Abstract
The adverse outcome pathway (AOP) framework can be used to help support the development of alternative testing strategies aimed at predicting adverse outcomes caused by triggering specific toxicity pathways. In this paper, we present a case-study demonstrating the selection of alternative in chemico assays targeting the molecular initiating events of established AOPs, and evaluate use of the resulting data to predict higher level biological endpoints. Based on two AOPs linking inhibition of the deiodinase (DIO) enzymes to impaired posterior swim bladder inflation in fish, we used in chemico enzyme inhibition assays to measure the molecular initiating events for an array of 51 chemicals. Zebrafish embryos were then exposed to 14 compounds with different measured inhibition potentials. Effects on posterior swim bladder inflation, predicted based on the information captured by the AOPs, were evaluated. By linking the two datasets and setting thresholds, we were able to demonstrate that the in chemico dataset can be used to predict biological effects on posterior chamber inflation, with only two outliers out of the 14 tested compounds. Our results show how information organized using the AOP framework can be employed to develop or select alternative assays, and successfully forecast downstream key events along the AOP. In general, such in chemico assays could serve as a first-tier high-throughput system to screen and prioritize chemicals for subsequent acute and chronic fish testing, potentially reducing the need for long-term and costly toxicity tests requiring large numbers of animals.
Keywords: thyroid hormone disruption, deiodinase inhibition assay, zebrafish embryo, swim bladder inflation, adverse outcome pathway
Graphical abstract
1. Introduction
Industry and regulatory bodies have expressed the need for developing alternative testing strategies for risk assessment and hazard identification, focusing on non-animal alternatives and the use of mechanistic information (Ankley et al., 2010). Fish are ideal sentinels for evaluating aquatic toxicity to vertebrates, but testing for chronic fish toxicity is resource and animal intensive. Although a number of in vitro assays based on fish cells or cell lines have been developed (Bols et al., 2005; Segner, 2004, 1998; Stadnicka-Michalak et al., 2014; Tan et al., 2008), fish embryos have also become a popular alternative model system in aquatic ecotoxicology (Braunbeck and Lammer, 2006; Scholz et al., 2008). The publication of OECD Testing Guideline (TG) 236, the “Fish Embryo Acute Toxicity (FET) Test” (OECD, 2013a), describing a 96 h fish embryo test, has greatly facilitated the use of fish embryos in toxicity studies. The testing guideline is currently limited to observations of lethal endpoints and hatching, but research has shown that more subtle toxic effects can also be reliably investigated using fish embryos (Braunbeck et al., 2014; Hagenaars et al., 2014; Hill et al., 2005; Michiels et al., 2017; Pype et al., 2015; Scholz et al., 2008; Selderslaghs et al., 2013; Stinckens et al., 2016; Verstraelen et al., 2016; Voelker et al., 2007). However, the development of alternative assays capable of capturing and representing the mechanisms underlying toxicity pathways at sub-organismal levels of biological organization requires a targeted approach. Adverse outcome pathways (AOPs) can assist in the identification of measurable processes at specific levels of biological organization, termed key events (KEs), that are essential in a given toxicity pathway (Ankley et al., 2010). In this way, the AOP framework can directly assist in assay development by guiding the selection of specific KEs which are likely to have a high predictive value for an AO of interest.
The aim of the present study was to demonstrate how in chemico assays targeting specific KEs of an established AOP were selected and used to predict higher biological endpoints. The selected AOP focuses on the role of thyroid hormones in embryonic development in fish. Thyroid hormones (THs) have been shown to play an important role in a wide range of biological processes in vertebrates and disruption of the thyroid axis can lead to ecologically relevant adverse outcomes. For example, THs are involved in development, especially in amphibian metamorphosis (Callery and Elinson, 2000), embryonic-to-larval transition (Liu and Chan, 2002) and larval-to-juvenile transition (Brown, 1997) in fish. The two primary THs are the prohormone thyroxin (T4) and the biologically more active 3,5,3′-triiodothyronine (T3) (Hulbert, 2000). The synthesis of these THs is a process that involves several steps, with thyroperoxidase (TPO) playing an essential role in the production of T4, and to a lesser extent of T3. The bioavailability of T3 in developing cells is regulated by several processes, including deiodination by enzymes called iodothyronine deiodinases (DIOs) (Darras and Van Herck, 2012; Gereben et al., 2008; Orozco and Valverde-R, 2005). To date, three types of iodothyronine deiodinases (DIO1-3) have been described in vertebrates. Type 2 deiodinase (encoded by the DIO2 gene) is capable of activating T4 into T3, as well as of converting reverse T3 (rT3) into 3,3′ T2. Deiodinase 3 can convert T4 and T3 to the inactive TH forms rT3 and 3,3′ T2 respectively. Type 1 deiodinase (encoded by the DIO1 gene) is capable of both outer and inner ring deiodination, and can therefore catalyze all four TH deiodination reactions (Darras and Van Herck, 2012; Gereben et al., 2008; Orozco and Valverde-R, 2005).
Numerous chemicals are known to disturb thyroid-related processes, for example by inhibiting the TPO and/or DIO enzymes, by upregulating metabolization pathways, or by inhibiting sodium/iodide symporter (NIS) mediated iodide uptake (Butt et al., 2011; Hallinger et al., 2017; Hornung et al., 2010; Kim et al., 2015; Paul et al., 2014; Visser et al., 1979). Previous work suggests that chemicals interfering with the conversion of (maternal) T4 to T3 (a reaction catalyzed by either Dio1 or Dio2) could inhibit inflation of the posterior swim bladder in fish, which may result in reduced swimming capacity, an adverse outcome that can affect feeding behavior and predator avoidance, ultimately resulting in lower survival probability and population trajectory decline (Czesny et al., 2005; Woolley and Qin, 2010). The swim bladder of the zebrafish is a gas-filled structure that consists of a posterior and an anterior chamber. While the posterior chamber inflates during early development (96-120 hours post fertilization, hpf), the anterior chamber only inflates around 20-21 days post fertilization (dpf). Both chambers are important for regulating buoyancy and body density, and the anterior chamber additionally has a role in hearing in fish species of the superorder Ostariophysi (which possess a Weberian apparatus) (Dumbarton et al., 2010; Lindsey et al., 2010; Roberston et al., 2007).
Building on our previous work focused on AOP development related to TH disruption in fish (Cavallin et al., 2017; Nelson et al., 2016; Stinckens et al., 2016), we aligned the present study with AOPs that are publicly available in the AOP-Wiki (aopwiki.org), an online database organizing the available knowledge and published research into individual AOP descriptions using a user friendly Wiki interface. More specifically, we focused on two AOPs linking the molecular initiating events (MIEs) Dio1 and Dio2 inhibition to impaired inflation of the posterior swim bladder chamber in fish during early life-stages (Figure 1; Bagci et al., 2015; Cavallin et al., 2017; Knapen et al., 2018; Nelson et al., 2016; Stinckens et al., 2016; Villeneuve et al., 2018). These AOPs were used to optimize in chemico assays to measure the potential of compounds to inhibit DIO1 and DIO2 enzyme activity, using porcine tissue. We then selected 14 compounds with different DIO inhibitory potencies to assess potential effects on posterior chamber inflation, using the zebrafish embryo as a model system. By linking both datasets, we evaluated the use of in chemico data to explain and ultimately predict the in vivo biological effects on posterior chamber inflation.
Figure 1.
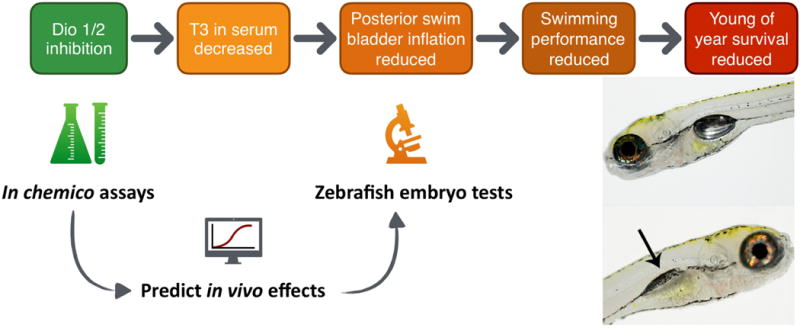
AOPs 157 (Dio1) and 155 (Dio2) describing the effects of the MIEs Dio1 and Dio2 inhibition on T3 in serum, leading to impaired posterior swim bladder inflation, reduced swimming performance and ultimately reduced young of year survival. See https://aopwiki.org/aops/155 and https://aopwiki.org/aops/157 for a graphical representation of these AOPs within a larger AOP network context. In chemico assays were optimized to measure the inhibitory potential of compounds on DIO1 and DIO2 enzyme activity. Zebrafish embryo tests were performed to assess the effect of a subset of compounds on posterior swim bladder inflation. Both datasets were linked to evaluate the use of the in chemico data for predicting the in vivo effects on posterior chamber inflation.
2. Materials and Methods
2.1. Ethics statement
The EU Directive 2010/63/EU and the Commission Implementing Decision 2012/707/EU state that fish are non-protected animals until they are free feeding, i.e. 120 hours post fertilization (hpf) for zebrafish (Strähle et al., 2012). All experiments of this study executed at the University of Antwerp (UA) exceeding 120 hpf were approved by the Ethical Committee for Animals of the University of Antwerp (project IDs 2014-29 and 2016-46). According to the Animal Research Advisory Committee Guidelines for the use of zebrafish in the National Institutes of Health Intramural Research Program 06/22/2016, fish are non-protected animals until 72 hpf. Therefore, all experiments of this study executed at the University of California, Riverside (UCR) exceeding 72 hpf were approved by the Institutional Animal Care and Use Committee (IACUC; animal use protocol (AUP) #20150035). Fish husbandry and all experiments were carried out in strict accordance with the EU Directive on the protection of animals used for scientific purposes (2010/63/EU) for work carried out at the UA, and in accordance with the IACUC-approved AUP (#20150035) for work carried out at the University of California, Riverside.
2.2. Test compounds
DIO1 and DIO2 in chemico enzyme activity inhibition was determined in the presence of 51 chemicals (Supplementary Table S1); fifteen of these chemicals have been described as TPO inhibitors (MMI, PTU, RCS, TCS, IOP, 2-TU, MBI, CMZ, NaPER, KPER, MBT, ETU, NP, AMT, BP2) and thirteen were reported DIO1 and/or DIO2 inhibitors (PTU, DEX, AMIO, PR, SA, NaSAL, IOP, 2-TU, PFOA, TBP, TCBPA, TBBPA, ANS). A number of the remaining compounds had been reported to have effects on other thyroid-related endpoints, such as inhibition of the iodide transport, affecting the metabolization of THs or disturbing the hormone receptor. For some of the test chemicals, no thyroid-related effects were reported in literature. They were either selected because of chemical similarity to TH active compounds, and/or because of environmental relevance.
2.3. In chemico screening for DIO enzyme inhibition
In chemico deiodinase assays were optimized to determine DIO1 and DIO2 enzyme activity by measuring the amount of free 125I released by conversion of 125iodine-labelled rT3 or T4 by DIO1 or DIO2, respectively (Ferreira et al., 2002; Forhead et al., 2006; Freyberger and Ahr, 2006; Pavelka, 2010). Pooled liver tissue of five male and five female pigs was used (Slaughterhouse RIMA Mechelen, Slachthuislaan 1, Belgium and Slaughterhouse Noordvlees Van Gool Kalmhout, Bloemstraat 56, Belgium) as the source of DIO. Porcine liver is readily available from slaughterhouses, and its use reduces the need for using laboratory animals. The tissue was minced in homogenization buffer (1 mL per 100 mg liver tissue, 100 mM potassium dihydrogen phosphate (K2PO4), 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM dithiothreitol (DTT), pH 7), using a Potter-Elvehjem and used in the assay at a final protein concentration of 1 mg/mL (Bradford assay; Bradford, 1976). An unlabeled rT3 mixture (final concentrations of 1 μM unlabeled rT3, 100 mM K2PO4, 1 mM EDTA, pH 7, 10 mM DTT), and an unlabeled T4 solution (final concentrations 1 nM unlabeled T4 and 100 nM unlabeled T3, 100 mM K2PO4, 1 mM EDTA, pH 7, 25 mM DTT) was used for the DIO1 and DIO2 assay respectively. Labelled rT3 and T4-solutions were prepared (50000 counts per minute (cpm) per μL 125I-rT3 (DIO1) or 125I-T4 (DIO2) in homogenization buffer; PerkinElmer Life Sciences, Boston, Massachusetts, United States). Per reaction, 1 μl of labelled solution was added to 99 μL unlabeled solution.
