Abstract
Background
Neuroendocrine tumours (NET) consist of a heterogeneous group of neoplasms with various organs of origin. At diagnosis 21% of the patients with a Grade 1 NET and 30% with a Grade 2 NET have distant metastases. Treatment with peptide receptor radionuclide therapy (PRRT) shows a high objective response rate and long median survival after treatment. However, complete remission is almost never achieved. The liver is the most commonly affected organ in metastatic disease and is the most incriminating factor for patient survival. Additional treatment of liver disease after PRRT may improve outcome in NET patients. Radioembolization is an established therapy for liver metastasis. To investigate this hypothesis, a phase 2 study was initiated to assess effectiveness and toxicity of holmium-166 radioembolization (166Ho-RE) after PRRT with lutetium-177 (177Lu)-DOTATATE.
Methods
The HEPAR PLUS trial (“Holmium Embolization Particles for Arterial Radiotherapy Plus 177Lu-DOTATATE in Salvage NET patients”) is a single centre, interventional, non-randomized, non-comparative, open label study. In this phase 2 study 30–48 patients with > 3 measurable liver metastases according to RECIST 1.1 will receive additional 166Ho-RE within 20 weeks after the 4th and last cycle of PRRT with 7.4 GBq 177Lu-DOTATATE. Primary objectives are to assess tumour response, complete and partial response according to RECIST 1.1, and toxicity, based on CTCAE v4.03, 3 months after 166Ho-RE. Secondary endpoints include biochemical response, quality of life, biodistribution and dosimetry.
Discussion
This is the first prospective study to combine PRRT with 177Lu-DOTATATE and additional 166Ho-RE in metastatic NET. A radiation boost on intrahepatic disease using 166Ho-RE may lead to an improved response rate without significant additional side-effects.
Trial registration
Clinicaltrials.gov NCT02067988, 13 February 2014. Protocol version: 6, 30 november 2016.
Keywords: NET, Neuroendocrine tumour, Liver metastasis, Radioembolization, Holmium-166, PRRT, Lutetium-177, 177Lu-DOTATATE
Background
In accordance with the most recent WHO/ENETS criteria, grade 1 and 2 neuroendocrine tumours (G1-/G2NET) are regarded as well- to moderately-differentiated tumours and grade 3 NET (G3NET) as poorly-differentiated NET or neuroendocrine carcinomas (NEC) [1, 2]. At diagnosis, 21% of all G1NET, 30% of all G2NET and 50% of all G3NET have distant metastases, of which the liver is most commonly affected [3, 4]. A correlation between the organ of origin and the likelihood of metastasis exists. For example, rectal NET has a slim chance of distant metastasis (5%) compared with pancreatic or colonic NET (respectively 64% and 53–86%) [3, 5]. Considering these numbers, many patients will be ineligible for curative treatment, which currently only includes surgical resection of the primary tumour.
Most G1-/G2NET have membrane receptors for somatostatin, allowing for targeted therapies, of which somatostatin-analogs are the most commonly used (e.g. octreotide). Treatment with somatostatin-analogs, chemotherapeutics and kinase inhibitors show only limited objective response rates in G1-/G2NET [6–12]. In addition, systemic therapies give rise to systemic side effects. In the last decade, the treatment of G1-/G2NET with peptide receptor radionuclide therapies (PRRT) has increased. High objective imaging response rates (CR + PR 29–58%) [13–17], clinical and biological response rates and a long median survival (95–128 months after diagnosis, 46 months after treatment) [13, 14] can be achieved after PRRT (Fig. 1).
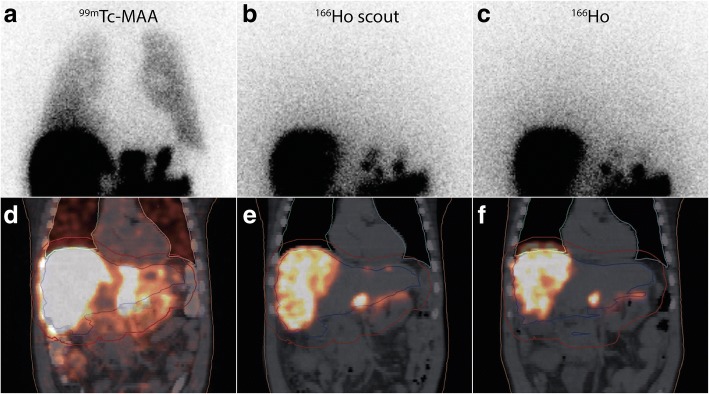
Fig. 1.
Example of 177Lu-DOTATATE in NET. Upper row: planar whole body 111In-pentetreotide scintigraphy. Lower row: venous phased CT of the liver. On the left baseline imaging and on the right imaging after 177Lu-DOTATATE treatment
All studies include high percentages of patients with liver metastases and show a dismal prognosis with increasing liver involvement (Table 1). As surgical resection techniques develop, some forms of hepatic involvement can be treated surgically. However, as three different patterns of hepatic metastases are described in NET, patients with the most common ‘diffuse pattern’ are not eligible for surgical resection (Table 2) [4]. Besides, most systemic therapies have a limited objective response rate. This indicates a need for improved treatment of extensive liver disease. In accordance with the ENETS guideline published in 2012, the treatment of choice in patients with NET liver metastases with a ‘diffuse’ or unresectable ‘complex pattern’, consists of systemic treatment followed by liver directed treatment [4]. Hepatic radioembolization (RE) is one of the liver directed treatments and it is an established minimal invasive treatment of patients with liver malignancies. RE has been demonstrated to be effective and well tolerated in primary, as well as secondary liver malignancies. A recent meta-analysis by Devcic et al. showed an average objective response rate (CR + PR) of 50% and an average disease control rate of 86% in a heterogeneous group of NET treated with RE [18]. 166Ho-RE is quite similar to 90Y-RE, but its distinct advantages will be discussed later on. Figure 2 shows an example of 166Ho-RE in a NET patient.
Table 1.
Liver involvement as a poor prognostic factor in different therapeutic studies
| Author | Treatment | N | Liver involvement | Median survival (months) | 5-year survival | p values |
|---|---|---|---|---|---|---|
| Chamberlain (2000) [46] | Surgical resection | 85 | 0–25% | – | 90% | |
| 25–50% | – | 83% | ||||
| 50–75% | 47 | 80% | ||||
| > 75% | 24 | – | ||||
| Yao (2001) [47] | Surgical resection | 16 | ≤4 liver metastases | 46 | – | < 0.05 |
| > 4 liver metastases | 20 | – | ||||
| Gupta (2005) [48] | TAE or TACE |
123 | 0–25% | 86 | – | |
| 25–50% | 30 | – | < 0.10 | |||
| 50–75% | 39 | – | < 0.17 | |||
| > 75% | 20 | – | < 0.05 | |||
| Kwekkeboom (2008) [14] | PRRT | 310 | None | > 48 | – | |
| Moderate | > 48 | – | ||||
| Extensive | 25 | – | < 0.01 |
Legend: TAE transarterial (bland) embolization, TACE transarterial chemoembolization, PRRT peptide receptor radionuclide therapy
Table 2.
