Abstract
Purpose
The role of appropriate therapy in breast cancer survival and survival disparities by race/ethnicity have not been fully elucidated. We investigated whether guideline-inconsistent therapy contributed to survival differences overall and among Hispanics relative to non-Hispanic white (NHW) women in a case-cohort study.
Methods
This study included a 15% random sample of female invasive breast cancer patients diagnosed from 1997–2009 in 6 New Mexico counties and all deaths due to breast cancer-related causes. Information was obtained from comprehensive medical chart reviews. National Comprehensive Cancer Network (NCCN®) guideline-consistent treatment was assessed among white women aged < 70 who were free of contraindications for recommended therapy, had stage I–III tumors, and had survived at least 12 months. Hazard ratios (HRs) and 95% confidence intervals (CIs) for breast cancer death were estimated using Cox proportional hazards models.
Results
The median survival was 101 months. The included women represented 4635 patients and 449 breast cancer deaths. Women who met specific NCCN treatment criteria but did not receive radiotherapy (HR 2.3; 95% CI 1.2–4.4) or endocrine therapy (HR 2.0; 95% CI 1.0–4.0) had an increased risk of breast cancer death relative to those who did receive these therapies. Guideline-consistent therapy receipt did not differ between Hispanic and NHW women for chemotherapy (84.2% vs. 81.3%, respectively), radiotherapy (89.2% vs. 91.1%, respectively) or endocrine therapy (89.2% vs. 85.8%, respectively), and it did not influence Hispanic survival disparities.
Conclusions
Guideline-concordant receipt of radiotherapy and endocrine therapy contributed to survival as strongly as other established prognostic indicators. Hispanic survival disparities in this population do not appear to be attributable to treatment differences.
Keywords: Breast Neoplasms, Guideline Adherence, Hispanic Americans, Healthcare Disparities, Survival Analysis
Introduction
Breast cancer treatment guidelines, such as those developed by the National Comprehensive Cancer Network® (NCCN®)[1], the National Cancer Institute (NCI)[2], the St. Gallen conference [3] and others, using the highest levels of evidence and consensus expert opinion, have evolved to play a definitive role in the selection of appropriate adjuvant therapy following breast cancer surgery. Only a handful of studies have evaluated the influence of guideline-concordant therapy on breast cancer survival: in those investigations, breast cancer patients who received treatment consistent with guidelines had a reduced risk of mortality [4], whereas those who did not were at an increased risk of recurrence [5] or overall mortality [6].
Guideline-consistent treatment may be relatively underutilized among underserved populations such as Hispanic or Black women. In several studies, such women have been less likely than other women to receive radiation after breast-conserving surgery or other adjuvant therapy [7–10], despite guideline eligibility. Lack of receipt of appropriate therapy is of great concern in light of the persistently elevated breast cancer-specific mortality in these populations[11,12]. Few studies have quantified the influence of differences in treatment on the gap in outcomes by race or ethnicity[13]. Therapy receipt, if a contributor to breast cancer survival disparities, is amenable to intervention at the patient, provider, and system levels[14–16].
Despite widespread dissemination of clinical practice guidelines, variations in breast cancer treatment may be attributable in some part to patient contraindications, such as comorbid conditions, or possibly to early mortality. In a population-based case-cohort study in New Mexico, using a framework that accounted for clinical reasons for the non-receipt of therapy, we sought to determine whether women who received guideline-inconsistent therapy had an increased risk of breast cancer-specific mortality and whether differential receipt of guideline-based treatment contributed to the 1.7-fold increased risk of breast cancer-specific mortality in Hispanic women.
Patients and Methods
We conducted a population-based case-cohort study of breast cancer-specific survival among all first invasive breast cancer cases diagnosed from 1997–2009 among white female residents of six New Mexico counties (Bernalillo, Sandoval, Santa Fe, Socorro, Torrance, and Valencia). We used information from the New Mexico Tumor Registry (NMTR), a National Cancer Institute (NCI)-funded Surveillance Epidemiology End Results (SEER) site, to randomly select 15% of all first invasive breast cancer diagnoses (sub-cohort) and all deaths due to breast cancer-related causes (cases) among all incident diagnoses (not diagnosed by autopsy or death certificate).
