Abstract
Background
Concentrations of antiretrovirals (ARVs) in the genital tract play a key role in pre-exposure prophylaxis. This study aims to describe rilpivirine (Edurant®) concentrations in the genital tract in pregnant and postpartum women.
Methods
International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1026s is an ongoing, prospective study of antiretroviral pharmacokinetics (PK) in HIV infected pregnant women that include a cohort receiving rilpivirine combination regimen. Intensive PK evaluations were performed at steady state during the second and third trimester, and postpartum. Plasma and directly-aspirated cervicovaginal fluid (CVF) samples were collected at 4 timepoints around an observed dose, and measured using high-performance liquid chromatography with ultraviolet detection, (plasma; lower limit of quantification (LLQ) = 10ng/mL) or liquid chromatography-tandem mass spectrometry (CVF; LLQ = 1ng/mL).
Results
A total of 24 women were included in the analysis. For all time points combined, median (Interquartile range, IQR) rilpivirine concentrations were 70ng/ml (23–121) in CVF and 92 ng/ml (49–147) in plasma. The CVF to plasma AUC (0-4) ratios were significantly higher in the 2nd (0.90, 90% CI 0.61–1.46) and 3rd trimesters of pregnancy compared to postpartum (0.40, 90% CI 0.19–0.87). Three of 189 (1.6%) plasma samples in two women were below the LLQ as well as the corresponding CVF concentrations. Seventeen additional CVF concentrations (10.6%) were below LLQ in 13 participants. No major safety concerns were noted.
Conclusions
Rilpivirine concentrations were higher in the CVF during pregnancy compared to postpartum. CVF Rilpivirine is likely to achieve inhibitory concentrations effective for preventing peripartum HIV transmission.
Keywords: Rilpivirine, pregnancy, cervicovaginal fluid, post-partum
INTRODUCTION:1
Many ARVs have been shown to reduce mother to child transmission of HIV.1,2 Genital tract concentrations of ARVs may play a key role in preventing perinatal HIV transmission by reducing viral replication in this compartment. 3 This is critical because several studies have demonstrated that HIV viral load in the female genital tract is independently associated with the risk of mother-to-child HIV transmission 1,2,4–6. While there has been considerable research describing ARV concentrations in the genital tracts of men and non-pregnant women, studies in pregnant women have been limited. 7,8. Although pharmacokinetic analyses of mucosal tissue drug concentrations typically involve invasive biopsies, these techniques limit the number of samples that can safely be obtained from pregnant women, increases cost and difficulty associated with sample collection and makes storage and processing for drug quantification difficult. Therefore, recent studies use CVF as surrogates to cervicovaginal tissue biopsy 9.
The physiological changes during pregnancy impact the pharmacokinetics of most ARVs, and some ARV’s may require dose adjustment during pregnancy in order to maintain optimal pharmacokinetic exposure 8. The extent of penetration through the genital tract in non-pregnant women has been previously shown to be constant regardless of the number of doses given, reflecting a constant relationship between systemic and genital drug exposure 10 In the only published study reporting female genital tract ARV concentrations during pregnancy, genital tract/plasma ratios for zidovudine and lopinavir were significantly lower than those in non-pregnant women, suggesting that genital tract drug concentrations from non-pregnant women cannot be extrapolated to pregnant women.8,10
Pregnancy PK data have been described for some of the newer antiretroviral agents. Currently available data suggest that with standard adult dosing, plasma concentrations of some ARVs (especially protease inhibitors) are reduced during the second and/or third trimesters 6,11,12. Rilpivirine is the newest of five non-nucleoside reverse transcriptase inhibitors (NNRTIs) approved by the Food and Drug Administration 13. Rilpivirine is recognized for its ability to inhibit HIV-1 replication, adaptability to reverse transcriptase (RT) mutations, high oral bioavailability and long half-life, which allows for 25mg once-daily oral dosing in antiretroviral naïve adults with HIV-1 RNA copies less than 100,000 copies/mL 14,15. In a PK study of rilpivirine in pregnant women, area under the curve (AUC) during the second and third trimester were reduced by 20–33% compared to postpartum 16,17. However, genital tract concentrations of rilpivirine have not been previously studied or described in pregnant women. The primary objective of this study was to investigate the concentrations of rilpivirine in the female genital tract and to compare the concentrations between pregnancy and postpartum.
