Abstract
Background
Bicuspid aortic valve (BAV) is associated with asymmetric dilatation of the proximal ascending aorta. We previously demonstrated increased susceptibility of smooth muscle cells to oxidative stress in the BAV-aneurysmal aorta and hypothesized that antioxidant expression is regionally defined and influenced by the BAV morphotype.
Methods
BAV valve morphology was defined according to number of raphes: type 0 (0 raphes), type 1 (1 raphe), or type 2 (2 raphes) and by the raphe location among the left (L), right (R) or non (N) coronary cusps. Ascending aortic specimens were partitioned into three regions corresponding to the sinuses of Valsalva, denoted R, N (greater curve), and L (lesser curve). Transcripts 1, 2, and 3 from the gene expressing superoxide dismutase (Sod) were quantified in all three regions. Results were compared with aneurysmal and nonaneurysmal aortic specimens from patients with a tricuspid aortic valve.
Results
Region-specific Sod1 upregulation and Sod2 downregulation were dependent on the BAV morphotype. Sod3 was uniformly downregulated in all regions in a morphotype-independent manner. Sod1 upregulation was noted in the R region of the nonaneurysmal type 1 L/R morphotype. Aortic valve regurgitation, but not stenosis, affected the expression of Sod isoforms in specimens of degenerative aneurysms.
Conclusions
Region-specific transcription profiles of Sod on the basis of BAV morphotype deepen our understanding of its associated aortopathy and provide biological insight on the asymmetric dilatation pattern. This work indicates regional differences exist in the oxidative stress biology of the proximal aortic wall, and this may lead to newer diagnostic techniques to adjudicate aortic catastrophe risk.
Bicuspid aortic valve (BAV) remains the most common congenital heart malformation [1] and conveys a predisposition to proximal ascending thoracic aortopathy and dilatation. Ascending thoracic aortic aneurysm (TAA) formation imparts an increased risk of rupture or aortic dissection, or both. Currently, the only treatment to prevent such aortic catastrophe is open elective replacement of the ascending aorta, and contemporary opinion of surgical experts dictates intervention when the maximal orthogonal aortic diameter reaches 55 mm [2]. The frequency (w50%) of patients with aortic dissection but an aortic diameter significantly less than 55 mm [3] underscores the current deficits in TAA risk assessment based on orthogonal diameter.
The aortopathy associated with BAV is phenotypically [4] and etiologically [5] diverse, as is the morphology of the BAV itself. The classification and prevalence of each BAV morphotype has been well described by Sievers and Schmidtke [6] according to the number and location of the raphe(s). BAV morphotypes are categorized as type 0 (0 raphe), type 1 (1 raphe), and type 2 (2 raphes). Subcategorization of type 1 BAVs refers to the location of the raphe between the left and right coronary cusp (L/R), the left and noncoronary cusp (L/N), and the right and noncoronary cusp (R/N). Despite this variability in BAV morphology, patients uniformly exhibit increased aortic diameters [7, 8] and abnormal elasticity [9], and thus aortopathy, compared with controls with tricuspid aortic valve (TAV) matched for age and sex.
BAV patients also incur an unspecified increased risk of aortic dissection or rupture, or both [10], which persists despite surgical correction of the BAV by valve repair or replacement [11]. A recent study by Sievers and colleagues [12] revealed weak and nonexclusive associations between type 0, type 1 L/R, type 1 R/N, and aortic root dilation and between type 1 L/R and aortic root and ascending aortic dilatation for regurgitant BAVs [12].
Considering the tendency for the asymmetric dilation of the proximal ascending aorta in BAV patients, we hypothesized that oxidative stress defense in the ascending aorta of BAV patients is spatially dependent and predicated by BAV morphotype. We evaluated the gene expression of three isoforms of superoxide dismutases (Sods) in three different regions of the proximal ascending aorta on the basis of their proximity to the aortic valve cusps. In this study, we quantified the expression profile of Sods in different circumferential regions of the proximal ascending aorta and identified differences among valve morphotypes.
Patients and Methods
Patients
Ascending aortic tissue from 157 patients was obtained during elective aortic valve/ascending aortic replacement and during heart transplants at the University of Pittsburgh Medical Center with approval of the Institutional Review Board and informed patient consent. Aortic valve morphology and BAV morphotype were determined by intraoperative inspection. Nonaneurysmal aortic specimens collected from patients with TAV morphology served as controls. For a subset (n = 74) of all samples, specimens of the proximal ascending aorta were harvested distally to the sinotubular junction and partitioned into three circumferential regions, corresponding to the sinuses of Valsalva, denoted as right (R), non (N) (which together approximate the greater curve), and left (L) (approximating the lesser curve). Ascending aortic specimens were collected from 12 nonaneurysmal BAV patients (<42 mm), and 32 aneurysmal patients (≥42 mm) and also from 10 TAV patients with normal-caliber aortas and 20 with degenerative aneurysms (TAV-TAA; Table 1). The adventitial and intimal layers of the tissue were removed, and the medial layer of each region was preserved in RNAlater solution (part #AM7021; Ambion, Foster City, CA) according to the manufacturer’s instructions.
Table 1.