NET Liver involvement patterns [4]
| Involvement | Incidence | |
|---|---|---|
| Simple pattern | One lobe or two adjacent lobes | 20–25% |
| Complex pattern | Primarily one lobe and smaller satellites contralaterally | 10–15% |
| Diffuse pattern | Multifocal disease | 60–70% |
Fig. 2.
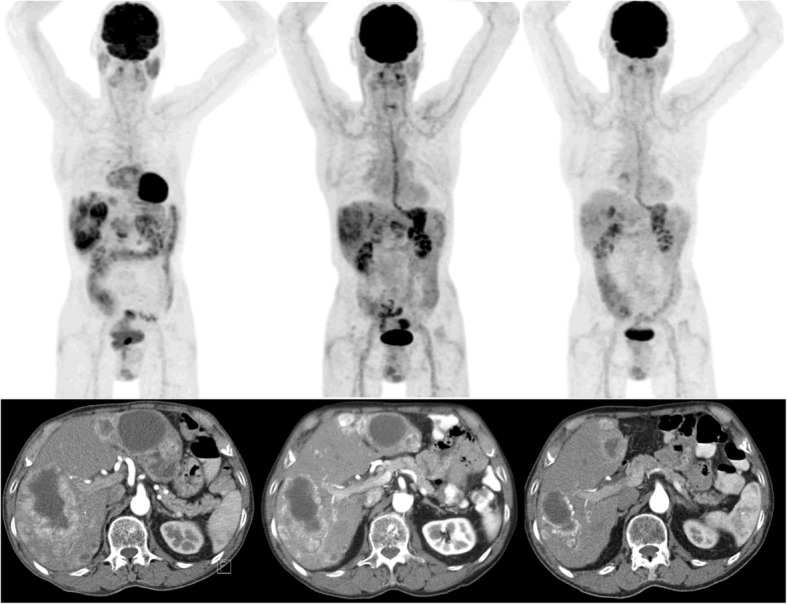
Example of 166Ho-radioembolization in NET. A patient with a grade 2 small intestinal NET according to the WHO-criteria, treated in the prior HEPAR 2 trial. On the left, the 18FDG-PET and venous phased CT at baseline. In the middle, the imaging studies 3 months after 166Ho-RE with partial metabolic 18FDG-PET response and some tumour reduction on CT. On the right, follow-up imaging studies 6 months after 166Ho-RE with significant partial metabolic response and significant tumour reduction on CT
In the current clinical setting, RE is used for liver dominant or liver isolated disease, often in a salvage setting. In this study, it is hypothesized that improved outcome can be obtained by escalating the treatment of liver metastases, the most significant prognostic factor for NET patients, additional to treatment of all extrahepatic disease in G1-/G2NET patients, by combining systemic PRRT with RE. In the presented study, patients with metastasized NET will receive PRRT in 4 cycles of 7.4 GBq with 177Lu-DOTATATE, followed by 166Ho-RE in the University Medical Centre Utrecht, the Netherlands, using 166Ho-microspheres. The following paragraphs will address the details of the study.
Methods
Study design
The HEPAR PLUS study is a single centre, interventional, non-randomized, non-comparative, open label study. In this phase 2 study all patients will receive additional 166Ho-RE after 177Lu-DOTATATE. Overall, 30–48 patients with metastasized NET will be investigated for efficacy and toxicity.
Subjects
Patients with NET and liver metastasis, who completed 4 cycles of 7.4 GBq 177Lu-DOTATATE, will receive additional 166Ho-RE within 20 weeks of the last/fourth cycle of 177Lu-DOTATATE. At time of recruitment, all included patients have no need for conventional treatment options like surgery or chemotherapy. In the Netherlands, 177Lu-DOTATATE is often a first- or second-line treatment. Previous treatments prior to 177Lu-DOTATATE were no exclusion criterium. Detailed inclusion and exclusion criteria are listed in Table 3.
Table 3.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Patient must have given a written informed consent | Brain metastases or spinal cord compression, unless irradiated > 4 weeks prior to 166Ho-RE and stable for at least 1 week without steroids |
| ≥ 18 years of age | Serum bilirubin > 1.5 x upper limit of normal |
| Confirmed histological diagnosis NET | Glomerular filtration rate < 35 ml/min |
| Prior treatment with 4 cycles of 7.4 GBq 177Lu-DOTATATE within 20 weeks before 166Ho-RE | Alkaline phosphatase, alanine aminotransferase or aspartate aminotransferase > 5 x upper limit of normal |
| Life expectancy > 12 weeks | Leucocytes < 2.0 × 109/l and/or platelet count < 50 × 109/l |
| WHO performance score 0–2 | Significant cardiac event within 3 months of inclusion |
| ≥ 3 measurable liver lesions according to RECIST 1.1 | Patients suffering from diseases with an increased chance of liver toxicity |
| Negative pregnancy test for women of childbearing potential | Patients declared incompetent or suffering from psychic disorders making comprehensive judgment impossible |
| No nursing activities for women of childbearing potential | Severe bile duct abnormalities: papillotomy, cholecystectomy, biliary stents and bilidigestive anastomosis are allowed |
| Acceptable method of contraception | Body weight > 150 kg |
| Severe contrast allergy | |
| Liver tumour involvement > 70% on CT |
Time schedule
Recruitment will take place between Augustus 2014 and January 2019. First participant was enrolled in November 2014.
Medical device
166Ho-microspheres are produced by incorporating non-radioactive 165Ho and its acetylacetonate complex (165HoAcAc) in a poly(L-lactic acid) matrix to form microspheres with an average diameter of 30 μm. By neutron-activation in a nuclear facility, the prescribed amount of radioactive 166Ho-microspheres are produced [19, 20]. The radionuclide 166Ho has a half-life of 26.8 h, is a beta-emitter (Εβmax = 1.85 MeV) and a gamma emitter (Εγ = 81 keV). Due to their additional photon emitting properties, 166Ho-microspheres can be visualized and quantified using SPECT imaging [21, 22].
Recruitment
All patients have previously been treated with four cycles of 177Lu-DOTATATE. After the fourth cycle patients are eligible for study inclusion. The study physician (AJATB) and principal investigator (MGEHL) inform all patients; thereafter informed consent will be obtained. On the informed consent form, participants can indicate whether they wish to receive a summary of the trial results, once the trial is completed.
