general, as positive findings are more likely to be published, we also tested for publication bias to estimate the possibility of distortion of synthesized meta-analysis results.26
Statistical analysis
Cumulative incidence (absolute risk, i.e., the total number of events divided by the total number of people at risk) of each study was initially calculated because of the different follow-up durations and formats used to measure events across studies.27 Heterogeneity was tested using Cochran’s Q statistic, along with summary estimates using the metan command. Due to non-significance of heterogeneity (χ2 =8.84, d.f.=7, p=0.26; I2= 20.9%), which suggested that the differences between the studies were explicable by random variation,28 we used the Mantel-Haenszel fixed-effects method with the metan command in Stata/IC 14 (StataCorp, College Station, TX, USA) to combine the different results and obtain a pooled estimate of the effect size.28,29 The cumulative incidence ratio (relative risk [RR]) was used as a pooled measure of association to interpret the synthesized impact of the prevalence of HIV coinfection on the risk of AG-induced hearing loss, with variance presented by 95% confidence intervals (CIs). The funnel plot—a graphic plot to diagnose publication bias and other small-study effects (the tendency for smaller studies in a meta-analysis to show larger treatment effects)—was used using the funnel command.28,29
RESULTS
Overview of studies included in the meta-analysis
This meta-analysis comprised eight studies that met the inclusion and exclusion criteria (Table). All eight studies were published between 2012 and 2016.13,24,30–35 Most were prospective and retrospective cohort studies; one study retrospectively collected study outcomes from medical records and then compared these to cross-sectional patient interview outcomes.24 The studies were conducted in specialist TB hospitals (n=7)13,24,30,32–35 and community-based HIV-TB clinical settings (n=1).31 Seven studies had a cohort sample of adults aged ≥14 years;13,24,31–35 only one study had a sample of children aged <15 years.30 Sample size was between 50 and 99 individuals in four studies,30–32,34 between 100 and 299 in two studies,13,24 and >300 in two studies.33,35 All studies were conducted in southern Africa: four studies were conducted in South Africa,13,24,30,31 two in Botswana33,34 and two in Namibia.32,35 NOS scores ranged between 5 and 8; the mean was 6.75 out of 8.
Table.
Descriptive Analysis of Included Studies
| Author, Year (Country) |
Design (NOS score), Sample Size, Age |
Study Purpose | Diagnostic Methods of HL |
Absolute risk of HL |
HIV Prev. |
ART Status |
Type of AGs (%) |
Major Findings |
|---|---|---|---|---|---|---|---|---|
| Harris et al.13 2012 (South Africa) | Prospective cohort (8) N= 153 Adults [range=14–70y] |
To document the incidence of ototoxicity in MDR-TB patients with and without HIV, and develop clinical guidelines relating to ototoxicity in such patients |
|
87/153 (57%) | 86/153 (56%) | 86/86 (100%) | AMK(1), KM (94), SM(4), CPM(1) |
|
| Seddon et al.30 2012 (South Africa) | Prospective cohort (8) N=93 (Confirmed MDR-TB n= 50) Children [IQR=20–110m] |
To determine the extent of hearing loss in children treated for MDR-TB |
|
23/93 (24%) | 28/93 (30%) | 20/28 (71%) | AMK(88), SM(10), CPM(1) |
|
| Brust et al.31 2013 (South Africa) | Retrospective cohort
(7) N=89 Adults [IQR= 29–41y] |