Demographics of Patients Enrolled in the Study
| Valve Type | TAV | BAV | |||||
|---|---|---|---|---|---|---|---|
| BAV subtype | – | – | 0 | 1 | 1 | 1 | 2 |
| Type 1 BAV morphotype | – | – | – | L/R | L/R | R/N | – |
| Aneurysm status | NA | TAA | TAA | NA | TAA | TAA | TAA |
| N | 10 | 20 | 8 | 12 | 14 | 7 | 3 |
| Sex (M/F) | 4/5 | 14/6 | 5/3 | 8/4 | 10/4 | 3/4 | 2/1 |
| Age, yr | 56.5 (26.3) | 65.0 (11.5) | 59.5 (17.5) | 55.8 (9.5) | 54.1 (15.0) | 55.0 (16.0) | 39.0 |
| Mean (IQR) | |||||||
| Aortic dia., mm | Normal | 52.5 (1.3) | 47.6 (1.7) | Normal | 51.7 (0.6) | 46.0 (1.3) | 48.7 (2.4) |
| Mean (SEM) | |||||||
| Diabetes | 0 | 3 | 2 | 1 | 2 | 0 | 1 |
| HTN | 5 | 13 | 6 | 8 | 9 | 5 | 2 |
| Smoking | 5 | 9 | 3 | 9 | 6 | 4 | 0 |
| Statin use | 3 | 9 | 5 | 8 | 5 | 3 | 0 |
| ACE inhibitor use | 1 | 5 | 3 | 5 | 3 | 2 | 1 |
BAV = bicuspid aortic valve; Dia = diameter; F = female; HTN = hypertension; IQR = interquartile range; L = left; M = male; NA = non-aneurysmal; N = non; R = right; SEM = standard error of the mean; TAA = thoracic aortic aneurysm; TAV = tricuspid aortic valve.
The sex of one patient in the TAV-NA (organ donor) was not recorded.
Echocardiography Studies and Computed Tomography Angiography
Preoperative computed tomography angiography was performed to determine the patients’ maximal orthogonal diameter of the ascending aorta to assess their indication for aortic replacement due to aneurysm. Patients also underwent preoperative transesophageal echocardiography to assess the degree of aortic insufficiency or stenosis, or both.
RNA Isolation and Real-Time Polymerase Chain Reaction Analysis
Tissue preserved in RNAlater was homogenized using the gentleMACS Dissociator (Miltenyi Biotech, San Diego, CA). Isolation of RNA was performed using the RNeasy Fibrous Tissue Mini Kit (part #74704; Qiagen, Valencia, CA) and quantified using Qubit 2.0 (Invitrogen, Carlsbad, CA). Real-time quantitative polymerase chain reaction was completed using the TaqMan RNA-Ct One-Step Kit (#4392938, Invitrogen) and inventoried TaqMan assay primer/probe sets (Supplemental Table S1) on an AB Prism 7900HT thermocycler (Invitrogen). Data analysis was performed using Sequence Detection Systems 2.2.2 software (Invitrogen). Relative expression of target genes was calculated from cycle threshold (Ct) values for each target gene, and normalized to HPRT1 as the endogenous control gene using the 2−ΔCt method.
Statistical Analysis
Comparisons were performed using the Mann-Whitney U nonparametric test in SPSS software (Version 21 Armonk, NY). A p value of 0.05 or less was considered statistically significant.
Results
Valve Morphotypes
Aortic valve morphology and BAV morphotype were determined intraoperatively in a series of 157 patients with BAV presenting to the Center for Thoracic Aortic Disease at the University of Pittsburgh Medical Center for surgical management of BAV-related pathology. Type 1 L/R was the most prevalent morphotype, comprising 65% of the BAV patients considered in our series. Frequency of the other BAV morphotypes was as follows: type 0, 16%; type 1 R/N, 13%; type 2, 5%; and rarely, type 1 L/N, 1%. Patient demographics, degree of valvulopathy, and comorbidities are reported in Table 1. There was only one specimen available each from aneurysmal type 1 L/N and from nonaneurysmal type 1 R/N, type 1 L/N, and type 2. No specimens were available from non-aneurysmal patients exhibiting the type 0 morphotype.
Analysis of Aneurysmal Specimens
Sod1 expression was upregulated in specimens from patients with the type 1 L/R morphotype only in the L region (p = 0.05) and type 1 R/N morphotypes only in the N region (p = 0.04; Fig 1A and B). No change was found in specimens from type 0 (p > 0.1 for all regions) or type 2 for any region (p > 0.1 for all regions), and no morphotypes exhibited any changes in the R region of the proximal ascending aorta.
Fig 1.