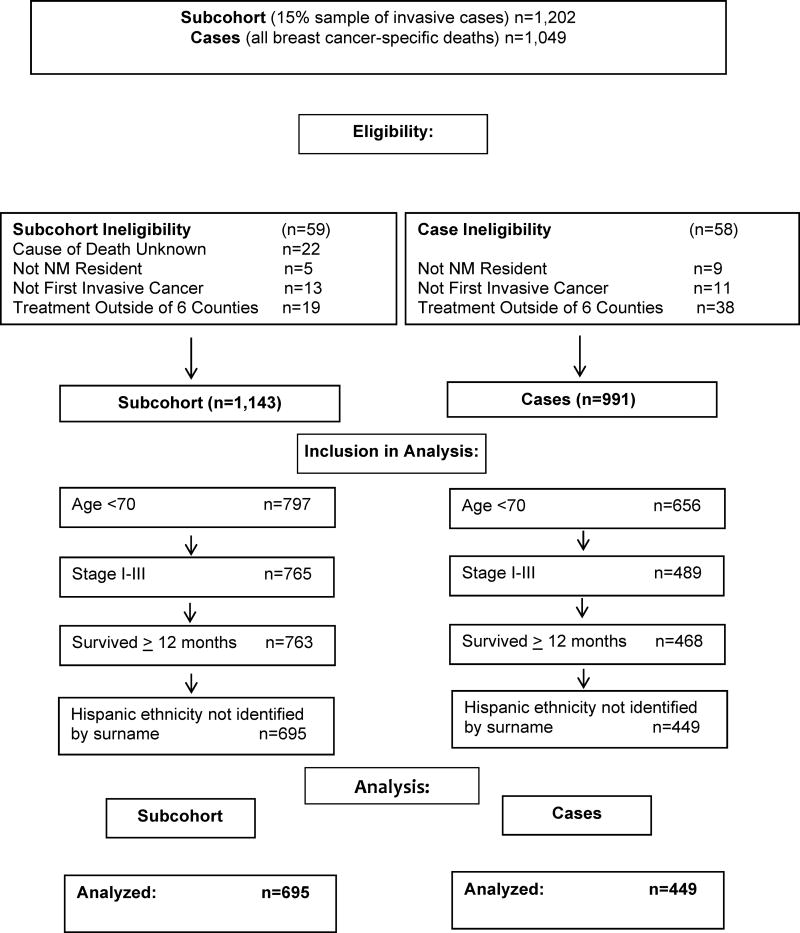
We excluded women who were not residents of New Mexico, who had an unknown cause of death, or who received treatment outside the six-county region (thus precluding the treatment assessment). Hispanic ethnicity was defined using the North American Association of Central Cancer Registries (NAACR) algorithm utilized in SEER, with the exclusion of women identified only by Spanish surname. Analyses were restricted to women with stage I–III disease who survived at least 12 months post-diagnosis, thus allowing a modicum of time for guideline-adherent treatment to be received. In accordance with NCCN guidelines, the included women were also restricted to age < 70 at diagnosis, which minimized non-receipt due to comorbidity or life expectancy (Figure 1).
Fig. 1.
Eligibility and Inclusion in Analyses: Population-Based Invasive Breast Cancer Cases Diagnosed from 1997–2009 in six New Mexico counties.
Data Collection
Initial inpatient and outpatient providers were identified through SEER. Because the counties surrounding the six counties are sparsely populated, most residents receive medical care within the study area. Medical record reviews were conducted by SEER-trained certified tumor registrars (CTR) or a registered health information technologist. Paper and electronic medical records were reviewed for surgical oncology, radiation oncology, medical oncology, and long-term follow-up care. SEER funding allows collection of only the first course of therapy[17,18], resulting in under ascertainment of treatment[19,20,18]. Thus, additional providers identified through referral notes, pathology information, or hospital or clinic record searches were also reviewed. Medical records were sought at an average of 2.3 hospitals or clinics per woman. The data collected included standard demographic variables, diagnosis information (tumor size, lymph node status, tumor receptor status), and detailed information about treatment, including surgery, neoadjuvant and adjuvant chemotherapy, radiation, endocrine, and biological therapy (types, dates of receipt, doses, and agents). For women who had bilateral synchronous cancers diagnosed, only the most advanced cancer was included. Information was sought regarding physician-stated contraindications for each therapy, patient refusals of treatment, and comorbidities in the Charlson Index[21,22]. Oncotype DX Breast Cancer Assay[23] (Genomic Health, Redwood City, CA) results were abstracted when available. SEER data (limited to treatment initiation only) were the sole source of information for 73/663 (11.0%) of the women who received chemotherapy, 71/703 (10.0%) who received radiation, and 50/638 (7.8%) who received endocrine therapies. The positive predictive value for SEER data in comparison with medical record reviews or claims data is high[18]. Medical chart reviews yielded information that SEER did not (SEER treatment initiation sensitivity: chemotherapy (89.3%), radiotherapy (76.1%), endocrine therapy (55.0%)). For n=45 women (included in the totals above), medical records were unavailable, and SEER was the sole source of information used.
Follow-up and ascertainment of vital status
Women were followed through January 1, 2013. Vital status and cause of death were determined by the New Mexico Tumor Registry, using probabilistic matching to the New Mexico State Vital Statistics Bureau files, and the National Death Index of the National Center for Health Statistics. Vital status was verified by the submission of files to the Centers for Medicare and Medicaid Services. Because Hispanic women have a lower age-adjusted all-cause mortality rate than non-Hispanic white women, which can mask any elevation in cause-specific mortality rates, only deaths attributed to breast cancer as an underlying cause on the death certificate were included as events in the analysis.
