Abstract
Exposure to ambient air pollution has been associated with greater risk of elevated blood pressure (BP) in adults and children. Recent evidence suggests that air pollution exposure in pregnancy may also portend increased risk for the next generation; however, few studies have examined this relationship. We conducted a prospective study of 1,293 mothers in the Boston Birth Cohort (enrolled 1998–2012) and their children who had follow up visits between 3–9 years of age and complete exposure and outcome data. Our primary exposure, ambient particulate matter ≤2.5 microns (PM2.5) concentration during pregnancy, was estimated by matching mother’s residential address to the U.S. Environmental Protection Agency’s air quality monitors. We defined our primary outcome child systolic BP (SBP) percentile according to U.S. reference (Fourth Report) and classified elevated BP as SBP ≥ 90th percentile. Our multivariable-adjusted cubic spline showed a sharp increase in offspring SBP percentile and risk for elevated BP when third-trimester PM2.5 concentration was ≥13 μg/m3. The highest vs. lowest tertile of third-trimester PM2.5 exposure was associated with a 4.85 (95% CI: 1.38–8.37) percentile increase in child SBP or a 1.61 (95% CI: 1.13–2.30) times higher risk of child elevated BP. A 5 μg/m3 increase in PM2.5 during the third trimester was associated with a 3.49 (95% CI: 0.71–6.26) percentile increase in child SBP or a 1.47 (95% CI:1.17–1.85) times higher risk of elevated BP. Our findings suggest that exposure to ambient PM2.5 during the third trimester of pregnancy is associated with elevated BP in children, ages 3–9 years.
Keywords: Air Pollution, Particulate Matter, Blood Pressure, Hypertension, Pregnancy, Maternal Exposure, Child Health
INTRODUCTION
High blood pressure (BP) is a major risk factor for cardiovascular disease and the leading modifiable cause of mortality, contributing to an estimated death of 7.5 million worldwide.1,2 Although great strides have been made to control BP, the global prevalence of elevated BP after age-standardization remains high at 20% to 25% and has recently increased among children and adolescents.3–5 High BP tracks from childhood to adulthood, and thus it is crucial to start prevention as early as possible.6
Air pollution, a significant contributor to morbidity and mortality worldwide,7 has been associated with elevated BP in both children and adults.8,9 Air pollution may also have transgenerational effects. Murine models have found that exposure to particulate matter ≤2.5 microns (PM2.5) in utero affects the development of offspring’s cardiovascular system, increasing the risk for elevated BP and other cardiovascular disease events.10–12 In humans, our team was the first to report the link between maternal exposure to air pollution with offspring low birthweight and shorter gestational age.13,14 More recently, we reported an association between PM2.5 and intrauterine inflammation,15 as well as a combined effect of maternal exposure to PM2.5 and pre-pregnancy obesity on childhood overweight or obesity.16 However, data on the transgenerational effects of air pollution on BP are sparse. Van Rossem et al. found PM2.5 exposure in the third trimester related with elevated BP among newborns.17 Breton and colleagues, on the other hand, did not find such association among 11-year-old teenagers using retrospectively collected pollutant data.18
In light of this literature gap, we sought to test the hypothesis that exposure to PM2.5 during pregnancy is associated with higher offspring systolic BP (SBP) in childhood, using longitudinal data from the Boston Birth Cohort, a large, predominantly urban, low income minority population.
METHODS
Our manuscript adheres to the American Heart Association Journals’ implementation of the Transparency and Openness Promotion (TOP) Guidelines. Dr. Xiaobin Wang, the Principal Investigator of the Boston Birth Cohort, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The data, analytic methods, and study materials that support the findings of this study will be available from Dr. Xiaobin Wang (xwang82@jhu.edu) upon request, after the request is submitted and formally reviewed and approved by the institutional review board of the Boston University Medical Center and Johns Hopkins Bloomberg School of Public Health.
STUDY PARTICIPANTS
Participants for our study are from the Boston Birth Cohort, an ongoing prospective birth cohort that began recruiting mother-child pairs in Boston University Medical Center starting from 1998 on a rolling basis. The cohort consists of a predominantly urban low-income minority population rich in preterm birth and low birth weight infants.
Detailed methods of recruitment and data collection have been described previously.19 The enrollment period for the current analytic data set was from 1998 to 2012. Recruitment was conducted 24–72 hours after the child were born with written consent from all mothers. A standardized questionnaire interview of mothers was used to collect information on socio-economic status, lifestyle and environmental factors. Multiple-gestation pregnancies and newborns with major birth defects were excluded from the study. Postnatal follow-up was limited to children who were enrolled in the study and received primary care at the Boston University Medical Center between January 2001 to December 2014.
Figure S1 (please see http://hyper.ahajournals.org) illustrates how participants were selected for our analysis. Of the 2,890 mothers under postnatal follow-up, 1,877 had their child’s BP measured on at least one well-child visit from 3 to 9 years old. We excluded 6 pairs who did not complete maternal questionnaire and 578 pairs who had missing data for PM2.5 exposure during preconception or any trimester during pregnancy, which reduced our sample size to 1,293 mother-child pairs.
The study was approved by the institutional review board of Boston University Medical Center and Johns Hopkins Bloomberg School of Public Health.
EXPOSURES
The primary exposure for this study was mothers’ exposure to ambient PM2.5 during pregnancy. Pregnancy exposure periods were divided into the first trimester (days 1–90 of pregnancy), the second trimester (days 91–180), third trimester (days 181-birth). We created a combined exposure from the first to third trimester as a proxy of exposure during entire pregnancy. We also examined the preconception (90 days before pregnancy) and postnatal (first two years of life) PM2.5 exposure.
