Abstract
Roux-en-Y gastric bypass (RYGB) surgery is a highly effective treatment for obesity but negatively affects the skeleton. Studies of skeletal effects have generally examined areal BMD by DXA, but DXA may be inaccurate in the setting of marked weight loss. Further, as a result of modestly sized samples of mostly premenopausal women and very few men, effects of RYGB by sex and menopausal status are unknown. We prospectively studied the effects of RYGB on skeletal health, including axial and appendicular volumetric BMD and appendicular bone microarchitecture and estimated strength. Obese adults (N=48; 27 premenopausal and 11 postmenopausal women, 10 men) with mean ±SD BMI 44 ±7 kg/m2 were assessed before and 6 and 12 months after RYGB. Participants underwent spine and hip DXA, spine QCT, radius and tibia HR-pQCT, and laboratory evaluation. Mean 12-month weight loss was 37 kg (30% of preoperative weight). Overall median 12-month increase in serum CTx was 278% (p<0.0001), with greater increases in postmenopausal than premenopausal women (p=0.049). Femoral neck BMD by DXA decreased by mean 5.0% and 8.0% over 6 and 12 months (p<0.0001). Spinal BMD by QCT decreased by mean 6.6% and 8.1% (p<0.0001); declines were larger among postmenopausal than premenopausal women (11.6% vs. 6.0% at 12 months, p=0.02). Radial and tibial BMD and estimated strength by HR-pQCT declined. At the tibia, detrimental changes in trabecular microarchitecture were apparent at 6 and 12 months. Cortical porosity increased at the radius and tibia, with more dramatic 12-month increases among postmenopausal than premenopausal women or men at the tibia (51.4% vs. 18.3% vs. 3.0%, p<0.01 between groups). In conclusion, detrimental effects of RYGB on axial and appendicular bone mass and microarchitecture are detectable as early as 6 months postoperatively. Postmenopausal women are at highest risk for skeletal consequences and may warrant targeted screening or interventions.
Keywords: bone mineral density, bone microarchitecture, bariatric surgery, gastric bypass surgery, biochemical markers of bone turnover
JBMR Keywords: ANALYSIS/QUANTITATION OF BONE: Bone QCT/μCT, DXA; BONE MODELING AND REMODELING: Biochemical Markers of Bone Turnover; CELL/TISSUE SIGNALING–Endocrine Pathways; NUTRITION; SYSTEMS BIOLOGY–Bone Interactors: Bone-fat interactions
Introduction
With obesity a continued public health crisis, there has been escalating interest in surgical weight loss (bariatric surgery). Bariatric surgery is a highly effective treatment for obesity, producing durable weight loss, improving obesity-related comorbidities, and decreasing mortality.(1-4) However, the commonly performed Roux-en-Y gastric bypass (RYGB) procedure may induce negative effects on bone metabolism, with increases in bone turnover, decreases in bone mass, and even increased fracture incidence.(5-9) These effects are attributed to a combination of factors, including the mechanical unloading of the skeleton with weight loss, nutritional deficiencies from calcium and vitamin D malabsorption, loss of muscle mass, bone marrow fat changes, and changes in fat-secreted hormones, sex steroids, and gut-derived hormones.(10-14)
Attempts to understand the skeletal consequences of RYGB have been hindered by imaging limitations. Most clinical studies have used DXA to assess BMD,(5, 6) but assessment of BMD by DXA may be biased in the setting of marked weight loss, due to changes in the composition of the soft tissue surrounding bone.(15, 16) Further, spurious increases in measured spinal BMD by DXA may occur in the setting of degenerative change. QCT is an established method for assessing volumetric BMD at the axial and appendicular skeleton, the latter sometimes undertaken by HR-pQCT, which can also examine cortical and trabecular bone microarchitecture and estimate bone strength. Although obesity and weight loss may also influence QCT assessments,(17) QCT avoids the biases of DXA stemming from 2-dimensional, single projection data acquisition. The effects of RYGB on bone mass and microarchitecture may begin very early in the postoperative period—as the bone resorption marker serum CTx has been shown to increase a mere 10 days after RYGB(18)—but no studies have included comprehensive imaging with DXA and axial and appendicular QCT as early as 6 months after surgery.
The relative skeletal effects of RYGB by sex and menopausal status are also uncertain. Men account for just under 20% of bariatric surgery patients nationwide,(19) and studies of postoperative skeletal changes have included few men(20-23) or have restricted enrollment to women.(24-27) Similarly, studies to date have included few postmenopausal women(21, 26, 28) or have excluded postmenopausal women altogether to decrease heterogeneity.(29, 30) However, postmenopausal women, for whom age and sex steroid-related bone metabolism changes are already a concern, may be particularly affected by RYGB. If this is the case, they may warrant special clinical attention to skeletal health pre- and postoperatively, with screening and preventive or therapeutic interventions.
We conducted a prospective cohort study of RYGB and skeletal health, the largest to date to examine axial and appendicular volumetric BMD and appendicular bone microarchitecture and estimated strength, including postmenopausal women and men. We determined postoperative changes in bone turnover markers, bone mass, and bone microarchitecture overall. Then, we examined changes by sex and menopausal status, hypothesizing that postmenopausal women would experience more substantial skeletal changes than premenopausal women or men.
Materials and Methods
Study population
We recruited women and men 25-70 years of age from two academic bariatric surgery centers (the University of California, San Francisco [UCSF] and the San Francisco Veterans Affairs Medical Center). Participants were eligible if they were scheduled for an upcoming RYGB procedure. Women were excluded if they were perimenopausal (defined as last menses >3 months but <5 years ago), in order to minimize skeletal changes unrelated to RYGB. Premenopausal women on stable hormonal contraception, postmenopausal women on stable menopausal hormone therapy, and men on stable testosterone were eligible. Participants were excluded if they used medications known to impact bone metabolism, including bisphosphonates or teriparatide (in the last year or for >12 months ever), oral glucocorticoids (>5 mg prednisone equivalent daily for >10 days in the last 3 months), and thiazolidinediones. Other exclusion criteria included prior bariatric surgery, weight >159 kg (the DXA scanner weight limit), estimated glomerular filtration rate <30 mL/min/1.73m2, and disorders of calcium or bone metabolism (e.g., primary hyperparathyroidism or Paget's disease).
Study protocol
Participants attended study visits preoperatively (within a month before surgery) and at 6 and 12 months postoperatively.
The research protocol included standardization of calcium intake and attention to vitamin D status, with vitamin D and chewable calcium citrate supplements supplied throughout the study. At enrollment, low 25OHD levels were repleted to a target level ≥30 ng/mL, and each participant's total daily calcium intake was brought to 1200 mg through individualized calcium citrate dosing, based on estimation of dietary intake with a validated questionnaire.(31) Postoperatively, 25OHD levels and estimated dietary calcium intake were monitored, and each participant's supplement doses were adjusted to maintain our vitamin D and calcium intake goals.
The RYGB procedure was performed in a standardized laparoscopic fashion at both academic bariatric surgery centers. This included a 30 mL gastric pouch, a gastrojejunal anastomosis created with a 25 mm circular stapler, an antecolic and antegastric Roux limb 100 to 150 cm in length, and an end-to-side jejunostomy.
Our institutional review board approved the study protocol, and all participants provided written informed consent. The study was registered at www.clinicaltrials.gov (NCT01330914).
DXA
Areal BMD (aBMD, g/cm2) of the lumbar spine (L1 to L4), proximal femur, and distal radius was measured by DXA (Hologic Discovery Wi densitometer, Bedford, MA, USA) preoperatively and 6 and 12 months postoperatively. Whole body scans were performed for assessment of body composition, including whole body fat and lean mass (grams). If a participant's body dimensions exceeded the scanning area width, the left arm was incompletely imaged during whole body scanning and then measured values for the right arm were used for the left during data analysis. The local coefficient of variation (CV) for spinal BMD derived from the manufacturer phantom is 0.437%.
