Abstract
Efficient absorption of nutrients by the intestine is essential for life. In mammals and birds, convolution of the intestinal surface into finger-like projections called villi is an important adaptation that ensures the massive surface area for nutrient contact that is required to meet metabolic demands. Each villus projection serves as a functional absorptive unit: it is covered by a simple columnar epithelium that is derived from endoderm and contains a mesodermally-derived core with supporting vasculature, lacteals, enteric nerves, smooth muscle, fibroblasts, myofibroblasts and immune cells. Seen in cross section, the consistency of structure in the billions of individual villi of the adult intestine is strikingly beautiful. Villi are generated in fetal life, and work over several decades has revealed that villus morphogenesis requires substantial “crosstalk” between the endodermal and mesodermal tissue components, with soluble signals, cell-cell contacts, and mechanical forces providing specific dialects for sequential conversations that orchestrate villus assembly. A key part of this process is the formation of sub-epithelial mesenchymal cell clusters that act as signaling hubs, directing overlying epithelial cells to cease proliferation, thereby driving villus emergence and simultaneously determining the location of future stem cell compartments. Interestingly, distinct species-specific differences govern how and when tissue-shaping signals and forces generate mesenchymal clusters and control villus emergence. As the details of villus development become increasingly clear, the emerging picture highlights a sophisticated local self-assembly cascade that underlies the reproducible elaboration of a regularly patterned field of absorptive villus units.
Introduction
Through the process of morphogenesis, developing organs acquire their characteristic structural and functional attributes. These morphogenic processes play out in the context of organ anlagen that have previously been endowed with the molecular instructions that dictate their eventual identity. In the case of the intestine, the anlage is a tube of endodermally-derived epithelial cells that expresses both SONIC and INDIAN HEDGEHOG (SHH and IHH). The epithelial tube is surrounded by a cylinder of loose mesenchyme, suspended in the coelomic cavity, and attached to the body wall by the dorsal mesentery. This structure forms from an initially flat epithelial sheet of endodermal cells that is transformed into a tube. However, even before tube closure is complete, anterior/posterior patterning has molecularly defined the CAUDAL TYPE HOMEBOX 2 (CDX2)-positive domain that will become small intestine1, 2. The early patterning events that encode this domain are the subject of several excellent reviews3, 4 and will not be discussed here.
The early intestinal epithelium has a flat luminal surface. Over several days, vascular elements become organized, enteric neurons from the neural crest invade, and visceral smooth muscle begins to differentiate within the mesenchyme. The process of villus morphogenesis initiates at embryonic day (E)14.5 in the mouse (19 day gestational period), E8 in the chick (21 day gestational period) and week 6 in the human (40 week gestational period). Emerged villi are first detectable at E15 (mouse), E16 (chick) and 8 weeks (human). In all of these species, the process of villus formation begins in the duodenum and progressively spreads to the distal ileum; the local cellular and molecular events that give rise to each villus unit are thus repeated over and over, as the morphogenic wave sweeps down the intestine5–11.
Studies in chick, mouse, and rat have shown that each emerging villus is associated with a tight cluster of mesenchymal cells that cling to the basement membrane beneath the epithelium11–14. These clusters form de novo in a temporally precise manner and appear to act as signaling centers that initiate villus outgrowth11, 13, 15. Epithelial cells directly above the clusters stop proliferating soon after cluster formation, and begin to differentiate; proliferation is thereby restricted to intervillus epithelium. Well after villi have emerged (indeed after birth in mouse and chick), these intervillus regions switch to a different type of morphogenesis, giving rise to flask-like crypts which house the adult stem cells that will maintain the constantly renewing epithelium throughout life. The process of crypt morphogenesis has yet to be described in detail at the cellular and molecular level and will not be a focus of this review.
To provide a structural backdrop for this review, we first highlight key findings from early anatomical and histological studies of villus morphogenesis in several species including chick, mouse, rat, pig, sheep and human. Since many of the cellular and molecular correlates of villus development have been worked out primarily in mouse and chick, we focus on recent data from these two model systems, highlighting the similarities and differences in villus morphogenesis and emphasizing several molecular players involved. In interpreting the striking differences between aspects of mammalian and avian villus formation, it is important to realize that the villus, as a solution to optimizing surface area within the gut, almost certainly evolved independently in these two species. We therefore also explore the complex range of intestinal epithelial morphologies in a variety of creatures to discuss the evolution of gut morphogenesis, and ultimately we consider the remaining mysteries and important next steps in understanding villus morphogenesis.
ANATOMY AND HISTOLOGY OF INTESTINAL VILLUS EMERGENCE
One of the most comprehensive early studies on the gross anatomy of adult and fetal intestinal mucosa was published in 1902 by William A. Hilton, who collated existing literature and surveyed patterns of mucosal folds and villi found in the small intestine of a variety of adult vertebrate species (mammals, birds, amphibians, reptiles and fish)5. Additionally, Hinton detailed the process of villus morphogenesis in the developing chick and rat, noting the proximal to distal formation of both folds and villi, as well as the fact that that both folds and villi emerge in multiple rounds, with new folds arising from formed folds and new villi emerging between existing villi. On the basis of his findings, Hilton proposed that villi can arise “phylogenetically and ontogenetically” from mucosal folds, but also noted several cases in which villi develop directly from a flat tube without going through a fold-like stage. This careful accounting by Hinton appears to be the first hint that there is more than one way to make a villus. Subsequent investigators have added more histological and molecular detail, confirming that interesting differences exist in the process of villus development among the vertebrates.
The structural basis of villus development in birds: from folds to zigzags to villi
At E4, the chick intestine consists of an epithelial tube that is surrounded by loose mesenchyme5, 6, 8, 9, 16 (Fig. 1). Its luminal surface is flat, and remains so until E8, when two to three longitudinal folds become visible in proximal regions, imparting a triangular appearance to the cross-sectioned epithelium5, 9, 16. Between E9–13, an increasing number of longitudinal folds or ridges develop proximally and extend distally, each round of fold formation doubling the number of ridges5, 8, 9,6, 7. By E12–13, 16 relatively sharp ridges have been formed by four sequential rounds of fold formation. Initially straight, these ridges become softly wavy by E12 and adopt acute zigzag shapes by E15, again, in a proximal to distal progression. The zigzags are formed by adjacent ridges folding in tight register with one another, such that the luminal surface of the intestine takes on a remarkably regular herringbone appearance just before villus emergence7. At E15–16, a distinct population of cells becomes visible atop the arms of the zigzags nearest the pylorus5, 7, 8; these represent the first villi, which fully emerge by E17 (Fig. 1). Interestingly, at least in the duodenum, as these first villi initiate on the ridge-tops, additional villi, referred to by Grey as “Set II” and by others as “round 5” arise directly from the base of the zigzags, in the absence of a fold-like precursor 6, 7. A typical chick intestinal cross section after E18 exhibits approximately 32 well-formed villi.
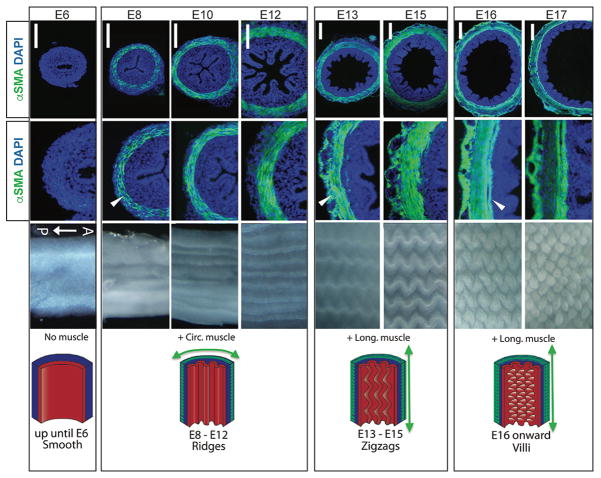
Figure 1. Progressive bending of the chick epithelium into ridges and then zigzags is driven by sequential differentiation of smooth muscle layers.
Prior to muscle formation (E6) the epithelium is flat. Ridges are formed following the differentiation of the inner circular muscle marked by anti-alpha-smooth muscle actin (green; E8–12). Zigzags evolve with the formation of the outer longitudinal muscle (E13–15). Villus emergence is concomitant with the formation of the muscular mucosa at E16. Scalebars are 100 μm. Arrowheads denote the new muscle layer formed at that stage. All images are reproduced with permission from16.
Seeking an explanation for the progressive folding that characterizes intestinal morphogenesis in the chick, Coulombre noted that at E8, as ridges appeared in the mucosa, the inner circular smooth muscle became obvious in the surrounding mesenchyme8. On this basis, he proposed that confinement of the growing epithelium by muscle is what forces initial ridge formation8. Moreover, he observed that the outer longitudinal muscle forms outside of the circular muscle by E12–13 and this corresponds with the initiation of zigzags, consistent with the possibility that the increase in longitudinal force (or resistance) could give rise to the zigzag bends. Indeed, direct measurements revealed that this temporal window corresponds with a plateau in lengthwise growth of the intestine, as if it is under lengthwise constraint8. On the basis of these findings, Coulombre proposed that the developing muscle layers might mechanically shape the progressive mucosal folding patterns.
