Introduction
Tympanic ears probably originated independently in lepidosaurs (lizards) and archosaurs (crocodiles and dinosaurs including birds). Despite their independent evolution, the middle ears of lepidosaurs and archosaurs share similar features: A thin and sensitive eardrum, a single, slender middle ear bone and an extracolumella connecting the columella to the eardrum. Most importantly for this review, both groups have internally coupled ears, which probably also evolved independently, possibly under selection for segregating sound sources (Fig. 1). In this review we will compare the structure and function of the coupled ears of lizards and crocodiles, since the bird ear is treated elsewhere (see Larsen et al., this volume). We will highlight what is currently known about computation of directional signals from the two ears for comparison with animals whose ears are uncoupled.
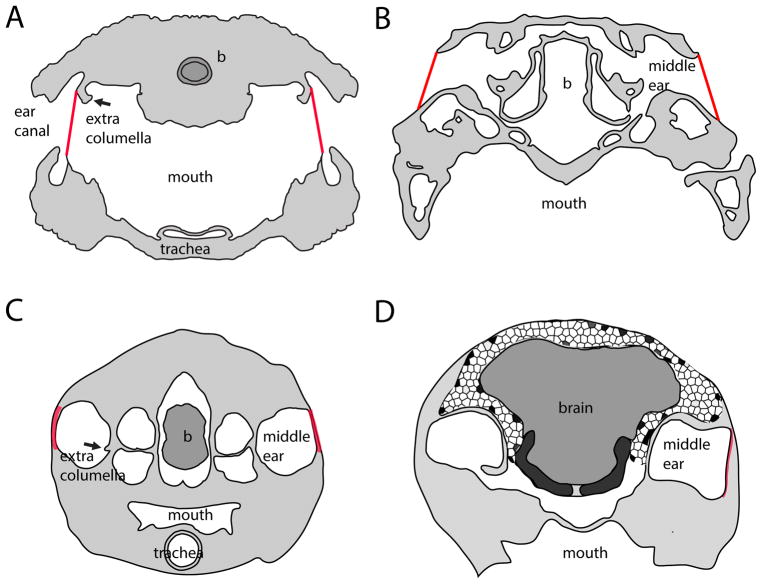
Figure 1. Coupling between the middle ears in Reptilia.
Cross-sections at the level of the middle ear through the heads or skulls of 4 Reptilia. A. Tokay gecko, B, American alligator (skull), C. red-eared slider turtle, and D. barn owl. A and C were traced from sections through decalcified skulls, B from CT images obtained from DigiMorph (University of Texas). The owl was imaged in a 7T micro-MRI at the Armed Forces Institue of Pathology (Rockville, MD) (Gans et al. 2012). Brains are shown in dark grey, bone and tissue in grey, approximate location of tympana as a red line, and sinuses as empty spaces. Note the direct coupling between the tympana in the gecko, both dorsal and ventral sinuses in the alligator, eustacian tubes in turtle (not shown) and a ventral sinus (interaural canal) in the barn owl. Dorsally, trabeculated bone connects the middle ears.
There actually appear to be three conditions; uncoupled ears, coupled ears, and partially coupled ears (Fig. 1). Most modern mammals have uncoupled ears (but see Mason, this volume), and thus must compute sound source location in the central nervous system. Lizard ears are coupled (Christensen-Dalsgaard 2005; Christensen-Dalsgaard and Manley 2005; Christensen-Dalsgaard and Manley 2008; Christensen-Dalsgaard et al. 2011) and in consequence all auditory responses in their brains should be directional, without a requirement for computation of sound source location. Crocodilians and birds have partially coupled ears (Bierman and Carr 2015), and the effects of this coupling on CNS computation of sound source direction vary with frequency. Coupling may improve the processing of low frequency directional signals in archosaurs, while higher frequency signals are progressively uncoupled.
I. Lizards, directly coupled ears
Internally coupled ears (ICE) are a defining feature of the tympanic ear of lepidosaurs (lizards). The ears are coupled acoustically through an essentially continuous connection with the mouth or the buccal cavity, so that the inner surface of the tympanic membrane is accessible to sound transmitted from the other ear or mouth (Christensen-Dalsgaard and Manley 2005; Christensen-Dalsgaard and Manley 2008) (Fig. 1A). This acoustical connection of the two middle ears, with almost perfect transmission from the contralateral ear, generates highly directional responses at the tympanum that are more pronounced in the lizards than in any other tetrapod studied. Coupling endows the auditory nerve with directional information, in addition to the typical coding of frequency, intensity and timing information found in animals with closed Eustachian tubes, like mammals and turtles (Fig. 1C).