To examine the frequency and severity of AEs in patients with MDR-TB and HIV coinfection treated at an integrated MDR-TB/HIV home-based treatment program |
|
31/89 (34%) | 76/89 (84%) | 66/76 (87%) | KM (100) |
|
|
24/35 (69%) | |||||||
| Sagwa et al.32 2013 (Namibia) | Retrospective cohort
(6) N=57 No age restriction [range= 11–55y] |
To compare the absolute risks and risk factors for commonly observed adverse events (occurring in >20 % of patients) during DR-TB treatment in HIV-infected and HIV-uninfected patients. |
|
13/57 (23%) | 31/57 (54%) | 13/31 (42%) | AMK(36), KM(51), SM(5), CPM(7) |
|
| Modongo et al.33 2014 (Botswana) | Retrospective cohort
(7) N=437 Adults [IQR= 31–49y] |
To determine the effect of amikacin on treatment outcomes and development of hearing loss in MDR-TB patients |
|
270/437 (62%) | 288/437 (66%) | 267/288 (93%) | AMK(100) |
|
|
147/437 (34%) | |||||||
|
123/437 (28%) | |||||||
| Modongo et al.34 2015 (Botswana) | Retrospective cohort
(6) N=28 Adult [mean(SD)= 44y(18)] |
To identify clinical factors, including amikacin concentration thresholds that predicted audiometry-confirmed ototoxicity among MDR pulmonary TB patients |
|
11/28 (39%) | 12/28 (43%) | 12/12 (100%) | AMK(100) |
|
|
7/28 (25%) | |||||||
| Sagwa et al.35 2015 (Namibia) | Retrospective cohort
(7) N=353 No age restriction [mean (SD)= 35.69y (9.56) in Am; 36.47y (11.57) in Km group] |
To compare the cumulative incidence of hearing loss among patients treated for MDR-TB with amikacin or kanamycin-based regimens, and to identify the most-at-risk patients, based on the real-life clinical practice experiences |
|
206/353 (58%) | 164/353 (46%) | 132/164 (80%) | AMK(14), KM(86) |
|
| Kelly et al.24 2016 (South Africa) | Retrospective cohort +
cross-sectional (5) N=121 Adults [range=17–63y] |
To describe concordance between patient report and clinician documentation of ADR from MDR-TB treatment |
|
39/121 (32%) | 90/121 (74%) | 79/90 (88%) | N/S |
|
|
32/121 (26%) |
NOS=Newcastle-Ottawa Quality Assessment Scale; HIV=human immunodeficiency virus; ART=antiretroviral therapy; AG=aminoglycoside; MDR-TB=multidrug-resistant tuberculosis; PTA=pure tone audiometry; AMK=amikacin; KM=kanamycin; SM=streptomycin; CPM=capreomycin; IQR=interquartile range; DPOAE=distortion product otoacoustic emissions; AE=adverse effect; DR-TB=drug-resistant tuberculosis; aOR=adjusted OR; CI=confidence interval; SD=standard deviation; AUC=area under the curve; OR=odds ratio; ADR=adverse drug reaction.
The outcomes of hearing loss diagnosis were categorized by audiometric hearing loss (n=3)13,30,35 and composite hearing loss, including both clinician-identified hearing loss confirmed using audiometry (n=4)24,31,33,34 and self-reported hearing loss (n=1).32 Audiometric hearing loss was assessed using either pure tone audiometry in adults and children aged >7 years or distortion product otoacoustic emissions in children aged <6 years.30 Of the five studies that used audiometry testing of both air and bone conductions to diagnose drug-induced sensorineural hearing loss, only two studies confirmed and differentiated conductive hearing loss by assessing outer and middle ears through tympanometry or otoscopy.13,30 Finally, the risk of hearing loss during injectable anti-tuberculosis treatment ranged from 23% to 69%. The prevalence of HIV coinfection at TB treatment initiation ranged from 30% to 83%.