Statistical analysis
This single arm open label study will have a sequential design. Stopping boundaries are determined such that an overall one-side alpha of at the most 0.05 is maintained in case the true tumour response is 20%. Early termination at a response interim analysis (after 30, 36 or 42 patients) is determined by pre-defined boundaries on the number of partial and complete responses according to RECIST 1.1 (Table 4). A superiority or futility boundary may be reached or crossed before 30 patients are reached, but the study will continue to at least 30 patients to allow estimation of the key secondary endpoints. The sequential design with boundaries as given in Table 4 will have a power of 90% to reach a positive tumour response decision in case the true target lesions tumour response is 40%. The exact overall one-sided type I error is 4.5%.
Table 4.
Stopping boundaries for early termination at interim analysis
| Analysis | Sample Size | Lower boundary | Upper boundary |
|---|---|---|---|
| 1 | 30 | 5 | 11 |
| 2 | 36 | 6 | 13 |
| 3 | 42 | 7 | 14 |
| 4 | 48 | 15 | 16 |
Interim analysis of toxicity with descriptive statistics (N, mean, median, etc.) will be performed for every 3 patients. All analysis will be performed in the Full Analysis Set (FAS), including all patients who received at least the scout dose procedure (see below). The Per Protocol Set (PPS) will include all patients who complied with the protocol up to at least 3 months. PPS analyses will be used for the primary endpoint. For the assessment of the primary objective at least 30 patients should have a 3 months follow-up CT-scan. If patients do not reach the 3 months follow-up CT-scan or receive a new treatment prior to the evaluation moment, a new patient will be included for the PPS analysis.
Monitoring
All safety interim analyses will be presented to our Independent Data Monitoring Commission (IDMC), consisting of one interventional radiologist, one nuclear medicine physician, one gastroenterologist and one biostatistician. All IDMC members are not involved in the trial and have no conflicting interests. Additionally, safety analysis will be performed every 3 months during the recruitment of the first 30 patients, after patient 36, patient 42 and patient 48, and evaluated by the IDMC.
Severe adverse (device) events will be reported to the Ethics Committee of the University Medical Center Utrecht and IDMC within 8 days. In accordance with Dutch regulations on research with medical devices, a summary of all severe adverse (device) events will be reported to the Dutch Health Care Inspectorate (in Dutch: Inspectie GezondheidsZorg en Jeugd in oprichting; IGJ) every 3 months.
Data management
All patient data collected in this trial, will be coded. All coded data will be entered in to an, in-house developed, electronic case report form (e-CRF) in a secure digital environment. All collected data is monitored and validated by an independent, external data monitor approximately every 3–4 months. In accordance with Dutch regulations, all collected data will be stored for a duration of 15 years. Trial data is only accessible to the study physician, principal investigator and external data monitor.
Ethical considerations
The study protocol has been approved by the Ethics Committee and the institutional radiation protection committee of the University Medical Centre Utrecht, the Netherland. This study will be performed in accordance with the Declaration of Helsinki (current version October 2013), the Medical Research Involving Human Patients Act (WMO, the Netherlands) and the requirements of International Conference on Harmonization (Good Clinical Practice). In accordance to regulations, all future protocol amendments need Ethics Committee approval. Unexpected harm to the participant during the trial, is covered by the institutions’ insurance for clinical trials.
Funding
This phase 2 study is funded by the Department of Radiology and Nuclear Medicine of the University Medical Center Utrecht. No external funding received.
Treatment
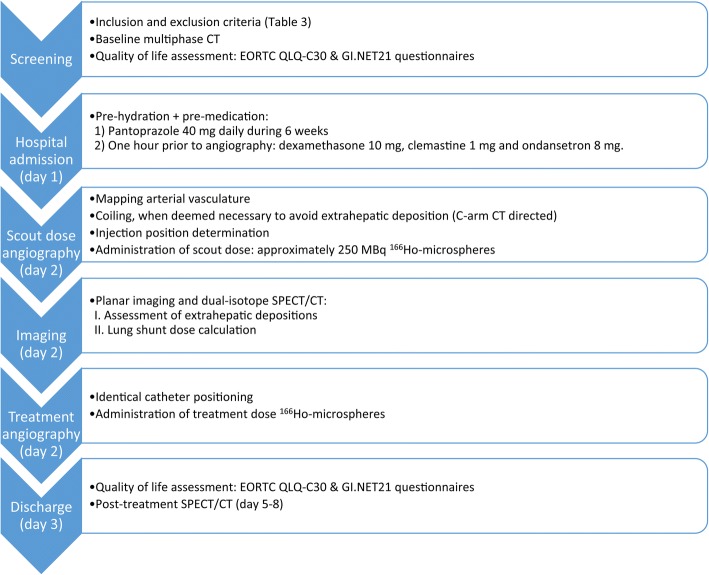
Screening
After obtaining informed consent, all study proceedings will occur in the University Medical Center Utrecht, Utrecht, The Netherlands. A screening visit will take place at the outpatient clinic prior to the first angiography. The study physician and principal investigator will check in- and exclusion criteria, perform a physical examination (including blood pressure, temperature and heart rate) and assess the WHO performance status of the patient. All patients are asked to fill out the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 and QLQ-GINET21 questionnaires. Additional tests include relevant laboratory testing (haematology, coagulation profile and serum chemistry) including a tumour marker (when present/measurable in the patient), electrocardiogram (ECG) and a contrast-enhanced CT. All contrast enhanced CT’s will be assessed by RECIST 1.1 criteria [23].
Angiography
Patients are admitted for 3 days (2 nights) starting the day prior to the angiography. After physical examination and relevant laboratory testing, patients are pre-hydrated to prevent kidney damage, and started on proton pump inhibitors for 6 weeks (pantoprazole once a day 40 mg). Premedication 1 h prior to the angiography consists of one dose of corticosteroids, antihistamines and anti-emetics (respectively dexamethasone 10 mg, clemastine 1 mg and ondansetron 8 mg), at the same time a tranquilizer is offered to the patient (oxazepam 10 mg). If the patient is familiar with a mild to moderate contrast allergy, additional corticosteroids and antihistamines will be given prior to the angiography according with national guidelines of the Central Accompaniment Institution (CBO in Dutch) [24].
A skilled and trained interventional radiologist will perform all angiographies of the upper abdominal vessels. A catheter is introduced via one of the femoral arteries by the Seldinger technique. After identifying all arteries supplying the liver, additional branches of these arteries that supply other organs than the liver are coiled, if needed. This usually involves the gastroduodenal artery (GDA) and right gastric artery (RGA).
Scout dose
Once successful identification of the supplying arteries and occlusion of additional branches has been performed, a scout dose of 250 MBq 166Ho-microspheres will be administered [20, 25]. Due to the photon emission of 166Ho, distribution of the microspheres, lung shunting and extrahepatic depositions can all be assessed using SPECT/CT. Planar imaging and SPECT/CT will be performed following the angiography and evaluated qualitatively as well as quantitatively. Extrahepatic deposition of activity is a contra-indication for treatment. Lung shunting will be assessed by planar imaging and SPECT/CT and should not exceed the maximum tolerable lung absorbed dose (i.e. 30 Gy).
