Abstract
A cross-sectional study was conducted to determine sero-prevalence of Peste des Petits Ruminants (PPR) among goat population of outbreak suspected areas; Kaski and Syangja districts of Nepal. A total of 460 goat serum samples were tested by using c-ELISA for the presence of antibodies against PPR. Out of the 460 samples tested, 380 samples were found positive, giving overall sero-positivity of 82.60%. Significantly higher sero-prevalence was found (*P < 0.05) in females (87.50%) compared to males (70.45%) and crossbreed goats were found highly susceptible than the pure breed goats (*P < 0.05). Likewise, higher sero-prevalence of PPR was observed in adults and cross breed goats compared to their counterparts and significantly higher sero-prevalence was observed in Syangja district compared to Kaski. Thus, adults, females and cross-breeds populations of goats are at higher risk of PPR whereas geographically, goat population of Syangja district were found significantly prone to PPR. Appropriate control measures, such as ring vaccination can be followed to prevent the potential outbreak situation.
Keywords: Goats, Peste des Petits Ruminants (PPR), Sero-prevalence, Nepal
Introduction
Peste des Petits Ruminants (PPR) also known as “goat plague”, is a contagious trans-boundary viral disease of both domestic and wild small ruminants which was first reported from Cote-d’Ivoire in 1942 [7]. The causative agent of PPR is Peste des Petits Ruminants virus (PPRV) belongs to the genus morbillivirus and family Paramyxoviridae. This virus can be categorized into four distinct lineages (I, II, III and IV) based on fusion (F) and nucleoprotein (N) gene sequencing [16]. PPR has been a constant threat to small ruminant farmers of Asia, Middle East and Africa since its first identification [11]. The challenge has been more intensified due to its highly destructive nature and constant emergence across the newer part of the world [12]. Therefore, the Food and Agriculture Organization (FAO) and World Organization for Animal Health (OIE) have declared 2030 target for PPR eradication from the world [27]. The morbidity and mortality of PPR is reported to go up to 100 and 90%, respectively, and sometimes in endemic area the mortality may be as low as 20% (http://www.fao.org/tempref/docrep/fao/003/X1703E/X1703E00.PDF.) [20]. According to FAO, 62.5% of the total global domestic small ruminants are at risk of PPR infection and it imposes significantly negative impact on country’s economy as an aftermath of outbreak situation, for this reason it has been enlisted as one of the prioritized disease of FAO Emergency Preventive System (EMPRES) program (http://www.fao.org/ag/againfo/resources/documents/AH/PPR_flyer.pdf.) [19]. Though commercial sero-diagnosis ELISA kits with high specificity and sensitivity are available in the market [9], the utility of these diagnostic procedures have been limited by their inability to distinguish between infected and vaccinated condition [32]. In this connection, a vaccine inducing equal level of immunity that can be distinguished from infected population could be a very good asset in PPR eradication program [32]. The PPR virus circulating in different countries of Asia i.e. Nepal, India, Bhutan, China, Iran, Iraq, Israel, Kuwait, Bangladesh, Pakistan, Saudi Arabia, Tajikistan, Turkey is of lineage (IV) [32] however, the F and N gene sequencing based study has shown that the virus found in Nepal, India and Bangladesh is more closely related than virus found in rest of the countries [16].
In Nepal, first time PPR outbreak was reported in 1995 [11, 16] from the Dhanusha, Mahottari, Bara, Sarlahi, Rauthat and Gorkha districts [21] and to the date 68 districts of Nepal have reported the PPR outbreaks covering all eco-zones and developmental regions [17]. A study done by Regional Agricultural Research Station (Goats), Bandipur under the National Agriculture Research Council in Syangja and one other district has reported PPR as the major infectious disease of goats and recorded 33.48% mortality [22]. In this study, investigative efforts were made to determine sero-prevalence of PPR in unvaccinated goat population of Syangja and Kaski districts and associated risk factors.
Material and methods
Study site
Syangja and Kaski Districts of Nepal were selected because of their central location and typical climatic condition. Syangja lies at latitude 28°4′60″ North and longitude 83°52′0″ East where as Kaski lies at latitude 28°16′00″ North and longitude 83°53′00″ East (Figs. 1, 2).
Fig. 1.
PPR sample collection sites
Fig. 2.
PPR Outbreak districts of Nepal during Jan–Dec 2015.