METHODS
Data were collected as part of International Maternal Pediatric Adolescent AIDS Clinical Trials Protocol P1026s, an ongoing, multicenter, non-blinded, prospective Phase IV study of the pharmacokinetics and safety of selected ARVs in HIV infected pregnant women that included an arm for pregnant women at US sites receiving rilpivirine.17 The study is registered in ClinicalTrials.gov [NCT00042289]. For eligibility, HIV-infected women (≥ 20 weeks gestation until 12 weeks postpartum), not on tuberculosis treatment, and receiving rilpivirine were included in the rilpivirine arm. Local institutional review boards approved P1026s at all participating sites, and the study followed all relevant human subject research guidelines. All participants provided signed informed consent before participation. HIV-infected pregnant women receiving rilpivirine 25 mg orally once daily as part of clinical care before the beginning of the 35th week of pregnancy and expected to continue on treatment until at least six weeks postpartum were eligible to enroll in the rilpivirine arm of P1026s. All antiretroviral medications were prescribed by primary care providers and dispensed by local pharmacies, as per the sites’ standard of care. Maternal exclusion criteria were current use of medications known to interfere with rilpivirine metabolism, including dexamethasone, omeprazole, and phenytoin, multiple gestation, or clinical or laboratory toxicity that, per site investigator, would require a change in the antiretroviral regimen. Mothers and their infants continued in the study until 6 months after delivery. Infant HIV status was evaluated at 24 weeks of life by physical examination and chart abstraction.
Clinical and laboratory monitoring
Maternal demographic and clinical information were extracted from the medical record, including maternal HIV-1 RNA, CD4+ lymphocyte count, maternal age, ethnicity, weight and concomitant medications. Background regimen were similar for all women throughout the evaluation period. Plasma HIV-1 RNA assays were performed locally. Study mothers and infants were followed for clinical and laboratory toxicities through six months after delivery. Neonatal gestational age at the time of delivery, birth weight and HIV infection status data were collected from the infant’s medical record. Physical examinations were performed on neonates after delivery, and infant laboratory evaluations were performed only as clinically indicated.
Sample collection and drug assays
Cervicovaginal fluid and plasma samples were collected pre-dose and at 1, 2, and 4 hours post-dose during second trimester (20–26 weeks of gestation), third trimester (30–38 weeks of gestation), and postpartum (6–12 weeks after delivery) visits. Cervicovaginal fluid samples were collected by direct aspiration and blood samples by venipuncture. On the day of sampling, rilpivirine was given as an observed dose with a meal consisting of at least 500 calories.
Cervicovaginal secretions were collected directly using a soft plastic aspirator (UNC Center for AIDS Research Vaginal Specimen Aspirator; CarTika Medical, Inc) or by other methods, such as a swipe with a gloved finger, for women who had difficulty using the aspirator. Cervicovaginal fluid samples were collected by the participant or by the clinician. Aspirates were placed into 2mL pre-weighed vials that were then reweighed at the sites. For the rilpivirine samples, mean sample weight was 0.13 grams, with range of 0.01 to 0.93 grams. The samples were stored at −70°C or colder. Rilpivirine concentrations in cervicovaginal fluid were measured by liquid chromatography-tandem mass spectrometry (LC-MS/MS) at the University of North Carolina Center for AIDS Research’s Clinical Pharmacology and Analytical Chemistry Core with a lower limit of quantitation of 1 ng/mL. The CVF assay utilized stable isotopically-labeled rilpivirine-d6 as an internal standard and was linear over the concentration range of 1–500ng/mL. Rilpivirine in CVF was analyzed in three runs with an average correlation coefficient of 0.9976. Quality control samples over the three runs demonstrated between-assay precision (%CV) from 3.87% to 6.58% coefficient of variation (CV), and accuracy ranging from −2.13% to 11.50% deviation, All calibration standards and quality control samples were within 15% of their nominal value.
