Abstract
Little is known regarding mechanisms that control vascular aging, particularly at the cell-specific level. Peroxisome proliferator-activated receptor-γ (PPARγ) exerts protective effects in the vasculature when activated pharmacologically. To gain insight into the cell-specfic impact of PPARγ, we examined the hypothesis that genetic interference with endothelial PPARγ would augment age-induced vascular dysfunction. We studied carotid arteries from adult (11.6±0.3 mo) and old (24.7±0.6 mo) mice with endothelial specific expression of a human dominant negative mutation in PPARγ driven by the vascular cadherin promoter (E-V290M), along with age-matched, non-transgenic (non-Tg) littermates. Acetylcholine (an endothelium-dependent agonist) produced similar relaxation in arteries from adult non-Tg and E-V290M mice and old non-Tg mice. In contrast, responses to acetylcholine were reduced by >50% in old male and female E-V290M mice (p<0.01). Endothelial function in old E-V290M mice was not altered by an inhibitor of cyclooxygenase, but was restored to normal by a superoxide scavenger, an inhibitor of NADPH oxidase, or inhibition of Rho kinase. Relaxation of arteries to nitroprusside, which acts directly on vascular muscle, was similar in all groups. Vascular expression of interleukin-6, Nox2 and CDKN2A (a marker of senescence) was significantly increased in old E-V290M mice compared to controls (p<0.05). These findings provide the first evidence that age-related vascular dysfunction, inflammation, and senescence is accelerated following interference with endothelial PPARγ via mechanisms involving oxidative stress and Rho kinase. The finding of an essential protective role for endothelial PPARγ has implications for vascular disease and therapy for vascular aging.
Keywords: cardiovascular risk factors, carotid artery disease, oxidative stress, nitric oxide, NADPH oxidase, Rho kinase, senescence
Introduction
Although aging is the greatest risk factor for vascular disease and subsequent clinical complications,1, 2 relatively little is known regarding the biology of vascular aging.1, 3, 4 A fundamental contributor to the initiation and progression of vascular disease, endothelial dysfunction is an independent predictor of vascular events in experimental models and humans.1, 3, 5, 6 While loss of endothelial health is critical in relation to vascular disease, studies using endothelial-specific models or manipulation combined with aging are rare. In experimental models and humans, two common features of vascular disease during aging are interacting oxidative-stress and low-grade inflammation.1, 4, 5, 7, 8 Our ability to slow or reverse the progression of vascular aging is limited, in part due to poor understanding of mechanisms that control these processes.
A member of the nuclear hormone receptor superfamily, PPARγ functions as a ligand-activated transcription factor,9 regulating expression of target genes by binding to PPARγ-response elements or other mechanisms.9 Clinically, PPARγ is the target of thiazolidinediones (TZDs), high-affinity ligands used to treat diabetes.9 Protective effects of TZDs include inhibition of inflammation and oxidative stress,9, 10 concepts that are largely based on effects in young or adult models where PPARγ was activated pharmacologically (and in all cell types) using TZDs. In contrast, the current study used a cell-specific approach incorporating a human mutation in PPARγ that produces clinical symptoms to increase the relevance of the preclinical model.11
The functional importance of PPARγ in relation to aging is hard to predict due to our incomplete understanding of its cell-specific effects along with potential gene-age interactions.12 We hypothesized that endothelial PPARγ normally protects against age-induced oxidative stress and endothelial dysfunction. To address this question, we used mice expressing a human dominant negative form of PPARγ expressed specifically in endothelial cells.13, 14
A common site of vascular disease is the carotid artery. The clinical consequences of carotid artery disease progress with age and are substantial, contributing to ischemic stroke and cognitive deficits.3, 15 Thus, we studied this segment of the circulation. Overall, our findings suggest that age-related oxidative stress, inflammation, senescence, and vascular dysfunction are significantly increased following endothelial-specific interference with PPARγ, suggesting a critical role for this cell and molecule in protecting against fundamental aspects of vascular aging.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Experimental animals
Protocols were approved by the Animal Care and Use Committee of the University of Iowa. We studied E-V290M mice (n=34) and non-Tg (n=33) littermates.13, 14 Littermates were used as they provide the most rigorous control in relation to genetics and environment (intrauterine and post-birth environment) along with diet and age. Both male and female mice were studied. Mice were fed standard chow and water ad libitum. Using radiotelemetry, we found previously that arterial pressure is normal in these mice.13 Care of mice met the standards set forth by the NIH for the care and use of experimental animals.
Statistical analysis
All data are expressed as means±SE. Vasodilator responses were expressed as percent relaxation to U46619-induced precontraction. Comparisons between groups were made using analysis of variance followed by Bonferroni or Tukey’s multiple comparison post-hoc test. Statistical significance was accepted at P<0.05.
RESULTS
Genetic interference with PPARγ in endothelial cells predisposes to endothelial dysfunction with aging
Both genotypes of adult (11.6±0.3 mo) and old (24.7±0.6 mo) mice were carefully matched with respect to age (Figure S1 in the online-only Data Supplement). Body weight was similar in all groups of mice (Figure S1).