Source: DoAH [17]
Study design
Across-sectional study design was used with purposive sampling in the outbreak suspected villages within the Kaski and Syangja Districts. The sampling areas were picked up by using the records of Regional Veterinary Laboratory (RVL) in association with District Livestock Services Office (DLSO) Kaski, and DLSO Syangja. Because of unavailability of complete list of animals within each village, animals were selected randomly for blood collection and blood collection was performed by researchers themselves.
Sample size calculation
The total goat population in Kaski and Syangja as stated in statistical yearbook, 2014 were 1, 05,553 and 1, 84,828, respectively. The sample size was calculated according to Daniel formula [15] which is further supported by Naing et al. [29] in which the use of 50% prevalence is suggested if the exact prevalence is unknown or ranging from 10 to 90%. According to the available literatures on prevalence of PPR in unvaccinated goat population of neighboring countries, it was found to be approximately equal to 50% thus, we calculated the sample size assuming 50% prevalence [15]:
where n = sample size; Z = 95% level of confidence in Z statistics (Z = 1.96); P = expected level of prevalence (i.e. P = 0.5); d = precision (d = 0.05).
The calculated sample size was 385; however, researchers have managed to take 460 samples to optimize the power of the test.
Sample collection
Samples were collected from Dhobadi, Sworek, Kafaldada and Chhangchhangdi VDCs of Syangja and Astham, Dharapani and Deorali VDCs of Kaski district over the duration of four months beginning from 1st September to 31st, December 2016. The blood was collected from Jugular veins using sterile 5 ml syringe and was kept undisturbed in the same syringe with the needle holding end positioned down until the clot was fully separated from the serum. The serum samples were then centrifuged to remove any blood cells left and stored in a freezer at − 20 °C until laboratory analysis was done.
Serological analysis
Serum samples were analyzed by using competitive enzyme linked immunosorbent assay (c-ELISA) kit, ID Screen© PPR Competition, which was manufactured by ID.vet innovative diagnostics, 310, rue Louis Pasteur, 34790 Grabels, France. This diagnostic kit was used for the detection of antibodies directed against the PPRV nucleoprotein to calculate apparent prevalence (AP). The entire assay was performed as per the manufacturer’s manual. True prevalence was calculated using Rogan and Gladen formula as follows:
where AP is apparent prevalence and Sp and Se are test kit specificity and sensitivity, respectively. According to the manufacturer’s manual, the ID Screen © PPR Competition c-ELISA kit has an average diagnostic specificity and relative sensitivity of 97.98 and 93.95% respectively. Although, the virus isolation is a gold standard for PPR diagnosis, virus neutralization test is considered to be an equal alternative for practical purposes.
Questionnaire survey
A questionnaire survey was conducted in the study area pertaining to risk factors of PPR in goats. Information related to age, sex, breed, geographical location and vaccination status were asked to respective farmers from where samples were collected.
Statistical analysis
The data entry and analysis were done using MS-Excel 2013 and SPSS Version 16. Univariate analysis by using Chi square test and Post hoc test LSD (least significance difference) were done to find out significant association between potential risk factors.
Results and discussion
Findings
The overall sero-prevalence of PPR was found to be 82.60% and the calculated true sero-prevalence was 87.65% with an average specificity and relative sensitivity of the test kit 97.98 and 93.95% respectively. The sex wise distribution of sero-prevalence of PPR among goat shows, 70.45% sero-positive in males and 87.50% seropositive in females (Fig. 3a). The sero-prevalence of female was found to be statistically significantly (*P < 0.05) higher than the sero-prevalence of male with an odd ratio of 0.341 indicating the higher risk of PPR among females than males by 0.341 times. Within age groups; sucklers (1–3 months) were found to be 69.86% sero-positive, young (4–12 months) were found to be 79.72% sero-positive and adults (> 12 months) were found to be 95.90% sero-positive. These observations indicate a statistically significant association (*P < 0.05) of age groups with PPR occurrence in adults followed by young and sucklers, respectively (Fig. 3b and Table 1). The breed was found to be significantly (*P < 0.05) associated with sero-prevalence of PPR; in which crossbreed goats showed the highest (90.62%) sero-prevalence of PPR followed by pure breeds; Khari (85.19%), Jamunapari (74.35%) and Sannen (73.91%) (Fig. 3c and Table 2). The location wise sero-prevalence was found to be higher in Syangja district than in Kaski district which was statistically highly significant (***P < 0.000) with an odd ratio 6.474 showing risk of PPR disease occurrence in Syangja is 6.474 times higher than in Kaski district (Fig. 3d and Table 3).