Treatment
If the pre-treatment assessment is successful, patients return to the angiography suite for the treatment angiography combined with the 166Ho-RE. This will take place on the same day as the pre-treatment angiography and scout dose procedure (see Fig. 3). Based on the results of the dose escalation study (i.e. HEPAR I trial), a whole liver absorbed dose of 60 Gy was determined to be safe. A whole liver absorbed dose of 60 Gy leads to the following equation for activity calculation:
Fig. 3.
Study protocol depicting the time line and study proceedings between inclusion and hospital discharge
As mentioned above, additional information derived from the scout dose SPECT/CT can change treatment planning, either performing a one session whole liver treatment or two session sequential whole liver treatment. A significant lung shunt dose (> 30 Gy) will lead to a reduction in treatment activity.
Radiation exposure rate
The radiation exposure rate of the patient will be measured from 1-m distance at t = 0 h and t = 24 h after the 166Ho-RE.
Follow-up
Follow-up visits
During 12 months after treatment, patients are followed at the outpatient clinic. The visits will take place after 3 weeks, 6 weeks, 3 months, 6 months, 9 months and 12 months (closing visit). During these visits patients will undergo a physical examination, laboratory testing, WHO performance status assessment and will be monitored for (serious) adverse (device) events. Prior to the 3 weeks, 6 weeks and 3 months visits, patients are asked to fill out the EORTC questionnaires (QLQ-C30 and QLQ-GI.NET21). Prior to the 3, 6, 9 and 12 months visits, a CT will be performed for response assessment according to the RECIST 1.1 criteria.
Primary objectives
Two distinct objectives are the focus of our study. Tumour response on CT at 3 months of follow-up will be the first primary objective. This is defined as complete response (CR = disappearance of all lesions) or partial response (PR = ≥30% decrease in the sum of the longest diameters of the target lesions, compared to baseline measurements). The second primary objective is to establish the safety and toxicity profile of treatment with 166Ho-RE as an additional treatment after 177Lu-DOTATATE, using the Common Terminology Criteria for Adverse Events (CTCAE version 4.03) [26].
Secondary objectives
Three secondary objectives have been defined. Anti-tumour effect will be assessed by relevant tumour markers (when available), expressed as a percentage of the pre-treatment values. Furthermore, Quality of Life (QoL) will be assessed using the EORTC questionnaires (QLQ-C30 and QLQ-GI.NET21) during the first 3 months after treatment. The impact of treatment on QoL will be compared to tumour response and other parameters.
Additionally, biodistribution and dosimetry will be evaluated using a dual isotope fusion SPECT/CT protocol. After the standard scout dose SPECT/CT and treatment dose SPECT/CT (i.e. 166Ho-SPECT), 50 MBq of 99mTc-phytacis (CIS bio, France) will be administered. Subsequently a dual-isotope SPECT/CT will be acquired, simultaneously providing a 166Ho-SPECT for assessment of microsphere distribution and a 99mTc-phytacis SPECT for the assessment of truly functional liver parenchyma.
Safety profile
The phase 1 study on 166Ho-RE (HEPAR I trial) [20] and its subsequent phase 2 study (HEPAR 2 trial) [27], demonstrated similar treatment-related effects as the current commercially available 90Y-microspheres. Common adverse events up to grade 1 or 2 of the CTCAE v4.03 included: fever, nausea, vomiting, abdominal discomfort, and fatigue, often called the post-embolization syndrome. These complaints were generally self-limiting within 4–6 weeks. More serious adverse events of RE in general were rare (< 1%) and included RE-induced liver disease (REILD) [28] and inadvertent extrahepatic distribution of activity [29].
Escape medication
The protocol ensures all patients are pre- and post-hydrated in order to minimize the chance of renal insufficiency caused by the vascular contrast agent, jodixanol (Visipaque®). After 166Ho-RE standard escape medication includes paracetamol up to 4000 mg / day and ondansetron up to 24 mg, as respectively oral analgesic and intravenous anti-emetic. If persisting nausea occurs, additional metoclopramide up to 120 mg / day will be used. In case of diarrhoea, patients will receive loperamide up to 16 mg / day. In this specific patient group, some patients might experience excessive release of NET-related hormones that could cause a ‘carcinoid syndrome’ or ‘carcinoid crisis’. These complaints can be prevented (to some extent) with octreotide intravenously, steroids and hydration.
Withdrawal of individual patients
Patients may be withdrawn from the study if a serious adverse event occurs.
Patients will be withdrawn from the study if 1) the investigator considers it in the best interest of the patient that he/she be withdrawn (e.g. progressive disease), 2) the patient withdraws consent or 3) the patient is unable to comply with the protocol procedures.
Discussion
Liver metastases significantly limit patient survival (Table 1). In the current study, the beneficial effect of additional 166Ho-RE within 12 weeks after systemic 177Lu-DOTATATE will be investigated. Combining these treatments may lead to an improved response rate for liver metastases, with acceptable and suppressible side effects, which may eventually lead to prolonged survival. Although the latter question is not an objective of the current phase 2 study, if significant efficacy and limited toxicity are shown, a subsequent phase 3 study might be initiated.
To date, Ezziddin et al. published the only report describing RE with 90Y-microspheres after PRRT with 177Lu-DOTATATE in a retrospective study [30]. They described a population of 23 patients in which RE was performed in a salvage setting. Patients had progressive or functionally uncontrolled disease after PRRT. Three months after RE, 30% had PR and 61% had stable disease without any serious toxicity, comparable with other reports on 90Y-microspheres in NET patients. The authors concluded that salvage RE after PRRT shows a toxicity profile similar to RE alone, despite the high cumulative activity administrated. Less than 15% experienced a CTCAE grade 3 toxicity (abdominal pain, fatigue, fever, nausea and vomiting) and one patient developed a gastroduodenal ulcer. The interval between PRRT and RE was not mentioned, but patients were only referred for RE in case they had progressive disease (i.e. salvage setting). In their study, a cumulative liver dose of 2–12 Gy was described after PRRT. Due to the hypervascular nature of NET, the absorbed dose on healthy liver parenchyma due to RE is (far) below the presumed toxicity limit of healthy liver tissue (i.e. 70 Gy; and 50 Gy in cirrhotic livers with 90Y resin microspheres) [31]. Thus, in theory, combining RE and PRRT can be safe. Nonetheless, concerns arise when implementing RE shortly after PRRT, due to the cumulative radiation dose and the short interval, potentially provoking REILD [28]. On the other hand in a recent case report, Filippi et al. described their treatment combination a patient with one hepatic metastasis, mesenteric metastasis and several bone metastases, diagnosed on a 68Ga-DOTATATE-PET/CT [32]. The hepatic metastasis was downstaged with a lobar RE procedure, followed by 4 cycles of PRRT to treat extrahepatic lesions (a mesenteric metastasis and several bone metastases). Restaging after 3 months with a 68Ga-DOTATATE-PET/CT showed a nearly complete remission of the extrahepatic metastases and an incomplete remission of the hepatic metastasis, thus another additional lobar RE procedure was performed to treat the hepatic lesion, with success [32]. They reported no significant adverse events of the combined treatments, complete symptomatic control and a survival of 42 months [32]. Additionally, they reported an absorbed dose on healthy liver parenchyma after the RE procedures of just 18 Gy and 20 Gy [32]. An example that the combination of RE and PRRT with short intervals can be safe.