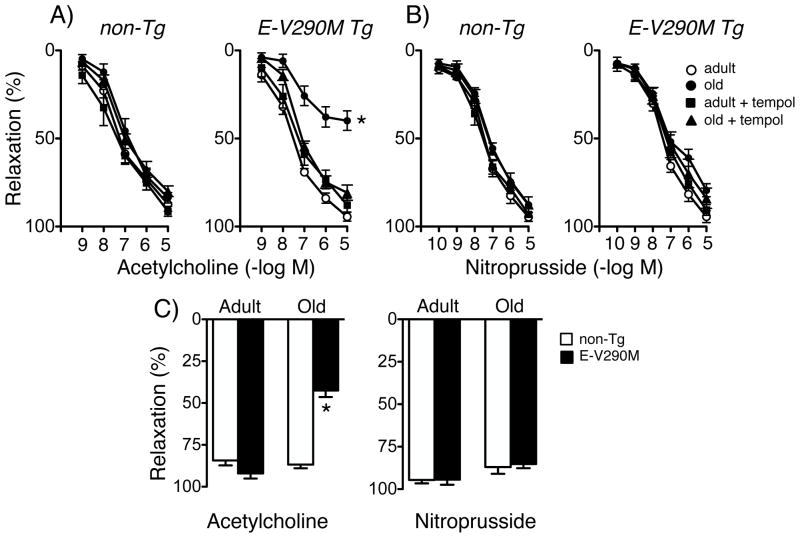
Acetylcholine and nitroprusside produced concentration-dependent relaxation of carotid arteries. Consistent with previous studies,16, 17 there was no significant change in endothelium-dependent relaxation in arteries from old non-Tg mice at the age studied (Figure 1). Vascular responses to acetylcholine were similar in adult and old non-Tg mice as well as adult E-V290M mice (Figure 1). In contrast, responses to acetylcholine were reduced by more than 50% in old E-V290M mice (Figure 1). Within the aged group, the magnitude of response was similar in female and male animals. For example, percent relaxation of carotid arteries to 10 μmol/L acetycholine was 84±2 (n=8) and 88±2% (n=14) in old female and old male non-Tg mice, but was 39±4 (n=8) and 44±4% (n=15) in old female and old male E-V290M mice, respectively. Relaxation of arteries to nitroprusside was similar in all groups (Figure 1B). For ease of comparison, maximum effects of acetylcholine and nitroprusside in all groups are presented in Figure 1C. These findings suggest that a form of vascular dysfunction in old E-V290M mice occurred at the level of endothelium and not vascular muscle. Thus, interference with PPARγ in endothelial cells did not affect endothelium-dependent relaxation in adult mice under baseline conditions but revealed a critical role for this nuclear receptor in protecting against endothelial dysfunction with aging.
Figure 1.
Response of carotid arteries to acetylcholine (A) and nitroprusside (B) in adult and old non-Tg and E-V290M mice, in the absence or presence of tempol. For both genotypes, n=6 adult and n=7 old mice. Panel C shows maximum responses for combined groups. For both genotypes, n=11 for acetylcholine in adults, n=22–23 in old mice. For nitroprusside, n=6 adults and n=16–18 old mice. Statistical differences were based on two-way ANOVA followed by Bonferroni post-hoc test. All data are mean±SE. * p<0.05 vs adult.
Superoxide and NADPH oxidase contribute to vascular dysfunction in aged E-V290M mice
We next examined mechanisms that could account for impaired endothelial function in old E-V290M mice. Relaxation of carotid arteries to acetylcholine was not affected by treatment with tempol (a scavenger of superoxide) in the organ bath in adult or old non-Tg mice or adult E-V290M mice (Figure 1). In contrast, reduced responses to acetylcholine in arteries from old E-V290M mice were restored to normal levels by tempol (Figure 1). Tempol did not affect vasorelaxation to nitroprusside in any group (Figure 1). These findings suggest superoxide plays an important role in age-induced vascular dysfunction in E-V290M mice.
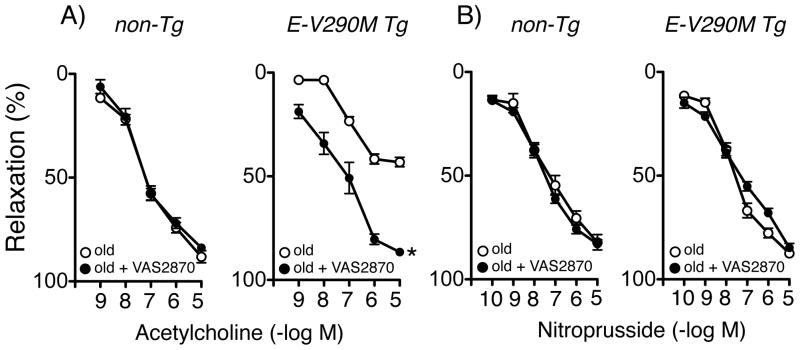
Activation of NADPH oxidase, a major source of superoxide in vascular cells, has been implicated in endothelial dysfunction in experimental models of aging and in arteries from aged humans.4, 7, 8 We next examined whether treatment with an inhibitor of NADPH oxidase (VAS-2870)14 would improve endothelial function in old E-V290M mice. Relaxation of the carotid artery to acetylcholine in old non-Tg mice was not affected by VAS-2870, but the response in old E-V290M mice was restored to normal levels by VAS-2870 (Figure 2). VAS-2870 did not affect the response to nitroprusside in any group (Figure 2). Thus, results with both tempol and VAS-2870 support the concept that oxidative stress and NADPH oxidase play a key role in vascular aging in E-V290M mice.
Figure 2.
