Abstract
Purpose of review
To examine the epidemiology and mechanistic underpinnings of heightened cardiovascular disease (CVD) risk among women living with HIV (WLHIV) in North America and Europe.
Recent Findings
WLHIV in North America and Europe exhibit high CVD incidence rates, on par with those of compatriot men living with HIV (MLHIV). Compared with uninfected women, WLHIV in these regions face a two- to four-fold increased relative risk for myocardial infarction (MI), stroke, and heart failure. HIV-associated CVD risk is fuelled by a negative synergy of traditional cardiometabolic risk factors and heightened systemic immune activation/inflammation. Among WLHIV, female sex and endogenous sex hormone production influence both traditional cardiometabolic risk factors and patterns of systemic immune activation/inflammation. WLHIV in North America and Europe may also experience heightened CVD risk in relation to a relatively increased prevalence of behavioral and psychosocial CVD risk factors, coupled with suboptimal therapeutic targeting of known traditional cardiometabolic risk factors.
Summary
Additional research on sex-specific mechanisms of HIV-associated CVD - based not only out of North America and Europe but also and especially out of Africa, Asia, and South America - will inform the development of CVD prediction algorithms and prevention guidelines clinically relevant to the 17 million women aging with HIV globally.
Keywords: HIV, Cardiovascular Disease, Women
INTRODUCTION
Availability of effective antiretroviral therapy (ART) across North America and Europe has, in these regions, transformed HIV into a chronic disease with extended life expectancy (1) (2). Progress notwithstanding, women living with HIV (WLHIV) in these regions evidence high incidence rates of cardiovascular disease (CVD) events, not dissimilar from those of compatriot men living with HIV (MLHIV)(3) (4) (5) (6) (7) (8). WLHIV in North America and Europe, as compared with women without HIV residing in these regions, confront a two- to four-fold increased relative risk for myocardial infarction (MI), stroke, and heart failure(3) (4) (5) (6) (7) (8) (9). In this review, we will consider large-scale epidemiologic studies situating the scope of the problem of heightened CVD risk among WLHIV in North America and Europe. Next, drawing from both epidemiologic and physiologic studies, we will consider sex-specific mechanisms underlying this risk. Finally, we will address future directions for HIV-associated CVD risk research relevant to women and ways research findings may be applied towards improving preventive cardiac care for WLHIV globally.
EPIDEMIOLOGY OF HEIGHTENED CVD RISK IN HIV – A FOCUS ON WOMEN
Findings from select large-scale epidemiologic studies, reviewed below, have helped to characterize and contextualize the heightened risk of CVD among WLHIV in North America and Europe.
Risk of Myocardial Infarction (MI)
Triant et al. published one of the earliest large-scale epidemiologic studies offering sex-stratified analyses of MI risk among individuals with and without HIV. As part of this study, 3,851 individuals with HIV (30.4% women) and 1,044,589 individuals without HIV (59.1% women) in the US Partners HealthCare System were followed from 1996 to 2004. Unadjusted MI rates by sex and HIV status were 12.71/1000 person-years (PY) among WLHIV, as compared with rates of 4.88/1000 PY for women without HIV, 10.48/1000 PY for MLHIV, and 11.44/1000 PY for men without HIV. Adjusted relative risk assessments for MI by comparator group (adjusted for traditional CVD risk factors) were 1.75 (95% CI 1.51–2.02; 2.98 (95%CI 2.33–3.75) for WLHIV vs. women without HIV; and 1.40 (95% CI 1.16–1.67) for MLHIV vs. men without HIV(3). Data from subsequent large-scale epidemiologic studies with later follow-up periods conducted in the US and in Europe corroborated similar trends – namely, a higher relative risk of MI among women with vs. without HIV as compared to that among men with vs. without HIV(4) (10) (11) (5). Moreover, sex- and age-stratified analyses from US and European studies revealed that among WLHIV (vs. age-matched women without HIV), the most prominently increased relative risk of MI was in women <50 years old (4) (5).
Risk of Stroke
Chow et al. published seminal large-scale epidemiologic studies offering sex-stratified analyses of ischemic and hemorrhagic stroke risk among individuals with and without HIV. In one study, 4,308 individuals with HIV (31% women) and 32,423 individuals without HIV (10:1 matched) receiving care in the US Partners HealthCare System were followed from 1996 to 2009. Unadjusted ischemic stroke incidence rates by sex and HIV status were 5.02/1000 PY for WLHIV, 2.31/1000 PY for women without HIV, 5.38/1000 PY for MLHIV, and 4.59/1000 PY for men without HIV. The unadjusted hazard ratios for ischemic stroke by comparator groups were 1.40 (95% CI 1.17 to 1.69) HIV vs. non-HIV; 2.16 (95% CI 1.53–3.04) WLHIV vs. women without HIV; and 1.18 (95% CI 0.95–1.47) MLHIV vs. men without HIV. The adjusted hazard ratios for ischemic stroke in HIV (adjusted for stroke risk factors) were also significantly increased among women but not among men(6). Sex- and age-stratified analyses revealed that among WLHIV (vs. age-matched women without HIV), the most prominently increased hazard ratios for ischemic stroke were among women < 55 years old (7). With respect to hemorrhagic stroke, Chow et al followed 4,251 individuals with HIV (31% women) and 35,268 individuals without HIV (10:1 matched) from 1996 to 2009. Unadjusted hemorrhagic stroke incidence rates by sex and HIV status were 2.24/1000 PY for WLHIV, 0.72/1000 PY for women without HIV, 2.31/1000 PY for MLHIV, and 1.53/1000 PY for men without HIV. The unadjusted incidence rate ratios for hemorrhagic stroke by comparator groups were 1.85 (95%CI 1.37–2.47) HIV vs. non-HIV; 3.09 (95%CI 1.71–5.34) WLHIV vs. women without HIV; and 1.50 (95%CI 1.04–2.12) MLHIV vs. men without HIV. The hazard of hemorrhagic stroke was lower for women vs. men in the cohort without HIV (0.49, 95% CI 0.36–0.66) but not in the cohort with HIV (1.01, 95% CI 0.57 – 1.77)(8).
