Abstract
The purpose of the study is to investigate the prognostic significance of PD-L1 expression and tumor infiltrating lymphocytes (TILs) in HER2+ breast cancer (BC). HER2+ BC cases (n=191) were collected between 1996 and 2013. Tissue microarray (TMA) slides were stained with two clones of PD-L1 antibodies (28-8 and 22C3) and the percentage of positive membranous staining was scored. TILs of the full sections were also scored using percentage scale. Clone 28-8 had expression in ≥1% of the tumor cells in 25.7% of the cases, while clone 22C3 in ≥1% of the tumor cells was expressed in 11.5% of the cases. In the multivariate analysis, higher expression of PD-L1 (clone 28-8) in tumor correlated with lower risk of tumor recurrence, with HR of 0.4 (p=0.033). Higher level of TILs (>15%) predicts better overall survival (OS) in all patients with HR of 0.35 (p=0.0046). In the group of patients who were treated with trastuzumab-based adjuvant chemotherapy, lower PD-L1 (clone 28-8) expression in TILs correlated with tumor recurrence (p=0.034). In the group of patients who were treated with non-trastuzumab-based adjuvant chemotherapy, lower TILs and lower PD-L1 (clone 28-8) expression in tumor had borderline statistical significance in association with tumor recurrence, p=0.064 and 0.083, respectively. In the group of patients who were treated with trastuzumab-based adjuvant chemotherapy, PD-L1 or TILs was not statistically significant to predict 5-year survival. In the group of patients who were treated with non-trastuzumab-based adjuvant chemotherapy, low TILs (p=0.009) correlated with 5-year death due to disease. We conclude that PD-L1 may have prognostic significance in HER2+ BCs.
INTRODUCTION
During the development and progression of human cancer cells, multiple genetic and epigenetic alterations occur. These alterations produce proteins which can be recognized by host immune system [1]. However, the cancer cells have developed multiple mechanisms of immune escape, one such mechanism is the suppressive tumor microenvironment [2, 3]. Recently, great advances have been made in the field of cancer immunotherapy. Focus has been on modulating tumor-immune interaction. Notably, immune response checkpoint inhibitors directed to programed cell death 1 (PD-1)/programed death ligand 1 (PD-L1) axis have demonstrated promising effects in melanoma, lung cancer and urothelial cancer [4–6].
Expressed on immune cells, PD-1 is a member of the B7-CD28 family of T-cell coregulatory receptors and plays an important role in maintaining immune homeostasis in normal physiological conditions [7]. PD-1 binds two cognate ligands, PD-L1and PD-L2. PD-L1 is constitutively expressed on antigen presenting cells (APC), DCs, activated monocytes and B cells, as well as non-lymphoid tissues of different organs [8, 9]. When PD-1 binds to its ligands PD-L1/PD-L2, cytotoxic T-cell activity is downregulated, thereby protecting normal cells from excessive damage [7]. In addition to normal cells, PD-L1 has been discovered to be expressed in a variety of cancers, including lung, breast, pancreas, esophagus, head and neck, and kidney [10]. Meanwhile, PD-L1 has been found to be expressed on tumor infiltrating lymphocytes (TILs), mostly CD3 positive T cells, especially in large, high grade, HER2-positive, and basal-like BC. PD-L1 positive BCs were more likely to contain PD-L1 positive TIL than PD-L1 negative BCs [11, 12].
In cancer, the inhibitory signals conveyed by PD-1/PD-L1 axis impede immune response, thus creating an attenuated antitumor microenvironment, or so called immune escape or immune tolerance [13, 14]. Therefore, inhibiting PD-1/PD-L1 axis could enhance anti-tumor activity of T cells and exert therapeutic effects. This notion has been proven in a variety of refractory solid tumors, including melanoma, renal, non-small cell lung carcinoma (NSCLC), and urothelial [4–6, 15]. Monoclonal antibodies to PD-1 or PD-L1 have shown significant and durable clinical responses in these tumors in clinical trials [4, 16, 17]. A few monoclonal antibodies have been used in advanced NSCLC to target this access including KEYTRUDA® (pembrolizumab) and OPDIVO® (nivolumab) among others. FDA approved antibodies provided by DAKO, clone 22C3 [5] and clone 28-8 [18] are linked to pembrolizumab and nivolumab, respectively[19].
Breast cancer (BC) is a heterogeneous disease that includes several molecular subtypes. Compared to the above mentioned solid tumors, BC has been regarded as relatively less immunogenic. However, within this heterogeneous disease, TILs have been associated with host immune response and favorable prognosis in triple negative breast cancer (TNBC) and HER2+ subtypes, despite a lack of detailed information on the immune subsets of the infiltrate [20–23]. These findings suggest that host immunity could still be exploited to combat BC, and TILs as a whole could be utilized as a prognostic factor and assessed using Hematoxylin & Eosin (H&E) sections without further immunohistochemistry studies to delineate the subgroup status.
The role of PD-L1 in BC has only been investigated in a few studies with contradictory results. While a study demonstrated PD-L1 expression in tumor as a negative prognostic factor in human BC [24], other studies showed that PD-L1 expression/upregulation was associated with better clinical outcomes [25, 26]. Currently it is still unclear whether PD-L1 expression in BC can be used a prognostic and/or predictive biomarker.
Moreover, published studies investigated either overall BC as a single disease or focused on triple negative BC (TNBC) [20, 24–30]. We elected to study HER2+ BC for two reasons: first, PD-L1 has been previously shown to be expressed in high percentage of HER2+ BC. As one of the described pathways of immune avoidance, it may correlate with TILs [12]; and second, PD-L1 expression might modulate trastuzumab therapeutic effects [31–33]. There is an ongoing debate of benefit from trastuzumab in HER2+ BC with high TILs [34].
