Abstract
Aims
We hypothesized that height-corrected abdominal size (supine sagittal abdominal diameter/height ratio [SADHtR] or waist circumference/height ratio [WHtR]) would associate more strongly than body mass index (BMI, weight/height2) with levels of fasting insulin, triglycerides, and three derived biomarkers of insulin resistance.
Methods
Anthropometry, including SAD by caliper, was collected on 4398 adults in the 2011–2014 National Health and Nutrition Examination Survey. For comparison purposes, each adiposity indicator was scaled to its population-based, sex-specific, interquartile range (IQR). For each biomarker we created four outcome groups based on equalsized populations with ascending values. Multivariable polytomous logistic regression modeled the relationships between the adiposity indicators and each biomarker.
Results
Highest-group insulin was associated with a one-IQR increment of BMI (RR 4.3 [95% CI 3.9–4.9]), but more strongly with a one-IQR increment of SADHtR (RR 5.7 [5.0–6.6]). For highest-group HOMA-IR the RR for BMI (4.2 [3.7–4.6]) was less than that of SADHtR (6.0 [5.1–7.0]). Similarly, RRs for BMI were smaller than those for SADHtR applying to highest-group triglycerides (RR 1.6 vs 2.1), triglycerides/HDL-cholesterol (RR 1.9 vs 2.4) and TyG index (RR 1.7 vs 2.2) (all p < .001). The RRs for WHtR were consistently between those for SADHtR and BMI. The top 25% of insulin resistance among US adults was estimated to lie above adiposity thresholds of 0.140 for SADHtR, 0.606 for WHtR, or 29.6 kg/m2 for BMI.
Conclusions
Relative abdominal size rather than relative weight may better define adiposity associated with homeostatic insulin resistance. These population-based, cross-sectional findings could improve anthropometric prediction of cardiometabolic risk.
Keywords: Anthropometry; Body mass index; Insulin resistance; Obesity, abdominal; Sagittal abdominal diameter; Waist-height ratio
1. Introduction
The body mass index (BMI, weight/height2) is commonly recommended for clinical and epidemiological categorization of human adiposity. Although adult BMI is associated with cardiometabolic disorders and mortality assessed at the population level, the BMI cannot distinguish between lean mass and various depots of adipose tissue. Thus, dependence on conventional categories of BMI sometimes misclassifies health risks for individuals. The exploration of alternative adiposity indicators has been recommended [1].
Guided by an emerging appreciation of the roles played by visceral (intra-abdominal) adiposity, various experts have evaluated the utility of the waist circumference or the waist-to-hip circumference ratio as candidate indicators suitable for identifying risks of mortality [2], type 2 diabetes [3], cardiovascular disease, hypertension [4], and cancer [5]. However, neither waist circumference nor waist-to-hip circumference ratio acknowledges an influence of height which corrects the waist value for general body size and thereby may improve the association of waist circumference with cardiometabolic risk variables [6]. We note also that increased height tends to identify adults with relatively longer legs. Greater leg length can support enlarged adipose depots in the lower extremities which are associated with reduced cardiometabolic risk [7–9]. Accordingly, many researchers have considered benefits of using the waist circumference/height ratio (WHtR) for estimating the risk of cardiometabolic disorders [10–14].
Since the supine sagittal abdominal diameter (SAD, also called “abdominal height”) correlates strongly with the amount of visceral fat [15,16] and with traditional cardiometabolic risk markers [17–19], several authors have extended consideration to the SAD/height ratio (SADHtR) as yet one more alternative to the BMI [10,11,20,21]. Beginning in 2011, the supine SAD has been measured routinely in the U.S. National Health and Nutrition Examination Survey (NHANES) along with waist circumference, height, and weight. Thus, recent NHANES information includes a large, representative sample of adults from which we can calculate simultaneous values of SADHtR, WHtR, and BMI.
Our primary purpose in this report is to clarify how strongly SADHtR, WHtR, and BMI are associated with “upstream” markers of insulin dynamics and dyslipidemia that may influence a variety of “downstream” cardiometabolic diseases. Thus, our biomarkers of interest are adverse levels of fasting insulin, triglycerides, and three derived homeostatic biomarkers that can be measured readily in population-based surveys. Approximately half of NHANES participants had their blood drawn in the fasting state during a morning encounter at the examination centers. Within this large sample, we hypothesized that adiposity indicators based on abdominal size relative to height (SADHtR and WHtR) would have greater utility than the BMI for identifying fundamental physiologic disturbances that contribute eventually to cardiometabolic disease.
2. Materials and methods
2.1. Participants and anthropometry
Our report is based on non-pregnant adults (age ≥ 20 years) participating in NHANES examinations during 2011–2014. The NHANES protocol was approved by the Research Ethics Review Board of the Centers for Disease Control and Prevention’s National Center for Health Statistics. Participants provided informed consent. Of 4664 non-pregnant adults attending the morning examinations, 4616 provided BMI values, 4494 provided WHtR, and 4405 provided SADHtR. We restricted our primary descriptive sample to include only the 4398 adults who had values for all three of the adiposity indicators (94.3% of morning attendees). With statistical weighting these examinees represented approximately 213.8 million US adults.