The 125I-rT3 or -T4 was purified prior to use using a Sephadex LH-20 column with 2 mL of 10% LH-20 (1 mL bed volume). The column was rinsed twice with 1 mL 0.1N HCl. Labelled rT3 or T4 solution (1:4 rT3/T4 solution to 0.1N HCl) was added. The column was washed twice with 1 mL 0.1 N HCL (elution of 125I−), twice with 1 mL water and once with 0.7 mL 0.1 M NH4OH in ethanol. The latter is just enough to displace water in the column with ethanol, but not enough to elute the labelled rT3 or T4. Finally, the column was eluted with 1 mL 0.1M NH4OH in ethanol.
Stock solutions of the test chemicals were prepared in 100% dimethyl sulfoxide (DMSO) (333.33 mM of test chemical). Working solutions, diluted to 6% DMSO in homogenization buffer, were stored at room temperature and protected from light. Preliminary tests showed that the use of a final concentration of 0.4 to 1.5% DMSO resulted in a deiodination efficiency of 95 to 100% compared to 0% DMSO, while final concentrations of 2-25% DMSO affected the efficiency with 57-90% compared to 0% DMSO (data not shown). Therefore, a final concentration of 1.5% DMSO was selected. The assay was performed using three replicates, each containing 50 μL test substance (final concentrations 0.001, 0.01, 0.1, 1, 10, 100, 1000 and 5000 μM in 1.5% DMSO), 50 μL homogenate and 100 μL of the substrate solution. Samples were incubated for 30 (DIO1) or 60 minutes (DIO2) at 37°C and the reaction was stopped by adding 100 μL 5% ice-cold bovine serum albumin. 500 μL 10% trichloroacetic acid was added to precipitate albumin-bound remaining 125I-T4 or 125I-rT3. After incubation on ice for 10 minutes, the tubes were centrifuged (10 min, 1500 g, 4°C) and 500 μL of the supernatant was used to determine the radioactivity, using a γ-counter (PerkinElmer Life Sciences, Boston, Massachusetts, United States). Furthermore, a blank (absence of test compounds and homogenate) was used to correct for non-enzymatic degradation of the tracer. Enzyme activities were expressed as picomoles (DIO1) or femtomoles (DIO2) of substrate deiodinated per minute per mg protein and if inhibition occurred, the half maximal inhibitory concentration (IC50) was calculated (see section 2.5 Data analysis).
2.4. In vivo screening using acute zebrafish embryo toxicity tests
Zebrafish embryos were exposed to 12 chemicals (methimazole, MMI; 6-propylthiouracil, PTU; iopanoic acid, IOP; nonafluorobutane-1-sulfonic acid, PFBS; pentadecafluorooctanoic acid, PFOA; bisphenol A, BPA; tetrachlorobisphenol A, TCBPA; benzophenone 2, BP2; sodium perchlorate, NaPER; 2,4,6-tribromophenol, TBP; salicylic acid, SA; 8-anilino-1-naphthalene sulfonic acid, ANS) at the University of Antwerp (UA), where posterior chamber inflation was assessed (Supplementary Table S2). Previously published data on two additional compounds generated in the same lab were added to the dataset (heptadecafluorooctane sulfonic acid, PFOS and 2-mercaptobenzothiazole, MBT; Hagenaars et al., 2014; Stinckens et al., 2016). Additional zebrafish embryo experiments were performed at the University of California, Riverside (UCR), using 10 of the 14 compounds to study the posterior chamber surface area in more detail.
2.4.1. Collection of eggs
For posterior chamber inflation assessment in zebrafish larvae at UA, adult zebrafish (in house wild type zebrafish line) were used for egg production and were accommodated in a ZebTEC standalone system (Tecniplast, Buguggiate, Italy). All non-exposed zebrafish and embryos were kept in reconstituted freshwater with a conductivity of 500 ± 15 μS/cm (Instant Ocean® Sea Salt, Blacksburg, USA), pH 7.5 ± 0.3 (NaHCO3), a constant temperature of 28 ± 0.2°C and a 14/10 h light/dark cycle. Concentrations of ammonium (NH4+), nitrite (NO2−) and nitrate (NO3−) were monitored twice weekly using Tetratest kits (Tetra Werke, Melle, Germany) and always remained below 0.25, 0.3 and 12.5 mg/L respectively. Adult zebrafish were fed three times per day, twice with granulated food (0.5% of their average wet weight, Biogran medium, Prodac International, Cittadella, Italy) and once with either frozen Chironomidae larvae, Artemia sp. nauplii, Chaoboridae larvae or Daphnia sp. (Aquaria Antwerp bvba, Aartselaar, Belgium). During weekends, fish were fed once daily with granulated food (1% of their average wet weight). In order to collect embryos, one female and two males were placed in breeding tanks with a perforated bottom, separated by a transparent divider. The divider was removed the next morning to allow spawning.
For posterior chamber surface area assessment in zebrafish larvae at UCR, an adult wild type zebrafish line referred to as strain 5D was maintained in a recirculating system with UV sterilization and mechanical/biological filtration units (Aquaneering, San Diego, CA, USA), also under a 14/10 h light/dark cycle at a water temperature of ~27-28°C, pH of ~7.2 (NaHCO3), and conductivity of ~900-950 μS (10 mM NaCl, 0.17 mM KCL, 0.66 mM CaCl2, 0.66 mM MgSO4). Water quality was constantly monitored for pH, temperature, and conductivity using a real-time water quality monitoring and control system. Ammonium, nitrate, nitrite, alkalinity, and hardness levels were manually monitored weekly by test strip (Lifeguard Aquatics, Cerritos, CA). Zebrafish were fed twice per day with dry diet (Gemma Micro 300, Skretting, Fontaine-lès-Vervins, France). Embryo production was performed as described previously (UA), however using 10 adult males and 10 females for breeding.
2.4.2. Test compound concentrations
To evaluate posterior chamber inflation at UA, zebrafish embryo experiments based on OECD TG 236 (OECD, 2013a), directly observing possible effects on posterior swim bladder chamber inflation were performed. Zebrafish embryos were exposed until 120 and/or 168 hpf to at least six concentrations of the test compounds compared to control and solvent control conditions. Concentrations resulting in 0 to 100% mortality and 0 to 100% effect on swim bladder inflation were selected, based on available data from literature and/or preliminary range finding experiments (data not shown). Zebrafish embryos were exposed to 12 chemicals: MMI (0-2500 mg/L), PTU (0-1200 mg/L), IOP (0-8 mg/L), PFBS (0-6400 mg/L), PFOA (0-1000 mg/L), BPA (0-30 mg/L), TCBPA (0-1.5 mg/L), BP2 (0-30 mg/L), NaPER (0-2000 mg/L), TBP (0-10 mg/L), SA (0-200 mg/L) and ANS (0-1000 mg/L) in separate experiments (see Supplementary Table S2 for detailed information on concentrations tested). To assess posterior chamber surface area at UCR, zebrafish embryos were exposed until 120 hpf to the EC10, EC30 and EC50 concentrations (based on experiments performed at UA) of MMI, PTU, PFBS, PFOA, BPA, NaPER, TBP, SA, MBT and ANS (Supplementary Table S3). Stock and working solutions of each chemical were prepared by dissolving chemicals in reconstituted freshwater. When compounds were not sufficiently soluble in water (BPA and TBP), a stock solution was prepared in 100% DMSO (final concentration of 0.1% DMSO). All solutions were prepared one day in advance of the experiment and stored in the dark, at room temperature until use.
2.4.3. Experimental design
Using a plastic pipette, 24 viable embryos were manually arrayed into polystyrene 24-well plates (one embryo/well; two mL/well). The first column of each plate consisted of internal negative control embryos (reconstituted fresh water), resulting in 20 exposed embryos per plate. For posterior inflation assessments at UA, two 24-well plates were used per condition (i.e., negative control or exposure concentration, n=40 per condition). 3,4-dichloroaniline (CAS 95-76-1, purity of 98%, Sigma-Aldrich, nominal concentrations 0.5, 1, 2 and 4 mg/L; 6 embryos/concentration; one mL/well) was used as positive control for lethality in a separate 24-well plate (OECD TG 236, OECD, 2013a). For posterior surface area assessments at UCR, two 24-well plates were used for the EC10 and EC30 conditions per compound (n=40) and four 24-well plates were used for the EC50 condition (n=80).
Two 24-well plates per experiment were used as solvent control if necessary (eight internal negative controls, 40 solvent controls: 0.1% DMSO in reconstituted water). A test was considered valid if ≥80% of the negative controls successfully hatched and ≥90% survived until the end of the 120/168 hpf exposure (OECD TG 236, OECD, 2013a). Furthermore, exposure to the highest tested concentration of the positive control (4 mg/L) should result in a minimum mortality of 30% at the end of the exposure (OECD TG 236, OECD, 2013a).
2.4.4. Exposure and assessment
Polystyrene 24-well plates (sterile tissue culture plates, Greiner Bio-One, Frickenhausen, Germany) were saturated by filling the plates with test solutions one day in advance of egg collection. The test media was subsequently renewed immediately before exposure of the eggs. Eggs were transferred to a beaker containing the test solution within 30 to 60 minutes after spawning. In order to reduce the dilution effect of transfer from reconstituted water to test solution, the eggs were transferred to a second beaker with the same test solution. Fertilized eggs were separated from non-fertilized eggs using a stereomicroscope (UA: Leica S8APO, Leica Microsystems GmbH, Germany; UCR: Leica MZ10 F stereomicroscope) and eggs with anomalies were discarded. Normally shaped fertilized eggs were then divided over the plates. Plates were sealed with parafilm (Parafilm®, Bemis Europe, Soignies, Belgium) and plastic lids and stored in an incubator (UA: MIR-254-PE, Panasonic, TCPS, Rotselaar, Belgium; UCR: Thermo Scientific Precision Plant Growth Chamber) with a day/night cycle of 14/10 h and a constant temperature of 28-28.5°C. All exposure solutions were renewed every 24 h, with the exception of NaPER which was renewed every 48 h (this compound is stable for at least 48 h, Mukhi and Patiño, 2007; Patiño et al., 2003). Every 24 h, mortality (coagulation, absence of heart beat, absence of somite formation, no tail detachment; OECD TG 236, OECD, 2013a), was evaluated using a stereomicroscope. When delayed hatching was observed as a result of compound exposure, embryonic chorions were manually removed at 52 hpf, i.e. at the time of natural hatching of the controls. Delayed hatching could interfere with swim bladder inflation, since non-hatched larvae are unable to reach the water surface and gulp for air, a process that is required for initial swim bladder inflation.
Since inflation of the posterior chamber of the swim bladder occurs around 96-120 hpf, even the slightest delay in either developmental speed in general, or in the development and inflation process of the swim bladder itself, would lead to incomplete swim bladder assessments if only a standard 120 hpf zebrafish embryo test would have been used. Therefore, extended fish embryo acute toxicity tests were performed until 168 hpf. At UA, posterior chamber inflation was recorded in a binary fashion (inflated or non-inflated) every 24 hpf, starting from initial inflation around 96 hpf. At the end of the experiment (168 hpf), larvae were euthanized using an overdose of 1g/L MS-222 adjusted to pH 7.5 using NaHCO3.
For the posterior surface area assessment at UCR, exposures were limited to 120 hpf, as we wanted to investigate whether an effect on posterior chamber surface area at 120 hpf could be a more sensitive endpoint compared to the binary scoring of posterior chamber inflation at 168 hpf, thereby also eliminating the need for using protected life-stages to assess possible effects on posterior chamber inflation. At 120 hpf, each surviving embryo was transferred to a 96-well plate and anesthetized using 100 mg/L MS-222 (tricaine methanesulfonate, Sigma–Aldrich) adjusted to pH 7.5. All embryos were imaged directly within each well of the 96-well plate using automated image acquisition protocols. Body length and swim bladder surface were assessed using an ImageXpress Micro XLS Widefield High-Content Screening System equipped with MetaXpress 6.0.3.1658 (Molecular Devices, Sunnyvale, CA), a 2X objective and transmitted light with a camera binning of 1 to acquire one frame per entire well. Custom data extraction and analysis pipelines within MetaXpress were used to quantify body length and posterior chamber surface area of live embryos at 120 hpf. At the end of the experiment, all embryos were euthanized by placing the plate and all its content at −30°C.