Response of carotid arteries to acetylcholine (A) and nitroprusside (B) in old non-Tg (n=6) and old E-V290M (n=6) mice, in the absence or presence of VAS2870. Statistical differences were based on two-way ANOVA followed by Bonferroni post-hoc test. * p<0.05 vs old.
Rho kinase (ROCK) contributes to endothelial dysfunction in aged E-V290M mice
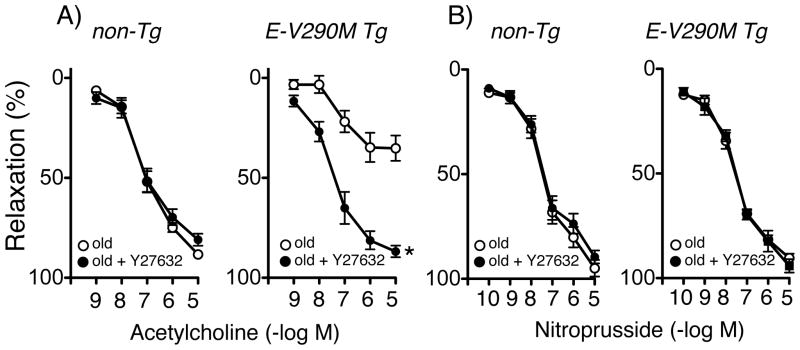
There are at least two reasons to hypothesize that ROCK may play a role in vascular aging. Oxidative stress or loss of suppression of activity by eNOS-derived NO can activate RhoA and ROCK, a potential mediator of vascular dysfunction.18, 19 In addition, several lines of evidence suggest that PPARγ in vascular muscle normally suppresses activation of RhoA/ROCK.20, 21 In the current experiments, we found that inhibition of ROCK with Y-27632 substantially improved endothelial function in old E-V290M mice without affecting responses in old non-Tg mice (Figure 3). Y-27632 did not affect the response to nitroprusside (Figure 3).
Figure 3.
Response of carotid arteries to acetylcholine (A) and nitroprusside (B) in old non-Tg (n=3) and old E-V290M (n=5) mice, in the absence or presence of Y27632. Statistical differences were based on two-way ANOVA followed by Bonferroni post-hoc test. * p<0.05 vs old.
Vascular dysfunction in old E-V290M mice is not mediated by COX
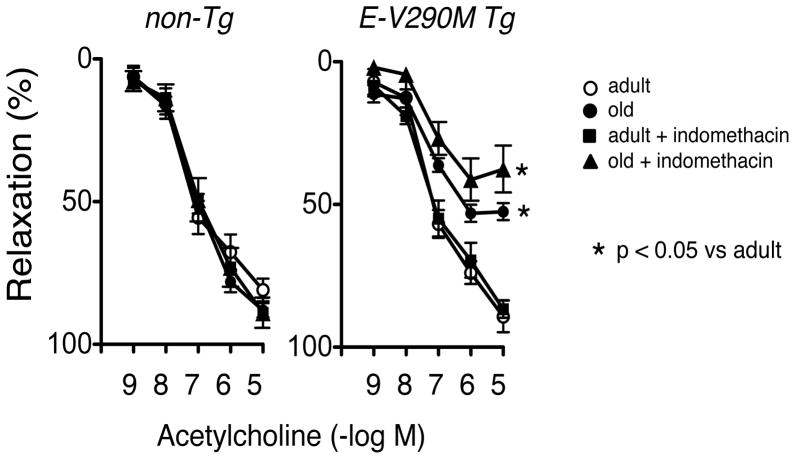
Interactions between oxidative stress and other mechanisms of vascular dysfunction can contribute to vascular disease.3 One such mechanism is COX-dependent production of endothelium-derived contracting factors (EDCF) or superoxide.3, 22 In further experiments, we evaluated whether such a mechanism was functional in old E-V290M mice using two approaches. Relaxation of carotid arteries to acetylcholine was not affected by indomethacin (an inhibitor of COX) in either strain of mice, adult or old (Figure 4). Another approach to study the impact of EDCF is to use the calcium ionophore A23187.22 In rings of arteries at resting tension, A23187 produced concentration-dependent contraction that was similar in adult non-Tg and E-V290M mice (Figure S2). Responses to A23187 tended to be greater in old mice of both strains, but these differences were not significant (Figure S2). Vascular responses to A23187 were not affected significantly by indomethacin in any group (Figure S2).
Figure 4.
Response of carotid arteries to acetylcholine in adult (n=5 for both genotypes) and old non-Tg (n=6) and E-V290M (n=5) mice, in the absence or presence of indomethacin. Statistical differences were based on two-way ANOVA followed by Bonferroni post-hoc test. * p<0.05 vs adult.