Weight, height, and waist circumference (standing position, by tape measure just above the uppermost lateral border of the ilium) were obtained by established methods [22]. WHtR was calculated as the waist circumference divided by height. The SAD measurement used a portable, sliding-beam caliper (Holtain, Ltd, Wales, UK) [22]. Supine participants rested on a lightly padded exam table with their hips in flexed position as the examiner marked the level of their iliac crests. The lower arm of the caliper was then positioned under the small of the back, and the upper arm was raised above the belly in alignment with their iliac-crest level. The examiner asked the participant to inhale gently, slowly let the air out, and then relax. The examiner then lowered the caliper’s upper arm, letting it lightly touch the abdomen but without compressing it (Fig. 1). The SAD value, recorded to the nearest 0.1 cm, was read directly from a tape measure fixed to the caliper shaft. For 94.4% of adults we defined SAD as the mean of two initial measurements; when the difference between the first and second measurements exceeded 0.5 cm (5.6%) we used the mean of up to 4 measurements [23]. SADHtR was calculated as SAD/height.
Fig. 1.
Measurement of the sagittal abdominal diameter (SAD) in a supine participant. Reprinted from Kahn HS et al. [50] with permission from Elsevier.
We estimated the sex-stratified distributions of anthropometric dimensions and adiposity indicators in the represented population, including the sex-specific interquartile range (IQR; 75th percentile [p75] minus 25th percentile [p25]) for SADHtR, WHtR, and BMI.
2.2. Dependent variables
Fasting insulin, triglycerides, and HDL cholesterol were assayed in serum, and fasting glucose was assayed in plasma by standard methods [24]. Based on these laboratory data, we identified five biomarkers as dependent variables reflecting underlying metabolic risk.
Two biomarkers (insulin and homeostasis model assessment of insulin resistance [HOMA-IR]) were analyzed from 4251 participants with complete data on fasting insulin and glucose. For each of these biomarkers we categorized four groups from their distributions estimated in the represented population. These ordinal outcome groups of the dependent variable were based on ascending quartile cut points for insulin (6.01, 9.45, 15.39 μU/mL) or for HOMA-IR (1.041, 2.343, 4.055).
Three biomarkers (fasting triglycerides, ratio of triglycerides/HDL-cholesterol, and TyG index) were analyzed from 4353 participants with complete data on fasting triglycerides, HDL cholesterol, and glucose. For each of these biomarkers we categorized four groups based on quartile cut points for fasting triglycerides (0.779, 1.13, 1.67 mmol/L) [69,100,148 mg/dL], for the ratio of triglycerides/HDL-cholesterol (1.215, 1.954, 3.223), or for the TyG index (8.116, 8.522, 8.968). Triglycerides/HDL-cholesterol is a proxy variable for identifying persons with insulin resistance and elevated cardiometabolic risk [25,26]. The TyG index, an estimator of insulin resistance and hepatic steatosis [25,27], was calculated as loge [((triglycerides, mg/dL) × (glucose, mg/dL))/2)] [28].
2.3. Statistical methods
NHANES selected participants through a complex, multistage-probability design requiring a sampling weight for each participant. We used SAS (release 9.3; SAS Institute Inc.) and SUDAAN (PROC DESCRIPT and PROC MULTILOG, release 11.0; RTI International) to account for the complex design and statistical weights for the fasting sample so that characteristics of the represented population could be correctly described.
For comparing the effects of SADHtR, WHtR, and BMI we standardized these adiposity indicators by scaling them to their sex-specific IQRs. We used multivariable polytomous logistic regression models with calculated predicted margins associated with an adiposity increment of one IQR to estimate the relative risk (RR) for being in each of the ordinal outcome groups (Q1, Q2, Q3, Q4) described above for five different biomarkers. Each of the regression models included adjustment for 7 categories of age (decades, of which the highest was 80+ years) and five mutually exclusive categories of ancestry as determined from self-reported ethnicity and race (non-Hispanic white, non-Hispanic black, non-Hispanic Asian, Hispanic, and “other, including multi-racial”). Some models were also adjusted for sex, use of injected insulin, or use of oral anti-hyperglycemic medication (by self report) when these covariates made a significant (p < .05) contribution. No models required adjustment for use of anti-cholesterolemic medication.
For practitioners who use adiposity indicators to categorize persons or populations with a high-risk physiologic status, we have estimated threshold values of SADHtR, WHtR, and BMI that could identify those likely to be in the most adverse range (25% of adults) for each of the five evaluated biomarkers. In this exercise, we estimated the distributions of each adiposity indicator among the most adverse and the less adverse (favorable) sub-populations. For each biomarker, our estimated adiposity threshold was the mean of adiposity indicator p25 in the most adverse group (low quantile) and adiposity indicator p75 in the less adverse group (high quantile) (see general illustration in Supplementary Material, Fig. S1). Quantitative details for threshold estimation of each adiposity indicator and each adverse biomarker level are provided in the Supplementary Material with Table S1.
2.4. Role of the funding source
This research received no specific grant from any funding agency.
3. Results
3.1. Comparisons between three scaled adiposity indicators
Sex-specific distributions of SADHtR, WHtR and BMI in the overall adult population are presented in Table 1. For each adiposity indicator, the IQR was larger for women compared to men. This observation supports the analytic strategy to employ sex-specific IQRs for scaling the adiposity indicators used in our comparative models.
Table 1.