In the current study, we set out to characterize the expression of PD-L1 in both tumor cells and TILs in HER2+ BC by using two antibodies currently in clinical use (clone 28-8 and 22C3), to investigate the correlation between PD-L1 expression and TILs, and to examine the prognostic significance of PD-L1 expression and TILs.
MATERIALS AND METHODS
Patient cohort, tissue microarray
The BC patient database between 1996 and 2013 at our institution was searched for eligible cases which were positive for HER2. HER2 scoring was conducted using the American Society of Clinical Oncology-College of American Pathologists (ASCO-CAP) 2007 guidelines, as these cases were selected prior to the release of ASCO-CAP 2013 guidelines. HER2 was considered positive by immunohistochemistry when the score was 3+ in more than 30% of the tumor cells or by fluorescence in situ hybridization (FISH) when the HER2/Cep17 ratio >2.2 [35, 36].
The following clinicopathologic variables were obtained: tumor size, lymph node status, Nottingham grade [37], tumor histologic subtype, patient’s age, stage of tumor, adjuvant chemotherapy (with and without trastuzumab), hormonal therapy, radiation therapy, recurrence free survival (RFS) and overall survival (OS). Tumor recurrence was defined as either local or distant recurrence within 5 years of initial diagnosis. Pathologic stage was determined using the seventh edition of the American Joint Committee on Cancer (AJCC) staging system for BC. Stage II was defined as T (0, 1, or 2) with N1, or T (2 or 3) with N0, while stage III was defined as any T with N2, T (3 or 4) with N1, T4 with N0, or any T with N3[38]. Estrogen receptor (ER) and progesterone receptor (PR) were scored using Allred scoring system from 0 to 8. A score >2 was considered positive [39]. A positive hormonal receptor (HR) was defined as ER+ and/or PR+. Patients who were treated in the neoadjuvant setting were excluded from the study.
Tissue microarray (TMA) blocks were constructed using Beecher tissue puncher and array system (Beecher Instruments, Silver Spring, MD). The core sites were randomly chosen in the tumor regions. Tumor cores were constructed in triplicate in a total of 7 TMA blocks. Clinical data was de-identified and controlled by an honest broker.
Immunohistochemistry
Anti-PD-L1 staining was performed using Dako pharmDx kits and Autostainer Link 48 as described by the manufacturer (Dako, Santa Clara, CA). Anti-PD-L1 antibodies (clone 28-8 and 22C3) in tumor and TILs were scored by two pathologists (TK, YL), independently from each other and blinded to the clinical variables and outcomes. The percentage of positive partial or complete membranous staining in the tumor cells and TILs was evaluated. The staining intensity was not graded. Then the final score was derived by averaging the scores between the two raters. A score ≥1% was considered positive. Cohen’s weighted kappa value for comparison of the two evaluators was 0.90 for PD-L1 clone 28-8 in tumor, 0.92 for PD-L1 clone 28-8 in TILs, 0.83 for PD-L1 clone 22C3 in tumor, and 0.97 for PD-L1 clone 22C3 in TILs.
Scoring tumor-infiltrating lymphocytes
Histopathologic scoring of TILs was performed on hematoxylin and eosin-stained full sections by two pathologists (TK, YL), independently from each other and blinded to the clinical variables and outcomes. TILs were scored following the International TILs Working Group 2014 recommendation by estimating the percentage of TILs occupying stroma [23]. The final score was derived by averaging the two scores. Cohen’s weighted kappa value for comparison of the two evaluators was 0.56 for TILs.
Statistical analysis
Univariate analysis was performed to examine the association between PD-L1 expression and clinical variables. Since there are no agreed on cutoffs for PD-L1, the estimated empirical distribution of the PD-L1 percentage was used in this study. An apparent bimodal gap at 1% in the distribution in our data naturally separated it to two groups, hence was chosen as the cutoff threshold for high PD-L1 expression vs. low expression. Fisher’s exact test was used for categorical variables and Wilcoxon non-parametric test for continuous variables. The associations of PD-L1 expression in the tumor and in TILs with RFS and OS were analyzed using Cox proportional hazard model with and without adjustment for clinical covariates. Because of the long time span of the cases included in the study (1997–2013), during which period trastuzumab was introduced and significantly improved the survival outcomes of HER2+ patients, to avoid bias due to the length of follow-up time, survival analysis was truncated at a maximum of 5 years from diagnosis. Analysis was first performed in the whole cohort, and then repeated in subgroups defined by HR status (HR− vs. HR+) and by Trastuzumab based chemotherapy (yes vs. no). All analyses were performed with R version 3.3.2 (http://www.r-project.org), using a nominal significance level of 0.05.
RESULTS
Patient characteristics
A total of 191 eligible patients were included in the study. Table 1 summarizes the clinical and pathological characteristics of the study population by anti-PD-L1 expression in tumors. 71% tumors were Nottingham grade 3, approximately half of the patients had T-stage at least 2, and 59.2% had at least a single positive lymph node. 60% tumors were HR+ and 39% HR−. 57% patients were treated with hormonal therapy, 74% treated with radiation therapy, and 60% with trastuzumab-based chemotherapy.
Table 1.