Risk of Heart Failure
To date, no large-scale epidemiologic studies have presented sex-stratified analyses of newly diagnosed heart failure among individuals with and without HIV. However, juxtaposition of separate cohort studies focused on incident heart failure in men or women with and without HIV permits for comparative inferences. Butt et al. first analyzed the risk of incident heart failure among men with and without HIV in the US Veterans Aging Cohort Study Virtual Cohort and the Large Health Study of Veteran Enrollees. As part of this study, 2391 men with HIV and 6095 men without HIV were followed from 2000 to 2007 The adjusted hazard ratio for heart failure among men with vs. without HIV (adjusted for heart failure risk factors) was 1.81 (95% CI 1.39–2.36) (12). Freiberg et al. returned to the US Veterans Aging Cohort Study Virtual Cohort (97% men) to examine the predominant types of heart failure among individuals with HIV. In this study, 98, 015 participants, 32.2% with HIV, were followed from 2003 to 2012. Among those MLHIV who developed heart failure, 34.6% developed heart failure with preserved ejection fraction (EF >/=50%), 15.5% developed borderline heart failure with preserved ejection fraction (EF 40–49%), 37.1% developed heart failure with reduced ejection fraction (EF <40%), and 12.8% developed heart failure of unknown type. Moreover, in this study, the relative risk of all 3 types of heart failure was increased by ~20 to ~60% among individuals with HIV, even after adjustment for heart failure risk factors(13). Most recently, Janjua et al. led a large-scale epidemiologic study focused on 1, 388 WLHIV and 13, 781 women without HIV (10:1) matched) receiving clinical care in the US Partners HealthCare System. Among WLHIV, the heart failure incidence rate was approximately 4x higher than that among women without HIV (2.5% cumulative or 0.27%/year for WLHIV vs. 0.74% cumulative or 0.07%/year for women without HIV). Among those WLHIV with heart failure, heart failure with preserved ejection fraction was far and away the predominant subtype (71%)(9).
SEX-SPECIFIC MECHANISMS UNDERLYING HEIGHTENED CVD RISK AMONG WLHIV
Broadly, traditional cardiometabolic risk factors, behavioral risk factors, and heightened systemic immune activation/inflammation contribute synergistically to the heightened risk of HIV-associated CVD (14) (15). Antiretroviral therapy exerts complex and regimen-specific effects on non-behavioral CVD risk factors, exacerbating select metabolic conditions and concomitantly dampening (without normalizing) indices of systemic immune activation/inflammation(14) (15). Discussed below are special considerations for WLHIV. Among WLHIV, female sex and endogenous sex hormone production influence both non-behavioral cardiometabolic risk factors and, importantly, patterns of systemic immune activation/inflammation. WLHIV in North America and Europe may also experience heightened CVD risk in relation to a relatively increased prevalence of behavioral /psychosocial CVD risk factors and suboptimal therapeutic targeting of known traditional cardiometabolic risk factors (Figure 1).
Figure 1.
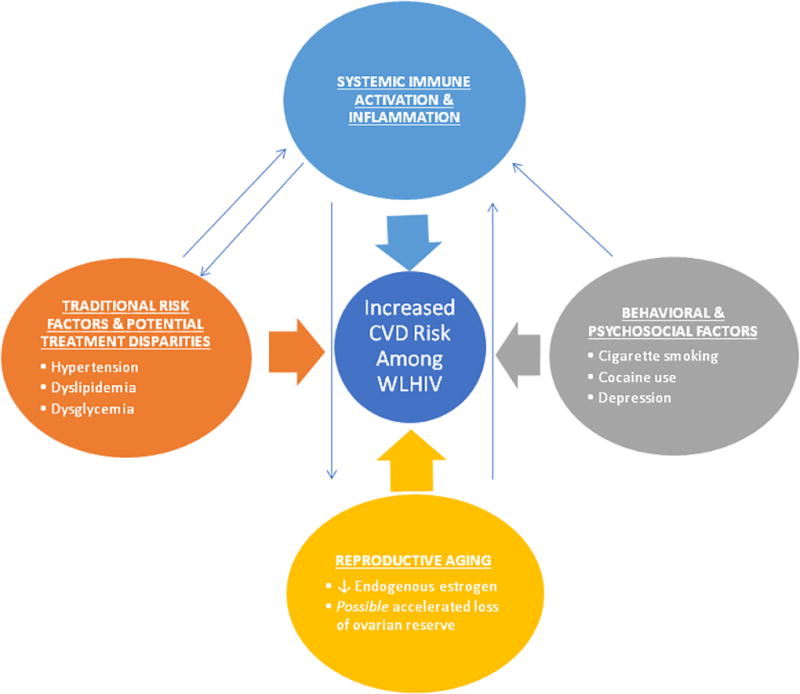
Mechanisms of Heightened Cardiovascular Disease Risk among Women Living with HIV in North America & Europe.