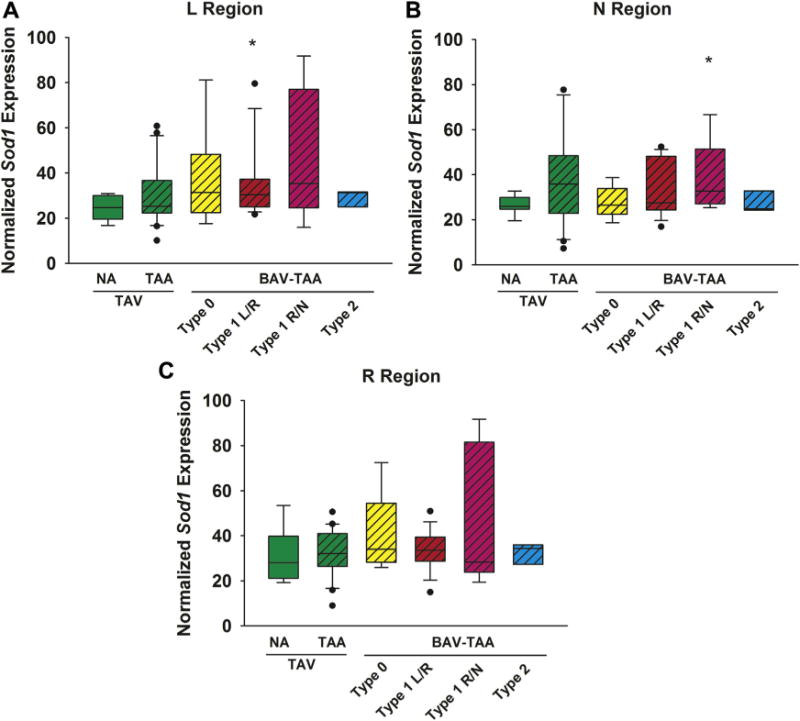
Expression of the superoxide dismutase isoform 1 (Sod1) gene in human ascending aorta in the (A) left (L), (B) non (N), and (C) right (R) regions (n = 76 patients). Box plots depict the median (middle line of the box), first quartile (lower line of the box) and third quartile (upper line of the box), with the whiskers representing 90th and 10th percentiles. The closed circles indicate outliers. *p < 0.05, significant from TAV-NA (normal). (BAV = bicuspid aortic valve; NA = nonaneurysmal; TAA = thoracic aortic aneurysm; TAV = tricuspid aortic valve.)
Although no change occurred in Sod1 expression in any region of specimens from degenerative aneurysms when all degrees of valvulopathy were considered together and compared with normal aortic specimens, some additional differences emerged in the setting of aortic regurgitation (AR). Patient specimens from regurgitant valves exhibited Sod1 upregulation in the R and N regions compared with specimens from patients with competent valves (R region: p = 0.02; N region: p = 0.003; Supplemental Table S2).
Sod2 expression was decreased in aneurysmal BAV patients compared with control specimens, with different expression profiles according to the BAV morphotype and the regions considered (Fig 2). Specifically, Sod2 was uniformly decreased in all three regions of aneurysmal specimens from BAV patients with the type 1 L/R morphotype (L region: p = 0.03; N region: p = 0.04; and R region: p = 0.03). However, a decrease in Sod2 expression was noted only in the L and R regions of specimens exhibiting type 0 morphotype (p = 0.03 and p = 0.01 respectively), whereas downregulation of Sod2 was localized solely to the R region of specimens (p = 0.04) from patients with type 1 R/N morphotype. The presence of AR was associated with less reduction in Sod2 expression only in the L region of aneurysmal specimens from patients exhibiting the type 1 L/R morphotype compared with specimens from patients with competent valves (p = 0.04; Supplemental Table S3).
Fig 2.
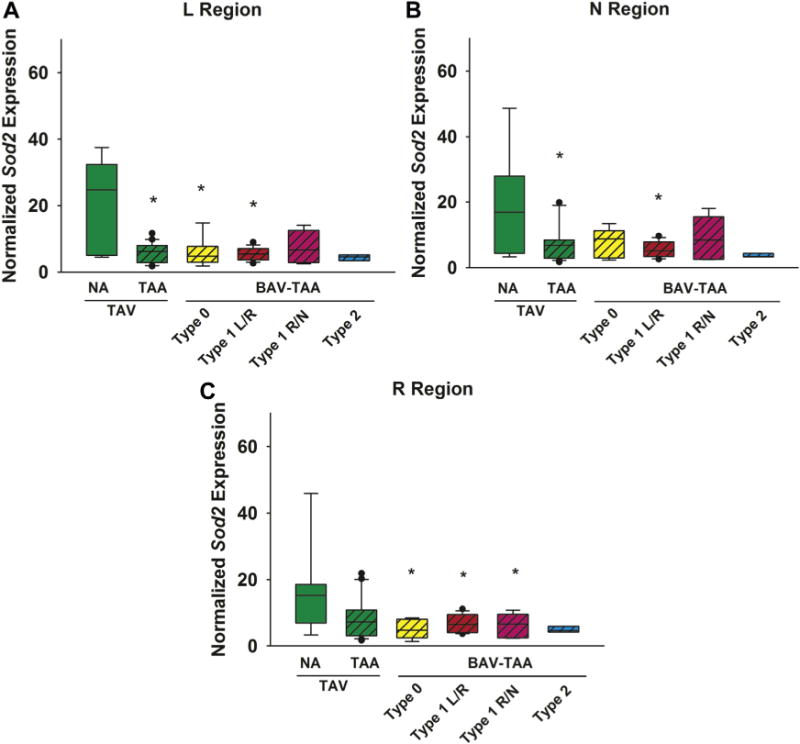
Expression of the superoxide dismutase isoform 2 (Sod2) gene in human ascending aorta in the (A) left (L) (B) non (N), and (C) right (R) regions (n = 76 patients). The box plots depict the median (middle line of the box), first quartile (lower line of the box) and third quartile (upper line of the box), with the whiskers representing the 90th and 10th percentiles. The closed circles indicate outliers. *p < 0.05 significant from TAV-NA (normal). (BAV = bicuspid aortic valve; NA = nonaneurysmal; TAA = thoracic aortic aneurysm; TAV = tricuspid aortic valve.)