QCT
Volumetric QCT of the L3 and L4 vertebrae was performed preoperatively and 6 and 12 months postoperatively (General Electrics VCT64 scanner, Milwaukee, WI, USA), as described previously.(32) With a calibration phantom (Mindways Software, Austin, TX, USA) beneath the participant, axial contiguous images were obtained using a standardized helical protocol with tube voltage of 120 kVp, tube load of 200 mAs, and slice thickness of 1.25 mm, reconstructed to 2.5 mm. Images were individually examined and rated regarding whether and to what extent abdominal soft tissue extended outside the 50-cm field of view and induced a bright artifact in doing so. Trabecular volumetric BMD (vBMD, g/cm3) was evaluated using QCTPro software (Mindways Software, Austin, TX, USA), with all scans evaluated by one experienced operator and longitudinal scans evaluated together. The root mean square CV (CVRMS) for trabecular volumetric BMD is 1.7%.(33) Visceral adipose tissue area (VAT, cm2) was measured using a single axial slice at the mid-L4 vertebra. The fascial borders of the internal abdominal wall were traced manually, using specialized software developed at UCSF,(34) and VAT was calculated by multiplying the number of pixels within the adipose attenuation threshold by the pixel area.
HR-pQCT: acquisition and analysis
Participants were imaged in a HR-pQCT system (XtremeCT, Scanco Medical, Brüttisellen, Switzerland) preoperatively and 6 and 12 months postoperatively, using the manufacturer's standard in vivo protocol (source potential 60 kVp, tube current 900 μA, isotropic 82 μm nominal resolution).(35-37) The nondominant forearm and ankle were scanned, with fixed scan regions starting at 9.5 mm and 22.5 mm proximal to the mid-jointline for the ultradistal radius and tibia, respectively, and extending proximally for 9.02 mm (110 slices). Images were individually examined and rated regarding whether and to what extent soft tissue extended outside the field of view and induced a bright artifact in doing so.
HR-pQCT images were analyzed using the manufacturer's standard clinical evaluation protocol(38-40) in Image Processing Language (IPL v5.08b, Scanco Medical). Contours identifying the periosteal perimeter of the bone were drawn semi-automatically using an edge-finding algorithm;(40) contours were examined manually and modified as necessary to delineate the boundary. A threshold-based process was used to segment cortical and trabecular regions for compartment-specific measurements of density, geometry, and structure.(40) Periosteal and endosteal contours were manually checked and corrected to ensure accurate segmentation. Trabecular structure was extracted using a threshold-based binarization process.(41) Cortical parameters were assessed using an extended cortical bone analysis that provides direct calculation of cortical thickness and measures of porosity.(42, 43) A skeletonization algorithm was applied to the cortical porosity at preoperative and 12-month time points to define the topology of pore structures, as described previously.(44) This included quantification of pore size and number of junctions (to assess pore interconnectedness), and classification of pore shape as slab-like or tube-like. Finally, linear elastic micro-finite element analysis (μFEA, Scanco FE Software, Scanco Medical) was performed to calculate apparent biomechanical properties, as described previously.(45-47) Precision errors have been published previously, with relevant CVRMS values <1.4% for densitometric parameters, 1.3%-8.9% for structural parameters, and 1.9%-4.3% for strength parameters.(48)
HR-pQCT: quantification of soft tissue effects
To assist in the interpretation of HR-pQCT data, we tested the accuracy of vBMD measurements in the context of changing soft tissue mass. Four models were constructed using an idealized tibial bone phantom(49) (trabecular insert density 180 mg HA/cm3, cortical shell thickness 2 mm and density 945.2 mg HA/cm3) and simulated soft tissue layers. Each model contained a purchased gel ice pack of similar density to human muscle (18 mg HA/cm3) wrapped around the bone phantom. Simulated fat layers of increasing thickness (1 cm to 3.5 cm) were created with vegetable shortening (-50 mg HA/cm3) inside poly plastic bags. Soft tissue surrounding the phantom artificially decreased apparent vBMD, and increasing the amount of soft tissue decreased vBMD further. Measured cortical vBMD was 930.9 mg HA/cm3 for the model with least simulated fat, lower than the true vBMD of 945.2 mg HA/cm3. Cortical vBMD then decreased by 5.2% further moving from least to greatest simulated fat models. Measured trabecular vBMD was 174.0 mg HA/cm3 for the model with least simulated fat, lower than the true vBMD of 180 mg HA/cm3. Trabecular vBMD then decreased by 4.2% further moving from least to greatest simulated fat models. These observations would be consistent with an apparent increase in vBMD when thickness of fat is reduced (i.e., during weight loss).
Other measures
Preoperatively and 6 and 12 months postoperatively, BMI was calculated as weight/height2 (kg/m2). Waist circumference was measured in the midaxillary line at the level of the lowest rib, and hip circumference at the maximum extension of the buttocks, viewed from the side. Comprehensive estimates of dietary intakes were obtained using the full-length Block food frequency questionnaire.(50) Physical activity was assessed using the International Physical Activity Questionnaire short-form.(51)
Serum samples were collected at each time point after an overnight fast. Basic chemistries and hemoglobin A1c (HbA1c) were measured, 25OHD was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS), and intact PTH was measured by automated chemiluminescent immunoassay (ADVIA Centaur, Siemens Healthineers, Erlangen, Germany), then serum was stored at -70° C until batch analyzed for other analytes in a central laboratory (Maine Medical Center Research Institute, Scarborough, ME). Bone turnover markers serum CTx, P1NP, bone-specific alkaline phosphatase (BAP), and osteocalcin (OC) were measured by automated immunoassay (iSYS, Immunodiagnostic Systems, Scottsdale, AZ), with inter- and intra-assay CVs 6.2% and 3.2%, 4.6% and 2.9%, 7.3% and 1.6%, and 6.1% and 2.5%, respectively. Total estradiol and total testosterone were measured by ELISA (Alpco Diagnostics, Salem, NH), with inter- and intra-assay CVs 8.7% and 7.8%, and 7.3% and 8.0%, respectively; testosterone was measured only in male participants. Sclerostin was measured by ELISA (R&D Systems, Minneapolis, MN), with inter- and intra-assay CVs 9.5% and 2.0%, respectively. Blood draws in premenopausal women were not timed to the menstrual cycle. A 24-hour urine sample was collected at each time point for urinary calcium determination.
Statistical analysis
Baseline characteristics were assessed for normality, with means ± SDs or medians (interquartile ranges, IQR) calculated. To determine whether baseline characteristics differed between premenopausal women, postmenopausal women, and men, linear regression, Fisher's exact test, or the nonparametric Mann-Whitney test were employed as indicated. For normally distributed characteristics (presented as means ± SDs), linear regression models were utilized; residuals were used to check the assumptions of normality and linearity and to check for influential points. The nonparametric Kruskal-Wallis test was used when appropriate to confirm results. For characteristics with skewed distributions (presented as medians and IQR), the Mann-Whitney test was utilized. Next, paired t-tests or Wilcoxon signed-rank tests were used to determine whether study outcomes changed between preoperative and 6-month or 12-month postoperative time points. We then tested for differences in 12-month changes between premenopausal women, postmenopausal women, and men, utilizing linear regression models or the Mann-Whitney test with the approach described above for baseline characteristics. For other pairwise comparisons, the t-test and Mann-Whitney test were utilized as appropriate. In this approach, we used calculated change variables and assessed the main effects of sex and menopausal status. Adjusted associations were estimated using regression models, examining which factors might account for differences in 12-month skeletal changes between the sex and menopausal groups; variables associated with sex and menopausal status were included as covariates, and normalizing log transformations were used if needed. Finally, Pearson's coefficient of correlation was computed to characterize the relationships between changes in skeletal parameters and baseline values or changes in metabolic parameters. Data were analyzed with Stata 13 software (StataCorp, College Station, TX).
At the time of study design, sample size calculations were based on DXA, as there were no published data about changes in QCT- or HR-pQCT-derived parameters after RYGB. Based on the SD of total hip aBMD change after RYGB in one published study,(20) a sample size of 46 provided 90% power to detect a change in aBMD as small as 2.3%.
Results
Baseline participant characteristics
Of 54 participants who underwent preoperative measurements, 3 had sleeve gastrectomy surgical procedures rather than RYGB, and 3 withdrew from the study due to lack of time, leaving 48 participants who contributed postoperative data.
Of the 48 participants, 27 (56%) were premenopausal women, 11 (23%) were postmenopausal women, and 10 (21%) were men (Table 1). Three postmenopausal women were on stable menopausal hormone therapy, and two men were on stable testosterone replacement. Participants were 46 ± 12 (mean ± SD) years old overall; on average, premenopausal women were younger than both postmenopausal women and men. BMI was similar across sex and menopause groups, with mean 44 ± 7 kg/m2. However, other body composition parameters differed between groups: Postmenopausal women had lower preoperative weight and total lean mass (kg) than premenopausal women and men. Men had lower percentage body fat but greater VAT area and waist-hip ratio than women.