Sample-weighted distributions, by sex, of anthropometric dimensions and adiposity indicators among US, non-pregnant adults aged 20+ years in 2011–14.
| Dimension, indicator | Men (sample n = 2189) | Women (sample n = 2209) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Mean | Percentiles | IQR | Percentiles | IQR | ||||||
|
|
|
|||||||||
| p25 | p50 | p75 | Mean | p25 | p50 | p75 | ||||
| SAD, cm | 23.3 | 20.1 | 22.8 | 25.7 | 5.6 | 22.0 | 18.4 | 21.3 | 24.7 | 6.3 |
| WC, cm | 101.4 | 90.6 | 100.0 | 109.7 | 19.1 | 97.0 | 84.5 | 95.0 | 106.9 | 22.4 |
| Weight, kg | 89.1 | 74.8 | 85.7 | 99.0 | 24.2 | 76.8 | 62.0 | 73.0 | 87.1 | 25.1 |
| Height, cm | 176.1 | 171.0 | 176.0 | 181.1 | 10.1 | 162.1 | 157.3 | 162.3 | 166.4 | 9.1 |
| SADHtR | 0.1322 | 0.1145 | 0.1294 | 0.1468 | 0.0323 | 0.1360 | 0.1138 | 0.1330 | 0.1543 | 0.0405 |
| WHtR | 0.5762 | 0.5167 | 0.5683 | 0.6250 | 0.1083 | 0.5994 | 0.5221 | 0.5872 | 0.6637 | 0.1416 |
| BMI, kg/m2 | 28.64 | 24.62 | 27.75 | 31.29 | 6.68 | 29.17 | 23.66 | 27.77 | 33.20 | 9.55 |
IQR, interquartile range (p75 minus p25).
WC, waist circumference.
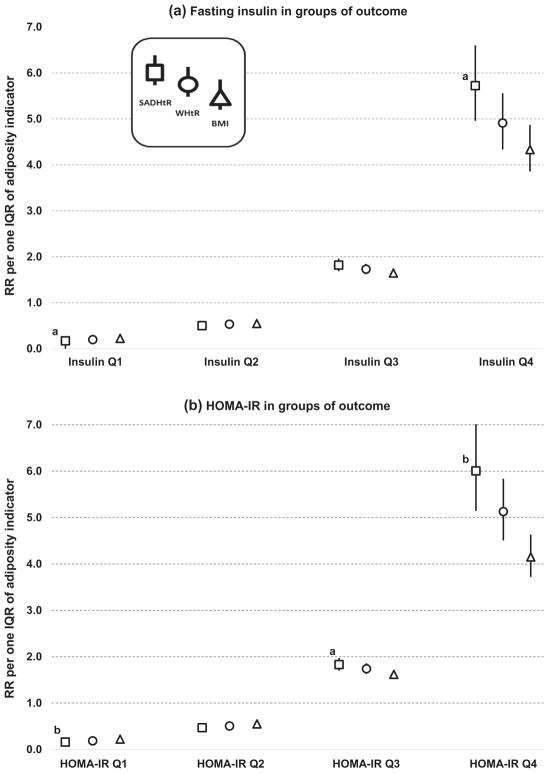
As applied to the modeling of insulin-based biomarkers, Fig. 2 displays smaller RRs per one IQR of BMI, as compared to one IQR of SADHtR, for being in outcome groups at the highest level (Q4) of metabolic risk. Fig. 2.a. shows that a one-IQR increment of the BMI was associated with an increased risk for being in the Q4 of insulin (RR 4.33 [95% CI3.86–4.87]), but a one-IQR increment of the SADHtR was associated with a larger increased risk for being in the Q4 of insulin (RR 5.72 [4.96–6.60]; p = .003 for difference with BMI). The scaled WHtR (RR 4.91 [4.34–5.56]) was associated with an estimate that was between the RR values for BMI and SADHtR. As seen in Fig. 2b, we found for HOMA-IR the same ranking of RRs for the adiposity indicators: 4.15 [3.72–4.63] for BMI; 5.13 [4.51–5.84] for WHtR (p = .013 for difference with BMI); and 6.01 [5.14–7.01] for SADHtR (p < .001 for difference with BMI).
Fig. 2.
Risk ratios associated with SADHtR, WHtR, or BMI for being in ascending biomarker groups (Q1 through Q4) of fasting insulin or HOMA-IR. Models (sample n = 4251) are adjusted for age, ancestry, use of injected insulin, and use of oral antiglycemic medication. Error bars show the 95% CI. a p < 0.01 for comparison to BMI. b p < 0.001 for comparison to BMI.
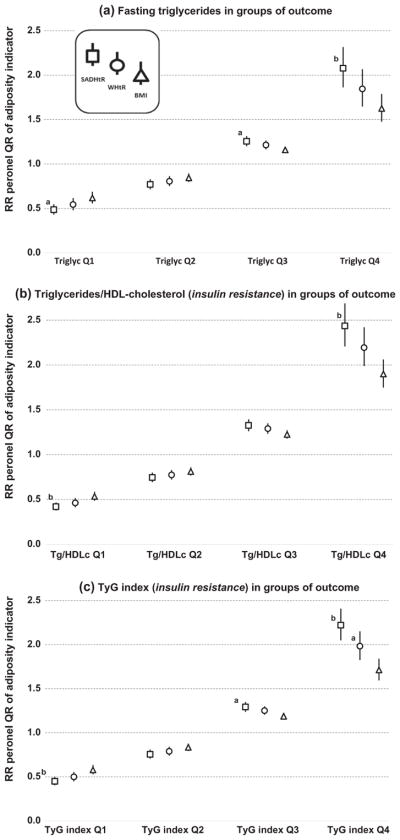
Among our models that predicted lipid-based, proxy biomarkers for insulin resistance we also found reduced RRs for scaled BMI as compared with scaled SADHtR (Fig. 3a, b, and c). The contrast between BMI and SADHtR was confirmed in the Q4 groups of triglycerides (RR 1.63 vs 2.08), of triglycerides/HDL-cholesterol (RR 1.90 vs 2.44) and of the TyG index (RR 1.71 vs 2.22) (all p < .001). In the Q3 groups of these biomarkers we also noted RR contrasts between BMI and SADHtR in models for triglycerides (RR 1.16 vs 1.26; p = .004), for triglycerides/HDL-cholesterol (RR 1.23 vs 1.33; p = .010) and for the TyG index (RR 1.19 vs 1.29; p = .002). When BMI was compared with WHtR in the Q4 groups, the RR for BMI was less in models for triglycerides/HDL-cholesterol (1.90 vs 2.19; p = .024) and for the TyG index (1.71 vs 1.98; p = .007).
