Abstract
Background
Patients undergoing salvage laryngectomy are predisposed to radiation-induced hypothyroidism and impaired wound healing secondary to the tissue effects of prior treatment. The impact of hypothyroidism on postoperative wound healing is not established.
Methods
A single-institution retrospective case series was performed. Inclusion criteria included preoperatively euthyroid adults who underwent salvage laryngectomy with concurrent neck dissection between 1997–2015 for persistent or recurrent laryngeal squamous cell carcinoma after radiation or chemoradiation therapy. (n=182). The principle explanatory variable was post-operative hypothyroidism (defined as TSH > 5.5 mIU/L); the primary endpoints were pharyngocutaneous fistulae and wounds requiring re-operation. Multivariate analysis was performed.
Results
The fistula rate was 47% in hypothyroid patients compared a 23% fistula rate in euthyroid patients. In the multivariate analysis, patients who developed hypothyroidism in the post-operative period had a 3.6 times higher risk of fistula (95% CI: 1.8 – 7.1, p = 0.0002). Hypothyroid patients had an 11.4 times greater risk of requiring reoperation (24.4% versus 5.4%) when compared to euthyroid patients (95% CI: 2.6 – 49.9, p = 0.001). The risk of fistula (p = 0.003) and reoperation (p=0.001) increased with increasing TSH. This corresponds to an approximately 12.5% incremental increase in the absolute risk of fistula and a 10% increase in the absolute risk of reoperation with each doubling of the TSH.
Conclusion
Postoperative hypothyroidism independently predicts postoperative wound healing complications. The association of hypothyroidism with fistula formation may yield opportunities to modulate wound healing with thyroid supplementation, or provide a biomarker of wound progression.
Keywords: Salvage Laryngectomy, Laryngeal Squamous Cell Carcinoma, Recurrent Laryngeal Cancer, Hypothyroidism, TSH, Thyroid, Pharyngocutaneous Fistula
INTRODUCTION
Laryngeal squamous cell carcinoma (SCC) has a significant disease burden with approximately 150,000 cases diagnosed annually.1 The Veterans Affairs Larynx Cancer study2 and subsequent Radiation Therapy Oncology Group3 studies have shifted the treatment paradigm towards non-surgical approaches with laryngeal preservation. As a result, radiotherapy (RT) or chemoradiotherapy (CRT) for laryngeal SCC has become an accepted standard of care with the goal of maintaining native speech and swallowing function while providing similar survival to primary laryngectomy.2–4 Unfortunately, disease recurrence or persistence after treatment requires laryngectomy in approximately one third of advanced stage patients.5–7 As a result, salvage laryngectomy has become a growing component of the head and neck surgeon’s practice.
Patients undergoing salvage laryngectomy are predisposed to impaired wound healing secondary to the tissue effects of RT and CRT, as it induces a hypoxic, hypocellular, and hypovascular environment.8 Breakdown of the pharyngeal closure leads to a pharyngocutaneous fistula in 30–75% of patients.9–14 Fistula formation increases short and long term morbidity, prolongs hospitalization, and is the most common cause of wound related death in the post-surgical head and neck cancer patient.12,14–16 In response, surgeons have developed a variety of reconstructive techniques to reduce the risk of fistula, with significant institutional and surgeon variation in reconstructive patterns employed during salvage surgery.17–31
Hypothyroidism, as indicated by elevated thyroid stimulating hormone (TSH), is a known contributor to poor wound healing, and head and neck cancer patients previously treated with RT and CRT have a high risk (up to 48%) of developing hypothyroidism.32 Radiation induces direct microvascular damage to the thyroid gland resulting in reduced synthetic and secretory capacity.33–35 Subsequently, during laryngectomy, oftentimes one or more of the thyroid lobes is resected with the surgical specimen. Even when the gland is not resected, it is manipulated during surgery.36 Furthermore, the gland is often devascularized as the superior thyroid artery is frequently used for microvascular anastomosis, or ligated during neck dissection. Central neck dissection further compromises the blood supply to the thyroid gland.
Hypothyroidism also has been associated with pharyngocutaneous fistula development.37 Spontaneous closure of persistent fistulas has been reported after thyroid hormone replacement therapy, and persistent fistulas have been correlated with ongoing hypothyroidism.38–40 To date, however, there have been no studies that explore the relationship between post-operative hypothyroidism and wound healing during salvage head & neck surgery. We hypothesize that the previously radiated and surgically manipulated thyroid gland is predisposed to decreased function, leading to an inability to compensate for increased metabolic demands and compromises wound healing following surgery.41–43 Therefore, the purpose of this study is to examine the relationship between hypothyroidism and wound healing after salvage laryngectomy.