Fig. 3.
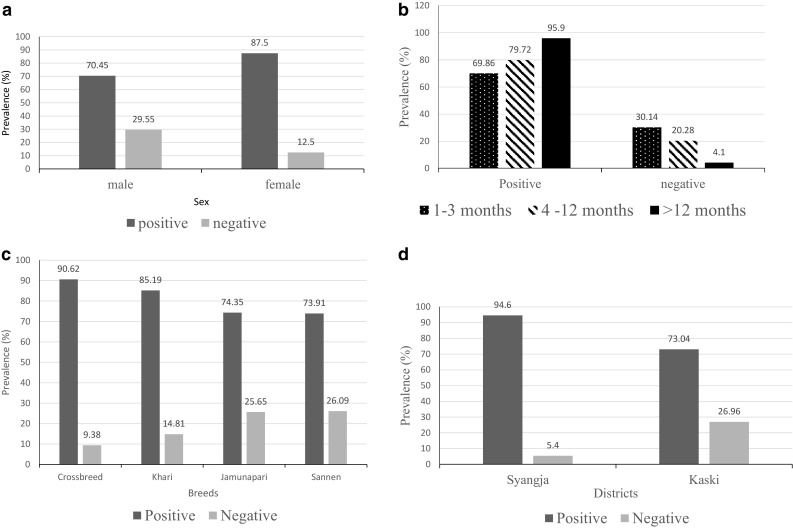
a Prevalence of PPR by sex, b age wise prevalence of PPR, c breed wise prevalence, d location wise prevalence of PPR
Table 1.
Post hoc (LSD) analysis of age group of goats
| Combination (age groups) | P value | Remarks |
|---|---|---|
| 1–3 months × 3–12 months | 0.008 | ** |
| 1–3 months ×>12 months | 0.000 | *** |
| >12 months × 3–12 months | 0.000 | *** |
NS non-significant
**P < 0.01; ***P < 0.001
Table 2.
Post hoc (LSD) analysis of Breeds group of goats
| Combination (BREED groups) | P value | Remarks |
|---|---|---|
| Khari × Jamunapari | 0.005 | ** |
| Khari × Sannen | 0.019 | * |
| Khari × Crossbreed | 0.334 | NS |
| Jamunapari × Sannen | 0.937 | NS |
| Jamunapari × Crossbreed | 0.011 | * |
| Sannen × Crossbreed | 0.017 | * |
NS non-significant
*P < 0.05; **P < 0.01
Table 3.
Risk factors for prevalence of Pestes des Petits Ruminants (PPR)
| Variables | Result | Total | 95% CI | χ2 value | OR | P value | |
|---|---|---|---|---|---|---|---|
| Positive (%) | Negative (%) | ||||||
| Sex of Animal | |||||||
| Male | 93 (70.45) | 39 (29.55) | 132 | (62.18–77.57; 22.43–37.82) | 19.035 | 0.341 | 0.000*** |
| Female | 287 (87.50) | 41 (12.50) | 328 | (83.48–90.65; 9.35–16.52) | |||
| Age | |||||||
| 1–3 | 102 (69.86) | 44 (30.14) | 146 | (61.99–76.72;23.28–38.01) | 38.387 | 0.000*** | |
| 12-Apr | 114 (79.72) | 29 (20.28) | 143 | (72.39–85.49; 14.51–27.61) | |||
| > 12 | 164 (95.90) | 7 (4.10) | 171 | (91.79–98.00; 2.00–8.21) | |||
| Breed | |||||||
| Crossbreed | 29 (90.62) | 3 (9.38) | 32 | (75.78–96.76; 3.24–24.22) | 8.965 | 0.030* | |
| Khari | 259 (85.19) | 45 (14.81) | 304 | (80.77–88.75; 11.25–19.23) | |||
| Jamunapari | 58 (74.35) | 20 (25.65) | 78 | (63.69–82.74; 17.26–36.31) | |||
| Sannen | 34 (73.91) | 12 (26.09) | 46 | (59.74–84.40; 15.60–40.26) | |||
| Location | |||||||
| Syangja | 193 (94.60) | 11 (5.40) | 204 | (90.60–96.96; 3.09–9.40) | 36.736 | 6.474 | 0.000*** |
| Kaski | 187 (73.04) | 69 (26.96) | 256 | (67.30–78.11;21.89–32.70) | |||
NS non-significant
*P < 0.05; ***P < 0.001
Overall prevalence of PPR compared to neighboring countries
The overall sero-prevalence 82.60% resulted in this study is in close approximation with the result of studies done in Nigerian goat population where they have reported 75% [18] and 73.8% [13] sero-prevalence of PPR. Similarly, a study to scan sero-prevalence status of PPR virus throughout Turkey has reported highest prevalence (82%) among goat on flock basis in Sakarya province [31]. The other study from Sudan has reported flock level true prevalence of 74% [5]. In contrast, several other studies from this region have reported lower sero-prevalence rate than the result of this study. In a study conducted in goat population of five different states of India has reported 34.54% sero-prevalence [9]; however within India as well different sero-prevalence rates have been reported. Sero-prevalence of 65.51% has been reported from Maharastra [14], 15.05% from Kerala [20], 28.70% from Northern state Jammu and Kasmir [26], and 11.63% overall sero-prevalence from 7 North East states of India [10]. On the other hand, country wide sero-prevalence of PPR in goat population of Pakistan is reported to be 27.53% [3]. Within Pakistan the sero-prevalence of 39.02% from Punjab [24], 34.5% from wildlife and domestic animal interface areas