Fig. 3.
Risk ratios associated with SADHtR, WHtR, or BMI for being in ascending biomarker groups (Q1 through Q4) of lipid-based biomarkers (proxy variables for insulin resistance). Models (sample n = 4353) are adjusted for age, ancestry, and sex. Error bars show the 95% CI. a p < 0.01 for comparison to BMI. b p < 0.001 for comparison to BMI.
3.2. Estimating adiposity thresholds for high-risk groups of each biomarker
As expected, for each of the five biomarkers measured in this study the distribution of adiposity indicators in the most adverse group (Q4) had higher values than the adiposity distribution in the remaining favorable population containing Q1, Q2, and Q3 (Table S1). Threshold adiposity values for distinguishing the most adverse from the favorable biomarker groups of insulin action were estimated to be 0.140 for SADHtR, 0.606 for WHtR, and 29.6 kg/m2 for BMI. Thresholds estimated between groups of lipid-based proxy variables had values similar to the thresholds for biomarkers of insulin action, but their uncertainty was greater.
3.3. Interactions by age and ancestry
In logistic models stratified according to participant age (20– 49 y [sample n = 2225–2277] or 50+ y [sample n = 2026–2076]), we found for the highest-risk outcome groups that the ranking of RRs among the adiposity indicators was unchanged by the age level (Table 2). Compared to the younger adults, however, older participants had RRs that tended to be weaker, and the confidence limits around the RRs for each adiposity indicator were more likely to overlap each other.
Table 2.
Stratification by age showing risk ratios associated with a one-IQR increment of SADHtR, WHtR, or BMI for being in the most adverse 25% (group Q4) of five biomarkers.
| Biomarker | 20–49 years | 50+ years | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| RR for adverse group of biomarker | 95% LL | 95% UL | RR for adverse group of biomarker | 95% LL | 95% UL | ||
| Fasting insulin | SADHtR | 6.37 | 5.59 | 7.26 | 5.02 | 3.78 | 6.66 |
| WHtR | 5.15 | 4.52 | 5.88 | 4.64 | 3.59 | 6.00 | |
| BMI | 4.53 | 3.99 | 5.14 | 4.08 | 3.09 | 5.39 | |
| HOMA-IR, insulin resistance | SADHtR | 6.58 | 5.71 | 7.59 | 5.07 | 3.84 | 6.69 |
| WHtR | 5.27 | 4.60 | 6.04 | 4.76 | 3.68 | 6.16 | |
| BMI | 4.42 | 3.88 | 5.04 | 3.86 | 3.00 | 4.96 | |
| Fasting triglycerides | SADHtR | 2.08 | 1.86 | 2.33 | 2.08 | 1.79 | 2.42 |
| WHtR | 1.83 | 1.63 | 2.05 | 1.91 | 1.62 | 2.25 | |
| BMI | 1.58 | 1.42 | 1.76 | 1.71 | 1.51 | 1.94 | |
| Triglycerides/HDLc, proxy insulin resistance | SADHtR | 2.44 | 2.20 | 2.70 | 2.43 | 2.05 | 2.87 |
| WHtR | 2.14 | 1.94 | 2.35 | 2.30 | 1.91 | 2.78 | |
| BMI | 1.83 | 1.67 | 2.02 | 2.00 | 1.73 | 2.31 | |
| Fasting TyG Index, proxy insulin resistance | SADHtR | 2.31 | 2.04 | 2.62 | 2.10 | 1.83 | 2.41 |
| WHtR | 2.04 | 1.83 | 2.27 | 1.95 | 1.71 | 2.22 | |
| BMI | 1.72 | 1.55 | 1.92 | 1.74 | 1.57 | 1.93 | |
All models were adjusted for age (in 4 or 3 decades) and ancestry. Models for fasting insulin and HOMA-IR were adjusted also for use of insulin and oral anti-glycemic medication; models for triglycerides and Tg/HDLc were adjusted also for sex; models for TyG index were adjusted also for sex and use of insulin and oral anti-hyperglycemic medication.
Among the ancestral categories identified in our various multivariable models, we noted that outlying regression coefficients occurred frequently only for the non-Hispanic black category (“black”). In models stratified as black (sample n = 851–885) or non-black (sample n = 3400–3468) we found for the highest-risk outcome groups that the ranking of RRs was unchanged between blacks and non-blacks (Supplementary Material, Table S2). Compared to the non-black population, the blacks had relatively weaker RRs and the confidence limits around their RRs were more likely to overlap.
With one minor exception, none of our models demonstrated any interaction with the sex covariate (p > .18). A weak interaction (sex × WHtR; p = .032) was found only in the model for triglyceride/HDL-cholesterol, but was judged insufficient to require sex-stratified analyses for the entire dataset.
