Abstract
Ethanol is one of the most commonly used substances in the world. Behavioral effects of alcohol are well described, however, cellular mechanisms of its action are poorly understood. There is an apparent contradiction between measurable behavioral changes produced by low concentrations of ethanol, and lack of evidence of synaptic changes at these concentrations. Furthermore, effects of ethanol on synaptic transmission in the neocortex are poorly understood. Here, we set to determine effects of ethanol on excitatory synaptic transmission in the neocortex. We show that 1–50 mM ethanol suppresses excitatory synaptic transmission to layer 2/3 pyramidal neurons in rat visual cortex in a concentration-dependent manner. To the best of our knowledge, this is the first demonstration of the effects of very low concentrations of ethanol (from 1 mM) on synaptic transmission in the neocortex. We further show that a selective antagonist of A1 adenosine receptors, 8-cyclopentyl-1,3-dipropylxanthine (DPCPX), blocks effects of 1–10 mM ethanol on synaptic transmission. However, the reduction in excitatory postsynaptic potential amplitude by 50 mM ethanol was not affected by DPCPX. We propose that ethanol depresses excitatory synaptic transmission in the neocortex by at least two mechanisms, engaged at different concentrations: low concentrations of ethanol reduce synaptic transmission via A1R-dependent mechanism and involve presynaptic changes, while higher concentrations activate additional, adenosine-independent mechanisms with predominantly postsynaptic action. Involvement of adenosine signaling in mediating effects of low concentrations of ethanol may have important implications for understanding alcohol’s effects on brain function, and provide a mechanistic explanation to the interaction between alcohol and caffeine.
Keywords: A1 receptor, adenosine, ethanol, neocortex, synaptic transmission
Introduction
Ethanol (EtOH) is one of the most commonly used substances in the world and has effects on diverse systems, including digestive, cardiovascular and nervous. Behavioral effects of different concentrations of ethanol are well described (e.g., Little, 1999; Zorumski et al., 2014; Dar, 2015). Intoxicating effects of ethanol on the nervous system start at about 5–20 mM (21.7 mM corresponds to 0.1% blood alcohol concentration (BAC); and about 23 mM corresponds to 1 g/kg), causing mood changes, excitation and impaired cognition (e.g., Little, 1999; Zorumski et al., 2014; http://pubs.niaaa.nih.gov/publications/AlcoholOverdoseFactsheet/Overdosefact.htm). Higher concentrations of alcohol (20–40 mM) typically impair motor coordination, and have an anxiolytic and sedation effects, while yet higher concentrations (60–100 mM) may affect breathing, heart rate and lead to coma, and are considered life-threatening. Despite detailed characterization of behavioral effects of ethanol, cellular mechanisms of its action are poorly understood. Specific receptors for ethanol have not been identified so far, rather, it affects a multitude of biochemical cascades, biological molecules and transmitter systems (e.g., Ariwodola & Weiner, 2004; Kelm et al., 2011; Förstera et al., 2016). An overall effect of ethanol on excitation and inhibition in the brain is a suppression of glutamatergic transmission and potentiation of GABAergic transmission. However, studies of ethanol effects in different brain regions report highly heterogeneous results, indicating that details of the actions of ethanol are non-uniform but structure specific. In CA1 pyramidal neurons of the hppocampus, NMDA-mediated synaptic responses were selectively inhibited by 25–100 mM ethanol (Lovinger et al., 1990; Hendricson et al., 2004; Proctor et al., 2006). In nucleus accumbens excitatory postsynaptic potential (EPSPs) consisting of predominantly α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-mediated components were reduced by 22–66 mM (but not by 11 mM) of ethanol (Nie et al., 1993). GABAergic transmission was enhanced by ethanol (40–100 mM) in hippocampal CA1 neurons, Purkinje cells in cerebellum, amygdala or substantia nigra (Ariwodola & Weiner, 2004; Proctor et al., 2006; Criswell et al., 2008; Kelm et al., 2011), but not in lateral septum and medial prefrontal cortex neurons (Criswell et al., 2008; Kelm et al., 2011).
Two gaps in our knowledge which hinder progress in understanding synaptic mechanisms of ethanol effects on brain function are evident. First is the lack of evidence for effects of low concentrations of ethanol on synaptic transmission. Significant effects on synaptic transmission were reported only at ethanol concentrations > 20 mM (typically from 40 to 50 mM), which is higher than concentrations at which clear behavioral effects can be measured (< 20 mM, typically from 5 to 15 mM, for example, Wallgren & Barry, 1970; Givens & McMahon, 1997; Little, 1999). Remarkably, a recent study (Rae et al., 2014) reports that ethanol at a yet lower concentration (0.1 mM) had significant effects on metabolism of a number of biologically active molecules, including glutamate and GABA, and 1 mM ethanol affected all metabolic parameters measured in this study (e.g., decreased incorporation of 13C-pyruvate and its products in Krebs cycle, glycolytic byproducts alanine and lactate, and decreased total metabolite pool sizes). Second is lack of data on effects of ethanol on synaptic transmission in the neocortex. Alterations of cognitive functions by ethanol (e.g., Givens & McMahon, 1997; Little, 1999) indicate that ethanol might affect cortical neurons and synapses, however, effects of low concentrations of ethanol on synaptic transmission in neocortex are not investigated.
One possible mechanism of ethanol’s action on synaptic transmission is via an adenosine pathway. Adenosine is a metabolite of adenosine triphosphate (ATP) and a ubiquitous neuromodulator in the brain. During vigorous neuronal activity, adenosine and ATP can be released from neurons and astrocytes into the extracellular space, where ATP is broken down to adenosine by ectonucleotidases (Wall & Dale, 2009; Lovatt et al., 2012; Pajski & Venton, 2012). Clearance of adenosine from extracellular space involves nucleoside transporters embedded in the cell membrane. One type of these transporters, equilibrative nucleoside transporter 1 (ENT1) is sensitive to ethanol, which acts as a transporter blocker. By inhibiting adenosine uptake, ethanol leads to an increase of extracellular adenosine tone (Nagy et al., 1990; Choi et al., 2004; Mailliard & Diamond, 2004; Allen-Gipson et al., 2009). Indeed, agonists of adenosine A1 receptors (A1R) accentuated ethanol-induced motor incoordination (Dar, 2001; Dar & Mustafa, 2002) and increased anxiolytic effect of ethanol (Prediger et al., 2004). Antagonists of A1R had an opposite effect, reducing both motor incoordination and anxiolytic effect of ethanol (Dar, 2001; Dar & Mustafa, 2002; Prediger et al., 2004). Similarly, natural decrease of adenosine tone after lasting sleep restriction decreased the motor-impairing effects of alcohol (Clasadonte et al., 2014). Hypnotic and ataxic effects of ethanol were reduced in ENT1-null mice compared to wild-type littermates (Choi et al., 2004). The equilibrative transporters as well as the adenosine A2A receptor (A2AR) are involved in regulation of ethanol tolerance and drinking behavior (Diamond et al., 1991; Choi et al., 2004; Nam et al., 2013a,b).