To determine ambient PM2.5 concentration, we matched each mother’s residential address by street level to the nearest U.S. Environmental Protection Agency’s (EPA) air quality monitor using Euclidean minimum distance and recorded daily PM2.5 concentration from this monitor.16 A map showing the locations of participants relative to the monitors can be found in our previous paper.15 If the participant moved away from the previous address during pregnancy, PM2.5 concentration was recorded from the nearest monitor matched to the new address since the date she moved. Individual exposure to PM2.5 during each period was calculated as the geometric mean of the daily concentration during this time. We treated PM2.5 concentration both as a continuous variable (scaled to 5μg/m3 increase) and as a categorical variable (deriving tertiles of exposure separately for each period).
OUTCOME
The primary outcome was child SBP at the last recorded well-child visit at the Boston University Medical Center, which fell between 3 and 9 years of age. Child BP was measured at the right brachial artery by the clinical staff using the validated automatic sphygmomanometer Masimo Set (2003–2008: the Welch Allyn 420 Spot Vital Signs monitor; 2008–2014, the Welch Allyn 45MT0 Spot Vital Signs LXi monitor). We transformed SBP for each child into percentile based on the U.S reference (The Fourth Report).20 We chose to use SBP in lieu of diastolic BP (DBP) because it is a better predictor of adult hypertension and adverse cardiovascular outcomes.21,22
We modeled SBP as a continuous variable and as a binary variable (having elevated BP or not). We defined elevated BP as SBP percentile ≥90 in accordance to the Fourth Report which is the reference standard for child hypertension diagnosis.20
COVARIATES
We extracted information on mother’s pre-pregnancy weight, height, race, education, smoking status and alcohol consumption from the standardized questionnaire. Maternal pre-pregnancy body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. We further categorized it as underweight (BMI < 18.5 kg/m2), normal weight (18.5 kg/m2 ≤ BMI < 25 kg/m2), overweight (25 kg/m2 ≤ BMI < 30 kg/m2) and obesity (BMI ≥ 30 kg/m2). Maternal race was categorized as Black, Hispanic and Other (including White, Asian, any other self-reported race or mixed race).
Covariates included maternal age at delivery, maternal hypertensive disorders, birth weight, gestational age, child sex and delivery type, all of which were extracted from electrical medical records. Maternal hypertensive disorder was defined by having chronic hypertension or one of the pregnancy-induced symptoms including gestational hypertension, eclampsia, pre-eclampsia or hemolysis, elevated liver enzymes, and low platelet count (HELLP syndrome).23 Gestational age was modelled both continuously and categorized as preterm birth (<37 weeks) or not (≥37 weeks).
DATA ANALYSIS
We first estimated the effect of PM2.5 exposure on SBP percentile using multivariable generalized linear regression model (GLM). Poisson regression with robust variance was used to examine the relative risk of elevated BP in relation to PM2.5 exposure.24 In all the models, PM2.5 exposure was treated as both a continuous variable scaled to 5μg/m3 increases and a categorical variable by tertile. We also examined the possible non-linear relationship using the restricted cubic spline regression model.25
To address confounding, we identified covariates related to both the PM2.5 exposure and the outcome of BP and which were not in the causal pathway between the exposure and the outcome. We began with the crude model, and then added confounders into the regression model, including maternal age at delivery (continuous), maternal self-reported race (Black/African American; Hispanic; other), maternal marital status (married; other), maternal education (middle school or below; high school; college or above), maternal smoking history (never smoking; quit smoking; continued smoking during pregnancy) and maternal alcohol intake (yes; no). All missing values for categorical variables were coded as a separate category. There were no missing values for the continuous variable maternal age at delivery.
Potential effect measure modifiers (EMM) considered included: maternal hypertensive disorders (yes; no), maternal pre-pregnancy BMI (normal weight; overweight; obesity), child sex (male; female), preterm birth (yes; no), birth weight for gestational age (small for gestational age; appropriate for gestational age; large for gestational age). We conducted subgroup analysis stratified by each potential EMM. Likelihood ratio test was conducted to compare two models with and without the interaction term (the product of the potential EMM and the continuous PM2.5 concentration scaled to 5μg/m3). All missing values for potential EMM were excluded from the analysis when conducting the stratified analysis and in the likelihood ratio test.
We then considered birthweight (continuous), gestational age (continuous) and child BMI-z score (continuous) as potential mediators since they may be in the causal pathway between prenatal PM2.5 exposure and child BP. We conducted mediation analysis by adding each mediator into the confounder model and estimated the degree of mediation individually and jointly.
To assess potential selection bias due to the missingness of the BP data, we conducted sensitivity analyses using the stabilized inverse probability weighting method by calculating the chance of having missing BP based on a set of baseline covariates and applying them to the regression model.26 In estimating the probability, we included all the potential confounders identified above and also maternal hypertensive disorders, maternal BMI, child sex, parity, low birth weight, preterm birth and delivery type. We used multiple imputation by chained equation method in the prediction model to deal with the missing values.27
All tests were based on a two-sided p<0.05 defined as statistically significant. Data management and analysis were conducted using Stata 14.2 (Stata Corp, College Station, TX) and SAS 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Table 1 provides characteristics of mother-child pairs according to PM2.5 levels in the third trimester (characteristics by PM2.5 levels during whole pregnancy is provided in Table S1 on http://hyper.ahajournals.org). Compared to mothers exposed to the lowest tertile of PM2.5 during the third trimester, those exposed to the highest tertile were more likely to be unmarried, have lower educational achievement, have maternal hypertensive disorders and have higher pre-pregnancy BMI. Children whose mothers were exposed to the highest PM2.5 level were more likely to have been born preterm, low birth weight, and tended to have higher BMI z-scores in childhood.