In contrast to other studies, 99mTc-macroaggregated albumin (99mTc-MAA) will not be used as a scout dose for treatment planning. Instead, a small number of 166Ho-microspheres will be used as a scout dose (approximately 250 MBq). This may overcome known limitations of 99mTc-MAA: 1) differences in flow dynamics caused by the randomly shaped 99mTc-MAA particles (90% between 10 and 90 μm) versus the spherically shaped 166Ho-microspheres (30 ± 5 μm), 2) differences in scanning protocols of pre- and post-procedural imaging (i.e. 99mTc-SPECT vs 90Y-PET versus 166Ho SPECT for both procedures), 3) employing a similar injection technique during both angiographies (i.e. bolus 99mTc-MAA versus intermittent injection of 166Ho microspheres), and 4) overestimation of the lung shunt using 99mTc-MAA.
As shown by Elschot et al., in patients treated with 166Ho-RE, using 99mTc-MAA as well as 166Ho-microspheres as pre-treatment imaging scout dose, 166Ho-SPECT/CT was the most accurate in predicting the lung absorbed dose after 166Ho-RE [33]. On 166Ho-SPECT/CT a median lung shunt dose of 0.02 Gy was calculated. This was significantly overestimated in lung shunt dose calculations based on 99mTc-MAA planar scintigraphy (5.5 Gy), 166Ho planar scintigraphy (10.4 Gy) and 99mTc-MAA SPECT/CT (2.5 Gy). An example of severe overestimation by 99mTc-MAA compared to 166Ho-microsperes is shown in Fig. 4. In the present study biodistribution / extrahepatic deposition assessment and lung shunt dose calculation will solely be evaluated by 166Ho-SPECT/CT.
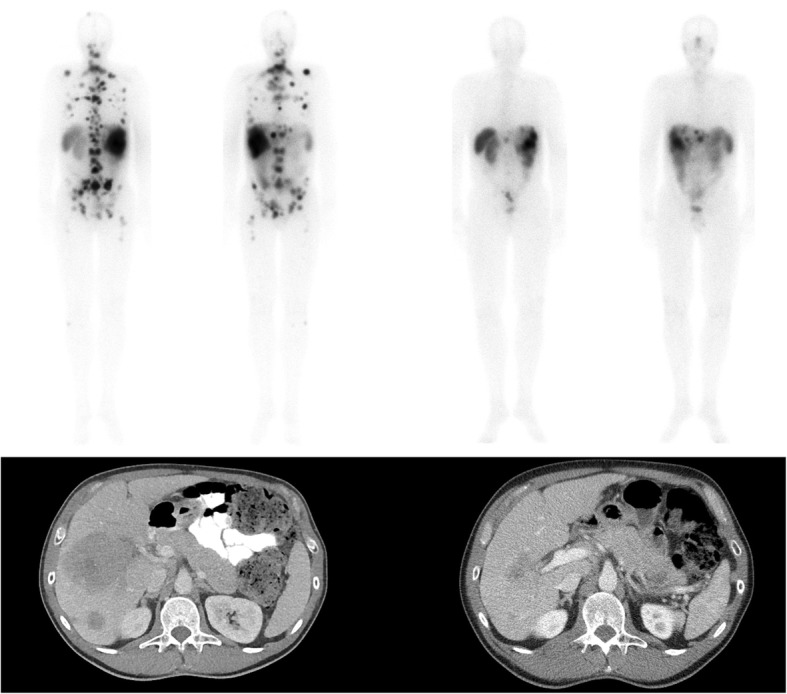
Fig. 4.
Example of lung shunt fraction overestimation by 99mTc-MAA. A patient with multiple liver metastases of a cholangiocarcinoma treated in the prior HEPAR 2 trial. Note the (visual) significant overestimation of 99mTc-MAA on planar imaging compared to the 166Ho-scout dose and 166Ho-treatment dose. Quantification of the lung shunt fraction on planar and SPECT/CT imaging confirmed the visual assessment: a 99mTc-planar = 13.4%, d 99mTc-SPECT = 6%, all 166Ho imaging modalities (b, c, e and f) with the scout dose and with the treatment dose showed a lung shunt fraction of < 1%
The safety of administrating 250 MBq of beta-emitting 166Ho-microspheres as a scout dose has been studied by Prince et al. [25] They predicted the amount of extrahepatic activity and radiation absorbed dose, using 99mTc-MAA SPECT/CT of 160 patients prior to 90Y-RE. Based on a prior study by Kao et al., they defined a dose exceeding 49 Gy as clinically significant [25, 34, 35]. Simulating the use of 250 MBq 166Ho-microspheres as a scout dose, only 1.3% of the patients had an extrahepatic deposition that could potentially be harmful (i.e. exceeded a mean dose of 49 Gy) [25]. Additionally, C-arm CT’s will be acquired at each injection position, prior to scout dose administration, to minimize the chance of extrahepatic depositions and partial tumour coverage, and to avoid extra angiography procedures [36, 37].
As mentioned in the ‘secondary objectives’ section, the application of the dual isotope SPECT/CT protocol will enable us to derive all relevant dosimetric parameters for treatment dose calculation. 99mTc-phytacis, like other radiocolloids, is extracted from the blood pool by the reticuloendothelial cells of the liver [38]. Solely the functional liver parenchyma is depicted, due to absence of reticuloendothelial cells in tumours. Validation of this dual-isotope protocol will be performed in a side-study. Previous studies by Lam et al. have shown the prognostic value of the combination of 99mTc-MAA and 99mTc-sulphur colloid imaging, showing a tumour dose-response correlation and healthy liver dose-toxicity correlation [39, 40].
As an additional advantage, holmium is one of the 14 lanthanide elements, making 166Ho-microspheres MRI-compatible for treatment imaging. In short, using estimated R2* value changes from multi-gradient echo data, the holmium concentration per voxel could be determined [41, 42]. Conversion into units of activity enables dosimetric calculations. A prior study by Smits et al. has shown its feasibility in clinical practice and its comparability to SPECT-based dosimetry [21]. Using a similar MR-sequence, real-time imaging of the 166Ho-microspheres during administration may become a future application [43]. However, the use of MRI is beyond the scope of this study, to minimize the study impact for patients.