In lizards, the middle ear cavity is a recess that is continuous with the buccal cavity. The columella may be seen in the open mouth, since it is exposed against the wall of recess and runs rostrally to insert into the oval window. Caudally, the columella contacts the extracolumella and tympanum (Werner and Wever 1972; Wever 1978; Saunders et al. 2000; Werner et al. 2008). Anatomical reconstructions of the whole head in geckos have provided accurate reconstructions of the cavity (Vossen et al. 2010; Christensen-Dalsgaard et al. 2011).
Laser vibrometry measurements
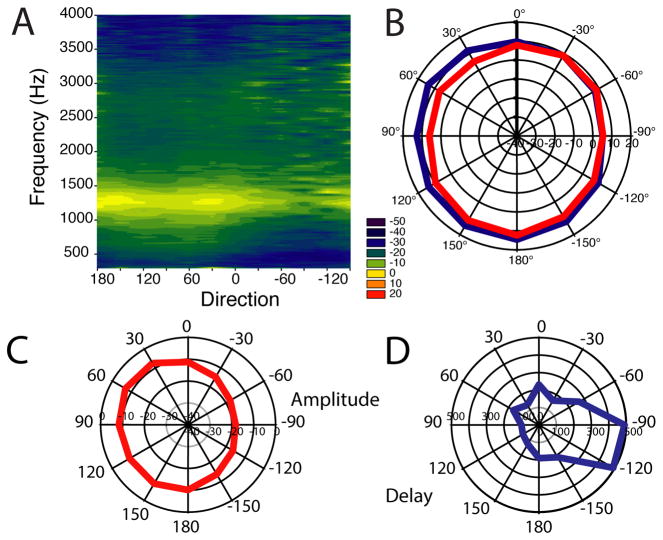
Laser vibrometry measurements of eardrum vibrations in response to free-field sound in a number of lizards show that the ear is very directional in a 2 kHz-wide band, with up to 40 dB differences between responses to ipsi- and contralateral stimulation (Christensen-Dalsgaard and Manley 2005; Christensen-Dalsgaard and Manley 2008). Furthermore, this directionality is asymmetrical across the midline, where there is a steep gradient (Fig. 2A). The phase of eardrum vibrations varies systematically with direction with maximal directional differences of 5.5 radians and the ipsilateral eardrum generally advanced in phase. Phase differences may be recalculated as eardrum delay differences by multiplying by 1/f*2π (see Fig. 2D in (Christensen-Dalsgaard et al. 2011). Delays increase systematically with direction and are largest at contralateral angles. The maximal recorded delay differences are about 500 μs in the geckos measured, thus creating enhanced ITD cues.
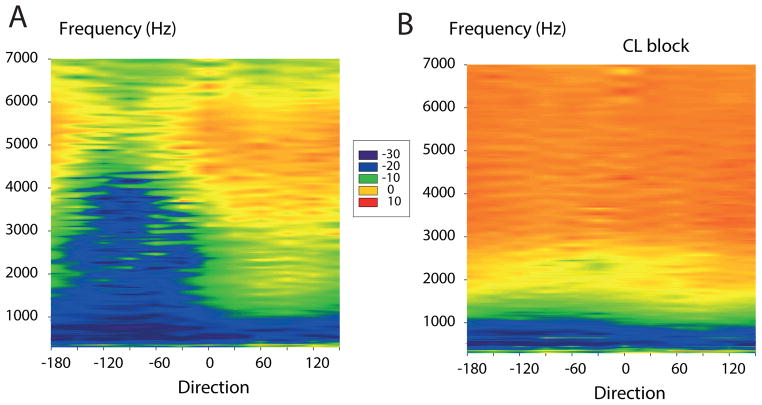
Figure 2. House gecko cylinder surface plots with and without an occluded contralateral eardrum.