METHODS
An IRB-approved single-institution retrospective case series was performed, informed by a prospectively maintained database of patients with laryngeal cancer (University of Michigan IRB HUM00081554). Adult patients who underwent salvage laryngectomy with concurrent neck dissection between 1997–2015 for persistent or recurrent laryngeal SCC after RT or CRT failure (n=182) at the University of Michigan were included. As hypothyroidism was the principle explanatory variable, patients who were hypothyroid and not supplemented preoperatively or did not have TSH levels post-operatively were excluded from the study. Patients were also excluded if a diverting pharyngostome was created at the time of the initial reconstruction.
Demographics, initial and recurrent clinical T classification and N classification, primary treatment modality, levels of neck dissections, and pathologic recurrent T and N classification, and pre-operative levothyroxine use were tabulated. Patients were staged in accordance to the 7th edition American Joint Committee on Cancer Staging System. Thyroid stimulating hormone (TSH) levels were collected. Patients were classified as hypothyroid if a post-operative TSH level were greater than 5.5 mIU/L, which is our lab value cutoff institutionally that defines hypothyroidism. The primary endpoints were fistula formation within 30 days of surgery and wound complications that required reoperation.
Statistics
Univariate analysis was performed and bivariate associations between clinical variables were tested with nonparametric tests (i.e. Fisher’s exact test, chi-square test with Monte Carlo estimates for error terms). The peak TSH value in the post-operative period was evaluated as a predictor in a logistic regression model of fistula and need for reoperation, and multivariate analysis was performed. Variables with a p-value of less than 0.1 in the bivariate analysis were included in the model. Additionally, reconstruction with vascularized tissue (regional or free flap) and prior chemotherapy were included in the model as these were felt to be important variables to help explain the model. The development of hypothyroidism was also evaluated. Bivariate analysis was performed and variables with a p-value of less than 0.1 in the bivariate analysis were included in the multivariate model. Univariate and bivariate statistics were performed using SPSS version 24 software (IBM; Armonk, NY) and logistic regression modeling was performed using Statistical Analytics Software (SAS Institute Inc.; Cary, NC). An alpha of 0.05 was used for all statistical tests to determine statistical significance.
RESULTS
Study Population
There were 182 patents in the cohort, the majority of whom were male with a mean age of 61 years (Table 1). The majority of patients were current (42.9%) or former (54.9%) smokers. In total, 53.3% of patients had recurrent tumors of the glottis, while 45.1% had tumors of the supraglottis with the remainder of tumors arising from the subglottis. In terms of previous treatment, 53.8% of patients were initially treated with RT alone, while 46.2% of patients were treated with CRT. Most tumors were advanced T classification (67.9%), and advanced overall stage (71.0%).
Table 1.
Baseline characteristics of the study cohort.
| Variable | Value (%) n=182 |
|---|---|
| Age, mean (st dev) | 61.4 ± 9.8 |
| Gender | |
| Male | 157 (86.3%) |
| Female | 25 (13.7%) |
| BMI, mean (st dev) | 24.9 ± 5.8 |
| Tobacco | |
| Never | 4 (2.2%) |
| Former | 100 (54.9%) |
| Current | 78 (42.9%) |
| Alcohol | |
| Never | 117 (65.7%) |
| Former | 28 (15.7%) |
| Current | 33 (18.5%) |
| MD* | 4 (2.2%) |
| Primary Therapy | |
| RT | 98 (53.8%) |
| CRT | 84 (46.2%) |
| Recurrent Site | |
| Supraglottis | 82 (45.1%) |
| Glottis | 97 (53.3%) |
| Subglottis | 3 (1.6%) |
| T Classification | |
| T1 | 9 (4.9%) |
| T2 | 63 (34.6%) |
| T3 | 52 (28.6%) |
| T4 | 58 (31.9%) |
| N Classification | |
| N0 | 155 (85.2%) |
| N1 | 12 (6.6%) |
| N2 | 15 (8.2%) |
| Stage | |
| I | 8 (4.4%) |
| II | 59 (32.4%) |
| III | 48 (24.6%) |
| IV | 67 (36.8%) |
Ninety-six percent of the population was euthyroid based on pre-operative TSH. Eight patients were noted to be hypothyroid prior to surgery and these patients were started on levothyroxine prior to their operation. An additional 23 patients were on chronic thyroid replacement. In total, 17% of patients were on levothyroxine prior to surgery. Patients that underwent total thyroidectomy during laryngectomy were started on levothyroxine post-operatively.
