Abstract
Vitamin D deficiency (VDD), 25-OHD levels <20 ng/ml, is prevalent among patients with sickle cell disease (SCD) and is linked to acute and chronic pain and bone fracture in this population. There is limited literature regarding VDD-associated risk factors for SCD. We examined potential clinical and genomic parameters associated with VDD in 335 adults with SCD in a cross-sectional study. VDD was present in 65% of adult SCD patients, and 25-OHD levels independently and positively correlated with older age (p < 0.001) and vitamin D supplementation (p < 0.001). 25-OHD levels were higher in SCD patients over 40 years of age compared to the general African-American population. Both lower 25-OHD levels and increased pain frequency were associated with increased expression of SLC6A5 encoding glycine transporter-2 (GlyT2), a protein involved in neuronal pain pathways. Lower 25-OHD levels were also associated with increased expression of CYP3A4, and with decreased expression of GC (also termed DBP) and VDR, three genes involved in vitamin D metabolism. We conclude that vitamin D supplementation should be an almost universal feature of the care of young adults with SCD, and that further research is warranted into genomic factors that regulate vitamin D metabolism in SCD.
Keywords: sickle, vitamin D, gene expression, SLC6A5, mortality
INTRODUCTION
Vitamin D is synthesized from 7-dehydrocholesterol when skin is exposed to ultraviolet light. 7-dehydrocholesterol is hydrolysed first to 25-hydroxyvitamin D (25-OHD) and then to 1,25-dihydroxyvitamin D (1,25-(OH)2D), the ligand for the nuclear vitamin D receptor (VDR) (Ramagopalan et al., 2010). VDR and 1,25-(OH)2D form a heterodimer with retinoid X receptor (RXR), which binds vitamin D response elements (VDREs) in genomic sequences and influences gene transcription. Vitamin D is involved in the regulation of multiple biological processes, such as cellular proliferation, differentiation and apoptosis, calcium metabolism and bone health (Arnson et al., 2007, Holick, 2007). Multiple genes are involved in the vitamin D metabolism pathway (Jolliffe et al., 2016). Cytochrome P450 2R1 (CYP2R1) and cytochrome P450 27B1 (CYP27B1) encode the most important hydroxylases. Cytochrome P450 24A1 (encoded by CYP24A1) catabolizes both 25-OHD and 1,25-(OH)2D. Vitamin D binding protein (encoded by GC, also termed DBP) binds to 25-OHD in plasma, and is hypothesized to serve as a reservoir and prolong the half-life of 25-OHD (Powe et al., 2013, Carpenter et al., 2013). In the general population, vitamin D deficiency (VDD), defined as a 25-OHD concentration less than 20 ng/ml, is a risk factor for acute/chronic pain syndromes (Shipton and Shipton, 2015), cardiovascular (Wang et al., 2012a), autoimmune (Gatenby et al., 2013) and pulmonary (Janssens et al., 2013, Gupta et al., 2011) diseases, and higher mortality as demonstrated in numerous studies (Schottker et al., 2013, Schottker et al., 2014, Chowdhury et al., 2014, Melamed et al., 2008, Signorello et al., 2013). Although most of these studies either had relatively small sample sizes or were meta-analysis or secondary analyses of existing cohorts, they imply that VDD may be a risk factor for various complications in patients with chronic diseases.
A systematic review reported that the prevalence of VDD in sickle cell disease (SCD) populations ranges from 56% to 96% (Nolan et al., 2015). Patients with SCD tend to have dark skin colour, limited sunlight exposure, poor nutrition and a high prevalence of renal dysfunction, which puts them at a higher risk of developing VDD (Forrest and Stuhldreher, 2011). VDD in SCD patients leads to lower bone density and increased risk of bone fracture (Sadat-Ali et al., 2011, Arlet et al., 2013), and is also associated with acute vaso-occlusive crisis (VOC) (Osunkwo et al., 2011, Lee et al., 2015) and use of pain medication (Han et al., 2016) although studies have only been small so far. Vitamin D supplementation resulted in fewer pain days in SCD patients in a small pilot trial (Osunkwo et al., 2012). Little is known about clinical and genomic risk factors for VDD in SCD. In this study, we evaluated clinical and genomic variables associated with VDD in an adult cohort of SCD patients at the University of Illinois at Chicago (UIC).
METHODS
Patient Population
A cohort of 335 adults (age ≥ 18 years old) with SCD followed at the UIC hospital was enrolled in a registry between 2010 and 2014. The study was approved by the Institutional Review Board prior to enrolment and all participants gave written informed consent for participation in the registry.
Data collection
Demographic information and laboratory data were collected from the electronic medical record charting system, Cerner PowerChart. Laboratory data, including 25-OHD levels, complete blood counts with differentials, liver function test and complete metabolic panel, were recorded from a steady-state clinic visit closest to the date when the patient consented to the study. A steady-state clinic visit was defined as an office visit at which the patient was not in an acute vaso-occlusive pain episode. For the mortality follow-up, the dates of death were collected from the medical charts, providers, or the Social Security Death Index search. The length of follow-up was measured using the reverse censoring method (Schemper and Smith, 1996). For subjects who had not been followed up at UIC during the past 6 months when the data collection was conducted and yielded no positive identification in the Social Security Death Index, the last follow-up date was used for the mortality analysis.
