The Beginning
This review highlights milestones leading to the discovery that 20-hydroxyeicosatetraenoic acid (20-HETE) plays a critical role in the regulation of renal function, vascular tone, and the development of hypertension and cardiovascular disease. Our interest in this pathway emerged 30 years ago. At that time, we were studying mechanisms of pressure natriuresis and factors that reset this response in hypertension. The results indicated that pressure natriuresis is associated with elevations in renal medullary blood flow and interstitial pressure that inhibit sodium transport in the proximal tubule (PT) and thin descending loop of Henle.1 The pressure-natriuretic response was shifted to higher pressures in spontaneously hypertensive rat (SHR) and Dahl salt-sensitive (S) rats. Resetting of the response in SHR was due to elevated renal vascular resistance. Renal hemodynamics were relatively normal in Dahl S rats, and the blunted natriuretic response was due to elevated sodium transport in the thick ascending loop of Henle (TALH).2 However, the factors that reset this relationship were unknown.
As presented in Figure 1, we were intrigued with the finding that arachidonic acid (AA) could be metabolized by renal cytochrome P450 (CYP) enzymes to 20-HETE.3,4 Prior to this, only cyclooxygenase and lipoxygenase enzymes were known to metabolize AA, and the CYP enzymes responsible for ω-hydroxylation of fatty acids were thought to be only expressed in the liver. Iwai et al. then reported that Cyp4a2 mRNA that produces 20-HETE is differentially expressed in the kidney of Wistar Kyoto and SHR,5 and Sacerdoti et al. found that synthesis of 20-HETE in the kidney was elevated in SHR.6 This was followed by a seminal report that treating SHR with SnCl2 reduced renal 20-HETE and attenuated hypertension.7 However, subsequent studies suggested that the observed fall in blood pressure might be due to induction of the heme oxygenase-carbon monoxide system that can dilate vessels via mechanisms in addition to inhibition of 20-HETE.8
Figure 1.
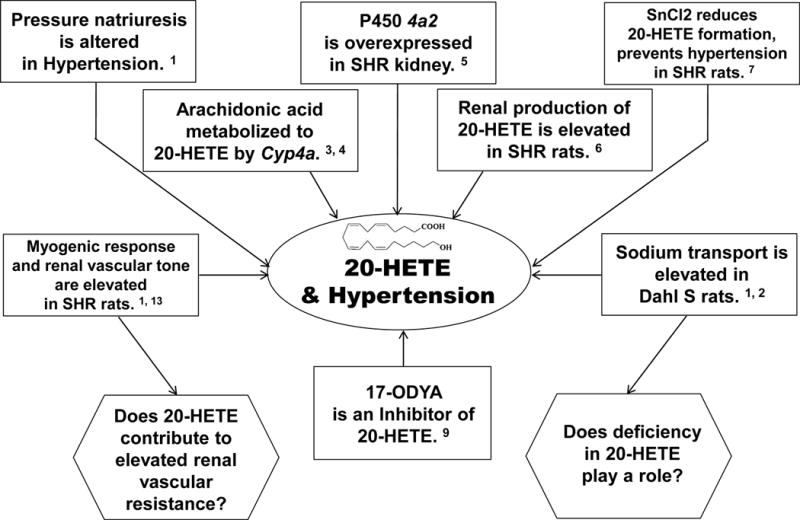
Initial discoveries implicating 20-HETE in the development of hypertension in the spontaneously hypertensive rat (SHR) and Dahl salt-sensitive (S) rats.
Serendipity and the fertile research environment at the Medical College of Wisconsin provided us the opportunity to study the role of CYP metabolites of AA in controlling renal tubular and vascular functions. We were working with Dr. Bettie Sue Masters characterizing the effects of new suicide substrate inhibitors and found that 17-octadecynoic acid (17-ODYA) inhibited formation of 20-HETE.9 This provided a tool to determine if 20-HETE promotes hypertension in SHR by altering vascular tone or the renal handling of sodium. We found that 20-HETE was produced by microsomes prepared from dog renal arterioles and that 20-HETE was a potent constrictor of these vessels.10 CYP inhibitors reduced myogenic tone in these vessels.11 In collaboration with David Harder, we found that the vasoconstrictor response to 20-HETE was associated with blockade of the large conductance potassium channel, membrane depolarization, and increase in intracellular calcium concentration.10,12 Follow-up studies indicated that 20-HETE production was elevated in the kidney and renal microvessels of SHR, which was associated with increased myogenic tone in the afferent arteriole that was normalized by inhibitors of 20-HETE formation.13 In contrast, 20-HETE synthesis was reduced in the kidney of Dahl S rats.14 These findings led to the hypothesis (Figure 1) that elevated renal vascular production of 20-HETE contributes to hypertension in SHR by resetting the pressure natriuretic relationship secondary to elevated renal vascular tone, while a deficiency in formation of 20-HETE attenuates pressure natriuresis in Dahl S rats by enhancing tubular sodium reabsorption.
20-HETE Effects on Renal and Vascular Functions
These initial findings triggered a remarkable series of discoveries highlighted in the timeline presented in Figure 2. Elevations in transmural pressure were found to increase formation of 20-HETE in cerebral arteries.15 Blockade of 20-HETE diminished myogenic tone in renal and cerebral arteries11,15–18 and autoregulation of renal and cerebral blood flow.15,17,19 The formation of 20-HETE in blood vessels is increased by angiotensin II (ANG II),20,21 endothelin,22 and serotonin.23 20-HETE inhibitors attenuated the vasoconstrictor responses to these agonists.24 20-HETE was shown to increase vascular tone by activating protein kinase C, mitogen-activated protein kinases, tyrosine kinases, Rho kinase,24 and promote Ca2+ influx by depolarizing the cell membrane secondary to blockade of the large conductance calcium sensitive potassium channel.10,12 20-HETE also increases conductance of the L-type calcium channel25 and activates the transient receptor potential canonical 6 channels.24 The production of 20-HETE is inhibited by nitric oxide, carbon monoxide, and superoxide that bind to heme in the catalytic site of CYP4A enzymes.24,26,27 The subsequent fall in 20-HETE levels mediates the cGMP-independent effects of nitric oxide to activate K+ channels and reduce vascular tone.27
Figure 2.