Table 1. Baseline Characteristics, Stratified by Sex and Menopausal Status.
| Characteristic | All participants (n=48) | Premenopausal women (n=27) | Postmenopausal women (n=11) | Men (n=10) |
|---|---|---|---|---|
| Age (yrs) | 46 ± 12 | 39 ± 9b | 55 ± 8a | 52 ± 13 |
| Race, n (%) | ||||
| White | 27 (56%) | 12 | 8 | 7 |
| Black | 9 (19%) | 7 | 2 | 0 |
| Asian | 3 (6%) | 2 | 0 | 1 |
| Hispanic/Latino | 6 (13%) | 5 | 1 | 0 |
| Other | 3 (6%) | 1 | 0 | 2 |
| Diabetes, n (%) | 19 (40%) | 7 (26%)b | 5 (45%) | 7 (70%) |
| Body composition parameters | ||||
| Weight (kg) | 122 ± 19 | 123 ± 18 | 110 ± 16a,b | 134 ± 15 |
| BMI (kg/m2) | 44 ± 7 | 45 ± 8 | 43 ± 5 | 42 ± 4 |
| Percentage body fat (%) | 47 ± 6 | 49 ± 3b | 49 ± 4b | 37 ± 6 |
| Lean mass (kg) | 61 ± 12 | 58 ± 7b | 52 ± 6a,b | 79 ± 8 |
| Waist circumference (cm) | 120 ± 14 | 120 ± 13 | 115 ± 15b | 129 ± 9 |
| Waist-hip ratio | 0.89 ± 0.10 | 0.86 ± 0.06b | 0.85 ± 0.11b | 1.03 ± 0.04 |
| Visceral adipose tissue area (cm2) | 192 ± 93 | 143 ± 64b | 231 ± 70a | 283 ± 100 |
| Laboratory parameters | ||||
| HbA1c (%) | 5.6 (5.4, 6.7) | 5.5 (5.2, 6.3)b | 6.2 (5.6, 6.7) | 6.5 (6.3, 9.3) |
| 25OHD upon enrollment (ng/mL) | 24 (18, 29) | 21 (18, 29) | 26 (17, 35) | 27 (24, 29) |
| 25OHD at preop study visit (ng/mL) | 42 (33, 50) | 43 (34, 53) | 45 (37, 49) | 33 (29, 43) |
| PTH (pg/mL)c | 42 (32, 54) | 41 (33, 49) | 53 (28, 68) | 43 (36, 53) |
| CTx (ng/mL)c | 0.25 (0.17, 0.34) | 0.25 (0.18, 0.31) | 0.27 (0.11, 0.46) | 0.22 (0.15, 0.41) |
| P1NP (ng/mL)c | 32 (26, 47) | 32 (26, 44) | 45 (23, 56) | 39 (28, 48) |
| BAP (μg/L) c | 13 (10, 18) | 12 (9, 17) | 19 (11, 25)a | 13 (13, 15) |
| OC (ng/mL) c | 11 (8, 14) | 11 (8, 13) | 12 (8, 17) | 10 (8, 18) |
| Areal BMD (DXA) (g/cm2) | ||||
| Femoral neck | 0.948 ± 0.131 | 1.002 ± 0.115 | 0.839 ± 0.139a | 0.925 ± 0.079 |
| Total hip | 1.120 ± 0.144 | 1.157 ± 0.152 | 1.020 ± 0.120a | 1.130 ± 0.098 |
| 1/3 distal radius | 0.727 ± 0.067 | 0.717 ± 0.036b | 0.672 ± 0.063a,b | 0.814 ± 0.054 |
| Ultradistal radius | 0.515 ± 0.090 | 0.513 ± 0.081b | 0.450 ± 0.048a,b | 0.593 ± 0.092 |
| Lumbar spine | 1.158 ± 0.147 | 1.177 ± 0.111 | 1.061 ± 0.163a,b | 1.213 ± 0.175 |
| Areal BMD (DXA) Z-scores | ||||
| Femoral neck | +1.1 ± 0.9 | +1.4 ± 0.9b | +0.9 ± 0.8 | +0.7 ± 0.6 |
| Total hip | +1.5 ± 1.0 | +1.8 ± 1.2b | +1.4 ± 0.6 | +1.0 ± 0.7 |
| Lumbar spine | +1.3 ± 1.3 | +1.3 ± 1.2 | +1.3 ± 1.4 | +1.6 ± 1.8 |
| Volumetric BMD (QCT) (g/cm3) | ||||
| Spine (L3-L4) | 0.159 ± 0.037 | 0.177 ± 0.033b | 0.128 ± 0.035a | 0.146 ± 0.019 |
Values are means ± SDs, counts (percentages), or medians (IQR).
p<0.05 vs. premenopausal women.
p<0.05 vs. men.
95% reference intervals provided by the test manufacturers: PTH, 14-72 pg/mL; CTx, 0.112-0.738 ng/mL; P1NP, 27.7-127.6 ng/mL; BAP, 4.7-27.0 μg/L; OC, 10.4-45.6 ng/mL
Upon initial enrollment, median (IQR) 25OHD level was 24 (18-29) ng/mL, and with individualized vitamin D repletion, median 25OHD rose to 42 ng/mL at the time of comprehensive preoperative study measurements. Vitamin D status and PTH level did not differ by sex or menopausal status. Postmenopausal women had higher BAP levels than premenopausal women, while levels of other bone turnover marker levels did not significantly differ between subgroups.
Preoperatively, mean aBMD at the proximal femur and distal radius was lowest among postmenopausal women. At the spine, postmenopausal women had lowest mean aBMD by DXA and vBMD by QCT; mean spinal aBMD by DXA was highest in men, but mean spinal vBMD by QCT was highest in premenopausal women.
Changes in body composition, metabolic, and dietary parameters after RYGB
All participants lost weight after RYGB, with a large mean decrease by 6 months (31 kg, or a 25% decline, p<0.0001) that continued through 12 months (37 kg, or a 30% decline from baseline, p<0.0001, Table 2). By 12 months, total fat mass declined by a mean 49% from its preoperative baseline, and total lean mass declined by a mean 14% (p<0.0001 for both).
Table 2. Changes in Body Composition, Laboratory, and Lifestyle Parameters After Roux-en-Y Gastric Bypass.
| Parameter | Preoperative value (n=48) | 6-month change (n=45)a | p-value | 12-month change (n=45)a | p-value |
|---|---|---|---|---|---|
| Body composition | |||||
|
| |||||
| Weight (kg) | 122 ± 19 | -31 ± 8 | <0.0001 | -37 ± 10 | <0.0001 |
| BMI (kg/m2) | 44 ± 7 | -11 ± 3 | <0.0001 | -13 ± 4 | <0.0001 |
| Total body fat (kg) | 56 ± 12 | -22 ± 6 | <0.0001 | -27 ± 8 | <0.0001 |
| Lean mass (kg) | 61 ± 12 | -8 ± 4 | <0.0001 | -9 ± 4 | <0.0001 |
| Visceral adipose tissue area (cm2) | 192 ± 93 | -87 ± 56 | <0.0001 | -108 ± 54 | <0.0001 |
|
| |||||
| Laboratory parameters: Metabolic | |||||
|
| |||||
| HbA1c (%) | 5.6 (5.4, 6.7) | -0.6 (-1.3, -0.3) | <0.0001 | -0.6 (-1.2, -0.2) | <0.0001 |
| Estradiol (pg/mL) | 62 (44, 99) | +3 (-18, +35) | 0.28 | -2 (-29, +26) | 0.97 |
| Testosterone (ng/dL)b | 359 (284, 461) | +124 (+70, +191) | <0.01 | +173 (+161, +232) | <0.01 |
|
| |||||
| Laboratory parameters: Calcium homeostasis | |||||
|
| |||||
| 25(OH)D (ng/mL) | 42 (33, 50) | -5 (-11, +2) | 0.02 | -5 (-13, 0) | <0.01 |
| 24-hour urinary calcium (mg) | 184 (114, 258) | -45 (-94, +11) | <0.01 | -53 (-95, +9) | <0.001 |
| PTH (pg/mL) | 42 (32, 54) | +7 (-1, +20) | <0.01 | +6 (-5, +24) | 0.01 |
| Sclerostin (pg/mL) | 128 (102, 170) | +42 (-15, +84) | <0.01 | +36 (-18, +67) | <0.01 |
|
| |||||
| Lifestyle parameters | |||||
|
| |||||
| Physical activity (met-min/wk) | 1022 (33, 2772) | +415 (-990, +1489) | 0.38 | +499 (-868, +1062) | 0.29 |
| Dietary protein (g/d) | 77 ± 31 | -24 ± 30 | <0.0001 | -29 ± 36 | <0.0001 |
| Dietary protein (% of total kcal) | 17 ± 2 | +4 ± 5 | <0.0001 | +1 ± 5 | 0.27 |
Values are means ± SDs or medians (IQR).