Gene Expression Analysis
RNA samples were isolated from patient peripheral blood mononuclear cells (PBMCs) within 60 days of the vitamin D level measurement. Messenger RNA was purified, labelled and hybridized to Affymetrix Gene 2.0 ST Array (Affymetrix, Santa Clara, CA, USA). Probe sequences were aligned to human genome assembly GRCh37 (https://www.ncbi.nlm.nih.gov/assembly/GCF_000001405.13) to select for probes with unique perfect alignment. Probes that interrogate multiple gene transcripts and that contain single nucleotide polymorphisms (SNPs) with ≥1% minor allele frequency in the SNP database (dbSNP: https://www.ncbi.nlm.nih.gov/SNP) dataset were removed. Probe intensities were log2 transformed, background corrected and quantile normalized. Probe intensity was extracted by the corresponding probe mean across samples. Gene expression level was summarized as mean intensity across probes within gene, using Gencode version 19 (https://www.gencodegenes.org/releases/19.html). Batch effects of RNA labelling and array hybridization were adjusted using an empirical Bayes method (Johnson et al., 2007). Patients treated with vitamin D supplement and hydroxycarbamide were excluded, and so 68 patients were analysed for 18,551 autosomal genes. Regression analysis for gene expression was performed on natural log-transformed vitamin D levels adjusting for age, gender and Hb genotype. The false discovery rate (FDR) was determined by Benjamini and Hochberg approach (Benjamini and Hochberg, 1995). Genes that are involved in the vitamin D metabolism (Jolliffe et al., 2016) were presented. Gene enrichment analysis for GO biological processes was performed using the National Institutes of Health (NIH) DAVID (Huang et al., 2009).
National Health and Nutrition Examination Survey (NHANES) Data Analysis
The 25-OHD levels from 2003–2004 and 2005–2006 NHANES laboratory data (https://www.cdc.gov/nchs/nhanes/index.htm) were pooled. Analyses were restricted to US-born African Americans aged 18–85 years old. One outlier with 25-OHD level >60 ng/ml was removed, resulting in 2050 subjects. The 25-OHD levels between NHANES and UIC cohorts for age categories were compared using a two-tailed Wilcoxon rank sum test. The two-tailed Cruzik’s test was used to estimate the trend of 25-OHD levels across age categories of ≤29, 30–39, 40–49 and ≥50 years for both the UIC and NHANES cohorts. To compare the trend difference, a linear regression model was used to test the interaction between disease status (SCD for UIC and normal for NHANES) and age categories (<40 and ≥40 years) on log-transformed 25-OHD levels.
Effects of Vitamin D Supplementation
Weekly oral ergocalciferol (50,000 units) is often prescribed to patients who had a 25-OHD level less than 20 ng/ml in our institution. Twenty-five of the patients prescribed ergocalciferol had a repeat measurement of 25-OHD levels within 180 days. The Wilcoxon signed-rank test was used to compare 25-OHD levels before and after supplementation.
Statistical Analysis
The SCD patients were divided into three groups based on 25-OHD levels: <20 ng/ml (VDD), 20–29 ng/ml (vitamin D insufficiency), or ≥30 ng/ml (vitamin D sufficiency) (Holick, 2007). The baseline characteristic differences based on 25-OHD levels were compared using the Jonckheere-Terpstra trend test for linear variables or Cochran trend test for categorical variables. The 25-OHD levels among different age groups and months were compared using the Kruskal–Wallis test and the Comparison Conover-Inman Pairwise Comparison Test. In the multivariable analysis, a stepwise linear regression was used. The backward regression had an entry probability of 0.2 and an exit probability of 0.1. The 25-OHD levels were transformed as loge(25-OHD). To measure the seasonal 25-OHD variations, a calendar year was categorized into four quarters (November to January, February to April, May to July and August to October) based on the duration of daylight (Astronomical Applications Dept., U. S. Naval Observatory, Washington, DC; http://aa.usno.navy.mil/data/docs/Dur_OneYear.php), and the Kruskal–Wallis test and the Conover-Inman Test for All Pairwise Comparisons were used to compare 25-OHD levels in different quarters. A stepwise Cox proportional hazards regression model was used to analyse the mortality risk. SYSTAT 13 (Systat Software Corporation, Chicago, IL, USA) and SAS 9.3 (SAS Institute Inc., Cary, NC, USA) were used for most analyses. For gene expression analysis, linear regression was applied to model vitamin D level (natural log transformed) using gene expression, age, gender, and SCD Hb genotype as explanatory variables. The significance of gene expression term was estimated using χ2 test with 1 degree of freedom. P-values were adjusted for multiple comparisons using Benjamini and Hochberg method (Benjamini and Hochberg, 1995).
RESULTS
Clinical Correlates of Vitamin D Deficiency
The 335 SCD adult patients who had 25-OHD levels measured included 252 with Hb SS or S/beta0thalassaemia (SS/Sbeta0), 63 with Hb SC and 20 with Hb S/beta+ thalassaemia. The median age (interquartile range [IQR]) was 32 (24–44) years. Thirty-nine percent were male, and 40% were taking hydroxycarbamide therapy at the time of 25-OHD levels measured. The 25-OHD levels were not normally distributed (one-sample Kolmogorov-Smirnov test p < 0.01), and the median 25-OHD level was 14 (IQR 9–23) ng/ml. The SCD patients were divided into three groups based on 25-OHD levels (<20 (VDD), 20–29, or ≥30 ng/ml). The majority of patients (65%) had VDD, and this proportion increased to 77% of those in the <40 years of age category. The patient characteristics in each group are summarized in Table I. All three groups had similar percentage of male patients and Hb SS/Sbeta0 patients. The median age was significantly older as 25-OHD levels increased (p < 0.001), whereas glomerular filtration rate (GFR) was reduced as 25-OHD levels increased (p = 0.008) due to the confounding effect of age. The groups with higher 25-OHD levels had more patients on vitamin D supplementation at the time the 25-OHD levels were measured (p =0.002). White blood cell (WBC) counts were higher in groups with lower 25-OHD levels (Table I). Multivariable regression analysis identified lower 25-OHD levels as being independently associated with younger age (p < 0.001) and lack of vitamin D supplementation (p < 0.001) (Table II).