One hundred and sixty patients underwent total laryngectomy without pharyngectomy, 9 patients underwent a total laryngectomy with partial pharyngectomy, and 13 patients underwent total laryngopharyngectomy. In terms of reconstruction of the pharynx after laryngectomy, 45.8% of patients were reconstructed with a vascularized flap, while 51.6% of patient underwent primary closure alone.
Risk Factors for Post-Operative Hypothyroidism
In total, 50% of patients developed hypothyroidism in the post-operative period. Recurrent advanced stage, tumor subsite, prior chemotherapy, vascularized flap reconstruction, central neck dissection, the development of post-operative hypocalcemia, previous tracheostomy, gender, and tobacco use were all predictive variables. In the binomial logistic regression, only advanced stage, flap reconstruction, and post-operative hypocalcemia were predictive of hypothyroidism. Patients with an advanced stage (III or IV) had a 3.2 times higher risk of developing hypothyroidism compared to patients with early stage disease (I or II), (95% CI: 1.4 – 7.3, p = 0.005). Similarly, patients reconstructed with a vascularized flap had a 2.8 times higher chance of developing post-operative hypothyroidism (95% CI: 1.3 – 6.2, p = 0.008). Patients with post-operative hypocalcemia were 6.7 times more likely to develop post-operative hypothyroidism (95% CI: 2.5 – 12.8, p < 0.001). However, central neck dissection alone did not correlate with the development of hypothyroidism (p = 0.183) (Figure 1).
Figure 1.
Multivariate analysis of developing hypothyroidism in the post-operative period with 95% confidence interval.
Fistula and Hypothyroidism
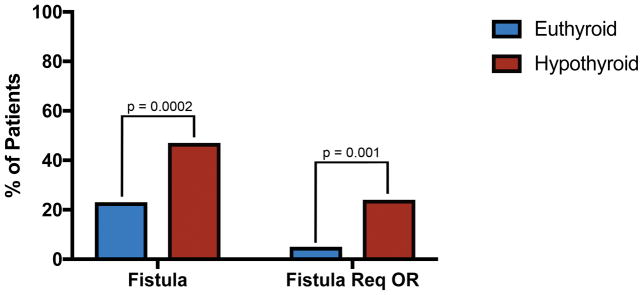
The overall pharyngocutaneous fistula rate in our cohort was 35%. Fifteen percent of patients developed a fistula that required reoperation. Reconstructive approach was not statistically related to fistula formation (p=0.752) or fistula requiring return to the operating room (OR) (p=0.487, Table 2). In the multivariate analysis, patients who developed hypothyroidism in the post-operative period had a 3.6 times higher risk of fistula (95% CI: 1.8 – 7.1, p = 0.0002). This corresponds to a 47% fistula rate in hypothyroid patients compared a 23% fistula rate in euthyroid patients (Figure 2). Similarly, hypothyroid patients had an 11.4 times greater risk of developing a fistula that required reoperation (24% versus 5%) when compared to euthyroid patients (95% CI: 2.6 – 49.9, p = 0.001) (Figure 2). Preoperative thyroid replacement therapy did not correlate with fistula formation (p = 0.17) or reoperation (p = 0.44). Neither a history of previous chemotherapy, or reconstruction using a vascularized flap significantly affect the fistula rate. There was an association of previous chemotherapy and reconstruction using a vascularized flap, (p<0.0001), but neither variable was predictive in the multivariate model.
Table 2.
Fistula and reoperation rates based on TSH level and reconstruction.
| Cohort | Thyroid Status | Fistula Rate | Reoperation Rate |
|---|---|---|---|
| Total Cohort (n=182) | Hypothyroid (n=90) | 46.7% | 24.4% |
| Normothyroid (n=92) | 22.8% | 5.4% | |
| p-value | 0.001 | <0.001 | |
|
| |||
| TSH>20 (n=31) | 54.8% | 29.0% | |
| TSH<20 (n=151) | 37.1% | 11.9% | |
| p-value | 0.07 | 0.02 | |
|
| |||
| Primary Closure (n=94) | Hypothyroid (n=30) | 63.3% | 26.7% |
| Normothyroid (n=64) | 25.0% | 6.3% | |
| p-value | <0.001 | 0.02 | |
|
| |||
| TSH>20 (n=8) | 75.0% | 25.0% | |
| TSH<20 (n=86) | 33.7% | 11.6% | |
| p-value | 0.05 | 0.27 | |
|
| |||
| Flap Reconstruction (n=88) | Hypothyroid (n=60) | 48.3% | 23.3% |
| Normothyroid (n=28) | 32.1% | 3.6% | |
| p-value | 0.17 | 0.03 | |
|
| |||
| TSH>20 (n=23) | 47.8% | 30.4% | |
| TSH<20 (n=65) | 41.5% | 12.3% | |
| p-value | 0.63 | 0.06 | |
Figure 2.