Plasma rilpivirine concentrations were measured by high-performance liquid chromatography with ultraviolet detection at the University of California, San Diego Pediatric Pharmacology Laboratory. Mean recovery of drug from plasma was 99.1 %. The plasma method was linear over the concentration range of 10–2560 ng/mL, with a lower limit of quantitation of 10 ng/mL. Linearity was evaluated over three days, and had an average correlation coefficient of 0.9992 from 3 curves. For all validation samples, the between-assay precision and accuracy ranged from 4.06% to 9.04% coefficient of variation (CV), and −9.39% to 7.62% deviation, respectively. Rilpivirine was stable in plasma stored at −70°C for 2 years.
Pharmacokinetic and statistical analysis
Cervicovaginal fluid and plasma rilpivirine concentrations were analyzed using standard descriptive statistics and are presented as medians with interquartile range. Areas under the concentration time curve (AUC) for cervicovaginal fluid and plasma from pre-dose concentration (C0) to 4 hours post dose (AUC0-4) was estimated using the trapezoidal rule. The ratio of cervicovaginal fluid AUC to plasma AUC at each study visit were determined. Within-participant comparisons (e.g., between second versus third trimester) was performed for continuous outcome measures using the Wilcoxon signed-rank test and for dichotomous outcome measures using McNemar’s test. Between-participant comparisons was performed for continuous outcome measures using the Wilcoxon rank-sum test and for dichotomous outcome measures using the chi-square or Fisher exact test. 90% confidence limits for the geometric mean ratio of the PK exposure parameters were calculated to describe the range of values that are consistent with the observed data to assess whether there was a clinically significant difference in exposure. The 90% rather than 95% confidence interval was used to match the usual practice in the pharmacokinetic literature. Pairwise comparisons of cervicovaginal AUC, plasma AUC and their ratio within each subject during the second trimester and third trimester compared to postpartum were performed using a two-sided Wilcoxon signed rank test with p < 0.01 considered statistically significant.
RESULTS
The study enrolled 24 women with cervicovaginal fluid and plasma concentration data available for ten women in the second trimester, seventeen in the third trimester and nineteen postpartum. Maternal demographic and clinical characteristics of the participants and the pregnancy outcomes are described in Table 1. Twelve of 24 (50.0%) of the mothers were black, eleven of the mothers were Hispanic (46%), and one woman was white (non-Hispanic), with a median age of 27 years (interquartile range [IQR], 17 to 38). No congenital anomalies were identified by prenatal ultrasound or physical examination at the time of birth. The mean gestational age at the time of sampling in the 2nd trimester was 24 weeks (interquartile range [IQR], 22 to 26 weeks). The mean gestational age at the time of sampling in the 3nd trimester was 34 weeks (interquartile range [IQR], 29 to 35 weeks), and median postpartum sampling time was 9 weeks after delivery (interquartile range [IQR], 6 to 12 weeks postpartum).
Table 1.
Participant demographics – Table 1 describes the demographics of participants that were recruited into the rilpivirine pharmacokinetic study.
| Age at delivery (years) | 26.8 (17.2–37.6) |
| Weight at delivery (kg) | 92 (60.9–131.8) |
| Race/Ethnicity | |
| White | 1 (4%) |
| Black | 12 (50%) |
| Hispanic | 11 (46%) |
| 2nd Trimester | |
| Gestational age | 23.6 (21.7 – 26.7) |
| HIV-1 RNA (≤ 50 copies/mL) | 7/10 (70%) |
| CD4+ cells (cells/mm3) | 565 (293–828) |
| 3rd Trimester | |
| Gestational age | 33.6 (29.3–35.0) |
| HIV-1 RNA (≤ 50 copies/mL) | 14/17 (82%) |
| CD4+ cells (cells/mm3) | 554 (297–1147) |
| Postpartum | |
| Weeks after delivery | 9.3 (6.1–12.4) |
| HIV-1 RNA (≤ 50 copies/mL) | 13/18 (72%) |
| CD4+ cells (cells/mm3) | 693 (185–1180) |
| Pregnancy outcomes | |
| Gestational age (weeks) | 38.9 (32.3–41.4) |
| Birth weight (grams) | 3075 (1570–4570) |
| Infection status | |
| Uninfected by best available data | 24/24 (100%) |
Interquartile ranges (IQR) are in brackets. 90% confidence intervals (CIs) were used for analysis.