A. Directionality of the eardrum in the house gecko, Hemidactylus. Eardrum transfer function data are shown as cylinder surface plots, interpolated contour plots of amplitude with direction and frequency as independent variables. Each horizontal line corresponds to a polar plot, and each vertical line corresponds to an amplitude spectrum of eardrum motion stimulated by sound from a certain direction. The X-axis shows direction angle in degrees (ipsilateral directions are positive, contralateral negative, 0° is frontal); y-axis frequency (Hz). The color scale is the eardrum vibration amplitude transfer function (decibel re. 1 mm/s/Pa).
B. Loss of directionality after occluding the contralateral eardrum with a dome of Vaseline (from (Christensen-Dalsgaard and Manley 2008).
This directionality depends on acoustical coupling of the eardrums, i.e., on the acoustical interference between the direct and indirect sound components at the eardrum, and could be abolished by occlusion of the contralateral eardrum (for example with Vaseline). The directional response of a house gecko (Hemidactylus frenatus, head width 10 mm) is shown in Fig. 2, and the color plot shows the eardrum vibration velocity transfer function, with negative angles being contralateral and positive ipsilateral. Note the large directional difference beginning with the frequencies above about 1500 Hz (Fig. 2A) and the reduced directionality after occlusion of the eardrum (Fig. 2B).
The correlation between the eardrum directionality and the strong acoustical coupling of the eardrums has been measured in five lizard species, by comparing the transfer function of the eardrum to the effects of ipsi- and contralateral local stimulation (Christensen-Dalsgaard and Manley 2008; Christensen-Dalsgaard et al. 2011). In all species, the frequency range of the maximal directionality transmission gain (contralateral transfer function divided by ipsilateral transfer function) is close to 0 dB, so sound arrives at the inner surface of the eardrum with almost the same amplitude as the sound arriving at the external surface. The phase spectrum of the transmission gain shows a linear dependence on frequency, indicating the fixed delay of the internal sound component. In the lizards studied, this delay is up to three times the delay corresponding to travel time across the head of the lizard (e.g., 100 μs instead of 30 μs for a gecko with a 1 cm head width).
Analytical models of the mouth cavity
These large measured delays are consistent with an analytical model of internally coupled ears that allows for calculation of a complete vibration profile of the tympanic membranes (Vossen et al. 2010). This model is based on casts of the mouth cavity, and allows for numerical calculation of the eigen-frequencies as well the prediction of a large internal delay (Vossen et al. 2010). Their analytical calculations show that the internally coupled ears increase the directional response, generating large directional internal amplitude and time differences. Numerical simulations of the eigen-functions in a realistically reconstructed mouth cavity provide additional insights into the effects of its complex geometry (Vossen et al. 2010). A simpler electrical analog model also captures the basic characteristics of the directionality produced by acoustical coupling (Fig. 3B), and when combined with EI-type processing, has been implemented in a robot that shows robust sound localization (Shaikh et al 2015, this volume, also see Fig. 5).
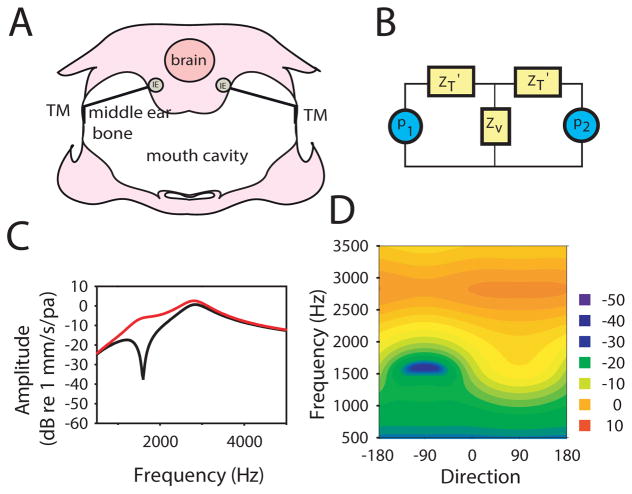
Figure 3. Basis of lizard ear directionality (modified from Christensen-Dalsgaard and Manley, 2005).
A: A simple model of lizard ear directionality (Fletcher 1992; Christensen-Dalsgaard and Manley 2005), using a diagram of a transverse section of a lizard head (Sceloporus; redrawn and altered from (Wever 1978). TM, tympanic membrane; C, columella; ET, Eustachian tube; MEC, middle ear cavity; RW, round window; OW, oval window (compare Fig. 1A).