Table 1.
Characteristics of mother-child pairs in the Boston Birth Cohort by PM2.5 level in the third trimester (n=1293)
| Variable, Number (%) | PM2.5 Level During Third Trimester | p-value | ||
|---|---|---|---|---|
|
| ||||
| Tertile 1 (3.79–9.57 μg/m3) | Tertile 2 (9.57–11.80 μg/m3) | Tertile 3 (11.80–28.81 μg/m3) | ||
| Number | 431 | 431 | 431 | |
| Maternal Characteristics | ||||
| Age at delivery, mean (SD) | 28.88 (6.76) | 28.39 (6.51) | 28.32 (6.78) | 0.41 |
| African American race | 191 (44.3%) | 182 (42.2%) | 188 (43.6%) | 0.82 |
| Married mothers | 140 (33.0%) | 155 (36.3%) | 124 (29.2%) | 0.09 |
| Low educational achievement* | 122 (28.4%) | 126 (29.3%) | 137 (32.0%) | 0.48 |
| Smoke during pregnancy | 43 (10.0%) | 44 (10.4%) | 47 (11.0%) | 0.90 |
| Alcohol intake during pregnancy | 35 (8.3%) | 31 (7.5%) | 27 (6.6%) | 0.67 |
| Maternal hypertensive disorder | 76 (17.6%) | 65 (15.1%) | 83 (19.3%) | 0.26 |
| Pre-pregnancy BMI (kg/m2), mean (SD) | 26.46 (6.41) | 26.37 (5.95) | 27.14 (6.43) | 0.16 |
| Children Characteristics | ||||
| SBP percentile, mean (SD) | 56.30 (26.28) | 57.35 (25.88) | 60.97 (25.78) | 0.02 |
| Boys | 209 (48.5%) | 216 (50.1%) | 205 (47.6%) | 0.75 |
| Nulliparous | 186 (43.3%) | 164 (38.1%) | 186 (43.2%) | 0.21 |
| Preterm birth | 122 (28.3%) | 98 (22.7%) | 133 (30.9%) | 0.02 |
| Low birth weight | 112 (26.0%) | 98 (22.7%) | 132 (30.6%) | 0.03 |
| Vaginal delivery | 279 (64.7%) | 270 (62.6%) | 280 (65.0%) | 0.74 |
| Gestational age in week, mean (SD) | 37.66 (3.32) | 38.14 (2.83) | 37.75 (3.09) | 0.06 |
| BMI z-score, mean (SD) | 0.62 (1.36) | 0.76 (1.24) | 0.97 (1.22) | <0.01 |
Low educational achievement defined as having the highest education “Middle school or below”
Abbreviations: PM2.5 = particulate matter ≤2.5 microns; SD = standard deviation; BMI = body mass index; SBP = systolic blood pressure; Ref = Reference group
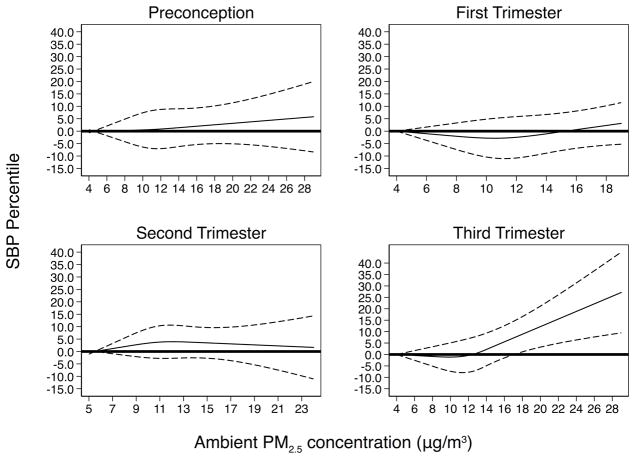
In Table 2, we show the results for the associations of maternal exposure to ambient PM2.5 concentrations in each period with childhood SBP percentile. Associations were significant for PM2.5 exposure in the third trimester. Compared to those in the lowest third trimester PM2.5 tertile, those in the highest tertile had a 4.62 (95% CI: 1.12, 8.12) percentile higher SBP after adjusting for confounders. This estimate increased to 4.79 (95% CI: 0.21, 9.37) after controlling postnatal PM2.5 concentration. Modelled as a continuous exposure, a 5 μg/m3 increase in PM2.5 concentration exposure in the third trimester was associated with a 3.39 (95% CI: 0.63, 6.15) percentile increase in child SBP. Based on multivariable cubic spline model (Figure 1), the SBP percentile increased monotonically and sharply after a threshold of 13 μg/m3 PM2.5 exposure in the third trimester. Childhood SBP percentile was not associated with exposure to ambient PM2.5 in the preconception period (adjusted β=1.05, 95% CI: −1.59, 3.68), first trimester (adjusted β =1.16, 95% CI: −1.63, 3.95) or second trimester (adjusted β =1.18, 95% CI: −1.75, 4.11).
Table 2.