Fistula and reoperation rates in patients with post-operative hypothyroidism compared to euthyroid patients.
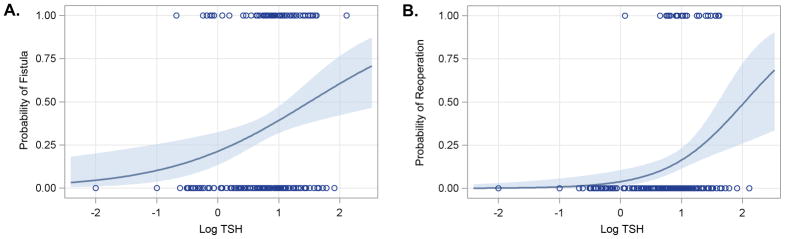
Thyroid stimulating hormone was also analyzed as a continuous variable as it relates to fistula and need for reoperation in a logistic regression model. When analyzing TSH as a continuous variable, the risk of fistula (p = 0.003) and fistula requiring reoperation (p=0.001) steadily increased with increasing TSH. This corresponds to an approximately 12.5% incremental increase in the absolute risk of fistula with each doubling of the TSH (Figure 3a) and a 10% increase in the absolute risk of fistula requiring reoperation with each doubling of the TSH (Figure 3b).
Figure 3.
Probability of developing a fistula (a.) or requiring reoperation (b.) based on the peak post-operative TSH. Shaded area represents the 95% confidence interval. TSH levels are log-transformed on the x-axis. Hypothyroid threshold is 0.74 (log(5.5)=0.74).
Hypothyroidism was associated with other wound complications in addition to fistula. In a bivariate analysis, patients with postoperative hypothyroidism were more likely to develop post-operative hemorrhage or hematoma (20.0% versus 9.8%, p=0.05), and were more likely to develop dehiscence of the neck closure (56.7% versus 35.9%, p = 0.005). Surgical site infection did not differ between the two groups (p = 0.161).
DISCUSSION
The increased use of RT and CRT for patients with laryngeal SCC has engendered an increase in salvage laryngeal surgeries. Salvage laryngectomy after RT or CRT places patients at significant risk for wound healing complications, the most notable of which is fistula development.44,45 The fistula rate in our study was 35%, consistent with other reports in the literature.45 This is contrasted with a 17% (12/72) fistula rate in patients undergoing primary laryngectomy (not previously treated with radiation or chemoradiation) at our institution over the same time period. Additionally, the reoperation rate in our cohort was 15%, compared to 4% in patients undergoing primary laryngectomy (3/72). Despite the numerous studies evaluating risk factors for fistula and other wound complications, few have identified a significant modifiable risk factor. This is the first and largest study to evaluate the effects of postoperative hypothyroidism on surgical wound healing. In this study, hypothyroidism was the only predictive factor in relation to pharyngocutaneous fistula. Additionally, this is the first study to demonstrate that the risk of fistula, and wound complications requiring return to the OR, both increase with increasing TSH. The contribution of hypothyroidism to wound healing is an important consideration when aiming to improve upon the persistently high rate of pharyngocutaneous fistulae, as hypothyroidism after salvage laryngectomy is becoming increasingly recognized.42,43 This is significant, as this is a potentially modifiable risk factor with dramatic opportunity for quality improvement in an era of such clinical scrutiny. Moreover, there is potential application to other surgical venues in which wound healing is a vexing issue.
Measurement of TSH is the most commonly used test to diagnose hypothyroidism as it reliably detects dynamic perturbations in thyroid hormone levels.46 Thyroid stimulating hormone synthesis is modulated by thyroid hormone levels, neuropeptides and neurotransmitters. Hypothalamic thyrotropine-releasing hormone (TRH) is the main stimulating factor of TSH synthesis, but it is a labile hormone that can be affected by non-thyroidal influences as it modulated by catecholamines, somatostatin and dopamine.47 It has been established that TSH levels can be altered during an acute illness.48 Though most hospitalized patients with acute illnesses have normal serum TSH concentrations, transient TSH abnormalities can be encountered in the absence of thyroid dysfunction.48 During an acute illness TSH levels are typically suppressed, however there are instances in which the TSH levels become mildly elevated. In light of this, there has been debate as to whether elevated TSH after salvage laryngectomy represents true hypothyroidism or rather an acute phase reactant indicating physiologic stress associated with major surgery. While the findings in this study are associative and thus cannot fully answer this question, we hypothesize that elevated TSH in this population represents true hypothyroidism in the majority of patients, and that this is a contributory factor in wound complications.