Traditional Cardiometabolic Risk Factors
Dyslipidemia, dysglycemia, and hypertension are well-established traditional cardiometabolic risk factors for MI, stroke, and heart failure in the general population(16). The question of how the cardiometabolic risk profile of WLHIV in North America and Europe compares to that among compatriot women without HIV, MLHIV, and men without HIV poses unique challenges. First, lifestyle choices, rooted in regional custom, influence the prevalence of traditional cardiometabolic risk factors. Second, several large cohort studies focused on WLHIV and MLHIV have purposefully recruited control subjects with similar lifestyle choices so as to isolate the effects of infection and treatment on HIV-associated morbidity. Consequently, the cardiometabolic risk profiles of control subjects in these cohorts may differ distinctly from those of the general population. Finally, few studies in unselected populations compare traditional cardiometabolic risk factors across groups stratified both by HIV status and by sex. Sex-stratified analyses on the prevalence of traditional cardiometabolic risk factors among the large number of patients with and without HIV receiving care in the US Partners Healthcare System have, however, been presented by Triant et al. We may glean insights from these analyses, cognizant that the patterns observed may be regionally specific (urban Northeast US) and not broadly generalizable. Among WLHIV, the prevalence of traditional cardiometabolic risk factors - including hypertension, diabetes, and dyslipidemia - tends to be higher than that among women without HIV and just slightly lower than that among MLHIV and men without HIV(3) (4).
Why might the prevalence of traditional non-behavioral cardiometabolic risk factors be relatively increased among WLHIV vs. women without HIV than among MLHIV vs. men without HIV? It is possible that sex differences in pharmacokinetics and pharmacodynamics(17) (18) render WLHIV particularly susceptible to ART-induced ectopic fat deposition and/or metabolic dysregulation. Prospective studies comparing differential effects of newly-initiated contemporary ART regimens on salient metabolic parameters among ART-naive women vs. men with HIV could test this hypothesis. A second consideration is that among WLHIV (vs. women without HIV), lower-level and/or less sustained endogenous sex hormone production may predispose to the development of cardiometabolic risk factors (see below, Altered Endogenous Sex Hormone Production).
Increased Systemic Immune Activation and Inflammation
HIV infection depresses immune function while simultaneously stimulating several systemic immune/inflammatory pathways relevant to carotid/coronary atherosclerosis and myocardial fibrosis(19) (15). Activated immune cells (particularly monocytes-turned-macrophages, but also T cells and other types of immune cells) participate meaningfully in atherosclerotic plaque formation and pathologic plaque remodeling(20). Several physiology studies performed in all-male or predominantly male (>90% male) cohorts have revealed relationships between systemic markers of monocyte activation (sCD163, sCD14) and surrogates of atherosclerotic cardiovascular disease (ASCVD) risk including subclinical carotid artery atherosclerosis(21), aortic inflammation(22) (23), subclinical coronary atherosclerotic plaque(24) (25) (26), and pathologically remodeled coronary atherosclerotic plaque(27) (28). Separate physiology studies among WLHIV have confirmed parallel relationships between systemic immune activation markers and ASCVD risk surrogates, including subclinical carotid atherosclerosis(29) (30) (31) and the percent of non-calcified coronary atherosclerotic plaque(32). Cells of monocyte/macrophage lineage may also engender myocardial edema and fibrosis, presaging diastolic dysfunction and heart failure with preserved ejection fraction(33) (34). Findings from an endomyocardial biopsy study suggest that among individuals with HIV, myocardial fibrosis is increased in association with in situ CD163+ macrophage infiltration and circulating levels of the monocyte activation marker sCD163(35). Moreover, mixed-sex cardiac MRI studies led by Holloway et al. and Thiara et al. confirmed among asymptomatic individuals with HIV an increased prevalence and extent of myocardial fibrosis in relation to diastolic dysfunction(36) (37).
Among WLHIV, several indices of systemic immune activation are particularly increased. Fitch et al. showed that levels of systemic monocyte activation markers relevant to HIV-associated CVD (sCD163, sCD14) are highest among asymptomatic WLHIV, as compared with levels among women without HIV, MLHIV, and men without HIV(32). In a two-group comparison of women with vs. without HIV, Alcaide et al. further demonstrated increased T cell activation and exhaustion among ART-treated WLHIV(38). There is a plausible biologic basis for heightened systemic immune activation/inflammation among WLHIV relating to sex-specific expression of key toll-like receptors (TLRs) and enhanced innate immune response to HIV infection(39). Acutely, innate immune hyper-responsiveness to HIV may enable women to better suppress the virus. Over time, this sex-specific response may be maladaptive, resulting in chronic immune cell activation and immunosenescence(39). Another possible explanation for heightened systemic immune activation/inflammation among WLHIV relates to the traditional and non-traditional cardiometabolic risk profile characteristic of this population. Behavioral risk factors (e.g. cigarette smoking, cocaine use) and co-morbid conditions (e.g. hepatitis C, depression) among WLHIV in North America and Europe may all be expected to stimulate systemic immune activation and inflammation.
Effects of therapeutic drugs on systemic immune activation markers differ by sex in HIV. Separate US studies among WLHIV and among MLHIV have confirmed that ART dampens select indices of systemic immune activation, albeit not down to levels seen in control subjects (40) (41) (42). Of note, however, an AIDS Clinical Trials Group study conducted in a resource-limited setting revealed that ART suppresses select indices of systemic immune activation to a lesser degree among WLHIV than among MLHIV (43). Just as ART exerts immunomodulatory effects in HIV, so too does statin therapy. Several US studies - including the mixed-sex SATURN-HIV study - have shown statins dampen key indices of systemic immune activation among asymptomatic PLWH (44) (45) (46). Intriguingly, in the mixed-sex INTREPID trial, pitavastatin (a newer statin which does not tend to have drug-drug interactions with ART)(47) decreased systemic levels of select monocyte activation markers relevant to HIV-associated CVD - more so among WLHIV than among MLHIV(48).