Downregulation of Sod2 was noted in the L and N regions of degenerative aneurysm specimens when all degrees of valvulopathy were considered together and compared with control specimens (p = 0.01 and p = 0.05, respectively; Fig 2). However, AR may influence Sod2 gene expression in degenerative aneurysmal specimens. In the R region, Sod2 gene expression was down-regulated in patients with AR compared with patients with competent valves (p = 0.05; Supplemental Table S3).
Decreased expression of Sod3 was noted in the L, N and R regions for specimens from patients with type 0 (p = 0.001, p = 0.004, and p = 0.008, respectively), and type 1 L/R morphotypes (p < 0.001, p = 0.001, and p < 0.001, respectively; Fig 3). However, Sod3 expression was downregulated solely in the L region for specimens from type 1 R/N (p = 0.04) and type 2 (p = 0.02) morphotypes. Decreased expression of Sod3 was also noted in all regions for specimens of degenerative aneurysms (p < 0.001 for all regions).
Fig 3.
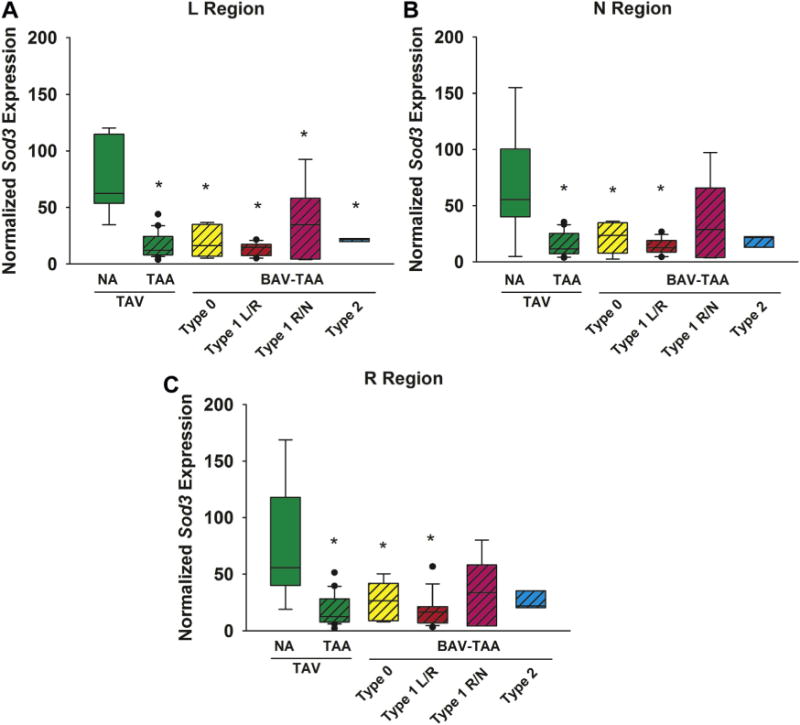
Expression of the superoxide dismutase isoform 3 (Sod3) gene in human ascending aorta in the (A) left (L), (B) non (N), and (C) right (R) regions (n = 76 patients). The box plots depict the median (middle line of the box), first quartile (lower line of the box) and third quartile (upper line of the box), with whiskers bars representing 90th and 10th percentiles. The closed circles indicate outliers. * p < 0.05, significant from TAV-NA (normal). (BAV = bicuspid aortic valve; NA = nonaneurysmal; TAA = thoracic aortic aneurysm; TAV = tricuspid aortic valve.)
Sod3 gene expression was habitually downregulated in all three regions of most pathologic aortic specimens. The presence of aortic regurgitation did not influence any of these above-described changes (Supplemental Table S4). Overall, Sod3 expression was mostly unchanged in each patient group when stenotic valves were directly compared with nonstenotic valves within each group (p > 0.06 for all; Supplemental Tables S5, S6, and S7).
Nonaneurysmal BAV (L/R) Findings and Comparison With BAV-TAA
Sod1 was upregulated in the R region (p = 0.02) of non-aneurysmal aortic specimens for patients with type 1 L/R morphotype compared with control specimens (Fig 4A). Sod1 was downregulated in the R region only of aneurysmal specimens from patients with the type 1 L/R morphotype compared with nonaneurysmal specimens (p = 0.03; Fig 4A). When nonaneurysmal specimens from patients were stratified based on the presence of aortic regurgitation, Sod1 expression was elevated in the L region in patients with AR compared with specimens from patients with competent valves (p = 0.01; Supplemental Table S2).
Fig 4.