Associations of ambient PM2.5 level and child SBP percentile in each exposure period (n=1293)
| Pregnancy Exposure Periods | PM2.5 Levels | Child SBP percentile | ||
|---|---|---|---|---|
|
| ||||
| Crude β (95% CI) | Adjusted β* (95% CI) | p-value | ||
| Preconception | Tertile 1 (4.48–9.90 μg/m3) | Ref. | Ref. | Ref. |
| Tertile 2 (9.90–12.08 μg/m3) | −1.43 (−4.91, 2.05) | −1.37 (−4.88, 2.13) | 0.442 | |
| Tertile 3 (12.10–29.00 μg/m3) | 1.85 (−1.63, 5.33) | 1.93 (−1.59, 5.46) | 0.282 | |
| Per 5μg/m3 increase | 0.94 (−1.64, 3.51) | 1.05 (−1.59, 3.68) | 0.437 | |
| First Trimester | Tertile 1 (4.17–9.72 μg/m3) | Ref. | Ref. | Ref. |
| Tertile 2 (9.72–11.93 μg/m3) | −1.41 (−4.89, 2.06) | −1.60 (−5.09, 1.90) | 0.370 | |
| Tertile 3 (11.93–19.15 μg/m3) | 3.02 (−0.45, 6.49) | 2.57 (−0.96, 6.09) | 0.153 | |
| Per 5μg/m3 increase | 1.39 (−1.36, 4.15) | 1.16 (−1.63, 3.95) | 0.415 | |
| Second Trimester | Tertile 1 (5.40–9.77 μg/m3) | Ref. | Ref. | Ref. |
| Tertile 2 (9.78–11.91 μg/m3) | 0.30 (−3.18, 3.78) | 0.12 (−3.39, 3.64) | 0.945 | |
| Tertile 3 (11.91–23.60 μg/m3) | 1.68 (−1.80, 5.17) | 1.57 (−1.95, 5.08) | 0.382 | |
| Per 5μg/m3 increase | 1.30 (−1.60, 4.20) | 1.18 (−1.75, 4.11) | 0.429 | |
| Third Trimester | Tertile 1 (3.79–9.57 μg/m3) | Ref. | Ref. | Ref. |
| Tertile 2 (9.57–11.80 μg/m3) | 1.05 (−2.42, 4.52) | 1.15 (−2.34, 4.64) | 0.519 | |
| Tertile 3 (11.80–28.81 μg/m3) | 4.68 (1.21, 8.15) | 4.62 (1.12, 8.12) | 0.010 | |
| Per 5μg/m3 increase | 3.48 (0.74, 6.23) | 3.39 (0.63, 6.15) | 0.016 | |
| Whole Pregnancy | Tertile 1 (6.15–9.94 μg/m3) | Ref. | Ref. | Ref. |
| Tertile 2 (9.94–11.70 μg/m3) | 1.50 (−1.98, 4.97) | 1.13 (−2.37, 4.63) | 0.528 | |
| Tertile 3 (11.70–18.43 μg/m3) | 3.56 (0.09, 7.04) | 3.34 (−0.19, 6.86) | 0.064 | |
| Per 5μg/m3 increase | 3.20 (−0.26, 6.66) | 2.99 (−0.51, 6.49) | 0.094 | |
Model adjusted for maternal age at delivery, self-reported race, marital status, education, smoking history and alcohol intake during pregnancy
Abbreviations: PM2.5 = particulate matter ≤2.5 microns; CI: confidence interval; SBP = systolic blood pressure; Ref = Reference group
Figure 1.
Relationship between child SBP percentile and maternal ambient PM2.5 level in each exposure period estimated by restricted cubic spline regression model
* Model adjusted for maternal age at delivery, self-reported race, marital status, education, smoking history and alcohol intake during pregnancy
† Abbreviations: PM2.5 = particulate matter ≤2.5 microns; SBP = systolic blood pressure
Associations for elevated childhood BP were similar (Table 3). In the third trimester, the relative risk (RR) of elevated BP was 1.60 (95% CI: 1.12, 2.27) times higher for a child whose mother was exposed to the highest PM2.5 tertile compared to the lowest tertile after adjustment. After scaling PM2.5 to continuous level, the relative risk is 1.46 (95% CI: 1.17, 1.83) times higher per 5μg/m3 increase in ambient PM2.5 concentration after adjustment. Our multivariable-adjusted cubic spline model (Please see the Figure S2 on http://hyper.ahajournals.org) showed that the risk for elevated BP significantly increased after exposure to a PM2.5 threshold of 13 μg/m3 in the third trimester. The adjusted RR of elevated BP comparing who have PM2.5 exposure ≥13 μg/m3 to those <13 μg/m3 during the third trimester was 1.80 (95% CI: 1.33, 2.44). Childhood elevated BP was not associated with 5 μg/m3 increase in ambient PM2.5 concentration in the preconception period (adjusted RR=0.87, 95% CI: 0.67, 1.13), first trimester (adjusted RR=1.19, 95% CI: 0.91, 1.56) or second trimester (adjusted RR =1.11, 95% CI: 0.85, 1.46).
Table 3.