of Lahore and Faisalabad [2],34.78% from Sindh province [30] and 15.36% from North West frontier provinces of Pakistan [28] have been reported. The other neighboring country of Nepal is Bangladesh which has reported 8.70% sero-prevalence of PPR among goat population [19]. Likewise, a published report on prevalence of PPR in goat population of China has reported 34.5% sero-prevalence in Tibet [35] which is the adjoining part of People’s Republic of China bordering to Nepal. The differences in sero-prevalence rate of PPR among different contiguous areas might be due to differences in geographical location, agro-climatic conditions, type of diagnostic test applied and sampling methods. On the other hand, the higher sero-prevalence might be due to higher population density of domestic and wild animals using common grazing/browsing and watering ground. Researches have shown that PPR prevalence is strongly associated with the climatic conditions like high rainfall and high wind speed [33]. Furthermore, both of the research districts are located on windward site of Annapurna range which is reported to be wettest part of Nepal with average annual precipitation more than 5400 mm; in which Kaski experiences highest rainfall occurrence out of entire country (http://www.dhm.gov.np/uploads/climatic/47171194ClimateandClimaticvariabilityofNepal-2015.pdf.) [21]. Similarly, porous border and unrestricted animal movement within the country during festive seasons (August to October) may also aid in increasing sero-prevalence. Also, there are reports of PPR outbreaks occurring most commonly during wet season i.e. April to October [8] and it was the sampling period of this study. Studies have suggested goats are more severely affected species by PPR virus resulting into low level of serum antibody and death while sheep manage to recover [24], since the research site also has significant number of sheep, cattle and buffalo which may be the source of PPR infection to goats. Moreover, there are several reports showing natural infection of PPR in cattle, buffalo [4, 24] which also increases the chance of transmission of PPRV from sub-clinically infected cattle and buffalo [9] to small ruminants due to shed sharing practices [4] that is common in the research site. Additionally, not all farmers of the research site are well educated and keep record of every vaccination they did with their animals. All the information obtained by researchers during the survey are memory based therefore, there might exist chance of considering vaccinated animal as an unvaccinated one, which may be a reason for increased sero-prevalence rate to some extent.
Host associated risk factors
The statistically significantly higher sero-prevalence of PPR among female goat observed in this study is in agreement with the findings of several other studies around the globe [1, 2, 3, 13, 25, 30, 33]. However, the result of this study contrasts to the findings of a study done in India [26] and Bangladesh [34]. The higher sero-prevalence found in females may be due to the livestock breeding pattern of Nepalese farmers in which females are kept longer for reproduction while most of the males are castrated and sold for meat purpose. The longer the females are kept for herd maintenance the more chances of exposure to the environment they get may result into more sero-prevalence.