In contrast to RE, PRRT’s main limitation is absorbed kidney dose. To reduce the dose, the most important preparatory measure is intravenous amino acid infusion prior, during and after 177Lu-DOTATATE administration. To overcome the disadvantage of the unavoidable kidney dose, intra-arterial administration of 177Lu-DOTATATE in patients with liver only disease could be a future application [44]. Several studies show a decreased absorbed kidney dose and an increased uptake in the liver metastases in most patients after intra-arterial administration. In a study by Pool et al., kidney absorbed dose decreased by 13% in addition to a 2.9-fold increase in liver metastases uptake [45]. In theory, a combination of intra-arterial PRRT and RE could be superior in patients with liver only disease.
Limitations of the study protocol are the small study cohort, non-comparative design, single center design and relatively short clinical follow-up for NET patients.
In conclusion, combining PRRT and RE could lead to improved treatment response and additional survival benefit: PRRT can be used to treat intra- and extrahepatic disease, whereas RE leads to an additional radiation boost on intrahepatic disease, the most incriminating factor in NET-patients’ survival. Based on this hypothesis, the HEPAR PLUS trial will include all patients treated with 177Lu-DOTATATE with significant intrahepatic disease.
Recruitment status
Ongoing: Currently recruiting patients.
Acknowledgements
The authors thank Tjitske Bosma (clinical research coordinator, University Medical Centre Utrecht) for her contribution to the study design and coordination, Remmert de Roos for his assistance in the production of the microspheres.
Funding
This phase 2 study is funded by the Department of Radiology and Nuclear Medicine of the University Medical Center Utrecht. No external funding received.
Abbreviations
- 166Ho
166Holmium isotope
- 177Lu
177Lutetium isotope
- 90Y
90Yttrium isotope
- 99mTc-MAA
99mTechnetium-macroalbumin aggregates
- CR
complete response
- CTCAE
Common Terminology Criteria for Adverse Events
- ECG
Electrocardiogram
- ENETS
European Neuroendocrine Tumour Society
- EORTC
European Organisation for Research and Treatment of Cancer
- GBq
Gigabecquerel
- Gy
Gray
- MBq
Megabecquerel
- NET
Neuroendocrine tumour
- PR
Partial response
- PRRT
Peptide receptor radionuclide therapy
- QoL
Quality of Life
- RE
Radioembolization
- RECIST
Response Evaluation Criteria In Solid Tumours
- SPECT/CT
Single photon emission computed tomography + computed tomography
- WHO
World Health Organization
Authors’ contributions
AJATB was involved in study design, writing of the manuscript, literature search, data gathering, clinical patient care and clinical image reconstruction. DJK was involved in study design and clinical patient care. BLRK was involved in study design and clinical patient care. JJMT was involved in study design and clinical patient care. WWH was involved in clinical patient care. KMAD was involved in and clinical patient care. RR was involved in clinical image reconstruction. GCK was involved in study design and in-house manufacturing of Ho-166-microspheres. HWAM was involved in study design and clinical image reconstruction. MAAJB involved in clinical patient care. MGEHL (principal investigator) was involved in study design and clinical patient care. All authors read, reviewed and approved the final manuscript.
Authors’ information
A.J.A.T. Braat, M.D., corresponding author and study physician. Department of Radiology and Nuclear Medicine, University Medical Centre Utrecht, Heidelberglaan 100, Huispostnummer E01.132, P.O. Box 85,500, 3584 CX Utrecht, the Netherlands.
Ethics approval and consent to participate
Phase 2 study was approved by the Medical Ethical Committee of the University Medical Centre Utrecht. Informed consent will be obtained of all participating patients.
Competing interests
The department of Radiology and Nuclear Medicine of the University Medical Centre Utrecht owns royalties for 166Ho-microspheres. MGEHL has acted as a consultant for BTG, Sirtex, Mirada and Bayer Healthcare. All other authors declared no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arthur J. A. T. Braat, Email: a.j.a.t.braat@umcutrecht.nl
Dik J. Kwekkeboom, Email: d.j.kwekkeboom@erasmusmc.nl
Boen L. R. Kam, Email: b.kam@erasmusmc.nl
Jaap J. M. Teunissen, Email: j.teunissen@erasmusmc.nl
Wouter W. de Herder, Email: w.w.deherder@erasmusmc.nl
Koen M. A. Dreijerink, Email: k.dreijerink@vumc.nl
Rob van Rooij, Email: r.vanrooij-3@umcutrecht.nl.
Gerard C. Krijger, Email: g.c.krijger@umcutrecht.nl
Hugo W. A. M. de Jong, Email: h.w.a.m.dejong@umcutrecht.nl
Maurice A. A. J. van den Bosch, Email: mbosch@umcutrecht.nl
Marnix G. E. H. Lam, Email: m.lam@umcutrecht.nl
References
- 1.Bosman FT, Carneiro F. World Health Organization classification of Tumours, pathology and genetics of Tumours of the digestive system. 4. Lyon: IARC Press; 2010. [Google Scholar]