Associations of ambient PM2.5 level and risk for elevated SBP in each exposure period (n=1293)
| Pregnancy Exposure Periods | PM2.5 Levels | Child Elevated SBP (defined by ≥90th percentile) | |||
|---|---|---|---|---|---|
|
| |||||
| Cases (%) | Crude RR (95% CI) | Adjusted* RR (95% CI) | p for adj-RR | ||
| Preconception | Tertile 1 (4.48–9.90 μg/m3) | 59 (13.69%) | Ref. | Ref. | Ref. |
| Tertile 2 (9.90–12.08 μg/m3) | 58 (13.46%) | 0.98 (0.70, 1.38) | 0.99 (0.71, 1.39) | 0.971 | |
| Tertile 3 (12.10–29.00 μg/m3) | 51 (11.83%) | 0.86 (0.61, 1.23) | 0.90 (0.63, 1.28) | 0.548 | |
| Per 5μg/m3 increase | N.A. | 0.84 (0.65, 1.09) | 0.87 (0.67, 1.13) | 0.292 | |
| First Trimester | Tertile 1 (4.17–9.72 μg/m3) | 52 (12.06%) | Ref. | Ref. | Ref. |
| Tertile 2 (9.72–11.93 μg/m3) | 43 (10.00%) | 0.83 (0.57, 1.21) | 0.83 (0.57, 1.21) | 0.336 | |
| Tertile 3 (11.93–19.15 μg/m3) | 73 (16.90%) | 1.40 (1.01, 1.95) | 1.38 (0.99, 1.92) | 0.056 | |
| Per 5μg/m3 increase | N.A. | 1.20 (0.91, 1.56) | 1.19 (0.91, 1.56) | 0.201 | |
| Second Trimester | Tertile 1 (5.40–9.77 μg/m3) | 52 (12.12%) | Ref. | Ref. | Ref. |
| Tertile 2 (9.78–11.91 μg/m3) | 54 (12.47%) | 1.03 (0.72, 1.47) | 1.00 (0.70, 1.44) | 0.978 | |
| Tertile 3 (11.91–23.60 μg/m3) | 62 (14.39%) | 1.19 (0.84, 1.67) | 1.20 (0.85, 1.69) | 0.297 | |
| Per 5μg/m3 increase | N.A. | 1.11 (0.85, 1.45) | 1.11 (0.85, 1.46) | 0.431 | |
| Third Trimester | Tertile 1 (3.79–9.57 μg/m3) | 44 (10.21%) | Ref. | Ref. | Ref. |
| Tertile 2 (9.57–11.80 μg/m3) | 54 (12.53%) | 1.23 (0.84, 1.79) | 1.24 (0.85, 1.81) | 0.257 | |
| Tertile 3 (11.80–28.81 μg/m3) | 70 (16.24%) | 1.59 (1.12, 2.26) | 1.60 (1.12, 2.27) | 0.009 | |
| Per 5μg/m3 increase | N.A. | 1.47 (1.17, 1.85) | 1.46 (1.17, 1.83) | 0.001 | |
| Whole Pregnancy | Tertile 1 (6.15–9.94 μg/m3) | 51 (11.83%) | Ref. | Ref. | Ref. |
| Tertile 2 (9.94–11.70 μg/m3) | 50 (11.60%) | 0.98 (0.68, 1.42) | 0.95 (0.66, 1.36) | 0.767 | |
| Tertile 3 (11.70–18.43 μg/m3) | 67 (15.55%) | 1.31 (0.94, 1.84) | 1.32 (0.94, 1.85) | 0.111 | |
| Per 5μg/m3 increase | N.A. | 1.45 (1.03, 2.04) | 1.45 (1.03, 2.05) | 0.034 | |
Model adjusted for maternal age at delivery, self-reported race, marital status, education, smoking history and alcohol intake during pregnancy
Abbreviations: PM2.5 = particulate matter ≤2.5 microns; CI: confidence interval; SBP = systolic blood pressure; RR = relative risk; adj-RR = Relative risk after adjusted; Ref = Reference group
In the mediation analysis, after separately adding birthweight, gestational age and child BMI-z score into the confounder model, the estimated SBP percentile increase changed from 3.39 (95% CI: 0.63, 6.15) to 3.08 (95% CI: 0.33, 5.82), 3.15 (95% CI: 0.40, 5.90) and 2.75 (95% CI: 0.01, 5.50) per 5 μg/m3 higher in ambient PM2.5 concentration, respectively. When both birthweight and BMI-z score were added to the model, they mediated 35% of the association, and the p-value for the association of PM2.5 and childhood SBP was no longer statistically significant (p=0.112); this finding suggests that the effects of PM2.5 on weight at birth and weight during childhood mediate part of the association of PM2.5 with childhood SBP.
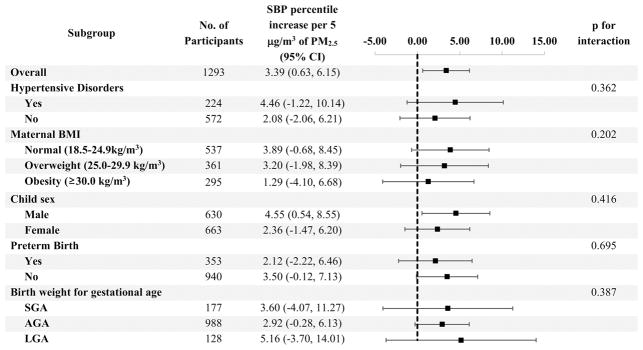
In the subgroup analysis stratified by potential EMMs, all subgroups show a positive increase of SBP percentile with increasing PM2.5 concentration (Figure 2) for the third trimester. The p-values for interaction suggests no EMM.
Figure 2.
Subgroup analysis by potential Effect Measure Modifiers
* Model adjusted for maternal age at delivery, self-reported race, marital status, education, smoking history and alcohol intake during pregnancy
† Abbreviations: PM2.5 = particulate matter ≤2.5 microns; CI: confidence interval; SBP = systolic blood pressure; BMI = body mass index; SGA = small for gestational age; AGA = average for gestational age; LGA = large for gestational age
Table S2 (please see http://hyper.ahajournals.org) shows the comparison of the characteristics between the 1293 pairs included in the analysis versus the 619 excluded from the study due to the missing BP measurement. In our sensitivity analyses, after applying stabilized inverse probability weights in the regression model, the association of PM2.5 with child SBP was further enhanced. For the third trimester, we see a 3.79 percentile increase in child SBP per 5 μg/m3 increase in PM2.5 concentration (95% CI: 0.96–6.62, p=0.009) and a 5.21 percentile increase comparing the highest tertile of PM2.5 exposure to the lowest tertile (95% CI: 1.70–8.72, p=0.004) after adjusting for potential confounders.