Classification of Therapy Receipt according to National Comprehensive Cancer Network Guidelines
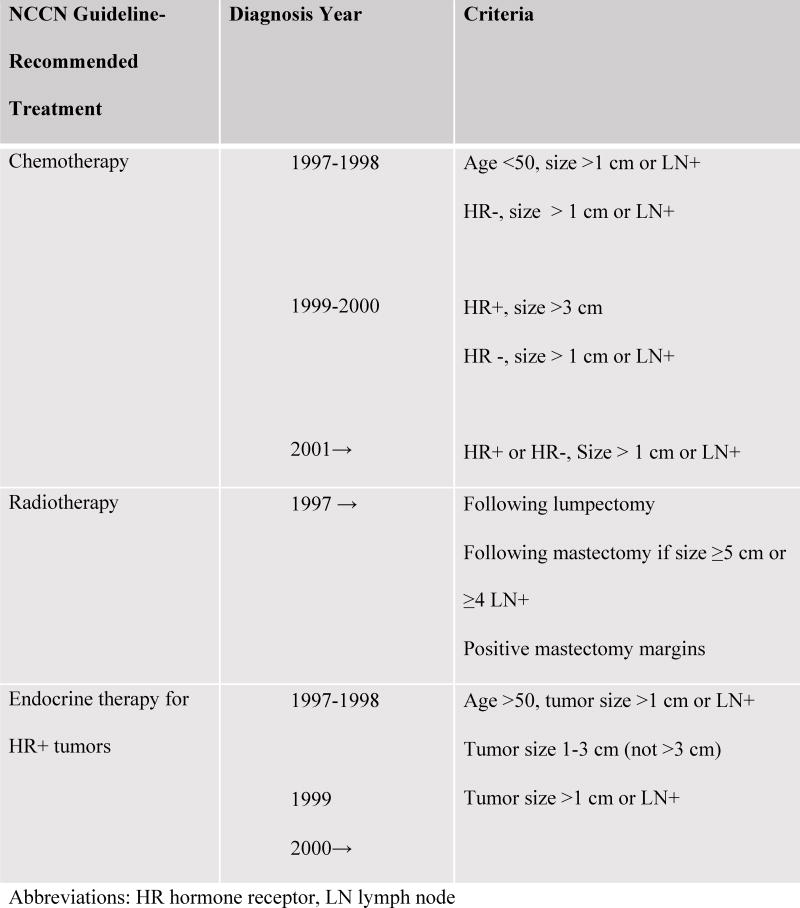
NCCN Clinical Practice Guidelines in Oncology® (NCCN Guidelines®) for breast cancer for 1997 through 2009[1] were reviewed to determine eligibility for treatment. Women were considered eligible for a particular therapy if their tumor characteristics met the guideline criteria for the year of diagnosis: category 1 for diagnoses from 1997 to 1999 and category 1 or 2A for 2000–2009 diagnoses (Figure 2). Women who had any physician-noted contraindications for a therapy were omitted from the analyses of guideline-concordant receipt of that therapy. Women missing tumor characteristics necessary to determine eligibility (< 5%; Table 1) were also excluded from the analysis of that therapy. For chemotherapy only, women with the following contraindications noted in medical records were also excluded from the guideline analysis: history of previous malignancy, heart failure, myocardial infarction, cerebrovascular accident, or an Oncotype DX score < 18. Women who met the guideline criteria for each therapy were classified as having received treatment if medical chart review yielded details of treatment (or if indicated in SEER records) and not treated otherwise.
Fig. 2.
National Comprehensive Cancer Network (NCCN) Guidelines for Invasive Breast Cancer Adjuvant Care, 1997–2009
Abbreviations: HR hormone receptor, LN lymph node
Table 1.
Characteristics of incident invasive breast cancer cases who survived 12 or more months (Subcohort – 15% sample of all eligible cases).
| Characteristica | Subcohort (15% sample weight 6.67) N=771 | Cases (Breast Cancer Deaths) N=449 | ||
|---|---|---|---|---|
| N | % | N | % | |
| Age Group | ||||
| <40 | 49 | 7.1 | 53 | 11.8 |
| 40–49 | 166 | 23.9 | 132 | 29.4 |
| 50–59 | 249 | 35.8 | 159 | 35.4 |
| 60–69 | 231 | 33.2 | 105 | 23.4 |
| Ethnicity | ||||
| Hispanic | 176 | 25.3 | 156 | 34.7 |
| Non-Hispanic | 519 | 74.7 | 293 | 65.3 |
| Year of Diagnosis | ||||
| 1997–2000 | 206 | 29.6 | 190 | 42.3 |
| 2001–2004 | 197 | 28.3 | 144 | 32.1 |
| 2005–2009 | 292 | 42.0 | 115 | 25.6 |
| Tumor Sizeb | ||||
| ≤ 1.0 cm | 160 | 24.5 | 27 | 6.4 |
| >1–3 cm | 356 | 54.6 | 204 | 48.5 |
| >3–5 cm | 72 | 11.1 | 85 | 20.1 |
| >5 cm | 32 | 4.9 | 40 | 9.5 |
| Chest wall/Skin | 32 | 4.9 | 65 | 15.4 |
| Missing | 42 | 28 | ||
| Number of Positive Nodes | ||||
| 0 | 454 | 66.6 | 151 | 34.6 |
| 1–3 | 159 | 23.4 | 165 | 37.8 |
| ≥4 | 68 | 10.0 | 121 | 27.6 |
| Missing | 14 | 12 | ||
| Tumor Grade | ||||
| Grade I | 182 | 28.1 | 37 | 8.9 |
| Grade II | 257 | 39.7 | 129 | 31.0 |
| Grade III/IV | 208 | 32.1 | 250 | 60.1 |
| Missing | 48 | 33 | ||
| Estrogen Receptor | ||||
| Positive | 553 | 82.4 | 297 | 68.6 |
| Negative | 118 | 17.6 | 136 | 31.4 |
| Missing | 24 | 16 | ||
| Progesterone Receptor | ||||
| Positive | 469 | 72.8 | 226 | 54.3 |
| Negative | 175 | 27.2 | 190 | 45.7 |
| missing | 51 | 33 | ||
| Her2/neu | ||||
| Positive | 94 | 16.6 | 53 | 23.8 |
| Negative | 471 | 83.3 | 170 | 76.2 |
| missing | 130 | 38 | ||
Tumor characteristics, including estrogen receptor positivity, are influenced by the study restriction to stages I–III and a minimum of 12 months survival.
Tumor size categorized to facilitate assessment according to National Comprehensive Cancer Network cut points (Figure 2). Note that most women with missing values had American Joint Commission on Cancer (AJCC) tumor size = T2 (2–5 cm) but actual size unknown; thus, they could not be assigned to the ≤ 3 cm or > 3 cm categories.