One particularly interesting finding in this study is that vascularized tissue reconstruction did not predict fistula formation. This conflicts with several multi-institutional studies looking at the beneficial effects of reconstructing using vascularized tissue.20,24,26,29,31,49–51 This is likely due to the fact that higher risk patients were significantly more likely in this cohort to require free tissue transfer, as indicated by the disparity in terms of chemotherapy exposure (63.6% versus 29.8%, p < 0.001), preoperative tracheostomy, (44.3% versus 21.3%, p = 0.001), and mean Body Mass Index (23.7 versus 26.1, p = 0.02). Thus, free flap potentially mitigates the risk associated with these other variables, and potentially explains why this was not significant in our dataset.
One interesting consideration is the recipient vessel choice when performing free tissue reconstruction. The superior thyroid artery is a common vessel used in microvascular reconstruction after total laryngectomy given its proximity to the defect and favorable geometry. In cases of prior irradiation or neck dissection, potential recipient blood vessels may be ligated or may be non-patent due to from prior treatment.23 In these cases, other arteries must be considered, such as the facial, transverse cervical, internal mammary, and dorsal scapular arteries.52,53 There are many factors to consider when deciding on recipient vessels, including vascular pedicle length, caliber, flow, treatment effects and pedicle geometry. The reconstructing surgeon should be cognizant, however, of the potential effects of using the superior thyroid artery on thyroid function and should monitor this post-operatively.
This study has notable limitations. The retrospective nature of this study does not allow us to establish causation. While this is the largest study evaluating hypothyroidism and wound healing after salvage head and neck surgery, the cohort remains relatively small. Additionally, the relationship of TSH to thyroxine (T4) and triiodothyronine (T3) levels could not be established as these lab values were not collected, which is a limitation of this retrospective study. Additionally, the hypothesis that elevated TSH is an acute phase response and not true hypothyroidism cannot be disproven, as we did not measure other acute phase response markers to draw correlations. The treatment of hypothyroidism was not uniform in timing or dosing in this cohort; thus the effect of thyroid replacement in mitigating wound complications cannot be fully understood with this data set.
Further research is needed to determine if wound complications can be mitigated with routine postoperative thyroid replacement algorithms, as well as to evaluate other acute phase markers. We are currently planning the development of such protocols in light of these hypothesis-generating data.
CONCLUSION
Postoperative hypothyroidism independently predicts complications with wound healing after surgery. While advanced tumor stage, flap reconstruction, and post-operative hypocalcemia were associated with hypothyroidism, hypothyroidism itself was the only variable predictive of pharyngocutaneous fistula formation. The association of hypothyroidism with fistula formation may yield opportunities to modulate wound healing with thyroid supplementation, or allow a blood based biomarker of wound progression in patients undergoing salvage laryngectomy.
SYNOPSIS.
Patients undergoing salvage laryngectomy are at high risk of hypothyroidism and wound healing complications. Postoperative hypothyroidism independently predicts postoperative wound healing complications after salvage laryngectomy in a dose dependent manner.
Footnotes
Presentation: This manuscript was presented as a poster at the 2016 American Head and Neck Society Meeting, Seattle, WA.