4. Discussion
The major results of this study support the hypothesis that adiposity indicators based on abdominal size relative to height, when compared to BMI, are more strongly associated with an adverse level of five fasting-state biomarkers. Our cross-sectional findings are robust for the indicator SADHtR, but less so for the WHtR. These observations in a large, contemporary, sample of U.S. adults could strengthen the applications of low-cost anthropometry in clinical, epidemiologic, and research practice.
Earlier cross-sectional studies have demonstrated that WHtR [12,13,21] or SADHtR [21] were similarly associated with traditional risk factors for cardiometabolic disease, and that these associations were generally stronger than those with BMI.
Our evaluations of BMI, WHtR, and SADHtR are novel in that our dependent variables of interest (five biomarkers) are neither classical cardiovascular risk factors nor incident clinical events. Rather, we used fasting-state data from NHANES to focus on adiposity’s cross-sectional associations with circulating insulin, triglycerides, and three derived biomarkers that estimate insulin resistance in the homeostatic condition. These biomarkers describe underlying metabolic dysfunctions that point generally to future diabetes, atherosclerotic diseases, fatty liver, and several types of cancer. NHANES does not include any longitudinal assessments, however, so our report is not a prospective study focused on the incidence of a specified clinical event.
By choosing to study biomarkers with a wide range of potential clinical sequelae, we remind ourselves that anthropometry’s purpose in clinical or epidemiologic settings need not be restricted to the prediction of just one disease at a time. Nevertheless, the ability to predict hard clinical outcomes is pertinent to clinical evaluations. Prospective studies have found that incident type 2 diabetes could be predicted by waist circumference in models that included height [29,30] or that WHtR predicted incident diabetes at least as well as BMI [31–34]. With regard to cardiovascular events and mortality, prospective reports found also that WHtR predicted incident outcomes at least as well as BMI [20,35–40]. Thus, there is ample reason to expand our use of WHtR.
There is less experience with the use of SADHtR in clinical or research practice. One prospective, population-based study of adults demonstrated that SAD contributed significantly to the prediction of incident diabetes in models including BMI [41], and a longitudinal study of patients with type 2 diabetes reported that SAD was superior to waist circumference or BMI for predicting incident cardiovascular events [42]. Other studies have documented associations between SAD and mortality in selected, high-risk populations [43,44].
Within our large, population-based dataset we found no evidence that the ranking of RRs across the three adiposity indicators (SADHtR >WHtR > BMI) would be influenced by sex or adult age group. Among those 50+ years old, compared to younger adults, we found a trend toward smaller RRs of each adiposity indicator in association with fasting insulin or HOMA-IR, but this age-related trend was not evident for the three lipid-based biomarkers. Smaller RRs among older participants may reflect a survivorship bias caused by excess mortality among high-adiposity participants as they advance through later decades of adulthood. If so, then the participants whose risk status was defined by insulin-based biomarkers may have been removed from the population earlier or more often than those whose risk was defined by lipid-based biomarkers. We found also that irrespective of the chosen biomarker, the ranking of adiposity RRs was the same for blacks and non-blacks, but the RRs tended consistently to be weaker among the blacks. This finding is consistent with other U.S. studies demonstrating that the effect of adiposity on mortality tended to be less among blacks than among non-blacks [37,38].
Although our study participants were strategically sampled to represent the U.S. population, the applications of our findings may be limited because NHANES recruitment excluded institutionalized persons such as those in nursing homes, hospitals, or prisons. Another limitation of our analyses is that NHANES employed only one protocol for measuring the waist circumference whereas other studies made waist measurements using alternative anatomic landmarks. It is possible that waist circumference measurements midway between lowest rib and iliac crest would provide more useful physiological information than measurements using the NHANES protocol [45].
Likewise, the SAD has been measured using different methods. Studies comparing SAD protocols suggest, however, that the NHANES protocol for SAD may be the most appropriate for identifying health risks [46,47]. A very similar protocol for SAD measurement was used successfully in a nationwide survey of over 6000 Finnish adults in 2000–2001 [48]. So long as it is possible for patients or survey participants to lie supine, the convenience and reliability of SAD measured by this standardized protocol may be no less than those of the waist circumferences or weights that are commonly obtained in clinical settings. A supine SAD measured by sliding-beam caliper, as compared to a standing waist circumference, avoids requirements that the tape measure be parallel to the ground and subjected to standardized degree of tension. In contrast to a standing weight, the SAD measurement can be readily interpreted for amputees, and it does not require adjustments for heavy garments, jewelry, medical appliances, or shoes. Such considerations of convenience and reliability, in addition to the advantages of SADHtR for estimation of fasting-state insulin resistance, could support the wider use of SADHtR in clinical settings where the BMI is usually collected. Normative reference values for WHtR and SADHtR calculated using NHANES anthropometric methods are available online for the U.S. adult population that was surveyed in 2011–2014 [49].
Doubts have been expressed about the universal application of traditional BMI threshold values that indicate high-risk health status. The designation of adult “obesity” when BMI reaches 30.0 kg/m2 might require modification for some persons depending on their ancestral group, age, or the health outcome under consideration. Similar doubts could be expressed about the estimation and application of universal, high-risk thresholds for WHtR or SADHtR. Our Supplementary Material provides examples of how adiposity thresholds could be estimated among contemporary US adults if the purpose were to screen for those whose homeostatic insulin resistance is in the top 25% of the population. In this exercise, our estimated BMI threshold of 29.6 kg/m2 was not far from the BMI value that conventionally defines adult “obesity”. By use of the same method, we estimated that a screening threshold for WHtR would be 0.606, a value considerably higher than the 0.500 threshold which has been recommended elsewhere [35]. Since more than 75% of US adults currently have WHtR values above 0.500 [49], we would advise caution before classifying so many people into a high-risk level of insulin resistance. Based on our analyses of abdominal adiposity indicators, a reasonable screening criterion for high-risk insulin resistance in the contemporary US adult population could be the SADHtR threshold value of 0.140.