Here, we set to determine the effects of ethanol on excitatory synaptic transmission in neocortex, and address a possible role of an adenosine pathway in these effects. As experimental model we have chosen excitatory transmission to layer 2/3 pyramidal neurons in visual cortex. Layer 2/3 pyramids mediate interaction between cortical areas, and thus play central role in cortical processing and integration. Furthermore, because prior research showed that adenosine modulates transmission at excitatory synapses to L2/3 pyramids and identified A1Rs as a major receptor mediating this modulation (Bannon et al., 2014), this experimental model allows us to address the possible involvement of adenosine-pathway into effects of ethanol. We ask how low to moderate concentrations of ethanol (0.1–50 mM) affect EPSPs in layer 2/3 pyramids, and whether effects of ethanol on synaptic transmission depend on an adenosine pathway, specifically on activity of A1Rs. We show that 1–50 mM of ethanol reduces EPSP amplitudes in L2/3 pyramidal neurons in a concentration-dependent manner. We further show that a selective antagonist of A1R, DPCPX, blocks effects of low concentrations of ethanol (1–10 mM) on synaptic transmission. However, reduction in EPSP amplitude by 50 mM of ethanol was not affected by DPCPX, indicating the involvement of mechanism(s) which are independent of A1R activation. We propose that ethanol depresses excitatory synaptic transmission in the neocortex by at least two mechanisms activated at different concentrations: low concentrations of ethanol reduce synaptic transmission via an A1R-dependent mechanism, while higher ethanol concentrations activate additional, adenosine-independent mechanisms.
Materials and methods
Slice preparation
All experimental procedures used in this study are in compliance with the US National Institutes of Health regulations and were approved by the Institutional Animal Care and Use Committee of the University of Connecticut. Details of slice preparation and recording were similar to those used in previous studies (Volgushev et al., 2000; Lee et al., 2012; Bannon et al., 2014). In this study, we used 34 male Wistar rats (18–32 day old) purchased from Charles-River or Harlan. Rats were anesthetized with isoflurane, decapitated, and the brain was quickly removed and placed into an ice-cold oxygenated artificial cerebrospinal fluid solution (ACSF), containing, in mM: 125 NaCl, 25 NaHCO3, 25 glucose, 3 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, bubbled with 95% O2/5% CO2, pH 7.4. Coronal slices (350-μm thickness) containing the visual cortex were prepared from the right hemisphere. Slices were allowed to recover for at least 1 h at room temperature. For recording, individual slices were transferred to a recording chamber mounted on an Olympus BX-50WI microscope equipped with infrared differential interference contrast (IR-DIC) optics. In the recording chamber slices were submerged in oxygenated ACSF at 28–32 °C.
Intracellular recording and synaptic stimulation
Layer 2/3 pyramidal cells from the visual cortex were selected for recording in the whole-cell configuration. Identification of pyramidal neurons using DIC microscopy was reliable as demonstrated in our previous work with biocytin labeling and morphological reconstruction of recorded neurons (Volgushev et al., 2000). Intracellular pipette solution contained, in mM: 130 K-Gluconate, 20 KCl, 10 HEPES, 10 Na-Phosphocreatine, 4 Mg-ATP, 0.3 Na2-guanosine triphosphate (pH 7.4 with KOH). Whole-cell recordings were made using patch electrodes (4–6 MΩ) in bridge mode of Axoclamp 2B (Axon Instruments, USA) or Dagan BVC-700A (Dagan Corporation, USA) amplifier. We opted for bridge mode over the voltage clamp because bridge mode allows to capture possible effects on resting membrane potential, and thus to address whether ethanol has a direct effect on the cell membrane. At the beginning of the recording, bridge compensation was set to zero, and capacitance neutralization about two turns back from maximal to avoid accidental overcompensation in the course of experiment. These settings were not changed throughout the recording. After amplification and low-pass filtering at 10 kHz membrane potential signals were digitized at 20 kHz and stored in computer using Digidata-1322A or 1440A interface and PCLAMP software (Molecular Devices, USA).
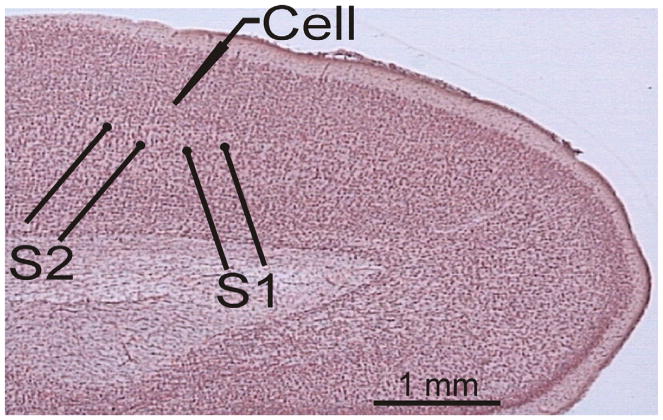
Two pairs of stimulating electrodes (S1 and S2) were placed in layer 4, below the layer 2/3 recording site (Fig. 1). Stimulation current intensities were adjusted to evoke monosynaptic excitatory postsynaptic potentials (EPSPs) in the recorded neuron. These EPSPs might be mediated by axons of layer 4 neurons or by ascending axons from deeper layers. We used a paired-pulse stimulation protocol with a 50-ms inter-pulse interval. Paired stimuli were applied to S1 and S2 in alternating sequence once per 7.5 s, so that each input was stimulated with paired pulses each 15 s. To test for the possible contribution of inhibition, evoked PSPs were recorded at membrane potentials between −50 and −40 mV, which is above the reversal potential for inhibitory responses. Only those PSPs that were still depolarizing at this membrane potential were considered excitatory and included in the analysis.
Fig. 1.
Scheme of the typical location of stimulation and recording electrodes in a slice of rat visual cortex. Recordings were made from layer 2/3 pyramidal neurons; the two stimulation electrodes (S1 and S2) were placed in layer 4 below the recording site. [Colour figure can be viewed at wileyonlinelibrary.com].
All drugs were bath applied. Experiments of the main series began by recording 60–80 test EPSPs to measure baseline responses. Ethanol (100%) was then micropipetted to the cylinder containing of extracellular medium to create a 0.1 mM solution of EtOH. Immediately after ethanol application 100–120 EPSPs were recorded. The same procedure was repeated for recording EPSPs under the 1, 10, and 50 mM EtOH, however, 80–100 sweeps were recorded in each condition. A washout was then performed by replacing the EtOH-containing solution with the original ACSF solution. 120–150 EPSPs were recorded in the course of washout (Fig. 2). In every EtOH concentration, IV relationship was recorded using 200-ms-long depolarizing and hyperpolarizing current steps of increasing amplitudes. Experiment on each cell lasted about 1.5 hours.
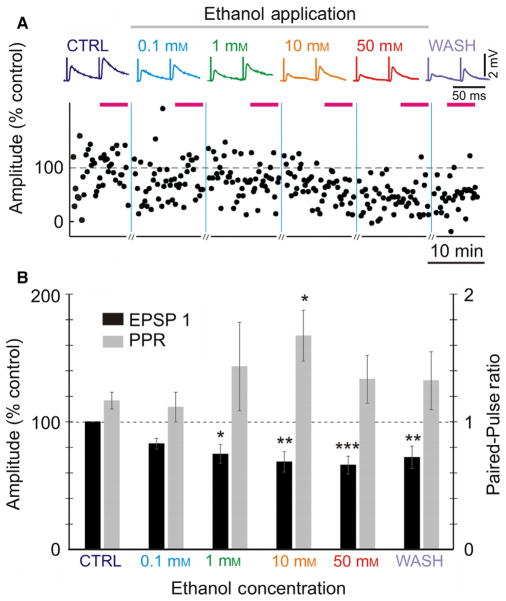
Fig. 2.
Ethanol attenuates evoked EPSP amplitudes and increases paired-pulse ratio (PPR) in a concentration-dependent manner. (A) Averaged EPSPs evoked in a layer 2/3 pyramidal neuron from the rat visual cortex by paired-pulse stimulation (50-ms inter-pulse interval) in control, and through increasing ethanol concentrations. Time course shows changes of the amplitude of individual EPSPs evoked by the first pulse in a pair (EPSP1, % of control). Horizontal bars above the plot indicate time intervals from which averaged EPSPs were calculated. Vertical lines show changes of ethanol concentration; recording of synaptic responses was briefly interrupted there for measurements of voltage–current relationships. (B) Averaged changes of EPSP1 amplitude and PPR in n = 17 inputs. EPSP amplitudes were normalized by the amplitude of EPSP1 in control. Significance denoted as *P < 0.05; **P < 0.01; ***P < 0.001. [Colour figure can be viewed at wileyonlinelibrary.com].