2.5. Data analysis
All statistical analyses were performed using GraphPad Prism version 7.00 (GraphPad Software, San Diego, CA) and data were considered significantly different when p-values were <0.05.
2.5.1. In chemico screening for DIO enzyme inhibition
The specific enzyme activity was expressed as picomole (DIO1) or femtomole (DIO2) substrate deiodinated per minute per milligram of protein. Enzymatic deiodination was corrected for non-enzymatic 125I− production as determined in blank incubations without enzyme and multiplied by a correction factor of 2 to account for the random labelling and deiodination of either the 3′ or the 5′ position of 125I-rT3 and 125I-T4. The percentage of enzyme activity compared to control activity (in the absence of inhibitor) was expressed as a function of the logarithmically transformed compound concentrations resulting in one concentration-response curve per compound constructed out of the three technical replicates (non-linear regressions; fitting a variable slope sigmoidal concentration-response model, with bottom and top constrained at 0 and 100% respectively). The IC50 concentration, the concentration at which 50% DIO-inhibition was observed, was calculated per compound. The threshold for positive DIO-inhibition was set to at least 20% inhibition compared to the negative control (i.e., no test compound present in homogenization buffer containing a final concentration of 1.5% DMSO, Paul et al., 2014). It must be noted that it was not possible to determine the IC50 values of compounds with a DIO-inhibition lower than 20% compared to controls as a concentration-response curve could not be fitted, and the IC50 concentration of these compounds was estimated to be 5000 μM (highest tested concentration) or higher.
2.5.2. In vivo screening using acute zebrafish embryo toxicity tests
Mortality rates and effects on posterior chamber inflation were analyzed as a function of logarithmic nominal exposure concentrations using a nonlinear regression estimate (variable slope, bottom and top constrained at 0 and 100%). LC50, concentration at which 50% mortality occurs, and EC50, concentration at which 50% effect on posterior chamber inflation occurs, values were calculated and the logarithm of these values compared using a sum-of-squares F test to determine significant differences between dose-response curves for mortality and swim bladder inflation. Swim bladder surface of inflated posterior chambers was determined using the MetaXpress 6.0 imaging analysis software and analyzed using a one-way analysis of variance (ANOVA) with a Tukey’s multiple comparisons test. Data normality was confirmed using the d’Agostino-Pearson and Kolmogorov-Smirnov normality test.
3. Results and discussion
As a case study of using the AOP framework for the development of alternative assays, we selected an AOP that describes the effects of Dio inhibition on posterior swim bladder inflation in fish. We first used in chemico assays for measuring the MIE (i.e., DIO inhibition). We then assessed the effects of exposure to a selection of DIO inhibiting compounds on posterior chamber inflation using zebrafish embryo assays. We linked both datasets to evaluate the potential for using AOP-based in chemico data as the basis for predicting biological effects at higher levels of biological organization.
3.1. In chemico screening for DIO enzyme inhibition
We characterized 51 compounds using in chemico DIO1 and DIO2 inhibition assays, using porcine tissue, to assess their thyroid hormone disrupting potential (Figure 2; Supplementary Table S4 and S5). In total, 28 compounds were identified as positive DIO1-inhibitors and 28 compounds were identified as positive DIO2-inhibitors. Several compounds inhibited both enzymes. Identification as a “positive” inhibitor at this stage is solely based on inhibition of porcine DIO activity by more than 20% relative to the negative control. It is not necessarily an indication of the compounds’ potential to cause biological effects by altering TH concentrations in vivo. We therefore distinguish between “DIO inhibitors” to refer to compounds that are in chemico enzyme inhibitors, and “TH disruptors” to refer to compounds actually causing DIO inhibition related in vivo effects on posterior chamber inflation. We attempted to define biological effect thresholds and associated uncertainties using the in vivo data (see section 3.2 and Figure 4) based on 14 compounds. Those thresholds were then used to group all 51 compounds that were screened using in chemico assays based on their likelihood to act as TH disruptors, bearing in mind that false positives and false negatives can occur (see section 3.3 for a detailed discussion).
Figure 2.
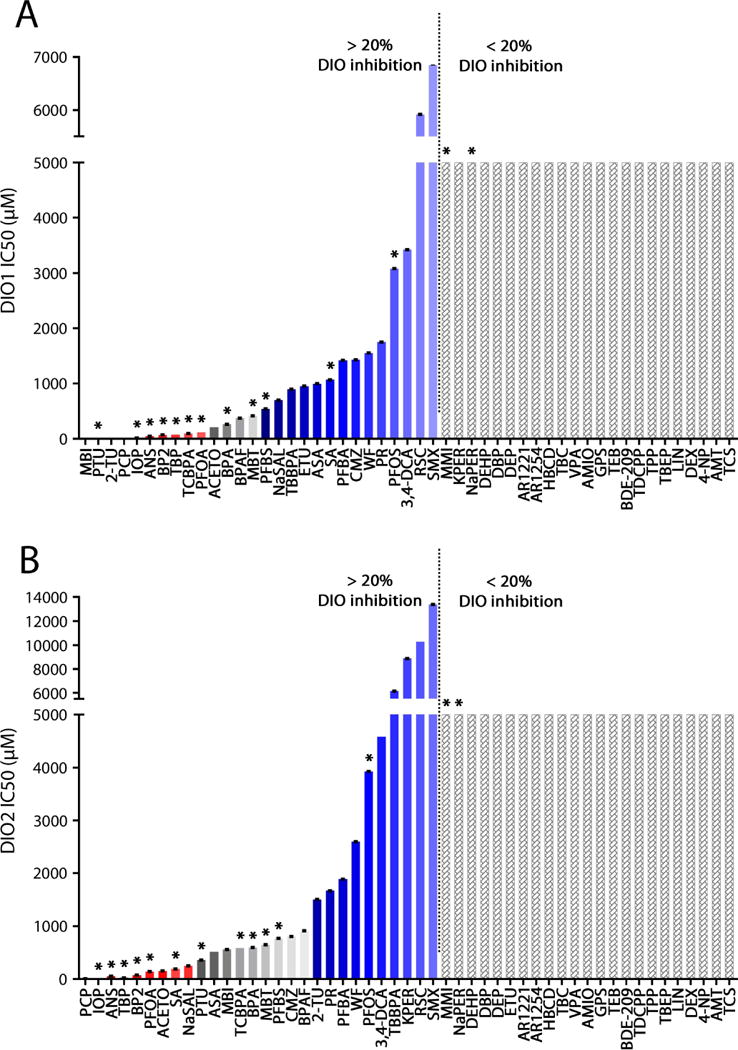
Mean IC50 values for DIO1 (A) and DIO2 (B) inhibition of 51 compounds (n=3). An estimate of 5000 μM is given for compounds which did not exceed the 20% inhibition threshold relative to the negative control. Red bars indicate strong DIO inhibitors that are likely to act as TH disruptors (although false positives can occur), and blue bars indicate weak DIO inhibitors unlikely to act as TH disruptors (although false negatives can occur). The zone in gray indicates the current uncertainty in defining biological effect thresholds: given the existing data, it is difficult to assess the likelihood for compounds in this zone to act as thyroid disruptors. See also Figure 4. * indicates compounds tested in in vivo zebrafish acute toxicity tests. Error bars indicate standard errors.
Figure 4.
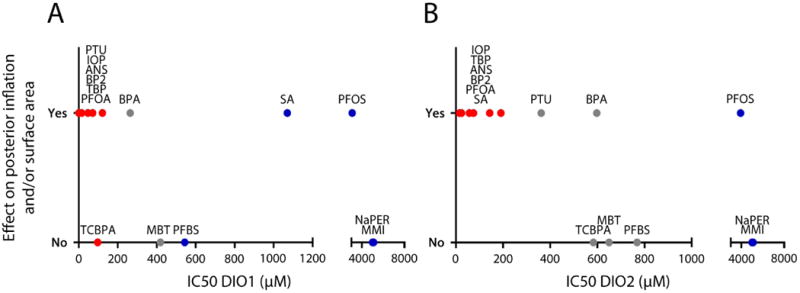
Summary of in chemico and in vivo datasets. DIO1 (A) and DIO2 (B) IC50 values as a function of the binary observation (yes or no) of effects on posterior chamber inflation and/or surface area. Compounds were grouped in 3 categories: (1) strong DIO inhibitors that are likely to act as TH disruptors (red), (2) weak DIO inhibitors unlikely to affect posterior chamber inflation (blue), and (3) zone of uncertainty (gray). See also Figure 2.
In general, it is important to critically assess the potential and robustness of in chemico to in vivo extrapolations. In our study, making a clear distinction between in chemico enzyme inhibition, and in vivo enzyme inhibition and its subsequent in vivo effects is therefore helpful and necessary to identify potential limitations of our approach. It is clear that in chemico data can function as an important first level screening tool, but many different factors can contribute to the reality that in chemico data are often only capable of partially explaining in vivo observations.
First of all, the occurrence and severity of in vivo effects are, of course, dependent on toxicokinetic and toxicodynamic processes, as well as on possible feedback and/or compensation mechanisms which are not captured by in chemico assays.
Also, consideration of other mechanisms than DIO inhibition may be required to fully explain observed in vivo effects. For example, Visser et al. (1979) examined the in chemico DIO inhibitory potential of several thiouracil (TU) and methimazole (MMI) analogues using rat liver microsomes, including 2-TU, MBI, MBT and MMI. In agreement with their results, we found a high DIO enzyme inhibition potential for 2-TU and MBI, while MBT and MMI showed weaker or no DIO inhibition. Weak in chemico DIO inhibition by MMI was also found by Taurog et al. (1994). However, MMI-treated tilapia and striped parrotfish displayed decreased T3 levels, associated with increased liver Dio1 and Dio2 activity and changes in dio1 and dio2 mRNA expression (Johnson and Lema, 2011; Mol, 1999; Van der Geyten et al., 2001). Overall, it seems likely that MMI does not directly affect Dio activity in fish, but rather causes hypothyroidism due to TPO inhibition, possibly activating a compensation mechanism at the level of Dio1 and Dio2 activity to increase the conversion of T4 to T3.
Importantly, chemical-induced perturbations can also vary among tissues, as well as across different species. For example, our in chemico data shows no effects of the PCB mixture Aroclor 1254 on DIO1 or DIO2 enzyme activity using porcine liver tissue. Coimbra et al. (2005) exposed fish (Nile tilapia) to Aroclor 1254 and in agreement with our in chemico results, there was no effect on Dio2 activity in liver. However, they did observe decreased Dio2 activity in the brain, which suggests tissue specific effects. Furthermore, the latter study found no effect on Dio1 and/or Dio2 activity in kidney, while Hood and Klaassen (2000) reported a decrease in kidney DIO1 activity after exposing rats to Aroclor 1254 (it should however be noted that the Aroclor 1254 mixture was purchased from a different supplier in these studies and may therefore slightly differ in composition). Overall, these results suggest possible taxonomic differences in addition to tissue specific effects. Taxonomic differences also become apparent when comparing our results, which show weak DIO1 and DIO2 inhibition for the brominated flame retardant TBBPA in porcine liver tissue, to the results of Butt et al. (2011), which reported a high DIO1 inhibitory capacity of TBBPA using human liver as source of DIO enzymes. As a last but noteworthy example in the context of our study, it has been shown that PTU, a drug designed to treat hyperthyroidism, effectively inhibits DIO1 in many higher vertebrate species (mammals, birds and reptiles; Kuiper et al., 2003; Toyoda et al., 1994; Van Der Geyten et al., 1997; Visser et al., 1978; Wassen et al., 2004), in agreement with our DIO1 in chemico data. However, the effects of PTU on Dio activity in fish are less clear. Some studies suggest a partial insensitivity of Dio1 to PTU in fish and amphibians (Finnson et al., 1999; Kuiper et al., 2006; Mol et al., 1998; Orozco et al., 2003; Sanders et al., 1997). To which extent these conclusions, which are mainly based on in chemico data and on a relatively limited number of species, can be extrapolated to all teleosts remains unclear. The amino acid sequence in the active center of deiodinase 1 appears to be conserved across different species, with the exception of fish and frogs (Orozco et al., 2012). A proline residue instead of a serine residue is found at position 128 in fish and position 132 in frog (Kuiper et al., 2006; Orozco et al., 2003; Sanders et al., 1997), which could explain the difference in PTU sensitivity in these species. Indeed, recombinant Xenopus laevis Dio1, with a substitution of the proline residue with serine, showed high sensitivity to PTU (Kuiper et al., 2006). However, the potential mechanism behind a lower sensitivity of Dio1 for inhibition by PTU in teleost species remains unknown, as a substitution of Pro128 by serine in tilapia did not affect PTU sensitivity (Orozco et al., 2003; Sanders et al., 1997).