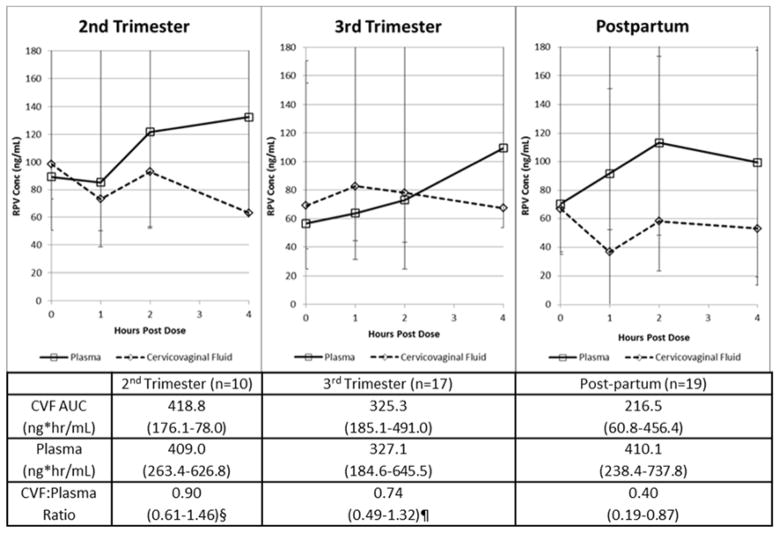
Maternal plasma HIV-1 RNA was noted to be less than or equal to 50 copies (≤ 50 copies/mL) in 7 of 10 (70%) participants during the second trimester, 13 of 17 (82%) participants during the third trimester and 13 of 18 (72%) participants postpartum. All infants in the cohort were uninfected. Three of 189 (1.6%) plasma samples in two women were below the quantitative limit for rilpivirine; the corresponding CVF concentrations were also below quantitation for rilpivirine. Seventeen additional CVF concentrations out of 189 (10.6%) were below quantitation in 13 women. When all time points were combined, median (IQR) rilpivirine concentrations were 70 ng/mL (23 – 121) in cervicovaginal fluids and 92 ng/mL (49–147) in plasma. Median cervicovaginal fluid and plasma rilpivirine concentrations for each study visit are shown in Figure 1.
Figure 1. Rilpivirine Curves (2nd trimester, 3rd trimester, postpartum).
Figure 1 shows the rilpivirine curves, including the cervicovaginal fluid AUC, plasma concentration and CVF/plasma ratios across the 2nd trimester, 3rd trimester and postpartum.
2nd trimester vs postpartum, p=0.02; 3rd trimester vs postpartum, p=0.06
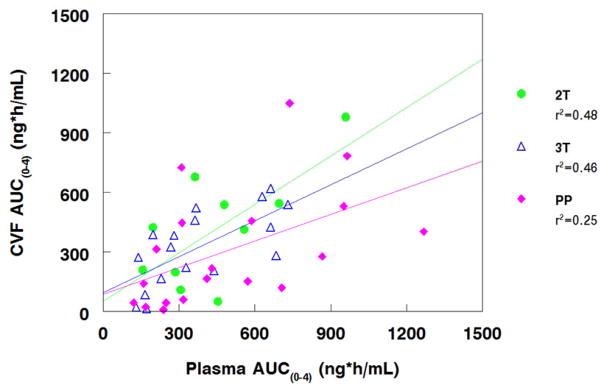
Median (IQR) rilpivirine cervicovaginal fluid and plasma AUC0-4 and their ratio for the second trimester, third trimester and postpartum visits are presented in Table 2. Median AUC (0-4) of rilpivirine in cervicovaginal fluid ranged from 419 ng*hr/mL in the second trimester of pregnancy to 217 ng*hr/mL postpartum. Plasma AUC (0-4) of rilpivirine ranged from 409 ng*hr/mL in the second trimester of pregnancy to 327 ng*hr/mL in third trimester and then 410 ng*hr/mL in postpartum. The cervicovaginal fluid to plasma AUC0-4 ratios were significantly higher in the 2nd and 3rd trimesters of pregnancy compared to postpartum due to differential increase in rilpivirine concentrations in cervicovaginal fluid compared to plasma (Figure 1, Table 2) (p<0.05 for second trimester vs postpartum, and p=0.04 for third trimester versus postpartum. Cervicovaginal and plasma AUC0-4 were moderately correlated antepartum and weakly correlated postpartum (Figure 2).