Table I. Patient Characteristics Based on Vitamin D Level at baseline.
The median of each variable was shown, and the patient characteristics were compared using Jonckheere-Terpstra trend test or Cochran trend test based on the variables. Medians with interquartile range are shown.
| 25-OHD level (ng/ml) | < 20 | 20–29 | ≥ 30 | p-value |
|---|---|---|---|---|
| N | 218 | 66 | 51 | |
| Age (years) | 29 (23–39) | 38.5 (26–50) | 46 (31–52) | <0.001 |
| Gender (%male) | 39 | 41 | 39 | 0.845 |
| GFR (ml/min) | 136 (104–175) | 117 (84–154) | 100 (69–135) | 0.008 |
| SCD genotype (%SS or Sbeta0) | 78 | 68 | 71 | 0.111 |
| Hydroxycarbamide (%) | 37 | 41 | 51 | 0.065 |
| Vitamin D supplementation (%) | 15 | 28 | 31 | 0.002 |
| 25-OHD level (ng/ml) | 10 (7–14) | 23 (21–26) | 37 (33–45) | <0.001 |
| WBC count (x 109/l) | 9.8 (7.8–12.3) | 8.6 (7.1–11.7) | 7.6 (6.7–10.5) | 0.012 |
GFR: glomerular filtration rate; SCD: sickle cell disease; WBC: white blood cell.
Table II.
Clinical Correlates of 25-OHD Levels.
| Correlate | % Change | 95% CI | p-value |
|---|---|---|---|
| Age (10-year period) | 20% increase per 10 years of age | 14–27% | <0.001 |
| Vitamin D supplementation | 39% higher level with vitamin D supplementation | 17–64% | <0.001 |
| Gender (male=1, female=0) | 19% higher level with male versus female gender | 3.6–36% | 0.010 |
| HbSS/Sbeta0Thalassaemia (Yes=0, No=1) | 20% lower level with severe Hb genotype | 2.7–40% | 0.032 |
A stepwise linear regression analysis of 25-OHD levels was performed. The covariates originally entered into the analysis were age, gender, SS/Sbeta0 SCD genotype, vitamin D supplementation, glomerular filtration rate, white blood cell counts, and hydroxycarbamide therapy. Age, gender, vitamin D supplementation, and HbSS/Sbeta0Thalassaemia remained in the final regression model. The backward regression had an entry probability of 0.2 and an exit probability of 0.1. The 25-OHD levels were transformed as loge(25-OHD). The exponentials of the coefficients and 95% confidence interval (CI) were calculated, and % change was presented. N=335.
Comparison of 25-OHD levels in the UIC SCD cohort and NHANES African Americans
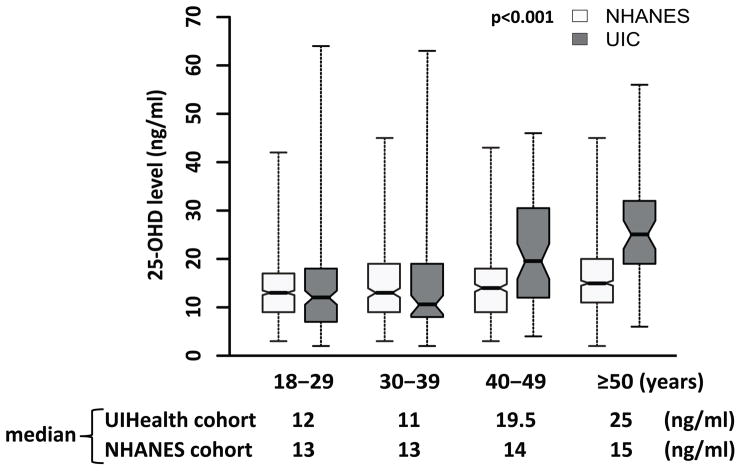
UIC SCD patients in the 40–49 years and ≥50 years old groups had higher levels of 25-OHD than younger age groups (p < 0.001) (Figure 1). NHANES data also showed a positive trend between 25-OHD levels and age in the general African-American population (p < 0.001). When the UIC cohort was compared to the NHANES cohort, there was no significant difference in 25-OHD levels for the 18–29 years (p = 0.31) or 30–39 years (p = 0.15) age groups, but the levels were higher in the UIC cohort in the 40–49 and ≥ 50 year age groups (p < 0.001). Older age was associated with increased 25-OHD levels in both SCD and normal individuals, but the magnitude of 25-OHD level increase with age was greater in SCD than the general African American population (p < 0.001).
Figure 1. The 25-OHD Levels in Different Age Groups.
The 25-OHD levels among different age groups were compared using the Kruskal–Wallis test and the Conover-Inman Test for All Pairwise Comparisons. Cruzik’s test was used to estimate the trend of 25-OHD levels across age groups of ≤29, 30–39, 40–49, and ≥50 years for both the University of Illinois at Chicago (UIC) cohort and National Health and Nutrition Examination Survey (NHANES) cohort. To test the differential effect of older age (≥40 years) on 25-OHD levels between sickle cell disease patients and normal individuals, log-transformed 25-OHD levels were regressed on age category, disease status and their interaction effect. The boxes denote the inter-quartile range and the whiskers denote the range of the data.
Seasonal 25-OHD Variation
Sun exposure leads to the photosynthesis of vitamin D (Holick, 2007), which may cause seasonal variation in 25-OHD levels. When 25-OHD levels were evaluated based on daylight duration, those in the quarter with the least sun exposure (November to January) were the lowest, which was significantly lower than those measured in the quarter with the most sun exposure (Table III), emphasizing the importance of vitamin D supplementation during the months with the least daylight.