Coulombre’s hypothesis was later tested by Burgess, who dissected the smooth muscle from one half of the pre-ridge intestinal tube or cut the intestine open lengthwise, two different strategies designed to interrupt the continuousness of the smooth muscle band9. In both cases, the intestine successfully formed ridges, though this process was delayed in the longitudinally cut intestines. Searching for other factors that could drive these morphological events, Burgess noted the presence of oriented microfilaments in the basal regions of the epithelial cells of villus tips and in apical regions of epithelial cells at the base of the ridges and proposed that dynamic epithelial cell shape changes (basal or apical constriction at fold tip or base, respectively) might be responsible for mucosal folding. Indeed, exposure of cultured intestines to cytochalasin B, which disrupts such actin filaments, prevented normal mucosal folding9. It is not clear however, what effects this drug might have had on muscle integrity.
Recently, more careful experiments by Shyer et al. have settled this controversy16. These investigators confirmed that formation of the inner circular, outer longitudinal, and muscularis mucosa muscle layers are temporally correlated with formation of ridges, zigzags, and villi, respectively (Fig. 1). To test the role of each muscle group in mucosal folding, they used two different small molecule inhibitors of muscle differentiation in defined temporal windows to block development of specific muscle layers in cultured intestines. When formation of the inner circular muscle was blocked in cultured E6 (pre-ridge) intestines, ridges failed to form. Furthermore, stimulation or inhibition of peristalsis did not affect ridge formation, suggesting that ridge formation might be a consequence of confinement of the growing epithelium by the circular muscle rather than secondary to muscle contraction. Indeed, completely stripping the muscle from the outside of the intestinal tube prevented folding, but artificial confinement of similar segments lacking muscle by threading them inside of a manufactured silk tube rescued ridge formation. Importantly, rescue occurred only when the calibre of the silk tube was small enough to constrain the growing intestinal piece16.
Similarly, adding small molecule inhibitors of muscle differentiation at E12 (after the inner circular muscle has formed and ridges are present) prevented formation of the second, outer longitudinal muscle layer and zigzags failed to form14. Finally, Shyer et al. noted that formation of the chick muscularis mucosa at E16 corresponds with the emergence of villi. Indeed, when differentiation of this final longitudinal muscle was inhibited (with both inner circular and outer longitudinal muscles remaining intact), zigzags remained, but villi failed to emerge. A mathematical model of sequential muscle constraint, based on experimentally determined measurements of diameter, thickness, length and stiffness parameters of epithelium (stiff) and mesenchyme (soft), accurately simulated the progression of fold, zigzag and villus formation observed in vivo, further supporting the idea that these mechanical factors can account for the morphological events that accompany chick villus morphogenesis14. Thus, in the chick, villus emergence is downstream of progressive deformation of the epithelium by the sequential development of smooth muscle layers.
Structural aspects of villus development in mammals: direct villus emergence from a flat epithelium
Studying fetal white rats, Hilton5 and others12 noted that the zigzag stage, which is so prominent in the chick and imparts the regular pattern to the villi, is entirely missing in mammals. Instead, villi develop either from soft mucosal folds, or directly from the flat epithelium. Others have corroborated these findings in rat17, mouse11, 18, sheep19, pig20 and human10, 21. Scanning electron microscopic images of developing chick, mouse, rat and human intestinal mucosa are shown in Figure 2.
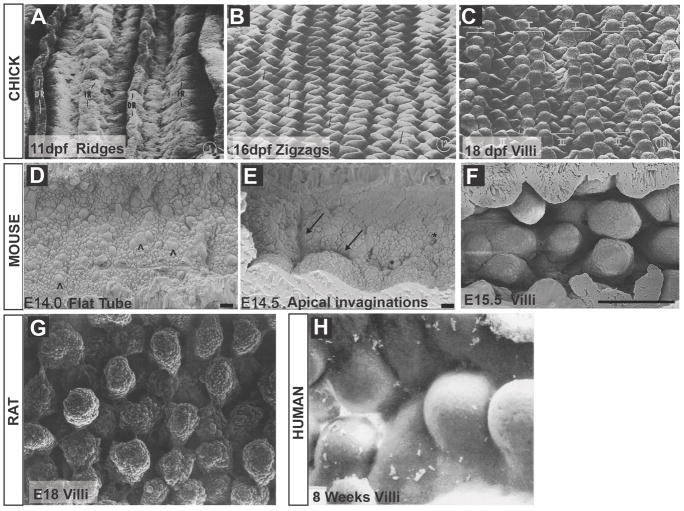
Figure 2. Surface views of the intestinal mucosa during development.
A–C) Chick at E11, E16 and E18, showing progressive formation of ridges, zigzags and villi. Images are reproduced with permission from7. DR and IR in panel A denote two different rounds of ridge formation. The arrows in B mark areas along the zigzags that are beginning to bulge where villi will emerge. I and II in panel C denote villi emerging atop alternating zigzags. D–F) Mouse intestinal surface at E14, E14.5 and E15.5, demonstrating the flat epithelial surface prior to villus formation. ^ in D indicate mitotically rounded cells along the flat apical surface. Apical invaginations begin to demarcate villi (arrows in E), which emerge as domes from the flat epithelium. Images D and E are reproduced with permission from24. Domes of newly emerged villi in the rat (G) and human (H), reproduced with permission from22 and 10. Scalebars are 10 μm in D and E and 100 μm in F.
A careful electron micrographic study by Mathan et al. describes the E15 rat previllus intestine as a highly proliferative, stratified epithelium containing 2–3 cell layers and a flat apical surface, surrounded by loose mesenchyme12, a description that is fully endorsed by Nakamura22. Sbarti describes a very similar morphology of the mouse pre-villus epithelium18 (Fig. 3A,B). However, more recent data reveal that this pre-villus epithelium is actually pseudostratified; that is, most cells touch both apical and basal surfaces23, 24 (Fig. 3D). The confusion with a stratified epithelium probably arose because nuclei in the pseudostratified epithelium lie at staggered positions (Fig. 3F,G). This is a consequence of their constant up and down movement within the epithelium, in a process known as interkinetic nuclear migration (IKNM). Nuclear movement occurs in accord with the cell cycle; S phase occurs when the nuclei are close to the basal lamina while mitosis occurs at the apical surface25 (Fig. 3H).
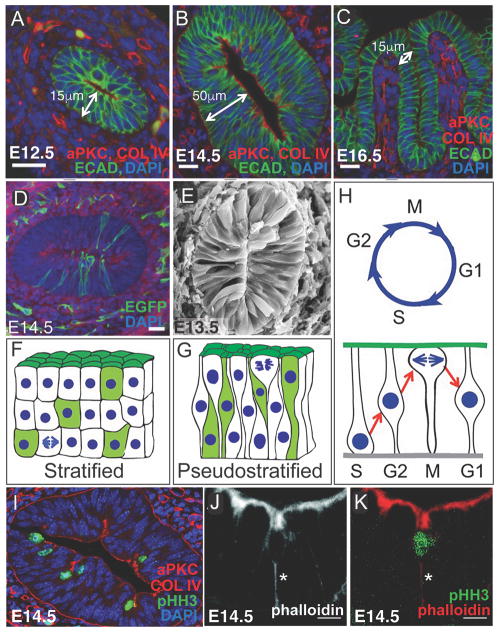
Figure 3. Cell shape during villus morphogenesis in the mouse.
A–B) Epithelium (ECADHERIN, green) thickens from 15 μm at E12.5 to 50 μm at E14.5. (C) Epithelial cells on the growing villi shorten and widen to become columnar. D) The pre-villus epithelium is pseudostratified, with cells touching both the apical and basal surfaces. Cells were sparsely labeled with myristylated EGFP to outline cell shapes. E) Scanning electron micrograph of an E13.5 intestine shows epithelial cells touching the apical and basal surfaces. Images A–E were reproduced with permission from23. F,G) Schematic representations of stratified and pseudostratified layers. Green illustrates expected cell shapes in each type of epithelium. Note that mitotic cells are basal in the stratified epithelium and apical in the pseudostratified epithelium. H) Nuclei of cells undergoing inter kinetic nuclear migration move between the apical and basal surface in accord with the cell cycle. F–H are reproduced with permission from30. I) Active apical invagination acts to demarcate villi. Apical and basal surfaces are marked by anti- aPKC and anti-COLLAGEN IV, respectively (red) and dividing cells are marked with anti-pHH3 (green). J–K) Constriction of the T invagination around an apically rounded cell (green in K, marked by anti-pHH3) is shown with phalloidin staining (white and red). * in J and K indicate the F-actin rich tether extending from the cell body to the basal surface. Images in J and K are reproduced with permission from24. Scalebars in A–D, J and K are 20 μm.
By E17, the walls of the developing rat intestine have grown in thickness, but still maintain a flat luminal surface. At this time, Mathan notes that “secondary lumens”, surrounded by unusually long junctional complexes, begin to appear between epithelial cells12. These spaces are not labelled by injection of a ferritin tracer into the intestinal lumen at E17, but can be accessed by the tracer at E18, leading Mathan to conclude that de novo formation of these small lumens and their progressive fusion with the main lumen helps to carve out the villus domains12. However, others have failed to detect secondary lumens in mammalian specimens20, 23. Indeed, three-dimensional reconstructions of the mouse epithelium reveal that all apparent secondary lumens visible in sections are actually connected to the main lumen at all times23, 24. These reconstructions also reveal an active process of apical membrane invagination (Fig. 3I–K) that accompanies villus emergence24, most likely accounting for the seemingly disconnected lumens and frequent junctional complexes seen in thin sections by Mathan et al. This process is discussed further below.
