Abstract
African Americans have the highest prevalence of hypertension in the US. Higher levels of Fibroblast growth factor-23 (FGF23) have been associated with worse cardiovascular outcomes. Whether FGF23 is associated with rising blood pressure (BP) and racial differences in incident hypertension is unclear. We studied 1758 adults (45.0±3.7 years, 57.8% female, 36.9% black) without hypertension or cardiovascular disease who participated in the Year 20 (2005–2006) follow-up exam of the Coronary Artery Risk Development in Young Adults (CARDIA) study. We investigated the associations of baseline (Year 20) c-terminal FGF23 levels with longitudinal BP patterns and incident hypertension (defined as being on antihypertensive medication, systolic BP ≥130 or diastolic BP ≥80 mmHg) over two follow-up visits (Year 25 and 30). During follow-up, 35.2% of participants developed hypertension. In multivariable linear mixed models, there were greater increases in systolic BP from Year 20–25 and Year 25–30 in the highest FGF23 quartile relative to the lowest quartile (+2.1 mmHg, p=0.0057 and +2.2 mmHg, p=0.0108, respectively for each time period) while there were greater increases in diastolic BP from Year 20–25 in the highest quartile relative to the lowest (+1.6 mmHg, p=0.0024). In multivariable modified Poisson regression analyses, the highest FGF23 quartile was associated with a 45% greater risk of developing hypertension during follow-up compared to the lowest quartile [Relative Risk: 1.45 (1.18, 1.77)]. Results did not vary by race. (pinteraction=0.1523). Higher FGF23 levels are independently associated with rising BP over time and an increased risk of incident hypertension, but not racial differences in hypertension.
Keywords: Fibroblast Growth Factor-23, Blood Pressure, Hypertension, African-American
INTRODUCTION
African Americans have the highest prevalence of hypertension in the United States and develop hypertension at earlier ages compared to whites.1 These differences are thought to contribute to an excess risk of hypertensive-related death, heart failure, stroke, and end stage renal disease. The mechanisms underlying racial differences in hypertension remain poorly characterized.
Fibroblast Growth Hormone-23 (FGF23) is an endocrine hormone released from the bone that acts on the kidney to stimulate phosphaturia and inhibit calcitriol synthesis.2 FGF23 levels rise in the presence of chronic kidney disease to maintain phosphate levels; however, elevated FGF23 levels are associated with worse cardiovascular outcomes and increased mortality.2–5 Elevated FGF23 levels have also been associated with increased left ventricular mass and incident heart failure in patients without known chronic kidney disease.6–8 FGF23 may upregulate the Renin-Angiotensin-Aldosterone System (RAAS) by decreasing calcitriol, a potential negative regulator of the RAAS system.9 In addition, experimental studies suggest that FGF23 may increase renal sodium uptake independent of the RAAS system leading to volume expansion and possible effects on blood pressure.10 Finally, some studies have suggested a correlation between African-American race and higher FGF23 levels after adjustment for demographic and clinic factors.11 Whether FGF23 could be mechanistically involved in the risk of developing hypertension remains in question.
We sought to characterize the potential association between FGF23 and longitudinal changes in blood pressure in a large multiethnic cohort of young and middle aged adults free of cardiovascular disease at baseline from the Coronary Artery Risk Development in Young Adults (CARDIA) Study. We hypothesized that higher FGF23 levels would be independently associated with greater increases in blood pressure over time and a higher risk of incident hypertension in African-Americans compared to whites.
METHODS
Data from the CARDIA study are not publically available. More information about CARDIA can be found at: https://www.cardia.dopm.uab.edu/.
Source Population
The CARDIA study is a longitudinal cohort study that enrolled 5,115 healthy young African-American and white adults aged 18–30 from 4 field centers (Birmingham, Alabama; Minneapolis, Minnesota; Chicago, Illinois; Oakland, California) from 1985–1986. The study was designed to investigate biological and behavioral factors contributing to the development of cardiovascular disease in young adults. Full details about the design of the CARDIA study have been previously published.12 To date, there have been 9 total examinations [baseline, years 2 (1987–1988), 5 (1990–1991), 7 (1992–1993), 10 (1995–1996), 15 (2000–2001), 20 (2005–2006), 25 (2010–2011), 30 (2015–2016)] spanning 30 years of follow-up. The institutional review board at each study site approved the study and all study participants provided informed consent.
Study Sample
FGF23 measures were first available at the year 20 exam. Of the 5115 CARDIA participants at baseline, 3547 participated in the year 20 exam. 3402 (95.9%) had adequate samples for FGF23 measurement at the year 20 exam. We excluded participants with prevalent hypertension, defined as taking antihypertensive medication, having a systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥80 mmHg at the time of year 20 visit (n = 1269). We also excluded participants with cardiovascular disease, defined as previous heart failure, myocardial infarction, coronary revascularization, angina, stroke, transient ischemic attack, or peripheral vascular disease (n = 251). Finally, we excluded patients with no follow-up blood pressure values (n = 124). The final study sample was composed of 1758 individuals.