Table III.
Quarterly Variations in 25-OHD Levels in SCD Patients.
| Quarter | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Month | Nov–Jan | Feb–Apr | May–July | Aug–Oct |
| N | 79 | 55 | 52 | 82 |
| 25-OHD (ng/ml) | 10 (7–20.5) | 12 (8.3–27.5) | 12.5 (8.5–20) | 15 (9–23) |
| Pairwise comparison vs. Quarter 1 | NA | 0.105 | 0.197 | 0.016 |
| Pairwise comparison vs. Quarter 2 | 0.105 | NA | 0.778 | 0.586 |
| Pairwise comparison vs. Quarter 3 | 0.197 | 0.778 | NA | 0.399 |
Quarters are based on the duration of daylight: 1. November to January; 2. February to April; 3. May to July; 4. August to October. Patients taking vitamin D supplementation when 25-OHD levels were measured were excluded from this analysis. The 25-OHD levels were compared using the Kruskal–Wallis test and the Conover-Inman Test for All Pairwise Comparisons.
Differential Gene Expression Associated with 25-OHD Levels
To investigate differential PBMC gene expression associated with 25-OHD levels, a genome-wide association study was conducted after adjusting for age, gender, and sickle cell Hb genotype. A total of 993 genes were positively associated with 25-OHD levels at FDR < 0.05, and 634 genes were negatively associated (Supplementary Tables 1 and 2). They were enriched for several pathways including Fc gamma R-mediated phagocytosis, inositol phosphate metabolism and olfactory transduction (Supplementary Table 3), but the implication of these pathways in the SCD pathophysiology is unknown.
The expression of three genes had the most stringent genome-wide correlation with 25-OHD levels at FDR <0.01. Expression of Solute Carrier Family 6 Member 5 (SLC6A5) was negatively associated with vitamin D levels (Supplementary Table 2). SLC6A5 encodes the glycine transporter-2 (GlyT2), a glycine transporter expressed in neurons that is involved in pain pathways. GlyT2 inhibitors have been investigated as analgesics in various pain conditions (Hermanns et al., 2008, Morita et al., 2008, Succar et al., 2007). Consistent with its potential function in pain transduction, the gene expression of SLC6A5 was positively correlated with pain frequency (Spearman’s r=0.16, p = 0.05, n=159) in the SCD patients in our cohort. Expression of Minichromosome Maintenance Complex Component 5 (MCM5) and Solute Carrier Family 38 Member 7 (SLC38A7) were positively associated with vitamin D levels. MCM5 is important in cell cycle regulation and is overexpressed in several cancers (Wang et al., 2018, Snyder et al., 2005). SLC38A7 encodes a glutamine transporter that is important for neuronal physiology and the growth of cancer cells (Verdon et al., 2017).
A list of 10 key genes involved in vitamin D metabolic and signalling pathways (Jolliffe et al., 2016) is presented in Table IV. After adjusting for age, gender and sickle cell Hb genotype, the expression levels of three of these 10 vitamin D metabolic pathway-related genes were associated with serum vitamin D levels at FDR < 0.05. This represents a 3.4-fold enrichment compared to all analysed genes: 1627 of 18,551 genes (Supplementary Tables 1 and 2) (binomial test p = 0.05). CYP3A4 expression significantly correlated with lower 25-OHD levels, and GC and VDR expression significantly correlated with higher 25-OHD levels in the genome-wide gene expression analysis (Table IV). CYP3A4 is one of the enzymes that catalyses the hydroxylation of vitamin D, causing clearance of vitamin D (Wang et al., 2013). DBP (encoded by GC) binds vitamin D in the plasma and prolongs the half-life of 25-OHD (Powe et al., 2013, Carpenter et al., 2013). VDR forms a complex with 1,25-(OH)2D regulating transcriptional responses of downstream targets (Ramagopalan et al., 2010).
Table IV.
Relationship of Serum 25-OHD Levels with Expression of Genes in Vitamin D Metabolic and Signalling Pathways.
| Gene | Gene Name | Beta | p-value | Adjusted p-value |
|---|---|---|---|---|
| DHCR7 | 7-Dehydrocholesterol Reductase | 1.330 | 0.1451 | 0.296 |
| CYP2R1 | Cytochrome P450 2R1 | −0.242 | 0.780 | 0.863 |
| CYP3A4 | Cytochrome P450 3A4 | −2.201 | 0.0004 | 0.027 |
| CYP27A1 | Cytochrome P450 27A1 | 0.312 | 0.1338 | 0.280 |
| CYP24A1 | Cytochrome P450 24A1 | −0.780 | 0.393 | 0.553 |
| GC | GC, vitamin D binding Protein | 1.168 | 0.0008 | 0.030 |
| LRP2 | Low Density Lipoprotein Receptor-Related Protein 2 | −1.033 | 0.137 | 0.286 |
| CYP27B1 | Cytochrome P450 27B1 | −0.170 | 0.868 | 0.920 |
| RXRA | Retinoid X Receptor Alpha | 0.489 | 0.198 | 0.359 |
| VDR | Vitamin D receptor | 2.011 | <0.0001 | 0.016 |
The correlation between the expressions of the listed genes involved in vitamin D metabolic pathways and loge(25-OHD) levels was examined after adjusting for age, gender and SCD Hb genotype.