Also at E18, Mathan describes subepithelial aggregates of mesenchymal cells that begin to “invade” the epithelium12. “Mesenchymal invasion” is flagged as first sign of villus emergence in the rat12, 17, mouse11, 24, 26, 27, pig20, sheep28 and human21 (Fig. 4). The mesenchymal aggregates noted by all of these investigators have been more recently studied at the molecular level (discussed below) and are now known to represent tight clusters of mesenchymal cells that act as signaling centers to drive villus emergence11, 13, 15. Indeed, signals from specialized clusters of mesenchymal cells appear to be an important component of villus outgrowth in birds as well14. Thus, in the mouse, the major structural hallmarks of villus emergence include “invasion” of the basal aspect of the epithelium by mesenchymal aggregates, accompanied by active membrane invagination at the apical surface.
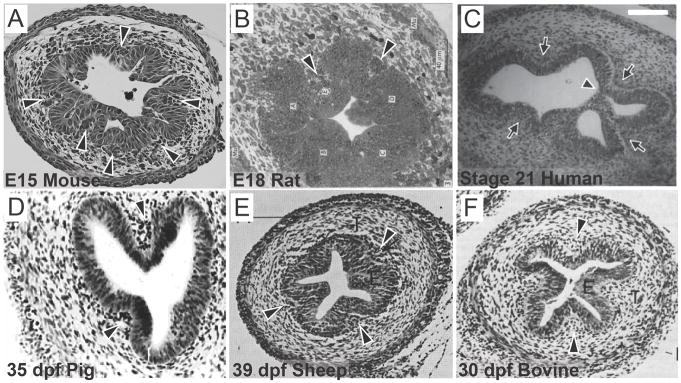
Figure 4. “Invasion” of the pseudostratified epithelium by mesenchyme (arrows) is an early feature of villus morphogenesis in mammals.
Arrowheads indicate invading mesenchymal clusters in developing intestines from A) mouse at E15 (Walton, K.D., unpublished), B) rat at E1817, C) human at stage 2121, D) pig at 35 dpf20, E) sheep at 39 dpf19 and F) cow at 30 dpf70. Images in B–F are reproduced with permission from the noted references.
Chick vs. Mouse: Similarities and differences in structural aspects of villus morphogenesis
In both chick and mouse, the early pre-villlus epithelium is pseudostratified and robustly proliferative as it is in human29; indeed, Ki67 labelling reveals that the vast majority of cells in such epithelia are actively cycling23, 25. Notably, in both chick and mouse, the process of villus emergence changes the pattern of epithelial proliferation: epithelial cells located at the base of emerged villi remain proliferative, while those on the villus proper withdraw from the cell cycle (Fig. 5). This event is critical as it fixes the presumptive stem cell population to the base of the villi in both species and presumably in humans, as this confinement of the proliferative zone is also observed in the developing human intestine29.
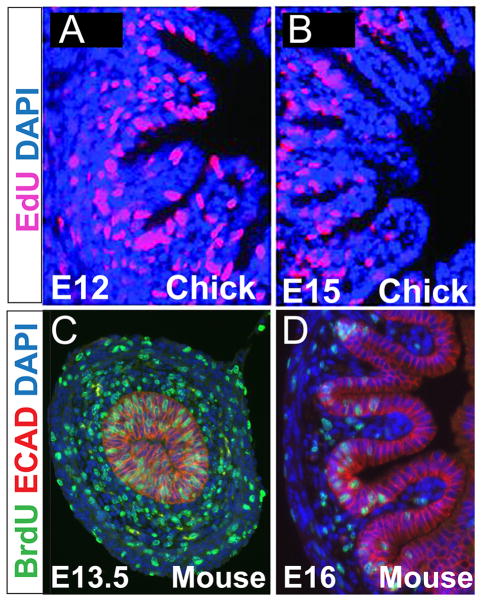
Figure 5. Changes in epithelial proliferation accompany villus morphogenesis in chick and mouse.
A,B) Top panels depict ridge (E12) and late zigzag/early villus (E15) stages in chick. EdU staining (pink) marks proliferative cells, which are seen throughout the epithelium at the ridge stage (A) and confined to the base of emerging villi at later stages(B) (reprinted with permission from14). C,D) Bottom panels illustrate robust proliferation, as marked by BrdU staining (green), throughout the pre-villus mouse pseudostratified epithelium (C, E13.5) and restriction of proliferative activity from the tips of emerging villi at E16 (D). Anti-ECADHERIN staining (red) outlines epithelial cells in C,D. DAPI staining marks the nuclei (blue).
Though the early epithelium of both chick and mouse is pseudostratified, the structure of this layer is quite different in the two species at the time that villi first emerge. In the mouse, the width of the epithelial tube wall is greatest at the time that villus formation initiates, measuring at least 50 μm from basal surface to lumen23 (Fig. 3B). Apposition of mesenchymal clusters to the basal side of this epithelium is followed by rapid shortening (to 10–15 μm) and widening of epithelial cells above the cluster, as they convert to a columnar shape15, 24 (Fig. 3C). Meanwhile, epithelial cells between clusters remain tall and pseudostratified and the apical surface is flat24. Such dramatic shrinkage of the murine epithelial cell height above clusters as villi emerge contrasts sharply with the situation in the chick where epithelial cell height is progressively attenuated during fold and zigzag formation, well before villus outgrowth. Indeed, chick epithelial cells measure only 10–12 μm between E13 and E186, a period that encompasses the timing of fourth rank ridge formation, zigzag folding, and initiation of villi. This thinner epithelial wall of the chick may facilitate the dramatic folding of this epithelium that precedes villus initiation.
Another major distinction between chick and mouse and humans is the differing role that smooth muscle layers play in villus patterning. In the chick, sequential development of three intestinal smooth muscle layers is temporally and mechanistically paired with the progressive stages of complex mucosal folding and villus emergence, as described above; removal of the muscle layer perturbs progressive epithelial deformation and villus formation16 (Fig. 1). In the mouse and human small intestine, however, these epithelial folding events do not occur and villus development and muscle layer maturation are unpaired. Also, interruption of the inner circular muscle continuity does not impair villus emergence 30, 31. Murine inner circular, outer longitudinal, and muscularis mucosa muscle layers form at E12, E15, and E18 respectively, while villus formation begins at E14.515. Similarly, in humans the inner circular muscle is already formed at the time that villi emerge, but the outer longitudinal muscle is only sparsely organized and the muscularis mucosa is absent29, 32–34. Although confinement forces imposed by the already formed inner circular muscle might play some role in the morphogenic process of villus development in these mammalian species, the other muscle layers are unlikely to contribute as patterning determinants of the emerging villi as is seen in the chick.
The mesenchymal cluster: a morphogenic signaling center
An early feature of villus development noted in multiple mammalian species is invasion of the thick pre-villus epithelium by mesenchymal condensations12, 17, 20, 21, 28 (Fig. 4). In the first molecular analysis of this invading mesenchyme, Karlsson et al. demonstrated that the clustered mesenchymal cells express the platelet-derived growth factor receptor, Pdgfra13. Indeed, prior to villus development, Pdgfra mRNA is found in scattered mesenchymal cells that are most concentrated immediately beneath the epithelium, while its ligand, Pdgfa, is uniformly expressed throughout the pseudostratified epithelium (Fig. 6A,C). As villi emerge, Pdgfa transcripts become restricted to epithelial cells that lie at the base of the growing villi, while receptor expression is most robust in mesenchymal condensations at the tips of the emerging villi and in smaller condensations underneath Pdgfa positive regions at the villus base, marking the initiation of a second round of villus formation13 (Fig. 6B,D). Later studies, using a nuclear EGFP-tagged Pdgfra allele35, confirmed that such clusters form in a proximal to distal wave in accord with the process of villus formation11. During the initial round of villus development, the formation of mesenchymal clusters deforms the basal region of the epithelium and this precedes the apical deformation that accompanies villus outgrowth11, 12, 15, 24. As the field of emerging villi spreads, smaller clusters are found in between larger clusters, marking multiple rounds of villus emergence11.
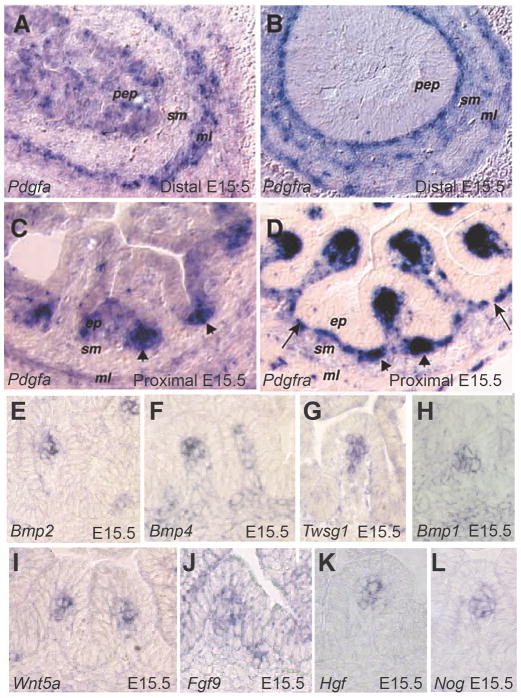
Figure 6. Mesenchymal clusters participate in epithelial/mesenchymal crosstalk.