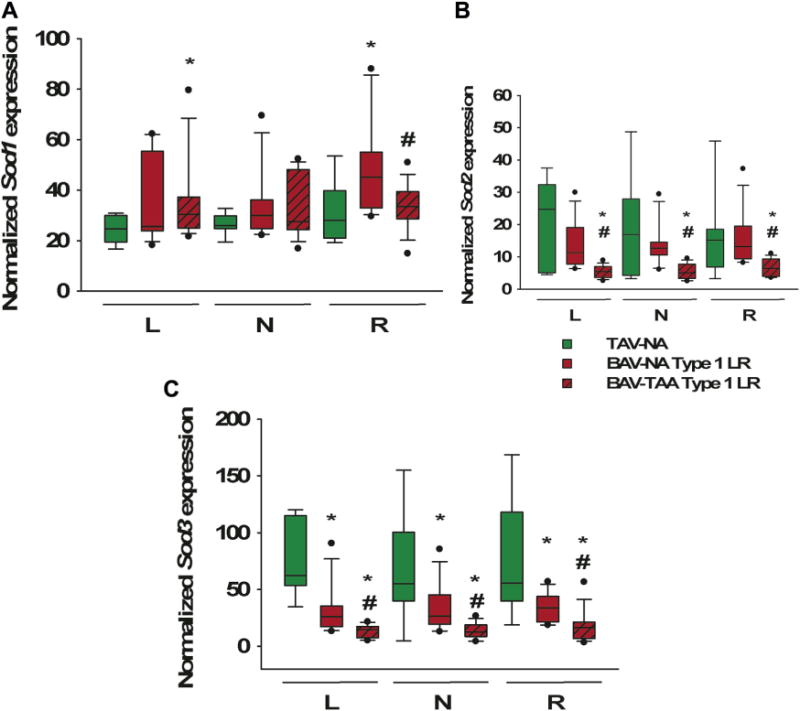
Expression of the superoxide dismutase (Sod) gene in bicuspid aortic valve (BAV) specimens by disease state. Total RNA from human ascending aorta was analyzed using real-time quantitative polymerase chain reaction. Results are shown for left (L), non (N), and right (R) regions each for (A) Sod1, (B) Sod2, and (C) Sod3. *p < 0.05, significant from TAV-NA (normal). (NA = nonaneurysmal; TAA = thoracic aortic aneurysm; TAV tricuspid = aortic valve.)
Sod2 expression was unchanged in nonaneurysmal specimens from patients with the type 1 L/R morphotype compared with control specimens (p > 0.46). However, aneurysmal specimens of patients with the type 1 L/R morphotype exhibited downregulation of Sod2 expression compared with nonaneurysmal specimens, regardless of regions (p < 0.001 for all regions; Fig 4B).
Downregulated Sod3 gene expression was detected in the L (p = 0.002), N (p = 0.02), and R (p = 0.02) regions of nonaneurysmal specimens from patients with the type 1 L/R morphotype compared with control specimens (Fig 4C). Interestingly, within the type 1 BAV L/R morphotype, aneurysmal patients showed further downregulation in Sod3 expression compared with non-aneurysmal BAV counterparts for all regions (p = 0.001 for all regions).
When the presence of AR was considered for the nonaneurysmal type 1 L/R specimens, there were no differences in Sod2 or Sod3 expression. Only one specimen in this subset was from a patient with a stenotic valve; therefore, given the low frequency of aortic stenosis, it cannot be completely excluded as a factor affecting Sod expression for type 1 L/R nonaneurysmal specimens.
Comment
The proximal ascending aorta tends to dilate asymmetrically in BAV patients, with more marked dilatation along the greater curve (N and R regions), and has an associated eccentric blood flow pattern, with regions of nonlaminar flow caused by this abnormal valve morphology [13]. Our group and others have made progress in predicting wall stress using constitutive modeling of aortic geometry in BAV patients [14–16], but little is known about morphotype-specific influences on the aortic wall. We addressed region-specific biology by focusing on antioxidant gene transcription profiles based on BAV morphotype (summary of results in Fig 5). We found that Sod1 and Sod2 displayed a unique expression profile that was dependent on both region and morphotype, whereas Sod3 expression was consistently reduced in all regions of pathologic specimens.
Fig 5.