Statistical Analysis
Cox proportional hazards models for case-cohorts were utilized [24] to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) using an alpha level of .05. Specifically, women in the subcohort were weighted by the inverse of the sampling fraction (100%/15% = 6.67). Women in the subcohort entered the study at 12 months post-diagnosis (staggered entry time) and were followed until death, loss to follow-up, or January 1, 2013. Death due to breast cancer-related causes was the end point of interest, and all other events were censored. Time to event was measured in months. To determine whether non-receipt of therapy was attributable in part to early mortality, we conducted additional analyses, restricting entry time to 24 months post-diagnosis. Confounding by indication for therapy was addressed by restricting each analysis only to women who met the NCCN guidelines for that therapy (women with contraindications were excluded). Such restriction to only one level of the confounder eliminates confounding. The contribution of guideline-concordant therapy to breast cancer survival was evaluated using likelihood ratio tests. To determine whether disparate breast cancer survival differences in Hispanic women were attributable in part to the differential receipt of therapy, we evaluated the change in hazard ratio with the addition of variables for appropriate treatment. Any decline in the hazard ratio for Hispanic ethnicity with the inclusion of treatment in the model would suggest that treatment was a contributor to survival disparities[25,26]. We also estimated whether the effect of therapy on survival differed among Hispanic women by including the main effects of Hispanic ethnicity and guideline-adherent therapy and an interaction term in Cox regression models. We calculated the Hispanic survival hazard ratio among strata defined by guideline-adherent treatment vs not. The proportional hazards assumption was verified using Schoenfeld residuals [27]. Analyses were adjusted for age (5-year age groups), tumor size (<1 cm, 1–<3 cm, 3–<5 cm, and ≥ 5 cm; skin/chest wall involvement, cut points determined by NCCN treatment guidelines-Figure 2), positive lymph nodes (0, 1–3, 4+) tumor grade (1, 2, 3/4), estrogen receptor (ER) status, progesterone receptor (PR) status, Her2/neu status, and Hispanic ethnicity. All analyses were conducted in SAS v 9.4 (Cary, N.C.), and multivariate-adjusted survival estimates were graphed using R software v 3.3.3 (Vienna, Austria). We received institutional review board approval for the study from the University of New Mexico Health Sciences Center under a Health Insurance Portability and Accountability Act (HIPAA) waiver of consent for previously collected data.
Results
This study included 695 women selected from the cohort (representing 4635 women in the full cohort, when weighted by 6.67, the inverse of the 15% sampling fraction) and 449 who died of breast cancer-related causes (Figure 1). On average, women who died were more likely to be younger, and as expected, had tumor characteristics associated with a poorer prognosis (Table 1). The median survival in the cohort was 101 months (8.4 years) post-diagnosis. Hispanic women comprised 25.3% of the cohort and 34.7% of breast cancer deaths. Overall, Hispanic women were 1.7-fold more likely to die of breast cancer-specific causes than non-Hispanic white women (95% CI 1.1–2.9).
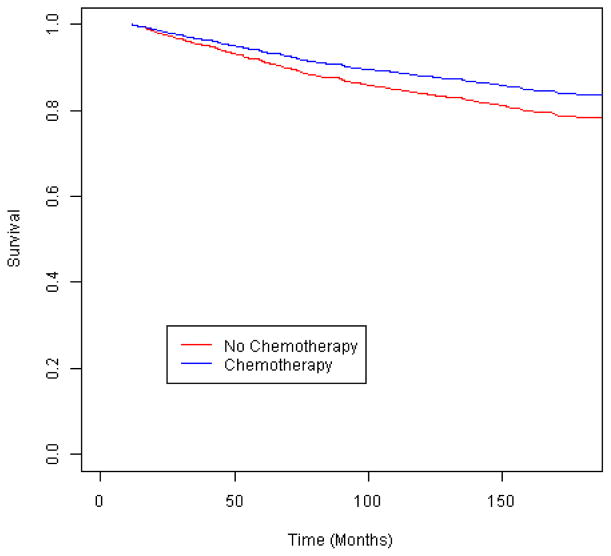
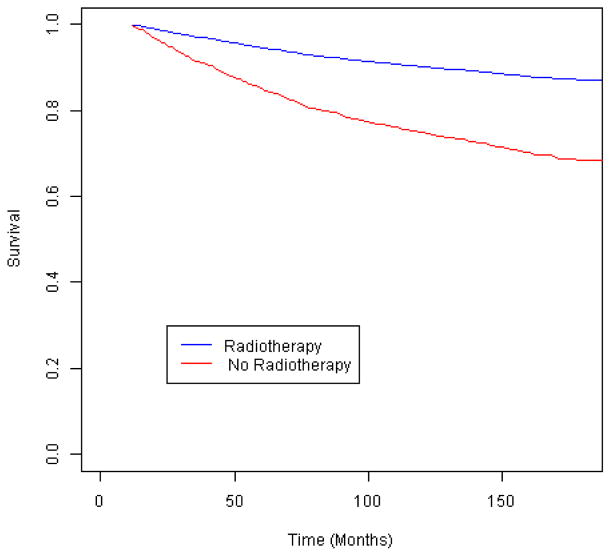
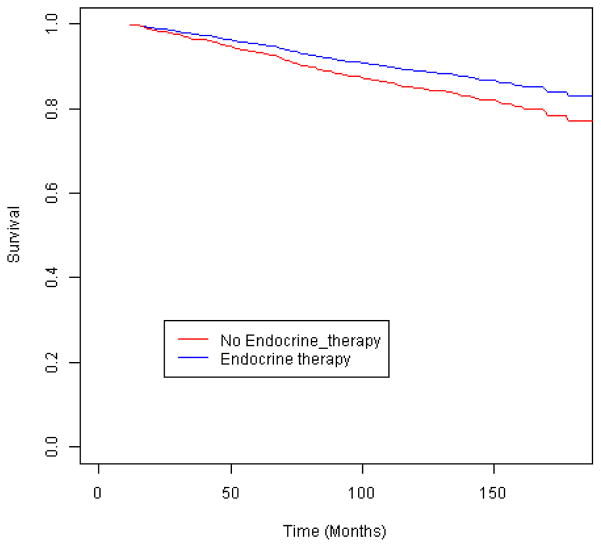