The higher prevalence among adults, followed by young and then sucklers obtained as a result of this study is in concordance with other studies [1, 26, 33] reporting decreasing sero-prevalence rate as the age decreases and it is because greater probability of older animals to be exposed to PPRV than younger. However, this finding disagrees with other study [13, 34] where young animals are reported to have more sero-prevalence rate than older animals showing more susceptible than adults. Additionally, a mixed result showing less risk to 1–3 years old animals than < 1 year old and more risk to > 3 years old animals than < 1 year old has also been reported [3]. The higher sero-prevalence among adults may be because long life time allowing more exposure to PPRV. On the other hand, passive immunity from dam to the sucklers might have influence on the result to some extent. However, poor nutritional status, parasitic diseases and stress conditions could be potential factors for higher sero-prevalence.
The strong association between the sero-prevalence of PPR and breeds observed in this study is also in accordance with the findings of other study [33, 34]. A study done by National Agriculture Research Station, Bandipur, Nepal has also reported Khapari (50% Khari and 50% Jamunapari) being more susceptible to PPR than Khari, the local breed of that area [23]. The higher prevalence observed in crossbreeds in this study may be due to the genetic variation and immune characteristics of animals with different degree of blood levels of pure breeds or may be due to relatively less sample size of other breeds.
PPR risk associated with geographical location of the site
The PPR prevalence varying with the geographical location of the site is also recorded in this study as reported by other researchers [33]. The higher sero-prevalence in Syangja district than in Kaski (statistically highly significant ***P < 0.000) with an odd ratio 6.474 showing higher risk of PPR in Syangja by 6.474 times is further supported by report from governmental body [6] in which the prevalence of PPR was reported to be 75 and 100% in Kaski and Syangja in outbreak samples. The higher prevalence seen in Syangja district might be due to sampling method and sample size.
PPR has been an endemic disease within the country since its first identification in 1994 from Bara district of eastern terai [17]. Despite very few published studies of this kind done within the country, this is the first study conducted in these districts that represent pre-dominant part of Nepal. These two adjoining districts are located at the center of political map of country extending from mid hills to the base of mountains. Not merely they represent most densely populated land of goat husbandry also exhibit typical climate and landscape features of entire Nation. Thus, the finding of this study is expected to portray overall status of natural infection and associated risk factors of PPR disease among goat population of whole country. Moreover, findings of this study will be helpful to future investigators interested at challenges of goat farming across the country. The key importance of these findings will be at effective implementation of control and prevention strategies to forestall outbreak situation and minimize loss. As this study unveils some of the important determinants and risk factors associated with PPR, government bodies, NGOs, INGOs and private parties involved directly or indirectly to goat production in these areas as well as across the country take advantage of it. Basically, it helps all stakeholders to be aware of imminent outbreak situation allowing them to become well prepared for impact mitigation.
To sum up it is important to note that PPR is rampant among goat population of central part of Nepal. It may negatively affect entire national small ruminant production practices if preventive strategies are not executed well ahead of time. This study provides important insights on endemic nature of PPR disease in the locality showing obvious association with some of the host factors i.e. age, sex and breed, and geographical location. Therefore, any suspected cases from these areas is strongly suggested to report to concerned authority as soon as possible because it may be an indicator case foreboding imminent outbreak situation. Further studies covering entire nation to determine sero-prevalence and titer level of PPR antibodies are strongly warranted which form the essential part of formulation of control and eradication strategies of PPR.
References
- 1.Abdalla AS, Majok AA, El Malik KH, Ali AS. Sero-prevalence of peste des petits ruminants virus (PPRV) in small ruminants in Blue Nile, Gadaref and North Kordofan States of Sudan. J Public Heal Epidemiol. 2012;4:59–64. [Google Scholar]
- 2.Abubakar M, Rasool MH, Manzoor S, Saqalein M, Rizwan M, et al. Evaluation of risk factors for peste des petits ruminants virus in sheep and goats at the wildlife-livestock interface in Punjab province, Pakistan. Biomed Res Int. 2016;2016:7826245. doi: 10.1155/2016/7826245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abubakar M, Zahur AB, Afzal M, Ali Q, Gonzales J. Peste des Petits Ruminants (PPR) in Pakistan: analysis of a national level serological data. Small Rumin Res. 2017;155:57–65. doi: 10.1016/j.smallrumres.2017.08.017. [DOI] [Google Scholar]
- 4.Abubakar M, Mahapatra M, Muniraju M, Arshed MJ, Khan EH, Banyard AC, et al. Serological detection of antibodies to peste des petits ruminants virus in large ruminants. Transbound Emerg Dis. 2017;64:513–519. doi: 10.1111/tbed.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Majali AM, Hussain NO, Amarin NM, Majok AA. Seroprevalence of, and risk factors for, peste des petits ruminants in sheep and goats in Northern Jordan. Prev Vet Med. 2008;85:1–8. doi: 10.1016/j.prevetmed.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Annual Technical Report [Internet]. Tripureshwor, Kathmandu, Central Veterinary Laboratory, Department of Livestock Services, Ministry of Livestock Development, Government of Nepal. 2015. http://www.cvl.gov.np/uploads/files/5324820354.pdf. Accessed 22 Dec 2017.