Apart from toxicokinetic and toxicodynamic processes, other mechanisms than DIO inhibition, tissue and species differences, effects of compounds on in chemico and/or in vivo DIO enzyme activity can also vary depending on developmental stage, gender, reproductive state, genetic background, etc. Therefore, it is important to determine the applicability domain of a given AOP, and more specifically of those KEs within that AOP that are used for predicting downstream effects. Continuing to expand the current thyroid AOP network to include additional taxa, life-stages, etc., will be helpful in more clearly defining and delineating the applicability domain of the DIO assays, especially in the context of in chemico to in vivo extrapolations (Knapen et al., 2018; Villeneuve et al., 2018).
3.2. In vivo acute zebrafish embryo toxicity tests
Based on the in chemico results, we selected 14 compounds with different potencies to inhibit DIO1 and/or DIO2 activity. We used these compounds to perform zebrafish embryo experiments based on OECD TG 236 (OECD, 2013a), directly observing possible effects on posterior swim bladder chamber inflation. Reduced inflation of the posterior chamber may manifest itself as either a complete failure to inflate or as a reduced chamber size. Therefore, we assessed both endpoints. It is expected that failure to properly inflate the swim bladder, especially the posterior chamber, would create increased oxygen and energy demands leading to decreased growth and ultimately young of year (young animals which have not yet reached one year of age) survival, as this chamber is thought to be involved in buoyancy control in most fish species (Lindsey et al., 2010).
Posterior chamber inflation normally occurs during a typical zebrafish embryo acute toxicity (ZFET) timeframe, around 96-120 hpf. Any delay in inflation, either caused by a direct impact on the inflation process or indirectly by affecting the overall growth rate, will result in the inflation process taking place beyond the EU limit of 5 dpf delineating the non-protected life-stage. Being able to predict this higher organismal endpoint based on in chemico assays would therefore in many realistic toxicological scenarios already be a valuable step towards replacing animal tests. In a follow-up study, we also plan to assess whether these in chemico assays can be used to predict endpoints in a 30 days fish early-life stage (FELS) chronic toxicity test, as the FELS test (OECD TG 210; OECD, 2013b) is one of the primary assays used to assess the chronic aquatic toxicity of chemicals in fish. Disruption of thyroid function has already been reported to affect anterior swim bladder chamber inflation (Cavallin et al., 2017; Godfrey et al., 2017; Nelson et al., 2016; Stinckens et al., 2016), a chronic adverse outcome which cannot be observed in acute ZFET experiments.
3.2.1. Effects on posterior chamber inflation
We tested seven compounds that were identified as strong DIO1 inhibitors (PTU, IOP, ANS, BP2, TBP, TCBPA and PFOA) and six compounds that were strong DIO2 inhibitors (IOP, ANS, TBP, BP2, PFOA, SA) and therefore likely to act as TH disruptors that affect posterior chamber inflation in fish (see Figure 2). Note that “DIO inhibition” is used here as defined earlier, i.e., referring to compounds that are in chemico enzyme inhibitors based on porcine liver tissue. Additionally, we evaluated six compounds (BPA, MBT, PFBS, PFOS, MMI, NaPER) for which a low or no DIO1 and DIO2 inhibitory capacity was found, and which we did not expect to affect posterior chamber inflation. Two of these compounds were previously tested in the same lab (PFOS and MBT; Hagenaars et al., 2014; Stinckens et al., 2016). Published data were therefore used in our analyses for these two compounds.
All compounds caused a concentration-dependent increase in mortality. After exposure to MBT, PFBS, MMI, NaPER, TCBPA and ANS, the posterior chamber inflated normally. Although MBT and MMI are known to inhibit TPO, and thus the synthesis of thyroid hormones, the absence of effects on posterior chamber inflation can likely be explained by maternal transfer of T4 into the eggs, as suggested by our previous work (Nelson et al., 2016; Stinckens et al., 2016). Exposure to PTU, IOP, BP2, TBP, PFOA, SA and BPA resulted in impaired posterior chamber inflation. Based on the resulting concentration-response curves (Supplementary Figure S1), EC50 values for posterior chamber inflation and LC50 values were calculated (Table 1). These values were determined at 168 hpf to eliminate possible effects of delayed inflation, with the exception of PTU, which was assessed at 120 hpf.
Table 1.
EC50 and LC50 values (168 hpf, with the exception of PTU 120 hpf), as well as the p-values indicating significant differences between LC50 and EC50 values, after exposure experiments (six concentrations per compound, 40 embryos per condition) at UA (effects on posterior chamber inflation).
| Compound | EC50 (mg/L) | LC50 (mg/L) | p-value | Observed impaired posterior chamber inflationa | Concentration at which effect on posterior chamber surface area (120 hpf) occursb |
|---|---|---|---|---|---|
| PTU | 259 | 555.9 | <0.0001 | X | EC50 |
| IOP | 2.791 | 3.471 | 0.0448 | X | No data |
| PFOA | 108.5 | 362.5 | 0.0150 | X | EC10, EC30, EC50 |
| BPA | 4.7 | 8 | 0.0427 | X | EC10, EC30, EC50 |
| BP2 | 10.55 | 15.48 | <0.0001 | X | No data |
| TBP | 0..42 | 0.84 | <0.0001 | X | EC10, EC30, EC50 |
| SA | 82.6 | 137.3 | <0.0001 | X | EC10, EC30, EC50 |
| PFOS (Hagenaars et al., 2016) | 2.12 | 8.35 | <0.0001 | X | No data |
| ANS | 112.2 | 124.7 | 0.0606 | – | EC10, EC30, EC50 |
| TCBPA | 0.43 | 0.41 | 0.0687 | – | No data |
| NaPER | 3435 | 2952.7 | <0.0001 | – | No effect |
| MMI | 772.0 | 894.8 | 0.1395 | – | EC10, EC30, EC50 *No effect at 168 hpf |
| PFBS | 830.3 | 921.9 | 0.3171 | – | No effect |
| MBT (Stinckens et al., 2016) | 3.2 | 4.2 | 0.1529 | – | No effect |
Observed effects on binary scoring of the absence of posterior chamber inflation (X = effect = significant difference between EC50 and LC50 values, UA) are given. Based on the exposure experiments at UA, EC10, EC30 and EC50 values were determined at 120 hpf and used to perform exposure experiments at UCR (three concentrations per compound, at least 40 embryos per condition, effects on posterior surface area).
Observed effects on posterior surface area (effect concentration, UCR, see section 3.2.2) are given.
For most compounds tested in this study, we did not observe any additional sublethal effects, with the exception of PFOA and BPA. Exposure to PFOA and BPA resulted in spinal curvature, oedema of the yolk and impaired larval growth. It is clear that certain morphological deviations such as a curvature of the spine or delay in overall larval growth could affect posterior chamber inflation, and it cannot be excluded that such effects contributed to the effect on posterior chamber inflation after PFOA and BPA exposure. These effects however were only observed at a concentration exceeding the EC50 value for posterior chamber inflation by a factor of 2 (250 mg/L for PFOA and 8 mg/L for BPA, see also Table 1) and are therefore unlikely to have had a large impact on the effect on swim bladder inflation.
3.2.2. Effects on posterior chamber surface area
To provide a few contrasting examples of how chemicals with different DIO inhibitory potential affect posterior chamber surface area, Figure 3 shows the results for one strong DIO1 inhibitor (PTU), one strong DIO1 and DIO2 inhibitor (ANS) and one compound with no DIO inhibition capacity (NaPER; see Supplementary Figures S2 and S3 for additional compounds). If posterior chambers were inflated, the surface area was smaller when exposed to PTU (Table 1, Figure 3B), as well as after exposure to PFOA, BPA, TBP, SA, ANS and MMI (Table 1, Supplementary Figure S3). Although ANS, a strong DIO inhibitor, did not completely prevent the posterior chamber to inflate, it clearly reduced the posterior chamber surface area. For PTU, only exposure to the EC50 resulted in a smaller surface area. For all other compounds that reduced posterior surface area, the effect was already detected after exposure to the EC10. MMI affected posterior inflation at 120 hpf, but this effect disappeared at 168 hpf (data not shown). As mentioned earlier, impaired swim bladder inflation is an ecologically relevant adverse effect relevant to risk assessment as it may affect swimming capacity, feeding behavior and predator avoidance, ultimately resulting in population trajectory decline (Czesny et al., 2005; Woolley and Qin, 2010). Although for certain compounds effects on posterior chamber inflation were observed at concentrations substantially lower than those causing mortality, it is important to note that nearly all tested compounds are lethal at concentrations that are only about 2-3 fold higher than those causing effects on swim bladder inflation. From an environmental risk assessment perspective, posterior chamber surface area could therefore be a more interesting endpoint compared to the binary scoring of posterior chamber inflation (i.e., inflated or non-inflated), since effects on surface area are already observed at lower concentrations for most compounds. However, it still remains to be established whether reduced swim bladder volume alone (as estimated by the surface area) is sufficient for a fish to be realistically disadvantaged in a natural environment.
Figure 3.
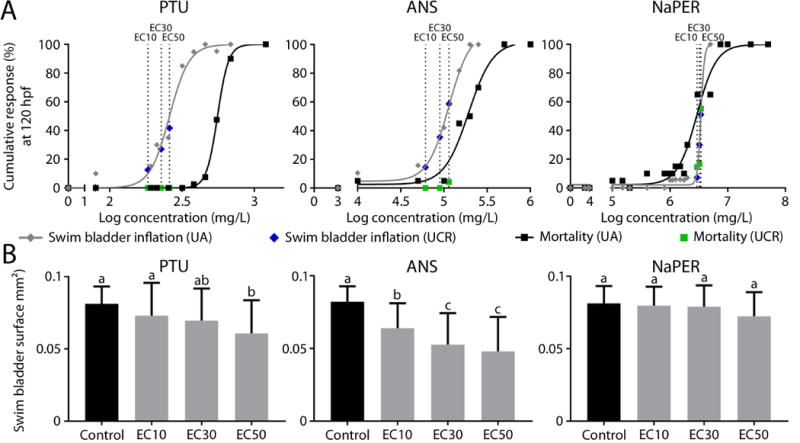
A. Concentration-response curves for mortality and percentage impaired posterior chamber inflation at 120 hpf (three concentrations per compound, at least 40 embryos per condition). Black squares and gray diamonds, associated with black and gray concentration-response curves, represent effects on mortality and posterior chamber inflation respectively after performing experiments at UA (data at 120 hpf in order to compare results with data obtained at UCR). EC10, EC30 and EC50 concentrations (dotted lines) were calculated based on these data and used to perform exposures at UCR. Green squares and blue diamonds represent effects on mortality and posterior chamber inflation respectively after performing experiments at UCR. Although zebrafish with different genetic backgrounds were used in the two laboratories, we observed a high similarity between labs for the frequencies of impaired posterior inflation and mortality. B. Mean posterior surface area at 120 hpf after exposure to 3 potentially thyroid disrupting compounds at UCR. Error bars indicate standard deviations. Different letters indicate a significant effect between the different groups (p<0.05).