We first examined outcomes among the women eligible for each treatment under NCCN guidelines. Among women who met the guideline criteria for chemotherapy (Figure 2), those who did not receive it were not at an elevated risk of breast cancer-specific mortality (Table 2) (Figure 3). However, when tumor characteristics were compared by chemotherapy receipt, the women who did not receive chemotherapy had more favorable prognostic indicators: a smaller tumor size (≤ 2 cm vs. larger, p=.01) and a greater proportion of negative nodes (p=.009). By contrast, the women who met the guidelines for radiotherapy but did not receive it had a 2.3-fold increased risk of breast cancer-specific mortality compared to those who received treatment (Figure 4), and that risk remained elevated more than 2-fold among those who survived 24 months. Similarly, the women who were eligible for endocrine therapy but did not receive it had 2.0-fold increased mortality relative to the treated women (Figure 5), with a similar mortality increase among those with 24 months or greater survival. Lack of receipt of guideline-concordant radiation and endocrine therapy independently contributed to statistical models of breast cancer survival, after adjustment for other prognostic indicators (Table 3).
Table 2.
Treatment initiation and breast cancer-specific mortality among incident invasive breast cancer cases for whom therapy was indicated by the National Comprehensive Cancer Network (NCCN) guidelines. Restricted to women who survived 12 or more months or 24 or more months.
| Received Treatment Indicated |
Subcohort (Weighted 6.67x) | Cases (Deaths ≥ 12 mo) | Survival HRa,b (≥12 mo post dx) | Survival HRa,b (≥ 24 mo post dx) | ||
|---|---|---|---|---|---|---|
| N | % | N | % | HR (95% CI) | HR (95% CI) | |
| Chemotherapy | ||||||
| Yes | 263 | 82.2 | 269 | 87.3 | 1.0 | 1.0 |
| No | 57 | 17.8 | 39 | 12.7 | 1.5 (0.8–2.7) | 1.3 (0.7–2.3) |
| Not indicated | 451 | 141 | ||||
| Radiation | ||||||
| Yes | 397 | 90.6 | 232 | 80.0 | 1.0 | 1.0 |
| No | 41 | 9.4 | 58 | 20.0 | 2.3 (1.2–4.4) | 2.1 (1.1–4.2) |
| Not indicated | 333 | 159 | ||||
| Endocrine Therapy | ||||||
| Yes | 278 | 86.6 | 172 | 81.1 | 1.0 | 1.0 |
| No | 43 | 13.4 | 40 | 18.9 | 2.0 (1.0–4.0) | 1.8 (0.9–3.7) |
| Not indicated | 450 | 237 | ||||
Adjusted for age, tumor size (National Comprehensive Cancer Network (NCCN) guideline categories < 1 cm, 1–<3 cm, 3–<5 cm, and ≥5 cm, chest wall/skin) positive lymph nodes (0, 1–3, 4+), tumor grade (1, 2, 3/4), estrogen receptor status, progesterone receptor status, Her2 status, and Hispanic ethnicity.
Abbreviations: HR – hazard ratio, CI – confidence interval
Fig. 3.
Breast cancer-specific survival according to receipt of chemotherapy among invasive breast cancer cases eligible for such treatment by the National Comprehensive Cancer Network (NCCN) guidelines
Fig. 4.
Breast cancer-specific survival according to receipt of radiotherapy among invasive breast cancer cases eligible for such treatment by the National Comprehensive Cancer Network (NCCN) guidelines
Fig. 5.
Breast cancer-specific survival according to receipt of endocrine therapy among invasive breast cancer cases eligible for such treatment by the National Comprehensive Cancer Network (NCCN) guidelines
Table 3.
Contribution of guideline-discordant treatment to breast cancer-specific mortality.
| Guideline Treatment |
Model 1: | Model 2: | Model 2 vs. 1 | |||
|---|---|---|---|---|---|---|
| Likelihood Ratio Chi-Square Base Modela | DFb | Likelihood Ratio Chi-Square Base Model a | ±Treatment DFb | Difference Chi-Square | p-value | |
|
|
|
|
|
|
|
|
| Chemotherapy | 211.7 | 15 | 214.9 | 16 | 3.2 | .07 |
| Radiation | 348.5 | 15 | 369.7 | 16 | 21.2 | <.0001 |
| Endocrine Therapy | 208.0 | 15 | 217.5 | 16 | 9.5 | .0021 |
Base model includes the variables age, tumor size, positive lymph nodes, tumor grade, estrogen receptor, progesterone receptor, Her2 status, and Hispanic ethnicity.
