Abstract
Multiple studies have confirmed a seemingly paradoxical finding that family caregivers have lower mortality rates than comparable samples of noncaregivers. Caregivers are often also found to report more symptoms of depression and higher stress levels, but psychological distress and mortality are rarely examined in the same study. This study tests a possible mechanism for the mortality effect by applying a theoretical model that posits psychological and physiological stress-buffering benefits from prosocial helping behaviors. Participants in the population-based REasons for Geographic and Racial Differences in Stroke (REGARDS) study included 3,580 family caregivers who were individually matched to 3,580 noncaregivers on 15 demographic, health history, and health behavior variables using a propensity score matching algorithm. Baseline measures of depressive symptoms and perceived stress levels were also collected. The results indicated that caregivers reported significantly more depressive symptoms and higher perceived stress levels than propensity-matched noncaregivers (ps < .0001). However, consistent with our previous analysis (Roth et al., 2013), an analysis of 7-year survival rates showed that caregivers had a 16.5% lower mortality rate than noncaregivers (hazard ratio = 0.835, 95% CI = 0.719, 0.970). Significant caregiving*psychological distress interaction effects supported the stress-buffering hypothesis. Both depressive symptoms and perceived stress scores were significant predictors of mortality for the matched noncaregivers (ps < .0001), but not for the caregivers (ps > .49). Family caregiving appears to be similar to other prosocial helping behaviors in that it provides stress-buffering adaptations that ameliorate the impact of stress on major health outcomes such as mortality.
Keywords: Caregiving, prosocial behavior, mortality, stress-buffering
A long history of research has shown that informal caregivers for family members with chronic illnesses and disabilities often report higher levels of psychological stress and depressive symptoms when compared to those who are not caregivers (National Academies of Sciences, Engineering, and Medicine, 2016; Pinquart & Sörensen, 2003; Schulz & Sherwood, 2008). Depression and other indicators of psychological distress are often linked to poorer physical health in the general population, including increased risks for mortality (Cuijpers et al., 2014; Prior et al., 2016). Consequently, a logical extension of these findings is that the increased stress associated with caregiving could extend into increased risks for physical illness that include elevated mortality rates among caregivers. In fact, one landmark study reported just such a finding – that spouse caregivers who reported some caregiving strain had 4-year mortality rates that were 63% higher than noncaregiving spouses (Schulz & Beach, 1999). This paper is widely cited, not only in the research literature, but also by advocacy groups and public policy documents that argue for more support services for the vulnerable caregiving population (Roth, Fredman, & Haley, 2015).
In spite of this seemingly coherent narrative that many caregivers are stressed and that this chronic stress places them at a heightened risk for increased mortality, at least seven subsequent population-based studies with larger and more diverse samples of caregivers than the Schulz and Beach (1999) investigation have found contradictory evidence, namely, that mortality rates are lower for caregivers than for noncaregiving control samples (Brown et al., 2009; Fredman et al., 2010; Fredman, Lyons, Cauley, Hochberg, & Applebaum, 2015; O’Reilly, Connolly, Rosato, & Patterson, 2008; O’Reilly, Rosato, & Maguire, 2015; Ramsay, Grundy, & O’Reilly, 2013; Roth et al., 2013). While these findings of lower mortality rates for caregivers appear to be replicable and robust, little research has examined the apparent contradictions implied – that a population at risk for depression and high stress mighty actually enjoy some physical health benefits and enhanced longevity – or examined potential mechanisms that might explain this reduced mortality finding among caregivers.
An important perspective on the possible physical health benefits of family caregiving is available from theory and research on the physiological and psychological effects of prosocial helping behaviors, such as volunteering. A growing body of research demonstrates that providing emotional and practical forms of help to others improves well-being and predicts decreased morbidity and mortality for the helper (Avlund, Damsgaard, & Holstein, 1998; Brown, Conseding, & Magai, 2005; Brown, Nesse, Vinokur, & Smith, 2003; Okun, Yeung, & Brown, 2013; Schwartz, Meisenhelder, Ma, & Reed, 2003). These findings have been incorporated into a new model of caregiving and prosocial behavior (Brown & Brown, 2015). In this model, certain physiological responses to prosocial helping behaviors appear to have evolved in humans and other social mammals and occur through the activation of neural circuits that were shaped by evolution to induce parental care (Numan, 2006). These neural circuits involve interactions between the medial pre-optic area of the hypothalamus, medial orbital frontal cortex, anterior cingulate, amygdala, nucleus accumbens, ventral pallidum, and the periaqueductal grey (Brown & Brown, 2015). The neurohormonal features of this circuit are hypothesized to promote immune system homeostasis through the regulation of sympathetic stress and the release of oxytocin and progesterone.
Two previous studies have tested the stress-buffering hypotheses developed under this new model. In a re-analysis of mortality data from Brown, Nesse, Vinokur and Smith (2003), which originally showed reduced mortality risk associated with helping behavior, Poulin and colleagues (2013) demonstrated that exposure to stressful life events moderated the association between providing instrumental help to others and mortality risk. Specifically, exposure to stressful life events increased mortality risk among non-helpers but had no influence on mortality risk for those who reported helping another person at least once in the prior year. In a separate study, Brown, House, Brown, & Smith (2008) showed a similar moderating effect of stress on the link between helping behavior and recovery from depressive symptoms that follow spousal bereavement. In that study, among the bereaved spouses who experienced high levels of grief (stress), a recent history of helping behavior was associated with faster recovery from bereavement-related depression. Taken together, these two studies suggest that helping-related benefits are more pronounced among individuals who have been exposed to stress and interrupt the association between stress and negative outcomes. Or, put differently, exposure to stress predicts worsened health and well-being among non-helpers, but has a markedly reduced influence on these same outcomes for helpers (Brown, Brown, House, & Smith, 2008; Poulin, Brown, Dillard, & Smith, 2013).
While these findings are interesting, they are limited in that they were based on a single report of prior helping behavior that could have been directed to anyone. The model has also been cited as a possible explanation for the longevity benefits of grandparents who provide non-custodial care to their grandchildren (Hilbrand, Coall, Gerstorf, & Hertwig, 2017), but it has not yet otherwise been employed to examine the health benefits of other helping behaviors within the context of family relationships. Family caregiving, defined as providing help on an ongoing basis to a family member with a chronic illness or disability, is a type of prosocial helping behavior that is relevant to this new model. Because family caregivers often report being burdened, stressed, or strained by their caregiving responsibilities, the informal caregiving context can provide one of the strongest and more interesting tests of the model. The model would predict, paradoxically, that caregivers may be specifically protected from the physical health consequences of the stressors and strains they endure in the caregiving relationship, whereas noncaregivers would not show this increased physical health resilience to other sources of stress and depressive symptoms.