B: The electrical analog circuit (ZV impedance of mouth cavity, ZT impedance of tympana, P1 and P2 sound inputs).
C: Vibration velocity spectra for ipsilateral (red curve) and contralateral (black curve) sound directions (compare Fig. 4B).
D: Cylinder surface plot of the model response (compare Fig. 3).
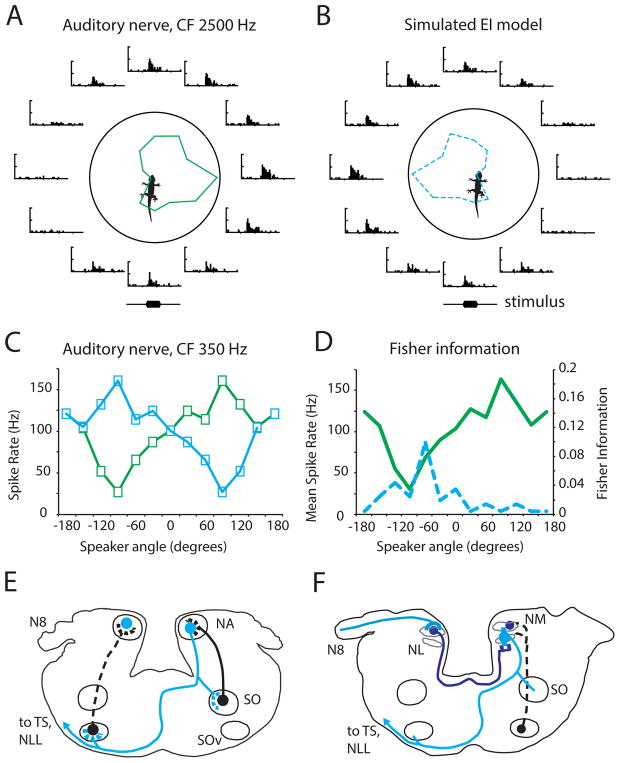
Figure 5. Binaural comparisons in free field responses from lizard auditory nerve.
A. Responses of a gecko auditory nerve fiber to free-field sound at CF (2500 Hz). The fiber was stimulated by 10 sweeps of 100 ms tone bursts from 12 directions, each shown as a PSTH. Blue line shows mean spike rate for each direction.
B. Simulated EI-response based on the data in Fig. 4A. The model assumes that the EI detector receives symmetrical input from the ipsilateral (inhibitory) and contralateral (excitatory) ear. The EI neuron spikes upon contralateral stimulation, but ipsilateral input blocks contralareral spiking arriving in the interval 0–1 ms. Spike trains are compared pair-wise, symmetrical across the midline, so each contralateral spike train is compared to each of the 10 spike trains from the symmetrical ipsilateral direction.
C. Responses of the same auditory nerve unit, plotting spike rate vs. speaker location. The unit responses are shown in green, then reflected to represent a similar response from the left auditory nerve (blue).
D. Same response as in C (green), plus Fisher information (dashed blue line) calculated for each 100 ms tone burst from each of the 12 directions.
E. Anatomical substrates for EI connections shown in a schematic cross section through the gecko brainstem at the level of the 8th nerve (N8), showing the cochlear nucleus angularis (NA) and its strong contralateral projection to the ventral superior olive (SOv). Descending connections from the ventral superior olive (SOv) and superior olive (SO) are shown in black. Modified from (Tang et al. 2012).
F. Anatomical substrates for EE connections shown in a schematic cross section through the gecko brainstem at the level of the 8th nerve (N8), showing the cochlear nucleus magnocellularis (NM) and its bilateral projections to nucleus laminaris (NL, dark blue). Nucleus laminaris neurons project to the olivary nuclei and to the midbrain torus (cyan). Descending connections from the ventral superior olive (SO) are shown in black. Modified from (Tang et al. 2012).