Effect of human immunodeficiency virus coinfection on aminoglycoside-induced hearing loss
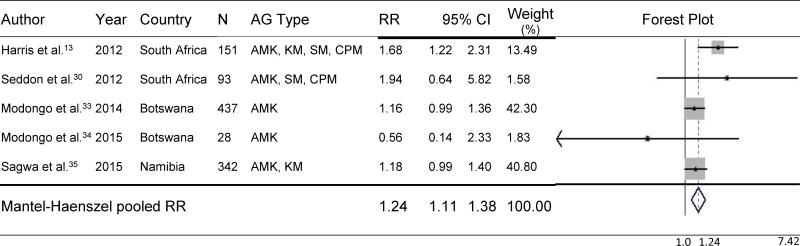
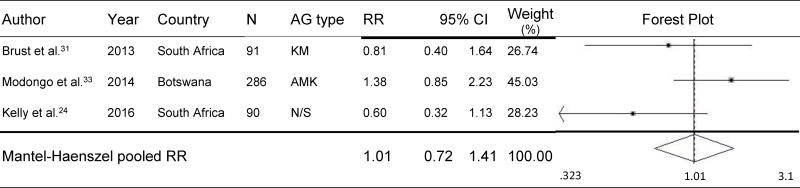
MDR-TB and HIV-coinfected individuals had a 22% higher risk of developing AG-induced hearing loss than non-HIV-infected individuals (pooled RR=1.22, 95% CI=1.10–1.36, p<.001) during MDR-TB treatment (Figure 1).13,24,30–35 No significant differences were found in subgroup analysis of studies for which audiometric hearing loss data were available (n=5). Such analyses demonstrated that the risk of hearing loss was 24% higher among HIV-coinfected individuals than among non-HIV-infected individuals (pooled RR=1.24, 95% CI=1.11–1.38, p<.001) (Figure 2). As shown in Figure 3, three studies compared the effect of participants’ ART status on AG-induced hearing loss, although the type of ART was not specified; the risk of developing AG-induced hearing loss did not differ, regardless of ART status in PLHIV (pooled RR=1.01, 95% CI=0.72–1.41, p=0.97).24,31,33 Baseline CD4 count was available from only one study, and patients whose baseline CD4 count was <200 cells/mm3 did not have a significantly increased risk of hearing loss compared with those with a baseline CD4 count ≥200 cells/mm3 (RR=1.16, 95% CI=0.95–1.42, p=0.15).33
Figure 1.
Effect of HIV Coinfection on Risk of AG-Induced Hearing Loss
HIV=human immunodeficiency virus; N=sample size; AG=aminoglycoside; RR=relative risk; CI=confidence interval; AMK=amikacin; KM=kanamycin; SM=streptomycin; CPM=capreomycin; N/S= Not specified.
Figure 2.
Effect of HIV Coinfection on Risk of AG-Induced Hearing Loss Confirmed by Audiometry
HIV=human immunodeficiency virus; N=sample size; AG=aminoglycoside; RR=relative risk; CI=confidence interval; AMK=amikacin; KM=kanamycin; SM=streptomycin; CPM=capreomycin.
Figure 3.
Effect of ART status on Risk of AG-induced Hearing Loss
ART=antiretroviral therapy; N=sample size; AG=aminoglycoside; RR=relative risk; CI=confidence interval; KM=kanamycin; AMK=amikacin; N/S= Not specified.
Publication bias
The asymmetric distribution funnel plots suggested some visual evidence of publication bias (Appendix Figure A.2); however, the effect size of AG-induced hearing loss was considered to be small. The homogeneity from Q statistics and significant P values for effect size supported the characteristics of stability, suggesting reasonably low levels of publication bias.
DISCUSSION
Questions are frequently raised about the risk of treatment-induced hearing loss. However, few studies have focused on the factors that might result in a higher risk of AG ototoxicity during MDR-TB treatment in sub-Saharan Africa. Although mitochondrial mutations in MT-RNR1 may increase genetic susceptibility,36,37 this is more prevalent in Europeans and Asians and not in sub-Saharan Black Africans, among whom the prevalence of this mutation is extremely low (0–0.09%).37–40
We found that individuals with MDR-TB and HIV coinfection had a higher risk of AG-induced hearing loss than non-HIV-infected MDR-TB patients. It is therefore likely that the high burden of HIV coinfection in sub-Saharan Africa may be the reason for the staggeringly high prevalence of AG-induced hearing loss (23–69%) compared with less burdened countries, such as the United States (13%),41 the Netherlands (18%),42 the United Kingdom (28%),43 and India (10–25%).44–46
We also revealed that AMK was the most common choice of AG for MDR-TB treatment across all eight studies. However, one of the included studies found that the risk of ototoxicity with AMK was four times higher than with KM (adjusted odds ratio 4.0, 95%CI 1.5–10.8).35 These findings will assist health care providers to develop personalized interventions, for example by choosing less ototoxic drugs, changing to an AG-sparing regimen, or scheduling more frequent hearing monitoring in PLHIV where AG is required for MDR-TB treatment, especially in sub-Saharan Africa.