Abbreviations: DF – degrees of freedom
We evaluated whether Hispanic women were less likely to receive guideline-appropriate therapy, which could plausibly influence breast cancer survival disparities. Contraindications for chemotherapy differed by ethnicity (19.7% in Hispanics vs. 29.3% in non-Hispanic whites; p=.04), whereas the contraindications for radiotherapy (6.0% vs. 4.7%) or endocrine therapy (20.6% vs. 14.0%) did not (Table 4). Hispanic women were slightly more likely than non-Hispanic white women to receive chemotherapy (84.2% vs. 81.3%, respectively) and endocrine therapy (89.2% vs. 85.8%, respectively) and were almost equally likely to receive radiation therapy (89.2% vs. 91.1%, respectively). Thus, treatment did not differ significantly by ethnicity. We next examined whether the Hispanic survival HR was altered by adjustment for variables indicating guideline-consistent treatment in Cox regression models, which would suggest that treatment receipt influenced disparities. Adjustment for each therapy did not materially alter the Hispanic survival disparity HR. In separate Cox multivariate models restricted to those guideline-eligible for each treatment, the interaction terms between Hispanic ethnicity and each treatment were non-significant, implying that the effect of treatment did not differ by ethnic group and that the Hispanic survival disparity did not differ by treatment. Hispanic women remained at an increased risk of breast cancer-related mortality in each stratum restricted to those who received guideline-appropriate therapy (Table 4).
Table 4.
Stratified analysis of treatment initiation and breast cancer-specific mortality according to Hispanic ethnicity among incident invasive breast cancer cases for whom treatment was indicated by the National Comprehensive Cancer Network (NCCN) guidelines.
| Received Treatment Indicated by NCCN Guidelines (Strata) |
Hispanic Subcohort | Non-Hispanic White Subcohort | |||
|---|---|---|---|---|---|
|
| |||||
| Contra-Indications | Therapy Eligible | Contra-Indications | Therapy Eligible | Stratum-Specific Hispanic Survival Hazard Ratioc | |
| N %a | N %b | N %a | N %b | ||
| Chemotherapy | |||||
| Yes | 80 (84.2) | 183 (81.3) | 1.5 (1.0–2.3) | ||
| No | 23 (19.7) | 15 (15.8) | 92 (29.3) | 42(18.7) | 1.4 (0.2–8.9) |
| Radiation | |||||
| Yes | 99 (89.2) | 298 (91.1) | 1.7 (1.1–2.4) | ||
| No | 7 (6.0) | 12 (10.8) | 16 (4.7) | 29 (8.9) | 2.4 (0.8–7.0) |
| Endocrine Therapy | |||||
| Yes | 66 (89.2) | 212 (85.8) | 1.9 (1.1–3.4) | ||
| No | 19 (20.6) | 8 (10.8) | 40 (14.0) | 35 (14.2) | 6.4 (0.8–49.4) |
Abbreviations: NCCN – National Comprehensive Cancer Network
Percent of all women eligible for therapy according to NCCN guidelines.
Percent of all eligible women after those with contraindications were omitted.
Adjusted for age, tumor size (National Comprehensive Cancer Network (NCCN) guideline categories < 1 cm, 1–<3 cm, 3–<5 cm, and ≥5 cm, chest wall/skin) positive lymph nodes (0, 1–3, 4+), tumor grade (1, 2, 3/4), estrogen receptor status, progesterone receptor status, Her2 status, and Hispanic ethnicity.
Discussion
We examined breast cancer survival disparities by ethnicity in a large population-based study of women diagnosed with breast cancer between 1997 and 2009 who were followed for a median of 8.4 years. We evaluated the treatment received using detailed information abstracted from medical records and applied NCCN-guideline criteria specific to diagnosis year to evaluate appropriate treatment. Our results suggest that women had a 2.0- to 2.3-fold elevated risk of breast cancer-related death if they did not receive guideline-consistent radiotherapy or endocrine therapy, implying that guideline-concordant therapy has a substantial influence on prognosis. Because Hispanic women were equally as likely to receive NCCN guideline-appropriate therapy as non-Hispanic white women, and because adjustment for treatment did not alter the Hispanic HR, treatment was not identified as a determinant of survival disparities.
Our results should be interpreted in light of the strengths and limitations of this study. We could not thoroughly evaluate therapy completion in all women; thus, only the initial receipt of guideline-consistent therapy was assessed. Additionally, although we reviewed both inpatient and outpatient medical charts and electronic and paper records to ascertain therapy, some forms of therapy may have been under-ascertained. Treatment determined using medical records, which frequently included dates of initiation, doses, and agents, is less likely to be over-ascertained. SEER information, which was utilized for a small proportion of the included women, also has a high positive predictive value for therapy[18]. The restriction of included women to those aged less than 70 years diagnosed with stage I–III disease who survived at least 12 months post-diagnosis provided a solid foundation to evaluate treatment receipt only among women who survived a sufficient time to initiate therapy and who were less likely than older women to omit treatment[28], including for comorbidities[16]. Other strengths of our study include the population-based case-cohort design, extensive detailed data collection, and the length of follow-up.
Our study is one of very few[4,6] to quantify the distinct survival disadvantages accrued by women who do not receive guideline-recommended care. Women who did not receive indicated endocrine therapy or radiotherapy had a 2.0- to 2.3-fold elevated risk of breast cancer mortality, independent of other risk factors for outcome. The likelihood ratio test (Table 3) suggests a contribution similar to that of other important prognostic indicators[29].