The present analysis extended previous analyses of data from caregivers and statistically-matched noncaregivers from a large national epidemiologic study in order to specifically address the stress-buffering hypothesis of caregiving derived from the Brown and Brown (2015) theoretical model. Caregivers were identified from the REasons for Geographic and Racial Differences in Stroke (REGARDS) project as participants who reported providing ongoing assistance to a family member with a chronic illness or disability. This type of caregiving assistance could be due to any condition and was not limited to stroke. In a previously published analysis, we used a propensity-score matching procedure to identify noncaregiving control participants from the same study and found that the caregivers enrolled in REGARDS had an 18% survival advantage compared to the matched noncaregivers (Roth et al., 2013). The propensity-score matching algorithm matched the caregiving and noncaregiving groups on age, gender, race, education, other demographic factors, health history questions, and health behaviors. The present analyses extended on the previous Roth et al. (2013) analyses in several important ways. First, we examined whether caregivers in REGARDS reported more depressive symptoms and higher perceived stress levels than propensity-matched noncaregivers. We hypothesized that these measures of psychological distress would be significantly higher among the caregivers. If this hypothesis is supported, it might then present a paradox in relation to our previous mortality findings (Roth et al. 2013), given that earlier studies have found that higher levels of both depressive symptoms (Cuijpers et al., 2014) and perceived stress (Prior et al., 2016) are associated with increased mortality. Second, we tested the stress-buffering hypothesis of caregiving by examining whether the effects of psychological distress (depressive symptoms, perceived stress levels) on subsequent mortality differed significantly as a function of caregiving status. We used the same propensity-matching variables and procedures as in our earlier analysis (Roth et al., 2013) but extended the follow-up time and examined survival over a subsequent 7-year post-enrollment time period for all participants. The stress-buffering hypothesis was explicitly tested by adding caregiving status by psychological distress interaction effects to the survival analysis models. We predicted, based on previous tests of the prosocial helping behaviors model, that the impact of depressive symptoms and perceived stress on mortality would be significantly smaller for caregivers than for noncaregivers.
Methods
REGARDS Study Design and Participants
Potential participants for the REGARDS study were randomly sampled from a commercially-available nationwide list. Exclusion criteria included age less than 45, race other than African American or White, previous diagnosis of cancer requiring chemotherapy, or residence in or on a waiting list for a nursing home. African Americans and residents from the southern “stroke belt” region of the United States were oversampled by design. The stroke belt consists of the states of Alabama, Arkansas, Georgia, Louisiana, Mississippi, North Carolina, South Carolina, and Tennessee. In addition, within the stroke belt region, further oversampling was done in the “stroke buckle” area, which consists of the coastal plains region of North Carolina, South Carolina, and Georgia (Howard et al., 1997). Enrollment began in 2003 and ended in 2007. Additional information on the design, sampling, and enrollment procedures for the REGARDS study have been described in detail elsewhere (Howard et al., 2005; Roth, Perkins, Wadley, Temple, & Haley, 2009; Roth et al., 2013). All procedures were reviewed and approved by the Institutional Review Board (IRB) of the University of Alabama at Birmingham and by the IRBs of each participating institution.
Trained interviewers contacted potential participants by telephone, established eligibility, and obtained verbal informed consent to participate in the project. A computer-assisted telephone interview (CATI) was then administered that collected extensive information on demographic variables and risk factors for stroke and cardiovascular disease. After the initial CATI, participants completed an in-home assessment during which directly observed health data were obtained and biologic specimens (e.g., blood, urine) were collected. A total of 30,239 participants completed both the baseline CATI and the in-home assessment, and semi-annual follow-up interviews were then conducted on these participants to ascertain incident events such as strokes, myocardial infarctions, and other outcomes. These semi-annual follow-up interviews continue at the present time for over 16,000 participants who are still actively participating in the REGARDS study.
Measures
The following measures were obtained from the baseline CATI:
Demographic Variables
Age was determined by the difference between the participant’s date of birth and the baseline CATI date. Gender and Race (African American, White) were dichotomous, self-report variables. Region was coded based on the stratified sampling categories that were used in the REGARDS sampling design (Stroke belt, Stroke buckle, Nonbelt). Participants were asked about the highest level of education they completed, a series of dichotomous questions about whether their annual household income was above or below certain reference values, and to report their current marital status. Responses were coded into categories as indicated in Table 1. Participants were asked if they had “any kind of healthcare coverage such as health insurance, an HMO, or a government plan like Medicare or Medicaid.” Those who answered “yes” were coded as having medical insurance coverage.
Table 1.
Comparisons of Caregivers and Noncaregivers Before and After Propensity-Score Matching
| Covariate/Matching Factor | Caregivers (N = 3,580) | All Non-Caregivers (N=25,471) | p | Propensity-Matched Non-Caregivers (N = 3,580) | p |
|---|---|---|---|---|---|
|
| |||||
| Age (M ± SD) | 63.62 ± 8.97 | 65.55 ± 9.44 | <.0001 | 63.57 ± 9.05 | .8359 |
|
| |||||
| Gender (% female) | 63.02 | 54.36 | <.0001 | 61.34 | .1437 |
|
| |||||
| Race (% African American) | 43.32 | 40.47 | .0017 | 43.72 | .7386 |
|
| |||||
| Region: | <.0001 | .6704 | |||
| (% Stroke Belt) | 37.91 | 34.30 | 38.52 | ||
| (% Stroke Buckle) | 21.68 | 20.93 | 22.09 | ||
| (% Rest of US) | 40.42 | 44.78 | 39.39 | ||
|
| |||||
| Education: | .0005 | .8311 | |||
| (% less than high school) | 10.78 | 12.38 | 10.34 | ||
| (% high school graduate) | 24.30 | 26.11 | 24.16 | ||
| (% some college) | 28.77 | 26.57 | 28.41 | ||
| (% college graduate) | 36.15 | 34.93 | 37.09 | ||
|
| |||||
| Income: | .0373 | .4884 | |||
| (% less than $20,000) | 16.62 | 17.88 | 15.11 | ||
| (% $20,00 to $34,000) | 25.22 | 23.96 | 25.39 | ||
| (% 35,000 to $74,000) | 31.28 | 29.73 | 32.37 | ||
| (% $75,000 or more) | 15.14 | 16.16 | 15.06 | ||
| (% refused to specify) | 11.73 | 12.27 | 12.07 | ||
|
| |||||
| Marital Status: | <.0001 | .6492 | |||
| (% married) | 66.82 | 58.07 | 66.82 | ||
| (% divorced) | 12.93 | 14.70 | 12.09 | ||
| (% single, never married) | 5.50 | 5.25 | 5.78 | ||
| (% widowed) | 12.40 | 19.64 | 11.68 | ||
| (% separated/refused) | 2.35 | 2.34 | 2.35 | ||
|
| |||||
| Medical Insurance (% yes) | 91.06 | 93.79 | <.0001 | 91.62 | .4007 |
|
| |||||
| Smoking: | <.0001 | .8211 | |||
| (% current) | 15.84 | 14.05 | 15.11 | ||
| (% former) | 35.84 | 40.63 | 36.31 | ||
| (% never) | 48.04 | 44.91 | 48.24 | ||
|
| |||||
| Current Alcohol Use: | .0249 | .5168 | |||
| (% heavy) | 3.41 | 4.05 | 3.91 | ||
| (% moderate) | 31.34 | 33.11 | 32.18 | ||
| (% none) | 63.38 | 60.93 | 61.96 | ||
|
| |||||
| 6-item Cognitive Screener | <.0001 | .7025 | |||
| (% 0 to 4 correct) | 6.79 | 9.47 | 6.25 | ||
| (% 5 correct) | 21.28 | 21.71 | 22.01 | ||
| (% 6 correct) | 71.93 | 68.82 | 71.65 | ||
|
| |||||
| Self-Rated Health: | .2937 | .6551 | |||
| (% excellent) | 15.20 | 16.24 | 15.17 | ||
| (% very good) | 30.22 | 30.76 | 31.45 | ||
| (% good) | 36.01 | 34.84 | 34.72 | ||
| (% fair) | 15.36 | 14.73 | 15.75 | ||
| (% poor) | 3.21 | 3.43 | 2.91 | ||
|
| |||||
| Hypertension (% yes) | 57.40 | 58.04 | .4674 | 57.21 | .8672 |
|
| |||||
| Diabetes (% yes) | 21.40 | 22.49 | .1407 | 21.54 | .8856 |
|
| |||||
| Cardiovascular Disease (% yes) | 18.69 | 23.16 | <.0001 | 19.72 | .2670 |
Health Behaviors
Participants were asked a series of questions about current and former smoking habits. Responses were used to classify participants into one of three categories: current smokers, former smokers, and those who never smoked. A series of questions were also asked about current and former alcohol use. Gender-specific guidelines from the National Institute on Alcohol Abuse and Alcoholism (2007) were applied to the question on current alcohol use within the past week (none, moderate use, heavy use).