A separate series of experiments was performed on the background of the adenosine A1 receptor antagonist 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX; Sigma, St. Louis MO, USA, cat# cat #C101, CAS #102146-07-6). DPCPX was dissolved in DMSO (Sigma, cat# D8418, CAS # 67-68-5) to a 1 mM stock solution, and added to the extracellular solution to create a final concentration of 30 nM DPCPX (3 μL stock solution per 100 mL bath solution; final concentration of the solvent DMSO in bath solution was ~ 0.003% or ~ 0.4 mM). The slice was kept in the DPCPX-containing solution for at least 20 minutes before recordings began to allow equilibration of DPCPX binding to the A1 receptor. The experiment with different ethanol concentrations was then performed in the same exact manner as described above.
In two series of control experiments we tested effects of application of 10 mM EtOH on the background of 0.4 mM DMSO, and the effect of application of high concentration of EtOH (50 mM). In each of these series only one concentration of EtOH was used, but washout period increased to 20–30 min. An experiment of these series on one cell lasted about 50–60 min.
Data analysis
Data analysis was made using custom-written programs in MatLab (The MathWorks, Natick, MA, USA). All inputs included in the analysis fulfilled the criteria of (i) stability of EPSP amplitudes during the control period, (ii) stability of the membrane potential throughout the recording, (iii) stability of the onset latency and kinetics of the rising slope of the EPSP, and (iv) the absence of inhibitory components. EPSP amplitudes were measured as the difference between the mean membrane potential during two time windows. The first time window was placed before the EPSP onset and the second time window was placed on the rising slope of the EPSP, just before the peak. Amplitude of the second EPSP in paired-pulse stimulation paradigm was measured using windows of the same duration, but shifted by the length of the inter-pulse interval (50 ms). For calculation of paired-pulse ratio (PPR), averaged amplitudes of 15–40 EPSPs were used. The PPR was calculated as the amplitude of the averaged EPSP evoked by the second pulse divided by the amplitude of the EPSP evoked by the first pulse in the paired-pulse stimulation paradigm.
Input resistance was measured either as a slope of the voltage–current relationship (membrane potential response to steps of positive and negative current of several different amplitudes; see Fig. 6), or calculated from membrane potential responses to small amplitude (5–20 pA) hyperpolarizing current steps applied before synaptic stimulation.
Significance was determined using a General Linear Model (repeated measures) with compound symmetry and Sidak post hoc tests. Covariance structure was chosen because it was the simplest structure which accurately captured the data and yielded the most favorable information criterion (Akaike’s information criterion) over other structures.
Correlations (Pearson’s r) and their significance were calculated using IBM SPSS Statistics package (PASW Statistics version 18.0.0). For comparisons between drug groups independent sample two-tailed t-tests were used, difference was considered significant with P < 0.05. Analysis using general linear model was done in R (version 3.2.3 (2015-12-10), The R Foundation for Statistical Computing), function lm.
Results
Low concentrations of ethanol attenuate evoked EPSP amplitude and increase paired-pulse ratio
To determine the effects of ethanol on excitatory synaptic transmission to layer 2/3 pyramidal neurons of the rat visual cortex, we recorded EPSPs evoked by paired-pulse electric stimuli in control conditions and during bath application of ethanol (addition to ACSF). Concentrations of ethanol ranged from 0.1 to 50 mM (Fig. 2). Figure 2A shows example EPSPs from one such experiment. EPSP amplitudes were reduced by ethanol starting from a concentration of 1 mM, the suppression becoming progressively stronger with increasing concentration to 10 and 50 mM. Washout of ethanol led to a partial recovery of responses (Fig. 2A). Averaged results from n = 17 experiments expressed similar pattern of amplitude changes (Fig. 2B). Averaged amplitude of test EPSPs in control was 1.03 ± 0.14 mV (n = 17). Application of 0.1 mM of ethanol led to a small (~ 17%) non-significant decrease in the EPSP amplitude compared to baseline. Increasing ethanol concentration led to a further decrease in EPSP amplitude. Decrease of EPSP amplitude was significant (P < 0.05, paired t-test) at concentrations of ethanol 1 mM and higher (Fig. 2A and B). Fifty millimolars of ethanol had the strongest effect on EPSP amplitude with a ~ 34% reduction compared to baseline (66.2 ± 7.0% of baseline, P < 0.001).
Two further statistical approaches demonstrated significance of the effect of ethanol on the EPSP amplitude. First, there was strong significant correlation between changes of EPSP amplitude and ethanol concentration (r = −0.34, P < 0.01, n = 70; pooled data from the above series and control experiments, see Fig. 5 and related text). Second, the linear model showed that EPSP amplitude changes, considered response, were significantly predicted by ethanol concentration (F1,68 = 8.74, P = 0.0043) (function lm in R version 3.2.3 (2015-12-10), the R Foundation for Statistical Computing).
Reduction of EPSP amplitude in 1 and 10 mM ethanol was accompanied by a progressive increase in the PPR (Fig. 2B). The PPR was calculated as the ratio of the averaged EPSP evoked by the second pulse in the paired-pulse paradigm to the EPSP evoked by the first pulse. PPR is a measure inversely related to the release probability, and is used for assessing pre-synaptic mechanisms (Stevens, 1993; Voronin, 1993). PPR changes were significantly correlated with the change in EPSP amplitude following ethanol exposure (all concentrations pooled, r = −0.53; n = 46, P < 0.001). Correlations between PPR changes and EPSP changes was also significant for the effects of 10 mM (r = −0.66, P < 0.001) and 50 mM (r = −0.53, P < 0.01) (see below, Fig. 5 and related text).
The increase in PPR is indicative of a decrease in release probability. These results suggest that a decrease in EPSP amplitude in low concentrations of ethanol might be at least partially mediated by a presynaptic mechanism, the decrease in release probability. Note that an even stronger reduction in EPSP amplitude during application of 50 mM ethanol was not associated with a further increase of PPR (Fig. 2B).
Blockade of adenosine A1 receptors prevents the effects of low, but not high, concentrations of ethanol on synaptic transmission
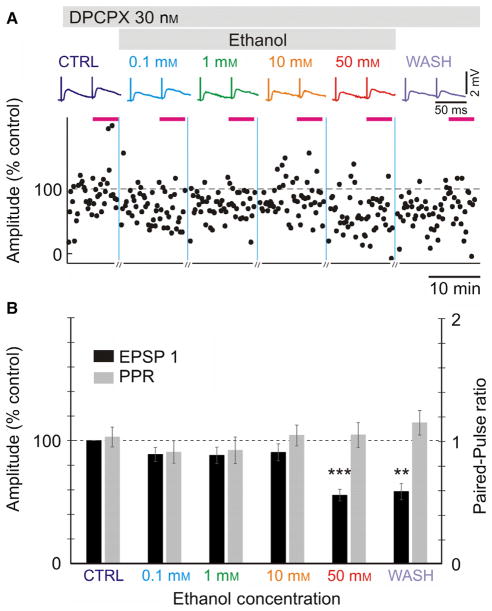
Two reasons suggest possible involvement of adenosine in mediating effects of low concentrations of ethanol on excitatory synaptic transmission to layer 2/3 pyramids. First, evidence from other structures shows that ethanol can block the re-uptake of adenosine by ENT-1 and thus lead to an increase in extracellular adenosine tone (Nagy et al., 1990; Choi et al., 2004; Mailliard & Diamond, 2004; Allen-Gipson et al., 2009). Second, the actions of low concentrations of ethanol described above were similar to the effects of adenosine on excitatory synaptic transmission which we observed in the same preparation (Bannon et al., 2014). Because reduction in EPSP amplitude by adenosine was mediated by adenosine A1 receptors (A1R) we next tested if the effects of ethanol were due to an increase in the A1R activation. To this end, we repeated experiments using the same paradigm as above, but in the presence of 30 nM of the selective A1R antagonist DPCPX in the recording medium throughout the experiment. Prior research revealed an increase in the frequency of miniature EPSPs (Bannon et al., 2014) and EPSP amplitudes (Kerr et al., 2013) after blockade of A1Rs. Therefore, before the start of recordings we incubated slices in extracellular solution with DPCPX for at least 20 min, so that the blockade of A1Rs reached steady state.