Altered Endogenous Sex Hormone Production and Effects on Cardiometabolic Parameters and Systemic Immune Activation/Inflammation
After menopause, women see a marked increase in the incidence of CVD risk factors (HTN, diabetes, and dyslipidemia) and CVD (MI, stroke, and heart failure) (49). Disentangling intertwined effects of chronological and reproductive aging on CVD risk in women has proven challenging(50) (51) (52). Select findings from large-scale US general population CVD research studies imply, indirectly, that robust and sustained endogenous estrogen production may protect women against CVD. For example, in the SWAN study, lower endogeneous estrogen levels among pre-menopausal/early peri-menopausal women predicted increased progression of subclinical atherosclerosis(53). Further, in the Nurses’ Health Study and the Framingham Heart Study, early age at natural menopause related to increased risk of coronary heart disease and ischemic stroke, respectively(54) (55).
WLHIV, however, seem to suffer disproportionately from perturbations along the hypothalamic-pituitary-gonadal axis, resulting in reduced premenopausal endogenous estrogen production and possibly in accelerated reproductive aging en route to terminal menses. Karim et al. showed through an analysis of data from the US Women’s Interagency HIV Study (WIHS) that estrogen and androgen levels were reduced among pre-menopausal WLHIV (vs. age-matched women without HIV)(56). Whether WLHIV experience menopause at an earlier age than women without HIV remains unsettled, despite a rigorous meta-analysis of available studies on the topic (57). The dilemma in defining menopause among WLHIV has for years hindered headway on this question. Traditional reproductive aging classification schemes rely on menstrual history, as well as FSH and E2 timed to menstrual cycle day 3, and such schemes are ill-suited to women with chronic illness or menstrual irregularity (58) (59). Among WLHIV, menstrual irregularity is not uncommon. Indeed, in the US WIHS, WLHIV (vs. similarly aged women without HIV) were approximately three times as likely to experience prolonged amenorrhea unrelated to ovarian failure(60). How, then, to assess reproductive aging in HIV?
Remarkably, the discovery and validation of a novel biomarker for ovarian reserve – Antimullerian hormone, or AMH – has ushered in a breakthrough for reproductive aging research in HIV. Antimullerian hormone is a protein encoded by the AMH gene and secreted by ovarian granulosa cells. A woman’s remaining egg supply is reflected in AMH levels, and AMH levels become undetectable prior to menopause(61) (62). Importantly, AMH levels are not cycle dependent(61) (62) (63). This lack of cycle dependency is crucial, as visits for female participants in clinical trials cannot always feasibly be timed to the menstrual cycle. Importantly, Scherzer et al. showed that among WLHIV in the WIHS, age-adjusted AMH levels were lower than among women without HIV(64). Scherzer et al. also demonstrated that among WLHIV, AMH levels predicted age at menopause(65). Most recently, Looby et al. used menstrual history and AMH levels to subdivide a US cohort of WLHIV into 3 reproductive aging groups – (1) women with menses and detectable AMH; (2) women with menses and undetectable AMH (reduced ovarian reserve); and (3) women without menses and with undetectable AMH (post-menopause). Reduced ovarian reserve among WLHIV was found to relate to a key CVD risk surrogate – noncalcified coronary atherosclerotic plaque – even after controlling for traditional CVD risk factors including age. Of note, the largest “step-up” for CVD risk (subclinical noncalcified coronary plaque prevalence) occurred between groups 1 and 2. This findings implies that WLHIV with reduced ovarian reserve who still undergo menstrual cycling should not necessarily be considered to harbor low CVD risk. In this study, among WLHIV, markers of systemic immune activation were also noted to increase across progressive categories of reproductive aging(66).
Behavioral and Psychosocial CVD risk factors and Treatment Disparities
Cigarette smoking and cocaine use represent known behavioral risk factors for MI, stroke, and heart failure in the general population(16). A rigorously conducted and representative large-scale US study reveals a markedly increased weighted/adjusted prevalence of current cigarette smoking among WLHIV (vs. women without) and among MLHIV (vs. men without): 34.6% (95% CI 30.6–38.9) WLHIV vs. 18.0 (95% CI 17.2–18.9) women without HIV, adjusted prevalence difference 16.6%; 40.9% (95% CI 38.6–43.2) MLHIV vs. 23.3% (95% CI 22.3–24.3) men without HIV, adjusted prevalence difference 17.6%(67). Alarmingly, large-scale European epidemiologic studies have shown that cigarette smoking disproportionately amplifies the risk of MI(68) and cardiovascular death (69) among individuals with vs. without HIV. There may also be a disproportionately high prevalence of cocaine use among WLHIV. In a special population (US Veterans), WLHIV evidenced a 13.5% prevalence of cocaine use – significantly higher than the 3.6 % prevalence of cocaine use among women without HIV(11). Psychosocial CVD risk factors such as depression are also increased among WLHIV (vs. women without) according to both US(70) and European studies(71) . Depression has been shown to relate to measures of CVD risk among WLHIV(70) and CVD event rates among women in the general population(72).
Data on whether WLHIV receive particularly suboptimal medical therapy for known traditional cardiometabolic risk factors or cardiac conditions are conflicting. A US WIHS study suggested that a higher percentage of WLHIV receive targeted therapeutics for known cardiometabolic risk factors as compared with women without HIV(73). In contrast, a US Partners Healthcare System database study revealed that a lower percentage of WLHIV with heart failure were prescribed standard-of-care therapy as compared with uninfected women with heart failure(9). Finally, in a different type of comparative study - examining therapeutic targeting of cardiometabolic risk factors among women vs. men with HIV participating in the large-scale European Observational Cohort study, D:A:D - Hatleberg et al. showed that WLHIV were less likely to receive therapeutic interventions for established cardiometabolic risk factors(74) .