Cognitive Function
The six-item screener of global cognitive status (Callahan, Unverzagt, Hui, Perkins, & Hendrie, 2002) was administered during telephone interviews that began in December of 2003. This measure was obtained from the baseline interview for 24,448 participants and from the first available semi-annual follow-up interview for the remaining 4,603 who were enrolled before this assessment was added to the baseline interview protocol. The number of correct responses (0 to 6) was included as a categorical predictor in the propensity-matching procedure.
Health and Disease History
Self-rated health was obtained by asking participants if their health, “in general,” was “excellent, very good, good, fair, or poor.” Participants were also asked several health history questions. A history of hypertension was recorded for participants who reported being told by a doctor or health professional that they had high blood pressure or hypertension, or who were taking medications for high blood pressure. A history of diabetes was recorded for participants who reported being told by a doctor or health professional that they had diabetes or “high blood sugar” or were taking medications specifically for diabetes. A history of cardiovascular disease was coded for any participants who reported a history of myocardial infarction, stroke, transient ischemic events, carotid endarterectomy, coronary intervention, repair of aortic aneurism, or peripheral arterial intervention. These same methods for recording a self-reported history of cardiovascular disease have been used in previous analyses (McKnight et al., 2011; Roth et al., 2013).
Depressive Symptoms
The 4-item short form of the Center for Epidemiologic Studies Depression (CES-D) scale was used to assess depressive symptoms (Melchior, Huba, Brown, & Reback, 1993). Participants were asked how many days in the past week they felt depressed, lonely, sad, or had crying spells. Each item was rated on 0 (less than 1 day in the past week) to 3 (5–7 days) scale. Scores ranged from 0 to 12, with higher scores indicating more depressive symptoms. Cronbach’s alpha measure of internal consistency was 0.82 for the analytic sample used in the present analyses.
Perceived Stress
Participants’ perceived stress scores were obtained using a 4-item short form of the Cohen’s Perceived Stress Scale (PSS; Cohen, Kamarck, & Mermelstein, 1983). The PSS is a widely-used measure of the degree to which participants appraise the events and circumstances of their lives as being stressful, uncontrollable, or beyond their resources to manage them sufficiently. Participants were specifically asked how often over the past month they felt unable to control important things in their lives, could not cope with all the things they had to do, felt difficulties were piling up so high that they could not overcome them, and were confident in their ability to handle personal problems. All questions were responded to on a 5-point scale (never, almost never, sometimes, fairly often, very often), and the last item was reversed scored so that higher overall scores indicated higher levels of perceived stress. The 4-item version has been commonly used for stress screening and telephone interviewing purposes. Previous analyses have supported the validity of this 4-item instrument (Karam et al., 2012; Warttig et al., 2013). Cronbach’s alpha was 0.68 for this measure in the present sample.
Caregiving Status
Toward the end of the baseline CATI, each participant was asked “are you currently providing care on an on-going basis to a family member with a chronic illness or disability? This would include any kind of help such as watching your family member, dressing or bathing this person, arranging care, or providing transportation.” Participants who answered affirmatively were categorized as “caregivers,” and were subsequently asked whether they lived with this person, their relationship with the care recipient, the number of hours of care per week they provided, and the amount of perceived strain associated with that care (none, some, a lot). No further caregiving information was obtained, including information on the disease condition(s) or functional impairments of the care recipients. While the parent REGARDS study focuses on stroke, the caregivers identified from the baseline CATI could have been providing care for any kind of illness or disability.
All-cause mortality
Preliminary dates of death were typically obtained from proxy reports when participants could not be reached for their scheduled semi-annual follow-up interviews. More specific information about the death including a death certificate was then requested from the participants’ family, and dates of death were further verified using the death certificates or the National Death Index. All death information, including date of death and specific cause of death, were adjudicated by two independent and trained clinicians.
Statistical Analysis
As in our previous analyses (Roth et al., 2013), a standard binary logistic regression analysis was used to predict caregiving status (Yes or No) with 15 other variables measured during the telephone interviews. These 15 variables were age at enrollment, sex, race, region of residence (stroke belt, stroke buckle, non-belt), education, income, marital status, health insurance coverage, smoking, current alcohol use, cognitive performance, self-rated health, and any self-reported history of hypertension, diabetes, or cardiovascular disease. A propensity score was obtained from this analysis that represented each participant’s predicted probability of being a caregiver based on that person’s covariate information. Each actual caregiver was then individually matched with a noncaregiver on this propensity score using a modified greedy matching algorithm without replacement (Parsons, 2001). In cases of tied propensity score differences, the matching noncaregiver was selected randomly from the pool of tied cases.
Propensity score matching provides a common method to balance non-randomized groups in an observational dataset on a number of potentially confounding factors (D’Agostino, 1998; Stuart, 2010). Descriptive comparisons between the caregivers and the propensity-matched noncaregivers were conducted to confirm balance between the two groups on these 15 demographic, health history, and health behavior variables. To facilitate comparisons with our earlier analyses (Roth et al., 2013), these are the exact same propensity matching variables and procedures that were used previously.
Independent samples t-tests were then used to compare the caregivers and propensity-matched noncaregivers on measures of depressive symptoms and perceived stress at baseline interview. These tests addressed the first goal of these extended analyses, which were to determine if caregivers had higher levels of psychological distress than noncaregivers after balancing the two groups on the 15 demographic, health behavior, and health history covariates used in the propensity-score matching.
Effects on 7-year mortality were then examined using a series of Cox proportional hazards survival analyses. These analyses were based on the number of days elapsed between the date of the baseline CATI and the date of death for the participants who died within 7 years of enrollment, or to the date exactly 7 years after enrollment for the cases who survived for that period of time. In survival analysis models that included depressive symptoms and perceived stress scores as predictors, these variables were standardized to Z scores (M = 0, SD = 1) based on the 7,160 participants who were included in those analyses.
The first survival analysis model compared the mortality rates of the caregivers and propensity-matched noncaregivers with no additional covariates or predictors. This analysis is conceptually similar to our previous findings (Roth et al., 2013), but includes a longer follow-up interval and more deaths. Subsequent survival analysis models tested the stress-buffering hypotheses unique to the present paper. The standardized depressive symptoms score was included as an additional predictor of subsequent mortality (along with caregiving status), and then a caregiving status*depressive symptoms interaction term was added to the model to test whether depressive symptoms had a differential predictive effect on subsequent mortality for caregivers than for noncaregivers. Similar models were run for perceived stress, with its standardized score and the caregiving status*perceived stress interaction terms added in separate models. In both the depressive symptoms and perceived stress analytic blocks, the interaction effects tested statistically whether self-reported levels of psychological distress were differentially predictive of subsequent mortality for caregivers compared to the propensity-matched noncaregivers. In analyses where those interaction effects were statistically significant (p < .05), subsequent stratified analyses were performed for caregivers and noncaregivers separately to estimate the group-specific mortality effects.