A new short-course MDR-TB treatment regimen recommended by the World Health Organization (WHO) reduces treatment from 20–24 months to 9–12 months; however, an injectable AG remains part of this recommendation, in part because of the low cost as well as potent antibacterial activities.2,4 To qualify for substitution of less or non-ototoxic drugs (e.g., bedaquiline) for AGs, many TB programs currently require evidence of treatment-related hearing loss. All patients’ hearing should therefore be carefully monitored while using second-line injectable AGs through routine audiological assessments for the early detection of hearing loss. Regular audiological assessments may prevent severe or complete hearing loss because, by the time a symptom of hearing loss is detected, it is often too late to reverse hair cell damage.4
In our meta-analysis, only three studies used an audiometric definition of hearing loss for all study participants,13,24,35 while others embraced self-reported or clinician-identified hearing loss as a surrogate outcome of hearing loss. Our meta-analysis also found that only two of eight studies conducted tympanometry and otoscopy to confirm drug-induced sensorineural hearing loss by differentiating it from conductive hearing loss.13,30 These findings suggest that regular and comprehensive audiological assessment may be impractical in many study sites due to insufficient resources.
The present study has several strengths. First, we used PRISMA criteria to increase the transparency of reporting and avoid selection bias during the study selection phase.23 Second, we conducted a comprehensive search of all potentially relevant studies with the help of an academic librarian to ensure a systematic approach to capture all the evidence that may pertain to the question of interest. Third, the NOS tool was used to assess the quality of all included studies so that results could be interpreted in the context of their quality. Finally, we used a meta-analysis, a rigorous statistical method, to consolidate research findings from studies addressing a similar topic but conducted in diverse settings.47,48 This approach enables the analysis to draw more decisive conclusions on effect size for a relationship between AG-induced hearing loss and HIV coinfection because of its greater statistical power and external validity.47
While our study findings contributed to the risk analyses of AG-induced hearing loss, there were several limitations. First, despite our expanded search criteria, only a small number of studies met the inclusion criteria due to the lack of published studies. As very few studies reported the ART status of participants, we were unable to conclude whether concomitant administration of ART affected the risk of AG-induced hearing loss during injectable MDR-TB treatment. Second, samples of included studies were not necessarily representative of the variety of people living in sub-Saharan Africa, as the geographical sites of the included studies were mostly limited to southern Africa, and participants were predominantly adults. Finally, this meta-analysis did not control for potential confounders, such as age or use of ototoxic or nephrotoxic drugs, during injectable treatment.
Future studies aiming to find AG-induced hearing loss risk factors or prevent AG-induced hearing loss must consider including a wide range of HIV-related variables, such as CD4 count, viral load, duration of living with HIV infection, as well as the specific ART combination given and its frequency. Future studies need to consider the influences of time-dependent variables, such as weight, serum creatinine, and AG accumulation on the risk of AG-induced hearing loss. Because conductive hearing loss commonly results from otitis media or cerumen impaction that can threaten construct validity, conductive hearing loss must be ruled out by comprehensive audiological assessment, including audiometry, tympanometry, and otoscopy.49 Finally, children need to receive more attention in AG-induced hearing loss studies, as children with hearing loss may suffer from delayed communicational development and literacy compared with children with normal hearing.50,51
CONCLUSION
The WHO recommends a new short-course MDR-TB treatment regimen, which includes an AG. The present study lends credibility to using injectable-sparing regimens and more frequent hearing monitoring—particularly in resource-limited settings for PLHIV in sub-Saharan Africa. Such strong evidence of AG-induced hearing loss risk may help health care providers to make clinical decisions when initiating MDR-TB treatment for PLHIV.
Supplementary Material
Acknowledgments
Research reported in this manuscript was supported by the National Institute of Allergy and Infectious Disease (R01 AI104488-01A1 to J. Farley) and the National Institute of Nursing Research (F31 NR016910-01A1 to H. Hong) of the National Institutes of Health; Sigma Theta Tau International Global Nursing Research Grant; Sigma Theta Tau International / Association of Nurses in AIDS Care Grant; and Global Korean Nursing Foundation Scientific Award. We would like to express our appreciation to a medical librarian, Stella Seal, for her assistance with article search. The content is solely the responsibility of the authors and does not necessarily represent the official views of the aforementioned organizations/institutions.