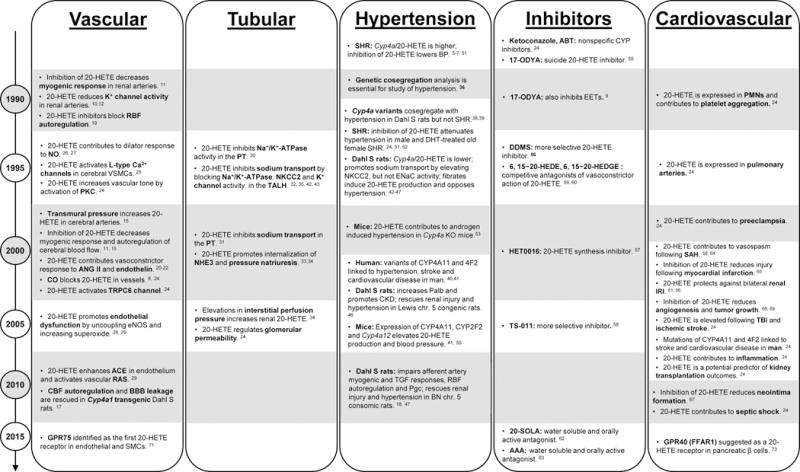
Timeline highlighting milestones leading to the discovery that 20-HETE plays a critical role in the regulation of renal function, vascular tone, hypertension and cardiovascular diseases
Increases in vascular 20-HETE production are associated with endothelial dysfunction in several models of hypertension. These include rats treated with a Cyp4a2 adenovirus, SHR, androgen-induced hypertensive rats, and Cyp4a12 transgenic and Cyp4a14 KO mice in which production of 20-HETE is elevated.24,28 20-HETE promotes endothelial dysfunction by uncoupling endothelial nitric oxide synthase and increasing formation of superoxide.28 More recent studies indicate that increases in vascular 20-HETE also activate the vascular renin-angiotensin system by increasing endothelial expression of angiotensin-converting enzyme.29
20-HETE is also a natriuretic agent (Figure 2) that inhibits Na+/K+-ATPase activity and sodium transport in the PT,30,31 as well as in the TALH.32 Elevations in renal perfusion pressure increase renal 20-HETE, which promotes internalization of the sodium–hydrogen exchanger 3 in the PT and pressure natriuresis.33,34 20-HETE inhibits sodium transport in the TALH by blocking the basolateral Na+/K+-ATPase and luminal Na+-K+-2Cl− cotransporter,32 as well as the luminal 70 pS K+ channel responsible for K+ backleak that sustains cotransporter activity.35
20-HETE and Hypertension
The advent of the genomic era transformed the field of hypertension. In 1991, J. Rapp argued that it was unacceptable to conclude that a gene plays a causal role in hypertension based on differential gene expression or blood pressure responses to agonists or antagonists in normotensive and hypertensive inbred strains.36 He proposed that genetic cosegregation analysis was necessary to establish or reject a causal role for a gene in hypertension. Working with Howard Jacob, who had just discovered that simple sequence length polymorphisms were common in rats,37 we developed polymorphic markers for Cyp4a2 from published sequences. We found that expression of CYP4A protein and renal formation of 20-HETE was reduced in Dahl S versus Lewis rats and elevated in SHR versus Brown Norway (BN) rats. Cyp4a2 genotype was associated with hypertension in an F2 cross of Dahl S and Lewis rats fed a high salt diet, but not in a cross of SHR and BN rats fed a normal salt diet.38,39 Inactivating variants in the coding region of CYP4A11 and 4F2 genes that produce 20-HETE in man were subsequently linked to hypertension, stroke, and cardiovascular disease in human genetic association studies.40,41
Expression of CYP4A protein and renal formation of 20-HETE in the kidney, and renal and cerebral vasculature is reduced in Dahl S rats relative to other strains. Dahl S rats do not increase renal epoxyeicosatrienoic acid levels in response to a high salt diet.41 The impaired pressure natriuretic response in Dahl S rats is mediated by elevated sodium transport in the TALH42,43 and epithelial sodium channel activity in the cortical collecting duct.44 Administration of 20-HETE normalized Na+-K+-2Cl− transport in the TALH; however, the elevated sodium transport in the collecting duct was related to decreased formation of epoxyeicosatrienoic acids. Induction of renal 20-HETE and EETs with fibrates45 or transfer of wild-type Cyp4a alleles from Lewis or BN rats increased renal 20-HETE, improved pressure natriuresis, and opposed hypertension and renal injury in consomic and congenic strains46,47 Vascular 20-HETE production is also reduced in Dahl S rats and this was associated with impaired myogenic and tubuloglomerular feedback responses in the afferent arteriole, autoregulation of renal blood flow and glomerular capillary pressure,18 increased albumin permeability of the glomerulus,46 and the development of proteinuria and kidney disease. Transfer of wild-type Cyp4a genes in chromosome 5 consomic and congenic Dahl S rats restored the myogenic response of the renal microcirculation and attenuated proteinuria and renal injury.46,47
While the original genetic cosegregation analysis excluded mutations in Cyp4a genes as a genetic cause of hypertension in SHR, considerable evidence now indicates that vascular 20-HETE serves as a downstream effector to raise blood pressure in various models. In this regard, renal and vascular 20-HETE production is elevated in SHR, and ANG II- and androgen-induced hypertensive rodents.24,41,48–50 20-HETE inhibitors attenuate hypertension in male51 and post-menopausal female SHR52 and in rats treated with dihydrotestosterone (DHT), which increases 20-HETE production.49 20-HETE inhibitors lower pressure in ANG II- and endothelin-induced hypertensive models.24,41 Expression of human CYP4A11 and CYP4F2 genes in transgenic mice increases 20-HETE production and blood pressure.40,41,50 Knockout of Cyp4a14, increases expression of Cyp4a12 and 20-HETE formation and produces salt-resistant hypertension in males secondary to raising testosterone levels.53,54 These effects are reversed by castration or 20-HETE inhibitors. Increased 20-HETE levels in a conditional Cyp4a12 transgenic mouse also produced hypertension.54 The hypertension is associated with elevated vascular reactivity to phenylephrine, increased oxidative stress, and endothelial dysfunction, all of which were reversed by a 20-HETE antagonist.54
A summary of the role of 20-HETE in hypertension is presented in Figure 3. Decreased renal formation of 20-HETE in Dahl S rats and patients with inactivating mutations in CYP4A11 and CYP4F2 promote salt-sensitive hypertension. This is associated with impaired myogenic and TGF responses in the afferent arteriole of Dahl S rats, which increases glomerular capillary pressure and renal injury that sustains hypertension. Autoregulation of cerebral blood flow is also impaired in Dahl S rats and this is associated with blood brain barrier leakage.17 Upregulation of the production of 20-HETE rescued cerebrovascular dysfunction in a Cyp4a1 transgenic Dahl S rat, suggesting that deficiencies in the formation of 20-HETE may contribute to loss of cognitive function with hypertension and aging.17
Figure 3.