Aging augments select vasoconstrictor responses
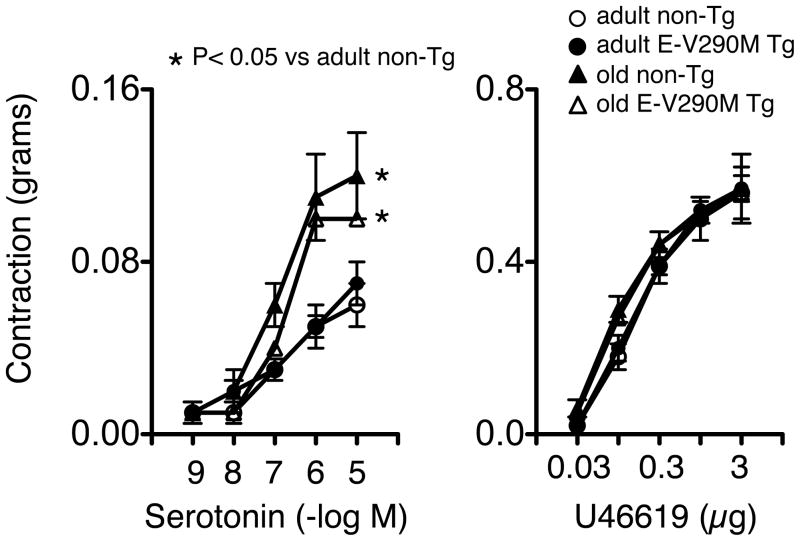
To determine if other vascular responses were affected by age or in E-V290M mice, we examined effects of serotonin and U46619. Via the actions of platelets or other mechanisms, both serotonin and thromboxane A2 receptors are known to contribute to vascular disease.3 Contraction of carotid arteries to serotonin was significantly increased with age to a similar extent in non-Tg and E-V290M mice (Figure 5). In contrast, vasoconstriction to U46619 was not affected by age or genotype (Figure 5).
Figure 5.
Contraction of carotid arteries to serotonin (n=5–6 in each group) and U46619 (n=6–7 in each group) in adult and old non-Tg and E-V290M mice. Statistical differences were based on two-way ANOVA followed by Bonferroni post-hoc test. * p<0.05 vs non-Tg adult.
Altered gene expression in the vasculature of E-V290M mice
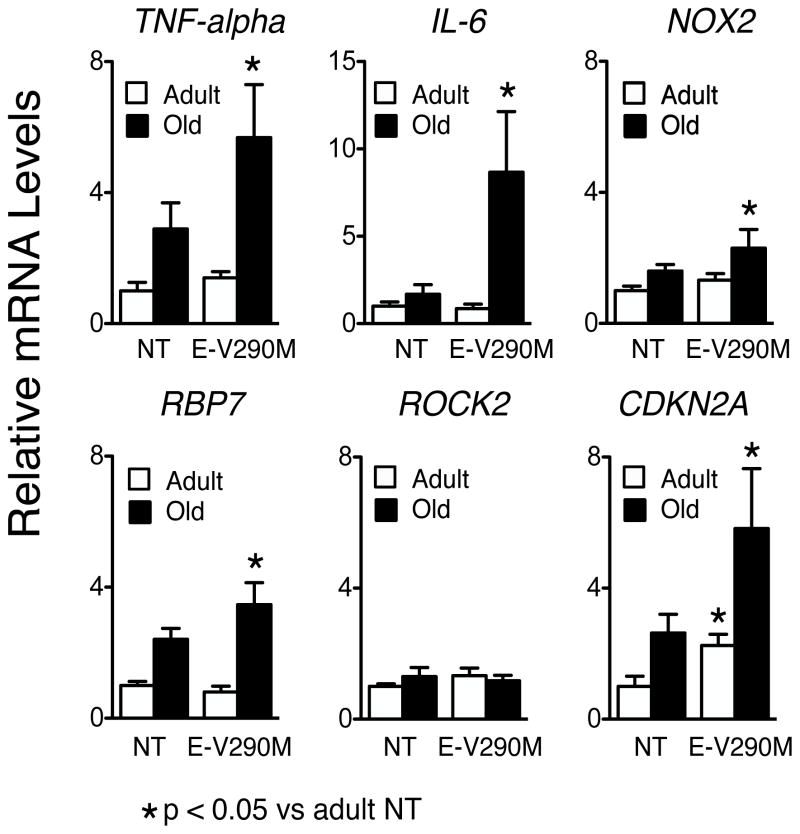
To gain additional insight into mechanisms that may contribute to vascular aging, we measured relative expression of several genes previously implicated in endothelial dysfunction and vascular disease (Figures 6 and S3). There were no significant differences in expression of these genes in adult non-Tg versus adult E-V290M mice. Compared to adult non-Tg mice, levels of mRNA for TNFα, Nox2, and RBP7 tended to increase in old non-Tg mice, increasing further in old E-V290M animals (Figure 6). RBP7 recently emerged as a protective molecule in endothelium, one that interacts with PPARγ.23 Increased expression of RBP7 in old E-V290M mice may reflect some type of compensatory response. Expression of IL-6, a pro-inflammatory cytokine implicated in vascular disease, was not altered in old non-Tg but was increased substantially in old E-V290M mice. Increases in TNFα and IL-6 are consistent with increased NF-κB mediated gene expression. Of note, mRNA levels of the senescence gene CDKN2A24 also tended to increase in old non-Tg, but increased signficantly in old E-V290M mice. These changes were selective as there were no significant differences in expression of eNOS, HGF, RhoA, ROCK2, or AT1-R in old E-V290M mice (Figure 6 and S3). Although the approach is common, a limitation of these data relates to the fact that expression was measured in intact vessels. With the exception of eNOS (and perhaps RPB7), the genes quantified are not specifically expressed in endothelial cells. For example, if changes in IL-6 in old E-V290M mice were confined to endothelium, we may be underestimating the true magnitude of change in that group.
Figure 6.
Relative expression levels of mRNA in aorta from adult and old non-transgenic (NT) and E-V290M mice. * P<0.05 versus adult mice, n = 6 in each group. Statistical differences were based on two-way ANOVA followed by Tukey’s multiple comparison post-hoc test.