In this series of experiments, the amplitude of test EPSP in control was 0.88 ± 0.12 mV (n = 21). We found that 30 nM of DPCPX completely abolished effects of low concentrations of ethanol (0.1–10 mM) on synaptic transmission. Neither the amplitude of evoked EPSP was reduced, nor was the PPR increased during application of 0.1–10 mM ethanol (Fig. 3, P > 0.1 for 0.1, 1 and 10 mM ethanol). However, 50 mM of ethanol still induced a significant decrease in EPSP amplitude by ~ 45% in the background of DPCPX (55.8 ± 4.7% of baseline, P < 0.001; Fig. 3B).
Fig. 3.
Antagonist of adenosine A1 receptor, DPCPX, prevents the actions of ethanol at lower concentrations but not at high concentrations. (A) Averaged EPSPs evoked in a layer 2/3 pyramidal neuron by paired-pulse stimuli (50-ms interpulse interval) in control, and through increasing ethanol concentrations. Time course of changes in the amplitude of individual EPSPs evoked by the first pulse in a pair (EPSP1, % of control). Specific antagonist of adenosine A1 receptor, DPCPX (30 nM) was present in the extracellular solution throughout the experiment. (B) Averaged changes in EPSP1 amplitude and PPR in n = 21 experiments with DPCPX. EPSP amplitudes were normalized by the amplitude of EPSP1 in control. Significance denoted as **P < 0.01; ***P < 0.001. Other conventions as in Fig. 2. [Colour figure can be viewed at wileyonlinelibrary.com].
We interpret these results as an indication that in layer 2/3 of the visual cortex ethanol exerts its actions through the activation of A1R at lower concentrations, but acts via different mechanism(s) at higher concentrations.
We ran two series of control experiments to address potential concerns regarding this interpretation (Fig. 4). The first concern is whether the abolishing of the effect of low concentrations of ethanol on EPSP amplitudes in experiments with DPCPX was due the blockade of A1Rs alone or if the solvent DMSO had a compounding effect. The second concern is poor washout: in the above experiments (Figs 2B and 3B) with and without DPCPX, the EPSP amplitudes increased during the washout by only a few percent, and recovered to only 60–70% of control values.
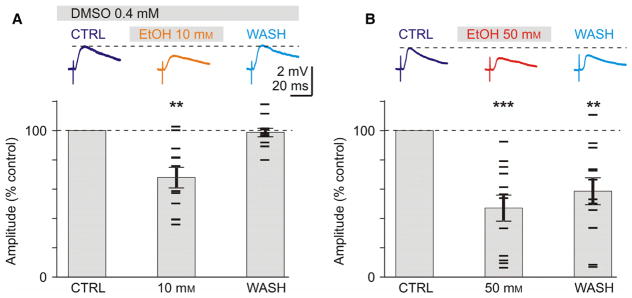
Fig. 4.
Control experiments: Successful wash out of effects of 10 mM, but not 50 mM ethanol, and validation of DMSO as a vehicle. (A) Reduction in EPSP amplitude by 10 mM ethanol is not changed in the presence of the solvent DMSO. Traces on the top show averaged EPSPs in control, during application of 10 mM EtOH and after washout of ethanol. DMSO was present in the extracellular solution throughout the experiment. Lower plot shows averaged changes of EPSP amplitudes, in percent of control, for n = 12 experiments. Mean ± SEM. Horizontal bar symbols show results from individual experiments. Note complete recovery of responses after washout of 10 mM ethanol. (B) Exposure to 50 mM concentration of ethanol leads to lasting reduction of EPSP amplitude. EPSPs from a representative experiment (top) and summary results of n = 12 experiments. Note only partial recovery of EPSP amplitude after washout (for > 20 min) of 50 mM ethanol. Significance denoted as **P < 0.01; ***P < 0.001. [Colour figure can be viewed at wileyonlinelibrary.com].
To address the first concern, we recorded EPSPs on the background of 0.4 mM DMSO (same final concentration as was used in experiments with A1R antagonist DPCPX). DMSO-containing extracellular solution was used throughout the experiment, and recordings were started after slices were incubated in this solution for at least 20 min. After recording control EPSPs (1.11 ± 0.12 mV, n = 12), we applied 10 mM of ethanol followed by washout. Application of 10 mM EtOH led to significant reduction in EPSP amplitudes to 67.9 ± 6.9% of control (n = 12, paired t-test, P < 0.01, Fig. 4A). This reduction was same as observed in the main experimental series in which 10 mM EtOH was applied after several lower concentrations (Fig. 2, 68.6 ± 8.0% of control in 10 mM EtOH). After washout for 15 min the effect of 10 mM ethanol was completely eliminated, and EPSP amplitudes recovered to 98.7 ± 2.8% of control (Fig. 4A). These results show that DMSO, at concentrations used in our experiments, did not have an effect on the reduction of EPSP amplitudes by ethanol.
To address the second concern, we tested if EPSP amplitudes could recover during washout after application of 50 mM of EtOH (Fig. 4B). We repeated the experiment as above except we excluded DMSO and applied 50 mM instead of 10 mM of ethanol. Application of 50 mM of ethanol led to a reduction in EPSPs (initial amplitude 1.56 ± 0.2 mV, n = 12) to 47.0 + 8.9% of control (n = 12, paired t-test, P < 0.001, Fig. 4B). In 4 of the 12 experiments, EPSP amplitudes were reduced dramatically to < 15% of control. After washout for 20 min, EPSPs recovered only partially, remaining significantly smaller than control (58.5 ± 9.2% of control, P < 0.01). These results are consistent with a partial recovery of EPSPs observed in the main series of experiments, in which ethanol concentration was gradually increased to 50 mM (Figs 2 and 3). Because the time frame in the two series of control experiments was similar and complete washout occurred after 10 mM ethanol, we conclude that incomplete recovery of EPSP amplitudes after application of 50 mM of ethanol might be due to the lasting effects of the high concentration of ethanol on neurons in visual cortex.
Ethanol affects synaptic transmission via adenosine-sensitive and adenosine-insensitive mechanisms
To dissociate adenosine-sensitive and adenosine-independent mechanisms of ethanol actions we compared effects of the same concentrations of ethanol in experiments with undisturbed A1R receptors, and in experiments with A1Rs blocked by DPCPX. Figure 5 shows breakdown and a side-by-side comparison of the data from Figs 2–4. Note that because the reduction in EPSP amplitudes in the main experiments with sequential use of several ethanol concentrations (Fig. 2) and in experiments with the use of only one concentration (Fig. 4) was the same, we pooled data for the respective concentrations in the analysis below.
Fig. 5.