Ascertainment of Incident Hypertension
Blood pressure was measured at each exam by trained CARDIA staff. For the Year 20, 25 and 30 exams, blood pressure was measured using Omron HEM907XL sphygmomanometers. Per CARDIA standardized protocol, participants had 3 blood pressure readings taken after sitting in a quiet room for 5 minutes. The second and third readings were then averaged. Incident hypertension was defined as any of the following at the Year 25 or Year 30 exam: 1) report of antihypertensive medication use; 2) systolic blood pressure ≥130 mmHg and/or diastolic blood pressure ≥80 mmHg per most recent 2017 guidelines.13
Measurement of Fibroblast Growth Factor-23
CARDIA study personnel collected blood samples after a fasting period and both serum and plasma samples were frozen to −70° centigrade for long-term storage based on a standardized protocol. For FGF23 analyses, samples were shipped to the University of Miami Biomarker and Immunoassay laboratory. We measured plasma c-terminal FGF23 (cFGF23) using a second generation enzyme-linked immunosorbent assay (ELISA) which detects epitopes within the carboxyl-terminal portion of FGF23 (Immutopics, Inc, Athens, Ohio; intra-assay coefficient of variation <3%). We analyzed 10% of the samples in duplicate for quality control purposes. We included a low and high value internal control sample for each run of the assay. As the assays were performed in two separate shipments, these procedures were done for each independently. For the first shipment of samples, the inter-assay and intra-assay variability were 3.9% and 4.1% for the low value control and 2.3% and 4.1% for the high value control respectively. For the second shipment of samples, the inter-assay and intra-assay variability were 4.3% and 4.8% for the low value control and 2.8% and 4.0% for the high value control respectively. We also measured intact FGF23 (iFGF23) using a second generation Immutopics ELISA based assay which detects epitopes within the amino and carboxyl portions of FGF23. We performed similar quality control testing for this assay. In vivo, a portion of synthesized FGF23 is cleaved into separate protein fragments. The c-terminal assay detects these fragments along with the full-length intact protein while the intact assay detects only the full-length protein.14
Other Study Variables
Standardized questionnaires were administered at each CARDIA exam and according to published protocols.12 Sex, race, education level, alcohol use, and smoking status were self-reported by participants. Body mass index (BMI) was calculated from standardized height and weight measurements. Physical activity was determined through a questionnaire and reported as exercise units.15, 16 Diabetes was defined as a fasting glucose ≥126 mg/dL or on a diabetic medication. Estimated glomerular filtration rate (eGFR) was calculated based on the CKD-EPI equation.17
Statistical Analysis
Baseline characteristics of the study population were summarized by FGF23 quartiles. Continuous variables were expressed as means and categorical variables as proportions. We used one-way ANOVA and the Kruskal-Wallis test to compare normally and non-normally distributed continuous variables, respectively. We used the chi-square test to compare categorical variables. In order to investigate the association of cFGF23 with systolic and diastolic blood pressure over time, we constructed linear mixed models and generated least squares means estimates of systolic and diastolic blood pressure at each of the three exams within each of the cFGF23 quartiles from fully adjusted models. We constructed two nested multivariable models. Model 1 adjusted for age, sex, race, education and study center. Model 2 additionally adjusted for body mass index (BMI), physical activity15, 16, smoking status, Triglyceride/high density lipoprotein ratio, estimated glomerular filtration rate (eGFR), and urine albumin-to-creatinine ratio (ACR). All covariables were included in the models as fixed effects. To account for the presence of anti-hypertensive therapy at the follow-up visits, we added 10 mmHg to the systolic and 5 mmHg to the diastolic blood pressure of participants taking antihypertensive therapy.18–20 Finally, because unlike systolic blood pressure, diastolic blood pressure tends to decrease with advanced age, we repeated analyses using pulse pressure (pulse pressure = systolic blood pressure – diastolic blood pressure).
In order to investigate the association of cFGF23 at baseline (year 20) with incident hypertension over the two follow-up visits (Year 25 and Year 30) we used a modified Poisson regression approach with robust error variance to estimate the relative risk of incident hypertension for each of the higher FGF23 quartiles compared to the lowest quartile.21 Multivariable models were adjusted for the same variables as aforementioned. In order to evaluate if any association between FGF23 and incident hypertension was modified by race, in fully adjusted models, we tested for interactions between FGF23 and race. We also tested for interactions between FGF23 and sex. Since chronic kidney disease is strongly associated with both higher FGF23 and hypertension, in sensitivity analyses, we excluded individuals with chronic kidney disease at baseline, which we defined as an eGFR < 60 ml/min/1.72 m2 or a urine ACR ≥ 30 mg/g. Finally, we repeated all analyses using iFGF23 measurements instead of cFGF23. A p-value of < 0.05 was considered statistically significant. We performed all statistical analyses using SAS software version 9.4 (SAS Institute Inc., Cary, North Carolina).