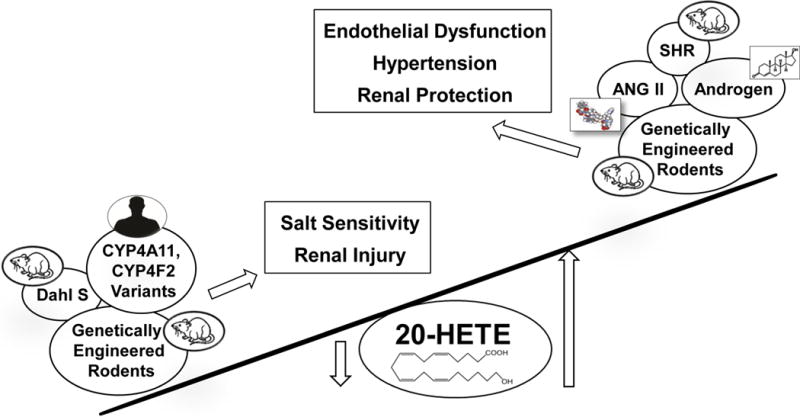
Role of 20-HETE in genetic and experimental models of hypertension. The renal and vascular production of 20-HETE is altered in various genetic and experimental models of hypertension and in CYP4A11, CYP4F2 transgenic and Cyp4a transgenic and KO rodents. In general, elevations in the vascular formation of 20-HETE increase renal and peripheral vascular resistance and produce salt-insensitive forms of hypertension, while decreases in renal 20-HETE increase sodium transport and promote salt-sensitive hypertension.
On the other hand, renal and vascular 20-HETE production are elevated in SHR, ANG II- and androgen-induced hypertensive rodents, and in Cyp4a14 knockout and Cyp4a12 and CYP4F2 transgenic mice. These models develop hypertension associated endothelial dysfunction and elevated vascular reactivity. The increase in renal vascular resistance likely shifts the pressure-natriuretic relationship to higher pressures. Increased 20-HETE levels in the kidney may also inhibit sodium reabsorption in the proximal tubule which can help explain the lack of salt-sensitivity of blood pressure in these models. The elevated renal vascular resistance that protects the glomerulus from increases in systemic pressure, likely explains the resistance to proteinuria and renal injury.
20-HETE Inhibitors and Antagonists
Ketoconazole, miconazole and 1-aminobenzotriazole, and other nonspecific agents were the first inhibitors used to reduce 20-HETE in early studies. However, they also inhibit formation of EETs and other CYP enzymes involved in drug metabolism. 17-ODYA was developed as a specific suicide inhibitor of 20-HETE formation,55 but this compound also inhibits formation of EETs.9 Dibromo-dodecenyl-methylsulfimide (DDMS) is a more selective 20-HETE inhibitor, but like 17-ODYA, it binds to plasma proteins, is not very soluble or useful for chronic studies.56
A major advance was the discovery of formamide derivatives (HET0016, and TS-011) as potent and highly selective inhibitors of 20-HETE formation.57,58 HET0016 is commercially available and remains the most widely used agent to block synthesis of 20-HETE. TS-011 is a more potent, however, the pharmacokinetic properties of these inhibitors are not favorable, which prevent their development as therapeutic agents. Dr. John Falck synthesized a number of 20-HETE analogs for structure-activity studies. The screening of these compounds revealed that some competitively blocked the vasoconstrictor actions of 20-HETE, while other analogs were stable agonists.59,60 This led to the emergence of 20-hydroxyeicosa-6(Z), 15(Z)-dienoic acid (6, 15−20-HEDE) and N-(20-hydroxyeicosa-6(Z), 15(Z)-dienoyl) glycine (6, 15−20-HEDGE) as competitive antagonists of vasoconstrictor actions of 20-HETE.61 However, these compounds are also highly bound to proteins and have a short half-life, which restricts their use to in vitro studies. More recently, Dr. Falck created new water-soluble 20-HETE antagonists, 2,5,8,11,14,17- hexaoxanonadecan-19-yl-20-hydroxyeicosa 6(Z), 15(Z)-dienoate (20-SOLA) and 6(Z),15(Z)-hydroxyeicoas-6,15-dienamido-diencoic acid (AAA).62,63 These compounds lowered blood pressure when given in drinking water to 20-HETE dependent hypertensive mouse models.
20-HETE in Cardiovascular Diseases
The availability of HET0016 and TS-011 fostered numerous studies on the role of 20-HETE in various diseases, including ischemic and hemorrhagic strokes, acute renal failure, toxemia of pregnancy, hepatorenal syndrome, vascular restenosis, angiogenesis, cardiac hypertrophy, myocardial infarction, renal ischemia-reperfusion injury, and shock.24,41 20-HETE is a potent constrictor of cerebral arteries that mimic vasospasm associated with subarachnoid hemorrhage (SAH).24 Clinical studies indicating that the levels of 20-HETE in cerebrospinal fluid are elevated in patients and experimental animals following SAH.64 Inhibition of the synthesis of 20-HETE prevented the fall in cerebral blood flow following SAH in rats and reversed delayed vasospasm in rats and dogs.58,64
20-HETE also plays a role in ischemic stroke. Levels of 20-HETE are elevated in cerebral tissue and plasma of patients and rats following cerebral ischemia. 20-HETE synthesis inhibitors reduce infarct size and improve neurological outcomes.64 Mutations in CYP4F2 and CYP4A11 have also been linked to an increased incidence of ischemic stroke in Chinese, Swedish, and Japanese populations.24,40
Other studies indicated that the concentration of 20-HETE in coronary venous blood is elevated following cardiac ischemia. Blockade of the synthesis of 20-HETE or administration of a 20-HETE antagonist reduced infarct size and potentiated the cardioprotective effects of ischemic preconditioning.65 These findings indicate that inhibitors of 20-HETE may be useful to reduce injury following myocardial infarction.