The first author conducted the study and led study design, data collection, data interpretation, article preparation, article review and correspondence as well as contributed to statistical analysis. The second author led statistical analysis and contributed to data collection, statistical analysis, data interpretation, and article review. The last author contributed to article preparation, data interpretation, and review. The authors declared no conflict of interest.
References
- 1.Republic of South Africa Department of Health. Management of drug-resistant tuberculosis: policy guidelines. Vol. 161. Pretoria, Republic of South Africa: Department of Health; 2013. [Google Scholar]
- 2.World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis, 2016 updates. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 3.Caminero JA, Sotgiu G, Zumla A, Migliori GB. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis. 2010;10:621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 4.Huth ME, Ricci AJ, Cheng AG. Mechanisms of aminoglycoside ototoxicity and targets of hair cell protection. Int J Otolaryngol. 2011;2011:937861. doi: 10.1155/2011/937861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olusanya BO, Neumann KJ, Saunders JE. The global burden of disabling hearing impairment: a call to action. Bull World Health Organ. 2014;92:367–373. doi: 10.2471/BLT.13.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sataloff RT. Hearing loss: economic impact. Ear Nose Throat J. 2012;91:10–12. doi: 10.1177/014556131209100103. [DOI] [PubMed] [Google Scholar]
- 7.Sha SH, Schacht J. Stimulation of free radical formation by aminoglycoside antibiotics. Hear Res. 1999;128:112–118. doi: 10.1016/s0378-5955(98)00200-7. [DOI] [PubMed] [Google Scholar]
- 8.Hirose K, Hockenbery DM, Rubel EW. Reactive oxygen species in chick hair cells after gentamicin exposure in vitro. Hear Res. 1997;104:1–14. doi: 10.1016/s0378-5955(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 9.Abi-Hachem RN, Zine A, Van De Water TR. The injured cochlea as a target for inflammatory processes, initiation of cell death pathways and application of related otoprotectives strategies. Recent Pat CNS Drug Discov. 2010;5:147–163. doi: 10.2174/157488910791213121. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov AV, Valuev-Elliston VT, Ivanova ON, et al. Oxidative stress during HIV infection: mechanisms and consequences. Oxid Med Cell Longev. 2016;2016:8910396. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Um JY, Jang CH, Kim HL, et al. Proinflammatory cytokine IL-1 beta polymorphisms in sudden sensorineural hearing loss. Immunopharmacol Immunotoxicol. 2013;35:52–56. doi: 10.3109/08923973.2012.719523. [DOI] [PubMed] [Google Scholar]
- 12.Simdon J, Watters D, Bartlett S, Connick E. Ototoxicity associated with use of nucleoside analog reverse transcriptase inhibitors: a report of 3 possible cases and review of the literature. Clin Infect Dis. 2001;32:1623–1627. doi: 10.1086/320522. [DOI] [PubMed] [Google Scholar]
- 13.Harris T, Bardien S, Schaaf HS, Petersen L, De Jong G, Fagan JJ. Aminoglycoside-induced hearing loss in HIV-positive and HIV-negative multidrug-resistant tuberculosis patients. S Afr Med J. 2012;102:363–366. doi: 10.7196/samj.4964. [DOI] [PubMed] [Google Scholar]
- 14.Harris T, Peer S, Fagan JJ. Audiological monitoring for ototoxic tuberculosis, human immunodeficiency virus and cancer therapies in a developing world setting. J Laryngol Otol. 2012;126:548–551. doi: 10.1017/S0022215112000357. [DOI] [PubMed] [Google Scholar]