Clinical and pathologic characteristics of the patient population overall and by PD-L1 (clone 28-8 and 22C3) expression in tumor
| Clone 28-8 in tumor
|
Clone 22C3 in tumor
|
||||||
|---|---|---|---|---|---|---|---|
| Overall | ≥1% | <1% | p-value | ≥1% | <1% | p-value | |
| (n=191) | (n=49) | (n=142) | (n=22) | (n=169) | |||
| Age, years, median (range) | 52 (25-86) | 52 (31-74) | 53 (25-86) | 0.35 | 53 (33-72) | 52 (25-86) | 0.70 |
| Nottingham grade, n (%) | 0.01 | 0.32 | |||||
| 1-2 | 55 (28.8) | 7 (14.3) | 48 (33.8) | 4 (18.2) | 51 (30.2) | ||
| 3 | 136 (71.2) | 42 (85.7) | 94 (66.2) | 18 (81.8) | 118 (69.8) | ||
| Tumor Size, mm, median (range) | 21 (1-80) | 25 (8-80) | 20 (1-70) | 0.01 | 22 (8-45) | 20 (1-80) | 0.20 |
| T-stage, n (%) | 0.05 | 0.07 | |||||
| 0 | 1 (0.5) | 0 (0) | 1 (0.7) | 0 (0) | 1 (0.6) | ||
| 1 | 94 (49.2) | 17 (34.7) | 77 (54.2) | 6 (27.3) | 88 (52.1) | ||
| 2 | 88 (46.1) | 28 (57.1) | 60 (42.3) | 14 (63.6) | 74 (43.8) | ||
| 3 | 6 (3.1) | 3 (6.1) | 3 (2.1) | 1 (4.5) | 5 (3.0) | ||
| 4 | 2 (1.1) | 1 (2.0) | 1 (0.7) | 1 (4.5) | 1 (0.6) | ||
| N-stage, n (%) | 0.92 | 0.39 | |||||
| 0 | 78 (40.8) | 21 (42.9) | 57 (40.1) | 8 (36.4) | 70 (41.4) | ||
| 1 | 72 (37.7) | 18 (36.7) | 54 (38.0) | 10 (45.5) | 62 (36.7) | ||
| 2 | 25 (13.1) | 7 (14.3) | 18 (12.7) | 4 (18.2) | 21 (12.4) | ||
| 3 | 16 (8.4) | 3 (2.0) | 13 (9.2) | 0 (0) | 16 (9.5) | ||
| Hormonal receptor, n (%) | 0.03 | 1.00 | |||||
| Positive | 75 (39.3) | 26 (53.1) | 49 (34.5) | 9 (40.9) | 66 (39.1) | ||
| Negative | 115 (60.2) | 23 (46.9) | 92 (64.8) | 13 (59.1) | 102 (60.4) | ||
| Hormonal Therapy, n(%) | 0.06 | 1.00 | |||||
| No | 72(37.7) | 24(49.0) | 48(33.8) | 8(36.4) | 64(37.9) | ||
| Yes | 109(57.1) | 22(44.9) | 87(61.3) | 12(54.5) | 97(57.4) | ||
| Radiation Therapy, n(%) | 0.85 | 0.294 | |||||
| No | 47(24.6) | 13(26.5) | 34(23.9) | 3(13.6) | 44(26.0) | ||
| Yes | 141(73.8) | 35(71.4) | 105(73.9) | 19(86.4) | 122(72.2) | ||
| Trastuzumab, n(%) | 0.066 | 0.010 | |||||
| No | 76(39.8) | 14(28.6) | 62(43.7) | 3(13.6) | 73(43.2) | ||
| Yes | 115(60.2) | 35(71.4) | 80(56.3) | 19(86.4) | 96(56.8) | ||
| TIL-Str, %, median (range) | 15 (0-85) | 30 (0-85) | 15 (0-65) | <0.001 | 40 (7.5-85) | 15 (0-65) | <0.001 |
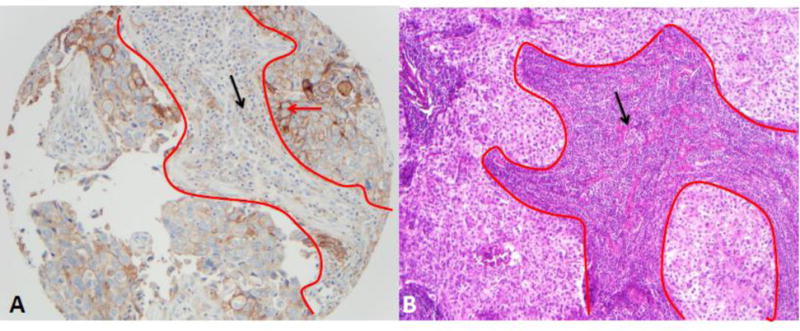
Clone 28-8 of PD-L1 was expressed in 25.7% of the tumors (Figure 1A), whereas clone 22C3 was expressed in 11.5% of the tumors. PD-L1 expression was also present on TILs (Figure 1A). Overall median and range of TILs was 15% (0% to 85%) (Figure 1B).
Figure 1.
Representative images of PD-L1 staining and TILs scores: A, PD-L1 (clone 28-8; 20× magnification), within red lines is stroma and outside is tumor, red arrow indicates membranous staining for PD-L1 in tumor cells (2 to 3 intensity in 80% of tumor cells), black arrow indicate PD-L1 expression in small lymphocytes (score 30%); B, TILs, within red line is TILs and indicated by black arrow, scored by one pathologist as 90% and by the other 80% with average of 85% (H&E 10×).
In univariate analyses, tumor PD-L1 expression (clone 28-8), using 1% cutoff, was associated with higher Nottingham grade (p=0.01), larger tumor size (p=0.01), HR− type (p=0.028), and higher level of TILs (p<0.001). Clone 22C3 of PD-L1 expression was correlated only with TILs (p<0.001) (Table 1).