BIBLIOGRAPHY
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal cancer. The Department of Veterans Affairs Laryngeal Cancer Study Group. The New England journal of medicine. 1991;324(24):1685–1690. doi: 10.1056/NEJM199106133242402. [DOI] [PubMed] [Google Scholar]
- 3.Forastiere AA, Goepfert H, Maor M, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. The New England journal of medicine. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 4.Urba S, Wolf G, Eisbruch A, et al. Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24(4):593–598. doi: 10.1200/JCO.2005.01.2047. [DOI] [PubMed] [Google Scholar]
- 5.Clark JR, de Almeida J, Gilbert R, et al. Primary and salvage (hypo)pharyngectomy: Analysis and outcome. Head & neck. 2006;28(8):671–677. doi: 10.1002/hed.20428. [DOI] [PubMed] [Google Scholar]
- 6.Paydarfar JA, Birkmeyer NJ. Complications in head and neck surgery: a meta-analysis of postlaryngectomy pharyngocutaneous fistula. Archives of otolaryngology--head & neck surgery. 2006;132(1):67–72. doi: 10.1001/archotol.132.1.67. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Lorenz RR, Khan MJ, et al. Salvage laryngectomy in patients with recurrent laryngeal cancer in the setting of nonoperative treatment failure. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2013;149(2):245–251. doi: 10.1177/0194599813486257. [DOI] [PubMed] [Google Scholar]
- 8.Marx RE. Osteoradionecrosis: a new concept of its pathophysiology. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 1983;41(5):283–288. doi: 10.1016/0278-2391(83)90294-x. [DOI] [PubMed] [Google Scholar]
- 9.Clark JR, Gilbert R, Irish J, Brown D, Neligan P, Gullane PJ. Morbidity after flap reconstruction of hypopharyngeal defects. Laryngoscope. 2006;116(2):173–181. doi: 10.1097/01.mlg.0000191459.40059.fd. [DOI] [PubMed] [Google Scholar]
- 10.Yu P, Robb GL. Pharyngoesophageal reconstruction with the anterolateral thigh flap: a clinical and functional outcomes study. Plast Reconstr Surg. 2005;116(7):1845–1855. doi: 10.1097/01.prs.0000191179.58054.80. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge JW, Spencer H, Moreno MA. Predictors for Perioperative Outcomes following Total Laryngectomy: A University HealthSystem Consortium Discharge Database Study. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2014;151(1):81–86. doi: 10.1177/0194599814528451. [DOI] [PubMed] [Google Scholar]
- 12.Hier M, Black MJ, Lafond G. Pharyngo-cutaneous fistulas after total laryngectomy: incidence, etiology and outcome analysis. The Journal of otolaryngology. 1993;22(3):164–166. [PubMed] [Google Scholar]
- 13.Sassler AM, Esclamado RM, Wolf GT. Surgery after organ preservation therapy. Analysis of wound complications. Archives of otolaryngology--head & neck surgery. 1995;121(2):162–165. doi: 10.1001/archotol.1995.01890020024006. [DOI] [PubMed] [Google Scholar]
- 14.Weber RS, Berkey BA, Forastiere A, et al. Outcome of salvage total laryngectomy following organ preservation therapy: the Radiation Therapy Oncology Group trial 91–11. Archives of otolaryngology--head & neck surgery. 2003;129(1):44–49. doi: 10.1001/archotol.129.1.44. [DOI] [PubMed] [Google Scholar]
- 15.Andrews BT, Smith RB, Hoffman HT, Funk GF. Orocutaneous and pharyngocutaneous fistula closure using a vacuum-assisted closure system. The Annals of otology, rhinology, and laryngology. 2008;117(4):298–302. doi: 10.1177/000348940811700410. [DOI] [PubMed] [Google Scholar]
- 16.Graboyes EM, Yang Z, Kallogjeri D, Diaz JA, Nussenbaum B. Patients undergoing total laryngectomy: an at-risk population for 30-day unplanned readmission. JAMA otolaryngology--head & neck surgery. 2014;140(12):1157–1165. doi: 10.1001/jamaoto.2014.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlson GW, Thourani VH, Codner MA, Grist WJ. Free gastro-omental flap reconstruction of the complex, irradiated pharyngeal wound. Head & neck. 1997;19(1):68–71. doi: 10.1002/(sici)1097-0347(199701)19:1<68::aid-hed13>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 18.Chahine KA, Chaffanjon P, Bettega G, Lebeau J, Reyt E, Righini CA. Gastro-omental free flap in the reconstruction of the unfavourable hypopharyngeal defects: a functional assessment. J Plast Reconstr Aesthet Surg. 2009;62(11):1367–1373. doi: 10.1016/j.bjps.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Chepeha DB, Annich G, Pynnonen MA, et al. Pectoralis major myocutaneous flap vs revascularized free tissue transfer: complications, gastrostomy tube dependence, and hospitalization. Archives of otolaryngology--head & neck surgery. 2004;130(2):181–186. doi: 10.1001/archotol.130.2.181. [DOI] [PubMed] [Google Scholar]