Several reports addressed the role of 20-HETE in renal injury. Blockade of 20-HETE with HET0016 increased, while administration of 20-HETE agonists (5,14-20-HEDGE and 5,14-20-HEDE) reduced bilateral renal ischemic injury.61 A recent study in Dahl S rats, in which the formation of 20-HETE is reduced, indicated that they are much more susceptible to renal ischemic injury than other strains. Transferring of the CYP4A genes on chromosome 5 from BN rats into the Dahl S genetic background normalized production of 20-HETE and susceptibility to renal injury.66
Formation of 20-HETE in carotid arteries was elevated following balloon injury, and chronic administration of HET0016 or other inhibitors of 20-HETE greatly reduced proliferation and migration of smooth muscle and neointimal formation.67 This suggests that stents or catheters coated with 20-HETE inhibitors may oppose restenosis. 20-HETE also induces angiogenesis. Inhibition of 20-HETE formation reduced corneal neovascularization produced by several angiogenic growth factors and following implantation of cancer cells.68 Subsequent studies confirming that 20-HETE inhibitors reduced the vascularization and growth of rat 9L and human U251 glioma tumors in the brain69 and other cancers has sparked interest in 20-HETE inhibitors as cancer chemotherapy agents.70
20-HETE Receptors
The existence of 20-HETE receptors was foretold by the discovery that inactive analogs of 20-HETE are antagonists of its vasoconstrictor actions.60 Other studies indicating that the vasoconstrictor effects of 20-HETE are phospholipase C/PKC dependent, and its effects on cell migration and proliferation, endothelial dysfunction, and inflammation are c-Src and MAPK pathway dependent, suggest that 20-HETE may act via a G-protein–coupled receptor (GPR).24 Garcia et al. recently reported that 20-HETE increases vascular tone and promotes endothelial dysfunction by activating a chemokine, RANTES/CCL5, G-protein receptor (GPR75) that signals through the Gαq/11/PLC/PKC and c-Src/EGFR pathways.71 It is the first demonstration that a member of this class of eicosanoids acts via a GPR. Activation of this receptor by RANTES was reported to protect the hippocampus from β-amyloid toxicity and to stimulate insulin secretion in pancreatic islet cells.72 More recently, high concentrations of 20-HETE has been reported to be a potent activator of a the long-chain fatty acid receptor (FFAR1, GPR40) to promote glucose-mediated insulin secretion.73 However, other studies indicated that 20-HETE inhibits promotes hyperglycemia in CYP4F2 transgenic mice by inhibiting insulin secretion and enhancing glycogenolysis.74,75 Overall, the new receptor data suggest that other 20-HETE receptors that mediate its multiple actions are yet to be discovered, analogous to multiple receptors that mediate the effects of prostaglandins.
Perspectives
CYPs of the 4A and 4F pathways and 20-HETE play an important role in hypertension and cardiovascular disease. CYP4A or CYP4F enzymes are highly expressed in vascular smooth muscle cells in small arteries and arterioles (<200 μm), the glomerulus, proximal tubule and thick ascending loop of Henle in the kidney, and the heart, liver, prostate, lung and pancreatic islet cells.24,41 The levels of 20-HETE are altered in numerous disease states. 20-HETE acts as a vasoconstrictor that promotes the myogenic response and autoregulation of RBF and CBF, and inhibits sodium reabsorption in the proximal tubule and thick ascending loop of Henle.24,41 20-HETE also serves as a second messenger in the regulation of endothelial dysfunction,49,50 vascular inflammation,50 angiogenesis, restenosis, and ischemic injury in the brain, heart, and kidney24 (Figure 4). Sequence variants in the genes that reduce the production of 20-HETE are associated with hypertension in man.40,41 GPR75 has recently been identified as the first 20-HETE receptor,71 and very selective synthesis inhibitors and orally active receptor antagonists are now available (Figure 4). The available data suggests the 20-HETE pathway is a drugable target for the treatment of indications, such as vascular restenosis, cerebral vasospasm and ischemic stroke, myocardial infarction, and cancer in which the duration of drug administration would be limited. However, given its myriad of actions, it remains to be determined whether 20-HETE inhibitors or antagonists could be safely used to treat chronic diseases, such as hypertension.
Figure 4.
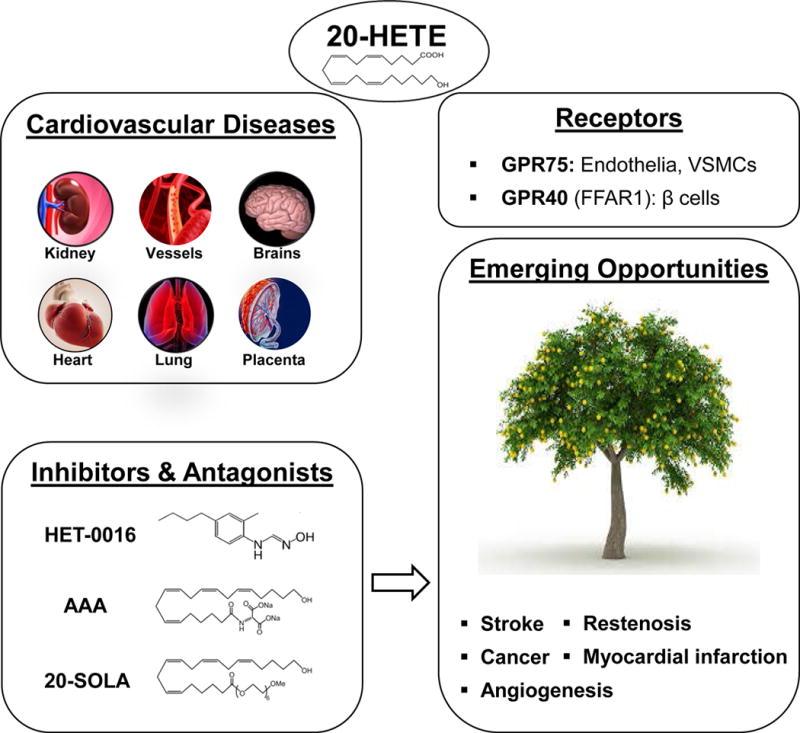
Emerging opportunities for 20-HETE inhibitors and antagonists in the treatment of cardiovascular disease and cancer.