FUTURE DIRECTIONS
In HIV-associated CVD research, as in general CVD research, findings from all-male studies should not be assumed to apply to women. CVD risk research relevant to women’s health requires conscientious study design, with plans to: 1) enroll and retain adequate numbers of women participants 2) assess sex-specific CVD risk factors and 3) present well-powered sex-stratified analyses on major scientific questions of interest(75). The ongoing international REPRIEVE study (Randomized Trial to Prevent Vascular Events in HIV) will help to elucidate mechanisms of cardiovascular disease risk and risk reduction among women and men with HIV. REPRIEVE is a randomized placebo control trial testing among 6500 individuals with HIV and low-to-moderate traditional CVD risk whether statin therapy (vs. placebo) reduces major adverse cardiovascular events through effects on lipids and/or systemic immune activation(76). To date, REPRIEVE has engaged >100 research sites in the US, Canada, Brazil, South Africa, Botswana, and Thailand, with additional international sites joining. The evidence-based REPRIEVE Follow YOUR Heart Campaign has been launched to educate WLHIV about heart disease and promote engagement with clinical trials like REPRIEVE(77). Moreover, objectives to study sex-specific mechanisms of cardiovascular disease risk and risk reduction in HIV have been integrated into the main REPRIEVE protocol.
CONCLUSIONS
Several forward-looking strategies will help improve cardiovascular health among WLHIV. The first is to invest resources in studying HIV-associated CVD where it afflicts the most women (please see Sliwa et al. “CVD in Women from Sub-Saharan Africa” in this same issue). Cross-comparative studies conducted on mechanisms of CVD risk among WLHIV in Africa, Asia, and South America – as well as North America and Europe – may elucidate region-specific genetic susceptibilities to CVD, as well as different relative contributions of traditional, behavioral, and psychosocial CVD risk factors. A second key strategy entails revisiting seminal observations from major CVD risk studies conducted among women in the general population(78) (79) and determining the extent to which these apply in HIV. Conversely, CVD research among WLHIV may inform sex-specific CVD research in the general population, as HIV represents an extreme model of the heightened immune reactivity to which women are generally prone. Finally, a third strategy centers on translating knowledge acquired from patient-oriented research into tailored CVD risk prediction algorithms and preventive care guidelines clinically relevant to the approximately 17 million women aging with HIV globally.
KEY POINTS.
Women living with HIV (WLHIV) in North America and Europe exhibit high cardiovascular disease (CVD) incidence rates, on par with those of compatriot men living with HIV (MLHIV). Compared with uninfected women, WLHIV in these regions face a two- to four-fold increased relative risk for myocardial infarction (MI), stroke, and heart failure.
Among WLHIV in North America and Europe, major factors contributing to increased CVD risk include traditional cardiometabolic risk factors and heightened systemic immune activation, both influenced by female sex and endogenous sex hormone production.
An increased prevalence of behavioral and psychosocial CVD risk factors, plus suboptimal therapeutic targeting of known traditional cardiometabolic risk factors, may also contribute to increased CVD risk among WLHIV in high-resource regions.
CVD risk research relevant to women’s health requires conscientious study design, with plans to: 1) enroll and retain adequate numbers of women participants; 2) assess sex-specific CVD risk factors; and 3) present well-powered sex-stratified analyses on major scientific questions of interest.
The ongoing international REPRIEVE trial, testing whether statin therapy prevents major adverse cardiovascular events in HIV, encourages WLHIV to participate through the Follow YOUR Heart Campaign and incorporates sex-specific objectives into the main study protocol.
Reducing women’s risk of HIV-associated CVD will require investing in targeted research across low- and high-resource regions globally and translating acquired knowledge into CVD risk prediction algorithms and preventive care guidelines tailored to women aging with HIV.
Acknowledgments
Financial Support and Sponsorship: This work was supported in part by the following grants/awards: Project grant 1R01AI123001(NIH/NIAID) to Drs. Zanni and Looby; Pilot and Feasibility Award to Dr. Zanni from the Harvard University Center for AIDS Research, as funded by P30 AI060354 (NIH/NIAID); Claflin Distinguished Scholar Awards from the MGH Executive Committee on Research to Drs. Zanni and Looby; Connell Extension Grant through the Yvonne L. Munn Center for Nursing Research to Dr. Looby.
Dr. Zanni received grant support to her institution from Gilead Sciences. She has also participated in a Scientific Advisory Board Meeting for Roche Diagnostics. Dr. Looby has given a talk for the Physician’s Research Network.
Footnotes
Conflicts of interest: Ms. Stone has no conflicts of interest to disclose.