- 7.Baazizi R, Mahapatra M, Clarke BD, Ait-Oudhia K, Khelef D, Parida S. Peste des petits ruminants (PPR): a neglected tropical disease in Maghreb region of North Africa and its threat to Europe. PLoS ONE. 2017;12:e0175461. doi: 10.1371/journal.pone.0175461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Balamurugan V, Saravanan P, Sen A, Rajak KK, Venkatesan G, Krishnamoorthy P, et al. Prevalence of peste des petits ruminants among sheep and goats in India. J Vet Sci. 2012;13:279–285. doi: 10.4142/jvs.2012.13.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balamurugan V, Krishnamoorthy P, Raju DSN, Rajak KK, Bhanuprakash V, Pandey AB, et al. Prevalence of Peste-des-petits-ruminant virus antibodies in cattle, buffaloes, sheep and goats in India. Virusdisease. 2014;25:85–90. doi: 10.1007/s13337-013-0177-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balamurugan V, Das S, Raju DSN, Chakravarty I, Nagalingam M, Hemadri D, et al. Prevalence of peste des petits ruminants in goats in North-East India. Virusdisease. 2014;25:488–492. doi: 10.1007/s13337-014-0237-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol. 2010;91:2885–2897. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- 12.Banyard AC, Wang Z, Parida S. Peste des petits ruminants virus, eastern Asia. Emerg Infect Dis. 2014;20:2176–2178. doi: 10.3201/eid2012.140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bello AM, Lawal JR, Dauda J, Wakil Y, Lekko YM, Mshellia ES, et al. Research for peste des petits ruminants (PPR) virus antibodies in goats, sheep and gazelle from Bauchi and Gombe states, North Eastern Nigeria. Direct Res J Agric Food Sci. 2016;4:193–198. [Google Scholar]
- 14.Bhaskar SR, Deshmukh VV, Chopade NA, Rautmare SS. Seroprevalence of Peste Des Petits Ruminants in Maharashtra. Indian J Anim Res. 2009;43:285–287. [Google Scholar]
- 15.Daniel WW, editor. Biostatistics: a foundation for analysis in the health sciences. 7. New York: Wiley; 1999. [Google Scholar]
- 16.Dhar P, Sreenivasa B, Barrett T, Corteyn M, Singh R, Bandyopadhyay S. Recent epidemiology of peste des petits ruminants virus (PPRV) Vet Microbiol. 2002;88:153–159. doi: 10.1016/S0378-1135(02)00102-5. [DOI] [PubMed] [Google Scholar]
- 17.DoAH. Annual Epidemiological Bulletin. Tripureshwor, Kathmandu: Veterinary Epidemiological Centre, Directorate of Animal Health; 2015.
- 18.El-Yuguda AD, Abubakar MB, Nabi AB, Andrew A, Baba SS. Outbreak of peste des petits ruminant in an unvaccinated Sahel goat farm in Maiduguri, Nigeria. Afr J Biomed Res. 2008;12:83–87. [Google Scholar]
- 19.Islam M, Hasan A, Yousuf A, Islam UK, Mahfuz M, Khan A. Seroprevalence of Peste des Petits Ruminant Virus specific antibody in goats in different regions of Bangladesh. J Adv Vet Anim Res. 2016;7710:127–133. doi: 10.5455/javar.2016.c140. [DOI] [Google Scholar]
- 20.Janus A, Tresamol PV, Saseendranath MR, Vijayakumar K, Pillai UN. Seroprevalence of PPR in goats in Kerala by cELISA. J Vet Anim Sci. 2009;40:15–16. [Google Scholar]
- 21.Jha VK, Singh DB, Thakuri KC, Gautam SP. National workshop. In: Gurung TB, Joshi BR, Singh UM, Paudel KP, Shrestha BS, Rijal KP et al (eds) Research & development strategies for goat enterprises in Nepal. 2013. https://www.researchgate.net/profile/Tek_Gurung/publication/257143203_Proceedings_of_the_National_Workshop_on_Research_and_Development_Strategies_for_Goat_Enterprises_in_Nepal/links/0deec524bc1267ea34000000/Proceedings-of-the-National-Workshop-on-Research. Accessed 9 Nov 2017
- 22.Khakural GP. Surveillance of goat diseases in the Western Hills of Nepal. Nepal J Sci Technol. 2003;5:37–40. [Google Scholar]
- 23.Khakural GP, Upreti CR. Proceedings of the 3rd national workshop on livestock and fisheries research in Nepal. 1999. p. 148–52. http://coin.fao.org/coin-static/cms/media/22/14376249192440/a_compendium_of_livestock_and_fisheries_research_highlights_in_nepal.pdf. Accessed 9 Nov 2017.