A–D) In situ hybridization analysis of the Pdgf signaling pathway, as demonstrated by Karlsson, et al.13 Pdgfa ligand transcripts are seen throughout the pre-villus pseudostratified epithelium and cells in the muscle layer (A) and are later concentrated in intervillus epithelium (C, arrows). The receptor, Pdgfra is highly expressed in the subepithelial mesenchyme in the pre-villus intestine (B) and later robustly expressed in mesenchymal clusters in the tips of emerged villi as well as in nascent forming clusters (D, arrows). A–D are reprinted with permission from13. Abbreviations are: pep, pseudostratified epithelium; sm, submucosal mesenchyme; ml, muscle layer; ep, epithelium. (E–L) Mesenchymal clusters are signaling centers that express transcripts corresponding to multiple soluble signaling proteins, including Bmp2 (E), Bmp4 (F), Twsg1 (G), Bmp1 (H), Wnt5a (I), Fgf9 (J), Hgf (K) and Nog (L). E, F,G,H, and L are reproduced with permission from15.
Thus, PDGFA and its receptor are positioned to participate in a paracrine signal transduction process between epithelium and mesenchyme. Indeed, loss of either the ligand or receptor leads to defects in villus formation and patterning13. Noting fewer mesenchymal clusters in Pdgfa null mice, Karlsson et al. found reduced proliferation in the sub-epithelial mesenchyme where Pdgfra-expressing cells are located, and concluded that the epithelial PDGFA signal promotes the proliferation of underlying Pdgfra-expressing mesenchymal cells 13. In mutant animals, reduced proliferation results in depletion of this cluster-forming cell population and this deficit may be responsible for defective villus development.
While continued formation of mesenchymal clusters requires Pdgf-driven mesenchymal cell proliferation, the cells that actually compose such clusters are post-mitotic11, 13. Suspecting active inhibition of the cell cycle in both the cluster and its associated epithelium, Karlsson et al. examined the expression of possible mitotic inhibitors and found that Bone morphogenetic protein (Bmp)4 and Bmp2 are both robustly expressed in clustered mesenchymal cells. Later work confirmed this finding14 and also demonstrated expression of additional members of the Bmp signaling family as well as several other secreted signaling factors, including: Bmp5, Bmp1, Twsg1, Noggin, Wnt5a, Fgf9 and Hgf15 (Fig. 6E–L). Thus, as first proposed by Karlsson et al., mesenchymal clusters represent signaling centers that could drive villus morphogenesis13.
The fact that Pdgf signaling mutants could readily form the first round of clusters13 suggested that another signaling program may be involved in cluster initiation. A good candidate is the Hedgehog (Hh) pathway, because transgenic mice expressing high levels of a soluble form of the Hh inhibitor (Hhip) fail to generate villi, retaining a partially pseudostratified epithelium at E18.536. Further analysis demonstrated that signaling by the Hh pathway is strictly paracrine at the time of villus initiation, with Shh and Ihh expressed in the endoderm and two well-established signaling targets, Gli1 and Patched 1(Ptch1), expressed at robust levels in clustered mesenchymal cells11, 26 (Fig. 7A–I). Indeed, while wild-type mouse intestines harvested at E13.5 will develop clusters and villi by 48-hours in culture, both features are lost in the presence of the Hh antagonist cyclopamine, or in the presence of anti-Hh monoclonal antibodies11. In accord with this finding, mice conditionally null for the main transducer of the Hh signal, Smoothened37, or for both Ihh and Shh38 exhibit short guts with no villi. Moreover, augmentation of Hh signaling by culturing E13.5 intestines with a SMOOTHENED agonist induces the formation of larger clusters that contain more PDGFRA-positive cells11. Mechanistically, it has been demonstrated that the epithelial Hh signal acts on PDGFRA-positive mesenchymal cells to promote their aggregation, rather than inducing localized cell proliferation to form clusters11.
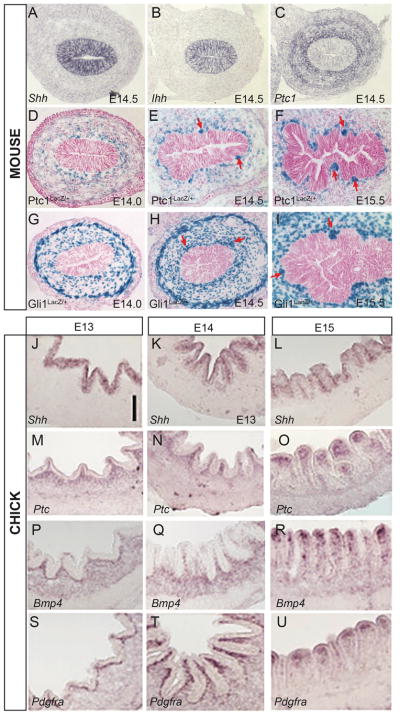
Figure 7. Epithelial/mesenchymal crosstalk via the Hedgehog (Hh) pathway in chick and mouse.
(A–C) In situ hybridization of transcripts for Shh (A), Ihh (B) and Ptch1 (C) in the mouse pre-villus epithelium. Hh transcripts are confined to the epithelium while the receptor is seen in surrounding mesenchyme and concentrated in the sub-epithelial region (reprinted with permission from26). (D–I) Eosin and X-Gal staining of intestinal sections from Ptc1LacZ/+ (D–F) or Gli1LacZ/+ (G–I) animals at E13.5 (D, G), E14.5 (E, H) and E15.5 (F, I). Red arrows indicate clustered mesenchymal cells that are receiving Hh signals (reprinted with permission from11). (J-R) In situ hybridization for Shh, Ptc, Bmp4 and Pdgfra in the developing chick intestine. The three columns represent early fold stage (J,M,P,S), zigzag stage (K,N,Q,T) and early villus (L,O,R,U). J-U are reproduced with permission from 14.
Together, these studies demonstrate that ligands expressed in the epithelia (Pdgf and Hh) signal to receptors in the mesenchyme (PDGFRA, PTCH1) to control the paracrine crosstalk that initiates villus outgrowth. While Pdgf signaling is needed for amplification of the mesenchymal compartment, Hh signaling is required for formation of the mesenchymal clusters themselves.
PATTERNING THE FIELD OF VILLI
As discussed above, mesenchymal clusters expressing PDGFRA, Bmp ligands, GLI1 and PTCH1 are critical for villus morphogenesis in the mouse11. Similarly, in the chick, mesenchymal clusters expressing these markers are present during villus outgrowth14 (Fig. 7M–U). Furthermore, in both mouse and chick, the formation of clusters requires Hh signals from the overlying epithelium11, 14 (Fig. 7A,B and J–L). These commonalities prompt an important question: considering that all cells of the pre-villus epithelium express SHH and IHH, how does this initially uniform signal give rise to a regularly spaced array of clusters that generate a patterned field of villi? Interestingly, the answer to this question is different for mouse and chick; recent work has shown that both the mechanism of formation of mesenchymal clusters and the means by which they are patterned is strikingly different in chick and mouse.
Villus patterning in the chick: epithelial deformation creates localized pockets of concentrated Hh ligand
The early pseudostratified chick intestinal epithelium uniformly expresses Hh ligands. Starting at E8, the mucosa begins to undergo the complex series of muscle-driven deformations that set the stage for villus emergence7, 8. Interestingly, this progressive epithelial folding affects the distribution of the originally uniform Hh signal that is secreted by the epithelium. As the folds become more angular at the late zigzag stage, Hh ligands become concentrated beneath fold peaks, creating localized pockets of particularly high Hh levels14. In these areas, mesenchymal cells form clusters that molecularly resemble the mesenchymal clusters described in the mouse11, 13. Thus, for the chick, progressive epithelial deformation driven by tensile forces from developing muscle is responsible for the eventual pattern of clusters (and villi) that are seen.
Cluster patterning in the chick mesenchyme quickly leads to reciprocal patterning of the epithelium as epithelial cells overlying the clusters begin to withdraw from the cell cycle. Signals from the clusters appear to drive this change in proliferation as inhibition of epithelial deformation (by turning intestinal segments inside out, or by inhibiting smooth muscle differentiation) inhibits both cluster formation and restriction of proliferation14. Shyer et al. showed that on a molecular level, phospho-SMAD is activated in the newly formed clusters, as well as in the epithelium overlying the clusters; the latter response restricts epithelial proliferation by suppressing Wnt signal reception in the overlying epithelial cells14. Inhibiting Bmp signaling (or inhibiting its upstream inducer, Hh) prevents the clusters from restricting proliferation, and epithelial Wnt target gene expression is expanded. Furthermore, addition of Hh or Bmp ligands broadly suppresses epithelial proliferation and Wnt target gene expression14. Therefore, in the chick, deformation of the epithelium by the developing muscle layers concentrates Hh signals in the underlying mesenchyme, causing cluster formation at regular intervals beneath the folds of the zigzags. These clusters then emit high levels of Bmp signals that inhibit Wnt-driven proliferation in the overlying epithelium. In this manner, proliferative cells at the villus base can drive further villus outgrowth14 (Fig. 8).
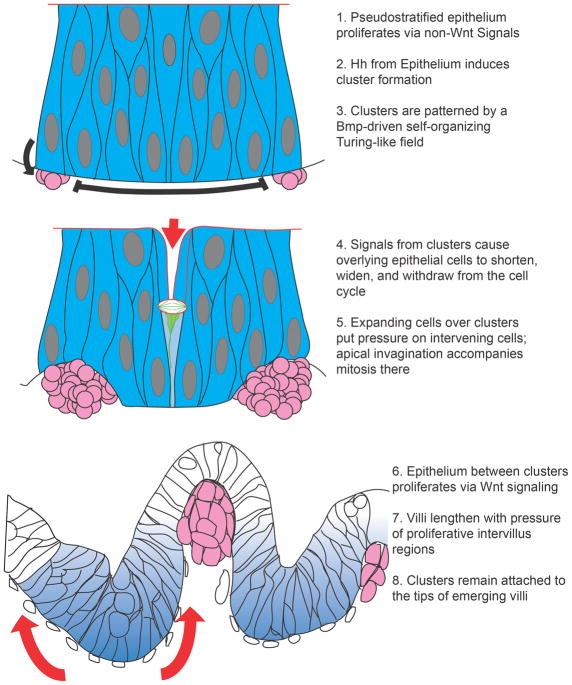
Figure 8. Schematic representation of the steps involved in villus emergence in the chick.