Of the 48 participants who contributed postoperative data, 3 did not participate in 6-month postoperative measurements, and 3 others did not participate in 12-month postoperative measurements, yielding n=45 available participants for each time interval.
Testosterone measured only in men (n=10).
Neither absolute nor percentage 12-month weight loss differed by sex or menopausal status (Supplemental Table 1). Men, who had greater lean mass than women preoperatively, had greater mean 12-month absolute decline in lean mass than women (12 kg vs. 8 kg, p=0.01), but percentage declines were similar (p=0.89). Similarly, men had greater absolute declines in VAT than women, but percentage declines were similar.
HbA1c decreased postoperatively (p<0.0001, Table 2). There was no statistically significant change in estradiol level over the study period, neither in the cohort as a whole nor in any of the 3 subgroups. Testosterone level, measured in men, increased (p<0.01). Physical activity level was highly variable and did not significantly change during the study period. Dietary protein intake (g/d) decreased, while the percentage of total kcal from protein increased at 6 months (p<0.0001).
Changes in calciotropic hormones and bone turnover after RYGB
Postoperatively, 25OHD level for the overall cohort decreased (Table 2) but remained robust with vitamin D supplementation, with median 35 (IQR 28 to 41) ng/mL at the 12-month postoperative time point. Median 24-hour urinary calcium level decreased, and serum PTH level increased. Twelve-month changes in these measures of calcium homeostasis did not differ by sex or menopausal status (Supplemental Table 1). Sclerostin level increased postoperatively; change did not differ among sex or menopausal subgroups.
Bone turnover markers increased markedly in the 6 months after RYGB and remained elevated (Table 3). Serum CTx, a marker of bone resorption, increased by a median 278% (IQR: +196 to +484%, p<0.0001) over the 12-month study period. Median 12-month percentage increases in bone formation markers serum P1NP, OC, and BAP were 111%, 176%, and 22%, respectively (p<0.0001 for all). Over 6 months, those with greater increases in PTH had greater increases in CTX (r=0.30, p=0.04). In analyses stratified by sex and menopausal status, 12-month increase in CTx was greater among postmenopausal than premenopausal women (median change +338% vs. +260%, p=0.049 for difference; median among men was +316%). Changes in bone formation markers were similar across sex and menopause groups (Supplemental Table 1).
Table 3. Percentage Changes in Bone Turnover Markers and in Areal and Volumetric Bone Mineral Density After Gastric Bypass.
| Parameter | 6-month % Change (n=45)a | p-value | 12-month % Change (n=45)a | p-value |
|---|---|---|---|---|
| Bone turnover markers | ||||
|
| ||||
| CTx | +276 (+166, +395) | <0.0001 | +278 (+196, +484) | <0.0001 |
| P1NP | +112 (+71, +153) | <0.0001 | +111 (+55, +163) | <0.0001 |
| OC | +137 (+83, +212) | <0.0001 | +176 (+101, +250) | <0.0001 |
| BAP | +18 (+4, +44) | <0.0001 | +22 (+5, +58) | <0.0001 |
|
| ||||
| Areal BMD (DXA) | ||||
|
| ||||
| Proximal femur | ||||
| Femoral neck | -5.0 ± 4.5 | <0.0001 | -8.0 ± 4.9 | <0.0001 |
| Total hip | -4.6 ± 4.8 | <0.0001 | -8.2 ± 5.2 | <0.0001 |
| Spine | ||||
| Lumbar spine | +0.0 ± 5.1 | 0.99 | -1.5 ± 5.1 | 0.06 |
| Radius | ||||
| Total radius | -0.7 ± 3.0 | 0.15 | -2.1 ± 2.7 | <0.0001 |
| 1/3 distal radius | +1.2 ± 2.2 | <0.01 | +0.7 ± 3.1 | 0.14 |
| Ultradistal radius | -3.4 ± 7.8 | <0.01 | -6.7 ± 7.8 | <0.0001 |
|
| ||||
| Volumetric BMD (QCT) | ||||
|
| ||||
| Spine (L3-L4) | -6.6 ± 5.0 | <0.0001 | -8.1 ± 6.7 | <0.0001 |
|
| ||||
| Volumetric BMD (HR-pQCT) | ||||
|
| ||||
| Radius | ||||
| Total | -1.2 ± 3.0 | 0.01 | -3.4 ± 3.2 | <0.0001 |
| Trabecular | -0.4 ± 1.7 | 0.17 | -2.0 ± 3.5 | 0.001 |
| Cortical | +0.1 ± 2.0 | 0.73 | -0.3 ± 2.7 | 0.48 |
| Tibia | ||||
| Total | -0.6 ± 1.7 | 0.04 | -2.7 ± 3.1 | <0.0001 |
| Trabecular | +0.4 ± 1.7 | 0.10 | -0.5 ± 3.8 | 0.42 |
| Cortical | -0.2 ± 1.6 | 0.32 | -1.6 ± 2.4 | 0.0001 |
Values are means ± SDs or medians (IQR).
Of the 48 participants who contributed postoperative data, 3 did not participate in 6-month postoperative measurements, and 3 others did not participate in 12-month postoperative measurements, yielding n=45 available participants for each time interval.
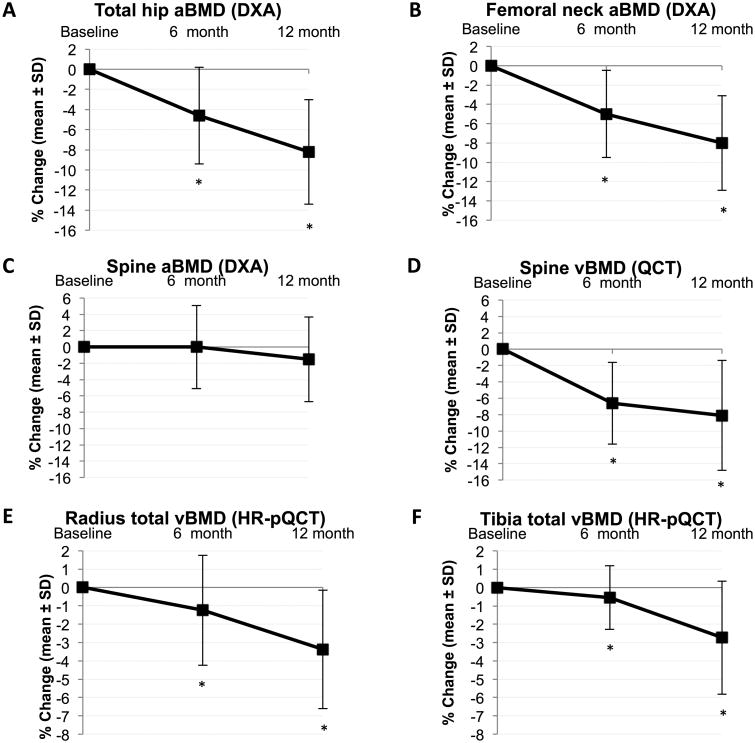
Changes in BMD after RYGB
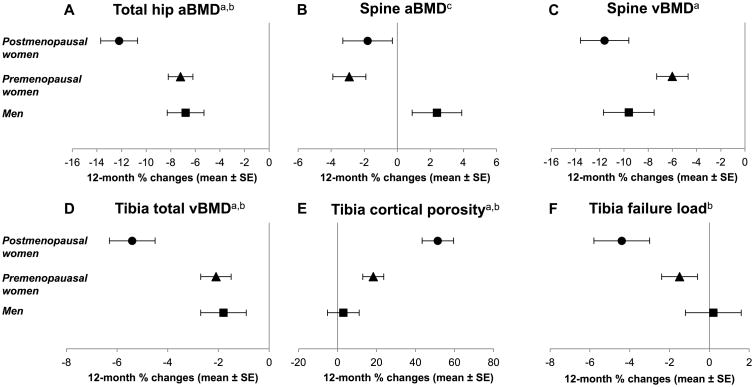
Areal BMD at the proximal femur (DXA) decreased progressively after RYGB (Table 3, Figure 1), with mean 6- and 12-month declines at the femoral neck of 5.0% and 8.0%, respectively (p<0.0001 for both). At the total hip, 12-month percentage decline in aBMD was larger for postmenopausal women than for premenopausal women or men (12.2% vs. 7.2% vs. 6.8%, respectively, p≤0.02; Figure 2; Supplemental Table 2). Overall, aBMD at the spine did not change postoperatively, but in analyses stratified by sex, women had a decrease in spinal aBMD over 12 months (mean 2.6%, p<0.001). Spinal vBMD (QCT) decreased by mean 6.6% and 8.1% over 6 and 12 months (p<0.0001); changes were significant within each sex and menopausal group but larger among postmenopausal than premenopausal women (-11.6% vs. -6.0% over 12 months, p=0.02 for difference; change among men was -9.6%). Declines in total vBMD at the radius and tibia (HR-pQCT) were smaller in magnitude but still statistically significant by 6 and 12 months, and by 12 months for trabecular vBMD at the radius and cortical vBMD at the tibia. At the tibia, 12-month change in total vBMD was greater for postmenopausal women than for premenopausal women or men (-5.4% vs. -2.1% vs. -1.8%, p<0.01 between groups). Sensitivity analyses excluding QCT and HR-pQCT image sets with soft tissue extension outside the field of view at the baseline scan, with and without associated bright radiographic artifacts, yielded similar results.