In conclusion, relative abdominal size (SADHtR, WHtR) rather than relative weight (BMI) may better define adult adiposity associated with homeostatic insulin resistance. These population-based, cross-sectional findings, if confirmed in prospective studies, could improve the anthropometric prediction of cardiometabolic risk.
Supplementary Material
Acknowledgments
The authors acknowledge the participants in the 2011–2014 NHANES, and the efforts of the NHANES field staff. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations
- BMI
body mass index
- IQR
interquartile range (p75 minus p25)
- nhanes
National Health and Nutrition Examination Survey
- RR
relative risk
- SADHtR
sagittal abdominal diameter-to-height ratio
- TyG index
triglyceride × fasting glucose
- WHtR
waist circumference-to-height ratio
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.diabres.2017.11.019.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest associated with this manuscript.
Author Contributions
Conceptualization: HSK YJC.
Data curation: HSK YJC.
Formal analysis: HSK YJC.
Methodology: HSK.
Visualization: HSK.
Writing – original draft: HSK.
Writing – review & editing: HSK YJC.
Data availability
The public-use data that support the findings of this study are available from the National Center for Health Statistics at http://wwwn.cdc.gov/nchs/nhanes/search/nhanes11_12.aspx and http://wwwn.cdc.gov/nchs/nhanes/search/nhanes13_14.aspx.
References
- 1.Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56:369–81. doi: 10.1016/j.pcad.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–20. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 3.Wannamethee SG, Papacosta O, Whincup PH, Carson C, Thomas MC, Lawlor DA, et al. Assessing prediction of diabetes in older adults using different adiposity measures: a 7 year prospective study in 6,923 older men and women. Diabetologia. 2010;53:890–8. doi: 10.1007/s00125-010-1670-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Waist circumference and waist– hip ratio: Report of a WHO expert consultation; Geneva. 8–11 December, 2008; Geneva, Switzerland: WHO; 2011. [cited 16 October 2017]. Available from: http://apps.who.int/iris/bitstream/10665/44583/1/9789241501491_eng.pdf. [Google Scholar]
- 5.Freisling H, Arnold M, Soerjomataram I, O’Doherty MG, Ordonez-Mena JM, Bamia C, et al. Comparison of general obesity and measures of body fat distribution in older adults in relation to cancer risk: meta-analysis of individual participant data of seven prospective cohorts in Europe. Br J Cancer. 2017;116:1486–97. doi: 10.1038/bjc.2017.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider HJ, Klotsche J, Silber S, Stalla GK, Wittchen HU. Measuring abdominal obesity: effects of height on distribution of cardiometabolic risk factors risk using waist circumference and waist-to-height ratio. Diabetes Care [Internet] 2011;34(1):e7. doi: 10.2337/dc10-1794. [cited 16 October 2017] Available from: http://care.diabetesjournals.org/content/34/1/e7.long. [DOI] [PubMed] [Google Scholar]
- 7.Snijder MB, Visser M, Dekker JM, Goodpaster BH, Harris TB, Kritchevsky SB, et al. Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 2005;48:301–8. doi: 10.1007/s00125-004-1637-7. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Hu EA, Wu H, Malik V, Sun Q. Associations of leg fat accumulation with adiposity-related biological factors and risk of metabolic syndrome. Obesity (Silver Spring, Md) [Internet] 2013;21(4):824–30. doi: 10.1002/oby.20028. [cited 16 October 2017] Available from: http://onlinelibrary.wiley.com/doi/10.1002/oby.20028/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotta LA, Gulati P, Day FR, Payne F, Ongen H, van de Bunt M, et al. Integrative genomic analysis implicates limited peripheral adipose storage capacity in the pathogenesis of human insulin resistance. Nat Genet. 2017;49:17–26. doi: 10.1038/ng.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumlin L, Dimberg L, Marin P. Ratio of abdominal sagittal diameter to height is strong indicator of coronary risk. BMJ. 1997;314:830. doi: 10.1136/bmj.314.7083.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn HS, Dunbar VG, Ziemer DC, Phillips LS. Diabetes in urban African Americans. XII. Anthropometry for assessing municipal hospital outpatients recently diagnosed with type 2 diabetes. Obes Res. 1998;6:238–45. doi: 10.1002/j.1550-8528.1998.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–53. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Ashwell M, Gunn P, Gibson S. Waist-to-height ratio is a better screening tool than waist circumference and BMI for adult cardiometabolic risk factors: systematic review and meta-analysis. Obes Rev. 2012;13:275–86. doi: 10.1111/j.1467-789X.2011.00952.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee CMY, Woodward M, Pandeya N, Adams R, Barrett-Connor E, Boyko EJ, et al. Comparison of relationships between four common anthropometric measures and incident diabetes. Diabetes Res Clin Pract. 2017;132:36–44. doi: 10.1016/j.diabres.2017.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sjostrom L, Kvist H. Regional body fat measurements with CT-scan and evaluation of anthropometric predictions. Acta Med Scand Suppl. 1988;723:169–77. doi: 10.1111/j.0954-6820.1987.tb05941.x. [DOI] [PubMed] [Google Scholar]