Results
Descriptive Comparisons and Propensity-Matching
Of the 30,239 participants enrolled in REGARDS, 1,188 (3.9%) had missing data on mortality status or on one of the 15 propensity-matching variables, leaving 29,051 participants with complete data for the present analyses. Table 1 summarizes the descriptive comparisons between the 3,580 caregivers, the 25,471 noncaregivers, and the 3,580 propensity-matched noncaregivers in REGARDS.
Among the 3,580 caregivers, additional descriptive analyses indicated that 845 (23.6%) were providing care to a spouse, 1,216 (34.0%) were providing care for a parent, and 1,519 (42.4%) were providing care to some other family member. A total of 1,891 caregivers (52.8%) were co-residing with their care recipients. Hours of caregiving per week was positively skewed with the lower quartile being 5 hours, a median of 14 hours, and an upper quartile of 40 hours per week. No caregiving strain was reported by 1,190 caregivers (33.2%), some strain was reported by 1,785 caregivers (49.9%), and high strain was reported by 591 (16.5%).
As we also demonstrated in our previous analyses (Roth et al., 2013), the 3,580 caregivers differed from all of the noncaregivers on most of the variables listed in Table 1 before propensity-score matching, with caregivers being slightly but significantly younger and more likely to be women, African American, and married. Caregivers were less likely to have health insurance and less likely to report a history of cardiovascular disease. After propensity-score matching, the 3,580 caregivers did not differ significantly from their 3,580 matched noncaregivers on any of the 15 balancing variables, confirming the success of the binary logistic regression model and greedy matching algorithm for identifying balanced groups of caregivers and matched noncaregivers for further analysis.
Effects of Caregiving on Measures of Distress
The independent samples t-tests revealed that caregivers reported significantly more depressive symptoms (M = 1.376, SD = 2.304) than propensity-matched noncaregivers (M = 1.025, SD = 1.923; t(df=7158) = 6.994, p < .0001) and significantly higher perceived stress levels (M = 3.639, SD = 3.093) than propensity-matched noncaregivers (M = 3.217, SD = 2.889; t(df=7158) = 5.970, p < .0001). These group differences, although highly significant statistically, were rather small in magnitude, representing 0.16 and 0.14 standard deviation units for depressive symptoms and perceived stress, respectively.
Effects of Caregiving on Mortality
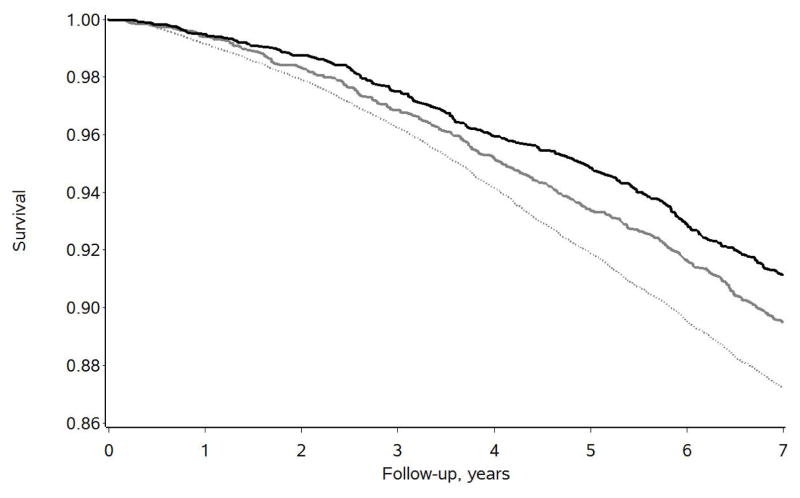
Figure 1 displays the descriptive 7-year survival curves for the 3,580 caregivers, for all 25,471 noncaregivers, and for the 3,580 propensity-matched noncaregivers. Of the 3,580 caregivers, 317 (8.9%) died over the 7-year follow-up period, whereas 3,257 of the 25,471 noncaregivers (12.8%) died over this same time period. After propensity-matching, 376 of the 3,580 matched noncaregivers (10.5%) died over the 7-year follow-up period.
Figure 1.
Seven-year survival curves for the caregivers (black line, n = 3,580), all noncaregivers (dotted gray line, n = 25,471), and propensity-matched noncaregivers (solid gray line, n = 3,580).
A preliminary survival analysis model indicated that the proportional hazards assumption was not violated (X2 (df=1) = 0.293, p = .589). The Cox proportional hazards model then indicated that the caregivers died at a 16.5% slower rate over the 7 year follow-up period than their individually-matched noncaregivers (hazard ratio (HR) = 0.835, p = .0180, 95% confidence interval (CI) = 0.719, 0.970).
Stress-Buffering Effects
When the measure of depressive symptoms was added to the model, it was found to significantly predict 7-year mortality across both caregivers and propensity-matched noncaregivers combined (adjusted HR = 1.116, p = .0012, 95% CI = 1.044, 1.193). A similar effect was found for perceived stress (adjusted HR = 1.119, p = .0023, 95% CI = 1.041, 1.202). For both measures, a one SD increase in psychological distress was associated with a 12% increase in mortality. When interaction effects with caregiving status were then added to these analytic models, statistically significant interaction effects were found between caregiving status and both depressive symptoms (X2 (df=1) = 5.258, p = .0231) and perceived stress (X2 (df=1) = 5.660, p = .0174).
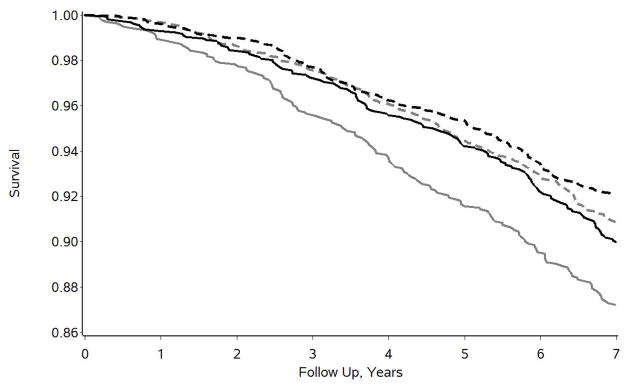
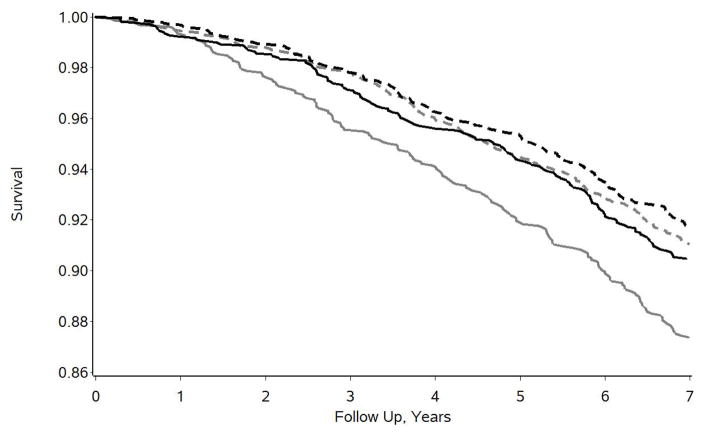
Subsequent group stratified analyses indicated that neither depressive symptoms (HR = 1.034, p = .4974, 95% CI = 0.938, 1.140) nor perceived stress (HR = 1.021, p = .6936, 95% CI = 0.919, 1.135) were statistically significant predictors of 7-year mortality for caregivers. However, both measures of psychological distress were highly significant predictors of increased 7-year mortality for propensity-matched noncaregivers (depressive symptoms: HR = 1.207, p < .0001, 95% CI = 1.102, 1,322; perceived stress: HR = 1.217, p < .0001, 95% CI = 1.103, 1.343). The nature of the caregiving status*psychological distress interaction effects is further illustrated in Figures 2 and 3 for depressive symptoms and perceived stress, respectively. For both measures, medians were determined and the sample was divided into high distress groups (above the median) and low distress groups (at or below the median). This was done for illustrative display purposes only; all statistical analyses were based on the standardized quantitative scores as described above. For the 4-item CES-D, the sample was divided into those who reported 1 or more symptoms (N = 2,887) and those who reported no symptoms (N = 4,273). For the PSS, the sample was divided into those who reported scores of 4 or more (N = 3,185) or 3 or less (N = 3,975). In Figure 2, the 1,329 matched noncaregivers who reported any depressive symptoms had the lowest survival (or highest mortality) over time. The other three groups – caregivers who reported some symptoms of depression (N = 1,558), and both caregivers (N = 2,022) and noncaregivers (N = 2,251) who reported no symptoms of depression – had similar and better survival curves over time. Very similar findings were observed for perceived stress as illustrated in Figure 3. That is, caregivers with high perceived stress scores did not show elevated mortality over time, but had survival curves that closely tracked along with both groups of low perceived stress participants. Noncaregivers with high perceived stress scores of 4 or more (N = 1,505) showed the highest rate of mortality over time.