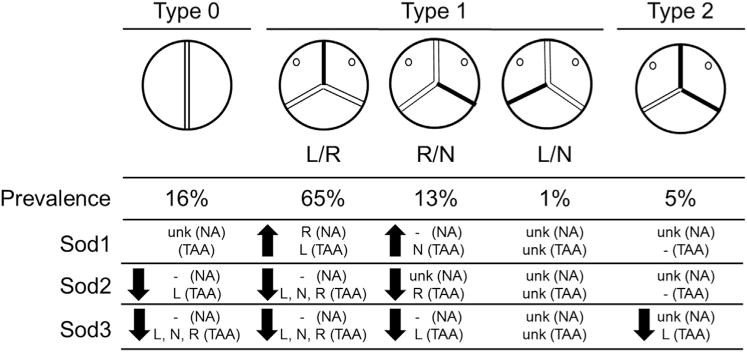
Schematic of overall findings. Prevalence of the bicuspid aortic valve (BAV) morphotype is displayed as a percentage of BAV patients (n = 157) presenting for elective replacement of a non-aneurysmal (NA) aortic valve or aortic replacement due to thoracic aortic aneurysm (TAA), or both. Sod transcriptions represent regionalized specimens from 74 patients, of which 44 BAV patients were a subset. Findings for BAV specimens are summarized as upregulation (↑) or downregulation (↓) by left (L), right (R), or non (N) region and aneurysmal status (NA or TAA) compared with NA tricuspid aortic valve (TAV) patients (TAV-NA). No change in expression when compared with TAV-NA is denoted by a dash (−). Sod transcript expression is unknown (ukn) for all patients with type 1 L/N morphotype, type 0 aneurysmal patients, and NA type 0, type 1 R/N, and type 2 patients due to low prevalence and sample sizes available.
The most striking difference found across all BAV morphotypes studied was the upregulated Sod1 transcription in the R region of nonaneurysmal BAV specimens with type 1 L/R morphotype—the region that tends to exhibit the greatest degree of asymmetric dilatation. Here, Sod1 upregulation may represent an early response given its presence before, or in the absence of, aortic dilatation. Downregulation of Sod2 transcription did not show the same degree of region specificity on the basis of BAV morphotype. We suspect that Sod1 expression plays an early role in the pathobiology leading to ascending aortic aneurysm formation but that downregulation of Sod2 expression occurs in the later stages of aneurysmal disease.
Sod3 expression was globally downregulated independent of the presence of a BAV or the presence of an aneurysm, suggesting that mishandling of extracellular oxidative stress occurs in the onset and the progression of aneurysm in both BAV-related aneurysms and degenerative aneurysms. Indeed, for the type 1 L/R morphotype, the nonaneurysmal specimens were downregulated in all regions, but the aneurysmal specimens were further downregulated compared with their nonaneurysmal counterparts. This step-wise decrease in Sod3 expression may be part of a progression of the BAV aortopathy in early through late disease. In contrast to Sod1 upregulation and Sod2 downregulation, which were localized to the R and N regions (greater curve), Sod3 downregulation was uniformly observed in all circumferential regions of the proximal aorta.
Our findings of altered Sod transcription are in agreement with a recent report from our group that revealed elevated nitric oxide (NO) synthase 3 expression at the transcript and protein levels despite unchanged levels of phosphorylation of vasodilator-stimulated phosphoprotein, a downstream target of NO signaling [17], suggesting that elevated endothelial NO synthase produces superoxide and thus interferes with NO signaling in the BAV aorta. Elevated Sod1 expression in the non-aneurysmal BAV aorta might be an early combative measure against elevated superoxide generation, whereas downregulation of Sod2 and Sod3 in dilated specimens is a consequence, contributing to an overall vulnerability to oxidative stress later in the disease process.
Results of our current study also concur with Arcucci and colleagues [18], who examined a limited number of aortic specimens from the greater curve and reported downregulation of SOD3 in specimens from BAV patients compared with normal patients. Because Sod3 is secreted, this uniform decrease in Sod3 expression could perpetuate elevated oxidative stress in the extracellular matrix and thus affect the final common pathway of extracellular matrix degeneration seen in the later stages of thoracic aortic disease.
Region-specific changes in expression of Sod1, Sod2, and Sod3 genes were unrelated to valve dysfunction in specimens from BAV patients. Conversely, only specimens from patients with regurgitant valves in the setting of a degenerative aneurysm displayed some of the same alterations in Sod expression as specimens from BAV patients. Hence, valve insufficiency was associated with region-specific changes in Sod1 and Sod2 in degenerative aneurysms only, and this association was limited to the R and N region for Sod1 expression and to the R region for Sod2 expression. Downregulation of Sod3 gene expression was uniformly observed in aortic samples from patients with normally functioning valves as well as regurgitant valves whether BAV or TAV. The presence of a stenotic valve did not influence the expression of any Sod isoform.
Despite clear perturbations in valvular hemodynamics due to the BAV [13], the associated aortopathy localized to the proximal ascending aorta involves cell-mediated mechanism(s) related to the oxidative stress defense of medial smooth muscle cells [19]. Prior work by others has revealed spatial differences (greater vs lesser curve) in the expression of matrix proteins such as collagens I and III, tenascin, laminin, and fibronectin, and increased expression of markers of smooth muscle cell apoptosis in aortic specimens from stenotic BAV patients in the absence of significant dilatation [20, 21]. Differential expression of extracellular matrix proteins [20] and smooth muscle cell phenotype [21] in the ascending aorta of BAV patients have been previously described for the greater vs lesser curve. However, a dilatation pattern has not been described for one particular BAV morphotype or another. To our knowledge, this is the first study to address regional aortic wall biology using the anatomy of the sinuses of Valsalva as circumferential fiduciary boundaries in the proximal ascending aorta.