Acknowledgments
The authors thank Dr. George Booz, PhD. for his valuable comments.
Sources of Funding: This study was supported by grants HL36279, HL138685 and DK104184 (Roman), AG050049 (Fan) and P20GM104357 (Roman and Fan) from the NIH and a grant 16GRNT31200036 (Fan) from the American Heart Association.
Footnotes
Disclosures
None.
References
- 1.Roman RJ. Alterations in renal medullary hemodynamics and the pressure-natriuretic response in genetic hypertension. Am J Hypertens. 1990;3:893–900. doi: 10.1093/ajh/3.11.893. [DOI] [PubMed] [Google Scholar]
- 2.Roman RJ, Kaldunski ML. Enhanced chloride reabsorption in the loop of Henle in Dahl salt-sensitive rats. Hypertension. 1991;17:1018–1024. doi: 10.1161/01.hyp.17.6.1018. [DOI] [PubMed] [Google Scholar]
- 3.Schwartzman ML, Abraham NG, Carroll MA, Levere RD, McGiff JC. Regulation of arachidonic acid metabolism by cytochrome P-450 in rabbit kidney. Biochem J. 1986;238:283–290. doi: 10.1042/bj2380283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lapuerta L, Chacos N, Falck JR, Jacobson H, Capdevila JH. Renal microsomal cytochrome P-450 and the oxidative metabolism of arachidonic acid. Am J Med Sci. 1988;295:275–279. doi: 10.1097/00000441-198804000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Iwai N, Inagami T. Isolation of preferentially expressed genes in the kidneys of hypertensive rats. Hypertension. 1991;17:161–169. doi: 10.1161/01.hyp.17.2.161. [DOI] [PubMed] [Google Scholar]
- 6.Sacerdoti D, Abraham NG, McGiff JC, Schwartzman ML. Renal cytochrome P-450-dependent metabolism of arachidonic acid in spontaneously hypertensive rats. Biochem Pharmacol. 1988;37:521–527. doi: 10.1016/0006-2952(88)90223-7. [DOI] [PubMed] [Google Scholar]
- 7.Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Kaide JI, Rodriguez-Mulero F, Abraham NG, Nasjletti A. Vasoregulatory function of the heme-heme oxygenase-carbon monoxide system. Am J Hypertens. 2001;14(6 Pt 2):62S–67S. doi: 10.1016/s0895-7061(01)02071-4. [DOI] [PubMed] [Google Scholar]
- 9.Zou AP, Ma YH, Sui ZH, Ortiz de Montellano PR, Clark JE, Masters BS, Roman RJ. Effects of 17-octadecynoic acid, a suicide-substrate inhibitor of cytochrome P450 fatty acid omega-hydroxylase, on renal function in rats. J Pharmacol Exp Ther. 1994;268:474–481. [PubMed] [Google Scholar]
- 10.Ma YH, Gebremedhin D, Schwartzman ML, Falck JR, Clark JE, Masters BS, Harder DR, Roman RJ. 20-Hydroxyeicosatetraenoic acid is an endogenous vasoconstrictor of canine renal arcuate arteries. Circ Res. 1993;72:126–136. doi: 10.1161/01.res.72.1.126. [DOI] [PubMed] [Google Scholar]
- 11.Kauser K, Clark JE, Masters BS, Ortiz de Montellano PR, Ma YH, Harder DR, Roman RJ. Inhibitors of cytochrome P-450 attenuate the myogenic response of dog renal arcuate arteries. Circ Res. 1991;68:1154–1163. doi: 10.1161/01.res.68.4.1154. [DOI] [PubMed] [Google Scholar]
- 12.Zou A-P, Fleming J, Falck J, Jacobs E, Gebremedhin D, Harder D, Roman R. 20-HETE is an endogenous inhibitor of the large-conductance Ca (2+)-activated K+ channel in renal arterioles. Am J of Physiol. 1996;270:R228–R237. doi: 10.1152/ajpregu.1996.270.1.R228. [DOI] [PubMed] [Google Scholar]
- 13.Imig JD, Falck JR, Gebremedhin D, Harder DR, Roman RJ. Elevated renovascular tone in young spontaneously hypertensive rats Role of cytochrome P-450. Hypertension. 1993;22:357–364. doi: 10.1161/01.hyp.22.3.357. [DOI] [PubMed] [Google Scholar]
- 14.Ma YH, Schwartzman ML, Roman RJ. Altered renal P-450 metabolism of arachidonic acid in Dahl salt-sensitive rats. Am J Physiol. 1994;267:R579–589. doi: 10.1152/ajpregu.1994.267.2.R579. [DOI] [PubMed] [Google Scholar]
- 15.Gebremedhin D, Lange AR, Lowry TF, Taheri MR, Birks EK, Hudetz AG, Narayanan J, Falck JR, Okamoto H, Roman RJ, Nithipatikom K, Campbell WB, Harder DR. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–65. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 16.Imig JD, Zou A, De Montellano PO, Sui Z, Roman RJ. Cytochrome P-450 inhibitors alter afferent arteriolar responses to elevations in pressure. Am J Physiol. 1994;266:H1879–H1885. doi: 10.1152/ajpheart.1994.266.5.H1879. [DOI] [PubMed] [Google Scholar]
- 17.Fan F, Geurts AM, Murphy SR, Pabbidi MR, Jacob HJ, Roman RJ. Impaired myogenic response and autoregulation of cerebral blood flow is rescued in CYP4A1 transgenic Dahl salt-sensitive rat. Am J Physiol. 2015;308:R379–390. doi: 10.1152/ajpregu.00256.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ge Y, Murphy SR, Lu Y, Falck J, Liu R, Roman RJ. Endogenously produced 20-HETE modulates myogenic and TGF response in microperfused afferent arterioles. Prostaglandins Other Lipid Mediat. 2013;102–103:42–48. doi: 10.1016/j.prostaglandins.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zou A, Imig JD, Kaldunski M, De Montellano PO, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol. 1994;266:F275–F282. doi: 10.1152/ajprenal.1994.266.2.F275. [DOI] [PubMed] [Google Scholar]