Our study results are consistent with those of other investigations that suggest that treatment accounts for little if any difference in breast cancer outcomes by race or ethnicity [13,30–33], but they are inconsistent with other studies. In randomized clinical trials, survival disparities by race have persisted despite equal treatment[30,31,33]. Among women with equal access to care through the Department of Defense or Medicare, breast cancer survival disparities continue [34], although not in early stage disease [35], and treatment accounted for only a limited proportion of racial differences in all-cause mortality [32]. However, Hispanic (or Black) women have been less likely to receive guideline-consistent treatment in several studies [7,9]. By contrast, in medical record data obtained for a population-based sample of SEER breast cancer cases, which allowed treatment evaluation beyond the initial first course usually available in SEER [17,18], Hispanic women were slightly more likely than non-Hispanic whites to receive guideline-concordant chemotherapy and radiotherapy, but not endocrine therapy[28]. In two additional studies that utilized self-report and medical record or SEER data, Hispanic or Black women were equally as likely to receive chemotherapy[36] or radiotherapy[37] as non-Hispanic white women. The proportion of women who received guideline-concordant therapy in our investigation was consistent with that reported in the latter three investigations[28,36,37].
In our population-based study in New Mexico, a state with the nation’s second highest uninsured rate during the included years[38], the proportion of women who received guideline-concordant care was high (>85% for non-chemotherapy), suggesting that clinicians and patients strongly adhere to quality of care recommendations and providing assurance that disparate survival in this setting is not arising from gaps in care. The increased risk of breast cancer-related deaths persisted even among Hispanic women who received full guideline-concordant treatment. Thus, equalizing receipt of standard of care and attempting to reduce treatment disparities may not be sufficient to address the disproportionate mortality evident in Hispanic women with breast cancer.
Acknowledgments
Funding information:
This study was supported by grants R01CA132877 and 2P30CA11810 from the National Cancer Institute (NCI) and NCI contract HSN26120130010I-Task Order HHSN26100005 to the Surveillance Epidemiology End Results (SEER) program.
We would like to thank Ms. Kimberly Cooke, Mr. Nicolas Eldredge, Ms. Rebecca Sando, Ms. Chanel Jim, Mr. Francisco Martinez, Ms. Nancy Brito, and Ms. Georgia Hufnagel for their assistance in conducting this study.
Footnotes
Conflict of interest information:
The authors declare that they do not have a conflict of interest in connection with this manuscript.
References
- 1.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Breast Cancer. 1997–2009 doi: 10.6004/jnccn.2009.0070. (Referenced with permission from The NCCN Clinical Practice Guidelines in Oncology® for Guidelines 1997–2009. © National Comprehensive Cancer Network, Inc 2010. All rights reserved. To view the most recent and complete version of the guideline, go online to www.nccn.org.) [DOI] [PubMed]
- 2.Institute NC. [Accessed December 2, 2015];Breast Cancer Treatment–for health professionals (PDQ®) 2015 [Google Scholar]
- 3.Coates AS, Winer EP, Goldhirsch A, Gelber RD, Gnant M, Piccart-Gebhart M, Thurlimann B, Senn HJ, Panel M. -Tailoring therapies-improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Annals of oncology : official journal of the European Society for Medical Oncology. 2015;26(8):1533–1546. doi: 10.1093/annonc/mdv221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wockel A, Wolters R, Wiegel T, Novopashenny I, Janni W, Kreienberg R, Wischnewsky M, Schwentner L group Bs. The impact of adjuvant radiotherapy on the survival of primary breast cancer patients: a retrospective multicenter cohort study of 8935 subjects. Annals of oncology : official journal of the European Society for Medical Oncology. 2014;25(3):628–632. doi: 10.1093/annonc/mdt584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badakhshi H, Gruen A, Sehouli J, Budach V, Boehmer D. The impact of patient compliance with adjuvant radiotherapy: a comprehensive cohort study. Cancer medicine. 2013;2(5):712–717. doi: 10.1002/cam4.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hebert-Croteau N, Brisson J, Latreille J, Rivard M, Abdelaziz N, Martin G. Compliance with consensus recommendations for systemic therapy is associated with improved survival of women with node-negative breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(18):3685–3693. doi: 10.1200/JCO.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Bickell NA, Wang JJ, Oluwole S, Schrag D, Godfrey H, Hiotis K, Mendez J, Guth AA. Missed opportunities: racial disparities in adjuvant breast cancer treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(9):1357–1362. doi: 10.1200/JCO.2005.04.5799. [DOI] [PubMed] [Google Scholar]
- 8.Haggstrom DA, Quale C, Smith-Bindman R. Differences in the quality of breast cancer care among vulnerable populations. Cancer. 2005;104(11):2347–2358. doi: 10.1002/cncr.21443. [DOI] [PubMed] [Google Scholar]
- 9.Freedman RA, Virgo KS, He Y, Pavluck AL, Winer EP, Ward EM, Keating NL. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–189. doi: 10.1002/cncr.25542. [DOI] [PubMed] [Google Scholar]
- 10.Smith GL, Shih YC, Xu Y, Giordano SH, Smith BD, Perkins GH, Tereffe W, Woodward WA, Buchholz TA. Racial disparities in the use of radiotherapy after breast-conserving surgery: a national Medicare study. Cancer. 2010;116(3):734–741. doi: 10.1002/cncr.24741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast cancer research and treatment. 2011;127(3):729–738. doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Archives of internal medicine. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 13.Warner ET, Tamimi RM, Hughes ME, Ottesen RA, Wong YN, Edge SB, Theriault RL, Blayney DW, Niland JC, Winer EP, Weeks JC, Partridge AH. Racial and Ethnic Differences in Breast Cancer Survival: Mediating Effect of Tumor Characteristics and Sociodemographic and Treatment Factors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(20):2254–2261. doi: 10.1200/JCO.2014.57.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bickell NA, LePar F, Wang JJ, Leventhal H. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(18):2516–2521. doi: 10.1200/JCO.2006.09.5539. [DOI] [PubMed] [Google Scholar]