We have previously described distinct microarchitectural features of the collagen and elastin fiber organization in the aorta of BAV patients [22–24]. The report by Ikonomidis and colleagues [25] was the first to show altered expression profiles of proteins regulating extracellular matrix homeostasis (matrix metalloproteinase and tissue-inhibitors of metalloproteinase) on the basis of BAV morphotype (albeit not regionally tested) and suggested that the type 1 L/R morphotype may be the most aggressive, given its prevalence. Our results are consistent with these previous findings, because most changes in Sod gene expression were noted for the type 1 L/R morphotype, and many occurred in nonaneurysmal and aneurysmal specimens alike. Others have contended that morphotype has little or no bearing on aneurysm geometry or extent from root to arch [26, 27] and may be more related to valve function than morphotype [12]. We found that valve function had no influence on regional Sod expression for the BAV-associated aortopathy, but regurgitant valves did appear to affect Sod expression profiles among TAV patients with degenerative aneurysms.
A limitation of the current study was inadequate sample sizes for certain patient subsets. Further studies are needed to better understand region-specific mechanisms contributing to cell and tissue phenomena associated with aneurysm formation in various BAV morphotypes. In our laboratory, we are attacking these questions by biological, computational, and biomechanical interrogatories [23], and we have recently shown unique matrix microarchitecture localized to the N and R regions of aneurysmal aortic specimens from BAV patients [28].
We conclude that region-specific antioxidant gene expression profiles are influenced by the presence and the location of the raphe and that these differential profiles may have a bearing on local material property changes in the proximal ascending aorta of BAV patients. The results indicate a likelihood of a heightened state of oxidative stress in the aorta as part of the pathophysiology of BAV-associated aortopathy, and defining the enzymatic source of free radical species is the focus of our ongoing work. To improve risk mitigation for aortic catastrophe, we hope to develop sophisticated multimodal approaches that combine biological and biomechanical data with clinical imaging to improve the management of BAV aortopathy.
Supplementary Material
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award HL-109132 (Dr Gleason). The AB Prism 7900HT thermocycler used to obtain the results for this publication is located in the Genomics Research Core at the University of Pittsburgh. The authors thank Andrew Althouse for biostatistical consultation and guidance and gratefully acknowledge the assistance of Drs Forozan Navid, Lawrence Wei, and Robert Kormos in the collection of ascending aortic tissue and Mr Michael Eskay in the processing of tissue specimens.
Footnotes
The Supplemental Tables can be viewed in the online version of this article [http://dx.doi.org/10.1016/j.athoracsur.2016.10.039] on http://www.annalsthoracicsurgery.org.
References
- 1.Roberts WC. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am J Cardiol. 1970;26:72–83. doi: 10.1016/0002-9149(70)90761-7. [DOI] [PubMed] [Google Scholar]
- 2.Svensson LG, Adams DH, Bonow RO, et al. Aortic valve and ascending aorta guidelines for management and quality measures: executive summary. Ann Thorac Surg. 2013;95:1491–505. doi: 10.1016/j.athoracsur.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 3.Parish LM, Gorman JH, 3rd, Kahn S, et al. Aortic size in acute type a dissection: implications for preventive ascending aortic replacement. Eur J Cardiothorac Surg. 2009;35:941–5. doi: 10.1016/j.ejcts.2008.12.047. discussion 945–6. [DOI] [PubMed] [Google Scholar]
- 4.Michelena HI, Prakash SK, Della Corte A, et al. Bicuspid aortic valve: identifying knowledge gaps and rising to the challenge from the International Bicuspid Aortic Valve Consortium (BAVCon) Circulation. 2014;129:2691–704. doi: 10.1161/CIRCULATIONAHA.113.007851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez B, Duran AC, Fernandez-Gallego T, et al. Bicuspid aortic valves with different spatial orientations of the leaflets are distinct etiological entities. J Am Coll Cardiol. 2009;54:2312–8. doi: 10.1016/j.jacc.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 6.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–33. doi: 10.1016/j.jtcvs.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Dore A, Brochu MC, Baril JF, Guertin MC, Mercier LA. Progressive dilation of the diameter of the aortic root in adults with a bicuspid aortic valve. Cardiol Young. 2003;13:526–31. [PubMed] [Google Scholar]
- 8.Keane MG, Wiegers SE, Plappert T, Pochettino A, Bavaria JE, Sutton MG. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation. 2000;102(19 Suppl 3):III35–9. doi: 10.1161/01.cir.102.suppl_3.iii-35. [DOI] [PubMed] [Google Scholar]
- 9.Nistri S, Grande-Allen J, Noale M, et al. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J. 2008;29:472–9. doi: 10.1093/eurheartj/ehm528. [DOI] [PubMed] [Google Scholar]