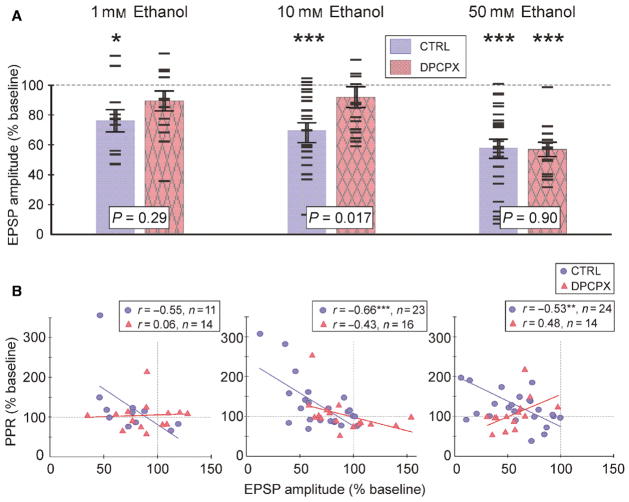
A1R-sensitive and A1R-independent mechanisms mediate suppression of EPSPs by different concentrations of ethanol. (A) Comparison EPSP amplitude changes in 1, 10 and 50 mM of ethanol for control experiments (blue; uniformly filled bars, circle symbols) and experiments with 30 nM of A1R antagonist DPCPX in the extracellular solution (pink; texture filled bars; triangle symbols). Bars show averaged EPSP amplitude changes (mean ± SEM), and horizontal dash symbols show results from individual experiments. Significance of difference to baseline is denoted above each bar (paired t-test). Significance of the difference between control and DPCPX experiments is shown for each concentration (t-test for two independent samples). (B) Changes in PPR plotted against changes in EPSP amplitude for 1, 10 and 50 mM ethanol in control series and DPCPX experiments. Significance denoted as *P < 0.05; **P < 0.01; ***P < 0.001. [Colour figure can be viewed at wileyonlinelibrary.com].
One millimolar of ethanol suppressed EPSP amplitudes by ~ 25% (74.7 ± 7.4% of baseline, P < 0.05, n = 11) in the control group, but led to only a ~ 12% decrease (88.0 ± 6.6%, n.s., n = 14) in the DPCPX group. The difference between EPSP reduction in these groups was not significant (P = 0.29, independent samples t-test).
Blockade of A1Rs with DPCPX had the strongest effect at 10 mM ethanol. In the pooled control group, 10 mM ethanol lead to a significant decrease in EPSP amplitude by ~ 32%, to 68.3 ± 5.1% of baseline (P < 0.001, n = 23). In the presence of DPCPX, 10 mM of ethanol did not significantly decrease the EPSP amplitude, which remained at 90.5 ± 7.0% of baseline (n.s. n = 16). The reduction of EPSP amplitude by 10 mM ethanol was significantly stronger in control as compared to experiments with DPCPX (P = 0.017, t-test for independent samples).
Application of 50 mM of ethanol led to a significant reduction in the EPSP amplitudes irrespective of the blockade of A1Rs. EPSP amplitudes were reduced to 56.6 ± 5.9% (P < 0.001, n = 24) in the control group, and to 55.6 ± 4.7% of baseline (P < 0.001, n = 14) in the presence of DPCPX. There is no significant difference between the two groups (P = 0.90).
Thus, low concentrations of ethanol (1 and 10 mM) led to a significant reduction in EPSP amplitudes via an adenosine A1R-dependent pathway. Higher concentration of ethanol (50 mM) reduced EPSP amplitudes to a similar extent in control experiments and in the presence of DPCPX and therefore employed A1R-independent mechanism(s).
This conclusion is further substantiated by a comparison of the relation between the changes in EPSP amplitude and changes in the PPR by ethanol in control and DPCPX experiments (Fig. 5B). In control experiments with 10 mM ethanol we found a strong negative correlation between EPSP and PPR changes (r = −0.66, n = 23, P < 0.001). Negative correlation between EPSP and PPR changes was also found in control experiments with 50 mM of ethanol application (r = −0.53, n = 24, P < 0.01). This negative correlation indicates that the decrease in EPSP amplitude was at least partially mediated by the reduction in release probability. In the application of 1 mM of ethanol in control conditions, negative correlation was just below significance level (r = −0.554, n = 11, P = 0.08). In the presence of DPCPX, no significant correlations were found between changes in EPSP amplitudes and PPR in any of the three concentration groups (Fig. 5B; 1, 10 and 50 mM in DPCPX).
Negative correlation between EPSP amplitude changes and PPR changes in low concentrations of ethanol (10 mM), and the abolishment of this correlation by the A1R antagonist, DPCPX, provide further support for the notion that low concentrations of ethanol act on presynaptic release via increased activation of A1R. The observation that such a correlation is present during the application of a high concentration of ethanol (50 mM) in control but is abolished in the presence of DPCPX, while EPSP amplitudes were reduced in both conditions, supports the notion that additional, A1R-independent and non-presynaptic mechanism(s) mediate actions of higher concentrations of ethanol on synaptic transmission.
Ethanol’s effect on input resistance and resting membrane potential
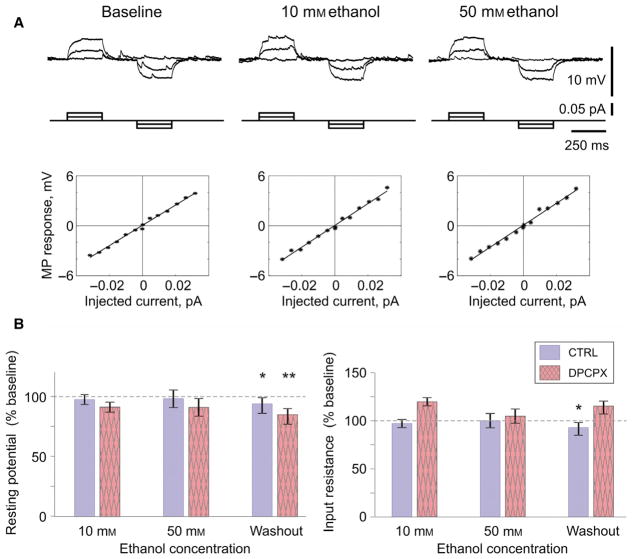
Input resistance and resting membrane potential remained stable throughout the experiment in both the control group and DPCPX group (Fig. 6). However, during washout resting membrane potential slightly depolarized, by ~ 5% of the initial baseline in control group (to 94.8 ± 2.3%, n = 24, P = 0.04) and by ~ 14% in the DPCPX group (to 85.7 ± 3.3%, n = 9, P < 0.01) (Fig. 6B).
Fig. 6.
Resting membrane potential and input resistance in layer 2/3 pyramids do not change during ethanol application. (A) Membrane potential response of a L2/3 pyramidal neuron to current steps in control experiments, during baseline and application of 10 and 50 mM of ethanol. Plots show voltage–current relationships: dependence of the membrane potential response on the amplitude of current step. Solid lines show linear regression. There was no change in the slope of the voltage–current relationship during ethanol application. (B) Average changes of the resting membrane potential (left) and input resistance (right) during application of 10 and 50 mM of ethanol and washout. The blue (uniformly filled) bars represent the control group and pink (texture filled) bars represent the DPCPX group. Significance denoted as *P < 0.05; **P < 0.01. [Colour figure can be viewed at wileyonlinelibrary.com].
Input resistance decreased during washout by ~ 8% in the control group (to 92.1 ± 3.9%, n = 24, P = 0.027) and increased by ~ 14% in the DPCPX group (to 114.2 ± 6%, n = 9, P = 0.053).
Note that Fig. 6 presents washout data for input resistance and resting membrane potential after prolonged exposure to increasing concentrations of ethanol (typically about 50 min), including high concentration (50 mM). Because neither membrane potential nor input resistance changed during washout in experiments with only 10 mM ethanol application (membrane potential 99 + 1.6%, input resistance 92.3 + 4.6%, n = 8, n.s.), we attribute these effects of 50 mM to a lasting action of high ethanol concentration. One possible scenario is that 50 mM of ethanol affected cell metabolism and caused changes which developed on a time scale longer than duration of our recordings. One further source of damaging effects of high ethanol concentration may be the increased osmolarity of the extracellular solution. These interpretations are supported by a poor washout of synaptic responses after application of 50 mM ethanol (Figs 2–4).