- 15.Bickell NA, Shastri K, Fei K, Oluwole S, Godfrey H, Hiotis K, Srinivasan A, Guth AA. A tracking and feedback registry to reduce racial disparities in breast cancer care. Journal of the National Cancer Institute. 2008;100(23):1717–1723. doi: 10.1093/jnci/djn387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bickell NA, Weidmann J, Fei K, Lin JJ, Leventhal H. Underuse of breast cancer adjuvant treatment: patient knowledge, beliefs, and medical mistrust. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(31):5160–5167. doi: 10.1200/JCO.2009.22.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Medical care. 2002;40(8 Suppl):IV-3–18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 18.Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL. Comparison of SEER Treatment Data With Medicare Claims. Medical care. 2014 doi: 10.1097/MLR.0000000000000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagsi R, Abrahamse P, Hawley ST, Graff JJ, Hamilton AS, Katz SJ. Underascertainment of radiotherapy receipt in Surveillance, Epidemiology, and End Results registry data. Cancer. 2012;118(2):333–341. doi: 10.1002/cncr.26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walker GV, Giordano SH, Williams M, Jiang J, Niu J, MacKinnon J, Anderson P, Wohler B, Sinclair AH, Boscoe FP, Schymura MJ, Buchholz TA, Smith BD. Muddy water? Variation in reporting receipt of breast cancer radiation therapy by population-based tumor registries. International journal of radiation oncology, biology, physics. 2013;86(4):686–693. doi: 10.1016/j.ijrobp.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Annals of epidemiology. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. The New England journal of medicine. 2004;351(27):2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 24.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50(4):1064–1072. [PubMed] [Google Scholar]
- 25.Rothman KJ, Greenland S. Measures of effect and measures of association. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. Lippincott Williams and Wilkins; Philadelphia (PA): 1998. pp. 55–56. [Google Scholar]
- 26.Brotman DJ. Mediators of the association between mortality risk and socioeconomic status. Jama. 2006;296(7):763–764. doi: 10.1001/jama.296.7.763-b. author reply 764. [DOI] [PubMed] [Google Scholar]
- 27.Xue X, Xie X, Gunter M, Rohan TE, Wassertheil-Smoller S, Ho GY, Cirillo D, Yu H, Strickler HD. Testing the proportional hazards assumption in case-cohort analysis. BMC Med Res Methodol. 2013;13:88. doi: 10.1186/1471-2288-13-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu X-C, Lund MJ, Kimmick GG, Richardson LC, Sabatino SA, Chen VW, Fleming ST, Morris CR, Huang B, Trentham-Dietz A, Lipscomb J. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(2):142–150. doi: 10.1200/JCO.2011.36.8399. [DOI] [PubMed] [Google Scholar]
- 29.Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, Zabaglo L, Mallon E, Green AR, Ellis IO, Howell A, Buzdar AU, Forbes JF. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(32):4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 30.Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, Saphner T, Wolff AC, Sledge GW, Jr, Wood WC, Davidson NE. Race and hormone receptor-positive breast cancer outcomes in a randomized chemotherapy trial. Journal of the National Cancer Institute. 2012;104(5):406–414. doi: 10.1093/jnci/djr543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. Journal of the National Cancer Institute. 2009;101(14):984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silber JH, Rosenbaum PR, Clark AS, Giantonio BJ, Ross RN, Teng Y, Wang M, Niknam BA, Ludwig JM, Wang W, Even-Shoshan O, Fox KR. Characteristics associated with differences in survival among black and white women with breast cancer. Jama. 2013;310(4):389–397. doi: 10.1001/jama.2013.8272. [DOI] [PubMed] [Google Scholar]
- 33.Hershman DL, Unger JM, Barlow WE, Hutchins LF, Martino S, Osborne CK, Livingston RB, Albain KS. Treatment quality and outcomes of African American versus white breast cancer patients: retrospective analysis of Southwest Oncology studies S8814/S8897. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27(13):2157–2162. doi: 10.1200/JCO.2008.19.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jatoi I, Becher H, Leake CR. Widening disparity in survival between white and African-American patients with breast carcinoma treated in the U. S. Department of Defense Healthcare system. Cancer. 2003;98(5):894–899. doi: 10.1002/cncr.11604. [DOI] [PubMed] [Google Scholar]
- 35.Rizzo JA, Sherman WE, Arciero CA. Racial disparity in survival from early breast cancer in the department of defense healthcare system. Journal of surgical oncology. 2015;111(7):819–823. doi: 10.1002/jso.23884. [DOI] [PubMed] [Google Scholar]
- 36.Silva A, Rauscher GH, Hoskins K, Rao R, Ferrans CE. Assessing racial/ethnic disparities in chemotherapy treatment among breast cancer patients in context of changing treatment guidelines. Breast cancer research and treatment. 2013;142(3):667–672. doi: 10.1007/s10549-013-2759-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jagsi R, Abrahamse P, Morrow M, Hawley ST, Griggs JJ, Graff JJ, Hamilton AS, Katz SJ. Patterns and correlates of adjuvant radiotherapy receipt after lumpectomy and after mastectomy for breast cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(14):2396–2403. doi: 10.1200/JCO.2009.26.8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Centers for Disease Control, National Center for Health Statistics. National Health Statistics Reports. State, Regional and National Estimates of Health Insurance Coverage for People Under 65 Years of Age. National Health Interview Survey. 2008 https://www.cdc.gov/nchs/data/nhsr/nhsr001.pdf. [PubMed]