Figure 1.
Figure 2.
In multivariable analysis, sex and menopausal differences in 12-month changes in total hip aBMD, spinal vBMD, and tibial vBMD were not explained by the baseline parameters or changes associated with sex or menopausal status in Table 1 or Supplemental Table 1. The sex and menopausal differences were observed not only for percentage changes but also for absolute changes in the skeletal parameters.
Participants with greater percentage weight loss had greater percentage declines in total hip aBMD (r=0.43, p<0.01 for 12-month changes); this association remained statistically significant after adjustment for sex and menopausal status (p<0.01). A similar trend was observed for femoral neck aBMD. Weight loss was not associated with decline in BMD at the spine, radius, or tibia. Baseline weight did not predict BMD changes. Declines in lean mass and fat mass were associated with decline in total hip aBMD over 12 months (r=0.33, p=0.03 and r=0.29, p=0.05, respectively), but these associations were not independent of overall weight loss. Participants with greater 6-month increases in PTH had greater 12-month percentage declines in femoral neck aBMD (r=-0.31, p=0.045). Those with greater 12-month increases in PTH had greater declines in total vBMD at the radius (r=-0.32, p=0.0496) but not tibia (r=-0.09, p=0.57).
Nonwhite participants experienced more negative 12-month changes in trabecular vBMD at the radius and tibia than white participants, with mean changes at the radius of -4.3% vs. -0.6%, respectively, and mean changes at the tibia of -2.3% vs. +0.7%, respectively (p<0.01 between groups at both radius and tibia). Changes in aBMD parameters and other vBMD parameters were not different between white and nonwhite participants.
Changes in bone microarchitecture and estimated strength after RYGB
Within the trabecular compartment, changes in microarchitecture associated with diminished skeletal strength were apparent by 6 and 12 months at the tibia, although not at the radius (Table 4). These included decreases in trabecular number and increases in trabecular separation and heterogeneity. Trabecular changes at the tibia were not different by sex or menopausal status (Supplemental Table 3). At the radius, there was no statistically significant trabecular microarchitectural change overall, but subgroup analysis revealed that this was because a decrease in trabecular number and an increase in trabecular separation in postmenopausal women were countered by opposite trends—trends towards favorable structural changes—in men.
Table 4. Percentage Changes in Bone Microarchitecture and Biomechanical Parameters After Gastric Bypass.
| Parameter | 6-month % Change (n=45)a | p-value | 12-month % Change (n=44)a | p-value |
|---|---|---|---|---|
| Radius | ||||
|
| ||||
| Trabecular geometry | ||||
|
| ||||
| Tb area | +0.7 ± 1.6 | <0.01 | +1.4 ± 1.2 | <0.0001 |
| Tb number | -0.3 ± 10.3 | 0.84 | -1.1 ± 10.7 | 0.53 |
| Tb thickness | +1.0 ± 9.8 | 0.51 | +0.2 ± 10.8 | 0.91 |
| Tb separation | +1.4 ± 10.5 | 0.39 | +2.7 ± 11.8 | 0.15 |
| Tb heterogeneity | +1.4 ± 13.4 | 0.51 | +5.4 ± 24.5 | 0.17 |
|
| ||||
| Cortical geometry | ||||
|
| ||||
| Ct thickness | -1.6 ± 4.6 | 0.03 | -2.8 ± 4.4 | <0.001 |
| Ct porosity | +12.8 ± 28.7 | <0.01 | +18.4 ± 53.3 | 0.03 |
| Ct pore size | -- | +8.6 ± 37.6 | 0.14 | |
| Ct pore junctions | -- | +39.7 ± 142.3 | 0.08 | |
| Ct pore slab/tube ratio | -- | +5.4 ± 19.6 | 0.08 | |
|
| ||||
| μFEA parameters | ||||
|
| ||||
| Failure load | +1.0 ± 4.5 | 0.14 | -1.7 ± 5.3 | 0.04 |
| Stiffness | +1.3 ± 5.7 | 0.16 | -1.9 ± 6.1 | 0.05 |
| Apparent modulus | +2.8 ± 6.3 | <0.01 | -0.2 ± 6.9 | 0.86 |
|
| ||||
| Tibia | ||||
|
| ||||
| Trabecular geometry | ||||
|
| ||||
| Tb area | +0.4 ± 0.7 | <0.001 | +1.2 ± 1.3 | <0.0001 |
| Tb number | -3.7 ± 8.5 | <0.01 | -4.6 ± 8.4 | <0.001 |
| Tb thickness | +5.0 ± 9.3 | <0.01 | +5.3 ± 9.9 | <0.01 |
| Tb separation | +4.5 ± 8.9 | <0.01 | +5.8 ± 8.9 | <0.001 |
| Tb heterogeneity | +7.0 ± 14.2 | <0.01 | +7.6 ± 12.4 | <0.001 |
|
| ||||
| Cortical geometry | ||||
|
| ||||
| Ct thickness | -0.8 ± 2.9 | 0.08 | -3.9 ± 4.8 | <0.0001 |
| Ct porosity | +5.6 ± 19.3 | 0.07 | +22.4 ± 30.4 | <0.0001 |
| Ct pore size | -- | +15.8 ± 27.3 | <0.001 | |
| Ct pore junctions | -- | +24.9 ± 42.8 | <0.001 | |
| Ct pore slab/tube ratio | -- | +7.8 ± 9.6 | <0.0001 | |
|
| ||||
| μFEA parameters | ||||
|
| ||||
| Failure load | +0.3 ± 3.5 | 0.65 | -1.8 ± 4.7 | 0.02 |
| Stiffness | +0.4 ± 4.1 | 0.56 | -1.8 ± 5.3 | 0.03 |
| Apparent modulus | +0.7 ± 4.2 | 0.26 | -1.6 ± 5.4 | 0.05 |
Values are means ± SDs. Tb, trabecular. Ct, cortical. μFEA, micro-finite element analysis. Cortical pore skeletonization analysis was performed on images from preoperative and 12-month postoperative but not 6-month postoperative time points.
Of the 48 participants who contributed postoperative data, 3 did not participate in 6-month postoperative measurements, 3 others did not participate in 12-month postoperative measurements, and one participant did not undergo HR-pQCT scanning—the focus of Table 4—at the 12-month visit.