Table 2.
Rilpivirine cervicovaginal fluid (CVF) and plasma pharmacokinetics – Table 2 describes the cervicovaginal fluid parameters in the 2nd and 3rd trimesters, including postpartum. Area under the curves (AUCs) were described across all the 2 trimesters and postpartum.
| Parameter median (IQR) | 2nd trimester | 3rd trimester | Postpartum |
|---|---|---|---|
| CVF AUC (ng* hr/mL) | 419 (176–578) | 325 (185–491) | 217 (61–456) |
| Plasma AUC (ng* hr/mL) | 409 (263–627) | 327 (185–646) | 410 (238–738) |
| CVF:Plasma AUC Ratio | 0.90 (0.61–1.46)* | 0.74 (0.49–1.32)** | 0.40 (0.19–0.87) |
AUC – Area under the curve
2nd trimester versus postpartum (p=0.02)
3rd trimester versus postpartum (p=0.04).
Figure 2. CVF and Plasma AUC Correlations.
Figure 2 shows the cervicovaginal fluid and plasma AUC correlations
DISCUSSION
This study included pregnant and postpartum women who are HIV infected and being treated with combination regimen that included a 25 mg rilpivirine tablet (Edurant®). For all time points combined, median (IQR) rilpivirine concentrations were higher in plasma (70ng/mL in cervicovaginal fluid and 92 ng/ml in plasma). Although rilpivirine plasma AUC concentrations decreased by 19.9% between the 2nd and 3rd trimesters of pregnancy, the cervicovaginal to plasma AUC(0-4) ratios of rilpivirine were significantly higher in the 2nd and 3rd trimesters of pregnancy compared to postpartum due to increased rilpivirine concentrations in cervicovaginal fluid. A prior study in non-pregnant women describing the pharmacokinetics of rilpivirine in CVF of women taking daily rilpivirine showed that rilpivirine concentrations in the CVF were sufficient for inhibiting HIV infection in this compartment.18 A similar effect of pregnancy on rilpivirine exposure was seen in analysis of the full pharmacokinetic profiles from the P1026s rilpivirine arm 17. The decrease in rilpivirine plasma AUC in our study between the 2nd and 3rd trimesters of pregnancy was less than 20%, and was not associated with virological failure or with mother to child transmission of HIV.
Quantifying drug concentrations in body compartments with which the neonate has exposure during pregnancy and the postpartum period (genital tract, cord blood plasma, and amniotic fluid) may aid in selecting drug regimens, and may have implications for mother to child transmission of HIV, as well as for pre-exposure prophylaxis. Findings from this study show that the cervicovaginal to plasma AUC0-4 ratios were statistically significantly higher in the 2nd and 3rd trimesters of pregnancy compared to postpartum (0.90, 0.74 and 0.40 respectively). However, in a pharmacokinetic compartmental analysis of genital tract, umbilical cord blood and amniotic fluid exposures study of seven older antiretroviral drugs (lamivudine, zidovudine, tenofovir, nelfinavir, lopinavir-ritonavir, nevirapine) during pregnancy and postpartum in HIV Type 1-infected women, no statistically significant differences in genital tract penetration were observed for any of the ARVs between the second and third trimesters, with only nelfinavir genital tract penetration being significantly higher postpartum compared to the second trimester and third trimester respectively 8. Tissue- specific pharmacokinetics may help explain these findings.