RESULTS
The mean age of the cohort was approximately 45 years (45.0 ± 3.7); 57.8% of the cohort was female and 36.9% black. The mean eGFR was 97.7 ± 16.0 ml/min/1.72 m2 and mean systolic blood pressure was 109.4 ± 9.3 mmHg. Few participants had an eGFR < 60 ml/min/1.72 m2 (0.1%). Baseline demographics categorized by cFGF23 quartile are presented in Table 1. The mean age was similar across cFGF23 quartiles. There were higher proportions of female and diabetic participants along with current smokers in the higher cFGF23 quartiles. BMI was also greater in higher cFGF23 quartiles. Baseline systolic and diastolic blood pressures were similar across cFGF23 quartiles. Participants in the highest cFGF23 quartile had higher median urine ACR levels as well as having the highest proportion of participants with a urine ACR ≥ 30 mg/g. Mean eGFR was similar across cFGF23 quartiles at baseline (Table 1) and in years 25 and 30 (Table S1 in the online-only Data Supplement).
Table 1.
Baseline (Year 20 Exam) Characteristics by Quartile of C-terminal Fibroblast Growth Factor-23
| Plasma C-Terminal Fibroblast Growth Factor-23 (RU/ml) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Characteristics | Overall | 29.8–62.0 | 62.1–77.0 | 77.1–103.2 | ≥103.3 | P Value |
| N | 1758 | 439 (25.0%) | 437 (24.9%) | 443 (25.2%) | 439 (25.0%) | |
| Age, years | 45.0 ± 3.7 | 44.9 ± 3.6 | 44.9 ± 3.6 | 45.2 ± 3.6 | 44.8 ± 3.8 | 0.8809 |
| Female | 1017 (57.8%) | 223 (50.8%) | 209 (47.8%) | 238 (53.7%) | 347 (79.0%) | <.0001 |
| Black | 648 (36.9%) | 169 (38.5%) | 141 (32.3%) | 149 (33.6%) | 189 (43.1%) | 0.1458 |
| Education, years | 15.4 ± 2.6 | 15.5 ± 2.6 | 15.7 ± 2.6 | 15.3 ± 2.5 | 15.1 ± 2.6 | 0.0036 |
| BMI, kg/m2 | 27.7 ± 6.2 | 27.0 ± 7.1 | 27.4 ± 5.5 | 28.1 ± 5.5 | 28.3 ± 6.3 | 0.0003 |
| Physical Activity Score* | 367.2 ± 285.2 | 383.0 ± 265.6 | 383.2 ± 291.8 | 371.4 ± 293.6 | 331.2 ± 286.7 | 0.0061 |
| Smoking Status | 0.0002 | |||||
| Former | 349 (20.0%) | 81 (18.6%) | 84 (19.3%) | 102 (23.2%) | 82 (19.0%) | |
| Current | 287 (16.5%) | 58 (13.3%) | 58 (13.3%) | 80 (18.2%) | 91 (21.1%) | |
| Alcohol, ml/day | 10.2 ± 19.4 | 11.8 ± 25.0 | 11.5 ± 20.1 | 9.8 ± 16.2 | 7.6 ± 14.5 | 0.0006 |
| Triglycerides, mg/dL | 99.0 ± 66.7 | 90.7 ± 57.5 | 104.4 ± 79.2 | 103.5 ± 69.0 | 97.5 ± 57.6 | 0.1710 |
| HDL cholesterol, mg/dL | 55.5 ± 16.6 | 56.6 ± 15.8 | 54.8 ± 17.8 | 53.9 ± 16.0 | 56.8 ± 16.7 | 0.9090 |
| Triglycerides/HDL Ratio | 1.5 (1.0–2.5) | 1.3 (0.9–2.1) | 1.6 (1.0–2.8) | 1.6 (1.0–2.7) | 1.5 (0.9–2.5) | 0.3075 |
| Glucose, mg/dL | 95.4 ± 15.5 | 94.6 ± 15.4 | 94.8 ± 10.9 | 96.7 ± 14.6 | 95.4 ± 19.7 | 0.2191 |
| Diabetes | 71 (4.1%) | 12 (2.7%) | 15 (3.4%) | 19 (4.3%) | 25 (5.7%) | 0.0209 |
| eGFR, ml/min/1.72m2 | 97.7 ± 16.0 | 99.6 ± 15.7 | 97.1 ± 15.6 | 95.2 ± 15.7 | 98.8 ± 16.8 | 0.2254 |
| eGFR < 60 | 2 (0.1%) | 1 (0.2%) | 0 (0.0%) | 0 (0.0%) | 1 (0.2%) | 0.9983 |
| Urine ACR, mg/g | 4.1 (2.9–6.1) | 4.1 (2.8–5.6) | 3.9 (2.7–5.7) | 4.0 (2.9–6.3) | 4.4 (3.1–6.9) | <.0001 |
| Urine ACR ≥ 30 | 61 (3.6%) | 10 (2.3%) | 10 (2.3%) | 14 (3.2%) | 27 (6.5%) | 0.0009 |
| SBP, mmHg | 109.4 ± 9.3 | 109.9 ± 9.3 | 109.4 ± 9.3 | 109.6 ± 9.3 | 108.7 ± 9.3 | 0.0918 |
| DBP, mmHg | 67.1 ± 7.2 | 67.0 ± 7.0 | 66.9 ± 7.3 | 67.5 ± 7.3 | 67.1 ± 7.3 | 0.6095 |
Values are expressed as n (%), mean (SD) and median (25th – 75th percentile). P values are for trend.