3.3. Linking in chemico data to in vivo data
We used two different AOPs (AOPs 155 and 157 in the AOP-Wiki; aopwiki.org) as the underlying framework for this study. The MIEs in these AOPs are Dio1 and Dio2 inhibition, which link to impaired swim bladder inflation in fish via altered TH concentrations in serum, through a series of hypothesized mechanisms such as impaired production of surfactant, impaired production of lactic acid, impaired tissue layer formation, etc. (Villeneuve et al., 2014). It is important to realize that within this network, the relative importance of Dio1 and Dio2 is not yet fully understood; their importance may differ among species, and could possibly even be different among different life-stages or tissues in a given species. It is therefore to be expected that any predictive model based on measuring the isoform-specific DIO inhibitory potentials of chemicals could be refined as knowledge on the involvement of these isoforms in specific processes grows. However, in general, we expected an impact of strong DIO inhibitors, regardless of the targeted isoform, on posterior chamber inflation and/or posterior chamber surface area, while compounds with low or no DIO inhibitory capacity were expected to have no such effect. We attempted to use our data to assess the relative importance of DIO1 and DIO2 in causing posterior chamber inflation effects, in addition to evaluating the use of our in chemico dataset for predicting in vivo effects and determining in chemico DIO inhibition threshold values (Figure 4).
3.3.1. Dio2 inhibition is likely more important for causing impaired swim bladder inflation
After exposure to seven strong DIO1 inhibitors (red symbols in Figure 4), six out of seven compounds impaired posterior chamber inflation. Exposure to strong DIO2 inhibitors on the other hand affected posterior chamber inflation and/or surface area in all cases. These results suggest that Dio2 enzymes may play a more important role in swim bladder inflation compared to Dio1 enzymes. This hypothesis is supported by the fact that (1) all compounds that are both strong DIO1 and DIO2 inhibitors have an effect on posterior chamber inflation or surface area, (2) one compound that is only a strong DIO2 inhibitor (SA) also had an effect on posterior chamber inflation, and (3) TCBPA, a strong DIO1 inhibitor but not a DIO2 inhibitor, did not affect posterior chamber inflation.
In a previous study we showed a peak of dio1 as well as dio2 mRNA in 5 day old normally developing zebrafish (Vergauwen et al., 2018), which does not suggest dominance of either isoform at that time point. However, it has been previously suggested that Dio2 is the major contributor to TH activation in developing zebrafish embryos (Darras et al., 2015; Walpita et al., 2010). It has been shown that a morpholino knockdown targeting dio1 mRNA alone did not affect embryonic development in zebrafish, while knockdown of dio2 delayed progression of otic vesicle length, head-trunk angle and pigmentation index (Houbrechts et al., 2016; Walpita et al., 2010, 2009). Dio1 inhibition may only become essential in hypothyroidal circumstances, for example when Dio2 is inhibited or in case of iodine deficiency, in zebrafish (Walpita et al., 2010) and mice (Galton et al., 2009; Schneider et al., 2006). Dio1 could therefore also be important during embryogenesis and metamorphosis of fish, when the demand for THs is high (Orozco et al., 2012). In fathead minnows exposed to IOP, a strong DIO1 and DIO2 inhibitor according to our data, during the period of anterior chamber inflation, dio2 mRNA levels increased, possibly as a compensatory response, while dio1 mRNA levels did not (Cavallin et al., 2017). Therefore, it seems plausible that Dio2 inhibition is more important for causing impaired posterior and anterior chamber inflation.
Overall, the in chemico DIO2 dataset seems to accurately predict in vivo effects on posterior chamber inflation (Figure 4B). All tested compounds with a low or no DIO2 inhibition capacity caused no effects, with the exception of the plasticizer and typical estrogenic xenobiotic bisphenol A (BPA) and the surfactant perfluorooctanesulfonic acid (PFOS). Although exposures to TCBPA (a flame retardant), MBT (a rubber vulcanizing agent) and the surfactant PFBS, all showing low DIO2 inhibition capacities, did not result in effects on posterior chamber inflation as expected, we included these compounds in our current DIO2 uncertainty zone along with BPA. These compounds have similar DIO1 and DIO2 inhibition capacity, but lead to a different effect on the posterior chamber compared to BPA. It has already been suggested that MBT is not a potent DIO inhibitor (Nelson et al., 2016; Stinckens et al., 2016; Visser et al., 1979). Furthermore, PTU was added to this uncertainty zone, as it has been demonstrated that DIO2 is less sensitive to PTU inhibition compared to DIO1 in rat, pig and human (Croteau et al., 1996; Maia et al., 2005; Salvatore et al., 1996; Silva et al., 1982; Visser et al., 1983; Wassen et al., 2004). In several fish species, Dio2 appears to be less sensitive to PTU exposure as well (Mol et al., 1993, 1998; Orozco et al., 1997).
3.3.2. The role of other non-DIO mechanisms: explaining unexpected results
BPA and PFOS did cause effects on posterior swim bladder inflation, but neither were in chemico DIO inhibitors. A prediction model based on only DIO1 and DIO2 enzyme inhibition data would therefore not be able to predict these effects (i.e., generate a false negative prediction). In addition to the fact that differences in predicted effects may be observed as a result of, for example, specific toxicokinetic and/or toxicodynamic properties, and taking into account that effects in fish can possibly be more accurately predicted using fish tissue-based assays rather than using porcine tissue, it is also highly plausible that many different toxicological mechanisms can lead to swim bladder inflation effects (Hagenaars et al., 2014; Li et al., 2011; Michiels et al., 2017; Sarnowski, 2004; Trotter et al., 2003; Villeneuve et al., 2014; Woolley and Qin, 2010; Yin et al., 2011). Some of these mechanisms may even be related to the hypothalamic-pituitary-thyroid (HPT) axis and disruption of TH balance but currently not be captured by our AOP network and in chemico assays (e.g., at the level of the sodium-iodine symporter, the production of thyroid stimulating hormone, etc.).
For example, BPA has been shown to affect multiple endocrine-related processes, as well as induce obesity, diabetes and affect reproductive capacity and neurodevelopment (ToxCast data, Rubin, 2011). Although Dio inhibition seems not to be the primary mechanism by which BPA impairs swim bladder inflation, this compound has been reported to be able to bind to and antagonize the T3 receptor (Moriyama et al., 2002; Zoeller et al., 2005). Secondly, BPA could also affect T3 signaling because of the indirect cross talk between the T3 and estrogenic pathways (Heimeier et al., 2009; Hogan et al., 2008, 2007; Rogers et al., 2013).
Many others reported on the endocrine disrupting properties of PFOS, including possible disruption of TH levels (Du et al., 2013, 2009; Hagenaars et al., 2014; Jain, 2013; Kim et al., 2011; Melzer et al., 2010; Shi et al., 2009; Wang et al., 2011; Yu et al., 2009). Specific evidence for PFOS affecting other thyroid-related processes is currently lacking. More than likely, a different mechanism is involved as well. For example, the surfactant properties of PFOS (Hagenaars et al., 2011) or the fact that PFOS exposure causes spinal curvatures in zebrafish larvae (Hagenaars et al., 2014) could affect swim bladder inflation. PFOS also has the potential to affect growth and development at early life stages, possibly contributing to the effect on posterior chamber inflation. Finally, PFOS is known to affect multiple enzymes and processes involved in developmental progression, immunotoxicity and hepatotoxicity (Ankley et al., 2005; Rosen et al., 2009; Shi et al., 2008; Wei et al., 2008; Yu et al., 2011; Zheng et al., 2011).
Exposure to PTU affected posterior chamber inflation and surface area in zebrafish. PTU is not a strong DIO2 inhibitor in pig according to our in chemico data, and also not in human, rat and fish according to literature (see section 3.3.1.), and can therefore not explain the observed effect in vivo. Although according to our in chemico data, this compound is a strong DIO1 inhibitor, this is likely less so in teleosts (see section 3.1) and it also may only become important in a hypothyroidal state. PTU however inhibits TPO (Paul et al., 2014), a mechanism that on its own is unlikely to cause impaired posterior chamber inflation because of maternally transferred thyroid hormones. TPO inhibition could therefore contribute to inducing a hypothyroidal state sufficiently severe for PTU to cause the in vivo effect through combined DIO1 and DIO2 inhibition after the larval thyroid gland is activated at 72 hpf (Chang et al., 2012; Elsalini et al., 2003). On the other hand, literature shows that Dio1 is partially insensitive to this compound in several fish species (see also section 3.1), although to our knowledge, the in chemico insensitivity of Dio1 and Dio2 to PTU has not yet been demonstrated in zebrafish. These sensitivities may be different from those measured in other fish species, similarly to what was reported for seabream (Klaren et al., 2005) and hagfish (McLeese et al., 2000). More importantly, the capacity of PTU to inhibit the deiodinase enzymes in vivo has currently not yet been explored. Lastly, other mechanisms than DIO inhibition could explain the effect of PTU in vivo as well. For example, PTU has been shown to induce alterations of thyroidal tissue and thyroid-stimulating hormone cell counts in the pituitary (Schmidt and Braunbeck, 2011).
4. Conclusion
We have performed a set of experiments to evaluate in chemico assays for predicting acute swim bladder inflation effects based on the AOP framework. Our results suggest that Dio2 may play a more important role in swim bladder inflation compared to Dio1. By linking the in chemico and in vivo datasets and setting threshold values, we were able to demonstrate that the DIO2 in chemico dataset can be used as a predictive tool for the biological effects on posterior chamber inflation, with only few outliers. In order to decrease the number of false negative predictions, the AOP network should be extended to include not only other HPT-related MIEs, but also MIEs that are related to the AO specifically, in our case toxicological mechanisms that can lead to swim bladder inflation effects. Further growing our thyroid AOP network to include additional taxa, life stages, etc., will be helpful to more clearly define and delineate the applicability domain of the DIO assays. The assays used in our study could then be implemented in a broader tiered testing strategy, as suggested by Volz et al. (2011). In tier 1, in chemico and/or in vitro high-throughput assays, in our case the in chemico DIO2 assay, can be used to screen for events at lower levels of biological organization along the selected AOPs. Tier 2 could involve modified short-term ZFET tests for whole-organism-based assessment of AOP-specific effects, in our case using posterior chamber inflation and/or surface area as adverse outcome. Finally, tier 3 could comprise chronic FELS tests. Tier 3 would only be implemented if required, for example if the effect of DIO and/or TPO inhibition on anterior chamber inflation would need to be investigated.
Supplementary Material
AOPs linking deiodinase inhibition to impaired swim bladder inflation in fish were previously developed.
Enzyme inhibition assays were selected based on these AOPs, targeting two molecular initiating events.
An array of 51 compounds was screened using these assays.
The in chemico dataset was used to accurately predict in vivo effects on swim bladder inflation in zebrafish.
This study illustrates how AOPs can guide alternative assay development.