PD-L1 expression with RFS and OS
Higher tumor expression of PD-L1 (clone 28-8) was associated with lower risk of disease recurrence. Patients with no recurrence had higher PD-L1 (clone 28-8) expression (p=0.055). Similar trend was observed with OS (p=0.618) (Table 2). After controlling for clinical prognostic factors, patients with tumor PD-L1 expression level >0% had an HR of 0.4 (95% CI 0.17–0.93, p=0.033). When a different cutoff point for PD-L1 expression was used (>1%), the results were similar but with only borderline significance (HR=0.4, 95% CI 0.15 to 1.03, p=0.058). In addition to tumor cells, PD-L1 expressed on TILs (clone 28-8) was also associated with RFS. Patients with no recurrence had higher PD-L1 expression than those with recurrence (p=0.011). For OS, similar trend was noted without reaching statistical significance (p=0.3) (Table 2).
Table 2.
Recurrence free survival and overall survival correlation with PD-L1 and stromal tumor infiltrating lymphocytes
| Recurrence Free Survival
|
Overall Survival
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Overall | Recurrence | No recurrence | p-value | Overall | Death | No death | p-value | |
| (n=182) | (n=30) | (n=152) | (n=191) | (n=20) | (n=171) | |||
| TIL-Str | 25 (0-85) | 16 (0-50) | 25 (0-85) | 0.101 | 24 (0-85) | 6 (3-85) | 25 (0-80) | 0.005 |
| 28-8 in tumor | 1.2 (0-85) | 0 (0-11.7) | 1.7 (0-85) | 0.055 | 1.2 (0-85) | 2.3 (0-66.7) | 0.8 (0-85) | 0.618 |
| 28-8 in TILs | 4.2 (0-91.7) | 0 (0-26.7) | 5 (0-91.7) | 0.011 | 4 (0-91.7) | 1 (0-60) | 5 (0-91.7) | 0.300 |
| 22C3 in tumor | 0 (0-73.3) | 0 (0-0.8) | 0 (0-73.3) | 0.149 | 0 (0-73.3) | 0 (0-51.7) | 0 (0-73.3) | 0.388 |
| 22C3 in TILs | 0.8 (0-66.7) | 0 (0-27) | 0.8 (0-66.7) | 0.566 | 0.8 (0-66.7) | 0 (0-61.7) | 0.8 (0-66.7) | 0.140 |
Correlation of stromal TILs with RFS and OS
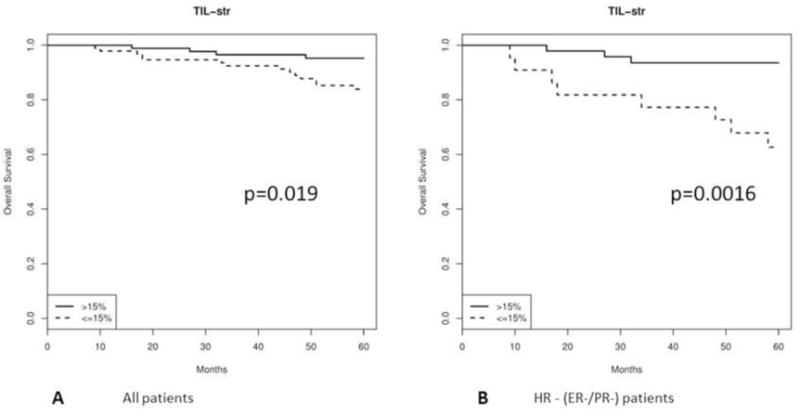
Higher Stromal TILs were found in patients without recurrence and patients who survived compared with patients with recurrence and patients who died, with the difference with OS reaching statistical significance (p=0.005) (Table 2). Kaplan-Meier analysis showed that patients whose tumor was enriched with TILs (>15%) had significantly better 5-year OS (p=0.019) (Figure 2A). In multivariate models, higher level of TILs (>15%) was associated with an HR of 0.35 after controlling for clinical prognostic factors (95% CI 0.17 – 0.73, p=0.005).
Figure 2.
Kaplan-Meier OS curves for high TILs (>15%) vs. low TILs (≤ 15%): A, All patients; B, In HR− type.
PD-L1 expression with RFS and OS by HR subtype (HR+ and HR−)
5-Year RFS analysis in two HR subtypes (HR+ and HR−)
In HR− type (n=71), lower TILs correlated with tumor recurrence with borderline statistical significance (p=0.077) (Table 3A). In HR + type (n=110), lower expression of PD-L1 (clone 28-8) in TILs correlated with high risk of tumor recurrence within 5 years (p=0.022) (Table 3B).
Table 3.