Vitamin D supplementation
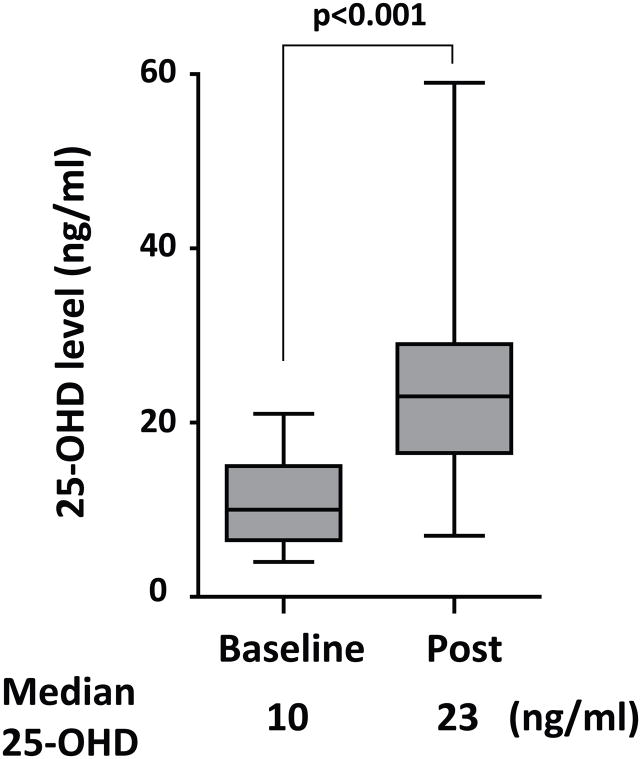
Twenty-five of the patients with VDD were prescribed weekly oral supplementation (50,000 units ergocalciferol) followed by a repeat measurement of 25-OHD levels within 180 days (Figure 2). The supplementation significantly improved 25-OHD levels (median 10 to 23 ng/ml, p < 0.001), and decreased the portion of patients with VDD from 96% (24/25) to 32% (8/25), showing that this supplementation method was an effective approach to increase 25-OHD levels.
Figure 2. Efficacy of Ergocalciferol Supplementation in Correcting vitamin D deficiency.
Weekly oral ergocalciferol (50,000 units) was given to sickle cell disease patients with VDDvitamin D deficiency for 12 weeks (N = 25), and 25-OHD levels were re-measured within 180 days (median 133 days, interquartile range 114 – 153 days) after starting supplementation. The Wilcoxon signed-rank test was used to analyse the data (p < 0.001).
Association between 25-OHD levels and mortality in SCD
To examine the impact of VDD on mortality in SCD, the 335 enrolled subjects were followed for a median of 51 months (IQR 31–63 months). Twenty-three patients (6.9%) died during the follow-up period. Kaplan-Meier analysis of the SCD patients who were divided into three groups based on 25-OHD levels (<20, 20–29, or ≥30 ng/mL) showed a survival benefit of higher 25-OHD levels with a trend towards statistical significance (log-rank p-value 0.069). When a stepwise Cox proportional hazards regression model was used to analyse the relationship between mortality and 25-OHD levels after adjusting for age, gender, SCD Hb genotype, GFR, WBC count, hydroxycarbamide treatment, and vitamin D supplementation, lower GFR was the only variable significantly associated with higher mortality risk (Hazard ratio [HR]: −0.01, 95% confidence interval [CI]: −0.002– −0.019, p = 0.016). Vitamin D level was not a significant correlate (HR: −0.292, 95% CI: −0.879 − 0.295, p = 0.329) after adjusting for GFR.
DISCUSSION
Although VDD is common in the general population, it is especially common among African Americans, probably due to difference in skin pigmentation, sun exposure and diet (Forrest and Stuhldreher, 2011). In our SCD cohort, 65% of the patients (218/335) had VDD. If patients already on vitamin supplementation at baseline are excluded, the prevalence of VDD is 69% (185/268). This is consistent with the published prevalence in SCD patients in a systematic review (Nolan et al., 2015). In addition to its known role in calcium homeostasis for maintaining skeletal health (Sadat-Ali et al., 2011, Arlet et al., 2013), vitamin D is important for non-skeleton functions in SCD. One retrospective study of 53 paediatric patients with SCD showed a correlation between chronic pain and lower 25-OHD levels (Osunkwo et al., 2011). Another cross-sectional study of 95 paediatric SCD patients associated of lower 25-OHD levels with acute pain (Lee et al., 2015). Lower 25-OHD levels are also associated with higher opioid usage in SCD patients, as shown in a retrospective study of 203 adult patients (Han et al., 2016), which seems correctable with high-dose vitamin D supplementation based on a small pilot trial (Osunkwo et al., 2012). Although most of the studies have been retrospective with small sample sizes, vitamin D levels can be potentially regarded as a health indicator (Schottker and Brenner, 2015, Chowdhury et al., 2014).
An important finding of our study is a strong relationship of SLC6A5 expression with both lower 25-OHD levels and increased pain frequency in the SCD patients in our study. Thus, targeting SLC6A5 and its product, GlyT2, is a potential novel approach to decreasing pain in SCD patients given the role of SLC6A5 in pain pathways (Hermanns et al., 2008, Morita et al., 2008, Succar et al., 2007). Among key genes involved in vitamin D metabolism (Table IV), CYP3A4 expression significantly correlated with lower 25-OHD levels in our study and the expression of DBP and VDD correlated with higher levels. CYP3A4 catalyses the hydroxylation of vitamin D leading to metabolic clearance of vitamin D (Wang et al., 2013). Higher CYP3A4 activity correlates with lower vitamin D levels (Prytula et al., 2016). Long term treatment with CYP3A4 expression-inducing drugs, such as rifampin, causes drug-induced vitamin D deficiency and osteomalacia (Wang et al., 2012b). An association between 25-OHD levels and SNPs in CYP3A4 has been observed in a non-African American cohort (Shao et al., 2017). GC expression correlated with higher vitamin D levels, and DBP seems to serve as a reservoir in the plasma and to prolong the half-life of 25-OHD (Powe et al., 2013, Carpenter et al., 2013). In a non-SCD African American cohort, two SNPs in GC were found to be significantly associated with 25-OHD levels (Signorello et al., 2011). In DBP-deficient mice, 25-OHD levels are lower and these mice are more susceptible to VDD manifestations when deprived of vitamin D (Safadi et al., 1999). VDR expression also correlated with higher 25-OHD levels in our study, which is consistent with previous findings that VDR is positively regulated by 1,25-(OH)2D in PBMCs and T cells (Yu et al., 1991, Baeke et al., 2010).VDR forms a transcription complex with 1,25-(OH)2D and RXR that regulates gene transcription of multiple downstream pathways, such as cellular proliferation, calcium metabolism and bone health (Bikle, 2014).