- 20.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol. 2002;283:R60–R68. doi: 10.1152/ajpregu.00664.2001. [DOI] [PubMed] [Google Scholar]
- 21.Fan F, Sun CW, Maier KG, Williams JM, Pabbidi MR, Didion SP, Falck JR, Zhuo J, Roman RJ. 20-Hydroxyeicosatetraenoic acid contributes to the inhibition of K+ channel activity and vasoconstrictor response to angiotensin II in rat renal microvessels. PLoS One. 2013;8:e82482. doi: 10.1371/journal.pone.0082482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hercule HC, Oyekan AO. Cytochrome P450 omega/omega-1 hydroxylase-derived eicosanoids contribute to endothelin(A) and endothelin(B) receptor-mediated vasoconstriction to endothelin-1 in the rat preglomerular arteriole. J Pharmacol Exp Ther. 2000;292:1153–1160. [PubMed] [Google Scholar]
- 23.Cambj-Sapunar L, Yu M, Harder DR, Roman RJ. Contribution of 5-hydroxytryptamine1B receptors and 20-hydroxyeiscosatetraenoic acid to fall in cerebral blood flow after subarachnoid hemorrhage. Stroke. 2003;34:1269–1275. doi: 10.1161/01.STR.0000065829.45234.69. [DOI] [PubMed] [Google Scholar]
- 24.Fan F, Ge Y, Lv W, Elliott MR, Muroya Y, Hirata T, Booz GW, Roman RJ. Molecular mechanisms and cell signaling of 20-hydroxyeicosatetraenoic acid in vascular pathophysiology. Front Biosci. 2016;21:1427–1463. doi: 10.2741/4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebremedhin D, Lange AR, Narayanan J, Aebly MR, Jacobs ER, Harder DR. Cat cerebral arterial smooth muscle cells express cytochrome P450 4A2 enzyme and produce the vasoconstrictor 20-HETE which enhances L-type Ca2+ current. J Physiol. 1998;507:771–781. doi: 10.1111/j.1469-7793.1998.771bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alonso-Galicia M, Sun CW, Falck JR, Harder DR, Roman RJ. Contribution of 20-HETE to the vasodilator actions of nitric oxide in renal arteries. Am J Physiol. 1998;275:F370–378. doi: 10.1152/ajprenal.1998.275.3.F370. [DOI] [PubMed] [Google Scholar]
- 27.Sun CW, Alonso-Galicia M, Taheri MR, Falck JR, Harder DR, Roman RJ. Nitric oxide-20-hydroxyeicosatetraenoic acid interaction in the regulation of K+ channel activity and vascular tone in renal arterioles. Circ Res. 1998;83:1069–79. doi: 10.1161/01.res.83.11.1069. [DOI] [PubMed] [Google Scholar]
- 28.Garcia V, Schwartzman ML. Recent developments on the vascular effects of 20-hydroxyeicosatetraenoic acid. Curr Opin Nephrol Hypertens. 2017;26:74–82. doi: 10.1097/MNH.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 29.Cheng J, Garcia V, Ding Y, Wu CC, Thakar K, Falck JR, Ramu E, Schwartzman ML. Induction of angiotensin-converting enzyme and activation of the renin-angiotensin system contribute to 20-hydroxyeicosatetraenoic acid-mediated endothelial dysfunction. Arterioscler Thromb Vasc Biol. 2012;32:1917–1924. doi: 10.1161/ATVBAHA.112.248344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nowicki S, Chen SL, Aizman O, Cheng XJ, Li D, Nowicki C, Nairn A, Greengard P, Aperia A. 20-Hydroxyeicosa-tetraenoic acid (20 HETE) activates protein kinase C. Role in regulation of rat renal Na+,K+-ATPase. J Clin Invest. 1997;99:1224–1230. doi: 10.1172/JCI119279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quigley R, Baum M, Reddy KM, Griener JC, Falck JR. Effects of 20-HETE and 19(S)-HETE on rabbit proximal straight tubule volume transport. Am J Physiol. 2000;278:F949–53. doi: 10.1152/ajprenal.2000.278.6.F949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu M, Lopez B, Dos Santos EA, Falck JR, Roman RJ. Effects of 20-HETE on Na+ transport and Na+-K+-ATPase activity in the thick ascending loop of Henle. Am Jl of Physiol. 2007;292:R2400–R2405. doi: 10.1152/ajpregu.00791.2006. [DOI] [PubMed] [Google Scholar]
- 33.Dos Santos EA, Dahly-Vernon AJ, Hoagland KM, Roman RJ. Inhibition of the formation of EETs and 20-HETE with 1-aminobenzotriazole attenuates pressure natriuresis. Am J Physiol. 2004;287:R58–68. doi: 10.1152/ajpregu.00713.2003. [DOI] [PubMed] [Google Scholar]
- 34.Williams JM, Sarkis A, Lopez B, Ryan RP, Flasch AK, Roman RJ. Elevations in renal interstitial hydrostatic pressure and 20-hydroxyeicosatetraenoic acid contribute to pressure natriuresis. Hypertension. 2007;49:687–694. doi: 10.1161/01.HYP.0000255753.89363.47. [DOI] [PubMed] [Google Scholar]
- 35.Wang W, Lu M. Effect of arachidonic acid on activity of the apical K+ channel in the thick ascending limb of the rat kidney. J Gen Physiol. 1995;106:727–743. doi: 10.1085/jgp.106.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapp JP. Disesecting the primary causes of genetic hypertension in rats. Hypertension. 1991;18(Suppl 3):18–28. doi: 10.1161/01.hyp.18.3_suppl.i18. [DOI] [PubMed] [Google Scholar]
- 37.Jacob HJ, Brown DM, Bunker RK, et al. A genetic linkage map of the laboratory rat, Rattus norvegicus. Nat Genet. 1995;9:63–69. doi: 10.1038/ng0195-63. [DOI] [PubMed] [Google Scholar]