Within the cortical compartment, cortical thickness declined at both the radius and tibia (Table 4). Cortical porosity increased at the radius and tibia, with more dramatic 12-month changes among postmenopausal than premenopausal women or men at the tibia (+51.4% vs. +18.3% vs. +3.0%, p<0.01 between groups; Figure 2; Supplemental Table 3). In conjunction, there was an increase in pore junctions (interconnectedness) at the tibia, the results of an increase in women but not men (+34.5% vs. -6.0%, p<0.01). Between-group differences were not explained by the baseline parameters or changes that were associated with sex or menopausal status in Table 1 or Supplemental Table 1.
Failure load and stiffness, both measures of estimated bone strength, decreased at the radius and tibia by 12 months (Table 4). At the radius, these decreases were not different by sex or menopausal status (Supplemental Table 3), but at the tibia, they were driven by decreases among postmenopausal women (Figure 2). In multivariable analysis, these between-group differences were not explained by the factors associated with sex or menopausal status in Table 1 or Supplemental Table 1.
Sex and menopausal differences were observed not only for percentage changes but also for absolute changes in the skeletal parameters. Baseline weight, weight loss, and change in PTH were not associated with change in bone microarchitecture or strength at the radius or tibia.
Discussion
We conducted a prospective cohort study of RYGB and skeletal health, the largest to date to examine axial and appendicular volumetric BMD and appendicular bone microarchitecture and estimated strength. We detected detrimental effects of RYGB on bone turnover, mass, structure, and strength just 6 months postoperatively, and these effects persisted throughout the 12-month study duration. Postmenopausal women not only had lower bone mass preoperatively than premenopausal women and men, but also they experienced more dramatic changes in skeletal health parameters, including greater increases in serum CTx, declines in BMD, and changes in bone microstructure.
Postoperative declines in BMD at the axial skeleton were substantial. Twelve months after RYGB, aBMD at the femoral neck had decreased by a mean of 8.0% in the overall cohort, and vBMD at the spine determined by QCT had similarly decreased by 8.1%. Interestingly, DXA did not detect a statistically significant change in spinal aBMD in the overall cohort, but further examination revealed that this was because the men had a mean measured aBMD change in the direction of an increase, despite a mean spinal vBMD decrease of 9.6%. Considering that spinal aBMD by DXA is known to be subject to artifactual elevation in the setting of degenerative disease and other processes,(52) we suspect that artifact confounded our spinal aBMD assessment. Published studies of the effects of RYGB on DXA-assessed aBMD have typically reported BMD declines that are greater at the proximal femur than at the spine,(5, 6) sometimes hypothesizing greater mechanical unloading at the proximal femur or greater susceptibility of the cortical bone which predominates at the femur, but our results indicate that artifact may contribute as well.
Spinal vBMD by QCT could be subject to its own biases.(17) In our cohort, a subset of baseline QCT images demonstrated abdominal soft tissue extension outside the field of view, with and without associated bright reconstruction artifacts. We performed sensitivity analyses excluding data from those scans, and we confirmed a vBMD decrease similar to that observed in the full cohort. We are also mindful of the radiographic phenomenon of beam hardening,(53) which, if incompletely corrected during QCT image acquisition and processing, could result in underestimation of vBMD at baseline and then an apparent vBMD increase as weight is lost. This would suggest that that our observed mean 12-month decline of 8.1% could be a conservative estimate. Our study joins 2 others that have utilized axial QCT after RYGB: Yu et al. reported a mean 6.1% 12-month decrease in trabecular spinal vBMD among 30 RYGB participants, significantly different than a -0.5% change among 20 nonsurgical controls.(54) Ivaska et al. reported no change in spinal vBMD in 21 bariatric surgery participants, 7 of whom underwent RYGB.(23)
Using HR-pQCT, we detected statistically significant decreases in total vBMD at the radius and tibia at the 6- and 12-month time points. At the radius, the decrease in total vBMD was driven by a decrease in trabecular vBMD, while at the tibia, the decrease was driven by vBMD change within the cortical compartment. The impact of mechanical unloading with weight loss could account in part for these site-specific differences in profile, as non-weight-bearing status after knee surgery has been shown to particularly impact the cortical compartment at the tibia.(47) Measured percentage declines in appendicular vBMD were smaller than at the axial skeleton, but our HR-pQCT phantom experiments (see Materials and Methods) indicate that HR-pQCT underestimates vBMD declines when fat mass is also declining. Detrimental changes in trabecular microarchitecture were detectable at 6 and 12 months at the tibia but not the radius; these included decreases in trabecular number and increases in trabecular separation and heterogeneity. An observed increase in mean trabecular thickness, which has also been reported in a study of healthy men undergoing prolonged bedrest,(55) may be the result of the disappearance of the thinnest trabeculae, perhaps with compensation by the remaining trabeculae left to bear more weight. At both radius and tibia, cortical thickness decreased and trabecular area increased, consistent with endocortical resorption. Cortical porosity increased dramatically, with 12-month increases at radius and tibia of 18% and 22%, respectively. These impairments in density and microarchitecture translated into declines in estimated strength at both radius and tibia. It is noteworthy that detrimental changes occurred at both the non-weight-bearing radius and weight-bearing tibia, as this highlights the systemic nature of the skeletal effects of RYGB. Comparing our findings to those of 3 other cohorts that have utilized HR-pQCT after RYGB, our results are similar those of Yu et al. and Shanbhogue et al. in the observation of cortical and trabecular changes at both appendicular sites;(22, 28) in contrast, Stein et al. reported changes to cortical but not trabecular bone in 22 bariatric surgery participants, 14 of whom underwent RYGB.(27)
Our study is unique in its examination of the relative skeletal effects of RYGB by sex and menopausal status. Approximately 80% of bariatric surgery patients nationwide are women,(19) and as a result, studies of postoperative skeletal changes have included few men(20-23) or have restricted enrollment to women.(24-27) Furthermore, studies to date have included few postmenopausal women(21, 26, 28) or have excluded postmenopausal women altogether to decrease heterogeneity.(29, 30) We found that the skeletal effects of RYGB were worse for postmenopausal women than for premenopausal women or men. Postmenopausal women had lower bone mass preoperatively, as one would expect, but also they experienced more dramatic postoperative changes in skeletal health parameters. This is consistent with the results of clinical trials of nonsurgical weight loss interventions: In the POUNDS LOST diet trial, postmenopausal women demonstrated decreases in DXA-assessed BMD at the spine and femoral neck, premenopausal women only at the femoral neck, and men at neither site.(56) Trials in obese older adults, but not trials in younger adults, have consistently shown BMD declines with moderate nonsurgical weight loss.(57) Our results suggest, then, that the postmenopausal skeleton is more vulnerable to the dramatic increase in bone resorption which characterizes RYGB-induced loss of bone mass. Possibly, the heightened vulnerability is due to the postmenopausal woman's distinct sex hormone milieu or lower muscle mass. Moreover, PTH levels rose after RYGB in our cohort in association with rise in CTx, making it likely that some of the high resorption was PTH-mediated, and murine models have demonstrated sex and aging differences in skeletal responses to hyperparathyroidism.(58) Meanwhile, for the male skeleton, negative skeletal effects could be mitigated by a postoperative increase in testosterone. In our analyses, neither changes in estradiol, lean mass, nor PTH explained the differences between sex and menopausal groups, and we suspect that subtle contributions from a combination of factors may be responsible. Of note, in our analyses of potential predictors of skeletal change other than sex and menopausal status, we found that participants with greater weight loss had greater decline in proximal femur aBMD, an association reported by several other groups.(21, 27, 59) Greater increase in PTH was associated with greater declines in femoral neck aBMD and total vBMD at the radius.