In this study, patients with VDD have higher WBC counts (Table I). Vitamin D modulates inflammatory diseases such as infection, asthma and chronic kidney disease (Yin and Agrawal, 2014, Zanetti et al., 2014). Acute and chronic inflammation contribute to the pathophysiology of SCD and its complications, such as pain crisis and acute chest syndrome (Hoppe, 2014). Our observation may provide a potential mechanism for how vitamin D may help to decrease chronic and acute pain in SCD (Lee et al., 2015, Osunkwo et al., 2011, Osunkwo et al., 2012) and emphasizes the importance of providing vitamin D supplementation to SCD patients with VDD. In our institution, weekly supplementation of oral ergocalciferol is usually prescribed for SCD patients with VDD for 12 weeks, and this has been an effective approach to correct 25-OHD levels (Figure 2), although some patients need longer duration of treatment or higher dose of supplementation. Weekly high dose oral cholecalciferol supplementation is also effective to restore 25-OHD levels in SCD patients (Wykes et al., 2014, Osunkwo et al., 2012).
This study is not without limitations. Our study is underpowered to detect a significant association between VDD and mortality. Gene expression was studied in a mixture of PBMCs rather than in purified blood cell fractions. We were unable to replicate our gene expression results in an independent data set. Nevertheless, the enrichment of independently selected vitamin D metabolic pathway genes in the genes associated with 25-OHD levels provides internal verification to our gene expression results. In conclusion, we found evidence that higher 25-OHD levels are associated with older age in adult patients with SCD, and that the differential expression of CYP3A4, GC and VDR may contribute to the difference in 25-OHD levels. Prospective clinical trials with longer follow-up are necessary to evaluate the possible association of VDD with increased SLC6A5 as a mediator of pain and with mortality in SCD, and to precisely characterize the clinical benefits of routine vitamin D supplementation.
Supplementary Material
Acknowledgments
Dr. Santosh Saraf is supported by the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health under Award Number K23HL125984. Dr. Robert Molokie is supported by grant number 1R01HL078536 from the National Institutes of Health, National Heart Lung and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, or the Department of Veterans Affairs.
Footnotes
Author contributions
J.H., X.Z., B.S. and T.A. performed the research; J.H., S.S., R.M. and V.G. designed the research study; J.H., X.Z. and V.G. analysed the data; M.G., R.M., Jo.Ha. and S.J. contributed essential reagents or tools; J.H., X.Z., S.S. and V.G. wrote the paper.
References
- ARLET JB, COURBEBAISSE M, CHATELLIER G, ELADARI D, SOUBERBIELLE JC, FRIEDLANDER G, DE MONTALEMBERT M, PRIE D, POUCHOT J, RIBEIL JA. Relationship between vitamin D deficiency and bone fragility in sickle cell disease: a cohort study of 56 adults. Bone. 2013;52:206–11. doi: 10.1016/j.bone.2012.10.005. [DOI] [PubMed] [Google Scholar]
- ARNSON Y, AMITAL H, SHOENFELD Y. Vitamin D and autoimmunity: new aetiological and therapeutic considerations. Annals of the rheumatic diseases. 2007;66:1137–42. doi: 10.1136/ard.2007.069831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAEKE F, KORF H, OVERBERGH L, VAN ETTEN E, VERSTUYF A, GYSEMANS C, MATHIEU C. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–7. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- BENJAMINI Y, HOCHBERG Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- BIKLE DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–29. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARPENTER TO, ZHANG JH, PARRA E, ELLIS BK, SIMPSON C, LEE WM, BALKO J, FU L, WONG BY, COLE DE. Vitamin D binding protein is a key determinant of 25-hydroxyvitamin D levels in infants and toddlers. J Bone Miner Res. 2013;28:213–21. doi: 10.1002/jbmr.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHOWDHURY R, KUNUTSOR S, VITEZOVA A, OLIVER-WILLIAMS C, CHOWDHURY S, KIEFTE-DE-JONG JC, KHAN H, BAENA CP, PRABHAKARAN D, HOSHEN MB, FELDMAN BS, PAN A, JOHNSON L, CROWE F, HU FB, FRANCO OH. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. BMJ. 2014;348:g1903. doi: 10.1136/bmj.g1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORREST KY, STUHLDREHER WL. Prevalence and correlates of vitamin D deficiency in US adults. Nutr Res. 2011;31:48–54. doi: 10.1016/j.nutres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- GATENBY P, LUCAS R, SWAMINATHAN A. Vitamin D deficiency and risk for rheumatic diseases: an update. Curr Opin Rheumatol. 2013;25:184–91. doi: 10.1097/BOR.0b013e32835cfc16. [DOI] [PubMed] [Google Scholar]