Discussion
We show that 1–50 mM of ethanol suppresses excitatory synaptic transmission to layer 2/3 pyramidal neurons in rat visual cortex. To the best of our knowledge, this is the first demonstration of the effects of very low concentrations of ethanol (starting from 1 mM) on synaptic transmission in the neocortex. We further show that ethanol acts on synaptic transmission by at least two mechanisms engaged at different concentrations of ethanol. One mechanism is adenosine and A1R dependent, involves presynaptic changes, and mediates reduction of EPSP amplitudes by low ethanol concentrations (1–10 mM). The other mechanism(s) of EPSP amplitude suppression is activated by higher concentrations of ethanol (50 mM), and neither depends on A1R, nor involves presynaptic changes.
Adenosine-dependent and adenosine-independent mechanisms of ethanol action on synaptic transmission
Low concentrations of ethanol (1–10 mM) suppress excitatory transmission to layer 2/3 pyramids. This suppression was blocked by a specific antagonist of the adenosine A1R, DPCPX, suggesting the involvement of adenosine and an A1R-dependent pathway. Indeed, A1Rs are common in the neocortex (Dixon et al., 1996; Fredholm et al., 2001), and are involved in suppression of excitatory and inhibitory synaptic transmission in the visual cortex by adenosine (Murakoshi et al., 2001; Kerr et al., 2013; Bannon et al., 2014; Zhang et al., 2015). Consistent with the presynaptic action of A1Rs on synaptic transmission in our preparation (Bannon et al., 2014; Zhang et al., 2015), suppression of EPSP amplitude by low concentrations of ethanol was associated with changes in PPR. Most probably, the adenosine pathway was activated due to the ability of ethanol to block activity of ENT1, which is involved in adenosine reuptake (Nagy et al., 1990; Choi et al., 2004; Mailliard & Diamond, 2004; Allen-Gipson et al., 2009). Resulting increase in adenosine tone and activation of A1Rs would then suppress synaptic transmission, similar to the effect of application of adenosine described in our prior research using the same preparation as in the present study (Bannon et al., 2014; Zhang et al., 2015). Notably, previous in vitro studies used high concentrations of ethanol (100–200 mM) to demonstrate blockade of ENT1 activity (Nagy et al., 1990; Allen-Gipson et al., 2009). We found that A1R-dependent suppression of EPSPs becomes evident already at 1 mM ethanol, suggesting that even very low concentrations of ethanol may block adenosine uptake. This conjecture is consistent with results of behavioral studies, which found that effects of low concentrations of ethanol (13–40 mM) are modulated by agonists and antagonists of adenosine receptors (Dar, 2001; Dar & Mustafa, 2002; Prediger et al., 2004), and by ENT1 expression (Choi et al., 2004). Further studies would be necessary for verifying that very low concentrations of ethanol (1–10 mM) are indeed sufficient for reducing ENT1 activity.
Higher concentrations of ethanol (50 mM) activated another adenosine-independent mechanism of suppression of excitatory transmission to L2/3 pyramids. EPSP suppression by this mechanism(s) was not blocked, and not even reduced, by the A1R antagonist DPCPX. These mechanisms may be similar to those mediating suppression of glutamatergic transmission observed in prior studies in neuronal cultures (Marszalec et al., 1998; Moriguchi et al., 2007) or brain slices from other structures (Lovinger et al., 1990; Nie et al., 1993; Hendricson et al., 2004; Proctor et al., 2006).
Results of the present study show that the switch between adenosine-dependent and adenosine-independent mechanisms of EPSP suppression occurs between 10 and 50 mM of ethanol. Further studies are necessary for determining individual concentration dependences of each of these mechanisms, and identification of the exact range of ethanol concentration at which adenosine-independent mechanism of suppression of synaptic transmission starts to dominate over the adenosine/A1R-dependent mechanism.
Physiologically relevant concentrations of ethanol: in the blood, in the brain and in experiments
In humans, alcohol level is measured using the BAC. Because alcohol is highly diffusible and passes through the blood–brain barrier (Crone, 1965), equilibrium concentration of alcohol in the brain (cerebrospinal fluid) is similar or same as in the blood (Nurmi et al., 1994). This allows us to relate BAC values (measured in %) and respective behavioral effects, to concentrations of ethanol (in mM) used in electrophysiological experiments.
Physiological actions of ethanol start from 0.02 BAC, which corresponds to 4.6 mM. The physiological effects of ethanol up to 0.05 BAC (~ 11 mM) include feelings of relaxation, euphoria, minor impairment of reasoning, anxiolytic effects and exacerbation of emotions (http://pubs.niaaa.nih.gov/publications/AlcoholOverdoseFactsheet/Overdosefact.htm). In most countries, BAC threshold for illegal driving is between 0 and 0.05. BAC of 0.1 (~ 23 mM) or higher is considered legally drunk. Our results show that the adenosine/ A1R pathway may mediate suppression of synaptic transmission in L2/3 pyramids from visual cortex by low concentrations of ethanol, in the range in which the first cognitive and behavioral effects are observed. Involvement of the adenosine pathway in mediating effects of low concentrations of ethanol is consistent with results of behavioral studies in animals considered above (Dar, 2001; Dar & Mustafa, 2002; Choi et al., 2004; Prediger et al., 2004).
Suppression of excitatory synaptic transmission by low concentrations of ethanol via an adenosine-sensitive mechanism, if it is not restricted to L2/3 pyramids from visual cortex but operates at other cortical synapses as well, allows us to reconcile an apparent contradiction between behavioral effects of low concentrations of ethanol (Wallgren & Barry, 1970; Givens & McMahon, 1997; Little, 1999; Dar, 2001; Dar & Mustafa, 2002; Prediger et al., 2004; Choi et al., 2004) and prior studies in slice preparations or neuron cultures, which reported changes in synaptic transmission only at higher concentrations, typically 40–50 mM and above (Lovinger et al., 1990; Nie et al., 1993; Marszalec et al., 1998; Ariwodola & Weiner, 2004; Hendricson et al., 2004; Proctor et al., 2006; Moriguchi et al., 2007; Criswell et al., 2008). When alcohol concentration in the blood is above 40–50 mM (44 mM corresponds to BAC 0.2) behavioral effects include feeling of nausea, severe motor impairments, significant memory impairment and increased risk of asphyxiation. BAC 0.4 (87 mM) is considered life-threatening. In rats, this range of concentrations (70–90 mM) induces severe hangover and place aversion (Morse et al., 2000; Prediger et al., 2006).
Outlook: possible implications for mechanisms of alcohols’ intoxicating effects in humans?
Existence of a mechanism mediating effects of low concentrations of ethanol on synaptic transmission, and dependence of this mechanism on adenosine signaling may have several important implications for understanding intoxicating effects of ethanol on the brain.