Figure 2.
Seven year survival curves for caregivers reporting some depressive symptoms (solid black line, n = 1,558), caregivers reporting no depressive symptoms (dashed black line, n = 2,022), propensity-matched noncaregivers reporting some depressive symptoms (solid gray line, n = 1,329), and propensity-matched noncaregivers reporting no depressive symptoms (dashed gray line, n = 2,251).
Figure 3.
Seven year survival curves for high perceived stress caregivers (solid black line, n = 1,680), low perceived stress caregivers (dashed black line, n = 1,900), high perceived stress propensity-matched noncaregivers (solid gray line, n = 1,505), and low perceived stress propensity-matched noncaregivers (dashed gray line, n = 2,075).
Supplemental Analyses of Caregiver Subgroups
In addition to overall caregiving effects, supplemental analyses were conducted for 15 different caregiver subgroups. The general purpose of the subgroup analyses was to explore whether the overall caregiving effects (including caregiving*distress interaction effects) were generally consistent across subgroups or varied substantially. The results of the subgroup analyses are summarized in Table 2.
Table 2.
Mortality and Interaction Effects for Caregiving Subgroups
| Caregiving Group | N | HR | 95% CI | CES-D interaction | 95% CI | PSS interaction | 95% CI |
|---|---|---|---|---|---|---|---|
| All | 3580 | 0.835 | (0.719, 0.970) | 0.857 | (0.750, 0.979) | 0.839 | (0.726, 0.970) |
| Women | 2256 | 0.924 | (0.738, 1.158) | 0.777 | (0.653, 0.925) | 0.720 | (0.584, 0.888) |
| Men | 1324 | 0.925 | (0.751, 1,138) | 0.964 | (0.780, 1.192) | 1.079 | (0.874, 1.332) |
| African Americans | 1551 | 0.942 | (0.754, 1,177) | 0.834 | (0.696, 1.001) | 0.918 | (0.747, 1.128) |
| Whites | 2029 | 0.918 | (0.744, 1.133) | 0.918 | (0.752, 1.121) | 0.845 | (0.680, 1.050) |
| Spouse Caregivers | 845 | 0.957 | (0.733, 1.250) | 0.870 | (0.681, 1.111) | 0.778 | (0.600, 1.011) |
| Adult Child Caregivers | 1216 | 0.659 | (0.464, 0.937) | 1.222 | (0.923, 1.617) | 0.963 | (0.688, 1.348) |
| No Strain | 1190 | 0.836 | (0.655, 1.065) | 0.987 | (0.774, 1.259) | 1.103 | (0.868, 1.1402) |
| Some Strain | 1785 | 0.889 | (0.706, 1.119) | 0.801 | (0.633, 1.014) | 0.787 | (0.620, 0.998) |
| High Strain | 591 | 0.729 | (0.518, 1.027) | 0.874 | (0.684, 1.116) | 0.712 | (0.525, 0.966) |
| ≥ 14 hours of care per week | 1627 | 0.783 | (0.629, 0.975) | 0.905 | (0.757, 1.083) | 0.915 | (0.745, 1.23) |
| < 14 hours of care per week | 1953 | 0.874 | (0.712, 1.072) | 0.830 | (0.684, 1.007) | 0.813 | (0.663, 0.996) |
| Age < 65 | 2110 | 0.868 | (0.665, 1.133) | 0.849 | (0.691, 1.043) | 0.891 | (0.700, 1.136) |
| Age ≥ 65 | 1470 | 0.823 | (0.687, 0.986) | 0.943 | (0.776, 1.145) | 0.905 | (0.752, 1.089) |
| Co-Residing Caregivers | 1891 | 0.762 | (0.633, 0.916) | 0.845 | (0.720, 0.991) | 0.881 | (0.736, 1.053) |
| Not Co-Residing | 1689 | 0.859 | (0.671, 1.100) | 1.005 | (0.777, 1.301) | 1.013 | (0.790, 1.300) |
Notes. HR = Hazard ratio. CI = Confidence interval. Quantities under interaction columns represent the quotient of the hazard ratio for caregivers divided by the hazard ratio for non-caregivers from the stratified analyses predicting mortality from each measure of psychological distress.
For each subgroup, the binary logistic regression and propensity-matching procedure was repeated to individually match each caregiver in that subgroup with a noncaregiver with a similar propensity score. Next, proportional hazards survival analyses were completed in the same sequence of steps used for the overall caregiving effects. Because each subgroup included smaller numbers of caregivers and matched noncaregivers than the overall analysis, power to detect survival effects and interaction effects was reduced. In some of the subgroup analyses, only 14 balancing covariates were used in the logistic regression propensity score calculation because both the caregivers and matched noncaregivers were restricted to just one class on the remaining demographic variable. The analysis of spouse caregivers, for example, was restricted to only married participants, and marital status was, therefore, not included as a predictor variable in the logistic regression analysis that calculated the propensity score.
In Table 2, the hazard ratios from the survival analysis models that compared mortality rates between caregiver subgroups and their own propensity-matched noncaregivers with no additional covariates or predictors are reported. Those analyses indicate that the point estimate of the hazard ratio was less than 1.0 for all 15 caregiver subgroups, and the 95% confidence intervals indicated that significantly reduced mortality for adult child caregivers, caregivers who provided 14 or more hours of care per week, caregivers 65 or more years of age, and caregivers who lived with their care recipients in comparison to each set of individually-matched non-caregivers.
The interaction tests in Table 2 represent a ratio of hazard ratios from the stratified analyses. For all caregivers for the depressive symptoms (CES-D) interaction effect, for example, 0.857 = 1.034/1.207. Consequently, quotients less than 1.0 indicate protective or buffering effects in which the predictive effect of that psychological distress measure on subsequent mortality was smaller for caregivers than for propensity-matched noncaregivers. Those analyses indicate general similarity of the interaction findings across the subgroups examined, with 25 of the 30 (83%) possible interaction effects examined showing a hazard ratio quotient of less than 1.0. Confidence intervals are also provided to allow the reader to examine statistical significance, although power is limited by the smaller sample sizes of the subgroups and by, in some cases, lower event rates (e.g., for younger caregivers).