- GUPTA A, SJOUKES A, RICHARDS D, BANYA W, HAWRYLOWICZ C, BUSH A, SAGLANI S. Relationship between serum vitamin D, disease severity, and airway remodeling in children with asthma. Am J Respir Crit Care Med. 2011;184:1342–9. doi: 10.1164/rccm.201107-1239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN J, SARAF SL, ZHANG X, GOWHARI M, MOLOKIE RE, HASSAN J, ALHANDALOUS C, JAIN S, YOUNGE J, ABBASI T, MACHADO RF, GORDEUK VR. Patterns of opioid use in sickle cell disease. Am J Hematol. 2016;91:1102–1106. doi: 10.1002/ajh.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERMANNS H, MUTH-SELBACH U, WILLIAMS R, KRUG S, LIPFERT P, WERDEHAUSEN R, BRAUN S, BAUER I. Differential effects of spinally applied glycine transporter inhibitors on nociception in a rat model of neuropathic pain. Neurosci Lett. 2008;445:214–9. doi: 10.1016/j.neulet.2008.09.012. [DOI] [PubMed] [Google Scholar]
- HOLICK MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- HOPPE CC. Inflammatory mediators of endothelial injury in sickle cell disease. Hematol Oncol Clin North Am. 2014;28:265–86. doi: 10.1016/j.hoc.2013.11.006. [DOI] [PubMed] [Google Scholar]
- HUANG DW, SHERMAN BT, LEMPICKI RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- JANSSENS W, DECRAMER M, MATHIEU C, KORF H. Vitamin D and chronic obstructive pulmonary disease: hype or reality? Lancet Respir Med. 2013;1:804–12. doi: 10.1016/S2213-2600(13)70102-4. [DOI] [PubMed] [Google Scholar]
- JOHNSON WE, LI C, RABINOVIC A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 2007;8:118–27. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- JOLLIFFE DA, WALTON RT, GRIFFITHS CJ, MARTINEAU AR. Single nucleotide polymorphisms in the vitamin D pathway associating with circulating concentrations of vitamin D metabolites and non-skeletal health outcomes: Review of genetic association studies. J Steroid Biochem Mol Biol. 2016;164:18–29. doi: 10.1016/j.jsbmb.2015.12.007. [DOI] [PubMed] [Google Scholar]
- LEE MT, LICURSI M, MCMAHON DJ. Vitamin D deficiency and acute vaso-occlusive complications in children with sickle cell disease. Pediatr Blood Cancer. 2015;62:643–7. doi: 10.1002/pbc.25399. [DOI] [PubMed] [Google Scholar]
- MELAMED ML, MICHOS ED, POST W, ASTOR B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Archives of internal medicine. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORITA K, MOTOYAMA N, KITAYAMA T, MORIOKA N, KIFUNE K, DOHI T. Spinal antiallodynia action of glycine transporter inhibitors in neuropathic pain models in mice. J Pharmacol Exp Ther. 2008;326:633–45. doi: 10.1124/jpet.108.136267. [DOI] [PubMed] [Google Scholar]
- NOLAN VG, NOTTAGE KA, COLE EW, HANKINS JS, GURNEY JG. Prevalence of vitamin d deficiency in sickle cell disease: a systematic review. PLoS One. 2015;10:e0119908. doi: 10.1371/journal.pone.0119908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSUNKWO I, HODGMAN EI, CHERRY K, DAMPIER C, ECKMAN J, ZIEGLER TR, OFORI-ACQUAH S, TANGPRICHA V. Vitamin D deficiency and chronic pain in sickle cell disease. Br J Haematol. 2011;153:538–40. doi: 10.1111/j.1365-2141.2010.08458.x. [DOI] [PubMed] [Google Scholar]
- OSUNKWO I, ZIEGLER TR, ALVAREZ J, MCCRACKEN C, CHERRY K, OSUNKWO CE, OFORI-ACQUAH SF, GHOSH S, OGUNBOBODE A, RHODES J, ECKMAN JR, DAMPIER C, TANGPRICHA V. High dose vitamin D therapy for chronic pain in children and adolescents with sickle cell disease: results of a randomized double blind pilot study. Br J Haematol. 2012;159:211–5. doi: 10.1111/bjh.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWE CE, EVANS MK, WENGER J, ZONDERMAN AB, BERG AH, NALLS M, TAMEZ H, ZHANG D, BHAN I, KARUMANCHI SA, POWE NR, THADHANI R. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369:1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRYTULA A, WALLE JV, VAN VLIERBERGHE H, KAUFMAN JM, FIERS T, DEHOORNE J, RAES A. Factors associated with 1,25-dihydroxyvitamin D3 concentrations in liver transplant recipients: a prospective observational longitudinal study. Endocrine. 2016;52:93–102. doi: 10.1007/s12020-015-0757-9. [DOI] [PubMed] [Google Scholar]
- RAMAGOPALAN SV, HEGER A, BERLANGA AJ, MAUGERI NJ, LINCOLN MR, BURRELL A, HANDUNNETTHI L, HANDEL AE, DISANTO G, ORTON SM, WATSON CT, MORAHAN JM, GIOVANNONI G, PONTING CP, EBERS GC, KNIGHT JC. A ChIP-seq defined genome-wide map of vitamin D receptor binding: associations with disease and evolution. Genome Res. 2010;20:1352–60. doi: 10.1101/gr.107920.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADAT-ALI M, AL-ELQ A, AL-TURKI H, SULTAN O, AL-ALI A, ALMULHIM F. Vitamin D level among patients with sickle cell anemia and its influence on bone mass. Am J Hematol. 2011;86:506–7. doi: 10.1002/ajh.22010. [DOI] [PubMed] [Google Scholar]