Although here we report results for L2/3 pyramidal neurons from visual cortex, necessary ‘hardware’ for ethanol–adenosine interactions is present in many key regions of the brain. In humans and rats, multiple brain structures, including neocortex, hippocampus, cerebellum, basal ganglia and thalamus, show expression of both ENT1 (Anderson et al., 1999; Jennings et al., 2001) and adenosine receptors (A1 and/or A2A; Dixon et al., 1996; Fredholm et al., 2001; Svenningsson et al., 1997; Dunwiddie & Masino, 2001; Fukumitsu et al., 2005). Therefore, very low concentrations of ethanol may affect synaptic transmission in these regions via activation of adenosine signaling pathways, in a similar way as we observed in the visual cortex. Our results show that an adenosine-dependent mechanism of the suppression of excitatory transmission is activated at concentrations of ethanol as low as 1 mM (~ 0.005 BAC), at which no behavioral effects are detected. The first implication of these results is that ethanol starts causing changes in synaptic transmission before we are even aware of it. Second, because adenosine tone increases with activity (Lovatt et al., 2012; Pajski & Venton, 2012; Wall & Dale, 2013; Van Gompel et al., 2014) and fluctuates during the sleep-wake cycle (Porkka-Heiskanen et al., 2000; Bjorness & Greene, 2009; Bjorness et al., 2009), it would modulate the effect of ethanol on synaptic transmission, and by extension on brain functions. Indeed, a recent study demonstrated that physiological decrease in adenosine tone after lasting sleep restriction decreased the impairment of motor function by ethanol (Clasadonte et al., 2014). Thus, the same concentration of ethanol may differentially affect synaptic transmission in different phases of sleep-wake cycle, and depending on the amount of prior activity. Finally, adenosine/ A1R dependence of the mechanism by which low concentrations of ethanol affect synaptic transmission may provide a mechanistic explanation for the interaction between alcohol and caffeine. In our experiments, blockade of A1Rs with DPCPX eliminated effects of 1 and 10 mM ethanol on synaptic transmission, and thus raised the effective threshold concentration to 50 mM. Caffeine, as a non-selective antagonist of adenosine receptors, may act in a similar way. By blocking adenosine receptors, caffeine might effectively and substantially raise the threshold for ethanol’s effects, bringing it into the range of high-risk concentrations.
Acknowledgments
This work was supported by the NIH grant RO1 MH087631 to MV and The PCLB Foundation, Inc. - Psychology Undergraduate Research Mini-Grant to LL. MV was partially supported by the Humboldt Research Award from the Alexander von Humboldt Foundation.
Abbreviations
- A1R
A1 Receptor
- A2AR
A2A Receptor
- ACSF
artificial cerebrospinal fluid
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ATP
adenosine triphosphate
- BAC
blood alcohol concentration
- DIC
differential interference contrast
- DMSO
dimethyl sulfoxide
- DPCPX
8-cyclopentyl-1,3-dipropylxanthine
- ENT1
equilibrative nucleoside transporter 1
- EPSP
excitatory post synaptic potential
- ETOH
ethanol
- GABA
gamma-amino butyric acid
- GTP
guanosine triphosphate
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic Acid
- IR-DIC
infrared differential interference contrast
- NMDA
N-methyl-D-aspartate
- PPR
paired pulse ratio
- PSP
post-synaptic potential
Footnotes
Conflict of interest
The authors declare no conflict of interests.
Author contributions
NB, MC and MV designed experiments; LL, NB and AR performed experiments; LL, NB, AR, MC and MV processed results; LL, NB, MC and MV prepared figures and wrote the manuscript.
Data accessibility
All data supporting this paper are stored in Volgushev Laboratory, University of Connecticut, Storrs and are available upon request (maxim.volgushev@uconn.edu).
References
- Allen-Gipson DS, Jarrell JC, Bailey KL, Robinson JE, Kharbanda KK, Sisson JH, Wyatt TA. Ethanol blocks adenosine uptake via inhibiting the nucleoside transport system in bronchial epithelial cells. Alcohol Clin Exp Res. 2009;33:791–798. doi: 10.1111/j.1530-0277.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Xiong W, Geiger JD, Young JD, Cass CE, Baldwin SA, Parkinson FE. Distribution of equilibrative, nitrobenzylthioinosine-sensitive nucleoside transporters (ENT1) in brain. J Neurochem. 1999;73:867–873. doi: 10.1046/j.1471-4159.1999.0730867.x. [DOI] [PubMed] [Google Scholar]
- Ariwodola OJ, Weiner JL. Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABAB receptors. J Neurosci. 2004;24:10679–10686. doi: 10.1523/JNEUROSCI.1768-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon NM, Zhang P, Ilin V, Chistiakova M, Volgushev M. Modulation of synaptic transmission by adenosine in layer 2/3 of the rat visual cortex in vitro. Neuroscience. 2014;260:171–184. doi: 10.1016/j.neuroscience.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, Greene RW. Adenosine and sleep. Curr Neuropharmacol. 2009;7:238–245. doi: 10.2174/157015909789152182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorness TE, Kelly CL, Gao T, Poffenberger V, Greene RW. Control and function of the homeostatic sleep response by adenosine A1 receptors. J Neurosci. 2009;29:1267–1276. doi: 10.1523/JNEUROSCI.2942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, et al. The Type 1 Equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7:855–861. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Clasadonte J, McIver SR, Schmitt LI, Halassa MM, Haydon PG. Chronic sleep restriction disrupts sleep homeostasis and behavioral sensitivity to alcohol by reducing the extracellular accumulation of adenosine. J Neurosci. 2014;34:1879–1891. doi: 10.1523/JNEUROSCI.2870-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Ther. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. The permeability of brain capillaries to non-electrolytes. Acta Physiol Scand. 1965;64(4):407–417. doi: 10.1111/j.1748-1716.1965.tb04198.x. [DOI] [PubMed] [Google Scholar]
- Dar MS. Modulation of ethanol-induced motor incoordination by mouse striatal A1 adenosinergic receptor. Brain Res Bull. 2001;55:513–520. doi: 10.1016/s0361-9230(01)00552-4. [DOI] [PubMed] [Google Scholar]
- Dar MS. Ethanol-induced cerebellar ataxia: cellular and molecular mechanisms. Cerebellum. 2015;14:447–465. doi: 10.1007/s12311-014-0638-4. [DOI] [PubMed] [Google Scholar]
- Dar MS, Mustafa SJ. Acute ethanol/cannabinoid-induced ataxia and its antagonism by oral/systemic/intracerebellar A1 adenosine receptor antisense in mice. Brain Res. 2002;957:53–60. doi: 10.1016/s0006-8993(02)03599-0. [DOI] [PubMed] [Google Scholar]
- Diamond I, Nagy L, Mochly-Rosen D, Gordon A. The role of adenosine and adenosine transport in ethanol-induced cellular tolerance and dependence. Ann NY Acad Sci. 1991;625:473–487. doi: 10.1111/j.1749-6632.1991.tb33878.x. [DOI] [PubMed] [Google Scholar]
- Dixon AK, Gubitz AK, Sirinathsinghji DJS, Richardson PJ, Freeman TC. Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol. 1996;118:1461–1468. doi: 10.1111/j.1476-5381.1996.tb15561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Förstera B, Castro PA, Moraga-Cid G, Aguayo LG. Potentiation of gamma aminobutyric acid receptors (GABAAR) by ethanol: how are inhibitory receptors affected? Front Cell Neurosci. 2016;10:114. doi: 10.3389/fncel.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- Fukumitsu N, Ishii K, Kimura Y, Oda K, Sasaki T, Mori Y, Ishiwata K. Adenosine A1 receptor mapping of the human brain by PET with 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine. J Nucl Med. 2005;46:32–37. [PubMed] [Google Scholar]
- Givens B, McMahon K. Effects of ethanol on nonspatial working memory and attention in rats. Behav Neurosci. 1997;111:275–282. doi: 10.1037//0735-7044.111.2.275. [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Sibbald JR, Morrisett RA. Ethanol alters the frequency, amplitude, and decay kinetics of Sr2+-supported, asynchronous NMDAR mEPSCs in rat hippocampal slices. J Neurophysiol. 2004;91:2568–2577. doi: 10.1152/jn.00997.2003. [DOI] [PubMed] [Google Scholar]