ANNOTATED REFERENCES
- 1.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lohse N, Obel N. Update of Survival for Persons With HIV Infection in Denmark. Ann Intern Med. 2016;165(10):749–750. doi: 10.7326/L16-0091. [DOI] [PubMed] [Google Scholar]
- 3.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92(7):2506–12. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Triant VA, Regan S, Grinspoon SK. MACE Incidence Among HIV and Non-HIV-Infected Patients in a Clinical Care Cohort. CROI. 2014 [Google Scholar]
- 5.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24(8):1228–30. doi: 10.1097/QAD.0b013e328339192f. [DOI] [PubMed] [Google Scholar]
- 6.Chow FC, Regan S, Feske S, Meigs JB, Grinspoon SK, Triant VA. Comparison of ischemic stroke incidence in HIV-infected and non-HIV-infected patients in a US health care system. J Acquir Immune Defic Syndr. 2012;60(4):351–8. doi: 10.1097/QAI.0b013e31825c7f24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow FRS, Looby S, Zanni MV, Meigs JB, Bushnell CD, Feske SK, Grinspook S, Triant VA. Persistently Increased Ischemic Stroke Risk in HIV-Infected Women. CROI. 2016 [Google Scholar]
- 8.Chow FC, He W, Bacchetti P, Regan S, Feske SK, Meigs JB, et al. Elevated rates of intracerebral hemorrhage in individuals from a US clinical care HIV cohort. Neurology. 2014;83(19):1705–11. doi: 10.1212/WNL.0000000000000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.Janjua SA, Triant VA, Addison D, Szilveszter B, Regan S, Staziaki PV, et al. HIV Infection and Heart Failure Outcomes in Women. J Am Coll Cardiol. 2017;69(1):107–108. doi: 10.1016/j.jacc.2016.11.013. This epidemiology study analyzes heart failure incidence and outcomes among US women with and without HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–22. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, et al. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3(5):e001035. doi: 10.1161/JAHA.114.001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butt AA, Chang CC, Kuller L, Goetz MB, Leaf D, Rimland D, et al. Risk of heart failure with human immunodeficiency virus in the absence of prior diagnosis of coronary heart disease. Arch Intern Med. 2011;171(8):737–43. doi: 10.1001/archinternmed.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freiberg MS, Chang CH, Skanderson M, Patterson OV, DuVall SL, Brandt CA, et al. Association Between HIV Infection and the Risk of Heart Failure With Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results From the Veterans Aging Cohort Study. JAMA Cardiol. 2017;2(5):536–546. doi: 10.1001/jamacardio.2017.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boccara F, Lang S, Meuleman C, Ederhy S, Mary-Krause M, Costagliola D, et al. HIV and coronary heart disease: time for a better understanding. J Am Coll Cardiol. 2013;61(5):511–23. doi: 10.1016/j.jacc.2012.06.063. [DOI] [PubMed] [Google Scholar]
- 15.Zanni MV, Schouten J, Grinspoon SK, Reiss P. Risk of coronary heart disease in patients with HIV infection. Nat Rev Cardiol. 2014;11(12):728–41. doi: 10.1038/nrcardio.2014.167. [DOI] [PubMed] [Google Scholar]
- 16.Mann D, Zipes D, Libby P, Bonow RO. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 2015 [Google Scholar]
- 17.Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF. Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol. 2004;44:499–523. doi: 10.1146/annurev.pharmtox.44.101802.121453. [DOI] [PubMed] [Google Scholar]
- 18.Umeh OC, Currier JS. Sex Differences in HIV: Natural History, Pharmacokinetics, and Drug Toxicity. Curr Infect Dis Rep. 2005;7(1):73–78. doi: 10.1007/s11908-005-0026-9. [DOI] [PubMed] [Google Scholar]
- 19.Hsue PY, Deeks SG, Hunt PW. Immunologic basis of cardiovascular disease in HIV-infected adults. J Infect Dis. 2012;205(Suppl 3):S375–82. doi: 10.1093/infdis/jis200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32(9):2045–51. doi: 10.1161/ATVBAHA.108.179705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. J Infect Dis. 2012;206(10):1558–67. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379–86. doi: 10.1001/jama.2012.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zanni MV, Toribio M, Wilks MQ, Lu MT, Burdo TH, Walker J, et al. Application of a Novel CD206+ Macrophage-Specific Arterial Imaging Strategy in HIV-Infected Individuals. J Infect Dis. 2017;215(8):1264–1269. doi: 10.1093/infdis/jix095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lo J, Abbara S, Shturman L, Soni A, Wei J, Rocha-Filho JA, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24(2):243–53. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204(8):1227–36. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Jr, Kingsley LA, et al. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211(8):1219–28. doi: 10.1093/infdis/jiu594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, et al. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27(8):1263–72. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller PE, Haberlen SA, Metkus T, Rezaeian P, Palella F, Kingsley LA, et al. HIV and coronary arterial remodeling from the Multicenter AIDS Cohort Study (MACS) Atherosclerosis. 2015;241(2):716–22. doi: 10.1016/j.atherosclerosis.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203(4):452–63. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaked I, Hanna DB, Gleissner C, Marsh B, Plants J, Tracy D, et al. Macrophage inflammatory markers are associated with subclinical carotid artery disease in women with human immunodeficiency virus or hepatitis C virus infection. Arterioscler Thromb Vasc Biol. 2014;34(5):1085–92. doi: 10.1161/ATVBAHA.113.303153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanna DB, Lin J, Post WS, Hodis HN, Xue X, Anastos K, et al. Association of Macrophage Inflammation Biomarkers With Progression of Subclinical Carotid Artery Atherosclerosis in HIV-Infected Women and Men. J Infect Dis. 2017 doi: 10.1093/infdis/jix082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, et al. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208(11):1737–46. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11(9):507–15. doi: 10.1038/nrcardio.2014.83. [DOI] [PubMed] [Google Scholar]