Discussion
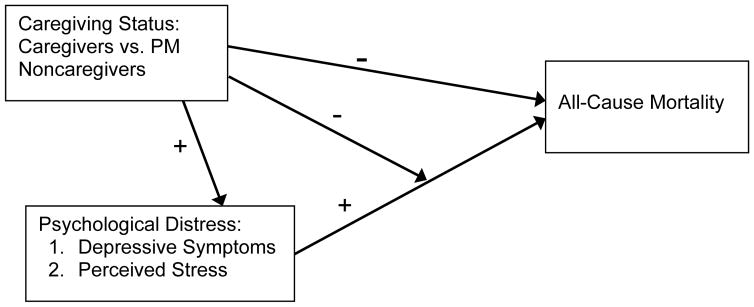
The present findings extend our previous analyses on the longevity benefits of family caregiving and are consistent with the growing body of literature demonstrating greater longevity in caregivers compared to suitable samples of noncaregivers. Our findings also add important new information on a possible mechanism that may be partly responsible for that effect. An overall summary of our findings is illustrated in Figure 4. Caregiving was positively associated with higher levels of both measures of psychological distress (illustrated by positive pathways in Figure 4) but was also associated with decreased mortality (illustrated by a negative pathway). High psychological distress scores were also associated with increased mortality (a positive pathway), but this effect was markedly diminished by caregiving (a negative pathway) and was actually not observed to be statistically significant among the family caregivers.
Figure 4.
Summary of the effects of caregiving and psychological distress on all-cause mortality over a 7-year period.
As predicted from the Brown and Brown (2015) theory of the physiological benefits of prosocial helping behaviors, we found significant interaction effects such that caregiving appeared to buffer or disconnect otherwise noteworthy predictive relationships between measures of psychological distress and subsequent all-cause mortality. These findings are even more remarkable when considering that the caregivers reported higher levels of both depressive symptoms and perceived stress than matched noncaregivers. However, higher psychological distress levels had no discernable associations with mortality among the caregivers, whereas a one standard deviation increase on either measure of psychological distress was associated with a more than 20% increase in mortality for the matched noncaregivers. Subgroup analyses revealed that the effects were similar in magnitude and generally consistent across many subgroups defined by gender, race, age of the caregiver, caregiving relationship, hours of care per week, caregiving strain levels, and whether the caregiver and care recipients lived together.
The present findings add to the growing body of literature that uses diverse samples and methods to examine associations among caregiving, distress, and mortality. Because both depression and stress are often elevated among caregivers (National Academies of Sciences, 2016; Pinquart & Sorensen, 2003; Roth, Perkins, Wadley, Temple, & Haley, 2009; Schulz & Sherwood, 2008) and have consistently been identified as risk factors for heightened mortality (Cuijpers et al., 2014; Prior et al., 2016), the widely-cited finding of elevated mortality among strained spouse caregivers by Schulz and Beach (1999) made sense and has been frequently cited by policy reports and caregiver support websites. Interestingly, the multiple subsequent population-based studies that have found the opposite pattern -- reduced mortality among caregivers -- have been cited much less frequently (Roth, Fredman, & Haley, 2015) and are sometimes either ignored or treated with skepticism (2016 Alzheimer’s disease facts and figures; 2016; National Academies of Sciences, 2016). The caregiving-mortality literature may be showing a pattern that is sometimes found in the social sciences whereby relatively small, initial studies with large effect sizes are highly cited but subsequent studies with larger samples and smaller effects or contradictory findings are cited less frequently (Fanelli, Costas, & Ioannidis, 2017). However, counterintuitive or internally inconsistent findings, such as the collective findings that family caregivers report more symptoms of depression and other forms of distress, but lower mortality rates than noncaregivers, do call out for additional explanation or qualifications.
Previous papers have provided a number of possible explanations for the findings that caregivers have reduced mortality rates compared to noncaregivers. One consideration is a possible self-selection factor such that healthier individuals may be more likely to take on caregiving roles than those in poorer health (Fredman et al., 2006; McCann et al., 2004). However, our health-related variables in the propensity-matching method should have minimized this possible explanation. In addition, caregivers may receive psychological benefits from caregiving such as satisfaction from helping others (Roth, Fredman, & Haley, 2015) or physical benefits from the activity of providing care (Fredman et al., 2009). The present study adds to these possible explanations and is unique in addressing a specific mechanistic hypothesis informed by a physiological model of the stress-buffering effects of prosocial helping behaviors. Interestingly, the overall mortality benefit found here for caregiving (16.5%) is similar to the range of overall effects (17%–26%) found in other population-based studies of the caregiving-mortality association (Roth, Fredman, & Haley, 2015) and also similar to the adjusted 24% mortality benefit reported from a recent meta-analysis of studies of organized volunteer activities (Okun, Yeung, & Brown, 2013). Caregiving within one’s own family and volunteerism (service to the community) can both be considered prosocial helping behaviors, and a recent paper that measured both caregiving and volunteering in a national census study from Northern Ireland found that both types of prosocial helping behaviors had independent associations with reduced mortality (O’Reilly et al., 2017). Adding to the overall survival benefits of caregiving observed in the present study are the specific stress-buffering effects that were supported by the present findings. The significant caregiving*distress interaction effects and the accompanying stratified analyses suggest that the caregiving experience alters one’s response to stress in such a way that psychological distress no longer has a detectable impact on the most fundamental of health outcomes – all-cause mortality.
The present findings represent a specific test of a hypothesis derived from a physiological model of the health benefits of prosocial helping behaviors. However, that model is not the only possible explanation for the effects on mortality that have been observed. More detailed analyses of the impact of caregiving on mortality were limited in the present study by the lack of longitudinal data on whether caregiving was sustained over time, or whether some noncaregivers became caregivers during the follow-up period. We also do not have any information on the care needs or clinical conditions of the care recipients, and we were unable, for example, to examine whether dementia caregivers might have different predictors of mortality or show a stress-buffering effect. We also have no information of which caregivers in our sample believed they had a choice in becoming caregivers, a factor that has been shown to affect caregiver well-being (Schulz et al., 2012) and may influence the mechanism(s) through which benefits are achieved from caregiving. Clinicians often report seeing caregivers at the upper extreme of caregiving responsibilities and strain, and often with a lack of resources. The kinds of health benefits that are being found in multiple, population-based studies of caregiving may not be as readily evident among highly stressed caregivers who are seeking help in clinical settings.
Future population-based studies of caregiving and its influence on mortality and other health outcomes should collect longitudinal data on changes in factors that are hypothesized to transmit the stress-buffering effects of caregiving. Studies that track changes in caregiving status, psychological distress, biomarkers, physical activity, and other measures such as benefit finding or positive aspects of caregiving should be informative for further identifying the causal pathways that may be involved. Analytic models should also be further informed by theoretical models that posit specific mechanisms to explain the links between prosocial helping relationships and health-related outcomes.