- 16.Yim JY, Kim D, Lim SH, Park MJ, Choi SH, Lee CH, et al. Sagittal abdominal diameter is a strong anthropometric measure of visceral adipose tissue in the Asian general population. Diabetes Care. 2010;33:2665–70. doi: 10.2337/dc10-0606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gustat J, Elkasabany A, Srinivasan S, Berenson GS. Relation of abdominal height to cardiovascular risk factors in young adults: The Bogalusa Heart Study. Am J Epidemiol. 2000;151:885–91. doi: 10.1093/oxfordjournals.aje.a010292. [DOI] [PubMed] [Google Scholar]
- 18.Ohrvall M, Berglund L, Vessby B. Sagittal abdominal diameter compared with other anthropometric measurements in relation to cardiovascular risk. Int J Obes Relat Metab Disord. 2000;24:497–501. doi: 10.1038/sj.ijo.0801186. [DOI] [PubMed] [Google Scholar]
- 19.Riserus U, de Faire U, Berglund L, Hellenius ML. Sagittal abdominal diameter as a screening tool in clinical research: cutoffs for cardiometabolic risk. J Obes [Internet] 2010;2010:757939. doi: 10.1155/2010/757939. [cited 16 October 2017] Available from: http://www.hindawi.com/journals/jobes/2010/757939/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlsson AC, Riserus U, Engstrom G, Arnlov J, Melander O, Leander K, et al. Novel and established anthropometric measures and the prediction of incident cardiovascular disease: A cohort study. Int J Obes (Lond) 2013;37:1579–85. doi: 10.1038/ijo.2013.46. [DOI] [PubMed] [Google Scholar]
- 21.Kahn HS, McKeever Bullard K. Beyond body mass index: advantages of abdominal measurements for recognizing cardiometabolic disorders. Am J Med. 2016;129(74–81):e2. doi: 10.1016/j.amjmed.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. Anthropometry Procedures Manual - National Health and Nutrition Examination Survey (NHANES) Hyattsville, MD: NCHS; 2013. [cited 16 October 2017]. Available from: http://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/2013_Anthropometry.pdf. [Google Scholar]
- 23.National Center for Health Statistics. NHANES 2013–2014 Data Documentation, Codebook, and Frequencies – Body Measures (BMX_H) Hyattsville, MD: NCHS; 2015. [cited 16 October 2017]. Available from: http://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/BMX_H.htm. [Google Scholar]
- 24.National Center for Health Statistics. 2013–2014 Lab Methods for NHANES. Hyattsville, MD: Centers for Disease Control and Prevention; 2013. [cited 16 October 2017]. Available from: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2013. [Google Scholar]
- 25.Abbasi F, Reaven GM. Comparison of two methods using plasma triglyceride concentration as a surrogate estimate of insulin action in nondiabetic subjects: triglycerides x glucose versus triglyceride/high-density lipoprotein cholesterol. Metabolism. 2011;60:1673–6. doi: 10.1016/j.metabol.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Sung KC, Reaven G, Kim S. Ability of the plasma concentration ratio of triglyceride/high-density lipoprotein cholesterol to identify increased cardio-metabolic risk in an east Asian population. Diabetes Res Clin Pract. 2014;105:96–101. doi: 10.1016/j.diabres.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Du T, Zhang J, Lu H, Lin X, Xie J, et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis [Internet] 2017;16(1):15. doi: 10.1186/s12944-017-0409-6. [cited 16 October 2017] Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5248473/pdf/12944_2017_Article_409.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 29.Schulze MB, Hoffmann K, Boeing H, Linseisen J, Rohrmann S, Mohlig M, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care. 2007;30:510–5. doi: 10.2337/dc06-2089. [DOI] [PubMed] [Google Scholar]
- 30.Kahn HS, Cheng YJ, Thompson TJ, Imperatore G, Gregg EW. Two risk-scoring systems for predicting incident diabetes mellitus in U.S. adults age 45 to 64 years. Ann Intern Med. 2009;150:741–51. doi: 10.7326/0003-4819-150-11-200906020-00002. [DOI] [PubMed] [Google Scholar]
- 31.Kodama S, Horikawa C, Fujihara K, Heianza Y, Hirasawa R, Yachi Y, et al. Comparisons of the strength of associations with future type 2 diabetes risk among anthropometric obesity indicators, including waist-to-height ratio: a meta-analysis. Am J Epidemiol. 2012;176:959–69. doi: 10.1093/aje/kws172. [DOI] [PubMed] [Google Scholar]
- 32.Huerta JM, Tormo M-J, Chirlaque M-D, Gavrila D, Amiano P, Arriola L, et al. Risk of type 2 diabetes according to traditional and emerging anthropometric indices in Spain, a Mediterranean country with high prevalence of obesity: results from a large-scale prospective cohort study. BMC Endocr Disord [Internet] 2013;13(1):7. doi: 10.1186/1472-6823-13-7. [cited 16 October 2017] Available from: http://www.biomedcentral.com/1472-6823/13/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartwig S, Kluttig A, Tiller D, Fricke J, Muller G, Schipf S, et al. Anthropometric markers and their association with incident type 2 diabetes mellitus: which marker is best for prediction? Pooled analysis of four German population-based cohort studies and comparison with a nationwide cohort study. BMJ Open [Internet] 2016;6(1):e009266. doi: 10.1136/bmjopen-2015-009266. [cited 16 October 2017] Available from: http://bmjopen.bmj.com/content/6/1/e009266.long. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janghorbani M, Amini M. The visceral adiposity index in comparison with easily measurable anthropometric markers did not improve prediction of diabetes. Can J Diabetes. 2016;40:393–8. doi: 10.1016/j.jcjd.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 35.Browning LM, Hsieh SD, Ashwell M. A systematic review of waist-to-height ratio as screening tool for the prediction of cardiovascular disease and diabetes: 0. 5 could be a suitable global boundary value. Nutr Res Rev. 2010;23:247–69. doi: 10.1017/S0954422410000144. [DOI] [PubMed] [Google Scholar]