Recurrence free survival and overall survival correlation with PD-L1 and stromal tumor infiltrating lymphocytes in HR− and HR+ type
| A. HR− type
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Recurrence Free Survival
|
Overall Survival
|
|||||||
| Overall | Recurrence | No recurrence | p-value | Overall | Death | No death | p-value | |
| (n=71) | (n=14) | (n=57) | (n=75) | (n=12) | (n=63) | |||
| TIL-Str | 36 (0-80) | 22 (5-50) | 40 (0-80) | 0.077 | 37 (0-80) | 10 (5-45) | 35 (0-80) | 0.001 |
| 28-8 in tumor | 1.7 (0-85) | 1.3 (0-11.7) | 1.7 (0-85) | 0.299 | 1.7 (0-85) | 4.1 (0-11.7) | 1.7 (0-85) | 0.416 |
| 28-8 in TILs | 5 (0-92) | 0.4 (0-26.7) | 5 (0-91.7) | 0.260 | 5 (0-91.7) | 0.6 (0-5.8) | 7.5 (0-91.7) | 0.209 |
| 22C3 in tumor | 0 (0-73.3) | 0 (0-0.8) | 0 (0-73.3) | 0.460 | 0 (0-73.3) | 0 (0-0.8) | 0 (0-73.3) | 0.293 |
| 22C3 in TILs | 2.5 (0-66.7) | 0.6 (0-18.8) | 3.1 (0-66.7) | 0.657 | 0.8 (0-61.7) | 0 (0-26.7) | 0.8 (0-61.7) | 0.072 |
|
B. HR+ type | ||||||||
|
Recurrence Free Survival
|
Overall Survival
|
|||||||
| Overall | Recurrence | No recurrence | p-value | Overall | Death | No death | p-value | |
| (n=110) | (n=15) | (n=95) | (n=115) | (n=8) | (n=107) | |||
|
| ||||||||
| TIL-Str | 18 (3-85) | 7 (5-45) | 23 (3-85) | 0.469 | 18 (3-85) | 4 (3-85) | 18 (3-65) | 0.085 |
| 28-8 in tumor | 0.8 (0-66.7) | 0 (0-0.8) | 0.8 (0-66.7) | 0.093 | 0.8 (0-66.7) | 0 (0-66.7) | 0.8 (0-50) | 0.424 |
| 28-8 in TILs | 3.3 (0-60) | 0 (0- 3.3) | 5 (0-60) | 0.022 | 3.3 (0-60) | 1.9 (0-60) | 3.3 (0-40) | 0.817 |
| 22C3 in tumor | 0 (0-51.7) | 0 (0-0.8) | 0 (0-51.7) | 0.219 | 0 (0-51.7) | 0 (0-51.7) | 0 (0-14.2) | 0.878 |
| 22C3 in TILs | 0.8 (0-61.7) | 0 (0-26.7) | 0.8 (0-61.7) | 0.815 | 0.8 (0-61.7) | 0 (0-61.7) | 0.8 (0-40) | 0.774 |
5-Year OS analysis in two HR subtypes (HR+ and HR−)
In HR− type (n=75), multivariate analysis showed higher level of TILs (>15%) was associated with an HR of 0.35 after controlling for clinical prognostic factors (95% CI 0.17 – 0.73, p=0.005). Lower TILs correlated with death due to disease (p=0.001) (Table 3A). Kaplan-Meier analysis showed that patients whose tumors were enriched with TILs had significantly better 5-year OS (p=0.0016) (Figure 2B). In the multivariate analysis, low level (≤15%) of TILs correlated with death due to disease with HR of 0.31 (95% CI 0.12 to 0.8l, p=0.015). In HR+ type (n=115), 8 (7%) died due to disease within 5 years. None of the variables was statistically significant in the univariate analysis.
PD-L1 expression with RFS and OS by trastuzumab therapy or not
5-Year RFS with and without trastuzumab based chemotherapy
In the group of patients who were treated with trastuzumab-based adjuvant chemotherapy (n=110), 16 (14.5%) developed tumor recurrence within 5 years. Lower PD-L1 (clone 28-8) expression in TILs (p=0.034) correlated with tumor recurrence (Table 4A). In the group of patients who were treated with non-trastuzumab-based adjuvant chemotherapy (n=72), 14 (19.4%) developed tumor recurrence within 5 years. Lower TILs and lower PD-L1 (clone 28-8) expression in tumor had borderline statistical significance in association with tumor recurrence, p=0.064 and 0.083, respectively (Table 4B).
Table 4.
Recurrence free survival and overall survival correlation with PD-L1 and stromal tumor infiltrating lymphocytes in patients treated with trastuzumab and non-trastuzumab based chemotherapy
| A. Trastuzumab based chemotherapy
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Recurrence Free Survival
|
Overall Survival
|
|||||||
| Overall | Recurrence | No recurrence | p-value | Overall | Death | No death | p-value | |
| (n=110) | (n=16) | (n=94) | (n=115) | (n=13) | (n=102) | |||
| TIL-Str | 25 (0-85) | 20 (8-45) | 25 (0-85) | 0.576 | 25 (0-85) | 14 (3-85) | 25 (0-80) | 0.102 |
| 28-8 in tumor | 1.7 (0-85) | 0.7 (0-11.7) | 1.7 (0-85) | 0.336 | 1.7 (0-85) | 3.8 (0-66.7) | 1.6 (0-85) | 0.747 |
| 28-8 in TILs | 5 (0-91.7) | 0.6 (0-26.7) | 8.1 (0-91.7) | 0.034 | 5 (0-91.7) | 1.9 (0-60) | 5.6 (0-91.7) | 0.305 |
| 22C3 in tumor | 0 (0-73.3) | 0 (0-0.8) | 0.6 (0-73.3) | 0.185 | 0 (0-73.3) | 0 (0-51.7) | 0 (0-73.3) | 0.534 |
| 22C3 in TILs | 2.5 (0-66.7) | 1.3 (0-26.7) | 2.5 (0-66.7) | 0.606 | 2.5 (0-66.7) | 0 (0-61.7) | 2.5 (0-66.7) | 0.407 |
|
B. Non-Trastuzumab based chemotherapy | ||||||||
|
Recurrence Free Survival
|
Overall Survival
|
|||||||
| Overall | Recurrence | No recurrence | p-value | Overall | Death | No death | p-value | |
| (n=72) | (n=14) | (n=58) | (n=76) | (n=7) | (n=69) | |||