Muscular development plays a major role in patterning villi in that species by deforming epithelium so as to cause pockets of high Hh concentration and cluster gene induction. Blue = Epithelium; Pink = underlying mesenchyme; Dark red = muscle groups.
Villus patterning in the mouse: Turing-like self-organization of villus domains
Just prior to the initiation of cluster and villus formation in the mouse, the flat pseudostratified epithelium is composed of tall, thin cells that are tightly packed in a tubular configuration around a small, slit-like lumen. The lateral surfaces of each cell measure 50–60 μm while the apical surfaces and basal surfaces are typically less than 5 μm. In a structure such as this, direct folding of the epithelium, as seen in the chick, would be difficult. Indeed, the first sign of morphogenic change in mouse is simultaneous with the formation of mesenchymal clusters in the sub-epithelial region11, 24, 26, 30. The forming clusters are associated with progressive deformation of the basal surface of the overlying epithelium, forming soft basal alcoves that are occupied by mesenchymal clusters; this initial basal deformation occurs in the absence of apical deformation15, 24. Both cluster formation and basal alcove formation are strictly dependent upon Hh signaling and enhanced by Hh agonists11.
Cluster and alcove formation begin in the duodenum and sweep in wave-like fashion down the intestine over 36 hours in the mouse. Given the length of the intestine at E15.5 (~30 mm), this wave must travel at a speed of approximately 15 μm/minute. Scanning electron micrographs of the apical surface at the wave-front of villus formation reveal a flat epithelium that transitions into clearly demarcated villus outlines over a span of 150 μm (Fig. 2D,E), indicating that new villi must be demarcated approximately every 10 minutes during this active process of morphogenesis24. The demarcations between villi are in the form of apical membrane invaginations, a process that early investigators erroneously attributed to secondary lumen formation12.
Like mesenchymal clusters themselves, apical invaginations are not seen prior to E14.5; after this point, they become visible in a proximal to distal gradient, deepening over time24. Quantitative assessment of the location of these apical invaginations indicates that they initiate preferentially in the epithelial territories between, rather than above, clusters. Matsumoto noted a similar event in the developing human intestine, writing, “the initial invasion site of the mesenchyme into the epithelium tended to be a point between the neighboring vacuoles…”21.
Interestingly, apical invaginations form in a manner that strikingly resembles the process of placode invagination in the Drosophila tracheal primordium, also a pseudostratified epithelium39. In that system, invaginations are facilitated by mitotic cells that round up in areas that are under circumferential intraepithelial pressure; downward movement of the mitotic cell “pulls” the apical surface inward, giving rise to a characteristic T-shaped invagination above the mitotic cell39. The rounded mitotic cells have a unique appearance, as they are located well below the apical surface, at the tip of the invaginating membrane; this contrasts with typical mitotic cells in a pseudostratified epithelium, which divide at the apical surface. Kondo et al. called this process “internalized cell rounding” and showed that these dividing cells drive rapid invagination of the tracheal placode.
Similarly, the apical invaginations seen during intestinal villus demarcation are frequently associated with rounded mitotic cells that are located well beneath the apical surface24. Analysis of epithelial cell shapes reveals that these internally rounded cells are located in regions of intraepithelial compression. Compressive forces appear to be secondary to shape changes in nearby epithelial cells that overlie mesenchymal clusters. These supra-cluster cells widen and shorten without changing volume, placing localized pressure on neighboring epithelial cells between clusters. As the rounded mitotic cells within the compressed regions move downward into the epithelium, they maintain connection to the apical surface via a T-shaped invagination. Mechanical modelling studies support the conclusion that, as in Drosophila, this sequence of mechanically driven invagination occurs on the time scale of minutes24, 39. For the mouse intestine, this process leads to rapid demarcation of patterned villus boundaries, even in the context of a thick, constrained epithelial substrate (Fig. 2D,E and Fig. 3).
But if clusters pattern apical invaginations by inducing uneven intraepithelial forces, how are the clusters themselves patterned? Using an in vitro culture system, Walton et al. demonstrated that agarose beads soaked with recombinant BMP2, BMP4, BMP5, BMP7, or heterodimerized forms BMP2/7 or BMP4/7, all potently inhibited cluster formation in the regions surrounding the beads30. BMP2 was the most potent of the ligands tested and interestingly also showed the most cluster-specific pattern of expression of all Bmp ligands tested. Several Bmp modulators are also expressed by mesenchymal clusters (e.g., Noggin, Twsg1 and Bmp1); beads soaked with these factors induce enlarging and merging of clusters rather than cluster clearing. Indeed, treatment of cultured intestines with the small molecule Bmp inhibitor, Dorsomorphin, causes a dramatic change in cluster pattern, resulting in enlarged clusters that merge into “stripes” rather than “spots”; this occurs without detectable differences in overall cell shape or Hh expression pattern in the epithelium, and without changes to the surrounding muscle15. Such alterations in cluster pattern can be reproduced by genetic ablation of Bmpr1a in the cluster cells (which produces large merged clusters) but not by loss of Bmpr1a in epithelial cells (which produces no modification of epithelial shape or proliferation), confirming that cluster pattern is controlled by reception of Bmp signals by the mesenchymal cluster cells themselves15.
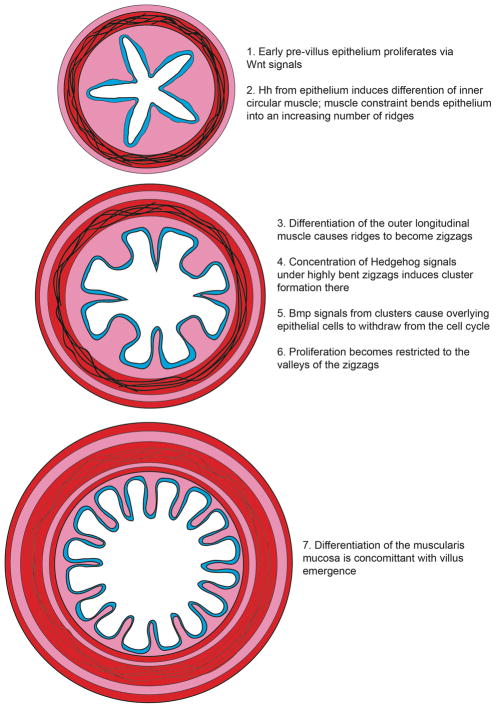
Mathematical modeling further demonstrates that the “spot to stripe” conversion seen in these experiments resembles a Turing activator/inhibitor reaction, with a Bmp inhibitor acting as the system “activator”15. Self-organizing behavior that obeys the Turing model and utilizes a Bmp inhibitor as the Turing activator has also been seen in feather40, 41 and vascular42 patterning. Of course, while this intestinal cluster patterning system closely resembles a Turing patterning field, pattern development may require more than just a single activator and inhibitor; the precise components that drive this system in vivo remain to be elucidated. Nevertheless, from the standpoint of cluster patterning, this Turing-like patterning program is quite different from the muscle-driven epithelial deformations that control cluster pattern in the chick. A summary of the cascade of events that lead to assembly of mouse villi is presented in Figure 9.
Figure 9. Schematic representation of the steps involved in villus emergence in the mouse.
Cluster patterning is via a Turing-like field, driven by Bmp signals. Clusters change overlying epithelial cell shape, resulting in regions of high intraepithelial pressure; cell division in these regions aid in apical invagination, demarcating villus boundaries. Blue = epithelium; Pink = mesenchymal clusters. In the lower panel, blue cells are proliferative; white cells are withdrawing from the cell cycle.
In summary, though similar signaling proteins are present in the mouse and chick, the means by which these signals function during villus emergence is somewhat different in the two species. As discussed above, in the chick conversion of the epithelium from pseudostratified to columnar occurs well before cluster formation and is therefore not cluster-dependent as it is in the mouse (Fig. 1). Also, suppression of epithelial proliferation in the chick is due to inhibition of Wnt activity by Bmp ligands that are secreted from the newly formed clusters14. In the mouse, signals from the patterned clusters direct both shortening and widening of epithelial cell shape and exit from the cell cycle; the nature of those signals has yet to be discovered. Though murine clusters do express robust levels of multiple Bmp ligands, inhibition of Bmp signaling, either by Dorsomorphin in vitro or by genetic removal of epithelial Bmpr1a in vivo does not change the proliferative landscape or the shapes of cells in the epithelium15.