DISCUSSION
In this study, we found that maternal exposure to ambient PM2.5 during the third trimester of pregnancy is associated with elevated child BP at 3 to 9 years old, even after adjustment for potential confounders and controlling for postnatal PM2.5 exposure. This association was consistent among children who were born healthy, preterm or with low birth weight. It was also consistent across different races and ethnicities. The association was partly mediated by the effects of PM2.5 on fetal growth and weight in childhood. As such, our study provides new insights on the underlying pathways by which prenatal PM2.5 exposure affects childhood SBP.
Our findings contribute to a very limited literature base on the transgenerational effects of maternal air pollution exposure on childhood BP. Consistent with our findings, in a cohort study of 1,131 mother-infant pairs, van Rossem et al. found that higher PM2.5 exposure in the third trimester, but not in the first or second trimesters, was associated with higher SBP in newborns.17 However, their outcome measure was neonatal BP which may not be a good approximation for effects on childhood BP. Also, their cohort predominantly contained white (68.7%) newborns, with few preterm births (4.2%) or small for gestational age newborns (mean gestational age: 39.7±1.4 weeks), which limits the generalizability of their conclusion. In another study, Breton et al. did not find any association between exposure to PM2.5 in pregnancy and BP in offspring at 11 years of age.18 However, the trimester-specific PM2.5 exposures were assigned retrospectively based on the birth certificate and 12% of the mothers moved during pregnancy, thus bias on exposure ascertainment may exist in this study. To our knowledge, our study is the first to investigate the association between prenatal PM2.5 exposure and childhood BP using prospectively collected data.
The potential mechanisms underlying the observed associations likely include altered fetal and childhood growth, as evidenced by our mediation analyses, along with inflammation, oxidative stress and/or endocrine disruption.9,28–30 Maternal exposure to PM2.5 in pregnancy has been shown to lead to altered trophoblast formation and abnormal vascularization of the placenta and cause defected in utero cardiovascular growth.31 Numerous epidemiology studies including our own have shown that in utero PM2.5 exposure is related to lower birth weight and preterm birth.32,33 Animal studies have further demonstrated that in utero exposure to PM2.5 may increase the risk for altered BP, heart failure and other cardiovascular events in offspring.10–12 More recent studies also suggest that mothers exposed to high-level PM2.5 may give birth to children with shorter telomere length,34 leading to higher cardiovascular risks.35
Our finding that maternal exposure to PM2.5 in the third trimester, but not the first or second trimester, was associated with elevated offspring BP is consistent with the findings by Rossem et al.,17 suggesting that this may be the most etiologically relevant time window for PM2.5 exposure. The third trimester is the time when a fetus gains most weight. According to the ultrasound estimation by World Health Organization (WHO), the median fetal weight is 1,039 grams at the end of the second trimester (27 weeks) and 3,617 grams at the end of the third trimester (40 weeks).36 Thus, one possible explanation is that PM2.5 affects child BP by affecting fetal weight gain and development in the third trimester. Again, this is supported by our finding that birthweight and child BMI mediate part of the association between PM2.5 and offspring SBP. A possible explanation for why third trimester but not postnatal exposure to PM2.5 was associated with offspring BP in our study may be that PM2.5 exposure in the third trimester is more critical to development than postnatal exposure. The lack of association for postnatal PM2.5 may also be due to the more variable postnatal exposures of a child. After a child is born, he or she might spend time in different locations (for example, in the day-care center) separate from his or her mother, and thus have different PM2.5 exposure than his or her mother. Further studies directly assessing PM2.5 in infants might help explain this hypothesis.
PM2.5 concentration in the third trimester (mean: 10.82 μg/m3, IQR: 8.86–12.41μg/m3) in our population was higher than the national average in the U.S. (7.77 μg/m3 in year 2016).37 It is estimated that 12.1 million people in the U.S. live in counties where the ambient PM2.5 concentrations exceed the EPA’s National Ambient Air Quality Standard of an annual mean of 12 μg/m3.38,39 Moreover, 92% of the global population live in places where the PM2.5 is higher than the WHO’s Ambient Air Quality Guidelines of 10 μg/m3 annual mean worldwide.40,41 Using these data sources and findings from our study, we estimated that approximately 2.38 million women in the U.S. and 1.51 billion women worldwide at child-bearing age (15–44 years old) are exposed to a PM2.5 concentration near or above a threshold that portends higher risk for the development of elevated childhood SBP in offspring.
There are several strengths of our study. First, our study is the first prospective study on this topic, allowing us to determine temporality of the association. Second, in addition to assessing the association of PM2.5 during pregnancy, our etiologically relevant time window, we also had data that allowed us to examine the association between preconception PM2.5 exposure and offspring SBP. This association, which was null, can be considered as a “negative control” and preconception exposure would not affect the fetus.42 Third, we had data on postnatal PM2.5 exposure, allowing us to determine that the association of third trimester PM2.5 was independent of postnatal exposure. Finally, the diversity of our cohort is a strength. Our study population consists more of African American (43.4%), low birth weight (26.5%) and preterm birth (27.3%) children, which improves the external validity of the conclusion.
There are also several limitations of our study. First, as an observational study, residual confounding may be present, although we tried to control for the main confounders. Second, there may be measurement error for child BP, specifically imprecision since it was only measured once at each well-child visit. However, such error would most likely be non-differential and attenuate findings. Another concern is that the PM2.5 data obtained from the EPA air quality monitors may not be precise enough and are likely to cause misclassifications of the PM2.5 exposure. However, a recent study by McGuinn et al. found that the monitors are nearly as accurate as any other PM2.5 calculating models for showing the long-term association between PM and cardiovascular disease in the urban setting.43 Moreover, more than 85% of the study participants lived within 10 km of the matched monitors, a distance within which the PM2.5 concentrations are spatially homogenous.15 Finally, although our findings are consistent with prior literature17, and the p-values for the third trimester are far less than 0.05, we still cannot rule out the possibility of false positive results due to multiple testing.