Acknowledgments
The aut0hors would like to thank Lobke Claes for her assistance with carrying out the deiodinase inhibition assays. This work was funded by the Cefic Long-range Research Initiative (http://www.cefic-lri.org/) project LRI-ECO20.2-UA (Development of an alternative testing strategy for the fish early life-stage test for predicting chronic toxicity: assay validation) with support of ECETOC. This work was further supported by the Society of Environmental Toxicology and Chemistry (SETAC)/Procter & Gamble Company Global Fellowship for Doctoral Research in Environmental Science 2016, sponsored by The Procter & Gamble Company. The views expressed in this paper are those of the authors and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ankley GT, Bennet RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL. Adverse outcome pathways: a conceptual framework to support ecotoxicoloy research and risk assessment. Environ Toxicol Chem. 2010;29:730–741. doi: 10.1002/etc.34. https://doi.org/10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- Ankley GT, Kuehl DW, Kahl MD, Jensen KM, Linnum A, Leino RL, Villeneuve DL. Reproductive and developmental toxicity and bioconcentration of perfluorooctanesulfonate in a partial life-cycle test with the fathead minnow (Pimephales promelas) Environ Toxicol Chem. 2005;24:2316–2324. doi: 10.1897/04-634r.1. https://doi.org/10.1080/753153338. [DOI] [PubMed] [Google Scholar]
- Bagci E, Heijlen M, Vergauwen L, Hagenaars A, Houbrechts AM, Esguerra CV, Blust R, Darras VM, Knapen D. Deiodinase knockdown during early zebrafish development affects growth, development, energy Metabolism, motility and phototransduction. PLoS One. 2015;10:e0123285. doi: 10.1371/journal.pone.0123285. https://doi.org/10.1371/journal.pone.0123285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bols NC, Dayeh VR, Lee LEJ, Schirmer K. Use of fish cell lines in the toxicology and ecotoxicology of fish. Piscine cell lines in environmental toxicology. Biochem Mol Biol Fishes. 2005;6:43–84. https://doi.org/10.1016/S1873-0140(05)80005-0. [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein - dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Braunbeck T, Kais B, Lammer E, Otte J, Schneider K, Stengel D, Strecker R. The fish embryo test (FET): origin, applications, and future. Environ Sci Pollut Res Int. 2014;22:16247–16261. doi: 10.1007/s11356-014-3814-7. https://doi.org/10.1007/s11356-014-3814-7. [DOI] [PubMed] [Google Scholar]
- Braunbeck T, Lammer E. Fish embryo toxicity assays, Background document on fish embryo toxicity assays. OECD; Dessau, Germany: 2006. [Google Scholar]
- Brown DD. The role of thyroid hormone in zebrafish and axolotl development. Proc Natl Acad Sci U S A. 1997;94:13011–13016. doi: 10.1073/pnas.94.24.13011. https://doi.org/10.1073/pnas.94.24.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicol Sci. 2011;124:339–347. doi: 10.1093/toxsci/kfr117. https://doi.org/10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callery EM, Elinson RP. Thyroid hormone-dependent metamorphosis in a direct developing frog. Proc Natl Acad Sci U S A. 2000;97:2615–2620. doi: 10.1073/pnas.050501097. https://doi.org/10.1073/pnas.050501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallin JE, Ankley GT, Blackwell BR, Blanksma CA, Fay KA, Jensen KM, Kahl MD, Knapen D, Kosian PA, Poole S, Randolph EC, Schroeder AL, Vergauwen L, Villeneuve DL. Impaired swim bladder inflation in early-life stage fathead minnows exposed to a deiodinase inhibitor, iopanoic acid. Environ Toxicol Chem. 2017;36:2942–2952. https://doi.org/10.1002/etc.3855. [Google Scholar]
- Chang J, Wang M, Gui W, Zhao Y, Yu L, Zhu G. Changes in Thyroid Hormone Levels during Zebrafish Development. Zoolog Sci. 2012;29:181–184. doi: 10.2108/zsj.29.181. https://doi.org/10.2108/zsj.29.181. [DOI] [PubMed] [Google Scholar]
- Coimbra AM, Reis-Henriques MA, Darras VM. Circulating thyroid hormone levels and iodothyronine deiodinase activities in Nile tilapia (Oreochromis niloticus) following dietary exposure to Endosulfan and Aroclor 1254. Comp Biochem Physiol - C Toxicol Pharmacol. 2005;141:8–14. doi: 10.1016/j.cca.2005.04.006. https://doi.org/10.1016/j.cca.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Croteau W, Davey JC, Galton VA, St Germain DL. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest. 1996;98:405–417. doi: 10.1172/JCI118806. https://doi.org/10.1172/JCI118806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czesny SJ, Graeb BDS, Dettmers JM. Ecological consequences of swim bladder noninflation for larval yellow perch. Trans Am Fish Soc. 2005;134:1011–1020. https://doi.org/10.1577/T04-016.1. [Google Scholar]
- Darras VM, Houbrechts AM, Van Herck SLJ. Intracellular thyroid hormone metabolism as a local regulator of nuclear thyroid hormone receptor-mediated impact on vertebrate development. Biochim Biophys Acta - Gene Regul Mech. 2015;1849:130–141. doi: 10.1016/j.bbagrm.2014.05.004. https://doi.org/10.1016/j.bbagrm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Darras VM, Van Herck SLJ. Iodothyronine deiodinase structure and function: From ascidians to humans. J Endocrinol. 2012;215:189–206. doi: 10.1530/JOE-12-0204. https://doi.org/10.1530/JOE-12-0204. [DOI] [PubMed] [Google Scholar]
- Du G, Hu J, Huang H, Qin Y, Han X, Wu D, Song L, Xia Y, Wang X. Perfluorooctane sulfonate (PFOS) affects hormone receptor activity, steroidogenesis, and expression of endocrine-related genes in vitro and in vivo. Environ Toxicol Chem. 2013;32:353–360. doi: 10.1002/etc.2034. https://doi.org/10.1002/etc.2034. [DOI] [PubMed] [Google Scholar]
- Du Y, Shi X, Liu C, Yu K, Zhou B. Chronic effects of water-borne PFOS exposure on growth, survival and hepatotoxicity in zebrafish: A partial life-cycle test. Chemosphere. 2009;74:723–729. doi: 10.1016/j.chemosphere.2008.09.075. https://doi.org/10.1016/j.chemosphere.2008.09.075. [DOI] [PubMed] [Google Scholar]
- Dumbarton TC, Stoyek M, Croll RP, Smith FM. Adrenergic control of swimbladder deflation in the zebrafish (Danio rerio) J Exp Biol. 2010;213:2536–2546. doi: 10.1242/jeb.039792. https://doi.org/10.1242/jeb.039792. [DOI] [PubMed] [Google Scholar]
- Elsalini OA, Von Gartzen J, Cramer M, Rohr KB. Zebrafish hhex, nk2.1a, and pax2.1 regulate thyroid growth and differentiation downstream of Nodal-dependent transcription factors. Dev Biol. 2003;263:67–80. doi: 10.1016/s0012-1606(03)00436-6. https://doi.org/10.1016/S0012-1606(03)00436-6. [DOI] [PubMed] [Google Scholar]
- Ferreira ACF, Lisboa PC, Oliveira KJ, Lima LP, Barros IA, Carvalho DP. Inhibition of thyroid type 1 deiodinase activity by flavonoids. Food Chem Toxicol. 2002;40:913–917. doi: 10.1016/s0278-6915(02)00064-9. https://doi.org/10.1016/S0278-6915(02)00064-9. [DOI] [PubMed] [Google Scholar]
- Finnson KW, McLeese JM, Eales JG. Deiodination and deconjugation of thyroid hormone conjugates and type I deiodination in liver of rainbow trout, Oncorhynchus mykiss. Gen Comp Endocrinol. 1999;115:387–397. doi: 10.1006/gcen.1999.7326. https://doi.org/10.1006/gcen.1999.7326. [DOI] [PubMed] [Google Scholar]
- Forhead AJ, Curtis K, Kaptein E, Visser TJ, Fowden AL. Developmental control of iodothyronine deiodinases by cortisol in the ovine fetus and placenta near term. Endocrinology. 2006;147:5988–5994. doi: 10.1210/en.2006-0712. https://doi.org/10.1210/en.2006-0712. [DOI] [PubMed] [Google Scholar]
- Freyberger A, Ahr HJ. Studies on the goitrogenic mechanism of action of N,N,N′,N′-tetramethylthiourea. Toxicology. 2006;217:169–175. doi: 10.1016/j.tox.2005.09.005. https://doi.org/10.1016/j.tox.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: Studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150:2957–2963. doi: 10.1210/en.2008-1572. https://doi.org/10.1210/en.2008-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gereben B, Zavacki AM, Ribich S, Kim BW, Huang SA, Simonides WS, Zeöld A, Bianco AC. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. https://doi.org/10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey A, Hooser B, Abdelmoneim A, Horzmann KA, Freemanc JL, Sepúlveda MS. Thyroid disrupting effects of halogenated and next generation chemicals on the swim bladder development of zebrafish. Aquat Toxicol. 2017;193:228–235. doi: 10.1016/j.aquatox.2017.10.024. https://doi.org/10.1016/j.aquatox.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Stinckens E, Vergauwen L, Bervoets L, Knapen D. PFOS affects posterior swim bladder chamber inflation and swimming performance of zebrafish larvae. Aquat Toxicol. 2014;157:225–235. doi: 10.1016/j.aquatox.2014.10.017. https://doi.org/10.1016/j.aquatox.2014.10.017. [DOI] [PubMed] [Google Scholar]
- Hagenaars A, Vergauwen L, De Coen W, Knapen D. Structure-activity relationship assessment of four perfluorinated chemicals using a prolonged zebrafish early life stage test. Chemosphere. 2011;82:764–772. doi: 10.1016/j.chemosphere.2010.10.076. https://doi.org/10.1016/j.chemosphere.2010.10.076. [DOI] [PubMed] [Google Scholar]
- Hallinger DR, Murr AS, Buckalew AR, Simmons SO, Stoker TE, Laws SC. Development of a screening approach to detect thyroid disrupting chemicals that inhibit the human sodium iodide symporter (NIS) Toxicol Vitr. 2017;40:66–78. doi: 10.1016/j.tiv.2016.12.006. https://doi.org/10.1016/j.tiv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- Heimeier RA, Das B, Buchholz DR, Shi YB. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology. 2009;150:2964–2973. doi: 10.1210/en.2008-1503. https://doi.org/10.1210/en.2008-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AJ, Teraoka H, Heideman W, Peterson RE. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol Sci. 2005;86:6–19. doi: 10.1093/toxsci/kfi110. https://doi.org/10.1093/toxsci/kfi110. [DOI] [PubMed] [Google Scholar]
- Hogan NS, Crump KL, Duarte P, Lean DRS, Trudeau VL. Hormone cross-regulation in the tadpole brain: Developmental expression profiles and effect of T3 exposure on thyroid hormone- and estrogen-responsive genes in Rana pipiens. Gen Comp Endocrinol. 2007;154:5–15. doi: 10.1016/j.ygcen.2007.02.011. https://doi.org/10.1016/j.ygcen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Hogan NS, Duarte P, Wade MG, Lean DRS, Trudeau VL. Estrogenic exposure affects metamorphosis and alters sex ratios in the northern leopard frog (Rana pipiens): Identifying critically vulnerable periods of development. Gen Comp Endocrinol. 2008;156:515–523. doi: 10.1016/j.ygcen.2008.03.011. https://doi.org/10.1016/j.ygcen.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Hood A, Klaassen CD. Effects of microsomal enzyme inducers on outer-ring deiodinase activity toward thyroid hormones in various rat tissues. Toxicol Appl Pharmacol. 2000;163:240–8. doi: 10.1006/taap.1999.8883. https://doi.org/10.1006/taap.1999.8883. [DOI] [PubMed] [Google Scholar]
- Hornung MW, Degitz SJ, Korte LM, Olson JM, Kosian PA, Linnum AL, Tietge JE. Inhibition of thyroid hormone release from cultured amphibian thyroid glands by methimazole, 6-propylthiouracil, and perchlorate. Toxicol Sci. 2010;118:42–51. doi: 10.1093/toxsci/kfq166. https://doi.org/10.1093/toxsci/kfq166. [DOI] [PubMed] [Google Scholar]