|
| ||||||||
| TIL-Str | 22 (0-75) | 13 (0-50) | 25 (5-75) | 0.064 | 22 (0-75) | 3 (5-10) | 20 (0-75) | 0.009 |
| 28-8 in tumor | 0.8 (0-15) | 0 (0-2.7) | 0.8 (0-15) | 0.083 | 0.2 (0-15) | 0.8 (0-8.3) | 0 (0-15) | 0.741 |
| 28-8 in TILs | 0.9 (0-35) | 0 (0-11.7) | 1.5 (0-35) | 0.185 | 0.8 (0-35) | 0.4 (0-1.1) | 0.8 (0-35) | 0.728 |
| 22C3 in tumor | 0 (0-10) | 0 (0-0.4) | 0 (0-10) | 0.688 | 0 (0-13.8) | 0 (0-0.1) | 0 (0-13.8) | 0.39 |
| 22C3 in TILs | 0 (0-35) | 0 (0-18.8) | 0 (0-35) | 0.925 | 0 (0-35) | 0 (0-0) | 0 (0-35) | 0.212 |
5-Year OS with and without trastuzumab Based Chemotherapy
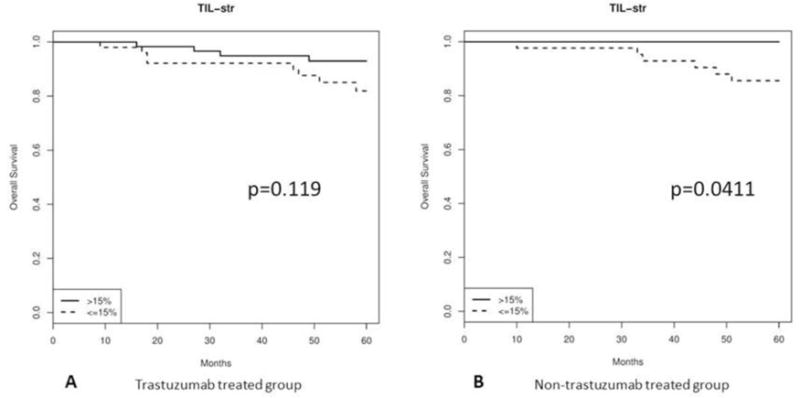
In the group of patients who were treated with trastuzumab-based adjuvant chemotherapy (n=115), 13 (11.3%) died due to disease within 5 years. Kaplan-Meier analysis showed that patients whose tumor was enriched with TILs had a trend of better 5-year OS without reaching statistical significance (p=0.119) (Figure 3A). In the group of patients who were treated with non-trastuzumab-based adjuvant chemotherapy (n=76), 7 (9.2%) died of disease within 5 years. Low TILs (p=0.009) correlated with death due to disease (Table 4B). Kaplan-Meier survival curve showed that patients whose tumor was enriched with TILs had significantly better 5-year OS (p=0.0411) (Figure 3B).
Figure 3.
Kaplan-Meier OS curves for high TILs (>15%) vs. low TILs (≤ 15%): A, trastuzumab treated patients; and B, non-trastuzumab treated patients.
DISCUSSION
Studies have shown that 21% to 59.5 % of BC cases demonstrate PD-L1 mRNA and/or protein expression on tumor cells, whereas not on adjacent normal breast epithelial cells [24–26, 30]. PD-L1 expression is associated with higher histological grade, and ER/PR negativity [12, 20, 28, 40]. That is consistent with our findings where PD-L1 expression (detected by clone 28-8) in BC cells correlated with higher Nottingham grade, larger tumor size, and HR− type.
TILs have also been found to express PD-L1, especially in large, high grade, HER2+, and basal-like tumors [11, 12]. TILs have been recognized as an independent favorable prognostic factor in TNBC and HER2+ BC, not so in luminal type [41, 42]. Specifically, higher TILs have been found to predict better outcomes in both early stage [34, 43] and advanced HER2+BCs [44].
Consistent with the majority of the published studies [25, 26, 30], we found that PD-L1 expression on tumor cells and TILs both correlated with improved OS in univariate analysis. In the multivariate analysis only higher PD-L1 expression in TILs (detected by clone 22C) found to correlate with improved OS. Although it is counterintuitive to consider PD-L1 as a favorable prognostic value given its immunosuppressive function, a biological explanation may be that PD-L1 expression in tumor corresponds to more active local immunity against the tumor. Increased PD-L1 represents the tumor cell’s attempt to damper the enhanced host immune activity [45]. In another word, expression of PD-L1 may not be an indicator of immunosuppression, but rather the host anti-tumor immunity. With that notion in mind, the logic underlying anti-PD-L1 therapy can be easily understood.
As a prototype medication in treating HER2+ BC, trastuzumab has been proposed to have additional mechanisms of action outside of its blocking HER2 signaling [46], specifically antibody-dependent cellular cytotoxicity (ADCC), which results in the activation of native immune cells including NK-T cell, macrophages and DCs [31–33]. We found that patients whose tumor was enriched with TILs had better OS in the non-trastuzumab treated group, while this prognostic significance was not seen in trastuzumab treated group. That is consistent with the findings of Perez et al in the N9831 clinical trial, which showed that when patients had higher preexisting TILs, trastuzumab did not provide additional therapeutic benefit to chemotherapy alone regimen, yet higher TILs correlated with better relapse free survival in patients treated with chemotherapy only. These observations suggest that patients whose tumor is enriched with TILs might lose the benefit of added trastuzumab to chemotherapy or hormonal therapy. However there are studies suggesting that targeting anti-PD-1/PD-L1 axis might restore/enhance the benefit of anti-HER2 agents through synergism of these two classes of agents[47, 48]. The detailed mechanism of this synergism is unclear, including possible actions on ADCC-mediating NK cells directly or other pathways in addition to ADCC.