If postmenopausal women are at highest risk for RYGB-induced skeletal complications, there are implications for clinical care. Guidelines for the care of the bariatric surgery patient currently include preoperative screening and postoperative monitoring of 25OHD and PTH levels with treatment of nutrient deficiencies, and postoperative calcium and vitamin D supplementation.(60-62) Guidelines differ in their approach to BMD assessment, variably recommending pre- and postoperative DXA(60, 61) or asserting that DXA should only be performed based on screening recommendations for the general population.(62) Other strategies that have been shown to attenuate the loss of the bone mass associated with non-surgical weight loss in older adults include exercise and higher protein intake.(63, 64) If postmenopausal women are particularly affected by RYGB, they might be targeted with screening and preventive or therapeutic interventions not deemed necessary for all RYGB patients. There is reason to believe that targeted interventions might be successful, as a randomized trial of a multipronged program of exercise, calcium, vitamin D, and protein supplementation was recently shown to attenuate postoperative declines in BMD, compared to no supplementation or obligatory exercise, in premenopausal women and men undergoing RYGB or sleeve gastrectomy.(65)
A limitation of our study is its 12-month duration, as we did not determine the longer-term skeletal effects of RYGB in our cohort. However, evidence from other cohorts suggests that skeletal parameters in our participants will not improve with time. In the longest prospective BMD study published to date, decreases in DXA-assessed BMD in the first postoperative year were followed by additional declines between years 1 and 3, despite mild weight regain during those years.(25) It is unclear whether and to what extent our observed changes translate to risk of fracture, although a number of studies have now documented increased fracture incidence after RYGB.(5-9) Our study is also limited by the absence of a nonsurgical control group. While our study is the largest to date to examine axial and appendicular vBMD and appendicular bone microarchitecture and strength, the sizes of the sex and menopausal subgroups were modest, and future studies should enroll larger groups of postmenopausal women and men.
In conclusion, RYGB negatively impacts axial and appendicular BMD, and appendicular bone microarchitecture and estimated strength. Effects are detectable as early as 6 months postoperatively and continue through 12 months. Postmenopausal women, for whom age and sex steroid-related bone metabolism changes already heighten risk for bone loss and fracture, are particularly affected by RYGB. Additional investigation should address strategies to avoid long-term skeletal consequences of this otherwise beneficial procedure, perhaps targeting postmenopausal women with screening and preventive or therapeutic interventions.
Supplementary Material
Acknowledgments
The authors thank Barbara Arnold for study coordination and data collection; Viva Tai, RD, MPH for DXA scan acquisition; Lisa Palermo, MA and Sheena Patel, MPH for their work in data management; Thomas Lang, PhD, Aldric Chau, and Dimitry Petrenko, DO for CT image analysis; Hanling Chang for her work on HR-pQCT phantom experiments; and Melissa Lu, Nooshin Yashar, MD, Nicole King, and Mariko Kamiya for their roles in study support and data coordination.
Grant support: This study was supported by the Department of Veterans Affairs (5 IK2 CX000549). Additional support was provided by the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through UCSF-CTSI grant UL1 TR000004, and by the National Institute of Diabetes, Digestive, and Kidney Diseases, NIH (R01 DK107629 and R21 DK112126). Manuscript contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. Laboratory assay kits were provided by Immunodiagnostic Systems (IDS), and calcium supplements were supplied by Bariatric Advantage.
Footnotes
Disclosure statement: The authors have nothing to disclose.
Authors' roles: Study design: ALS, DMS, and DMB. Study conduct: ALS, GJK, LS, SJR, JTC, AMP, DMS. Data collection: ALS, GJK. Data analysis: ALS, GJK, EV, CP. Data interpretation: ALS, GJK, TYK, DMS, DMB. Drafting manuscript: ALS. Revising manuscript content: All authors. Approving final version of manuscript: All authors. ALS takes responsibility for the integrity of the data analysis.
References
- 1.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 2.Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 3.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 4.Arterburn DE, Olsen MK, Smith VA, et al. Association between bariatric surgery and long-term survival. JAMA. 2015;313:62–70. doi: 10.1001/jama.2014.16968. [DOI] [PubMed] [Google Scholar]
- 5.Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol. 2014;2:165–74. doi: 10.1016/S2213-8587(13)70183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu EW. Bone metabolism after bariatric surgery. J Bone Miner Res. 2014;29:1507–18. doi: 10.1002/jbmr.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu CW, Chang YK, Chang HH, et al. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. Medicine (Baltimore) 2015;94:e2087. doi: 10.1097/MD.0000000000002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rousseau C, Jean S, Gamache P, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. BMJ. 2016;354:i3794. doi: 10.1136/bmj.i3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu EW, Lee MP, Landon JE, Lindeman KG, Kim SC. Fracture risk after bariatric surgery: Roux-en-Y gastric bypass versus adjustable gastric banding. J Bone Miner Res. 2017;32:1229–36. doi: 10.1002/jbmr.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folli F, Sabowitz BN, Schwesinger W, Fanti P, Guardado-Mendoza R, Muscogiuri G. Bariatric surgery and bone disease: from clinical perspective to molecular insights. Int J Obes (Lond) 2012;36:1373–9. doi: 10.1038/ijo.2012.115. [DOI] [PubMed] [Google Scholar]
- 11.Brzozowska MM, Sainsbury A, Eisman JA, Baldock PA, Center JR. Bariatric surgery, bone loss, obesity and possible mechanisms. Obes Rev. 2013;14:52–67. doi: 10.1111/j.1467-789X.2012.01050.x. [DOI] [PubMed] [Google Scholar]
- 12.Hage MP, El-Hajj Fuleihan G. Bone and mineral metabolism in patients undergoing Roux-en-Y gastric bypass. Osteoporos Int. 2014;25:423–39. doi: 10.1007/s00198-013-2480-9. [DOI] [PubMed] [Google Scholar]
- 13.Scibora LM. Skeletal effects of bariatric surgery: examining bone loss, potential mechanisms and clinical relevance. Diabetes Obes Metab. 2014;16:1204–13. doi: 10.1111/dom.12363. [DOI] [PubMed] [Google Scholar]
- 14.Schafer AL, Weaver CM, Black DM, et al. Intestinal calcium absorption decreases dramatically after gastric bypass surgery despite optimization of vitamin D status. J Bone Miner Res. 2015;30:1377–85. doi: 10.1002/jbmr.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tothill P, Hannan WJ, Cowen S, Freeman CP. Anomalies in the measurement of changes in total-body bone mineral by dual-energy X-ray absorptiometry during weight change. J Bone Miner Res. 1997;12:1908–21. doi: 10.1359/jbmr.1997.12.11.1908. [DOI] [PubMed] [Google Scholar]
- 16.Van Loan MD. Is dual-energy X-ray absorptiometry ready for prime time in the clinical evaluation of body composition? Am J Clin Nutr. 1998;68:1155–6. doi: 10.1093/ajcn/68.6.1155. [DOI] [PubMed] [Google Scholar]
- 17.Yu EW, Thomas BJ, Brown JK, Finkelstein JS. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J Bone Miner Res. 2012;27:119–24. doi: 10.1002/jbmr.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu EW, Wewalka M, Ding SA, et al. Effects of gastric bypass and gastric banding on bone remodeling in obese patients with type 2 diabetes. J Clin Endocrinol Metab. 2016;101:714–22. doi: 10.1210/jc.2015-3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuchs HF, Broderick RC, Harnsberger CR, et al. Benefits of bariatric surgery do not reach obese men. J Laparoendosc Adv Surg Tech A. 2015;25:196–201. doi: 10.1089/lap.2014.0639. [DOI] [PubMed] [Google Scholar]
- 20.Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL. Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab. 2004;89:1061–5. doi: 10.1210/jc.2003-031756. [DOI] [PubMed] [Google Scholar]
- 21.Fleischer J, Stein EM, Bessler M, et al. The decline in hip bone density after gastric bypass surgery is associated with extent of weight loss. J Clin Endocrinol Metab. 2008;93:3735–40. doi: 10.1210/jc.2008-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu EW, Bouxsein ML, Putman MS, et al. Two-year changes in bone density after Roux-en-Y gastric bypass surgery. J Clin Endocrinol Metab. 2015;100:1452–9. doi: 10.1210/jc.2014-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ivaska KK, Huovinen V, Soinio M, et al. Changes in bone metabolism after bariatric surgery by gastric bypass or sleeve gastrectomy. Bone. 2017;95:47–54. doi: 10.1016/j.bone.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Carrasco F, Ruz M, Rojas P, et al. Changes in bone mineral density, body composition and adiponectin levels in morbidly obese patients after bariatric surgery. Obes Surg. 2009;19:41–6. doi: 10.1007/s11695-008-9638-0. [DOI] [PubMed] [Google Scholar]