- 2.Capelli P, Fassan M, Scarpa A. Pathology - grading and staging of GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26(6):705–717. doi: 10.1016/j.bpg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26(18):3063–3072. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 4.Pavel MBE, Couvelard A, et al. ENETS consensus guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinol. 2012;95(2):157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 5.Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best Pract Res Clin Gastroenterol. 2012;26(6):691–703. doi: 10.1016/j.bpg.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Yao JC, Lombard-Bohas C, Baudin E, Kvols LK, Rougier P, Ruszniewski P, Hoosen S, St Peter J, Haas T, Lebwohl D, Van Cutsem E, Kulke MH, Hobday TJ, O'Dorisio TM, Shah MH, Cadiot G, Luppi G, Posey JA, Wiedenmann B. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28(1):69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quintela-Fandino M, Krzyzanowska M, Duncan G, Young A, Moore MJ, Chen EX, Stathis A, Colomer R, Petronis J, Grewal M, Webster S, Wang L, Siu LL. In vivo RAF signal transduction as a potential biomarker for sorafenib efficacy in patients with neuroendocrine tumours. Br J Cancer. 2013;108(6):1298–1305. doi: 10.1038/bjc.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faiss S, Pape UF, Böhmig M, Dörffel Y, Mansmann U, Golder W, Riecken EO, Wiedenmann B, International Lanreotide and Interferon Alfa Study Group Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the international Lanreotide and interferon alfa study group. J Clin Oncol. 2003;21(14):2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 9.Pavel ME, Hainsworth JD, Baudin E, Peeters M, Hörsch D, Winkler RE, Klimovsky J, Lebwohl D, Jehl V, Wolin EM, Oberg K, Van Cutsem E, Yao JC. RADIANT-2 study group: Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 10.Rinke A, Müller HH, Schade-Brittinger C, Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker M, Harder J, Arnold C, Gress T, Arnold R, PROMID Study Group Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID study group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 11.Kvols LK, Oberg KE, O'Dorisio TM, Mohideen P, de Herder WW, Arnold R, Hu K, Zhang Y, Hughes G, Anthony L, Wiedenmann B. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: results from a phase II study. Endocr Relat Cancer. 2012;19(5):657–666. doi: 10.1530/ERC-11-0367. [DOI] [PubMed] [Google Scholar]
- 12.Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG, Eastern Cooperative Oncology Group Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: eastern cooperative oncology group study E1281. J Clin Oncol. 2005;23(22):4897–4904. doi: 10.1200/JCO.2005.03.616. [DOI] [PubMed] [Google Scholar]
- 13.Imhof A, Brunner P, Marincek N, Briel M, Schindler C, Rasch H, Mäcke HR, Rochlitz C, Müller-Brand J, Walter MA. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29(17):2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 14.Kwekkeboom DJ, de Herder WW, Kam BLR, van Eijck CH, van Essen M, Kooij PP, Feelders RA, van Aken MO, Krenning EP. Treatment with the radiolabeled somatostatin analog [177Lu-DOTA0,Tyr3]octreotate: toxicity, efficacy, and survival. J Clin Oncol. 2008;26(13):2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 15.Swärd C, Bernhardt P, Ahlman H, Wängberg B, Forssell-Aronsson E, Larsson M, Svensson J, Rossi-Norrlund R, Kölby L. [177Lu-DOTA0-Tyr3]-octreotate treatment in patients with disseminated gastroenteropancreatic neuroendocrine tumors: the value of measuring absorbed dose to the kidney. World J Surg. 2010;34(6):1368–1372. doi: 10.1007/s00268-009-0387-6. [DOI] [PubMed] [Google Scholar]
- 16.Sansovini M, Severi S, Ambrosetti A, Monti M, Nanni O, Sarnelli A, Bodei L, Garaboldi L, Bartolomei M, Paganelli G. Treatment with the radiolabelled somatostatin analog Lu-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinol. 2013;97(4):347–354. doi: 10.1159/000348394. [DOI] [PubMed] [Google Scholar]
- 17.Chinol M, Bodei L, Cremonesi M, Paganelli G. Receptor-mediated radiotherapy with 90Y-DOTA-DPhe1-Tyr3-octreotide: the experience of the European Institute of Oncology Group. Semin Nucl Med. 2002;32(2):141–147. doi: 10.1053/snuc.2002.31563. [DOI] [PubMed] [Google Scholar]
- 18.Devcic Z, Rosenberg J, Braat AJAT, Techasith T, Banerjee A, Sze DY, Lam MGEH. The efficacy of hepatic 90Y resin Radioembolization for metastatic neuroendocrine tumors: a meta-analysis. J Nucl Med. 2014;55(9):1404–1410. doi: 10.2967/jnumed.113.135855. [DOI] [PubMed] [Google Scholar]
- 19.Zielhuis SW, Nijsen JF, de Roos R, Krijger GC, van Rijk PP, Hennink WE, van het Schip AD. Production of GMP-grade radioactive holmium loaded poly(L-lactic acid) microspheres for clinical application. Int J Pharm. 2006;311(1–2):69–74. doi: 10.1016/j.ijpharm.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Smits MLJ, Nijsen JFW, van den Bosch MAAJ, Lam MGEH, Vente MA, Mali WP, van het Schip AD, Zonnenberg BA. Holmium-166 radioembolisation in patients with unresectable, chemorefractory liver metastases (HEPAR trial): a phase 1, dose-escalation study. Lancet Oncol. 2012;13(10):1025–1034. doi: 10.1016/S1470-2045(12)70334-0. [DOI] [PubMed] [Google Scholar]
- 21.Smits MLJ, Elschot M, van den Bosch MAAJ, et al. In vivo dosimetry based on SPECT and MR imaging of 166Ho-microspheres for treatment of liver malignancies. J Nucl Med. 2013;54(12):2093–2100. doi: 10.2967/jnumed.113.119768. [DOI] [PubMed] [Google Scholar]
- 22.Elschot MSM, Nijsen JFW, Lam MGEH, Zonnenberg BA, van den Bosch MAAJ, et al. Quantitative Monte Carlo-based holmium-166 SPECT reconstruction. Med Phys. 2013;40:112502. doi: 10.1118/1.4823788. [DOI] [PubMed] [Google Scholar]
- 23.Response Evaluation Criteria In Solid Tumors [www.recist.com]. Accessed Dec 2016.
- 24.Central Accompaniment Institution; directive precautions with iodinated contrast / Centraal BegeleidingsOrgaan; Richtlijn Voorzorgsmaatregelen bij jodiumhoudende contrastmiddelen [http://www.nvpc.nl/uploads/stand/2617_12_07_Voorzorgsmaatregelen.pdf]. Accessed Dec 2016.
- 25.Prince JF, van Rooij R, Bol GH, de Jong HWAM, van den Bosch MAAJ, Lam MGEH. Safety of a scout dose preceding hepatic radioembolization with holmium-166 microspheres. J Nucl Med. 2015;56(6):817–823. doi: 10.2967/jnumed.115.155564. [DOI] [PubMed] [Google Scholar]
- 26.Common Terminology Criteria for Adverse Events version 4.03 (CTCAE v4.03) [http://www.hrc.govt.nz/sites/default/files/CTCAE%20manual%20-%20DMCC.pdf]. Accessed Dec 2016.