Given association of TILs, PD-1/PD-L1 expression and efficacy of anti-HER2 agents, further studies to determine TILs as predictive biomarker for immune related therapies and to delineate the details of participating immune subgroups are warranted.
We observed a difference of PD-L1 labeling between 28-8 and 22C3, similar to studies in NSCLC. While 28-8 is a rabbit antibody, and 22C3 is derived from mouse, they both target extracellular domain of PD-L1 molecule [49]. Since the details of antibodies are proprietary information, we can only speculate that the difference with the two antibodies may be due to different affinities, specificity, or distinct epitopes. Another explanation is due to the heterogeneity of the breast cancer and the focal nature of PD-L1 expression, so that the expression profile is different form one area of tumor to another, especially when we used TMA tissue for evaluation.
In summary, our study demonstrated that PD-L1 expression in HER2+ BC is associated with TILs, and correlates with favorable outcomes. Increased TILs correlate with better OS only in non-trastuzumab treated patients, suggesting TILs are not only a prognostic biomarker with regards to overall prognosis, but also has a potential of predicting benefit from trastuzumab therapy and thus indicator to combine anti-PD-1/PD-L1 treatment with anti-HER2 agents. Our study also highlights the challenges of studying TILs and PD1/PD-L1 expression in solid tumors due to changing definitions between studies and differing results between most commonly used antibodies. There is a continuing need for standardization of evaluation of patients in order to address if immunomodulatory therapies could lead to improved clinical outcomes in HER2+ BC.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
Data archiving is not mandated but data will be made available on reasonable request.
References
- 1.Hanahan D, Weinberg Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Current opinion in immunology. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science (New York, NY) 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 4.Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, Brahmer JR, Lawrence DP, Atkins MB, Powderly JD, et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. Journal of Clinical Oncology. 2014;32(10):1020–1030. doi: 10.1200/JCO.2013.53.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;372(21):2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 6.Drake CG, Bivalacqua TJ, Hahn NM. Programmed Cell Death Ligand-1 Blockade in Urothelial Bladder Cancer: To Select or Not to Select. Journal of Clinical Oncology. 2016;34(26):3115–3116. doi: 10.1200/JCO.2016.68.4696. [DOI] [PubMed] [Google Scholar]
- 7.Okazaki T, Honjo T. The PD-1–PD-L pathway in immunological tolerance. Trends in Immunology. 2006;27(4):195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the Pd-1 Immunoinhibitory Receptor by a Novel B7 Family Member Leads to Negative Regulation of Lymphocyte Activation. The Journal of Experimental Medicine. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annual review of immunology. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular cancer therapeutics. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
- 11.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia (New York, NY) 2006;8(3):190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, et al. PD-L1 (B7-H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas()() Human pathology. 2016;47(1):52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 14.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Science translational medicine. 2012;4(127):127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstock M, McDermott D. Targeting PD-1/PD-L1 in the treatment of metastatic renal cell carcinoma. Therapeutic Advances in Urology. 2015;7(6):365–377. doi: 10.1177/1756287215597647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer JR, Tykodi SS, Chow LQM, Hwu W-J, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. New England Journal of Medicine. 2012;366(26):2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, Activity, and Immune Correlates of Anti–PD-1 Antibody in Cancer. New England Journal of Medicine. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. New England Journal of Medicine. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen JT. Companion diagnostic assays for PD-1/PD-L1 checkpoint inhibitors in NSCLC. Expert review of molecular diagnostics. 2016;16(2):131–133. doi: 10.1586/14737159.2016.1117389. [DOI] [PubMed] [Google Scholar]
- 20.Wimberly H, Brown JR, Schalper K, Haack H, Silver MR, Nixon C, Bossuyt V, Pusztai L, Lannin DR, Rimm DL. PD-L1 Expression Correlates with Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy in Breast Cancer. Cancer immunology research. 2015;3(4):326–332. doi: 10.1158/2326-6066.CIR-14-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, Martino S, Wang M, Jones VE, Saphner TJ, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(27):2959–2966. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoury T, Nagrale V, Opyrchal M, Peng X, Wang D, Yao S. Prognostic Significance of Stromal Versus Intratumoral Infiltrating Lymphocytes in Different Subtypes of Breast Cancer Treated With Cytotoxic Neoadjuvant Chemotherapy. Applied immunohistochemistry & molecular morphology : AIMM. 2017 doi: 10.1097/PAI.0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26(2):259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muenst S, Schaerli AR, Gao F, Daster S, Trella E, Droeser RA, Muraro MG, Zajac P, Zanetti R, Gillanders WE, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast cancer research and treatment. 2014;146(1):15–24. doi: 10.1007/s10549-014-2988-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(10):2773–2782. doi: 10.1158/1078-0432.CCR-13-2702. [DOI] [PubMed] [Google Scholar]