The findings might also have implications for the development of interventions and other support services for family caregivers. Many such programs focus on alleviating stress and managing the burdens of caregiving, but benefit finding, or a focus on positive aspects of caregiving, is a particularly interesting potential psychological mechanism for future work on stress resilience in caregiving. Folkman (2007) articulated a theoretical model positing that highly stressful circumstances, including caregiving, can yield meaning-based coping, including positive reappraisal, which can be adaptive. Research on caregivers of people with cancer, dementia, and stroke has repeatedly shown that benefit finding is common, and that caregivers who report more positive aspects of caregiving or benefit finding report lower levels of psychological distress (Carbonneau, Caron, & Desrosiers, 2010; Haley et al., 2009; Kim, Schulz, & Carver 2007; Li & Yoke, 2013). In addition, several recent studies have found that adding an emphasis on helping caregivers to recognize and appreciate the psychological benefits of caregiving (or increasing gain-focused reappraisal) is more effective for reducing caregiver depressive symptoms than psychoeducational intervention without this component (Cheng et al., 2014; Cheng et al., 2017). In sum, benefits are commonly reported by caregivers, and benefit finding may be an important component that can be enhanced with interventions and incorporated more broadly in existing caregiver support services.
With the worldwide growth of the older adult population, family caregiving is becoming more common and has increasingly become a topic with major policy implications (Qualls, 2016; Redfoot, Feinberg, & Houser, 2013; Talley & Crews, 2007). Many discussions of aging and caregiving focus on the risks of increased burden, stress, poor health, and depression, but our findings indicate that caregiving, although associated with elevated psychological distress, is a prosocial helping role that leads to resilience and often softens the health impacts of that distress. While caregiving is often portrayed as burdensome and something that can get worse over time, a recent paper found that caregivers in 2015 reported significantly lower levels of strain and more use of respite services than did caregivers in 1999 (Wolff et al., in press). At a societal and policy level, it is important to continue to study caregiving in a more balanced way, as a positive and important family role that can be stressful but often also leads to personal gains and enhanced health outcomes.
Acknowledgments
Funding
This work was supported by cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke (NINDS), National Institutes of Health, Department of Health and Human Service. Additional funding was provided by an investigator-initiated grant (RF1 AG050609) from the National Institute on Aging (NIA). The content is solely the responsibility of the authors and does not necessarily represent the official views of NINDS, NIA, or the National Institutes of Health. Representatives of NINDS have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Contributor Information
David L. Roth, Johns Hopkins University
Stephanie L. Brown, Stony Brook University
J. David Rhodes, University of Alabama at Birmingham.
William E. Haley, University of South Florida
References
- 2016 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Avlund K, Damsgaard MT, Holstein BE. Social relations and mortality. An eleven year follow-up study of 70-year-old men and women in Denmark. Social Science and Medicine. 1998;47:635–643. doi: 10.1016/s0277-9536(98)00122-1. [DOI] [PubMed] [Google Scholar]
- Brown SL, Brown RM. Connecting prosocial behavior to improved physical health: Contributions from the neurobiology of parenting. Neuroscience and Biobehavioral Reviews. 2015;55:1–17. doi: 10.1016/j.neubiorev.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Brown SL, Brown RM, House JS, Smith DM. Coping with spousal loss: potential buffering effects of self-reported helping behavior. Personality and Social Psychology Bulletin. 2008;34:849–861. doi: 10.1177/0146167208314972. [DOI] [PubMed] [Google Scholar]
- Brown SL, Nesse RM, Vinokur AD, Smith DM. Providing social support may be more beneficial than receiving it: results from a prospective study of mortality. Psychological Science. 2003;14:320–327. doi: 10.1111/1467-9280.14461. [DOI] [PubMed] [Google Scholar]
- Brown SL, Smith DM, Schulz R, Kabeto MU, Ubel PA, Poulin M, … Langa KM. Caregiving behavior is associated with decreased mortality risk. Psychological Science. 2009;20:488–494. doi: 10.1111/j.1467-9280.2009.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WM, Consedine NS, Magai C. Altruism relates to health in an ethnically diverse sample of older adults. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2005;60:P143–P152. doi: 10.1093/geronb/60.3.P143. [DOI] [PubMed] [Google Scholar]
- Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Medical Care. 2002;40:771–781. doi: 10.1097/01.MLR.0000024610.33213.C8. [DOI] [PubMed] [Google Scholar]
- Carbonneau H, Caron C, Desrosiers J. Development of a conceptual framework of positive aspects of caregiving in dementia. Dementia. 2010;9:327–353. doi: 10.1177/1471301210375316. [DOI] [Google Scholar]
- Cheng ST, Lau RW, Mak EP, Ng NS, Lam LC. Benefit-finding intervention for Alzheimer caregivers: conceptual framework, implementation issues, and preliminary efficacy. Gerontologist. 2014;54:1049–1058. doi: 10.1093/geront/gnu018. [DOI] [PubMed] [Google Scholar]
- Cheng ST, Mak EP, Fung HH, Kwok T, Lee DT, Lam LC. Benefit-finding and effect on caregiver depression: A double-blind randomized controlled trial. Journal of Consulting and Clinical Psychology. 2017 doi: 10.1037/ccp0000176. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. doi: 10.2307/2136404. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Vogelzangs N, Twisk J, Kleiboer A, Li J, Penninx BW. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. American Journal of Psychiatry. 2014;171:453–462. doi: 10.1176/appi.ajp.2013.13030325. [DOI] [PubMed] [Google Scholar]
- D’Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Statistics in Medicine. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Fanelli D, Costas R, Ioannidis JPA. Meta-assessment of bias in science. Proceedings of the National Academy of Sciences. 2017;114:3714–3719. doi: 10.1073/pnas.1618569114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkman S. Positive psychological states and coping with severe stress. Social Science & Medicine. 1997;45:1207–1221. doi: 10.1016/s0277-9536(97)00040-3. [DOI] [PubMed] [Google Scholar]
- Fredman L, Cauley JA, Hochberg M, Ensrud KE, Doros G Study of Osteoporotic F. Mortality associated with caregiving, general stress, and caregiving-related stress in elderly women: results of caregiver-study of osteoporotic fractures. Journal of the American Geriatrics Society. 2010;58:937–943. doi: 10.1111/j.1532-5415.2010.02808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman L, Doros G, Ensrud KE, Hochberg MC, Cauley JA. Caregiving intensity and change in physical functioning over a 2-year period: results of the caregiver-study of osteoporotic fractures. American Journal of Epidemiology. 2009;170:203–210. doi: 10.1093/aje/kwp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman L, Lyons JG, Cauley JA, Hochberg M, Applebaum KM. The relationship between caregiving and mortality after accounting for time-varying caregiver status and addressing the healthy caregiver hypothesis. The Journals of Gerontology Series A, Biological Sciences and Medical sciences. 2015;70:1163–1168. doi: 10.1093/gerona/glv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley WE, Allen JY, Grant JS, Clay OJ, Perkins M, Roth DL. Problems and benefits reported by stroke family caregivers: Results from a prospective epidemiological study. Stroke. 2009;40:2129–2133. doi: 10.1161/strokeaha.108.545269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilbrand S, Coall DA, Gerstorf D, Hertwig R. Caregiving within and beyond the family is associated with lower mortailty for the caregiver: A prospective study. Evolution and Human Behavior. 2017;38:397–403. doi: 10.1016/j.evolhumbehav.2016.11.010. [DOI] [Google Scholar]