DISCUSSION
There are several new findings in this study. At the ages studied, endothelial function was similar in adult and old non-Tg mice. Genetic interference with PPARγ in endothelium had no significant effect on vascular function in adult mice but produced substantial endothelial dysfunction in old E-V290M mice. Thus, the impact of endothelial PPARγ on endothelium-dependent vasodilation was context dependent and unmasked by aging. Despite no difference in biological age, interference with endothelial PPARγ predisposed to fundamental features of vascular aging. The magnitude of endothelial dysfunction in aged E-V290M mice was sex-independent. Mechanistic studies suggested that age-induced endothelial dysfunction did not involve COX, but required activity of NADPH oxidase, superoxide, and ROCK (Figure S4). Consistent with these functional data, vascular expression of Nox2 and a molecular marker of cellular senescence were increased following endothelial interference with PPARγ in aged mice. To our knowledge, this is the first data for any portion of the vasculature to suggest that endothelial PPARγ plays an essential role protecting against age-related oxidative stress, inflammation, senescence, and endothelial dysfunction. While we studied the carotid artery and aorta in the present study, we assume these protective effects of endothelial PPARγ are not unique to these vessels.
Endothelial function and aging
Endothelial cells impact the biology of large and small vessels in part through effects they exert on other cells, vascular and non-vascular.3, 25 An example of this influence is their effect on smooth muscle and vascular tone. Examination of vasomotor responses controlled by endothelial cells is common in both basic science and clinical trials. Studies of this type provide insight into the regulation of vascular tone while providing an index of endothelial ‘health’, a predictor of vascular events.3, 6 This endpoint also provides insight into the effectiveness of therapeutic interventions. Thus, we focused primarily on effects of age and PPARγ interference on endothelial function.
Endothelial dysfunction occurs with aging.1, 3–5, 7 While the loss of endothelial function progresses with increasing age, the temporal pattern depends on the species and the specific vessel.4, 26, 27 In non-Tg mice, we found little change in endothelial function in carotid arteries at the ages studied, results consistent with previous studies that used mice at approximately the same age.16, 17 Endothelial dysfunction in carotid artery and aorta of control mice become evident as the animals grow even older.26, 28 In contrast to non-Tg mice, substantial impairment of endothelium-dependent vasodilation was present in old E-V290M mice. These changes were selective for endothelium because responses to an endothelium-independent vasodilator were not significantly altered in old E-V290M mice.
Impact of PPARγ within the vasculature
Although it was initially thought to primarily function within adipocytes, it is now apparent that PPARγ can be active in multiple cell types with cell-specific effects.9 PPARγ is expressed in endothelium, including in the carotid artery.9, 29 In various portions of the circulation, systemic TZD treatment affects vascular structure and has beneficial effects on vasomotor tone and vascular permeability in models of disease.3, 9 Findings such as these, along with other lines of evidence suggest that pharmacological activation of PPARγ has protective effects on vascular cells.9, 10
Patients with dominant negative mutations in the ligand-binding domain of PPARγ (V290M or P467L) exhibit early-onset hypertension and diabetes.11 Using mice expressing these same mutations provides a selective genetic approach to study the impact of PPARγ, avoid off-target effects of TZDs, and gain insight into PPARγ-dependent effects driven by endogenous ligands. Mice expressing the murine equivalent of the P467L mutation (P465L) in all cells share features of human disease including abnormal fat distribution, changes in circulating metabokines, and elevated blood pressure.30, 31 In contrast, when these genetic manipulations are targeted to endothelial cells, there are no significant changes in body weight, fat distribution, arterial pressure, plasma glucose or cholesterol.13, 32, 33 Because we did not measure arterial pressure in the current study, we cannot exclude possibility that arterial pressure was increased in old E-V290M mice and that such changes could have contributed to changes in vascular function.
A few studies suggested that PPARγ may play a role in aging. For example, a global reduction in PPARγ expression reduces lifespan in mice.34 Treatment of mesenteric arteries from elderly humans with a PPARγ agonist improved endothelial function.10 Previously we found that aging unmasked endothelial dysfunction in mice that express the P465L mutation in PPARγ in all cells.17 Such data support the concept that PPARγ protects the vasculature but provided no insight into the cell type(s) or mechanisms involved. The current studies provide direct evidence that vascular endothelium is a key cell type where PPARγ normally acts to inhibit mechanisms that promote oxidative stress and vascular aging (Figure S4). Collectively, the findings indicate that PPARγ is protective during aging, being functionally important in the absence of any pharmacological manipulation.
Oxidative stress and vascular aging
Studies in animal models and aged humans suggest reactive oxygen species (ROS) and oxidative stress plays an important role in age-dependent changes within the vasculature. For example, both pharmacological and genetic approaches indicate that oxidative stress underlies impairment of endothelium-dependent vasodilation, a key feature of vascular aging.3, 4, 7, 8, 16, 26, 27 Most evidence supports the concept that interactions between NO and superoxide, with resulting loss of NO-mediated signaling is the mechanistic basis for this form of dysfunction.27, 35–37
Although antioxidant effects have been described based upon systemic treatment with TZDs or genetic interference with PPARγ in all cells, the cellular site(s) that mediates these effects have not been defined and is not easy to predict. For example, genetic interference with PPARγ in smooth muscle has profound effects on vascular function without any apparent contribution by oxidative stress.20, 32
NADPH oxidases are a prominent source of superoxide in vascular cells including endothelium.3, 4, 8 Multiple lines of evidence suggest this enzyme complex plays an essential role in vascular abnormalities in models of aging and in arteries from older humans4, 7, 8. Our findings that a scavenger of superoxide or an inhibitor of NADPH oxidase acutely restored endothelial function to normal levels in old E-V290M mice supports the concept that oxidative stress is a key contributor to this vascular abnormality. The finding that Nox2 expression was higher in these mice supports these findings as well. Because we were limited in the number of aged E-V290M mice available, we did not measure ROS levels or activity of NADPH oxidase. This represents a limitation of the study.