DIVERSITY OF FOLDS AND VILLUS PATTERNS
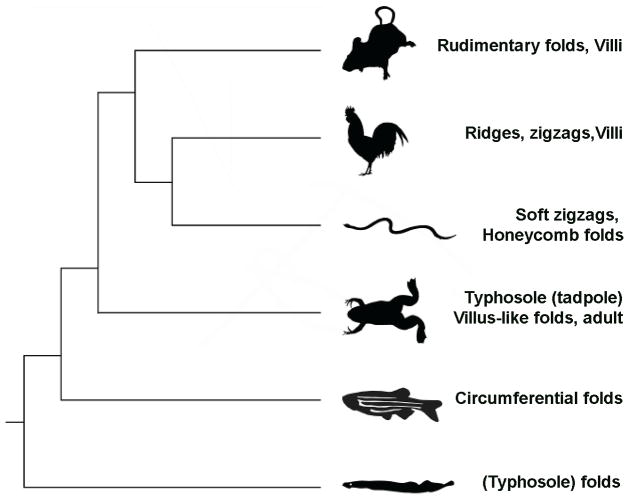
Interestingly, there is considerable diversity in the morphology of the intestinal epithelium across vertebrate species. In human, chick, and mouse, the intestinal lumen is lined with finger-like projections, but in other species the luminal surface can take the form of ridges (fish), zigzags (frogs), or honeycombs (snakes), among others10, 11, 16, 43–45 (Fig. 10). Phylogenetic considerations indicate that villi likely evolved independently in the mammalian and avian lineages, a notion supported by the different mechanisms used to make a villus in these species (described above). Intriguingly, the honeycomb pattern of some intestinal mucosal surfaces also seems to have independently evolved in multiple species, as this pattern is shared by the reticulum of ruminants such as cattle, and the intestine of marine turtles46, 47. What these various epithelial structures have in common is that they all serve to increase the surface area of the intestinal lumen available for nutrient absorption. The relative adaptive significance of the different epithelial morphologies remains to be elucidated. However, it is notable that the most metabolically active taxa, mammals and birds, possess the structure that provides the greatest increase in total surface area, the villus.
Figure 10. Evolutionary relationships among species and the appearance of the intestinal mucosa in those species.
The appearance of villi may have arisen independently three times during evolutionary time, but the survey needs broadening to clarify this issue.
The over-all intestinal structure observed in basal species such as the lamprey, and many early extinct fishes, is called the typhlosole44, 48. It appears in extant species ranging from the lamprey to sharks and rays, and the pre-metamorphic frog (tadpole). In mature animals, it twists on itself, forming what is termed a spiral valve. This can be more complex, as it is in sharks or rays, or minimal, as it is in lamprey49. During metamorphosis of the lamprey Petromyzon marinus, additional longitudinal folds appear along the anterior intestine and not on the typhlosole. These folds correspond to an elevation of the epithelium and the underlying muscle and connective tissue. As smooth muscle is present at the time of this folding, it may play a role in shaping the intestinal epithelium. By later stages, primary, secondary, and tertiary folds emerge on the entire epithelium including the typhlosole44. Since there is no muscle layer directly constraining the growth of folds along the typhlosole, they are not generated through physical forces, at least as produced in the chick. Interestingly stem cell marker expression and cell proliferation is restricted to the base of the typhlosole in larval lamprey50, similar to the localization of stems cells to the base of the villi in the developing mouse and chick.
In frogs, the typhlosole is only observed prior to metamorphosis, however villi and crypts are present in the adult form51. It is clear thyroid hormone (TH) acts to induce smooth muscle differentiation as well as Hh and Bmp signaling during frog metamorphosis, although the relative timing and interplay of these developmental events has yet to be explored51. The presence of villi in the adult frog raises the possibility that villi have arisen three independent times during vertebrate evolution. Further work on more taxa will be required to understand the exact mechanisms that underlie the development of villi in the adult frog, and how these mechanisms compare to those established in the avian and mammalian lineages.
In the zebrafish (Danio rerio) and other fish species, the intestinal epithelium forms circumferentially-oriented folds. In contrast to mouse and chick, zebrafish only form two smooth muscle layers: inner circumferential and outer longitudinal52, 53. Fold formation follows the anterior to posterior direction of muscle development52 and mutations that impair smooth muscle development prevent fold formation53. However, these mutations also eliminate changes to epithelial cell shape, preventing formation of columnar cells that normally appear closer to the top of folds53. In mouse, the epithelial cells above mesenchymal clusters fated to become villi take on a columnar shape while the intervening cells remain pseudostratified and not columnar15. Ectopic activation of the Hh signaling pathway increases mesenchymal cluster size resulting in more epithelial cells taking on a columnar shape and larger villi11. Therefore, it is unclear if changes in smooth muscle formation or cell shape, or some combination of the two, drives fold formation in fish.
Shyer et al. found that formation of the circumferential folds seen in fish, the zigzags in frogs, and the honeycomb in the proximal intestine of snakes, could be simulated by a computational model based on physical forces that modulate the number of muscle layers, stiffness of the tissue layers, and relative rates of proliferation16. For example, the honeycomb pattern of the snake could be simulated by increasing mesenchymal growth rate and decreasing endodermal growth rate in relation to growth rate of smooth muscle. While the potential for the signaling-based mechanism defined in mice to generate the range of epithelial forms found in nature has yet to be explored, one of the hallmarks of Turing mechanisms is their ability to produce a wide range of patterns. Thus, the combination of physical forces and molecular signals may provide flexible systems for generating a variety of epithelial structures that can be selected through evolution.
Conclusions and Outstanding Questions
As this review has highlighted, a hallmark of villus morphogenesis in all species studied is its reliance on sequential morphogenic events that are driven by epithelial-mesenchymal interactions. Classic tissue recombination experiments performed several decades ago hinted at the remarkable self-organizing activity of the intestinal mesoderm and endoderm, as well as the instructive ability of intestinal mesoderm (reviewed in54, 55). For example, Gumpel-Pinot et al. demonstrated that grafts composed of isolated mesenchyme from pre-villus chick small intestine, combined with 5-day endoderm dissected from esophagus, proventriculus, gizzard or colon, all take on an intestinal morphology56 (see also57). The degree of morphogenic organization seen in these grafts, by both endoderm (villus formation and cytodifferentiation) and mesoderm (muscle formation) provides substantial evidence for crosstalk between the two tissue layers that is clearly bi-directional. Now that we understand some molecular features of this amazing crosstalk, it would be fascinating to revisit these classic recombination studies using modern lineage tracing, transcriptomic and epigenomic tools, to further explore the underpinnings of villus assembly.
Though signals from mesenchymal clusters and mechanical forces from smooth muscle have been emphasized here as key aspects of villus morphogenesis, other features of the developing intestine are likely critical. For example, a basement membrane (BM) forms between the epithelial and mesenchymal components and likely influences cross-tissue communication (reviewed in58). Indeed, recent studies in Drosophila egg chambers revealed that regulation of BM stiffness can instruct tissue shape59. Signaling proteins, along with planar polarity contribute to generate this stiffness gradient, which is required for proper elongation of the tissue. The possibility that differential BM stiffness could aid in villus elongation has yet to be explored. Additionally, the possible contribution of vasculature and nerves to villus morphogenesis needs to be investigated. Even the smallest developing mesenchymal clusters contain vascular elements. Indeed, this early association may insure that each emerging villus contains a well-vascularized core, but it is possible that vascular and/or nervous elements play signaling roles as well. In this regard, it is interesting to consider the intestinal organoids that can be developed from progressive differentiation of human pluripotent stem cells. Such organoids do not form villi in culture, even when generated by progressive differentiation of mesendoderm tissue that can give rise to both endoderm and mesoderm60. However, after transplantation and ingrowth of host vessels (and likely nerves), well-ordered villi are observed61.
An important aspect of villus emergence is the simultaneous patterning of epithelial proliferation. After villi emerge and throughout adult life, Wnt signals are known to drive proliferation of intervillus regions (reviewed in62). However, regulation of proliferation in the early pre-villus epithelium may differ in mouse and chick. In the chick, low levels of Lgr5 (Leucine rich repeat containing G protein coupled receptor 5) and Sox9 (Sex-determining region Y-Box9), both Wnt target genes and markers of the adult stem cell zone, are expressed uniformly in the early pseudostratified epithelium and during progressive ridge and zigzag formation14. As zigzags compact and clusters begin to form, Wnt signals are strongly up-regulated at the base of the zigzags and down-regulated at the tips14. As discussed above, Bmp signals from clusters play an important role in suppressing Wnt signals in villus tips. In contrast, in the mouse, Chin et al. recently demonstrated that conditional deletion of either Ctnnb1 (β-catenin) or the Wnt co-receptors, Lrp5/6 at E12 does not alter cell division in the pseudostratified epithelium, confirming that Wnt signaling does not drive proliferation in the pre-villus stages63. Molecularly, Wnt targets (Lgr5, CD44 and Axin2) are strikingly absent from the mouse pre-villus epithelium, while several Wnt inhibitors (e.g., Sfrp1/2/5 and Robo1/2) are highly expressed64. Nigmatullina et al. suggest that the progressive down-regulation of Inhibitor of DNA binding 2 (Id2), a Wnt inhibitor that is expressed at high levels in the early epithelium, is responsible for the gradual emergence of a Wnt-driven proliferative state in intervillus regions as villi develop64. Accordingly, mice lacking Id2 in the intestinal epithelium show precocious activation of Wnt markers in the pseudostratified epithelium64. At this time, it is not clear what factors drive proliferation in the pseudostratified state in the murine intestine, although both Fgf and Retinoic acid signals have been speculated to play a role63.