- 24.Khan HA, Siddique M, Sajjad-Ur-Rahman, Abubakar M, Ashraf M. The detection of antibody against peste des petits ruminants virus in Sheep, Goats, Cattle and Buffaloes. Trop Anim Health Prod. 2008;40:521–527. doi: 10.1007/s11250-008-9129-2. [DOI] [PubMed] [Google Scholar]
- 25.Kihu SM, Gachohi JM, Ndungu EK, Gitao GC, Bebora LC, John NM, et al. Sero-epidemiology of Peste des petits ruminants virus infection in Turkana County, Kenya. BMC Vet Res. 2015;11:87. doi: 10.1186/s12917-015-0401-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahajan S, Agrawal R, Kumar M, Mohan A, Pande N. Risk of seroconversion to peste des petits ruminants (PPR) and its association with species, sex, age and migration. Small Rumin Res. 2012;104:195–200. doi: 10.1016/j.smallrumres.2011.10.009. [DOI] [Google Scholar]
- 27.Mariner JC, Jones BA, Rich KM, Thevasagayam S, Anderson J, Jeggo M, et al. The opportunity to eradicate peste des petits ruminants. J Immunol. 2016;196:3499–3506. doi: 10.4049/jimmunol.1502625. [DOI] [PubMed] [Google Scholar]
- 28.Mehmood A, Ali Q, Gadahi JA, Malik SA, Shah SI. Detection of peste des petits ruminants (PPR) virus antibodies in sheep and goat populations of the north west frontier province (NWFP) of Pakistan by competitive elisa (cELISA) Vet World. 2009;2:333–336. [Google Scholar]
- 29.Naing L, Winn T, Rusli BN. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006;1:9–14. [Google Scholar]
- 30.Nizamani AR, Nizamani ZA, Umrani AP, Dewani P, Vandiar MA, Gandahi JA, et al. Prevalence of peste des petits ruminants virus antibodies in small Ruminantsin Sindh, Pakistan. J Anim Plant Sci. 2015;25:1515–1519. [Google Scholar]
- 31.Ozkul A, Akca Y, Alkan F, Barrett T, Karaoglu T, Dagalp SB, et al. Prevalence, distribution, and host range of Peste des petits ruminants virus, Turkey. Emerg Infect Dis. 2002;8:708–712. doi: 10.3201/eid0807.010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parida S, Muniraju M, Mahapatra M, Muthuchelvan D, Buczkowski H, Banyard AC. Peste des petits ruminants. Vet Microbiol. 2015;181:90–106. doi: 10.1016/j.vetmic.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salih H, Elfadil A, Saeed I, Ali Y. Seroprevalence and risk factors of Peste des Petits Ruminants in sheep and goats in Sudan. J Adv Vet Anim Res. 2014;1:42–49. doi: 10.5455/javar.2014.a12. [DOI] [Google Scholar]
- 34.Sarker S, Islam H. Prevalence and risk factor assessment of peste des petits ruminants in goats in Rajshahi, Bangladesh. Vet World. 2011;4:546–549. doi: 10.5455/vetworld.2011.546-549. [DOI] [Google Scholar]
- 35.Wang Z, Bao J, Wu X, Liu Y, Li L, Liu C, et al. Peste des petits ruminants virus in Tibet, China. Emerg Infect Dis. 2009;15:299–301. doi: 10.3201/eid1502.080817. [DOI] [PMC free article] [PubMed] [Google Scholar]