PERSPECTIVES
Maternal exposure to ambient PM2.5 during the third trimester is associated with elevated BP in children aged 3 to 9 years. The observed association between maternal PM2.5 and offspring SBP association appears to be partly mediated by the effects of PM2.5 on fetal and childhood weight gain. If further confirmed, our findings provide new insight into early life origins of high blood pressure and opportunities for early screening and primary prevention of hypertension in childhood and beyond.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
We found that the third trimester during pregnancy may be a critical period during which high maternal exposure to ambient PM2.5 (≥13μg/m3) portends risk of elevated BP during childhood in offspring.
What Is Relevant?
Our study adds to the literature on the in-utero antecedents of cardiovascular disease, suggesting that reducing maternal exposure to ambient PM2.5 may represent an early opportunity for primordial prevention of childhood elevated blood pressure.
Summary
Maternal exposure to ambient PM2.5 during the third trimester of pregnancy is associated with elevated BP in children aged 3 to 9 years.
Acknowledgments
We would like to extend our sincerest thanks to all of the study participants, the Boston University Medical Center Labor and Delivery Nursing Staff, and the Boston Birth Cohort field team for their support and help with the Boston Birth Cohort Study.
SOURCES OF FUNDING
The Boston Birth Cohort (the parent study) was supported in part by the March of Dimes grants (PERI 20-FY02-56, #21-FY07-605), and the National Institutes of Health (NIH) grants (R21ES011666, 2R01HD041702, R21HD066471). The follow-up study is supported in part by the NIH grants (U01AI090727, R21AI079872, R01HD086013); and Maternal and Child Health Bureau (R40MC27443, UJ2MC31074).
Footnotes
CONFLICT(S) OF INTEREST/DISCLOSURE(S)
None.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raised blood pressure. WHO; [Accessed October 21, 2017]. http://www.who.int/gho/ncd/risk_factors/blood_pressure_prevalence_text/en/ [Google Scholar]
- 3.Din-Dzietham R, Liu Y, Bielo MV, Shamsa F. High blood pressure trends in children and adolescents in national surveys, 1963 to 2002. Circulation. 2007;116(13):1488–1496. doi: 10.1161/CIRCULATIONAHA.106.683243. [DOI] [PubMed] [Google Scholar]
- 4.Muntner P, He J, Cutler JA, Wildman RP, Whelton PK. Trends in blood pressure among children and adolescents. JAMA. 2004;291(17):2107–2113. doi: 10.1001/jama.291.17.2107. [DOI] [PubMed] [Google Scholar]
- 5.Worldwide trends in blood pressure from 1975 to 2015: a pooled analysis of 1479 population-based measurement studies with 19·1 million participants. Lancet (London, England) 2017;389(10064):37–55. doi: 10.1016/S0140-6736(16)31919-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet (London, England) 2017;389(10082):1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilenko N, van Rossem L, Brunekreef B, Beelen R, Eeftens M, Hoek G, Houthuijs D, de Jongste JC, van Kempen E, Koppelman GH, Meliefste K, Oldenwening M, Smit HA, Wijga AH, Gehring U. Traffic-related air pollution and noise and children’s blood pressure: results from the PIAMA birth cohort study. European Journal of Preventive Cardiology. 2015;22(1):4–12. doi: 10.1177/2047487313505821. [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, Rajagopalan S, Pope CA, 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Jr, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 10.Tanwar V, Gorr MW, Velten M, Eichenseer CM, Long VP, 3rd, Bonilla IM, Shettigar V, Ziolo MT, Davis JP, Baine SH, Carnes CA, Wold LE. In Utero Particulate Matter Exposure Produces Heart Failure, Electrical Remodeling, and Epigenetic Changes at Adulthood. Journal of the American Heart Association. 2017;6(4) doi: 10.1161/JAHA.117.005796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanwar V, Adelstein JM, Grimmer JA, Youtz DJ, Sugar BP, Wold LE. PM2.5 exposure in utero contributes to neonatal cardiac dysfunction in mice. Environmental Pollution (Barking, Essex : 1987) 2017;230:116–124. doi: 10.1016/j.envpol.2017.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weldy CS, Liu Y, Liggitt HD, Chin MT. In utero exposure to diesel exhaust air pollution promotes adverse intrauterine conditions, resulting in weight gain, altered blood pressure, and increased susceptibility to heart failure in adult mice. PloS one. 2014;9(2):e88582. doi: 10.1371/journal.pone.0088582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X, Ding H, Wang X. Acute effects of total suspended particles and sulfur dioxides on preterm delivery: a community-based cohort study. Archives of Environmental Health. 1995;50(6):407–415. doi: 10.1080/00039896.1995.9935976. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Ding H, Ryan L, Xu X. Association between air pollution and low birth weight: a community-based study. Environ Health Perspect. 1997;105(5):514–520. doi: 10.1289/ehp.97105514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nachman RM, Mao G, Zhang X, Hong X, Chen Z, Soria CS, He H, Wang G, Caruso D, Pearson C, Biswal S, Zuckerman B, Wills-Karp M, Wang X. Intrauterine Inflammation and Maternal Exposure to Ambient PM2.5 during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ Health Perspect. 2016;124(10):1608–1615. doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mao G, Nachman RM, Sun Q, et al. Individual and Joint Effects of Early-Life Ambient Exposure and Maternal Prepregnancy Obesity on Childhood Overweight or Obesity. Environ Health Perspect. 2017;125(6):067005. doi: 10.1289/EHP261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Rossem L, Rifas-Shiman SL, Melly SJ, Kloog I, Luttmann-Gibson H, Zanobetti A, Coull BA, Schwartz JD, Mittleman MA, Oken E, Gillman MW, Koutrakis P, Gold DR. Prenatal air pollution exposure and newborn blood pressure. Environ Health Perspect. 