- SAFADI FF, THORNTON P, MAGIERA H, HOLLIS BW, GENTILE M, HADDAD JG, LIEBHABER SA, COOKE NE. Osteopathy and resistance to vitamin D toxicity in mice null for vitamin D binding protein. J Clin Invest. 1999;103:239–51. doi: 10.1172/JCI5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEMPER M, SMITH TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–6. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- SCHOTTKER B, BRENNER H. Vitamin D as a Resilience Factor, Helpful for Survival of Potentially Fatal Conditions: A Hypothesis Emerging from Recent Findings of the ESTHER Cohort Study and the CHANCES Consortium. Nutrients. 2015;7:3264–78. doi: 10.3390/nu7053264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOTTKER B, HAUG U, SCHOMBURG L, KOHRLE J, PERNA L, MULLER H, HOLLECZEK B, BRENNER H. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97:782–93. doi: 10.3945/ajcn.112.047712. [DOI] [PubMed] [Google Scholar]
- SCHOTTKER B, JORDE R, PEASEY A, THORAND B, JANSEN EH, GROOT L, STREPPEL M, GARDINER J, ORDONEZ-MENA JM, PERNA L, WILSGAARD T, RATHMANN W, FESKENS E, KAMPMAN E, SIGANOS G, NJOLSTAD I, MATHIESEN EB, KUBINOVA R, PAJAK A, TOPOR-MADRY R, TAMOSIUNAS A, HUGHES M, KEE F, BOBAK M, TRICHOPOULOU A, BOFFETTA P, BRENNER H. Vitamin D and mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. doi: 10.1136/bmj.g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAO B, JIANG S, MUYIDULI X, WANG S, MO M, LI M, WANG Z, YU Y. Vitamin D pathway gene polymorphisms influenced vitamin D level among pregnant women. Clin Nutr. 2017 Nov 7; doi: 10.1016/j.clnu.2017.10.024. pii: S0261-5614(17)31397-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- SHIPTON EE, SHIPTON EA. Vitamin D Deficiency and Pain: Clinical Evidence of Low Levels of Vitamin D and Supplementation in Chronic Pain States. Pain Ther. 2015;4:67–87. doi: 10.1007/s40122-015-0036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGNORELLO LB, HAN X, CAI Q, COHEN SS, COPE EL, ZHENG W, BLOT WJ. A prospective study of serum 25-hydroxyvitamin d levels and mortality among African Americans and non-African Americans. American journal of epidemiology. 2013;177:171–9. doi: 10.1093/aje/kws348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIGNORELLO LB, SHI J, CAI Q, ZHENG W, WILLIAMS SM, LONG J, COHEN SS, LI G, HOLLIS BW, SMITH JR, BLOT WJ. Common variation in vitamin D pathway genes predicts circulating 25-hydroxyvitamin D Levels among African Americans. PLoS One. 2011;6:e28623. doi: 10.1371/journal.pone.0028623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SNYDER M, HE W, ZHANG JJ. The DNA replication factor MCM5 is essential for Stat1-mediated transcriptional activation. Proc Natl Acad Sci U S A. 2005;102:14539–44. doi: 10.1073/pnas.0507479102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUCCAR R, MITCHELL VA, VAUGHAN CW. Actions of N-arachidonyl-glycine in a rat inflammatory pain model. Mol Pain. 2007;3:24. doi: 10.1186/1744-8069-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERDON Q, BOONEN M, RIBES C, JADOT M, GASNIER B, SAGNE C. SNAT7 is the primary lysosomal glutamine exporter required for extracellular protein-dependent growth of cancer cells. Proc Natl Acad Sci U S A. 2017;114:E3602–E3611. doi: 10.1073/pnas.1617066114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG D, LI Q, LI Y, WANG H. The role of MCM5 expression in cervical cancer: Correlation with progression and prognosis. Biomed Pharmacother. 2018;98:165–172. doi: 10.1016/j.biopha.2017.12.006. [DOI] [PubMed] [Google Scholar]
- WANG L, SONG Y, MANSON JE, PILZ S, MARZ W, MICHAELSSON K, LUNDQVIST A, JASSAL SK, BARRETT-CONNOR E, ZHANG C, EATON CB, MAY HT, ANDERSON JL, SESSO HD. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012a;5:819–29. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Z, LIN YS, ZHENG XE, SENN T, HASHIZUME T, SCIAN M, DICKMANN LJ, NELSON SD, BAILLIE TA, HEBERT MF, BLOUGH D, DAVIS CL, THUMMEL KE. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol. 2012b;81:498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WANG Z, SCHUETZ EG, XU Y, THUMMEL KE. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. J Steroid Biochem Mol Biol. 2013;136:54–8. doi: 10.1016/j.jsbmb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WYKES C, ARASARETNAM A, O’DRISCOLL S, FARNHAM L, MONIZ C, REES DC. Vitamin D deficiency and its correction in children with sickle cell anaemia. Ann Hematol. 2014;93:2051–6. doi: 10.1007/s00277-014-2144-7. [DOI] [PubMed] [Google Scholar]
- YIN K, AGRAWAL DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YU XP, HUSTMYER FG, GARVEY WT, MANOLAGAS SC. Demonstration of a 1,25-dihydroxyvitamin D3-responsive protein in human lymphocytes: immunologic crossreactivity and inverse regulation with the vitamin D receptor. Proc Natl Acad Sci U S A. 1991;88:8347–51. doi: 10.1073/pnas.88.19.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZANETTI M, HARRIS SS, DAWSON-HUGHES B. Ability of vitamin D to reduce inflammation in adults without acute illness. Nutr Rev. 2014;72:95–8. doi: 10.1111/nure.12095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.