Total Physical Activity Score in exercise units.
BMI, body mass index; eGFR, estimated glomerular filtration rate; HDL, high density lipoprotein; ACR, albumin to creatinine ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Association of Fibroblast Growth Factor-23 and Blood Pressure Levels Over Time
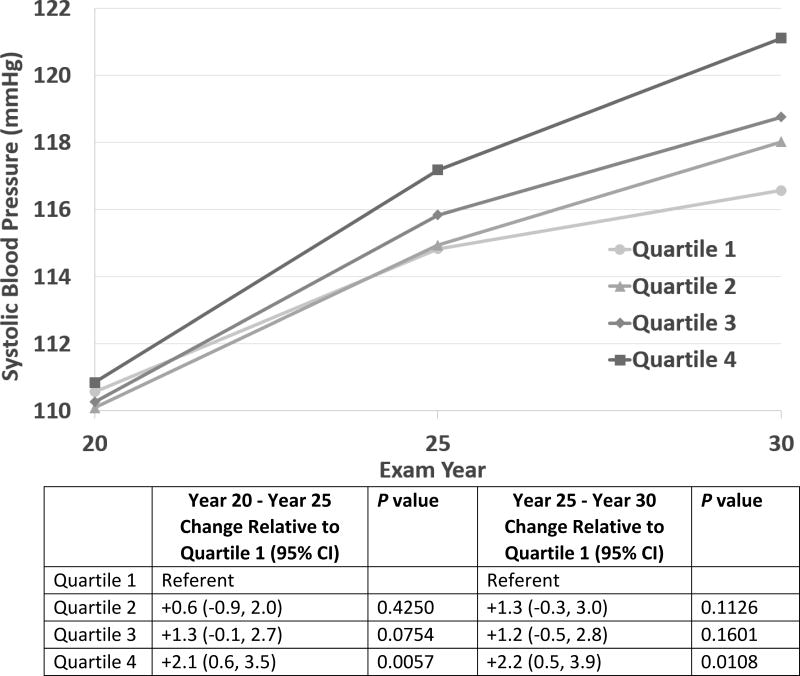
In fully adjusted mixed models (including all Model 2 co-variables), higher cFGF23 quartiles were associated with increasing systolic blood pressure over time (Figure 1). Increases in systolic blood pressure were significantly greater in the highest cFGF23 quartile relative to the lowest quartile during the Year 20 to 25 time period. (+2.1 mmHg for Quartile 4 relative to Quartile 1 during the Year 20 to 25 time period, p = 0.0057) as well as during the year 25 to 30 period (+2.2 mmHg for Quartile 4 relative to Quartile 1, p = 0.0108). By Year 30, the estimated adjusted mean systolic blood pressures for participants in cFGF23 quartile 4 exceeded 120 mmHg whereas this was not the case in the lower quartiles.
Figure 1.
Adjusted Mixed Model Least Squares Means Estimates for Systolic Blood Pressure by C-terminal Fibroblast Growth Factor-23 (cFGF23) Quartile. There were greater increases in estimated systolic blood pressure during both time periods in the highest cFGF23 quartile relative to the lowest quartile. CI, Confidence Interval.
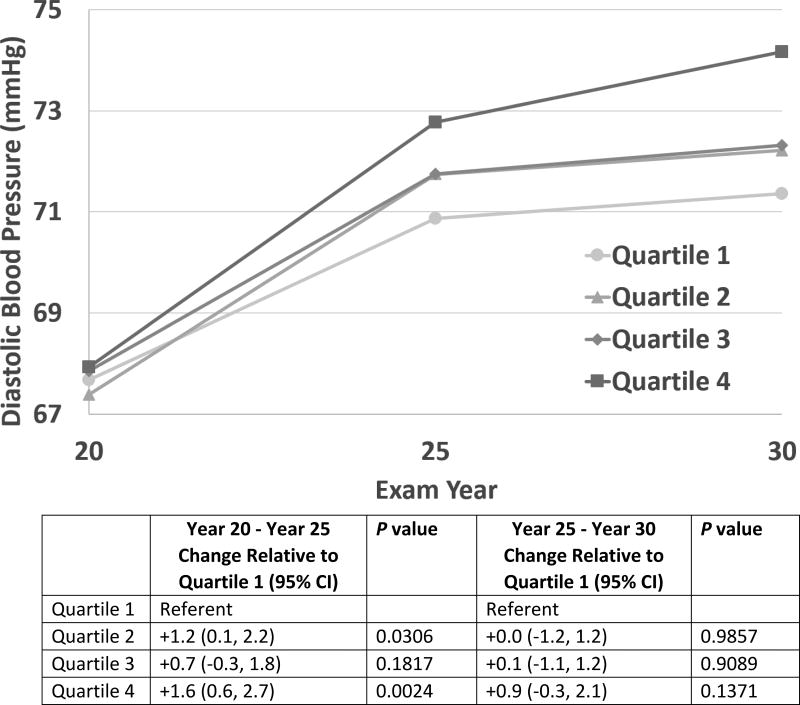
Increases in diastolic blood pressure were also greater in the highest cFGF23 quartile relative to the lowest quartile during the Year 20 to 25 time period (Figure 2: +1.6 mmHg for Quartile 4 relative to Quartile 1, p = 0.0024). There were no statistically significant differences in the change in diastolic blood pressure for Quartile 3 relative to Quartile 1 between Year 20 and Year 25 but a significant difference for Quartile 2 relative to Quartile 1. Between Year 25 and Year 30, diastolic blood pressure slopes in the top three quartiles were similar to the lowest quartile. However, during this Year 25 to 30 time period, there was a significantly greater increase in pulse pressure in the highest cFGF23 quartile relative to the lowest quartile (Figure S1).