- 25.Vilarrasa N, San Jose P, Garcia I, et al. Evaluation of bone mineral density loss in morbidly obese women after gastric bypass: 3-year follow-up. Obes Surg. 2011;21:465–72. doi: 10.1007/s11695-010-0338-1. [DOI] [PubMed] [Google Scholar]
- 26.Casagrande DS, Repetto G, Mottin CC, et al. Changes in bone mineral density in women following 1-year gastric bypass surgery. Obes Surg. 2012;22:1287–92. doi: 10.1007/s11695-012-0687-z. [DOI] [PubMed] [Google Scholar]
- 27.Stein EM, Carrelli A, Young P, et al. Bariatric surgery results in cortical bone loss. J Clin Endocrinol Metab. 2013;98:541–9. doi: 10.1210/jc.2012-2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shanbhogue VV, Stoving RK, Frederiksen KH, et al. Bone structural changes after gastric bypass surgery evaluated by HR-pQCT: a two-year longitudinal study. Eur J Endocrinol. 2017;176:685–93. doi: 10.1530/EJE-17-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrasco F, Basfi-Fer K, Rojas P, et al. Changes in bone mineral density after sleeve gastrectomy or gastric bypass: relationships with variations in vitamin D, ghrelin, and adiponectin levels. Obes Surg. 2014;24:877–84. doi: 10.1007/s11695-014-1179-0. [DOI] [PubMed] [Google Scholar]
- 30.Muschitz C, Kocijan R, Marterer C, et al. Sclerostin levels and changes in bone metabolism after bariatric surgery. J Clin Endocrinol Metab. 2015;100:891–901. doi: 10.1210/jc.2014-3367. [DOI] [PubMed] [Google Scholar]
- 31.Hacker-Thompson A, Robertson TP, Sellmeyer DE. Validation of two food frequency questionnaires for dietary calcium assessment. J Am Diet Assoc. 2009;109:1237–40. doi: 10.1016/j.jada.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lang TF, Li J, Harris ST, Genant HK. Assessment of vertebral bone mineral density using volumetric quantitative CT. J Comput Assist Tomogr. 1999;23:130–7. doi: 10.1097/00004728-199901000-00027. [DOI] [PubMed] [Google Scholar]
- 33.Engelke K, Mastmeyer A, Bousson V, Fuerst T, Laredo JD, Kalender WA. Reanalysis precision of 3D quantitative computed tomography (QCT) of the spine. Bone. 2009;44:566–72. doi: 10.1016/j.bone.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Harris TB, Launer LJ, Eiriksdottir G, et al. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res. 2008;23:392–9. doi: 10.1359/jbmr.071108. [DOI] [PubMed] [Google Scholar]
- 36.Melton LJ, 3rd, Riggs BL, van Lenthe GH, et al. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res. 2007;22:1442–8. doi: 10.1359/jbmr.070514. [DOI] [PubMed] [Google Scholar]
- 37.Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res. 2007;22:425–33. doi: 10.1359/jbmr.061206. [DOI] [PubMed] [Google Scholar]
- 38.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90:6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 39.Khosla S, Riggs BL, Atkinson EJ, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21:124–31. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laib A, Hauselmann HJ, Ruegsegger P. In vivo high resolution 3D-QCT of the human forearm. Technol Health Care. 1998;6:329–37. [PubMed] [Google Scholar]
- 41.Laib A, Ruegsegger P. Comparison of structure extraction methods for in vivo trabecular bone measurements. Comput Med Imaging Graph. 1999;23:69–74. doi: 10.1016/s0895-6111(98)00071-8. [DOI] [PubMed] [Google Scholar]
- 42.Buie HR, Campbell GM, Klinck RJ, MacNeil JA, Boyd SK. Automatic segmentation of cortical and trabecular compartments based on a dual threshold technique for in vivo micro-CT bone analysis. Bone. 2007;41:505–15. doi: 10.1016/j.bone.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Burghardt AJ, Buie HR, Laib A, Majumdar S, Boyd SK. Reproducibility of direct quantitative measures of cortical bone microarchitecture of the distal radius and tibia by HR-pQCT. Bone. 2010;47:519–28. doi: 10.1016/j.bone.2010.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tjong W, Nirody J, Burghardt AJ, Carballido-Gamio J, Kazakia GJ. Structural analysis of cortical porosity applied to HR-pQCT data. Med Phys. 2014;41:013701. doi: 10.1118/1.4851575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Macneil JA, Boyd SK. Bone strength at the distal radius can be estimated from high-resolution peripheral quantitative computed tomography and the finite element method. Bone. 2008;42:1203–13. doi: 10.1016/j.bone.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Mueller TL, Christen D, Sandercott S, et al. Computational finite element bone mechanics accurately predicts mechanical competence in the human radius of an elderly population. Bone. 2011;48:1232–8. doi: 10.1016/j.bone.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Kazakia GJ, Tjong W, Nirody JA, et al. The influence of disuse on bone microstructure and mechanics assessed by HR-pQCT. Bone. 2014;63:132–40. doi: 10.1016/j.bone.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonaretti S, Vilayphiou N, Chan CM, et al. Operator variability in scan positioning is a major component of HR-pQCT precision error and is reduced by standardized training. Osteoporos Int. 2017;28:245–57. doi: 10.1007/s00198-016-3705-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sekhon K, Kazakia GJ, Burghardt AJ, Hermannsson B, Majumdar S. Accuracy of volumetric bone mineral density measurement in high-resolution peripheral quantitative computed tomography. Bone. 2009;45:473–9. doi: 10.1016/j.bone.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–35. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 51.Kim Y, Park I, Kang M. Convergent validity of the international physical activity questionnaire (IPAQ): meta-analysis. Public Health Nutr. 2013;16:440–52. doi: 10.1017/S1368980012002996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones G, Nguyen T, Sambrook PN, Kelly PJ, Eisman JA. A longitudinal study of the effect of spinal degenerative disease on bone density in the elderly. J Rheumatol. 1995;22:932–6. [PubMed] [Google Scholar]
- 53.Van Gompel G, Van Slambrouck K, Defrise M, et al. Iterative correction of beam hardening artifacts in CT. Med Phys. 2011;38(Suppl 1):S36. doi: 10.1118/1.3577758. [DOI] [PubMed] [Google Scholar]
- 54.Yu EW, Bouxsein ML, Roy AE, et al. Bone loss after bariatric surgery: discordant results between DXA and QCT bone density. J Bone Miner Res. 2014;29:542–50. doi: 10.1002/jbmr.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belavy DL, Beller G, Ritter Z, Felsenberg D. Bone structure and density via HR-pQCT in 60d bed-rest, 2-years recovery with and without countermeasures. J Musculoskelet Neuronal Interact. 2011;11:215–26. [PubMed] [Google Scholar]
- 56.Tirosh A, de Souza RJ, Sacks F, Bray GA, Smith SR, LeBoff MS. Sex differences in the effects of weight loss diets on bone mineral density and body composition: POUNDS LOST Trial. J Clin Endocrinol Metab. 2015;100:2463–71. doi: 10.1210/jc.2015-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schafer AL. Decline in bone mass during weight loss: a cause for concern? J Bone Miner Res. 2016;31:36–9. doi: 10.1002/jbmr.2754. [DOI] [PubMed] [Google Scholar]
- 58.Cheng Z, Liang N, Chen TH, et al. Sex and age modify biochemical and skeletal manifestations of chronic hyperparathyroidism by altering target organ responses to Ca2+ and parathyroid hormone in mice. J Bone Miner Res. 2013;28:1087–100. doi: 10.1002/jbmr.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maghrabi AH, Wolski K, Abood B, et al. Two-year outcomes on bone density and fracture incidence in patients with T2DM randomized to bariatric surgery versus intensive medical therapy. Obesity (Silver Spring) 2015;23:2344–8. doi: 10.1002/oby.21150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95:4823–43. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 61.Mechanick JI, Youdim A, Jones DB, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient--2013 update: cosponsored by American Association of Clinical Endocrinologists, The Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21(1):S1–27. doi: 10.1002/oby.20461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim J, Brethauer S ASMBS Clinical Issues Committee. Metabolic bone changes after bariatric surgery. Surg Obes Relat Dis. 2014 doi: 10.1016/j.soard.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 63.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–9. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sukumar D, Ambia-Sobhan H, Zurfluh R, et al. Areal and volumetric bone mineral density and geometry at two levels of protein intake during caloric restriction: a randomized, controlled trial. J Bone Miner Res. 2011;26:1339–48. doi: 10.1002/jbmr.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Muschitz C, Kocijan R, Haschka J, et al. The Impact of vitamin D, calcium, protein supplementation, and physical exercise on bone metabolism after bariatric surgery: The BABS Study. J Bone Miner Res. 2016;31:672–82. doi: 10.1002/jbmr.2707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.