- 27.Prince JF, van den Bosch MAAJ, Nijsen JFW, Smits MLJ, van den Hoven AF, Nikolakopoulos S, Wessels FJ, Bruijnen RCG, Braat MNGJA, Zonnenberg BA, Lam MGEH. Efficacy of radioembolization with holmium-166 microspheres in salvage patients with liver metastases: a phase 2 study. J Nucl Med. 2018;59(4):582–8. [DOI] [PubMed]
- 28.Sangro B, Gil-Alzugaray B, Rodriguez J, Sola I, Martinez-Cuesta A, Viudez A, Chopitea A, Iñarrairaegui M, Arbizu J, Bilbao JI. Liver disease induced by radioembolization of liver tumors: description and possible risk factors. Cancer. 2008;112(7):1538–1546. doi: 10.1002/cncr.23339. [DOI] [PubMed] [Google Scholar]
- 29.Lam MGEH, Banerjee S, Louie JD, Abdelmaksoud MH, Iagaru AH, Ennen RE, Sze DY. Root cause analysis of gastroduodenal ulceration after yttrium-90 radioembolization. Cardiovasc Intervent Radiol. 2013;36(6):1536–1547. doi: 10.1007/s00270-013-0579-1. [DOI] [PubMed] [Google Scholar]
- 30.Ezziddin S, Meyer C, Kahancova S, Haslerud T, Willinek W, Wilhelm K, Biersack HJ, Ahmadzadehfar H. 90Y Radioembolization after radiation exposure from peptide receptor radionuclide therapy. J Nucl Med. 2012;53(11):1663–1669. doi: 10.2967/jnumed.112.107482. [DOI] [PubMed] [Google Scholar]
- 31.SIRTeX Medical . Package insert. 2013. [Google Scholar]
- 32.Filippi L, Ciorra A, Sardella B, Schillaci O, Bagni O. Sequential use of (90)Y microspheres Radioembolization and (177)Lu-Dotatate in Pluri-metastatic neuroendocrine tumors: a case report. Nucl Med Mol Imaging. 2014;48(4):321–325. doi: 10.1007/s13139-014-0292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elschot M, Nijsen JFW, Lam MEGH, Smits MLJ, Prince JF, Viergever MA, van den Bosch MAAJ, Zonnenberg BA, de Jong HWAM. 99mTc-MAA overestimates the absorbed dose to the lungs in radioembolization: a quantitative evaluation in patients treated with 166Ho-microspheres. Eur J Nucl Med Mol Imaging. 2014;41(10):1965–1975. doi: 10.1007/s00259-014-2784-9. [DOI] [PubMed] [Google Scholar]
- 34.Kao YH, Steinberg JD, Tay YS, et al. Post-radioembolization yttrium-90 PET/CT - part 1: diagnostic reporting. EJNMMI Res. 2013;3(1):56. doi: 10.1186/2191-219X-3-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kao YH, Steinberg JD, Tay YS, et al. Post-radioembolization yttrium-90 PET/CT - part 2: dose-response and tumor predictive dosimetry for resin microspheres. EJNMMI Res. 2013;3(1):57. doi: 10.1186/2191-219X-3-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Hoven AFPJ, de Keizer B, Vonken EJPA, Bruijnen RCG, Verkooijen HM, et al. Use of C-arm cone beam CT during hepatic Radioembolization: protocol optimization for extrahepatic shunting and parenchymal enhancement. Cardiovasc Intervent Radiol. 2015;39(1):64–73. doi: 10.1007/s00270-015-1146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Louie JDKN, Kuo WT, Hwang GL, Hofmann LV, Goris ML, et al. Incorporating cone-beam CT into the treatment planning for Yttrium-90 Radioembolization. J Vasc Interv Radiol. 2009;20:606–613. doi: 10.1016/j.jvir.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 38.Krishnamurthy GT, Krishnamurthy S. Nuclear hepatology: a textbook of hepatobiliary diseases. 2. New York: Springer; 2000. [Google Scholar]
- 39.Lam MGEH, Goris ML, Iagaru AH, Mittra ES, Louie JD, Sze DY. Prognostic utility of 90Y Radioembolization dosimetry based on fusion 99mTc-macroaggregated albumin–99mTc-sulfur colloid SPECT. J Nucl Med. 2013;54(12):2055–2061. doi: 10.2967/jnumed.113.123257. [DOI] [PubMed] [Google Scholar]
- 40.Lam MGEH, Banerjee A, Goris ML, Iagaru AH, Mittra ES, Louie JD, Sze DY. Fusion dual-tracer SPECT-based hepatic dosimetry predicts outcome after radioembolization for a wide range of tumour cell types. Eur J Nucl Med Mol Imaging. 2015;42(8):1192–1201. doi: 10.1007/s00259-015-3048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van de Maat GH, Seevinck PR, Elschot M, et al. MRI-based biodistribution assessment of holmium-166 poly(L-lactic acid) microspheres after radioembolisation. Eur Radiol. 2013;23(3):827–835. doi: 10.1007/s00330-012-2648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seevinck PR, Seppenwoolde JH, de Wit TC, Nijsen JF, Beekman FJ, van Het Schip AD, Bakker CJ. Factors affecting the sensitivity and detection limits of MRI, CT, and SPECT for multimodal diagnostic and therapeutic agents. Anti Cancer Agents Med Chem. 2007;7(3):317–334. doi: 10.2174/187152007780618153. [DOI] [PubMed] [Google Scholar]
- 43.Smits MLJ, Elschot M, Sze DY, Kao YH, Nijsen JF, Iagaru AH, de Jong HWAM, van den Bosch MAAJ, Lam MGEH. Radioembolization dosimetry: the road ahead. Cardiovasc Intervent Radiol. 2015;38(2):261–269. doi: 10.1007/s00270-014-1042-7. [DOI] [PubMed] [Google Scholar]
- 44.Bergsma H, van Vliet EI, Teunissen JJ, Kam BL, de Herder WW, Peeters RP, Krenning EP, Kwekkeboom DJ. Peptide receptor radionuclide therapy (PRRT) for GEP-NETs. Best Pract Res Clin Gastroenterol. 2012;26(6):867–881. doi: 10.1016/j.bpg.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 45.Pool SE, Kam BL, Koning GA, Konijnenberg M, Ten Hagen TL, Breeman WA, Krenning EP, de Jong M, van Eijck CH. [(111)in-DTPA]octreotide tumor uptake in GEPNET liver metastases after intra-arterial administration: an overview of preclinical and clinical observations and implications for tumor radiation dose after peptide radionuclide therapy. Cancer Biother Radiopharm. 2014;29(4):179–187. doi: 10.1089/cbr.2013.1552. [DOI] [PubMed] [Google Scholar]
- 46.Chamberlain RS, Canes D, Brown KT, Saltz L, Jarnagin W, Fong Y, Blumgart LH. Hepatic neuroendocrine metastases: does intervention Alter outcomes? J Am Coll Surg. 2000;190(4):432–445. doi: 10.1016/S1072-7515(00)00222-2. [DOI] [PubMed] [Google Scholar]
- 47.Yao KA, Talamonti MS, Nemcek A, Angelos P, Chrisman H, Skarda J, Benson AB, Rao S, Joehl RJ. Indications and results of liver resection and hepatic chemoembolization for metastatic gastrointestinal neuroendocrine tumors. Surgery. 2001;130(4):677–682. doi: 10.1067/msy.2001.117377. [DOI] [PubMed] [Google Scholar]
- 48.Gupta S, Johnson MM, Murthy R, Ahrar K, Wallace MJ, Madoff DC, McRae SE, Hicks ME, Rao S, Vauthey JN, Ajani JA, Yao JC. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors variables affecting response rates and survival. Cancer. 2005;104(8):1590–1602. doi: 10.1002/cncr.21389. [DOI] [PubMed] [Google Scholar]