Tissue-specific pharmacokinetics of drugs for HIV prevention may help us distinguish populations that are more likely to benefit from their use 19. For example, in a 2009 pharmacokinetic study, maraviroc cervicovaginal fluid concentrations 72 hours after dose and plasma concentrations 12 hours after dose were similar 20. In our study, in addition to the cervicovaginal to plasma AUC0-4 ratios being statistically significantly higher in the 2nd and 3rd trimesters of pregnancy compared to postpartum, the median CVF rilpivirine concentration was noted to be more than 100 fold above the protein-free EC90 for rilpivirine (0.66 ng/mL). Protein binding needs to be taken into account, as the free rilpivirine concentration in cervicovaginal fluid needs to exceed the EC90, not just the EC50, to develop full antiviral effect 21. It is plausible that these finding might not be a true reflection of rilpivirine concentrations in the genital tract because binding proteins are altered in the female genital tract compared to plasma during pregnancy 22. Hence, protein free EC90 may be more reflective of inhibitory concentrations of rilpivirine (as rilpivirine is 99.7% bound to plasma proteins)23. Accumulation of rilpivirine in cervicovaginal fluid may also be driven by physicochemical properties, in particular by lipophilicity 23. Since rilpivirine is highly lipophilic (pKa of 5.6, log P = 4.86) and has high accumulation levels in multiple cell types, it shows high cellular penetration 13.
Differential drug metabolism and drug transport in the female genital tract relative to plasma can also be a possible explanation for the difference in rilpivirine concentrations in cervicovaginal fluid versus plasma. Recent studies of mRNA expression of CYP enzymes in cervical tissues demonstrated that cytochrome P450 CYP activity present in cervical and vaginal tissue are markedly different from those expressed in the liver 24. Rilpivirine is primarily metabolized by cytochrome P450 (CYP)3A, and drugs that induce or inhibit CYP3A may thus affect the clearance of rilpivirine. Co-administration of rilpivirine and drugs that induce CYP3A may result in decreased plasma concentrations of rilpivirine and loss of virologic response and possible resistance to rilpivirine. Co-administration of rilpivirine and drugs that inhibit CYP3A may result in increased plasma concentrations of rilpivirine.
Our study has several strengths. This is the first study to report the cervicovaginal concentrations of rilpivirine in pregnancy. The participants in our study were followed longitudinally over time, and the collection of clinical findings related to rilpivirine exposure occurred at regular time intervals, so recall error or bias, systematic bias and confounding by genetic, sociodemographic and other individual characteristics were minimized. Any random measurement error that arises from the study would tend to diminish apparent effect size, causing estimates to be conservative. There was a high rate of follow up for mothers and neonates. The collection of samples followed a strict protocol with observed dosing to minimize errors due to sample collection.
This study had its limitations. First, the population studied within this network is mainly black or Hispanic, with only a limited number of non-Hispanic white patients included, so that limitations of generalizability may exist. Second, although methods for collecting samples were standardized, there may be still be limitations associated with some technical aspects of collecting cervicovaginal specimens from patients due to human related factors. For example, differences in collecting cervicovaginal samples with or without a vaginal speculum; swiping with a gloved finger versus using an aspirator for women who had problems with the aspirator. Third, there is still limited information available regarding protein binding in cervicovaginal fluid, which may limit interpretation of such data in the context of related plasma concentration data, although it is possible to make some educated guesses based on what is known about the concentrations of albumin and alpha1-acid glycoprotein (AAG) in cervicovaginal fluid and what proteins rilpivirine binds to. In addition, we did not measure unbound concentrations, thus limiting our ability to assess pharmacologically active rilpivirine. Fourth, we did not study the effects of genital inflammation on rilpivirine levels. Vaginal microbiome has been shown to affect the levels of ARVs when used topically, and bacterial vaginosis is common during pregnancy and postpartum. It is possible that genital inflammation in these women might have contributed to lower levels of rilpivirine in the CVF.25 Fifth, we did not assess the effect of co-administration of rilpivirine with other medications in pregnancy, especially antacids (women on antacids were excluded from the study). In prior pharmacokinetic studies, rilpivirine AUC concentration decreased by 76% with famotidine 40 mg, and by 40% with omeprazole (proton pump inhibitors cannot be coadministered with rilpivirine and H2 antagonists can be used only if taken more than 12 h before or 4 h after rilpivirine).26,27 Sixth, a major drawback of our study includes a small sample size.