- 20.Fung K, Teknos TN, Vandenberg CD, et al. Prevention of wound complications following salvage laryngectomy using free vascularized tissue. Head & neck. 2007;29(5):425–430. doi: 10.1002/hed.20492. [DOI] [PubMed] [Google Scholar]
- 21.Genden EM, Rinaldo A, Shaha AR, Bradley PJ, Rhys-Evans PH, Ferlito A. Pharyngocutaneous fistula following laryngectomy. Acta oto-laryngologica. 2004;124(2):117–120. doi: 10.1080/00016480310015191. [DOI] [PubMed] [Google Scholar]
- 22.Hanasono MM. Use of reconstructive flaps following total laryngectomy. JAMA otolaryngology--head & neck surgery. 2013;139(11):1163. doi: 10.1001/jamaoto.2013.2768. [DOI] [PubMed] [Google Scholar]
- 23.Hanasono MM, Lin D, Wax MK, Rosenthal EL. Closure of laryngectomy defects in the age of chemoradiation therapy. Head & neck. 2012;34(4):580–588. doi: 10.1002/hed.21712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paleri V, Drinnan M, van den Brekel MW, et al. Vascularized tissue to reduce fistula following salvage total laryngectomy: a systematic review. The Laryngoscope. 2014;124(8):1848–1853. doi: 10.1002/lary.24619. [DOI] [PubMed] [Google Scholar]
- 25.Patel RS, Makitie AA, Goldstein DP, et al. Morbidity and functional outcomes following gastro-omental free flap reconstruction of circumferential pharyngeal defects. Head & neck. 2009;31(5):655–663. doi: 10.1002/hed.21016. [DOI] [PubMed] [Google Scholar]
- 26.Patel UA, Moore BA, Wax M, et al. Impact of pharyngeal closure technique on fistula after salvage laryngectomy. JAMA otolaryngology--head & neck surgery. 2013;139(11):1156–1162. doi: 10.1001/jamaoto.2013.2761. [DOI] [PubMed] [Google Scholar]
- 27.Punthakee X, Zaghi S, Nabili V, Knott PD, Blackwell KE. Effects of salivary bypass tubes on fistula and stricture formation. JAMA facial plastic surgery. 2013;15(3):219–225. doi: 10.1001/jamafacial.2013.791. [DOI] [PubMed] [Google Scholar]
- 28.Selber JC, Xue A, Liu J, et al. Pharyngoesophageal reconstruction outcomes following 349 cases. Journal of reconstructive microsurgery. 2014;30(9):641–654. doi: 10.1055/s-0034-1376887. [DOI] [PubMed] [Google Scholar]
- 29.Teknos TN, Myers LL, Bradford CR, Chepeha DB. Free tissue reconstruction of the hypopharynx after organ preservation therapy: analysis of wound complications. The Laryngoscope. 2001;111(7):1192–1196. doi: 10.1097/00005537-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Wadsworth JT, Futran N, Eubanks TR. Laparoscopic harvest of the jejunal free flap for reconstruction of hypopharyngeal and cervical esophageal defects. Archives of otolaryngology--head & neck surgery. 2002;128(12):1384–1387. doi: 10.1001/archotol.128.12.1384. [DOI] [PubMed] [Google Scholar]
- 31.Withrow KP, Rosenthal EL, Gourin CG, et al. Free tissue transfer to manage salvage laryngectomy defects after organ preservation failure. The Laryngoscope. 2007;117(5):781–784. doi: 10.1097/MLG.0b013e3180332e39. [DOI] [PubMed] [Google Scholar]
- 32.Miller MC, Agrawal A. Hypothyroidism in postradiation head and neck cancer patients: incidence, complications, and management. Current opinion in otolaryngology & head and neck surgery. 2009;17(2):111–115. doi: 10.1097/moo.0b013e328325a538. [DOI] [PubMed] [Google Scholar]
- 33.Bhandare N, Kennedy L, Malyapa RS, Morris CG, Mendenhall WM. Primary and central hypothyroidism after radiotherapy for head-and-neck tumors. International journal of radiation oncology, biology, physics. 2007;68(4):1131–1139. doi: 10.1016/j.ijrobp.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 34.Turner SL, Tiver KW, Boyages SC. Thyroid dysfunction following radiotherapy for head and neck cancer. International journal of radiation oncology, biology, physics. 1995;31(2):279–283. doi: 10.1016/0360-3016(93)E0112-J. [DOI] [PubMed] [Google Scholar]
- 35.Tell R, Sjodin H, Lundell G, Lewin F, Lewensohn R. Hypothyroidism after external radiotherapy for head and neck cancer. International journal of radiation oncology, biology, physics. 1997;39(2):303–308. doi: 10.1016/s0360-3016(97)00117-x. [DOI] [PubMed] [Google Scholar]