- 38.Stec DE, Trolliet MR, Krieger JE, Jacob HJ, Roman RJ. Renal cytochrome P4504A activity and salt sensitivity in spontaneously hypertensive rats. Hypertension. 1996;27:1329–36. doi: 10.1161/01.hyp.27.6.1329. [DOI] [PubMed] [Google Scholar]
- 39.Stec DE, Deng AY, Rapp JP, Roman RJ. Cytochrome P4504A genotype cosegregates with hypertension in Dahl S rats. Hypertension. 1996;27:564–568. doi: 10.1161/01.hyp.27.3.564. [DOI] [PubMed] [Google Scholar]
- 40.Fava C, Ricci M, Melander O, Minuz P. Hypertension, cardiovascular risk and polymorphisms in genes controlling the cytochrome P450 pathway of arachidonic acid: A sex-specific relation? Prostaglandins Other Lipid Mediat. 2012;98:75–85. doi: 10.1016/j.prostaglandins.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Fan F, Roman RJ. Effect of Cytochrome P450 Metabolites of Arachidonic Acid in Nephrology. JASN. 2017;28:2845–2855. doi: 10.1681/ASN.2017030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zou A-P, Drummond HA, Roman RJ. Role of 20-HETE in elevating loop chloride reabsorption in Dahl SS/Jr rats. Hypertension. 1996;27:631–635. doi: 10.1161/01.hyp.27.3.631. [DOI] [PubMed] [Google Scholar]
- 43.Ito O, Roman RJ. Role of 20-HETE in elevating chloride transport in the thick ascending limb of Dahl SS/Jr rats. Hypertension. 1999;33:419–423. doi: 10.1161/01.hyp.33.1.419. [DOI] [PubMed] [Google Scholar]
- 44.Pavlov TS, Ilatovskaya DV, Levchenko V, Mattson DL, Roman RJ, Staruschenko A. Effects of cytochrome P-450 metabolites of arachidonic acid on the epithelial sodium channel (ENaC) Am J Physiol. 2011;301:F672–81. doi: 10.1152/ajprenal.00597.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alonso-Galicia M, Frohlich B, Roman RJ. Induction of P4504A activity improves pressure-natriuresis in Dahl S rats. Hypertension. 1998;31:232–236. doi: 10.1161/01.hyp.31.1.232. [DOI] [PubMed] [Google Scholar]
- 46.Williams JM, Sarkis A, Hoagland KM, Fredrich K, Ryan RP, Moreno C, Lopez B, Lazar J, Fenoy FJ, Sharma M, Garrett MR, Jacob HJ, Roman RJ. Transfer of the CYP4A region of chromosome 5 from Lewis to Dahl S rats attenuates renal injury. Am J Physiol. 2008;295:F1764–1777. doi: 10.1152/ajprenal.90525.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol. 2012;302:R1209–1218. doi: 10.1152/ajpregu.00604.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sodhi K, Wu CC, Cheng J, Gotlinger K, Inoue K, Goli M, Falck JR, Abraham NG, Schwartzman ML. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension. 2010;56:871–878. doi: 10.1161/HYPERTENSIONAHA.110.154559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev. 2014;22:1–12. doi: 10.1097/CRD.0b013e3182961659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garcia V, Schwartzman ML. Recent developments on the vascular effects of 20- hydroxyeicosatetraenoic acid. Curr Opin Nephrol Hypertens. 2017;26:74–82. doi: 10.1097/MNH.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 51.Su P, Kaushal KM, Kroetz DL. Inhibition of renal arachidonic acid omega-hydroxylase activity with ABT reduces blood pressure in the SHR. Am J Physiol. 1998;275:R426–38. doi: 10.1152/ajpregu.1998.275.2.R426. [DOI] [PubMed] [Google Scholar]
- 52.Yanes LL, Lima R, Moulana M, Romero DG, Yuan K, Ryan MJ, Baker R, Zhang H, Fan F, Davis DD. Postmenopausal hypertension: role of 20-HETE. Am J Physiol. 2011;300:R1543–R1548. doi: 10.1152/ajpregu.00387.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Holla VR, Adas F, Imig JD, Zhao X, Price E, Jr, Olsen N, Kovacs WJ, Magnuson MA, Keeney DS, Breyer MD, Falck JR, Waterman MR, Capdevila JH. Alterations in the regulation of androgen-sensitive Cyp 4a monooxygenases cause hypertension. Proc Natl Acad Sci. 2001;98:5211–5216. doi: 10.1073/pnas.081627898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR, Capdevila JH, Schwartzman ML. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12-20-HETE synthase. JASN. 2013;24:1288–1296. doi: 10.1681/ASN.2012070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ortiz de Montellano PR, Reich NO. Specific inactivation of hepatic fatty acid hydroxylases by acetylenic fatty acids. J Biol Chem. 1984;259:4136–4141. [PubMed] [Google Scholar]
- 56.Wang MH, Brand-Schieber E, Zand BA, Nguyen X, Falck JR, Balu N, Schwartzman ML. Cytochrome P450-derived arachidonic acid metabolism in the rat kidney:characterization of selective inhibitors. J Pharmacol Exp Ther. 1998;284:966–973. [PubMed] [Google Scholar]
- 57.Miyata N, Taniguchi K, Seki T, Ishimoto T, Sato-Watanabe M, Yasuda Y, Doi M, Kametani S, Tomishima Y, Ueki T, Sato M, Kameo K. HET0016, a potent and selective inhibitor of 20-HETE synthesizing enzyme. Br J Pharmacol. 2001;133:325–329. doi: 10.1038/sj.bjp.0704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyata N, Seki T, Tanaka Y, Omura T, Taniguchi K, Doi M, Bandou K, Kametani S, Sato M, Okuyama S, Cambj-Sapunar L, Harder DR, Roman RJ. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N′-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Therap. 2005;314:77–85. doi: 10.1124/jpet.105.083964. [DOI] [PubMed] [Google Scholar]