2015;123(4):353–359. doi: 10.1289/ehp.1307419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breton CV, Yao J, Millstein J, Gao L, Siegmund KD, Mack W, Whitfield-Maxwell L, Lurmann F, Hodis H, Avol E, Gilliland FD. Prenatal Air Pollution Exposures, DNA Methyl Transferase Genotypes, and Associations with Newborn LINE1 and Alu Methylation and Childhood Blood Pressure and Carotid Intima-Media Thickness in the Children’s Health Study. Environ Health Perspect. 2016;124(12):1905–1912. doi: 10.1289/EHP181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, Pearson C, Wang MC, Zuckerman B, Cheng TL, Wang X. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311(6):587–596. doi: 10.1001/jama.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl 4th Report):555–576. [PubMed] [Google Scholar]
- 21.Mourad JJ. The evolution of systolic blood pressure as a strong predictor of cardiovascular risk and the effectiveness of fixed-dose ARB/CCB combinations in lowering levels of this preferential target. Vascular Health and Risk Management. 2008;4(6):1315–1325. doi: 10.2147/vhrm.s4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119(2):237–246. doi: 10.1542/peds.2006-2543. [DOI] [PubMed] [Google Scholar]
- 23.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American Journal of Obstetrics and Gynecology. 2000;183(1):S1–s22. [PubMed] [Google Scholar]
- 24.Zou G. A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 25.Durrleman S, Simon R. Flexible regression models with cubic splines. Statistics in Medicine. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 26.Robins JM, Rotnitzky A, Zhao LP. Estimation of Regression Coefficients When Some Regressors are not Always Observed. Journal of the American Statistical Association. 1994;89(427):846–866. [Google Scholar]
- 27.Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? International Journal of Methods in Psychiatric Research. 2011;20(1):40–49. doi: 10.1002/mpr.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vadillo-Ortega F, Osornio-Vargas A, Buxton MA, Sánchez BN, Rojas-Bracho L, Viveros-Alcaráz M, Castillo-Castrejón M, Beltrán-Montoya J, Brown DG, O’Neill MS. Air pollution, inflammation and preterm birth: a potential mechanistic link. Medical Hypotheses. 2014;82(2):219–224. doi: 10.1016/j.mehy.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannan S, Misra DP, Dvonch JT, Krishnakumar A. Exposures to Airborne Particulate Matter and Adverse Perinatal Outcomes: A Biologically Plausible Mechanistic Framework for Exploring Potential Effect Modification by Nutrition. Environ Health Perspect. 2006;114(11):1636–1642. doi: 10.1289/ehp.9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferguson KK, Chin HB. Environmental chemicals and preterm birth: Biological mechanisms and the state of the science. Current Epidemiology Reports. 2017;4(1):56–71. doi: 10.1007/s40471-017-0099-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roberts JM, Taylor RN, Goldfien A. Clinical and biochemical evidence of endothelial cell dysfunction in the pregnancy syndrome preeclampsia. American Journal of Hypertension. 1991;4(8):700–708. doi: 10.1093/ajh/4.8.700. [DOI] [PubMed] [Google Scholar]
- 32.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environmental Research. 2012;117:100–111. doi: 10.1016/j.envres.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 33.Lamichhane DK, Leem JH, Lee JY, Kim HC. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environmental Health and Toxicology. 2015:30. doi: 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martens DS, Cox B, Janssen BG, Clemente DBP, Gasparrini A, Vanpoucke C, Lefebvre W, Roels HA, Plusquin M, Nawrot TS. JAMA Pediatrics. 2017;171(12):1160. doi: 10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ (Clinical research ed) 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiserud T, Piaggio G, Carroli G, et al. The World Health Organization Fetal Growth Charts: A Multinational Longitudinal Study of Ultrasound Biometric Measurements and Estimated Fetal Weight. PLoS medicine. 2017;14(1):e1002220. doi: 10.1371/journal.pmed.1002220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Particulate Matter (PM2.5) Trends. EPA; [Accessed October 26, 2017]. https://www.epa.gov/air-trends/particulate-matter-pm25-trends#pmnat. Published July 18, 2017. [Google Scholar]
- 38.NAAQS Table. EPA; [Accessed October 25, 2017]. https://www.epa.gov/criteria-air-pollutants/naaqs-table. Published December 20, 2016. [Google Scholar]
- 39.Air Quality - National Summary. EPA; [Accessed January 31, 2018]. https://www.epa.gov/air-trends/air-quality-national-summary. Published July 26, 2017. [Google Scholar]
- 40.WHO releases country estimates on air pollution exposure and health impact. World Health Organization; [Accessed January 31, 2018]. http://www.who.int/mediacentre/news/releases/2016/air-pollution-estimates/en/ [Google Scholar]
- 41.Ambient (outdoor) air quality and health. World Health Organization; [Accessed October 26, 2017]. http://www.who.int/mediacentre/factsheets/fs313/en/. Published September 2016. [Google Scholar]
- 42.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology (Cambridge, Mass) 2010;21(3):383–388. doi: 10.1097/EDE.0b013e3181d61eeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McGuinn LA, Ward-Caviness C, Neas LM, Schneider A, Di Q, Chudnovsky A, Schwartz J, Koutrakis P, Russell AG, Garcia V, Kraus WE, Hauser ER, Cascio W, Diaz-Sanchez D, Devlin RB. Fine particulate matter and cardiovascular disease: Comparison of assessment methods for long-term exposure. Environmental Research. 2017;159:16–23. doi: 10.1016/j.envres.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.