- 15.Alomar MJ. Factors affecting the development of adverse drug reactions (Review article) Saudi Pharm J. 2014;22:83–94. doi: 10.1016/j.jsps.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koethe JR, Chi BH, Megazzini KM, Heimburger DC, Stringer JS. Macronutrient supplementation for malnourished HIV-infected adults: a review of the evidence in resource-adequate and resource-constrained settings. Clin Infect Dis. 2009;49:787–798. doi: 10.1086/605285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anema A, Vogenthaler N, Frongillo EA, Kadiyala S, Weiser SD. Food insecurity and HIV/AIDS: current knowledge, gaps, and research priorities. Curr HIV/AIDS Rep. 2009;6:224–231. doi: 10.1007/s11904-009-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cederholm T, Jagren C, Hellstrom K. Outcome of protein-energy malnutrition in elderly medical patients. Am J Med. 1995;98:67–74. doi: 10.1016/S0002-9343(99)80082-5. [DOI] [PubMed] [Google Scholar]
- 19.Sitar ME, Aydin S, Cakatay U. Human serum albumin and its relation with oxidative stress. Clin Lab. 2013;59:945–952. [PubMed] [Google Scholar]
- 20.Blot SI, Pea F, Lipman J. The effect of pathophysiology on pharmacokinetics in the critically ill patient—concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev. 2014;77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Khare M, Mohanty C, Das BK, Jyoti A, Mukhopadhyay B, Mishra SP. Free radicals and antioxidant status in protein energy malnutrition. Int J Pediatr. 2014;2014:254396. doi: 10.1155/2014/254396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gogtay NJ, Kshirsagar NA, Dalvi SS. Therapeutic drug monitoring in a developing country: an overview. Br J Clin Pharmacol. 2001;52(Suppl 1):S103–S108. doi: 10.1046/j.1365-2125.2001.0520s1103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Kelly AM, Smith B, Luo Z, et al. Discordance between patient and clinician reports of adverse reactions to MDR-TB treatment. Int J Tuberc Lung Dis. 2016;20:442–447. doi: 10.5588/ijtld.15.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa, ON, Canada: The Ottawa Hospital Research Institute; 2014. [Accessed April 2018]. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 26.Joober R, Schmitz N, Annable L, Boksa P. Publication bias: what are the challenges and can they be overcome? J Psychiatr Neurosci. 2012;37:149–152. doi: 10.1503/jpn.120065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gordis L. Epidemiology. 5. Philadelphia, PA, USA: Saunders; 2014. [Google Scholar]
- 28.Sterne J. Meta-analysis: an updated collection from the Stata Journal. College Station, TX, USA: Stata Press; 2009. [Google Scholar]
- 29.StataCorp. Stata Statistical Software: Release 14. College Station, TX, USA: StataCorp LP; 2015. [Google Scholar]
- 30.Seddon JA, Thee S, Jacobs K, Ebrahim A, Hesseling AC, Schaaf HS. Hearing loss in children treated for multidrug-resistant tuberculosis. J Infect. 2013;66:320–329. doi: 10.1016/j.jinf.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Brust JC, Shah NS, van der Merwe TL, et al. Adverse events in an integrated home-based treatment program for MDR-TB and HIV in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;62:436–440. doi: 10.1097/QAI.0b013e31828175ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sagwa E, Ruswa N, Musasa JP, Mantel-Teeuwisse AK. Adverse events during treatment of drug-resistant tuberculosis: a comparison between patients with or without human immunodeficiency virus co-infection. Drug Saf. 2013;36:1087–1096. doi: 10.1007/s40264-013-0091-1. [DOI] [PubMed] [Google Scholar]
- 33.Modongo C, Sobota RS, Kesenogile B, et al. Successful MDR-TB treatment regimens including amikacin are associated with high rates of hearing loss. BMC Infect Dis. 2014;14:542. doi: 10.1186/1471-2334-14-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Modongo C, Pasipanodya JG, Zetola NM, Williams SM, Sirugo G, Gumbo T. Amikacin concentrations predictive of ototoxicity in multidrug-resistant tuberculosis patients. Antimicrob Agents Chemother. 2015;59:6337–6343. doi: 10.1128/AAC.01050-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sagwa EL, Ruswa N, Mavhunga F, Rennie T, Leufkens HG, Mantel-Teeuwisse AK. Comparing amikacin and kanamycin-induced hearing loss in multidrug-resistant tuberculosis treatment under programmatic conditions in a Namibian retrospective cohort. BMC Pharmacol Toxicol. 