- 34.Borlaug BA. Heart failure with preserved and reduced ejection fraction: different risk profiles for different diseases. Eur Heart J. 2013;34(19):1393–5. doi: 10.1093/eurheartj/eht117. [DOI] [PubMed] [Google Scholar]
- 35.Walker JABG, Miller AD, Burdo TH, Williams KC. sCD163 correlates with IMT and macrophages in aorta and heart with HIV infection. CROI. 2015 [Google Scholar]
- 36.Holloway CJ, Ntusi N, Suttie J, Mahmod M, Wainwright E, Clutton G, et al. Comprehensive cardiac magnetic resonance imaging and spectroscopy reveal a high burden of myocardial disease in HIV patients. Circulation. 2013;128(8):814–22. doi: 10.1161/CIRCULATIONAHA.113.001719. [DOI] [PubMed] [Google Scholar]
- 37.Thiara DK, Liu CY, Raman F, Mangat S, Purdy JB, Duarte HA, et al. Abnormal Myocardial Function Is Related to Myocardial Steatosis and Diffuse Myocardial Fibrosis in HIV-Infected Adults. J Infect Dis. 2015;212(10):1544–51. doi: 10.1093/infdis/jiv274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alcaide ML, Parmigiani A, Pallikkuth S, Roach M, Freguja R, Della Negra M, et al. Immune activation in HIV-infected aging women on antiretrovirals--implications for age-associated comorbidities: a cross-sectional pilot study. PLoS One. 2013;8(5):e63804. doi: 10.1371/journal.pone.0063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Addo MM, Altfeld M. Sex-based differences in HIV type 1 pathogenesis. J Infect Dis. 2014;209(Suppl 3):S86–92. doi: 10.1093/infdis/jiu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan RC, Landay AL, Hodis HN, Gange SJ, Norris PJ, Young M, et al. Potential cardiovascular disease risk markers among HIV-infected women initiating antiretroviral treatment. J Acquir Immune Defic Syndr. 2012;60(4):359–68. doi: 10.1097/QAI.0b013e31825b03be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29(4):463–71. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zanni MV, Toribio M, Robbins GK, Burdo TH, Lu MT, Ishai AE, et al. Effects of Antiretroviral Therapy on Immune Function and Arterial Inflammation in Treatment-Naive Patients With Human Immunodeficiency Virus Infection. JAMA Cardiol. 2016;1(4):474–80. doi: 10.1001/jamacardio.2016.0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **43.Mathad JS, Gupte N, Balagopal A, Asmuth D, Hakim J, Santos B, et al. Sex-Related Differences in Inflammatory and Immune Activation Markers Before and After Combined Antiretroviral Therapy Initiation. J Acquir Immune Defic Syndr. 2016;73(2):123–9. doi: 10.1097/QAI.0000000000001095. This patient-oriented research study illustrates sex-related differences in immunomodulatory effects of antiretroviral therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funderburg NT, Jiang Y, Debanne SM, Storer N, Labbato D, Clagett B, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58(4):588–95. doi: 10.1093/cid/cit748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209(8):1156–64. doi: 10.1093/infdis/jiu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Funderburg NT, Jiang Y, Debanne SM, Labbato D, Juchnowski S, Ferrari B, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(4):396–404. doi: 10.1097/QAI.0000000000000478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aberg JA, Sponseller CA, Ward DJ, Kryzhanovski VA, Campbell SE, Thompson MA. Pitavastatin versus pravastatin in adults with HIV-1 infection and dyslipidaemia (INTREPID): 12 week and 52 week results of a phase 4, multicentre, randomised, double-blind, superiority trial. Lancet HIV. 2017 doi: 10.1016/S2352-3018(17)30075-9. [DOI] [PubMed] [Google Scholar]
- *48.Toribio M, Fitch KV, Sanchez L, Burdo TH, Williams KC, Sponseller CA, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS. 2017;31(6):797–806. doi: 10.1097/QAD.0000000000001427. This study presents data from a clinical trial revealing differential effects of pitavastatin on systemic markers of monocyte activation among women vs. men with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crandall CJ, Barrett-Connor E. Endogenous sex steroid levels and cardiovascular disease in relation to the menopause: a systematic review. Endocrinol Metab Clin North Am. 2013;42(2):227–53. doi: 10.1016/j.ecl.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 50.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–73. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chae CU, Derby CA. The menopausal transition and cardiovascular risk. Obstet Gynecol Clin North Am. 2011;38(3):477–88. doi: 10.1016/j.ogc.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 52.Barrett-Connor E. Menopause, atherosclerosis, and coronary artery disease. Curr Opin Pharmacol. 2013;13(2):186–91. doi: 10.1016/j.coph.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis. 2012;225(1):180–6. doi: 10.1016/j.atherosclerosis.2012.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hu FB, Grodstein F, Hennekens CH, Colditz GA, Johnson M, Manson JE, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–6. doi: 10.1001/archinte.159.10.1061. [DOI] [PubMed] [Google Scholar]
- 55.Lisabeth LD, Beiser AS, Brown DL, Murabito JM, Kelly-Hayes M, Wolf PA. Age at natural menopause and risk of ischemic stroke: the Framingham heart study. Stroke. 2009;40(4):1044–9. doi: 10.1161/STROKEAHA.108.542993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karim R, Mack WJ, Kono N, Tien PC, Anastos K, Lazar J, et al. Gonadotropin and sex steroid levels in HIV-infected premenopausal women and their association with subclinical atherosclerosis in HIV-infected and -uninfected women in the women's interagency HIV study (WIHS) J Clin Endocrinol Metab. 2013;98(4):E610–8. doi: 10.1210/jc.2012-3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Imai K, Sutton MY, Mdodo R, Del Rio C. HIV and Menopause: A Systematic Review of the Effects of HIV Infection on Age at Menopause and the Effects of Menopause on Response to Antiretroviral Therapy. Obstet Gynecol Int. 2013;2013:340309. doi: 10.1155/2013/340309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76(5):874–8. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 59.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Fertil Steril. 2012;97(4):843–51. doi: 10.1016/j.fertnstert.2012.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cejtin HE, Kalinowski A, Bacchetti P, Taylor RN, Watts DH, Kim S, et al. Effects of human immunodeficiency virus on protracted amenorrhea and ovarian dysfunction. Obstet Gynecol. 2006;108(6):1423–31. doi: 10.1097/01.AOG.0000245442.29969.5c. [DOI] [PubMed] [Google Scholar]