In addition to oxidative stress, other processes can potentially interact with ROS and thus contribute to changes with aging.3 While activation of a COX-dependent mechanism is one such process,3 our data did not support such a possibility in old E-V290M mice. These findings in mice are consistent with data obtained in aged humans where inhibition of COX did not affect impaired endothelium.37 Rather it appears that the NO-component of the vasodilator response is the key mechanism affected.37
A key role for ROCK during vascular aging
As part of our effort to evaluate mechanisms in old E-V290M mice, we examined the role of ROCK. ROCK is the effector or target of RhoA, a small GTPase activated by diverse stimuli including ROS.19 In combination with RhoA, ROCK exerts important effects in the vasculature but its impact varies regionally and is cell-specific.19 In vascular muscle, ROCK is a major determinant of vascular tone due to effects on calcium sensitivity and the regulatory myosin light chain.19 Several levels of interaction between ROCK and eNOS/NO-dependent signaling have been described.19 In endothelial cells, ROCK inhibits eNOS expression and activity (via effects on mRNA stability and protein phosphorylation). Y27632 inhibits both isoforms of ROCK equally effectively.18 We found that endothelial dysfunction in old E-V290M mice were reversed by this inhibitor. While our data support the concept that ROCK plays an important role in vascular aging, it does not provide insight into which isoform was involved. Recent experiments suggest that ROCK2 plays the dominant role in endothelial dysfunction produced by angiotensin II.18 The present study is consistent with the concept that PPARγ in endothelial cells normally inhibits activation of RhoA/ROCK, thus supporting endothelium-dependent vasodilation. Chronic inhibition of ROCK by PPARγ is potentially significant because activity of Rho kinase is positively associated with cardiovascular events including stroke.38
Perspectives and significance
Although the presence of vascular disease is a well-known feature of aging, its biology has been greatly understudied relative to its clinical impact. When cell-specific mechanisms are taken into consideration, this gap becomes even greater. While it is not uncommon in the study of atherosclerosis to perform cell-specific experimental manipulations, such approaches have been rarely applied to the study of vascular aging.
Data from experimental models and people suggest that interacting inflammatory and oxidative-dependent mechanisms are driving forces responsible for age-induced endothelial dysfunction along with stiffening of large arteries, vascular cell senescence, and atherosclerosis.5, 39 The impact of endothelial PPARγ in vascular aging is difficult to predict when diversity of gene targets along with possible gene-age interations are taken into account.12 The present study provides the first evidence that age-related vascular dysfunction, inflammation, and senescence is accelerated following interference with endothelial PPARγ, supporting the concept that endothelial PPARγ normally protects against such changes. In relation to these findings, it is interesting that the CANTOS trial has provided outcome data that targeting inflammation in patients with vascular disease, most of whom were over 60 years of age, is beneficial.40 The finding of an essential protective role for endothelial PPARγ has implications for vascular pathophysiology as well as therapeutic approaches for vascular aging.
Caloric restriction is perhaps the most consistent experimental approach known to delay aging.41 The discovery of caloric restriction mimetics is a topic of interest due to their potential therapeutic applications. In this regard, one of the molecules with the strongest caloric restriction mimetic activity is PPARγ.42 The current findings related to vascular function and changes in expression of CDK2NA are consistent with this emerging concept. Thus, the study of PPARγ, along with target molecules that control these processes, may provide better insight into vascular abnormalities that occur with aging or other cardiovascular risk factors.
Supplementary Material
Novelty and Significance.
1. What Is New?
Genetic interference with PPARγ in endothelium did not affect vascular function under baseline conditions, but augmented key features of vascular aging in mice.
Age-induced endothelial dysfunction following interference with PPARγ involved oxidant- and inflammatory-signaling (NADPH oxidase, superoxide) along with a significant contribution by ROCK.
Despite no difference in biological age, interference with endothelial PPARγ predisposed to fundamental features of vascular aging.
2. What Is Relevant?
Although changes during vascular aging reflect integrated mechanisms, the impact of cell-specific mechanisms in these changes are very poorly defined.
In contrast to activation of PPARγ with pharmacological ligands, much less is known regarding the importance of PPARγ when driven by endogenous ligands.
When challenged with angiotensin II, endothelial PPARγ influences vascular function through mechanisms that include oxidative stress. The present findings suggest there may be common underlying mechanisms related to endothelial PPARγ in both hypertension and aging.
Little is known regarding the impact of ROCK in vascular aging.
3. Summary
This study supports the concept that key features of vascular aging are augmented by genetic interference with PPARγ within endothelial cells, while providing new insight into mechanisms involved. These changes appear to involve oxidant- and inflammatory-related mechanisms, interacting with ROCK. In addition to its influence on vascular tone, endothelial PPARγ may be a determinant of cellular senescence. Loss of these beneficial effects of endothelial PPARγ due to disease, genetic, or protein alterations has implications for severity and therapeutic approaches for vascular aging.