The finding that two different programs regulate proliferation in the mouse epithelium before and after villus emergence adds another conundrum: how is proliferation suppressed at the emerging villus tips? It is possible that the entire epithelium switches to a Wnt-driven proliferation program just prior to cluster formation and signals from the clusters then shut down this Wnt program in the overlying epithelial cells. Alternatively, clusters may shut down the unknown pseudostratified-specific proliferative program in overlying cells, while Wnt signaling is simultaneously activated de novo only in intervillus regions. In this regard, it is noteworthy that as villi emerge, cells in the intervillus regions become highly apically constricted, a shape change that has the potential to activate new gene expression patterns. Exactly this type of epithelial deformation in the Drosophila anterior midgut causes activation of armadillo (β-catenin) and twist in the apically constricted cells65, 66. β-catenin is also responsive to mechano-activation in the mouse during bone and joint development67, 68. Thus, it is possible that the deformation of the intervillus cells that accompanies progressive cluster-driven buckling of the emerging villi activates a β-catenin-driven proliferative program in the intervillus cells via mechanical signaling. Additional work is needed to differentiate between these two alternatives.
Both mouse and chick exhibit multiple rounds of villus formation, but the mechanisms underlying development and patterning of later rounds of villi are not entirely clear. Indeed, the first round of cluster formation in the mouse initiates along the smooth basement membrane of the pseudostratified tube, but as these first villi emerge, the geometry of the landscape becomes more complex. All subsequent rounds of cluster formation begin at the proliferative intervillus bases. These intervillus epithelial cells are apically constricted and progressively more columnar, compared to the tall pseudostratified cells of the first round. Moreover, as discussed above, different signals control their proliferation3, 64. In fact, the entire epithelial transcriptome undergoes a dramatic switch as villus formation initiates64, 69. It is presently unclear whether or how such epithelial changes might affect the formation or patterning of the later rounds of clusters and villi. Similarly, in the chick, the Set II villi that develop at the base of the tightly folded zigzags clearly utilize different assembly mechanisms, as muscle formation is completed and this set of villi arises directly, without underlying folds, but in a highly patterned manner7.
In summary, villus morphogenesis in both chick and mouse is driven by a series of back and forth messages, each prompting the next, interdigitated with mechanical signals produced by tissue growth, tension from surrounding muscles and cell shape change. At the level of each individual villus, this cascade of events promotes cell cycle quiescence, basal constriction and the beginnings of cytodifferentiation in epithelial cells at the villus tip, while simultaneously driving apical constriction and compartmentalization of a proliferative future stem cell population at the villus base. Using similar instructional signaling programs (Hh, Bmp, Wnt), the mouse and chick have evolved distinct structural mechanisms to form and pattern their villi. Finally, robust underlying patterning mechanisms, muscle-driven in the chick and Turing-like in the mouse, insure the production of a tightly packed array of identical villus units for maximal absorptive function.
Footnotes
Notes
NONE
Contributor Information
Katherine D. Walton, Department of Cell and Developmental Biology, University of Michigan, Ann Arbor, MI 48109-2200, No conflicts of interest
Darcy Mishkind, Department of Genetics, Harvard Medical School, Boston, MA, 02115, No conflicts of interest.
Misty R. Riddle, Department of Genetics, Harvard Medical School, Boston, MA, 02115, No conflicts of interest
Clifford J. Tabin, Department of Genetics, Harvard Medical School, Boston, MA, 02115, No conflicts of interest
Deborah L. Gumucio, Department of Cell and Developmental Biology, University of Michigan, Ann Arbor, MI 48109-2200; No conflicts of interest
References
- 1.Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–971. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- 2.Suh E, Chen L, Taylor J, Traber PG. A homeodomain protein related to caudal regulates intestine-specific gene transcription. Mol Cell Biol. 1994;14:7340–7351. doi: 10.1128/mcb.14.11.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chin AM, Hill DR, Aurora M, Spence JR. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin Cell Dev Biol. 2017 doi: 10.1016/j.semcdb.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev Dyn. 2011;240:501–520. doi: 10.1002/dvdy.22540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hilton WH. The morphology and development of intetinal folds and villi in vertebrates. Am J Anat. 1902;1:459–505. [Google Scholar]
- 6.Hinni JB, Watterson RL. Modified Development of the Duodenum of Chick Embryos Hypophysectomized by Partial Decapitation. J Morphol. 1963;113:381–425. doi: 10.1002/jmor.1051130306. [DOI] [PubMed] [Google Scholar]
- 7.Grey RD. Morphogenesis of intestinal villi. I. Scanning electron microscopy of the duodenal epithelium of the developing chick embryo. J Morphol. 1972;137:193–213. doi: 10.1002/jmor.1051370206. [DOI] [PubMed] [Google Scholar]
- 8.Coulombre AJ, Coulombre JL. Intestinal development. I. Morphogenesis of the villi and musculature. J Embryol Exp Morphol. 1958;6:403–411. [PubMed] [Google Scholar]
- 9.Burgess DR. Morphogenesis of intestinal villi. II. Mechanism of formation of previllous ridges. J Embryol Exp Morphol. 1975;34:723–740. [PubMed] [Google Scholar]
- 10.Lacroix B, Kedinger M, Simon-Assmann P, Haffen K. Early organogenesis of human small intestine: scanning electron microscopy and brush border enzymology. Gut. 1984;25:925–930. doi: 10.1136/gut.25.9.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walton KD, Kolterud A, Czerwinski MJ, Bell MJ, Prakash A, Kushwaha J, Grosse AS, Schnell S, Gumucio DL. Hedgehog-responsive mesenchymal clusters direct patterning and emergence of intestinal villi. Proc Natl Acad Sci U S A. 2012;109:15817–15822. doi: 10.1073/pnas.1205669109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathan M, Moxey PC, Trier JS. Morphogenesis of fetal rat duodenal villi. Am J Anat. 1976;146:73–92. doi: 10.1002/aja.1001460104. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson L, Lindahl P, Heath JK, Betsholtz C. Abnormal gastrointestinal development in PDGF-A and PDGFR-(alpha) deficient mice implicates a novel mesenchymal structure with putative instructive properties in villus morphogenesis. Development. 2000;127:3457–3466. doi: 10.1242/dev.127.16.3457. [DOI] [PubMed] [Google Scholar]
- 14.Shyer AE, Huycke TR, Lee C, Mahadevan L, Tabin CJ. Bending gradients: how the intestinal stem cell gets its home. Cell. 2015;161:569–580. doi: 10.1016/j.cell.2015.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walton KD, Whidden M, Kolterud A, Shoffner SK, Czerwinski MJ, Kushwaha J, Parmar N, Chandhrasekhar D, Freddo AM, Schnell S, et al. Villification in the mouse: Bmp signals control intestinal villus patterning. Development. 2016;143:427–436. doi: 10.1242/dev.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shyer AE, Tallinen T, Nerurkar NL, Wei Z, Gil ES, Kaplan DL, Tabin CJ, Mahadevan L. Villification: how the gut gets its villi. Science. 2013;342:212–218. doi: 10.1126/science.1238842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunn JS. The fine structure of the absorptive epithelial cells of the developing small intestine of the rat. J Anat. 1967;101:57–68. [PMC free article] [PubMed] [Google Scholar]
- 18.Sbarbati R. Morphogenesis of the intestinal villi of the mouse embryo: chance and spatial necessity. J Anat. 1982;135:477–499. [PMC free article] [PubMed] [Google Scholar]
- 19.Trahair J, Robinson P. The development of the ovine small intestine. Anat Rec. 1986;214:294–303. doi: 10.1002/ar.1092140309. [DOI] [PubMed] [Google Scholar]
- 20.Dekaney CM, Bazer FW, Jaeger LA. Mucosal morphogenesis and cytodifferentiation in fetal porcine small intestine. Anat Rec. 1997;249:517–523. doi: 10.1002/(SICI)1097-0185(199712)249:4<517::AID-AR12>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto A, Hashimoto K, Yoshioka T, Otani H. Occlusion and subsequent re-canalization in early duodenal development of human embryos: integrated organogenesis and histogenesis through a possible epithelial-mesenchymal interaction. Anat Embryol (Berl) 2002;205:53–65. doi: 10.1007/s00429-001-0226-5. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura K, Komuro T. A three-dimensional study of the embryonic development and postnatal maturation of rat duodenal villi. J Electron Microsc (Tokyo) 1983;32:338–347. [PubMed] [Google Scholar]
- 23.Grosse AS, Pressprich MF, Curley LB, Hamilton KL, Margolis B, Hildebrand JD, Gumucio DL. Cell dynamics in fetal intestinal epithelium: implications for intestinal growth and morphogenesis. Development. 2011;138:4423–4432. doi: 10.1242/dev.065789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freddo AM, Shoffner SK, Shao Y, Taniguchi K, Grosse AS, Guysinger MN, Wang S, Rudraraju S, Margolis B, Garikipati K, et al. Coordination of signaling and tissue mechanics during morphogenesis of murine intestinal villi: a role for mitotic cell rounding. Integr Biol (Camb) 2016;8:918–928. doi: 10.1039/c6ib00046k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norden C. Pseudostratified epithelia - cell biology, diversity and roles in organ formation at a glance. J Cell Sci. 2017;130:1859–1863. doi: 10.1242/jcs.192997. [DOI] [PubMed] [Google Scholar]