- 61.Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, Fanchin R, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20(3):370–85. doi: 10.1093/humupd/dmt062. [DOI] [PubMed] [Google Scholar]
- 62.de Kat AC, Broekmans FJ, Laven JS, van der Schouw YT. Anti-Mullerian Hormone as a marker of ovarian reserve in relation to cardio-metabolic health: a narrative review. Maturitas. 2015;80(3):251–7. doi: 10.1016/j.maturitas.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Seifer DB, Golub ET, Lambert-Messerlian G, Springer G, Holman S, Moxley M, et al. Biologic markers of ovarian reserve and reproductive aging: application in a cohort study of HIV infection in women. Fertil Steril. 2007;88(6):1645–52. doi: 10.1016/j.fertnstert.2007.01.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **64.Scherzer R, Bacchetti P, Messerlian G, Goderre J, Maki PM, Seifer DB, et al. Impact of CD4+ lymphocytes and HIV infection on Anti-Mullerian Hormone levels in a large cohort of HIV-infected and HIV-uninfected women. Am J Reprod Immunol. 2015;73(3):273–84. doi: 10.1111/aji.12332. This seminal study compares levels of antimullerian hormone among age-matched women with and without HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **65.Scherzer R, Greenblatt RM, Merhi ZO, Kassaye S, Lambert-Messerlian G, Maki PM, et al. Use of antimullerian hormone to predict the menopausal transition in HIV-infected women. Am J Obstet Gynecol. 2017;216(1):46 e1–46 e11. doi: 10.1016/j.ajog.2016.07.048. This study offers critical insights into the role of antimullerian hormone in predicting menopause among women living with HIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *66.Looby SE, Fitch KV, Srinivasa S, Lo J, Rafferty D, Martin A, et al. Reduced ovarian reserve relates to monocyte activation and subclinical coronary atherosclerotic plaque in women with HIV. AIDS. 2016;30(3):383–93. doi: 10.1097/QAD.0000000000000902. This clinical physiology study introduces a new paradigm for classifying reproductive aging among women with HIV. In this study, reproductive aging among women with HIV is related to indices of systemic immune activation and to subclinical coronary atherosclerosis, a surrogate for cardiovascular disease risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162(5):335–44. doi: 10.7326/M14-0954. [DOI] [PubMed] [Google Scholar]
- 68.Rasmussen LD, Helleberg M, May MT, Afzal S, Kronborg G, Larsen CS, et al. Myocardial infarction among Danish HIV-infected individuals: population-attributable fractions associated with smoking. Clin Infect Dis. 2015;60(9):1415–23. doi: 10.1093/cid/civ013. [DOI] [PubMed] [Google Scholar]
- 69.Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, et al. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56(5):727–34. doi: 10.1093/cid/cis933. [DOI] [PubMed] [Google Scholar]
- 70.Schwartz RM, Mansoor A, Wilson TE, Anastos K, Everson-Rose SA, Golub ET, et al. Chronic depressive symptoms and Framingham coronary risk in HIV-infected and HIV-uninfected women. AIDS Care. 2012;24(3):394–403. doi: 10.1080/09540121.2011.608791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jallow A, Ljunggren G, Wandell P, Carlsson AC. Prevalence, incidence, mortality and co-morbidities amongst human immunodeficiency virus (HIV) patients in Stockholm County, Sweden - the Greater Stockholm HIV Cohort Study. AIDS Care. 2015;27(2):142–9. doi: 10.1080/09540121.2014.963012. [DOI] [PubMed] [Google Scholar]
- 72.Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, et al. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J Am Coll Cardiol. 2007;50(21):2044–50. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- 73.Hanna DB, Jung M, Xue X, Anastos K, Cocohoba JM, Cohen MH, et al. Trends in Nonlipid Cardiovascular Disease Risk Factor Management in the Women's Interagency HIV Study and Association with Adherence to Antiretroviral Therapy. AIDS Patient Care STDS. 2016;30(10):445–454. doi: 10.1089/apc.2016.0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hatleberg CI, Ryom L, El-Sadr W, Mocroft A, Reiss P, de Wit S, et al. Gender differences in HIV-positive persons in use of cardiovascular disease-related interventions: D:A:D study. J Int AIDS Soc. 2014;17(4 Suppl 3):19516. doi: 10.7448/IAS.17.4.19516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *75.Wenger NK, Ouyang P, Miller VM, Bairey Merz CN. Strategies and Methods for Clinical Scientists to Study Sex-Specific Cardiovascular Health and Disease in Women. J Am Coll Cardiol. 2016;67(18):2186–8. doi: 10.1016/j.jacc.2016.03.504. This opinion piece offers practical advice for clinical scientists interested in studying sex-specific mechanisms of cardiovascular disease risk. [DOI] [PubMed] [Google Scholar]
- 76.Mitka M. Exploring Statins to Decrease HIV-Related Heart Disease Risk. JAMA. 2015;314(7):657–9. doi: 10.1001/jama.2015.5498. [DOI] [PubMed] [Google Scholar]
- 77.Zanni MV, Fitch K, Rivard C, Sanchez L, Douglas PS, Grinspoon S, et al. Follow YOUR Heart: development of an evidence-based campaign empowering older women with HIV to participate in a large-scale cardiovascular disease prevention trial. HIV Clin Trials. 2017;18(2):83–91. doi: 10.1080/15284336.2017.1297551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, et al. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part I: gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol. 2006;47(3 Suppl):S4–S20. doi: 10.1016/j.jacc.2005.01.072. [DOI] [PubMed] [Google Scholar]
- 79.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, et al. Insights from the NHLBI-Sponsored Women's Ischemia Syndrome Evaluation (WISE) Study: Part II: gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol. 2006;47(3 Suppl):S21–9. doi: 10.1016/j.jacc.2004.12.084. [DOI] [PubMed] [Google Scholar]