- 26.Baptista MZ, Sarian LO, Derchain SF, Pinto GA, Vassallo J. Prognostic significance of PD-L1 and PD-L2 in breast cancer. Human pathology. 2016;47(1):78–84. doi: 10.1016/j.humpath.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Hasan A, Ghebeh H, Lehe C, Ahmad R, Dermime S. Therapeutic targeting of B7-H1 in breast cancer. Expert opinion on therapeutic targets. 2011;15(10):1211–1225. doi: 10.1517/14728222.2011.613826. [DOI] [PubMed] [Google Scholar]
- 28.Zawlik I, Gablo N, Szymanska B, Pawlowska Z, Chudobinski C, Chalubinska-Fendler J, Morawiec Z, Zielinska-Blizniewska H, Morawiec-Sztandera A, Kolacinska A. Immune checkpoints in aggressive breast cancer subtypes. Neoplasma. 2016;63(5):768–773. doi: 10.4149/neo_2016_514. [DOI] [PubMed] [Google Scholar]
- 29.Adams S, Diamond J, Hamilton E, Pohlmann P, Tolaney S, Molinero L, Zou W, Liu B, Waterkamp D, Funke R, et al. Abstract P2-11-06: Safety and clinical activity of atezolizumab (anti-PDL1) in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer. Cancer Research. 2016;76(4 Supplement):P2-11-06–P12-11-06. [Google Scholar]
- 30.Bertucci F, Finetti P, Birnbaum D, Mamessier E. The PD1/PDL1 axis, a promising therapeutic target in aggressive breast cancers. OncoImmunology. 2016;5(3):e1085148. doi: 10.1080/2162402X.2015.1085148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barok M, Isola J, Palyi-Krekk Z, Nagy P, Juhasz I, Vereb G, Kauraniemi P, Kapanen A, Tanner M, Vereb G, et al. Trastuzumab causes antibody-dependent cellular cytotoxicity-mediated growth inhibition of submacroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Molecular cancer therapeutics. 2007;6(7):2065–2072. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 32.Denkert C, Darb-Esfahani S, Loibl S, Anagnostopoulos I, Johrens K. Anti-cancer immune response mechanisms in neoadjuvant and targeted therapy. Seminars in immunopathology. 2011;33(4):341–351. doi: 10.1007/s00281-011-0261-0. [DOI] [PubMed] [Google Scholar]
- 33.Savas P, Caramia F, Teo ZL, Loi S. Oncogene addiction and immunity: clinical implications of tumour infiltrating lymphocytes in breast cancers overexpressing the HER2/neu oncogene. Current opinion in oncology. 2014;26(6):562–567. doi: 10.1097/CCO.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 34.Perez EA, Ballman KV, Tenner KS, Thompson EA, Badve SS, Bailey H, Baehner FL. Association of Stromal Tumor-Infiltrating Lymphocytes With Recurrence-Free Survival in the N9831 Adjuvant Trial in Patients With Early-Stage HER2-Positive Breast Cancer. JAMA oncology. 2016;2(1):56–64. doi: 10.1001/jamaoncol.2015.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Archives of pathology & laboratory medicine. 2014;138(2):241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast Cancer Subtypes Based on ER/PR and Her2 Expression: Comparison of Clinicopathologic Features and Survival. Clinical Medicine & Research. 2009;7(1–2):4–13. doi: 10.3121/cmr.2009.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 38.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 39.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1999;17(5):1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 40.Ghebeh H, Tulbah A, Mohammed S, Elkum N, Bin Amer SM, Al-Tweigeri T, Dermime S. Expression of B7-H1 in breast cancer patients is strongly associated with high proliferative Ki-67-expressing tumor cells. International journal of cancer. 2007;121(4):751–758. doi: 10.1002/ijc.22703. [DOI] [PubMed] [Google Scholar]
- 41.Denkert C, Minckwitz Gv, Brase JC, Sinn BV, Gade S, Kronenwett R, Pfitzner BM, Salat C, Loi S, Schmitt WD, et al. Tumor-Infiltrating Lymphocytes and Response to Neoadjuvant Chemotherapy With or Without Carboplatin in Human Epidermal Growth Factor Receptor 2–Positive and Triple-Negative Primary Breast Cancers. Journal of Clinical Oncology. 2015;33(9):983–991. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 42.Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, de Azambuja E, Eidtmann H, Ellis CE, Baselga J, et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated With Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA oncology. 2015;1(4):448–454. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Denkert C, Loibl S, Salat C, Sinn B, Schem C, Endris V, Klare P, Schmitt W, Blohmer J-U, Weichert W, et al. Abstract S1-06: Increased tumor-associated lymphocytes predict benefit from addition of carboplatin to neoadjuvant therapy for triple-negative and HER2-positive early breast cancer in the GeparSixto trial (GBG 66) Cancer Research. 2013;73(24 Supplement):S1-06–S01-06. [Google Scholar]
- 44.Luen SJ, Salgado R, Fox S, Savas P, Eng-Wong J, Clark E, Kiermaier A, Swain SM, Baselga J, Michiels S, et al. Tumour-infiltrating lymphocytes in advanced HER2-positive breast cancer treated with pertuzumab or placebo in addition to trastuzumab and docetaxel: a retrospective analysis of the CLEOPATRA study. The Lancet Oncology. 18(1):52–62. doi: 10.1016/S1470-2045(16)30631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25(8):1544–1550. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 46.Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. The Lancet Oncology. 2014;15(2):e58–68. doi: 10.1016/S1470-2045(13)70477-7. [DOI] [PubMed] [Google Scholar]
- 47.Muntasell A, Cabo M, Servitja S, Tusquets I, Martínez-García M, Rovira A, Rojo F, Albanell J, López-Botet M. Interplay between Natural Killer Cells and Anti-HER2 Antibodies: Perspectives for Breast Cancer Immunotherapy. Frontiers in Immunology. 2017;8:1544. doi: 10.3389/fimmu.2017.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, Teng MW, Smyth MJ. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(17):7142–7147. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu H, Lin G, Huang C, Zhu W, Miao Q, Fan X, Wu B, Zheng X, Lin X, Jiang K, et al. Assessment of Concordance between 22C3 and SP142 Immunohistochemistry Assays regarding PD-L1 Expression in Non-Small Cell Lung Cancer. Scientific Reports. 2017;7:16956. doi: 10.1038/s41598-017-17034-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