- Jennings LL, Hao C, Cabrita MA, Vickers MF, Baldwin SA, Young JD, Cass CE. Distinct distribution of human equilibrative nucleoside transport proteins 1 and 2 (hENT1 and hENT2) in the central nervous system. Neuropharmacology. 2001;40:722–731. doi: 10.1016/s0028-3908(00)00207-0. [DOI] [PubMed] [Google Scholar]
- Kelm MK, Criswell HE, Breese GR. Ethanol-enhanced GABA release: a focus on G protein-coupled receptors. Brain Res Rev. 2011;65:113–123. doi: 10.1016/j.brainresrev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr MI, Wall MJ, Richardson MJ. Adenosine A1 receptor activation mediates the developmental shift at layer 5 pyramidal cell synapses and is a determinant of mature synaptic strength. J Physiol. 2013;591:3371–3380. doi: 10.1113/jphysiol.2012.244392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CM, Stoelzel C, Chistiakova M, Volgushev M. Heterosynaptic plasticity induced by intracellular tetanization in layer 2/3 pyramidal neurons in rat auditory cortex. J Physiol. 2012;590:2253–2271. doi: 10.1113/jphysiol.2012.228247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little HJ. The contribution of electrophysiology to knowledge of the acute and chronic effects of ethanol. Pharmacol Therapeut. 1999;84:333–353. doi: 10.1016/s0163-7258(99)00040-6. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci USA. 2012;109:6265–6270. doi: 10.1073/pnas.1120997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, White G, Weight FF. NMDA receptor-mediated synaptic excitation selectively inhibited by ethanol in hippocampal slice from adult rat. J Neurosci. 1990;10:1372–1379. doi: 10.1523/JNEUROSCI.10-04-01372.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailliard WS, Diamond I. Recent advanced in the neurobiology of alcoholism: the role of adenosine. Pharmacol Therapeut. 2004;101:39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Marszalec W, Aistrup GL, Narahashi T. Ethanol modulation of excitatory and inhibitory synaptic interactions in cultured cortical neurons. Alcohol Clin Exp Res. 1998;22:1516–1524. [PubMed] [Google Scholar]
- Moriguchi S, Zhao X, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on excitatory and inhibitory synaptic transmission in rat cortical neurons. Alcohol Clin Exp Res. 2007;31:89–99. doi: 10.1111/j.1530-0277.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- Morse AC, Schulteis G, Holloway FA, Koob GF. Conditioned place aversion to the “Hangover” phase of acute ethanol administration in the rat. Alcohol. 2000;22:19–24. doi: 10.1016/s0741-8329(00)00099-9. [DOI] [PubMed] [Google Scholar]
- Murakoshi T, Song SY, Konishi S, Tanabe T. Multiple G-protein-coupled receptors mediate presynaptic inhibition at single excitatory synapses in the rat visual cortex. Neurosci Lett. 2001;309:117–120. doi: 10.1016/s0304-3940(01)02051-1. [DOI] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–1951. [PubMed] [Google Scholar]
- Nam HW, Hinton DJ, Kang NY, Kim T, Lee MR, Oliveros A, Adams C, Ruby CL, et al. Adenosine transporter ENT1 regulates the acquisition of goal-directed behavior and ethanol drinking through A2A receptor in the dorsomedial striatum. J Neurosci. 2013a;33:4329–4338. doi: 10.1523/JNEUROSCI.3094-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam HW, Bruner RC, Choi DS. Adenosine signaling in striatal circuits and alcohol use disorders. Mol Cells. 2013b;36:195–202. doi: 10.1007/s10059-013-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie Z, Yuan X, Madamba SG, Siggins GR. Ethanol decreases glutamatergic synaptic transmission in rat nucleus accumbens in vitro: naloxone reversal. J Pharmacol Exp Ther. 1993;266:1705–1712. [PubMed] [Google Scholar]
- Nurmi M, Kiianmaa K, Sinclair D. Brain ethanol in AA, ANA and Wistar rats monitored with one minute dialysis. Alcohol. 1994;11:315–321. doi: 10.1016/0741-8329(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Pajski ML, Venton BJ. The mechanism of electrically stimulated adenosine release varies by brain region. Purinerg Signal. 2012;9:167–174. doi: 10.1007/s11302-012-9343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porkka-Heiskanen T, Strecker RE, McCarley RW. Brain site-specificity of extracellular adenosine concentration changes during sleep deprivation and spontaneous sleep: an in vivo microdialysis study. Neuroscience. 2000;99:507–517. doi: 10.1016/s0306-4522(00)00220-7. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, Batista LC, Takahashi RN. Adenosine A1 receptors modulate the anxiolytic-like effect of ethanol in the elevated plus-maze in mice. Eur J Pharmacol. 2004;499:147–154. doi: 10.1016/j.ejphar.2004.07.106. [DOI] [PubMed] [Google Scholar]
- Prediger RDS, Silva GE, Batista LC, Bittencourt AL, Takahashi RN. Activation of adenosine A1 receptors reduces anxiety-like behavior during acute ethanol withdrawal (Hangover) in mice. Neuropsychopharmacology. 2006;31:2210–2220. doi: 10.1038/sj.npp.1301001. [DOI] [PubMed] [Google Scholar]
- Proctor WR, Diao L, Freund RK, Browning MD, Wu PH. Synaptic GABAergic and glutamatergic mechanisms underlying alcohol sensitivity in mouse hippocampal neurons. J Physiol. 2006;575:145–159. doi: 10.1113/jphysiol.2006.112730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae CD, Davidson JE, Maher AD, Rowlands BD, Kashem MA, Nasrallah FA, Rallapalli SK, Cook JM, et al. Ethanol, not detectably metabolized in brain, significantly reduces brain metabolism, probably via action at specific GABA(A) receptors and has measureable metabolic affects at very low concentrations. J Neurochem. 2014;129:304–314. doi: 10.1111/jnc.12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF. Quantal release of neurotransmitter and long-term potentiation. Neuron. 1993;10:55–63. doi: 10.1016/s0092-8674(05)80028-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Hall H, Sedvall G, Fredholm BB. Distribution of adenosine receptors in the postmortem human brain: an extended autoradiographic study. Synapse. 1997;27:322–335. doi: 10.1002/(SICI)1098-2396(199712)27:4<322::AID-SYN6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Van Gompel JJ, Bower MR, Worrell GA, Stead M, Chang S, Goerss SJ, Kim I, Bennet KE, et al. Increased cortical extracellular adenosine correlates with seizure termination. Epilepsia. 2014;55:233–244. doi: 10.1111/epi.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volgushev M, Vidyasagar TR, Chistiakova M, Eysel UT. Synaptic transmission in the neocortex during reversible cooling. Neuroscience. 2000;98:9–22. doi: 10.1016/s0306-4522(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Voronin LL. On the quantal analysis of hippocampal long-term potentiation and related phenomena of synaptic plasticity. Neuroscience. 1993;56(2):275–304. doi: 10.1016/0306-4522(93)90332-a. [DOI] [PubMed] [Google Scholar]
- Wall M, Dale N. Activity-dependent release of adenosine: a critical re-evaluation of mechanism. Curr Neuropharmacol. 2009;6:329–337. doi: 10.2174/157015908787386087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall MJ, Dale N. Neuronal transporter and astrocytic ATP exocytosis underlie activity-dependent adenosine release in the hippocampus. J Physiol. 2013;591:3853–3871. doi: 10.1113/jphysiol.2013.253450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallgren H, Barry H., III . Actions of Alcohol, Vol. 1: Biochemical, Physiological and Psychological Aspects. Elsevier Press; Amsterdam: 1970. pp. 367–376. [Google Scholar]
- Zhang P, Bannon NM, Ilin V, Chistiakova M, Volgushev M. Adenosine effects on inhibitory synaptic transmission and excitation-inhibition balance in the rat neocortex. J Physiol. 2015;593:825–841. doi: 10.1113/jphysiol.2014.279901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorumski CF, Mennerick S, Izumi Y. Acute and chronic effects of ethanol on learning-related synaptic plasticity. Alcohol. 2014;48:1–17. doi: 10.1016/j.alcohol.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]