- 59.Alonso-Galicia M, Falck JR, Reddy KM, Roman RJ. 20-HETE agonists and antagonists in the renal circulation. Am J Physiol. 1999;277:F790–F796. doi: 10.1152/ajprenal.1999.277.5.F790. [DOI] [PubMed] [Google Scholar]
- 60.Yu M, Alonso-Galicia M, Sun CW, Roman RJ, Ono N, Hirano H, Ishimoto T, Reddy YK, Katipally KR, Reddy KM, Gopal VR,Yu J, Takhi M, Falck JR. 20-hydroxyeicosatetraenoic acid (20-HETE):structural determinants for renal vasoconstriction. Bioorg Med Chem. 2003;11:2803–21. doi: 10.1016/s0968-0896(03)00192-5. [DOI] [PubMed] [Google Scholar]
- 61.Regner KR, Zuk A, Van Why SK, Shames BD, Ryan RP, Falck JR, Manthati VL, McMullen ME, Ledbetter SR, Roman RJ. Protective effect of 20-HETE analogues in experimental renal ischemia reperfusion injury. Kidney Int. 2009;75:511–7. doi: 10.1038/ki.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pandey V, Garcia V, Gilani A, Mishra P, Zhang FF, Paudyal MP, Falck JR, Nasjletti A, Wang WH, Schwartzman ML. The Blood Pressure-Lowering Effect of 20-HETE Blockade in Cyp4a14(−/−) Mice Is Associated with Natriuresis. J Pharmacol Exp Ther. 2017;363:412–418. doi: 10.1124/jpet.117.243618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Savas U, Wei S, Hsu MH, Falck JR, Guengerich FP, Capdevila JH, Johnson EF. 20-Hydroxyeicosatetraenoic Acid (HETE)-dependent Hypertension in Human Cytochrome P450 (CYP) 4A11 Transgenic Mice. J Biol Chem. 2016;291:16904–16919. doi: 10.1074/jbc.M116.732297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roman RJ, Renic M, Dunn KM, Takeuchi K, Hacein-Bey L. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol Res. 2006;28:738–749. doi: 10.1179/016164106X152016. [DOI] [PubMed] [Google Scholar]
- 65.Nithipatikom K, Endsley MP, Moore JM, Isbell MA, Falck JR, Campbell WB, Gross GJ. Effects of selective inhibition of cytochrome P-450 omega-hydroxylases and ischemic preconditioning in myocardial protection. Am J Physiol. 2006;290:H500–505. doi: 10.1152/ajpheart.00918.2005. [DOI] [PubMed] [Google Scholar]
- 66.Muroya Y, Fan F, Regner KR, Falck JR, Garrett MR, Juncos LA, Roman RJ. Deficiency in the Formation of 20-Hydroxyeicosatetraenoic Acid Enhances Renal Ischemia-Reperfusion Injury. JASN. 2015;26:2460–2469. doi: 10.1681/ASN.2014090868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orozco LD, Liu H, Perkins E, Johnson DA, Chen BB, Fan F, Baker RC, Roman RJ. 20-Hydroxyeicosatetraenoic acid inhibition attenuates balloon injury-induced neointima formation and vascular remodeling in rat carotid arteries. J Pharmacol Exp Ther. 2013;346:67–74. doi: 10.1124/jpet.113.203844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen P, Guo M, Wygle D, Edwards PA, Falck JR, Roman RJ, Scicli AG. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am J Pathol. 2005;166:615–624. doi: 10.1016/S0002-9440(10)62282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo M, Roman RJ, Fenstermacher JD, Brown SL, Falck JR, Arbab AS, Edwards PA, Scicli AG. 9L gliosarcoma cell proliferation and tumor growth in rats are suppressed by N-hydroxy-N′-(4-butyl-2-methylphenol) formamidine (HET0016), a selective inhibitor of CYP4A. J Pharmacol Exp Ther. 2006;317:97–10. doi: 10.1124/jpet.105.097782. [DOI] [PubMed] [Google Scholar]
- 70.Johnson AL, Edson KZ, Totah RA, Rettie AE. Cytochrome P450 ω-Hydroxylases in Inflammation and Cancer. Adv Pharmacol. 2015;74:223–62. doi: 10.1016/bs.apha.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Garcia V, Gilani A, Shkolnik B, Pandey V, Zhang FF, Dakarapu R, Gandham SK, Reddy NR, Graves JP, Gruzdev A, Zeldin DC, Capdevila JH, Falck JR, Schwartzman ML. 20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension. Circ Res. 2017;120:1776–1788. doi: 10.1161/CIRCRESAHA.116.310525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fan F, Roman RJ. GPR75 Identified as the First 20-HETE Receptor: A Chemokine Receptor Adopted by a New Family. Circ Res. 2017;120:1696–1698. doi: 10.1161/CIRCRESAHA.117.311022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tunaru S, Bonnavion R, Brandenburger I, Preussner J, Thomas D, Scholich K, Offermanns S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat Commun. 2018 Jan 12;9(1):177. doi: 10.1038/s41467-017-02539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lai G, Wu J, Liu X, Zhao Y. 20-HETE induces hyperglycemia through thecAMP/PKA-PhK-GP pathway. Mol Endocrinol. 2012;26:1907–1916. doi: 10.1210/me.2012-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang B, Lai G, Wu J, Sun R, Xu R, Yang X, Qi Y, Zhao Y. 20-HETE attenuates the response of glucose-stimulated insulin secretion through the AKT/GSK-3β/Glut2 pathway. Endocrine. 2016;54:371–382. doi: 10.1007/s12020-016-1031-5. [DOI] [PubMed] [Google Scholar]