Figure 2.
Adjusted Mixed Model Least Squares Means Estimates for Diastolic Blood Pressure by C-terminal Fibroblast Growth Factor-23 (cFGF23) Quartile. There was a greater increase in estimated diastolic blood pressure between Year 20 and Year 25 in the highest cFGF23 quartile relative to the lowest quartile.
Association of Fibroblast Growth Factor-23 with Incident Hypertension
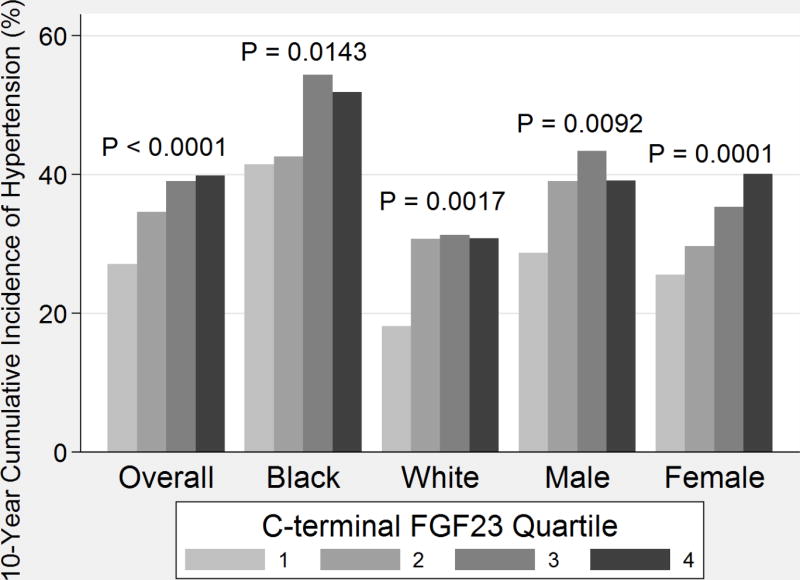
During follow-up, 35.2% (618) of participants developed hypertension. The crude 10-year incidence of hypertension by cFGF23 quartile for the cohort overall and stratified by race and sex is presented in Figure 3. Hypertension developed in more participants in higher cFGF23 quartiles (p for trend <0.0001). African-Americans had a higher overall incidence of hypertension (47.7%, 309/648) compared to whites (27.8%, 309/1110). When stratified by race, greater rates of incident hypertension continued to be present in higher FGF23 quartiles.
Figure 3.
Comparative 10-year Cumulative Incidence of Hypertension by C-terminal Fibroblast Growth Factor 23 Quartile, Overall and Stratified by Race and Gender. P values shown are for trend
In fully adjusted analyses incorporating BMI, smoking status and markers of renal function, cFGF23 levels in the highest quartile were associated with a 45% greater risk of developing hypertension compared to the lowest quartile. [Table 2, Relative Risk (RR): 1.45 (1.18, 1.77)]. There were also statistically significant associations with incident hypertension for the middle two FGF23 quartiles compared to the lowest quartile of FGF23 in fully adjusted models. The risk of developing incident hypertension was greater in the highest quartiles (Table 2: p for trend = 0.0002)
Table 2.