2015;16:36. doi: 10.1186/s40360-015-0036-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hobbie SN, Akshay S, Kalapala SK, Bruell CM, Shcherbakov D, Bottger EC. Genetic analysis of interactions with eukaryotic rRNA identify the mitoribosome as target in aminoglycoside ototoxicity. Proc Natl Acad Sci USA. 2008;105:20888–20893. doi: 10.1073/pnas.0811258106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch J, Lebeko K, Nziale JJ, Dandara C, Makubalo N, Wonkam A. In search of genetic markers for nonsyndromic deafness in Africa: a study in Cameroonians and Black South Africans with the GJB6 and GJA1 candidate genes. OMICS. 2014;18:481–485. doi: 10.1089/omi.2013.0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabahuma RI, Ouyang X, Du LL, et al. Absence of GJB2 gene mutations, the GJB6 deletion (GJB6-D13S1830) and four common mitochondrial mutations in nonsyndromic genetic hearing loss in a South African population. Int J Pediatr Otorhinolaryngol. 2011;75:611–617. doi: 10.1016/j.ijporl.2011.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lasisi AO, Bademci G, Foster J, 2nd, Blanton S, Tekin M. Common genes for non-syndromic deafness are uncommon in sub-Saharan Africa: a report from Nigeria. Int J Pediatr Otorhinolaryngol. 2014;78:1870–1873. doi: 10.1016/j.ijporl.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wonkam A, Bosch J, Noubiap JJ, Lebeko K, Makubalo N, Dandara C. No evidence for clinical utility in investigating the connexin genes GJB2, GJB6 and GJA1 in non-syndromic hearing loss in black Africans. S Afr Med J. 2015;105:23–26. doi: 10.7196/samj.8814. [DOI] [PubMed] [Google Scholar]
- 41.Marks SM, Flood J, Seaworth B, et al. Treatment practices, outcomes, and costs of multidrug-resistant and extensively drug-resistant tuberculosis, United States, 2005–2007. Emerg Infect Dis. 2014;20:812–821. doi: 10.3201/eid2005.131037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Jager P, van Altena R. Hearing loss and nephrotoxicity in long-term aminoglycoside treatment in patients with tuberculosis. Int J Tuberc Lung Dis. 2002;6:622–627. [PubMed] [Google Scholar]
- 43.Sturdy A, Goodman A, Jose RJ, et al. Multidrug-resistant tuberculosis (MDR-TB) treatment in the UK: a study of injectable use and toxicity in practice. J Antimicrob Chemother. 2011;66:1815–1820. doi: 10.1093/jac/dkr221. [DOI] [PubMed] [Google Scholar]
- 44.Isaakidis P, Varghese B, Mansoor H, et al. Adverse events among HIV/MDR-TB co-infected patients receiving antiretroviral and second line anti-TB treatment in Mumbai, India. PLOS ONE. 2012;7:e40781. doi: 10.1371/journal.pone.0040781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma V, Bhagat S, Verma B, Singh R, Singh S. Audiological evaluation of patients taking kanamycin for multidrug resistant tuberculosis. Iranian J Otorhinolaryngol. 2016;28:203–208. [PMC free article] [PubMed] [Google Scholar]
- 46.Duggal P, Sarkar M. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear, Nose, And Throat Disord. 2007;7:5. doi: 10.1186/1472-6815-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riley RD, Price MJ, Jackson D, et al. Multivariate meta-analysis using individual participant data. Res Synthesis Methods. 2015;6:157–174. doi: 10.1002/jrsm.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14(Suppl 1):29–37. [PMC free article] [PubMed] [Google Scholar]
- 49.Grove S, Burns N, Gray J. The practice of nursing research: appraisal, synthesis, and generation of evidence. St. Louis, MO, USA: Saunders; 2013. [Google Scholar]
- 50.Moeller MP. Current state of knowledge: psychosocial development in children with hearing impairment. Ear Hear. 2007;28:729–739. doi: 10.1097/AUD.0b013e318157f033. [DOI] [PubMed] [Google Scholar]
- 51.Moeller MP, Tomblin JB, Yoshinaga-Itano C, Connor CM, Jerger S. Current state of knowledge: language and literacy of children with hearing impairment. Ear Hear. 2007;28:740–753. doi: 10.1097/AUD.0b013e318157f07f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.