Acknowledgments
SOURCES OF FUNDING
This work was supported by funding from the NIH (HL-113863, NS-096465, HL-125603 and HL-131689), the Department of Veteran’s Affair’s (BX001399), the Fondation Leducq (Transatlantic Network of Excellence), and the National Health and Medical Research Council of Australia (1053786).
Footnotes
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
References
- 1.Wang M, Jiang L, Monticone RE, Lakatta EG. Proinflammation: the key to arterial aging. Trends Endocrinol Metab. 2014;25:72–79. doi: 10.1016/j.tem.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothwell PM, Coull AJ, Silver LE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 3.Faraci FM. Protecting against vascular disease in brain. Am J Physiol. 2011;300:H1566–1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Manas L, El-Assar M, Vallejo S, Lopez-Doriga P, Solis J, Petidier R, Montes M, Nevado J, Castro M, Gomez-Guerrero C, Peiro C, Sanchez-Ferrer CF. Endothelial dysfunction in aged humans is related with oxidative stress and vascular inflammation. Aging Cell. 2009;8:226–238. doi: 10.1111/j.1474-9726.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- 5.Martens CR, Seals DR. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J Physiol. 2016;594:7177–7195. doi: 10.1113/JP272348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emdin CA, Khera AV, Klarin D, et al. Phenotypic consequences of a genetic predisposition to enhanced nitric oxide signaling. Circulation. 2018;137:222–232. doi: 10.1161/CIRCULATIONAHA.117.028021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayhan WG, Arrick DM, Sharpe GM, Sun H. Age-related alterations in reactivity of cerebral arterioles: Role of oxidative stress. Microcirculation. 2008;15:225–236. doi: 10.1080/10739680701641421. [DOI] [PubMed] [Google Scholar]
- 8.Park L, Anrather J, Girouard H, Zhou P, Iadecola C. Nox2-derived reactive oxygen species mediate neurovascular dysregulation in the aging mouse brain. J Cerebral Blood Flow Metabol. 2007;27:1908–1918. doi: 10.1038/sj.jcbfm.9600491. [DOI] [PubMed] [Google Scholar]
- 9.Ketsawatsomkron P, Pelham CJ, Groh S, Keen HL, Faraci FM, Sigmund CD. Does peroxisome proliferator-activated receptor-γ (PPARγ) protect from hypertension directly through effects in the vasculature? J Biol Chem. 2010;285:9311–9316. doi: 10.1074/jbc.R109.025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angulo J, Vallejo S, El Assar M, Garcia-Septiem J, Sanchez-Ferrer CF, Rodriguez-Manas L. Age-related differences in the effects of α and γ peroxisome proliferator-activated receptor subtype agonists on endothelial vasodilation in human microvessels. Exp Gerontol. 2012;47:734–740. doi: 10.1016/j.exger.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AF, Chatterjee VK, O’Rahilly S. Dominant negative mutations in human PPARγ associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 12.Simino J, Shi G, Bis JC, et al. Gene-age interactions in blood pressure regulation: A large-scale investigation with the CHARGE, Global BPgen, and ICBP Consortia. Am J Hum Genet. 2014;95:24–38. doi: 10.1016/j.ajhg.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, Sigmund CD. Endothelium-specific interference with peroxisome proliferator activated receptor γ causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Silva TM, Hu C, Kinzenbaw DA, Modrick ML, Sigmund CD, Faraci FM. Genetic interference with endothelial PPAR-γ (peroxisome proliferator-activated receptor-γ) augments effects of angiotensin II while impairing responses to angiotensin 1–7. Hypertension. 2017;70:559–565. doi: 10.1161/HYPERTENSIONAHA.117.09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wendell CR, Zonderman AB, Metter EJ, Najjar SS, Waldstein SR. Carotid intimal medial thickness predicts cognitive decline among adults without clinical vascular disease. Stroke. 2009;40:3180–3185. doi: 10.1161/STROKEAHA.109.557280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Didion SP, Kinzenbaw DA, Schrader LI, Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension. 2006;48:1072–1079. doi: 10.1161/01.HYP.0000247302.20559.3a. [DOI] [PubMed] [Google Scholar]
- 17.Modrick ML, Kinzenbaw DA, Chu Y, Sigmund CD, Faraci FM. Peroxisome proliferator-activated receptor-γ protects against vascular aging. Am J Physiol. 2012;302:R1184–1190. doi: 10.1152/ajpregu.00557.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Silva TM, Kinzenbaw DA, Modrick ML, Reinhardt LD, Faraci FM. Heterogeneous impact of ROCK2 on carotid and cerebrovascular function. Hypertension. 2016;68:809–817. doi: 10.1161/HYPERTENSIONAHA.116.07430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawada N, Liao JK. Rho/Rho-associated coiled-coil forming kinase pathway as therapeutic targets for statins in atherosclerosis. Antioxidants Redox Signaling. 2014;20:1251–1267. doi: 10.1089/ars.2013.5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Silva TM, Modrick ML, Ketsawatsomkron P, Lynch C, Chu Y, Pelham CJ, Sigmund CD, Faraci FM. Role of peroxisome proliferator-activated receptor-γ in vascular muscle in the cerebral circulation. Hypertension. 2014;64:1088–1093. doi: 10.1161/HYPERTENSIONAHA.114.03935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, Sigmund CD. Interference with PPARγ function in smooth muscle causes vascular dysfunction and hypertension. Cell Metabol. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katusic ZS, Vanhoutte PM. Superoxide anion is an endothelium-derived contracting factor. Am J Physiol. 1989;257:H33–37. doi: 10.1152/ajpheart.1989.257.1.H33. [DOI] [PubMed] [Google Scholar]