- 36.Tucker HM. Total Laryngectomy: Technique. Operative Techniques in Otolaryngology - Head and Neck Surgery. 1990;1(1):42–44. [Google Scholar]
- 37.Gal RL, Gal TJ, Klotch DW, Cantor AB. Risk factors associated with hypothyroidism after laryngectomy. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2000;123(3):211–217. doi: 10.1067/mhn.2000.107528. [DOI] [PubMed] [Google Scholar]
- 38.Alexander MV, Zajtchuk JT, Henderson RL. Hypothyroidism and wound healing: occurrence after head and neck radiation and surgery. Archives of otolaryngology. 1982;108(5):289–291. doi: 10.1001/archotol.1982.00790530025007. [DOI] [PubMed] [Google Scholar]
- 39.Talmi YP, Finkelstein Y, Zohar Y. Pharyngeal fistulas in postoperative hypothyroid patients. The Annals of otology, rhinology, and laryngology. 1989;98(4 Pt 1):267–268. doi: 10.1177/000348948909800405. [DOI] [PubMed] [Google Scholar]
- 40.Bohannon IA, Carroll WR, Magnuson JS, Rosenthal EL. Closure of post-laryngectomy pharyngocutaneous fistulae. Head & neck oncology. 2011;3:29. doi: 10.1186/1758-3284-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavelle RJ. Thyroid function after radiotherapy and total larynbecomy in the treatment of carcinoma of the larynx. The Annals of otology, rhinology, and laryngology. 1971;80(4):593–598. doi: 10.1177/000348947108000424. [DOI] [PubMed] [Google Scholar]
- 42.Kojima R, Tsukahara K, Motohashi R, et al. Extent of thyroid resection and thyroid function after postoperative radiotherapy following total laryngectomy or total pharyngo-laryngo-esophagectomy. Int J Clin Oncol. 2017 doi: 10.1007/s10147-016-1082-x. [DOI] [PubMed] [Google Scholar]
- 43.Negm H, Mosleh M, Fathy H, Awad A. Thyroid and parathyroid dysfunction after total laryngectomy in patients with laryngeal carcinoma. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2016;273(10):3237–3241. doi: 10.1007/s00405-016-4105-3. [DOI] [PubMed] [Google Scholar]
- 44.Hasan Z, Dwivedi RC, Gunaratne DA, Virk SA, Palme CE, Riffat F. Systematic review and meta-analysis of the complications of salvage total laryngectomy. Eur J Surg Oncol. 2017;43(1):42–51. doi: 10.1016/j.ejso.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 45.Dedivitis RA, Aires FT, Cernea CR, Brandao LG. Pharyngocutaneous fistula after total laryngectomy: systematic review of risk factors. Head Neck. 2015;37(11):1691–1697. doi: 10.1002/hed.23804. [DOI] [PubMed] [Google Scholar]
- 46.Ladenson PW, Singer PA, Ain KB, et al. American Thyroid Association guidelines for detection of thyroid dysfunction. Arch Intern Med. 2000;160(11):1573–1575. doi: 10.1001/archinte.160.11.1573. [DOI] [PubMed] [Google Scholar]
- 47.Economidou F, Douka E, Tzanela M, Nanas S, Kotanidou A. Thyroid function during critical illness. Hormones (Athens) 2011;10(2):117–124. doi: 10.14310/horm.2002.1301. [DOI] [PubMed] [Google Scholar]
- 48.Stockigt JR. Guidelines for diagnosis and monitoring of thyroid disease: nonthyroidal illness. Clin Chem. 1996;42(1):188–192. [PubMed] [Google Scholar]
- 49.Righini C, Lequeux T, Cuisnier O, Morel N, Reyt E. The pectoralis myofascial flap in pharyngolaryngeal surgery after radiotherapy. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2005;262(5):357–361. doi: 10.1007/s00405-004-0827-8. [DOI] [PubMed] [Google Scholar]
- 50.Sayles M, Grant DG. Preventing pharyngo-cutaneous fistula in total laryngectomy: a systematic review and meta-analysis. The Laryngoscope. 2014;124(5):1150–1163. doi: 10.1002/lary.24448. [DOI] [PubMed] [Google Scholar]
- 51.Smith TJ, Burrage KJ, Ganguly P, Kirby S, Drover C. Prevention of postlaryngectomy pharyngocutaneous fistula: the Memorial University experience. The Journal of otolaryngology. 2003;32(4):222–225. doi: 10.2310/7070.2003.41697. [DOI] [PubMed] [Google Scholar]
- 52.Hanasono MM, Barnea Y, Skoracki RJ. Microvascular surgery in the previously operated and irradiated neck. Microsurgery. 2009;29(1):1–7. doi: 10.1002/micr.20560. [DOI] [PubMed] [Google Scholar]
- 53.Rosko AJ, Ryan JT, Wizauer EJ, et al. Dorsal scapular artery as a recipient vessel in the vessel-depleted neck during free tissue transfer in head and neck reconstruction. Head & neck. 2017;39(7):E72–E76. doi: 10.1002/hed.24785. [DOI] [PubMed] [Google Scholar]