- 26.Kolterud A, Grosse AS, Zacharias WJ, Walton KD, Kretovich KE, Madison BB, Waghray M, Ferris JE, Hu C, Merchant JL, et al. Paracrine Hedgehog signaling in stomach and intestine: new roles for hedgehog in gastrointestinal patterning. Gastroenterology. 2009;137:618–628. doi: 10.1053/j.gastro.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micheletti RCaPA. Selection of a Chemically Defined Medium for Culturing Fetal Mouse Small Intestine. In Vitro. 1978;17:331–344. doi: 10.1007/BF02618145. [DOI] [PubMed] [Google Scholar]
- 28.Toofanian F. Histological development of the small intestinal mucosa in the ovine fetus. Res Vet Sci. 1976;21:349–353. [PubMed] [Google Scholar]
- 29.Arsenault P, Menard D. Cell proliferation in developing human jejunum. Biol Neonate. 1987;51:297–304. doi: 10.1159/000242668. [DOI] [PubMed] [Google Scholar]
- 30.Walton KD, Freddo AM, Wang S, Gumucio DL. Generation of intestinal surface: an absorbing tale. Development. 2016;143:2261–2272. doi: 10.1242/dev.135400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvert R, Micheletti PA. Selection of a chemically defined medium for culturing fetal mouse small intestine. In Vitro. 1981;17:331–344. doi: 10.1007/BF02618145. [DOI] [PubMed] [Google Scholar]
- 32.Fu M, Tam PK, Sham MH, Lui VC. Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryol (Berl) 2004;208:33–41. doi: 10.1007/s00429-003-0371-0. [DOI] [PubMed] [Google Scholar]
- 33.Le Guen L, Marchal S, Faure S, de Santa Barbara P. Mesenchymal-epithelial interactions during digestive tract development and epithelial stem cell regeneration. Cell Mol Life Sci. 2015;72:3883–3896. doi: 10.1007/s00018-015-1975-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallace AS, Burns AJ. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367–382. doi: 10.1007/s00441-004-1023-2. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor alpha receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madison BB, Braunstein K, Kuizon E, Portman K, Qiao XT, Gumucio DL. Epithelial hedgehog signals pattern the intestinal crypt-villus axis. Development. 2005;132:279–289. doi: 10.1242/dev.01576. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, Cotton JL, Wang Y, Rajurkar M, Zhu LJ, Lewis BC, Mao J. Specific requirement of Gli transcription factors in Hedgehog-mediated intestinal development. J Biol Chem. 2013;288:17589–17596. doi: 10.1074/jbc.M113.467498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mao J, Kim BM, Rajurkar M, Shivdasani RA, McMahon AP. Hedgehog signaling controls mesenchymal growth in the developing mammalian digestive tract. Development. 2010;137:1721–1729. doi: 10.1242/dev.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kondo T, Hayashi S. Mitotic cell rounding accelerates epithelial invagination. Nature. 2013;494:125–129. doi: 10.1038/nature11792. [DOI] [PubMed] [Google Scholar]
- 40.Mou C, Pitel F, Gourichon D, Vignoles F, Tzika A, Tato P, Yu L, Burt DW, Bed’hom B, Tixier-Boichard M, et al. Cryptic patterning of avian skin confers a developmental facility for loss of neck feathering. PLoS Biol. 2011;9:e1001028. doi: 10.1371/journal.pbio.1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris MP, Williamson S, Fallon JF, Meinhardt H, Prum RO. Molecular evidence for an activator-inhibitor mechanism in development of embryonic feather branching. Proc Natl Acad Sci U S A. 2005;102:11734–11739. doi: 10.1073/pnas.0500781102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garfinkel A, Tintut Y, Petrasek D, Bostrom K, Demer LL. Pattern formation by vascular mesenchymal cells. Proc Natl Acad Sci U S A. 2004;101:9247–9250. doi: 10.1073/pnas.0308436101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferri S, Junqueira LC, Medeiros LF, Mederios LO. Gross, microscopic and ultrastructural study of the intestinal tube of Xenodon merremii Wagler, 1824 (Ophidia) J Anat. 1976;121:291–301. [PMC free article] [PubMed] [Google Scholar]
- 44.Youson JHC, KL Development of longitudinal mucosal folds in intestine of anadromous sea lamprey, petromyzon-marinus I, during metamorphosis. Canadian Journal of Zoology-Revue Canadienne De Zoologie. 1978;56:2364–2371. doi: 10.1139/z78-080. [DOI] [PubMed] [Google Scholar]
- 45.McAvoy JW, Dixon KE. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: structural aspects. J Anat. 1978;125:155–169. [PMC free article] [PubMed] [Google Scholar]
- 46.Budras KDHRE. Bovine Anatomy: An Illustrated Text. 2. Schluetersche; 2011. [Google Scholar]
- 47.Magalhaes MSS, AJB, da Silva NB, de Moura CEB. Anatomy of the digestive tube of sea turtles. Zooloogiae. 2012;29:70–76. [Google Scholar]
- 48.Argyriou T, Clauss M, Maxwell EE, Furrer H, Sanchez-Villagra MR. Exceptional preservation reveals gastrointestinal anatomy and evolution in early actinopterygian fishes. Sci Rep. 2016;6:18758. doi: 10.1038/srep18758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harder W. Anatomy of Fishes. 2. Stuttgart: Schweizerbart; 1975. [Google Scholar]
- 50.Aghaallaei N, Gruhl F, Schaefer CQ, Wernet T, Weinhardt V, Centanin L, Loosli F, Baumbach T, Wittbrodt J. Identification, visualization and clonal analysis of intestinal stem cells in fish. Development. 2016;143:3470–3480. doi: 10.1242/dev.134098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schreiber AM, Mukhi S, Brown DD. Cell-cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331:89–98. doi: 10.1016/j.ydbio.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seiler C, Abrams J, Pack M. Characterization of zebrafish intestinal smooth muscle development using a novel sm22alpha-b promoter. Dev Dyn. 2010;239:2806–2812. doi: 10.1002/dvdy.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wallace KN, Akhter S, Smith EM, Lorent K, Pack M. Intestinal growth and differentiation in zebrafish. Mech Dev. 2005;122:157–173. doi: 10.1016/j.mod.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Simon-Assmann P, Kedinger M. Heterotypic cellular cooperation in gut morphogenesis and differentiation. Semin Cell Biol. 1993;4:221–230. doi: 10.1006/scel.1993.1026. [DOI] [PubMed] [Google Scholar]
- 55.McLin VA, Henning SJ, Jamrich M. The role of the visceral mesoderm in the development of the gastrointestinal tract. Gastroenterology. 2009;136:2074–2091. doi: 10.1053/j.gastro.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Gumpel-Pinot M, Yasugi S, Mizuno T. Differentiation of the endodermal epithelium associated with the splanchnic mesoderm. C R Acad Sci Hebd Seances Acad Sci D. 1978;286:117–120. [PubMed] [Google Scholar]
- 57.Duluc I, Freund JN, Leberquier C, Kedinger M. Fetal endoderm primarily holds the temporal and positional information required for mammalian intestinal development. J Cell Biol. 1994;126:211–221. doi: 10.1083/jcb.126.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon-Assmann P, Spenle C, Lefebvre O, Kedinger M. The role of the basement membrane as a modulator of intestinal epithelial-mesenchymal interactions. Prog Mol Biol Transl Sci. 2010;96:175–206. doi: 10.1016/B978-0-12-381280-3.00008-7. [DOI] [PubMed] [Google Scholar]
- 59.Crest J, Diz-Munoz A, Chen DY, Fletcher DA, Bilder D. Organ sculpting by patterned extracellular matrix stiffness. Elife. 2017:6. doi: 10.7554/eLife.24958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, Poling HM, Schweitzer JI, Vallance JE, Mayhew CN, Sun Y, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–1314. doi: 10.1038/nm.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Clevers H, Loh KM, Nusse R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science. 2014;346:1248012. doi: 10.1126/science.1248012. [DOI] [PubMed] [Google Scholar]
- 63.Chin AM, Tsai YH, Finkbeiner SR, Nagy MS, Walker EM, Ethen NJ, Williams BO, Battle MA, Spence JR. A Dynamic WNT/beta-CATENIN Signaling Environment Leads to WNT-Independent and WNT-Dependent Proliferation of Embryonic Intestinal Progenitor Cells. Stem Cell Reports. 2016;7:826–839. doi: 10.1016/j.stemcr.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nigmatullina L, Norkin M, Dzama MM, Messner B, Sayols S, Soshnikova N. Id2 controls specification of Lgr5+ intestinal stem cell progenitors during gut development. EMBO J. 2017;36:869–885. doi: 10.15252/embj.201694959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Farge E. Mechanical induction of Twist in the Drosophila foregut/stomodeal primordium. Curr Biol. 2003;13:1365–1377. doi: 10.1016/s0960-9822(03)00576-1. [DOI] [PubMed] [Google Scholar]
- 66.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 67.Hens JR, Wilson KM, Dann P, Chen X, Horowitz MC, Wysolmerski JJ. TOPGAL mice show that the canonical Wnt signaling pathway is active during bone development and growth and is activated by mechanical loading in vitro. J Bone Miner Res. 2005;20:1103–1113. doi: 10.1359/JBMR.050210. [DOI] [PubMed] [Google Scholar]
- 68.Kahn J, Shwartz Y, Blitz E, Krief S, Sharir A, Breitel DA, Rattenbach R, Relaix F, Maire P, Rountree RB, et al. Muscle contraction is necessary to maintain joint progenitor cell fate. Dev Cell. 2009;16:734–743. doi: 10.1016/j.devcel.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Li X, Madison BB, Zacharias W, Kolterud A, States D, Gumucio DL. Deconvoluting the intestine: molecular evidence for a major role of the mesenchyme in the modulation of signaling cross talk. Physiol Genomics. 2007;29:290–301. doi: 10.1152/physiolgenomics.00269.2006. [DOI] [PubMed] [Google Scholar]
- 70.Toofanian F. Histological observations on the developing intestine of the bovine fetus. Res Vet Sci. 1976;21:36–40. [PubMed] [Google Scholar]