- 10.Fedak PW, Verma S, David TE, Leask RL, Weisel RD, Butany J. Clinical and pathophysiological implications of a bicuspid aortic valve. Circulation. 2002;106:900–4. doi: 10.1161/01.cir.0000027905.26586.e8. [DOI] [PubMed] [Google Scholar]
- 11.Yasuda H, Nakatani S, Stugaard M, et al. Failure to prevent progressive dilation of ascending aorta by aortic valve replacement in patients with bicuspid aortic valve: comparison with tricuspid aortic valve. Circulation. 2003;108(Suppl 1):II291–4. doi: 10.1161/01.cir.0000087449.03964.fb. [DOI] [PubMed] [Google Scholar]
- 12.Sievers HH, Stierle U, Hachmann RM, Charitos EI. New insights in the association between bicuspid aortic valve phenotype, aortic configuration and valve haemodynamics. Eur J Cardiothorac Surg. 2016;49:439–46. doi: 10.1093/ejcts/ezv087. [DOI] [PubMed] [Google Scholar]
- 13.Hope MD, Hope TA, Meadows AK, et al. Bicuspid aortic valve: four-dimensional MR evaluation of ascending aortic systolic flow patterns. Radiology. 2010;255:53–61. doi: 10.1148/radiol.09091437. [DOI] [PubMed] [Google Scholar]
- 14.Nathan DP, Xu C, Plappert T, et al. Increased ascending aortic wall stress in patients with bicuspid aortic valves. Ann Thorac Surg. 2011;92:1384–9. doi: 10.1016/j.athoracsur.2011.04.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okamoto RJ, Xu H, Kouchoukos NT, Moon MR, Sundt TM., 3rd The influence of mechanical properties on wall stress and distensibility of the dilated ascending aorta. J Thorac Cardiovasc Surg. 2003;126:842–50. doi: 10.1016/s0022-5223(03)00728-1. [DOI] [PubMed] [Google Scholar]
- 16.Pasta S, Rinaudo A, Luca A, et al. Difference in hemodynamic and wall stress of ascending thoracic aortic aneurysms with bicuspid and tricuspid aortic valve. J Biomech. 2013;46:1729–38. doi: 10.1016/j.jbiomech.2013.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotlarczyk MP, Billaud M, Green BR, et al. Regional disruptions in endothelial nitric oxide pathway associated with bicuspid aortic valve. Ann Thorac Surg. 2016;102:1274–81. doi: 10.1016/j.athoracsur.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arcucci A, Ruocco MR, Albano F, et al. Analysis of extracellular superoxide dismutase and Akt in ascending aortic aneurysm with tricuspid or bicuspid aortic valve. Eur J Histochem. 2014;58:2383. doi: 10.4081/ejh.2014.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phillippi JA, Klyachko EA, Kenny JP, 4th, Eskay MA, Gorman RC, Gleason TG. Basal and oxidative stress-induced expression of metallothionein is decreased in ascending aortic aneurysms of bicuspid aortic valve patients. Circulation. 2009;119:2498–506. doi: 10.1161/CIRCULATIONAHA.108.770776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cotrufo M, Della Corte A, De Santo LS, et al. Different patterns of extracellular matrix protein expression in the convexity and the concavity of the dilated aorta with bicuspid aortic valve: preliminary results. J Thorac Cardiovasc Surg. 2005;130:504–11. doi: 10.1016/j.jtcvs.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Della Corte A, Quarto C, Bancone C, et al. Spatiotemporal patterns of smooth muscle cell changes in ascending aortic dilatation with bicuspid and tricuspid aortic valve stenosis: focus on cell-matrix signaling. J Thorac Cardiovasc Surg. 2008;135:8–18. 18 e11–12. doi: 10.1016/j.jtcvs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Phillippi JA, Green BR, Eskay MA, et al. Mechanism of aortic medial matrix remodeling is distinct in patients with bicuspid aortic valve. J Thorac Cardiovasc Surg. 2014;147:1056–64. doi: 10.1016/j.jtcvs.2013.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsamis A, Pal S, Phillippi JA, Gleason TG, Maiti S, Vorp DA. Effect of aneurysm on biomechanical properties of “radially-oriented” collagen fibers in human ascending thoracic aortic media. J Biomech. 2014;47:3820–4. doi: 10.1016/j.jbiomech.2014.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsamis A, Phillippi JA, Koch RG, et al. Fiber microarchitecture in the longitudinal-radial and circumferential-radial planes of ascending thoracic aortic aneurysm media. J Biomech. 2013;46:2787–94. doi: 10.1016/j.jbiomech.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikonomidis JS, Ruddy JM, Benton SM, Jr, et al. Aortic dilatation with bicuspid aortic valves: cusp fusion correlates to matrix metalloproteinases and inhibitors. Ann Thorac Surg. 2012;93:457–63. doi: 10.1016/j.athoracsur.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazel SS, Mallidi HR, Lee RS, et al. The aortopathy of bicuspid aortic valve disease has distinctive patterns and usually involves the transverse aortic arch. J Thorac Cardiovasc Surg. 2008;135:901–7. 907 e901–2. doi: 10.1016/j.jtcvs.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Jackson V, Petrini J, Caidahl K, et al. Bicuspid aortic valve leaflet morphology in relation to aortic root morphology: a study of 300 patients undergoing open-heart surgery. Eur J Cardiothorac Surg. 2011;40:e118–24. doi: 10.1016/j.ejcts.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 28.Tsamis A, Phillippi JA, Koch RG, et al. Extracellular matrix fiber microarchitecture is region-specific in bicuspid aortic valve-associated ascending aortopathy. J Thorac Cardiovasc Surg. 2016;151:1718–28.e1715. doi: 10.1016/j.jtcvs.2016.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