- 36.Petursson H, Sigurdsson JA, Bengtsson C, Nilsen TI, Getz L. Body configuration as a predictor of mortality: Comparison of five anthropometric measures in a 12 year follow-up of the Norwegian HUNT 2 Study. PLoS One [Internet] 2011;6(10):e26621. doi: 10.1371/journal.pone.0026621. [cited 16 October 2017] Available from: https://doi.org/10.1371/journal.pone.0026621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn HS, Bullard KM, Barker LE, Imperatore G. Differences between adiposity indicators for predicting all-cause mortality in a representative sample of United States non-elderly adults. PLoS One [Internet] 2012;7(11):e50428. doi: 10.1371/journal.pone.0050428. [cited 16 October 2017] Available from: https://doi.org/10.1371%2Fjournal.pone.0050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katzmarzyk PT, Mire E, Bray GA, Greenway FL, Heymsfield SB, Bouchard C. Anthropometric markers of obesity and mortality in white and African American adults: the Pennington Center longitudinal study. Obesity (Silver Spring, Md) 2013;21:1070–5. doi: 10.1002/oby.20151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carmienke S, Freitag MH, Pischon T, Schlattmann P, Fankhaenel T, Goebel H, et al. General and abdominal obesity parameters and their combination in relation to mortality: a systematic review and meta-regression analysis. Eur J Clin Nutr. 2013;67:573–85. doi: 10.1038/ejcn.2013.61. [DOI] [PubMed] [Google Scholar]
- 40.Ashwell M, Mayhew L, Richardson J, Rickayzen B. Waist-to-height ratio is more predictive of years of life lost than body mass index. PLoS One [Internet] 2014;9(9):e103483. doi: 10.1371/journal.pone.0103483. [cited 16 October 2017] Available from: https://doi.org/10.1371%2Fjournal.pone.0103483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pajunen P, Rissanen H, Laaksonen MA, Heliovaara M, Reunanen A, Knekt P. Sagittal abdominal diameter as a new predictor for incident diabetes. Diabetes Care. 2013;36:283–8. doi: 10.2337/dc11-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radholm K, Tengblad A, Dahlen E, Lanne T, Engvall J, Nystrom FH, et al. The impact of using sagittal abdominal diameter to predict major cardiovascular events in European patients with type 2 diabetes. Nutr Metab Cardiovasc Dis. 2017;27:418–22. doi: 10.1016/j.numecd.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Chalkias A, Nitsotolis T, Papalexandrou A, Mikros S, Iacovidou N, Xanthos T. Sagittal abdominal diameter may effectively predict future complications and increased mortality in intensive care unit patients with severe sepsis. J Crit Care. 2013;28:964–9. doi: 10.1016/j.jcrc.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 44.Lee MJ, Shin DH, Kim SJ, Yoo DE, Ko KI, Koo HM, et al. Sagittal abdominal diameter is an independent predictor of all-cause and cardiovascular mortality in incident peritoneal dialysis patients. PLoS One [Internet] 2013;8(10):e77082. doi: 10.1371/journal.pone.0077082. [cited 16 October 2017] Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0077082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ma WY, Yang CY, Shih SR, Hsieh HJ, Hung CS, Chiu FC, et al. Measurement of waist circumference: Midabdominal or iliac crest? Diabetes Care. 2013;36:1660–6. doi: 10.2337/dc12-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anunciacao PC, Ribeiro RCL, Pereira MQ, Comunian M. Different measurements of waist circumference and sagittal abdominal diameter and their relationship with cardiometabolic risk factors in elderly men. J Hum Nutr Diet [Internet] 2014;27(2):162–7. doi: 10.1111/jhn.12201. [cited 16 October 2017] Available from: http://onlinelibrary.wiley.com/enhanced/doi/10.1111/jhn.12201/ [DOI] [PubMed] [Google Scholar]
- 47.de Almeida Paula HA, de Cassia Lanes Ribeiro R, de Lima Rosado LEFP, Abranches MV, do Carmo Castro Franceschini S. Relationship between waist circumference and supine abdominal height measured at different anatomical sites and cardiometabolic risk factors in older women. J Hum Nutr Diet. 2012;25:563–8. doi: 10.1111/j.1365-277X.2012.01267.x. [DOI] [PubMed] [Google Scholar]
- 48.Kahn HS, Rissanen H, Bullard KM, Knekt P. The population distribution of the sagittal abdominal diameter (SAD) and SAD/height ratio among Finnish adults. Clin Obes. 2014;4:333–41. doi: 10.1111/cob.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kahn HS, Bullard KM. Indicators of abdominal size relative to height associated with sex, age, socioeconomic position and ancestry among US adults. PLoS One [Internet] 2017;12(3):e0172245. doi: 10.1371/journal.pone.0172245. [cited 16 October 2017]; Available from: https://doi.org/10.1371/journal.pone.0172245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kahn HS, Austin H, Williamson DF, Arensberg D. Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol. 1996;49:1017–24. doi: 10.1016/0895-4356(96)00113-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.