Estimated Relative Risk for Incident Hypertension at Year 25 or Year 30 Follow-up Visits by C-terminal Fibroblast Growth Factor-23 (cFGF23) Quartile
| cFGF23 (RU/ml) quartile |
Incident Hypertension n (%) |
Unadjusted RR (95% CI) |
Model 1* RR (95% CI) |
Model 2† RR (95% CI) |
|---|---|---|---|---|
| 29.8–62.0 | 119/439 (27.1%) | Ref | Ref | Ref |
| 62.1–77.0 | 151/437 (34.6%) | 1.27 (1.04, 1.56) | 1.32 (1.08, 1.61) | 1.31 (1.07, 1.60) |
| 77.1–103.2 | 173/443 (39.1%) | 1.44 (1.19, 1.75) | 1.41 (1.16, 1.72) | 1.41 (1.16, 1.72) |
| ≥103.3 | 175/439 (39.9%) | 1.47 (1.21, 1.78) | 1.48 (1.22, 1.79) | 1.45 (1.18, 1.77) |
| P for Trend | <0.0001 | <0.0001 | 0.0002 |
Model 1 is adjusted for age, sex, race, education, and study center.
Model 2 adjusted for the Model 1 variables plus body mass index, smoking status, physical activity, Triglyceride/high density lipoprotein ratio, estimated glomerular filtration rate and urine albumin to creatinine ratio.
RR, Relative Risk; CI, Confidence Interval
Although African Americans had a higher incidence of hypertension than whites, there was no significant interaction by race (p = 0.1523) Likewise, there was no significant interaction by sex (p = 0.3726). With CKD patients excluded from the cohort (n = 63), the association between the highest quartiles of FGF23 and increased risk of incident hypertension persisted in fully adjusted models. [RR for Quartile 4 with Quartile 1 as referent: 1.63 (1.17, 2.27)].
Analyses repeated with iFGF23 did not demonstrate a similar independent association with incident hypertension (Table S2) or blood pressure over time.
DISCUSSION
In this study, we investigated whether FGF23 was associated with changes in blood pressure and incident hypertension in a large multiethnic population of young and middle aged adults. We demonstrated that higher FGF23 levels were associated with increasing systolic and diastolic blood pressure during follow-up despite similar baseline readings and that FGF23 was also associated with incident hypertension even after adjustment for potential confounders—including BMI, smoking status and markers of renal function. Although there were more black and female participants in the highest FGF23 quartile, the association of FGF23 with incident hypertension did not vary based on these demographic characteristics. Additionally, the association did not vary based on the presence of underlying kidney disease, which was only present in a small percentage of the study population.
Our findings of an association between FGF23 and rising systolic and diastolic blood pressure over time lend support to experimental evidence that suggests there are mechanisms by which FGF23 could affect blood pressure. Experimental data suggest that in chronic kidney disease, increased FGF23 contributes to RAAS activation by decreasing calcitriol synthesis which in turn may lead to increased renin production,9 in addition to other potential mechanisms by which FGF23 may modulate the RAAS system, such as suppression of Angiotensin Converting Enzyme 2.22–24 Experimental data also suggest that FGF23 may increase renal sodium uptake independent of the RAAS system.10 A previous study examining a possible association of FGF23 with incident hypertension in a primarily white population of older adults suggested that individuals in the highest FGF23 decile of values had a modestly increased risk of developing hypertension.25 Our study extends prior analyses in two significant ways. By the time US adults reach ages 55–64, the prevalence of hypertension already exceeds 50%.1 Given that the incidence of hypertension and other CVD increases with age, we focused on a younger population that was free of CVD. Second, we investigated the association of FGF23 with changes in both systolic and diastolic blood pressure measurements in a longitudinal fashion rather than only examining incident hypertension as an outcome alone. It is notable that even though our population would overall be expected to have less comorbid conditions than older populations, we demonstrate a significant association between higher FGF23 and incident hypertension risk not explained by other known risk factors for cardiovascular disease. Our findings further suggest that FGF23 may be an independent marker of risk of developing hypertension.
Our findings also have other potentially significant implications. Increasing blood pressure levels not meeting criteria for hypertension are nonetheless associated with worse cardiovascular outcomes.1, 26 In addition, there is evidence to suggest that antecedent blood pressure contributes significantly to future cardiovascular risk even when taking into account current blood pressure and hypertensive status.27, 28 Small numeric differences in blood pressure can have significant meaning for outcomes on a population level. Thus, the differential rises in blood pressure associated with higher FGF23 levels may have potential implications in and of themselves for future cardiovascular risk regardless of the presence of clinically diagnosed hypertension.
Strengths of our study include use of a large, well-characterized prospective cohort of African-American and white individuals. Nevertheless, this study has important limitations. We did not measure FGF23 levels at the baseline (first) exam, so we could not study incident hypertension at earlier ages. Other racial/ethnic groups were not part of the CARDIA cohort. Thus, our findings may not be generalizable to all racial/ethnic groups or age groups. We adjusted for important co-variables including BMI, smoking and markers of renal function. Although we think this would account for most confounding, we did not have other metabolic correlates such as serum renin, aldosterone or urine sodium levels. Thus, residual confounding from these and other factors cannot be excluded. In addition, not having such indices available limited our evaluation of mechanisms which could help explain an association between FGF23 and blood pressure. Such mechanistic insights will be important to further characterizing this association, and in turn might have implications for potential future therapeutic interventions. Studies of pharmacologic interventions to reduce FGF23 such as phosphate binders and calcimimetics have primarily been in the setting of chronic kidney disease.29 Monoclonal antibodies inhibiting FGF23 activity are also under investigation, but studies have been primarily experimental with only limited clinical data.30 Whether any pharmacologic interventions would be safe and effective in the setting of increased FGF23 and elevated blood pressure is unknown and beyond the scope of this study. Finally, we did not find an association between iFGF23 and incident hypertension. This finding illustrates that the choice of assay may be important when investigating possible associations of FGF23 with cardiovascular disease and other outcomes. Previous data have suggested that cFGF23 has less intra-individual variation than iFGF2314 yet associations between iFGF23 and cardiovascular disease have nonetheless been found previously.7, 25 Our study is the first to evaluate the association of both an intact and c-terminal assay for incident hypertension. Further study is needed to clarify the importance of type of assay when investigating possible associations with cardiovascular risk.