- 23.Hu C, Keen HL, Lu KT, Liu X, Wu J, Davis DR, Ibeawuchi SC, Vogel S, Quelle FW, Sigmund CD. Retinol-binding protein 7 is an endothelium-specific PPARγ cofactor mediating an antioxidant response through adiponectin. JCI Insight. 2017;2:e91738. doi: 10.1172/jci.insight.91738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roos CM, Hagler M, Zhang B, Oehler EA, Arghami A, Miller JD. Transcriptional and phenotypic changes in aorta and aortic valve with aging and MnSOD deficiency in mice. Am J Physiol. 2013;305:H1428–1439. doi: 10.1152/ajpheart.00735.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flammer AJ, Luscher TF. Three decades of endothelium research: from the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Weekly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 26.Brown KA, Didion SP, Andresen JJ, Faraci FM. Effect of aging, MnSOD deficiency, and genetic background on endothelial function: evidence for MnSOD haploinsufficiency. Arterioscler Thromb Vasc Biol. 2007;27:1941–1946. doi: 10.1161/ATVBAHA.107.146852. [DOI] [PubMed] [Google Scholar]
- 27.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol. 2009;296:H1914–1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx N, Bourcier T, Sukhova GK, Libby P, Plutzky J. PPARγ activation in human endothelial cells increases plasminogen activator inhibitor type-1 expression: PPARγ as a potential mediator in vascular disease. Arterioscler Thromb Vasc Biol. 1999;19:546–551. doi: 10.1161/01.atv.19.3.546. [DOI] [PubMed] [Google Scholar]
- 30.Pendse AA, Johnson LA, Kim HS, McNair M, Nipp CT, Wilhelm C, Maeda N. Pro- and antiatherogenic effects of a dominant-negative P465L mutation of peroxisome proliferator-activated receptor-γ in apolipoprotein E-null mice. Arterioscler Thromb Vasc Biol. 2012;32:1436–1444. doi: 10.1161/ATVBAHA.112.248682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai YS, Kim HJ, Takahashi N, Kim HS, Hagaman JR, Kim JK, Maeda N. Hypertension and abnormal fat distribution but not insulin resistance in mice with P465L PPARγ. J Clin Invest. 2004;114:240–249. doi: 10.1172/JCI20964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelham CJ, Keen HL, Lentz SR, Sigmund CD. Dominant negative PPARγ promotes atherosclerosis, vascular dysfunction, and hypertension through distinct effects in endothelium and vascular muscle. Am J Physiol. 2013;304:R690–701. doi: 10.1152/ajpregu.00607.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu C, Lu KT, Mukohda M, Davis DR, Faraci FM, Sigmund CD. Interference with PPARγ in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol Genomics. 2016;48:124–134. doi: 10.1152/physiolgenomics.00087.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argmann C, Dobrin R, Heikkinen S, Auburtin A, Pouilly L, Cock TA, Koutnikova H, Zhu J, Schadt EE, Auwerx J. Pparγ2 is a key driver of longevity in the mouse. PLoS Genet. 2009;5:e1000752. doi: 10.1371/journal.pgen.1000752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toth P, Tarantini S, Tucsek Z, Ashpole NM, Sosnowska D, Gautam T, Ballabh P, Koller A, Sonntag WE, Csiszar A, Ungvari Z. Resveratrol treatment rescues neurovascular coupling in aged mice: role of improved cerebromicrovascular endothelial function and downregulation of NADPH oxidase. Am J Physiol. 2014;306:H299–308. doi: 10.1152/ajpheart.00744.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA, Donato AJ. Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age. 2014;36:559–569. doi: 10.1007/s11357-013-9585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eisenach JH, Gullixson LR, Allen AR, Kost SL, Nicholson WT. Cyclo-oxygenase-2 inhibition and endothelium-dependent vasodilation in younger vs. older healthy adults. Br J Clin Pharmacol. 2014;78:815–823. doi: 10.1111/bcp.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kajikawa M, Noma K, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Liao JK, Higashi Y. Rho-associated kinase activity is a predictor of cardiovascular outcomes. Hypertension. 2014;63:856–864. doi: 10.1161/HYPERTENSIONAHA.113.02296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhayadia R, Schmidt BM, Melk A, Homme M. Senescence-induced oxidative stress causes endothelial dysfunction. J Gerontology. 2016;71:161–169. doi: 10.1093/gerona/glv008. [DOI] [PubMed] [Google Scholar]
- 40.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 41.Balasubramanian P, Howell PR, Anderson RM. Aging and caloric restriction research: A biological perspective with translational potential. EBioMedicine. 2017;21:37–44. doi: 10.1016/j.ebiom.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barger JL, Vann JM, Cray NL, Pugh TD, Mastaloudis A, Hester SN, et al. Identification of tissue-specific transcriptional markers of caloric restriction in the mouse and their use to evaluate caloric restriction mimetics. Aging Cell. 2017;16:750–760. doi: 10.1111/acel.12608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.