In conclusion, our findings confirm that concentrations of rilpivirine in the genital tract correlate with plasma concentrations, with higher concentrations in the cervicovaginal fluid during pregnancy than during the postpartum period. This could be pertinent not only to viral suppression during pregnancy and delivery and prevention of mother to child transmission of HIV (PMTCT), but, could also be an important new prevention drug for uninfected pregnant women for pre-exposure prophylaxis.
Acknowledgments
We would like to thank all the women who participated in the rilpivirine arm of the P1026s protocol, the sites that participated in this study and all the members of the P1026s protocol team. We would also like to thank Ruili Wang for her help in performing the vaginal secretions assays.
Footnotes
The abstract of this paper was presented at the Conference on Retroviruses and Opportunistic Infections (CROI), Hynes Convention Center, Boston, MA; February 22–25th, 2016.
- Angela Kashuba, Professor of Pharmacotherapy and Experimental Therapeutics is funded by The University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410.
- Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Network (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
- For the remaining authors none were declared.
References
- 1.Cu-Uvin S, DeLong AK, Venkatesh KK, et al. Genital tract HIV-1 RNA shedding among women with below detectable plasma viral load. AIDS. 2010;24(16):2489–2497. doi: 10.1097/QAD.0b013e32833e5043. [DOI] [PubMed] [Google Scholar]
- 2.Cu-Uvin S, Snyder B, Harwell JI, et al. Association between paired plasma and cervicovaginal lavage fluid HIV-1 RNA levels during 36 months. J Acquir immune Def Syndr. 2006;42(5):584–587. doi: 10.1097/01.qai.0000229997.52246.95. [DOI] [PubMed] [Google Scholar]
- 3.Clavel C, Peytavin G, Tubiana R, et al. Etravirine concentrations in the cervicovaginal compartment in HIV-1-infected women receiving etravirine-containing antiretroviral therapy: DIVA 02 study. Antimicrob Agents Chemother. 2012;56(7):4018–4020. doi: 10.1128/AAC.06474-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Kahle E, Lingappa JR, et al. Genital HIV-1 RNA predicts risk of heterosexual HIV-1 transmission. Sci Transl Med. 2011;3(77):77ra29. doi: 10.1126/scitranslmed.3001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia-Bujalance S, Ruiz G, De Guevara CL, et al. Quantitation of human immunodeficiency virus type 1 RNA loads in cervicovaginal secretions in pregnant women and relationship between viral loads in the genital tract and blood. Eur J of Clin Microb Infect Dis. 2004;23(2):111–115. doi: 10.1007/s10096-003-1058-4. [DOI] [PubMed] [Google Scholar]
- 6.Patterson KB, Dumond JB, Prince HA, et al. Protein binding of lopinavir and ritonavir during 4 phases of pregnancy: implications for treatment guidelines. J Acquir Immune Def Syndr. 2013;63(1):51–58. doi: 10.1097/QAI.0b013e31827fd47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the fetal compartment (placenta and amniotic fluid) Antivi Ther. 2011;16(8):1139–1147. doi: 10.3851/IMP1918. [DOI] [PubMed] [Google Scholar]
- 8.Yeh RF, Rezk NL, Kashuba AD, et al. Genital tract, cord blood, and amniotic fluid exposures of seven antiretroviral drugs during and after pregnancy in human immunodeficiency virus type 1-infected women. Antimicrob Agents Chemother. 2009;53(6):2367–2374. doi: 10.1128/AAC.01523-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cottrell ML, Prince HM, Allmon A, et al. Cervicovaginal and Rectal Fluid as a Surrogate Marker of Antiretroviral Tissue Concentration: Implications for Clinical Trial Design. J Acquir immune Def Syndr. 2016;72(5):498–506. doi: 10.1097/QAI.0000000000000996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Else LJ, Taylor S, Back DJ, Khoo SH. Pharmacokinetics of antiretroviral drugs in anatomical sanctuary sites: the male and female genital tract. Antivir Ther. 2011;16(8):1149–1167. doi: 10.3851/IMP1919. [DOI] [PubMed] [Google Scholar]
- 11.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir immune Def Syndr. 2010;54(4):381–388. doi: 10.1097/qai.0b013e3181d6c9ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirochnick M, Best BM, Stek AM, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir immune Def Syndr. 2008;49(5):485–491. doi: 10.1097/QAI.0b013e318186edd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Usach I, Melis V, Peris JE. Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. J Int AIDS Soc. 2013;16:1–14. doi: 10.7448/IAS.16.1.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das K, Bauman JD, Clark AD, Jr, et al. High-resolution structures of HIV-1 reverse transcriptase/TMC278 complexes: strategic flexibility explains potency against resistance mutations. Proc Natl Acad Sci USA. 2008;105(5):1466–1471. doi: 10.1073/pnas.0711209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvey L, Winston A. Rilpivirine: a novel non-nucleoside reverse transcriptase inhibitor. Expert Opin Investig Drugs. 2009;18(7):1035–1041. doi: 10.1517/13543780903055056. [DOI] [PubMed] [Google Scholar]
- 16.OARAC. [Accessed 8/22, 2017];Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. http://aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf.