- Howard G, Anderson R, Johnson NJ, Sorlie P, Russell G, Howard VJ. Evaluation of social status as a contributing factor to the stroke belt region of the United States. Stroke. 1997;28:936–940. doi: 10.1161/01.str.28.5.936. [DOI] [PubMed] [Google Scholar]
- Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, … Howard G. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- Karam F, Bérard A, Sheehy O, Huneau M-C, Briggs G, Chambers C … OTIS Research Committee. Reliability and validity of the 4-item perceived stress scale among pregnant women: Results from the OTIS antidepressants study. Research in Nursing & Health. 2012;35:363–375. doi: 10.1002/nur.21482. [DOI] [PubMed] [Google Scholar]
- Kim Y, Schulz R, Carver CS. Benefit-finding in the cancer caregiving experience. Psychosomatic Medicine. 2007;69:283–291. doi: 10.1097/PSY.0b013e3180417cf4. [DOI] [PubMed] [Google Scholar]
- Li Q, Loke AY. The positive aspects of caregiving for cancer patients: a critical review of the literature and directions for future research. Psychooncology. 2013;22:2399–2407. doi: 10.1002/pon.3311. [DOI] [PubMed] [Google Scholar]
- McCann JJ, Hebert LE, Bienias JL, Morris MC, Evans DA. Predictors of beginning and ending caregiving during a 3-year period in a biracial community population of older adults. American Journal of Public Health. 2004;94:1800–1806. doi: 10.2105/ajph.94.10.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight KK, Wellons MF, Sites CK, Roth DL, Szychowski JM, Halanych JH, … Safford MM. Racial and regional differences in age at menopause in the United States: findings from the REasons for Geographic And Racial Differences in Stroke (REGARDS) study. American Journal of Obstetrics & Gynecology. 2011;205:353e351–358. doi: 10.1016/j.ajog.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchior LA, Huba GJ, Brown VB, Reback CJ. A short depression index for women. Educational and Psychological Measurement. 1993;53:1117–1125. doi: 10.1177/0013164493053004024. [DOI] [Google Scholar]
- Schulz R, Eden J, editors. National Academies of Sciences, Engingeering, and Medicine. Families caring for an aging America. Washington DC: The National Academies Press; 2016. [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism (U.S.) Helping patients who drink too much: a clinician’s guide: updated 2005 edition. Rockville, Md: U.S. Dept. of Health and Human Services, National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; 2007. Rev. Jan. 2007. [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behavioral and Cognitive Neuroscience Reviews. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- O’Reilly D, Connolly S, Rosato M, Patterson C. Is caring associated with an increased risk of mortality? A longitudinal study. Social Science & Medicine. 2008;67:1282–1290. doi: 10.1016/j.socscimed.2008.06.025. [DOI] [PubMed] [Google Scholar]
- O’Reilly D, Rosato M, Ferry F, Moriarty J, Leavy G. Caregiving, volunteering or both? Comparing effects on health and mortality using census-based records from almost 250,000 people aged 65 and over. Age and Ageing. 2017;46:821–826. doi: 10.1093/ageing/afx017. [DOI] [PubMed] [Google Scholar]
- O’Reilly D, Rosato M, Maguire A. Caregiving reduces mortality risk for most caregivers: A census-based record linkage study. International Journal of Epidemiology. 2015;44:1959–1969. doi: 10.1093/ije/dyv172. [DOI] [PubMed] [Google Scholar]
- Okun MA, Yeung EW, Brown S. Volunteering by older adults and risk of mortality: A meta-analysis. Psychology and Aging. 2013;28:564–577. doi: 10.1037/a0031519. [DOI] [PubMed] [Google Scholar]
- Parsons LS. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Cary, NC: SAS Institute; 2001. [Google Scholar]
- Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychology and Aging. 2003;18:250–267. doi: 10.1037/0882-7974.18.2.250. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Brown SL, Dillard AJ, Smith DM. Giving to others and the association between stress and mortality. American Journal of Public Health. 2013;103:1649–1655. doi: 10.2105/AJPH.2012.300876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prior A, Fenger-Gron M, Larsen KK, Larsen FB, Robinson KM, Nielsen MG, … Vestergaard M. The association between perceived stress and mortality among people with multimorbidity: A prospective population-based cohort study. American Journal of Epidemiology. 2016;184:199–210. doi: 10.1093/aje/kwv324. [DOI] [PubMed] [Google Scholar]
- Qualls SH. Caregiving families within the long-term services and support system for older adults. American Psychologist. 2106;71:283–293. doi: 10.1037/a0040252. [DOI] [PubMed] [Google Scholar]
- Ramsay S, Grundy E, O’Reilly D. The relationship between informal caregiving and mortality: An analysis using the ONS Longitudinal Study of England and Wales. Journal of Epidemiology and Community Health. 2013;67:655–660. doi: 10.1136/jech-2012-202237. [DOI] [PubMed] [Google Scholar]
- Redfoot D, Feinberg L, Houser A. The aging of the baby boom and the growing care gap. A look at future declines in the availability of family caregivers. Washington, DC: AARP Public Policy Institute; 2013. [Google Scholar]
- Roth DL, Fredman L, Haley WE. Informal caregiving and its impact on health: A reappraisal from population-based studies. Gerontologist. 2015;55:309–319. doi: 10.1093/geront/gnu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DL, Haley WE, Hovater M, Perkins M, Wadley VG, Judd S. Family caregiving and all-cause mortality: Findings from a population-based propensity-matched analysis. American Journal of Epidemiology. 2013;178:1571–1578. doi: 10.1093/aje/kwt225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DL, Perkins M, Wadley VG, Temple EM, Haley WE. Family caregiving and emotional strain: Associations with quality of life in a large national sample of middle-aged and older adults. Quality of Life Research. 2009;18:679–688. doi: 10.1007/s11136-009-9482-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Beach SR. Caregiving as a risk factor for mortality: The Caregiver Health Effects Study. Journal of the American Medical Association. 1999;282:2215–2219. doi: 10.1001/jama.282.23.2215. [DOI] [PubMed] [Google Scholar]
- Schulz R, Beach SR, Cook TB, Martire LM, Tomlinson JM, Monin JK. Predictors and consequences of perceived lack of choice in becoming an informal caregiver. Aging and Mental Health. 2012;16:712–721. doi: 10.1080/13607863.2011.651439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, Sherwood PR. Physical and mental health effects of family caregiving. The American Journal of Nursing. 2008;108:23–27. doi: 10.1097/01.NAJ.0000336406.45248.4c. quiz 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz C, Meisenhelder JB, Ma Y, Reed G. Altruistic social interest behaviors are associated with better mental health. Psychosomatic Medicine. 2003;65:778–785. doi: 10.1097/01.psy.0000079378.39062.d4. [DOI] [PubMed] [Google Scholar]
- Stuart EA. Matching methods for causal inference: A review and a look forward. Statistical Science: A Review Journal of the Institute of Mathematical Statistics. 2010;25:1–21. doi: 10.1214/09-STS313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley RC, Crews JE. Framing the public health of caregiving. American Journal of Public Health. 2007;97:224–228. doi: 10.2105/AJPH.2004.059337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warttig SL, Forshaw MJ, South J, White AK. New, normative, English-sample data for the Short Form Perceived Stress Scale (PSS-4) Journal of Health Psychology. 2013;18:1617–1628. doi: 10.1177/1359105313508346. [DOI] [PubMed] [Google Scholar]
- Wolff JL, Mulcahy J, Huang J, Roth DL, Covinsky K, Kasper JD. Family caregivers of older adults, 1999–2015: Trends in characteristics, circumstances, and role-related appraisal. The Gerontologist. doi: 10.1093/geront/gnx093. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]