In conclusion, higher FGF23 levels are independently associated with rising blood pressure over time and an increased risk of incident hypertension in young and middle aged adults, but do not explain the disparate burden of hypertension in African Americans. Further study is necessary to elucidate the mechanisms by which higher FGF23 may contribute to unfavorable blood pressure trajectories.
PERSPECTIVES
Hypertension is a well-known risk factor for cardiovascular disease that is prevalent around the world. In this study, we demonstrated that higher FGF23 levels are independently associated with both increasing blood pressure over time and incident hypertension in a population of young and middle-aged adults without a high burden of chronic kidney disease and other significant comorbidities. Our findings suggest that FGF23 could have a clinical role as a novel marker in helping to identify individuals at higher risk of developing hypertension, beyond known risk factors. Defining the potential mechanisms by which FGF23 contributes to higher blood pressure may also have future implications for determining therapeutic options. Additional study of FGF23 in other young adult populations and of the mechanisms by which it may increase blood pressure are necessary to further explore these potentially clinically important hypotheses.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What is New?
Fibroblast Growth Factor-23 (FGF23) is a hormone in the body that has been associated with cardiovascular disease. It is not known whether FGF23 is associated with high blood pressure in young and middle-aged adults.
What is relevant?
This study suggests that higher FGF23 levels in the blood are associated with increasing blood pressure over time and the development of high blood pressure.
Summary
FGF23 may be a novel marker of increased risk of developing high blood pressure among young and middle aged adults.
Acknowledgments
The Coronary Artery Risk Development in Young Adults Study (CARDIA) is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with the University of Alabama at Birmingham (HHSN268201300025C & HHSN268201300026C), Northwestern University (HHSN268201300027C), University of Minnesota (HHSN268201300028C), Kaiser Foundation Research Institute (HHSN268201300029C), and Johns Hopkins University School of Medicine (HHSN268200900041C). CARDIA is also partially supported by the Intramural Research Program of the National Institute on Aging (NIA) and an intra-agency agreement between NIA and NHLBI (AG0005). This manuscript has been reviewed by CARDIA for scientific content. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
SOURCES OF FUNDING
This research was also supported in part by a grant from the American Heart Association (15SFDRN25080331).
Dr. Isakova received grant support from Shire and consulting honorarium from Bayer. Dr. Wolf has received grant support from Shire and consulted and/or received honoraria from Amag, Amgen, Ardelyx, DiaSorin, Incyte, Keryx, Lilly, Phizer, Sanofi, Ultragenyx, and ZS Pharma
Footnotes
DISCLOSURES
The remaining authors have no disclosures relevant to the content of this manuscript.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart Disease and Stroke Statistics-2017 Update: A Report from the American Heart Association. Circulation. 2017;135:e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–47. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gutierrez OM, Januzzi JL, Isakova T, Laliberte K, Smith K, Collerone G, Sarwar A, Hoffmann U, Coglianese E, Christenson R, Wang TJ, deFilippi C, Wolf M. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119:2545–52. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–9. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in CKD. J Am Soc Nephrol. 2014;25:349–60. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ix JH, Katz R, Kestenbaum BR, de Boer IH, Chonchol M, Mukamal KJ, Rifkin D, Siscovick DS, Sarnak MJ, Shlipak MG. Fibroblast growth factor-23 and death, heart failure, and cardiovascular events in community-living individuals: CHS (Cardiovascular Health Study) J Am Coll Cardiol. 2012;60:200–7. doi: 10.1016/j.jacc.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kestenbaum B, Sachs MC, Hoofnagle AN, Siscovick DS, Ix JH, Robinson-Cohen C, Lima JA, Polak JF, Blondon M, Ruzinski J, Rock D, de Boer IH. Fibroblast growth factor-23 and cardiovascular disease in the general population: the Multi-Ethnic Study of Atherosclerosis. Circ Heart Fail. 2014;7:409–17. doi: 10.1161/CIRCHEARTFAILURE.113.000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lutsey PL, Alonso A, Selvin E, Pankow JS, Michos ED, Agarwal SK, Loehr LR, Eckfeldt JH, Coresh J. Fibroblast growth factor-23 and incident coronary heart disease, heart failure, and cardiovascular mortality: the Atherosclerosis Risk in Communities study. J Am Heart Assoc. 