- 17.Tran AH, Best BM, Stek A, et al. Pharmacokinetics of Rilpivirine in HIV-Infected Pregnant Women. J Acquir immune Def Syndr. 2016;72(3):289–296. doi: 10.1097/QAI.0000000000000968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dezzutti CS, Else LJ, Yandura SE, et al. Distinct Pharmacodynamic Activity of Rilpivirine in Ectocervical and Colonic Explant Tissue. Antimicrob Agents Chemother. 2016;60(5):2765–2770. doi: 10.1128/AAC.00167-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rizk ML, Zou L, Savic RM, Dooley KE. Importance of Drug Pharmacokinetics at the Site of Action. Clin Transl Sci. 2017;10(3):133–142. doi: 10.1111/cts.12448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dumond JB, Patterson KB, Pecha AL, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir immune Def Syndr. 2009;51(5):546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Else LJ, Tjia J, Jackson A, et al. Quantification of rilpivirine in human plasma, cervicovaginal fluid, rectal fluid and genital/rectal mucosal tissues using liquid chromatography-tandem mass spectrometry. Bioanalysis. 2014;6(14):1907–1921. doi: 10.4155/bio.14.59. [DOI] [PubMed] [Google Scholar]
- 22.Zegels G, Van Raemdonck GA, Tjalma WA, Van Ostade XW. Use of cervicovaginal fluid for the identification of biomarkers for pathologies of the female genital tract. Proteome Sci. 2010;8:63. doi: 10.1186/1477-5956-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen PA, Lewi PJ, Arnold E, et al. In search of a novel anti-HIV drug: multidisciplinary coordination in the discovery of 4-[[4-[[4-[(1E)-2-cyanoethenyl]-2,6-dimethylphenyl]amino]-2- pyrimidinyl]amino]benzonitrile (R278474, rilpivirine) J Med Chem. 2005;48(6):1901–1909. doi: 10.1021/jm040840e. [DOI] [PubMed] [Google Scholar]
- 24.To EE, Hendrix CW, Bumpus NN. Dissimilarities in the metabolism of antiretroviral drugs used in HIV pre-exposure prophylaxis in colon and vagina tissues. Biochem Pharmacol. 2013;86(7):979–990. doi: 10.1016/j.bcp.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebenberg LJP, Archary D, Sivro A, Kwon DS. Bugs, drugs, and HIV: the role of the vaginal microbiome in HIV risk and antiretroviral efficacy for HIV prevention. Genome Med. 2017;9(1):74. doi: 10.1186/s13073-017-0469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Heeswijk RHR, Kestens D, et al. The pharmacokinetic (PK) interaction between famotidine and TMC278, a next generation non-nucleoside reverse transcriptase inhibitor (NNRTI), in HIV-negative subjects. 4th IAS Conf. HIV Pathog., Treat., Prev; 2007; Sydney Australia. [Google Scholar]
- 27.Crauwels HMvHP, Kestens D, et al. The pharmacokinetic (PK) interaction between omeprazole and TMC278, an investigational non-nucleoside reverse transcriptase inhibitor (NNRTI) J Int AIDS Soc. 2008;11(Suppl 1):239. [Google Scholar]