- Houbrechts AM, Delarue J, Gabriëls IJ, Sourbron J, Darras VM. Permanent deiodinase type 2 deficiency strongly perturbs zebrafish development, growth, and fertility. Endocrinology. 2016;157:3668–3681. doi: 10.1210/en.2016-1077. https://doi.org/10.1210/en.2016-1077. [DOI] [PubMed] [Google Scholar]
- Hulbert AJ. Thyroid hormones and their effects: a new perspective. Biol Rev Camb Philos Soc. 2000;75:519–631. doi: 10.1017/s146479310000556x. https://doi.org/10.1017/S146479310000556X. [DOI] [PubMed] [Google Scholar]
- Jain RB. Association between thyroid profile and perfluoroalkyl acids: Data from NHNAES 2007-2008. Environ Res. 2013;126:51–59. doi: 10.1016/j.envres.2013.08.006. https://doi.org/10.1016/j.envres.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Lema SC. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri) Gen Comp Endocrinol. 2011;172:505–517. doi: 10.1016/j.ygcen.2011.04.022. https://doi.org/10.1016/j.ygcen.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Kim S, Ji K, Lee S, Lee J, Kim J, Kim S, Kho Y, Choi K. Perfluorooctane sulfonic acid exposure increases cadmium toxicity in early life stage of zebrafish, Danio rerio. Environ Toxicol Chem. 2011;30:870–877. doi: 10.1002/etc.443. https://doi.org/10.1002/etc.443. [DOI] [PubMed] [Google Scholar]
- Kim S, Jung J, Lee I, Jung D, Youn H, Choi K. Thyroid disruption by triphenyl phosphate, an organophosphate flame retardant, in zebrafish (Danio rerio) embryos/larvae, and in GH3 and FRTL-5 cell lines. Aquat Toxicol. 2015;160:188–96. doi: 10.1016/j.aquatox.2015.01.016. https://doi.org/10.1016/j.aquatox.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Klaren PHM, Haasdijk R, Metz JR, Nitsch LMC, Darras VM, Van Der Geyten S, Flik G. Characterization of an iodothyronine 5′-deiodinase in gilthead seabream (Sparus auratus) that is inhibited by dithiothreitol. Endocrinology. 2005;146:5621–5630. doi: 10.1210/en.2005-0050. https://doi.org/10.1210/en.2005-0050. [DOI] [PubMed] [Google Scholar]
- Knapen D, Angrish MM, Fortin MC, Katsiadaki I, Leonard M, Margiotta-Casaluci L, Munn S, O’Brien JM, Pollesch N, Smith LC, et al. Adverse outcome pathway networks I: development and applications. Environ Toxicol Chem. 2018 doi: 10.1002/etc.4125. In press. https://doi.org/10.1002/etc.4125. [DOI] [PMC free article] [PubMed]
- Kuiper GGJM, Klootwijk W, Dubois GM, Destree O, Darras VM, Van Der Geyten S, Demeneix B, Visser TJ. Characterization of recombinant Xenopus laevis type I iodothyronine deiodinase: Substitution of a proline residue in the catalytic center by serine (Pro132Ser) restores sensitivity to 6-propyl-2-thiouracil. Endocrinology. 2006;147:3519–3529. doi: 10.1210/en.2005-0711. https://doi.org/10.1210/en.2005-0711. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Wassen F, Klootwijk W, Van Toor H, Kaptein E, Visser TJ. Molecular Basis for the Substrate Selectivity of Cat Type I Iodothyronine Deiodinase. Endocrinology. 2003;144:5411–5421. doi: 10.1210/en.2003-0728. https://doi.org/10.1210/en.2003-0728. [DOI] [PubMed] [Google Scholar]
- Li W, Zha J, Yang L, Li Z, Wang Z. Regulation of iodothyronine deiodinases and sodium iodide symporter mRNA expression by perchlorate in larvae and adult Chinese rare minnow (Gobiocypris rarus) Mar Pollut Bull. 2011;63:350–355. doi: 10.1016/j.marpolbul.2011.02.006. https://doi.org/10.1016/j.marpolbul.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Smith FM, Croll RP. From inflation to flotation: contribution of the swimbladder to whole-body density and swimming depth during development of the zebrafish (Danio rerio) Zebrafish. 2010;7:85–96. doi: 10.1089/zeb.2009.0616. https://doi.org/10.1089/zeb.2009.0616. [DOI] [PubMed] [Google Scholar]
- Liu YW, Chan WK. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation. 2002;70:36–45. doi: 10.1046/j.1432-0436.2002.700104.x. https://doi.org/10.1046/j.1432-0436.2002.700104.x. [DOI] [PubMed] [Google Scholar]
- Maia AL, Kim BW, Huang SA, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is the major source of plasma T3 in euthyroid humans. 2005;115:1–10. doi: 10.1172/JCI25083. https://doi.org/10.1172/JCI25083.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeese JM, Wright GM, Youson JH, Eales JG. Deiodination activity in extrathyroidal tissues of the Atlantic hagfish, Myxine glutinosa. J Exp Zool. 2000;287:445–452. doi: 10.1002/1097-010x(20001101)287:6<445::aid-jez6>3.0.co;2-a. https://doi.org/10.1002/1097-010X(20001101)287:6<445::AID-JEZ6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Melzer D, Rice N, Depledge MH, Henley WE, Galloway TS. Association between serum perfluorooctanoic acid (PFOA) and thyroid disease in the U.S. National Health and Nutrition Examination Survey. Environ Health Perspect. 2010;118:686–692. doi: 10.1289/ehp.0901584. https://doi.org/10.1289/ehp.0901584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels EDG, Vergauwen L, Hagenaars A, Fransen E, Van Dongen S, Van Cruchten SJ, Bervoets L, Knapen D. Evaluating complex mixtures in the zebrafish embryo by reconstituting field water samples : A metal pollution case study. Int J Mol Sci. 2017;18:1–16. doi: 10.3390/ijms18030539. https://doi.org/10.3390/ijms18030539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol K. Effects of experimental hypo- and hyperthyroidism on iodothyronine deiodinases in Nile tilapia, Oreochromis niloticus. Fish Physiol Biochem. 1999;20:201–207. https://doi.org/10.1023/A:1007739431710. [Google Scholar]
- Mol KA, Van der Geyten S, Burel C, Kuhn ER, Boujard T, Darras VM. Comparative study of iodothyronine outer ring and inner ring deiodinase activities in five teleostean fishes. Fish Physiol Biochem. 1998;18:253–266. https://doi.org/10.1023/a:1007722812697. [Google Scholar]
- Mol K, Kaptein E, Darras VM, de Greef WJ, Kühn ER, Visser TJ. Different thyroid hormone-deiodinating enzymes in tilapia (Oreochromis niloticus) liver and kidney. FEBS Lett. 1993;321:140–144. doi: 10.1016/0014-5793(93)80095-c. https://doi.org/10.1016/0014-5793(93)80095-C. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–5190. doi: 10.1210/jc.2002-020209. https://doi.org/10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Mukhi S, Patiño R. Effects of Prolonged Exposure to Perchlorate on Thyroid and Reproductive Function in Zebrafish. Toxicol Sci. 2007;96:246–254. doi: 10.1093/toxsci/kfm001. https://doi.org/10.1093/toxsci/kfm001. [DOI] [PubMed] [Google Scholar]
- Nelson KR, Schroeder AL, Ankley GT, Blackwell BR, Blanksma C, Degitz SJ, Flynn K, Jensen KM, Johnson RD, Kahl MD, Knapen D, Kosian PA, Milsk RY, Randolph EC, Saari T, Stinckens E, Vergauwen L, Villeneuve DL. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part I: Fathead minnow. Aquat Toxicol. 2016;173:204–217. doi: 10.1016/j.aquatox.2015.12.024. https://doi.org/10.1016/j.aquatox.2015.12.023. [DOI] [PubMed] [Google Scholar]
- OECD. Test No 236: Fish Embryo Acute Toxicity (FET) Test, OECD Guidelines for the Testing of Chemicals. 2013a https://doi.org/10.1787/9789264203709-en.
- OECD. Test No 210: Fish, Early-life Stage Toxicity Test, OECD Guidelines for the Testing of Chemicals. 2013b https://doi.org/10.1787/9789264203785-en.
- Orozco A, Silva JE, Valverde-r C. Rainbow trout liver expresses two iodothyronine phenolic ring deiodinase pathways with the characteristics of mammalian types I and II 5′-deiodinas. Endocrinology. 1997;138:254–258. doi: 10.1210/endo.138.1.4878. [DOI] [PubMed] [Google Scholar]
- Orozco A, Valverde-R C. Thyroid hormone deiodination in fish. Thyroid. 2005;15:799–813. doi: 10.1089/thy.2005.15.799. https://doi.org/10.1089/thy.2005.15.799. [DOI] [PubMed] [Google Scholar]
- Orozco A, Valverde RC, Olvera A, García GC. Iodothyronine deiodinases: A functional and evolutionary perspective. J Endocrinol. 2012;215:207–219. doi: 10.1530/JOE-12-0258. https://doi.org/10.1530/JOE-12-0258. [DOI] [PubMed] [Google Scholar]
- Orozco A, Villalobos P, Jeziorski MC, Valverde-R C. The liver of Fundulus heteroclitus expresses deiodinase type 1 mRNA. Gen Comp Endocrinol. 2003;130:84–91. doi: 10.1016/s0016-6480(02)00570-1. https://doi.org/10.1016/S0016-6480(02)00570-1. [DOI] [PubMed] [Google Scholar]
- Patiño R, Wainscott M, Cruz-Li E, Balakrishnan S, McMurry C, Blazer V, Anderson T. Effects of ammonium perchlorate on the reproductive performance and thyroid follicle histology of zebrafish. Environ Toxicol Chem. 2003;22:1115–1121. https://doi.org/10.1002/etc.5620220520. [PubMed] [Google Scholar]
- Paul KB, Hedge JM, Rotroff DM, Hornung MW, Crofton KM, Simmons SO. Development of a thyroperoxidase inhibition assay for high-throughput screening. Chem Res Toxicol. 2014;27:387–399. doi: 10.1021/tx400310w. https://doi.org/10.1021/tx400310w. [DOI] [PubMed] [Google Scholar]
- Pavelka S. Radiometric enzyme assays: Development of methods for extremely sensitive determination of types 1, 2 and 3 iodothyronine deiodinase enzyme activities. J Radioanal Nucl Chem. 2010;286:861–865. https://doi.org/10.1007/s10967-010-0798-8. [Google Scholar]
- Pype C, Verbueken E, Saad MA, Casteleyn CR, Van Ginneken CJ, Knapen D, Van Cruchten SJ. Incubation at 32.5°C and above causes malformations in the zebrafish embryo. Reprod Toxicol. 2015;56:56–63. doi: 10.1016/j.reprotox.2015.05.006. https://doi.org/10.1016/j.reprotox.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Roberston GN, McGee CAS, Dumbarton TC, Croll RP, Smith FM. Development of the swim bladder and its innervation in the zebrafish, Danio rerio. J Morphol. 2007;268:967–985. doi: 10.1002/jmor.10558. https://doi.org/10.1002/jmor. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Metz L, Yong VW. Review: Endocrine disrupting chemicals and immune responses: A focus on bisphenol-A and its potential mechanisms. Mol Immunol. 2013;53:421–430. doi: 10.1016/j.molimm.2012.09.013. https://doi.org/10.1016/j.molimm.2012.09.013. [DOI] [PubMed] [Google Scholar]
- Rosen MB, Lau C, Corton JC. Does exposure to perfluoroalkyl acids present a risk to human health? Toxicol Sci. 2009;111:1–3. doi: 10.1093/toxsci/kfp142. https://doi.org/10.1093/toxsci/kfp142. [DOI] [PubMed] [Google Scholar]
- Rubin BS. Bisphenol A: An endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. https://doi.org/10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Salvatore D, Bartha T, Harney JW, Larse PR. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology. 1996;137:3308–3315. doi: 10.1210/endo.137.8.8754756. [DOI] [PubMed] [Google Scholar]
- Sanders JP, Van Der Geyten S, Kaptein E, Darras VM, Kühn ER, Leonard JL, Visser TJ. Characterization of a propylthiouracil-insensitive type I iodothyronine deiodinase. Endocrinology. 1997;138:5153–5160. doi: 10.1210/endo.138.12.5581. https://doi.org/10.1210/en.138.12.5153. [DOI] [PubMed] [Google Scholar]
- Sarnowski P. The effects of metals on swimbladder inflation of common carp (Cyprinus Carpio L.) larvae. Electron J polish Agric Univ. 2004;7 [Google Scholar]