2014;3:e000936. doi: 10.1161/JAHA.114.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Borst MH, Vervloet MG, ter Wee PM, Navis G. Cross talk between the renin-angiotensin-aldosterone system and vitamin D-FGF-23-klotho in chronic kidney disease. J Am Soc Nephrol. 2011;22:1603–9. doi: 10.1681/ASN.2010121251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andrukhova O, Slavic S, Smorodchenko A, Zeitz U, Shalhoub V, Lanske B, Pohl EE, Erben RG. FGF23 regulates renal sodium handling and blood pressure. EMBO Mol Med. 2014;6:744–59. doi: 10.1002/emmm.201303716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haring R, Enserro D, Xanthakis V, Mitchell GF, Benjamin EJ, Hamburg NM, Sullivan L, Nauck M, Wallaschofski H, Vasan RS. Plasma Fibroblast Growth Factor 23: Clinical Correlates and Association with Cardiovascular Disease and Mortality in the Framingham Heart Study. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, Liu K, Savage PJ. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 13.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017 Nov 7; doi: 10.1016/j.jacc.2017.11.006. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 14.Smith ER, Cai MM, McMahon LP, Holt SG. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab. 2012;97:3357–65. doi: 10.1210/jc.2012-1811. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs DRHL, Haskell WL, Pirie P, Sidney S. Validity and reliability of short physical activity history: CARDIA and the Minnesota Heart Health Program. J Cardiopulm Rehabil. 1989;9:448–459. doi: 10.1097/00008483-198911000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidney S, Jacobs DR, Jr, Haskell WL, Armstrong MA, Dimicco A, Oberman A, Savage PJ, Slattery ML, Sternfeld B, Van Horn L. Comparison of two methods of assessing physical activity in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 1991;133:1231–45. doi: 10.1093/oxfordjournals.aje.a115835. [DOI] [PubMed] [Google Scholar]
- 17.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, Coresh J, Levey AS. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–9. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui JS, Hopper JL, Harrap SB. Antihypertensive treatments obscure familial contributions to blood pressure variation. Hypertension. 2003;41:207–10. doi: 10.1161/01.hyp.0000044938.94050.e3. [DOI] [PubMed] [Google Scholar]
- 19.Tobin MD, Sheehan NA, Scurrah KJ, Burton PR. Adjusting for treatment effects in studies of quantitative traits: antihypertensive therapy and systolic blood pressure. Stat Med. 2005;24:2911–35. doi: 10.1002/sim.2165. [DOI] [PubMed] [Google Scholar]
- 20.Balakrishnan P, Beaty T, Young JH, Colantuoni E, Matsushita K. Methods to estimate underlying blood pressure: The Atherosclerosis Risk in Communities (ARIC) Study. PLoS One. 2017;12:e0179234. doi: 10.1371/journal.pone.0179234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–6. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 22.Boehm M, Nabel EG. Angiotensin-converting enzyme 2--a new cardiac regulator. The N Engl J Med. 2002;347:1795–7. doi: 10.1056/NEJMcibr022472. [DOI] [PubMed] [Google Scholar]
- 23.Dai B, David V, Martin A, Huang J, Li H, Jiao Y, Gu W, Quarles LD. A comparative transcriptome analysis identifying FGF23 regulated genes in the kidney of a mouse CKD model. PLoS One. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crackower MA, Sarao R, Oudit GY, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–8. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 25.Fyfe-Johnson AL, Alonso A, Selvin E, Bower JK, Pankow JS, Agarwal SK, Lutsey PL. Serum fibroblast growth factor-23 and incident hypertension: the Atherosclerosis Risk in Communities (ARIC) Study. J Hypertens. 2016;34:1266–72. doi: 10.1097/HJH.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 27.Lee DS, Massaro JM, Wang TJ, Kannel WB, Benjamin EJ, Kenchaiah S, Levy D, D'Agostino RB, Sr, Vasan RS. Antecedent blood pressure, body mass index, and the risk of incident heart failure in later life. Hypertension. 2007;50:869–76. doi: 10.1161/HYPERTENSIONAHA.107.095380. [DOI] [PubMed] [Google Scholar]
- 28.Bonifonte A, Ayer T, Veledar E, Clark A, Wilson PW. Antecedent blood pressure as a predictor of cardiovascular disease. J Am Soc Hypertens. 2015;9:690–696.e1. doi: 10.1016/j.jash.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 29.Pool LR, Wolf M. FGF23 and Nutritional Metabolism. Annu Rev Nutr. 2017;37:247–268. doi: 10.1146/annurev-nutr-071816-064620. [DOI] [PubMed] [Google Scholar]
- 30.Kinoshita Y, Fukumoto S. X-linked hypophosphatemia and FGF23-related hypophosphatemic diseases -Prospect for new treatment. Endocr Rev. 2018 Jan 26; doi: 10.1210/er.2017-00220. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.