- Schmidt F, Braunbeck T. Alterations along the Hypothalamic-Pituitary-Thyroid Axis of the Zebrafish (Danio rerio) after Exposure to Propylthiouracil. J Thyroid Res. 2011;2011:1–17. doi: 10.4061/2011/376243. https://doi.org/10.4061/2011/376243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider MJ, Fiering SN, Thai B, Wu SY, St Germain E, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 1 selenodeiodinase gene (Dio1) results in marked changes in thyroid hormone economy in mice. Endocrinology. 2006;147:580–589. doi: 10.1210/en.2005-0739. https://doi.org/10.1210/en.2005-0739. [DOI] [PubMed] [Google Scholar]
- Scholz S, Fischer S, Gündel U, Küster E, Luckenbach T, Voelker D. The zebrafish embryo model in environmental risk assessment - Applications beyond acute toxicity testing. Environ Sci Pollut Res. 2008;15:394–404. doi: 10.1007/s11356-008-0018-z. https://doi.org/10.1007/s11356-008-0018-z. [DOI] [PubMed] [Google Scholar]
- Segner H. Cytotoxicity assays with fish cells as an alterntive to the acute lethality test with fish. Altern to Lab Anim. 2004;32:375–382. doi: 10.1177/026119290403200409. [DOI] [PubMed] [Google Scholar]
- Segner H. Fish cell lines as a tool in aquatic toxicology. Fish Ecotoxicology. 1998:1–38. doi: 10.1007/978-3-0348-8853-0_1. [DOI] [PubMed] [Google Scholar]
- Selderslaghs IWT, Hooyberghs J, Blust R, Witters HE. Assessment of the developmental neurotoxicity of compounds by measuring locomotor activity in zebrafish embryos and larvae. Neurotoxicol Teratol. 2013;37:44–56. doi: 10.1016/j.ntt.2013.01.003. https://doi.org/10.1016/j.ntt.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Shi X, Du Y, Lam PKS, Wu RSS, Zhou B. Developmental toxicity and alteration of gene expression in zebrafish embryos exposed to PFOS. Toxicol Appl Pharmacol. 2008;230:23–32. doi: 10.1016/j.taap.2008.01.043. https://doi.org/10.1016/j.taap.2008.01.043. [DOI] [PubMed] [Google Scholar]
- Shi X, Liu C, Wu G, Zhou B. Waterborne exposure to PFOS causes disruption of the hypothalamus-pituitary-thyroid axis in zebrafish larvae. Chemosphere. 2009;77:1010–1018. doi: 10.1016/j.chemosphere.2009.07.074. https://doi.org/10.1016/j.chemosphere.2009.07.074. [DOI] [PubMed] [Google Scholar]
- Silva JE, Leonard JL, Crantz R, Larsen PR. Evidence for two tissue-specific pathways for in vivo thyroxine 5′-deiodination in the rat. J Clin Invest. 1982;69:1176–1184. doi: 10.1172/JCI110554. https://doi.org/10.1172/JCI110554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadnicka-Michalak J, Tanneberger K, Schirmer K, Ashauer R. Measured and modeled toxicokinetics in cultured fish cells and application to in vitro - in vivo toxicity extrapolation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0092303. https://doi.org/10.1371/journal.pone.0092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinckens E, Vergauwen L, Schroeder AL, Maho W, Blackwell BR, Witters H, Blust R, Ankley GT, Covaci A, Villeneuve DL, Knapen D. Impaired anterior swim bladder inflation following exposure to the thyroid peroxidase inhibitor 2-mercaptobenzothiazole part II: Zebrafish. Aquat Toxicol. 2016;173:204–217. doi: 10.1016/j.aquatox.2015.12.023. https://doi.org/10.1016/j.aquatox.2015.12.023. [DOI] [PubMed] [Google Scholar]
- Strähle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T. Zebrafish embryos as an alternative to animal experiments-A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod Toxicol. 2012;33:128–132. doi: 10.1016/j.reprotox.2011.06.121. https://doi.org/10.1016/j.reprotox.2011.06.121. [DOI] [PubMed] [Google Scholar]
- Tan F, Wang M, Wang W, Lu Y. Comparative evaluation of the cytotoxicity sensitivity of six fish cell lines to four heavy metals in vitro. Toxicol Vitr. 2008;22:164–170. doi: 10.1016/j.tiv.2007.08.020. https://doi.org/10.1016/j.tiv.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Taurog A, Dorris ML, Guziec LJ, Guziec FS. The selenium analog of methimazole, measurement of its inhibitory effect on type I 5′-deiodinase and of its antithyroid activity. Biochem Pharmacol. 1994;48:1447–1453. doi: 10.1016/0006-2952(94)90569-x. [DOI] [PubMed] [Google Scholar]
- Toyoda N, Harney JW, Berry MJ, Larsens PR. Identification of critical amino acids for 3, 5, 3′-triiodothyronine deiodination by human type 1 deiodinase based on comparative functional-structural analyses of the human, dog, and rat enzymes. J Biol Chem. 1994;8:1–6. [PubMed] [Google Scholar]
- Trotter AJ, Battaglene SC, Pankhurst PM. Effects of photoperiod and light intensity on initial swim bladder inflation, growth and post-inflation viability in cultured striped trumpeter (Latris lineata) larvae. Aquaculture. 2003;224:141–158. https://doi.org/10.1016/S0044-8486(03)00212-6. [Google Scholar]
- Van Der Geyten S, Sanders JP, Kaptein E, Darras VM, Kühn ER, Leonard JL, Visser TJ. Expression of chicken hepatic type I and type III iodothyronine deiodinases during embryonic development. Endocrinology. 1997;138:5144–5152. doi: 10.1210/endo.138.12.5599. https://doi.org/10.1210/en.138.12.5144. [DOI] [PubMed] [Google Scholar]
- Van der Geyten S, Toguyeni A, Baroiller JF, Fauconneau B, Fostier A, Sanders JP, Visser TJ, Kühn ER, Darras VM. Hypothyroidism induces type I iodothyronine deiodinase expression in tilapia liver. Gen Comp Endocrinol. 2001;124:333–42. doi: 10.1006/gcen.2001.7722. https://doi.org/10.1006/gcen.2001.7722. [DOI] [PubMed] [Google Scholar]
- Vergauwen L, Cavallin JE, Ankley GT, Bars C, Gabriëls IJ, Michiels EDG, Nelson KR, Periz-Stanacev J, Randolph EC, Robinson SL, Saari TW, Schroeder AL, Stinckens E, Swintek J, Van Cruchten SJ, Verbueken E, Villeneuve DL, Knapen D. Gene transcription ontogeny of hypothalamic-pituitary-thyroid axis development in early-life stage fathead minnow and zebrafish. Gen Comp Endocrinol. 2018 doi: 10.1016/j.ygcen.2018.05.001. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstraelen S, Peers B, Maho W, Hollanders K, Remy S, Berckmans P, Covaci A, Witters H. Phenotypic and biomarker evaluation of zebrafish larvae as an alternative model to predict mammalian hepatotoxicity. J Appl Toxicol. 2016;36:1194–1206. doi: 10.1002/jat.3288. https://doi.org/10.1002/jat.3288. [DOI] [PubMed] [Google Scholar]
- Villeneuve D, Volz DC, Embry MR, Ankley GT, Belanger SE, Léonard M, Schirmer K, Tanguay R, Truong L, Wehmas L. Investigating alternatives to the fish early-life stage test: A strategy for discovering and annotating adverse outcome pathways for early fish development. Environ Toxicol Chem. 2014;33:158–169. doi: 10.1002/etc.2403. https://doi.org/10.1002/etc.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve DL, Angrish M, Fortin M, Katsiadaki I, Léonard M, Margiotte-Casaluci L, Munn S, O’Brien J, Pollesch N, Smith L, Zhang X, Knapen D. Adverse Outcome Pathway Networks II : Network Analytics. Environ Toxicol Chem. 2018 doi: 10.1002/etc.4124. In press. https://doi.org/10.1002/etc.4124. [DOI] [PMC free article] [PubMed]
- Visser TJ, Fekkes D, Docter R, Hennemann G. Sequential deiodination of thyroxine in rat liver homogenate. Biochem J. 1978;174:221–9. doi: 10.1042/bj1740221. https://doi.org/10.1042/bj1740221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser TJ, Kaplan MM, Leonard JL, Larsen PR. Evidence for two pathways of iodothyronine 5′-deiodination in rat pituitary that differ in kinetics, propylthiouracil sensitivity, and response to hypothyroidism. J Clin Invest. 1983;71:992–1002. doi: 10.1172/JCI110854. https://doi.org/10.1172/JCI110854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser TJ, Van Overmeeren E, Fekkes D, Docter R, Hennemann G. Inhibition of iodothyronine 5′-deiodinase by thioureylenes; Structure-activity relationship. FEBS Lett. 1979;103:314–318. doi: 10.1016/0014-5793(79)81352-6. https://doi.org/10.1016/0014-5793(79)81352-6. [DOI] [PubMed] [Google Scholar]
- Voelker D, Vess C, Tillmann M, Nagel R, Otto GW, Geisler R, Schirmer K, Scholz S. Differential gene expression as a toxicant-sensitive endpoint in zebrafish embryos and larvae. Aquat Toxicol. 2007;81:355–364. doi: 10.1016/j.aquatox.2006.12.013. https://doi.org/10.1016/j.aquatox.2006.12.013. [DOI] [PubMed] [Google Scholar]
- Volz DC, Belanger S, Embry M, Padilla S, Sanderson H, Schirmer K, Scholz S, Villeneuve D. Adverse outcome pathways during early fish development: A conceptual framework for identification of chemical screening and prioritization strategies. Toxicol Sci. 2011;123:349–358. doi: 10.1093/toxsci/kfr185. https://doi.org/10.1093/toxsci/kfr185. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Crawford AD, Darras VM. Combined antisense knockdown of type 1 and type 2 iodothyronine deiodinases disrupts embryonic development in zebrafish (Danio rerio) Gen Comp Endocrinol. 2010;166:134–141. doi: 10.1016/j.ygcen.2009.09.011. https://doi.org/10.1016/j.ygcen.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Crawford AD, Janssens EDR, Van Der Geyten S, Darras VM. Type 2 lodothyronine deiodinase is essential for thyroid hormone-dependent embryonic development and pigmentation in zebrafish. Endocrinology. 2009;150:530. doi: 10.1210/en.2008-0457. https://doi.org/10.1210/en.2008-0457. [DOI] [PubMed] [Google Scholar]
- Wang M, Chen J, Lin K, Chen Y, Hu W, Tanguay RL, Huang C, Dong Q. Chronic zebrafish PFOS exposure alters sex ratio and maternal related effects in F1 offspring. Environ Toxicol Chem. 2011;30:2073–2080. doi: 10.1002/etc.594. https://doi.org/10.1002/etc.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassen FWJS, Klootwijk W, Kaptein E, Duncker DJ, Visser TJ, Kuiper GGJM. Characteristics and thyroid state-dependent regulation of iodothyronine deiodinases in pigs. Endocrinology. 2004;145:4251–4263. doi: 10.1210/en.2004-0356. https://doi.org/10.1210/en.2004-0356. [DOI] [PubMed] [Google Scholar]
- Wei Y, Liu Y, Wang J, Tao Y, Dai J. Toxicogenomic analysis of the hepatic effects of perfluorooctanoic acid on rare minnows (Gobiocypris rarus) Toxicol Appl Pharmacol. 2008;226:285–297. doi: 10.1016/j.taap.2007.09.023. https://doi.org/10.1016/j.taap.2007.09.023. [DOI] [PubMed] [Google Scholar]
- Woolley LD, Qin JG. Swimbladder inflation and its implication to the culture of marine finfish larvae. Rev Aquac. 2010;2:181–190. https://doi.org/10.1111/j.1753-5131.2010.01035.x. [Google Scholar]
- Yin A, Korzh S, Winata CL, Korzh V, Gong Z. Wnt signaling is required for early development of zebrafish swimbladder. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018431. https://doi.org/10.1371/journal.pone.0018431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu WG, Liu W, Jin YH. Effects of perfluorooctane sulfonate on rat thyroid hormone biosynthesis and metabolism. Environ Toxicol Chem. 2009;28:990–996. doi: 10.1897/08-345.1. https://doi.org/10.1897/08-345.1. [DOI] [PubMed] [Google Scholar]
- Yu WG, Liu W, Liu L, Jin YH. Perfluorooctane sulfonate increased hepatic expression of OAPT2 and MRP2 in rats. Arch Toxicol. 2011;85:613–621. doi: 10.1007/s00204-010-0613-x. https://doi.org/10.1007/s00204-010-0613-x. [DOI] [PubMed] [Google Scholar]
- Zheng XM, Liu HL, Shi W, Wei S, Giesy JP, Yu HX. Effects of perfluorinated compounds on development of zebrafish embryos. Environ Sci Pollut Res. 2011;19:2498–2505. doi: 10.1007/s11356-012-0977-y. https://doi.org/10.1007/s11356-012-0977-y. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. https://doi.org/10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