Auditory nerve physiology reflects laser vibrometry measurements
The basic characteristics of lizard auditory nerve responses are similar to those recorded in other Reptilia, frogs and mammals, while the special properties of the coupled ear are best seen with dichotic or free-field stimulation. Gecko auditory nerve units are sensitive to both interaural level differences (ILDs) and interaural time differences (ITDs), and binaural auditory stimulation using stimuli delivered through earphones reveals sensitivity to ITDs that are about 3 times larger than would be predicted from the gecko’s head width (Fig. 4) (Christensen-Dalsgaard et al. 2011). ITD tuning was found in fibers with best frequencies between 200 and 3,600 Hz, including frequencies above 800 Hz, where the phase locking is insignificant.
Figure 4. Transmission gain and effects of coupling on the tympanum and auditory nerve (from Christensen-Dalsgaard et al., 2011).
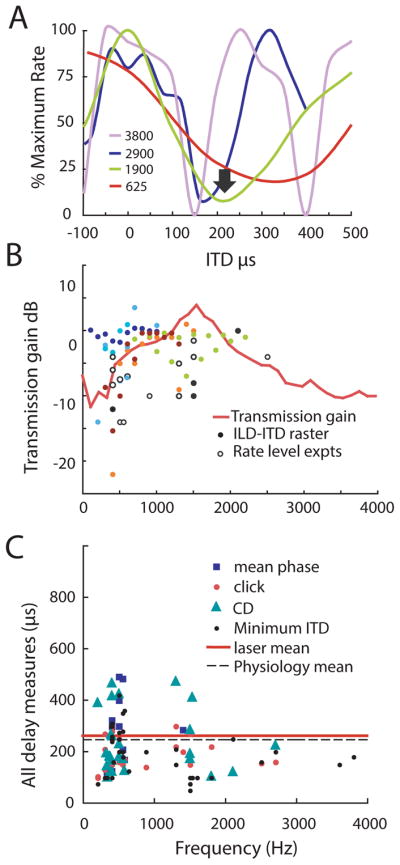
Physiological recordings from the auditory nerve all revealed similar interaural delays, consistent with the strong coupling between the eardrums (Christensen-Dalsgaard and Manley 2008; Vossen et al. 2010).
A: Dichotic stimulation reveals ITD sensitive responses in the auditory nerve. Plots of normalized firing rate from four nerve fibers with best frequencies between 400 and 3,800 Hz illustrate the common ITD that evokes a response minimum (arrow, 245 μs). The ITD response mimima most likely reflect the cancellation of eardrum motion by direct and indirect sound components impinging on both sides of the eardrum, as shown by the close correspondence between the response minima and the biophysical measures of interaural delay.
B: Auditory nerve recordings show strong coupling between the two eardrums, with almost equal sensitivity to ipsi- and contralateral stimulation over a wide frequency range, and comparable biophysical (red line) and neurophysiological transmission gains. Transmission gains were measured from rate-level curves with ipsilateral and contralateral stimulation (magenta symbols, rate- level experiments) and ITD-ILD minima (all other symbols, ITD-ILD raster). Different color symbols are from ITD-ILD experiments in different animals.
C. Three different physiological techniques yielded similar measures of interaural delays with respect to best frequency of all auditory nerve recordings. Dashed line shows the mean (245 us) from all 3 measures click delays, mean phase differences, and characteristic delays, while the red line shows the median, computed from biophysical measurements of interaural transmission gain phase (Christensen-Dalsgaard and Carr 2011).
ITD tuning recorded from gecko auditory nerve in response to earphone stimulation is largely indistinguishable from that recorded from binaural nuclei in birds and mammals, with the exception that gecko binaural delays are fixed, and determined by head size. The ITD tuning is caused by acoustical interaction of ipsi- and contralateral sound that, depending on the phase differences between ipsi- and contralateral sound, may cancel or amplify eardrum movements. As stated above, the special construction of the coupled ears generates a much larger internal delay than that predicted from the arrival-time delay at the eardrums. In the Tokay gecko (Christensen-Dalsgaard and Manley 2008; Christensen-Dalsgaard et al. 2011), delays are approximately three times the maximal arrival-time delay of 73 μs for a typical Tokay head width of 2.5 cm, or about 220 μs. The observed ITD response minima reflect the cancellation of contralateral eardrum motion from the summing both external sound pressures and internal sound pressures via the other side of the eardrum (Fig. 4A). Also, the neurophysiological measurements show strong crosstalk, with similar sensitivity for ipsi- and contralateral stimulation in a wide frequency band (Fig. 4B).
There are several key tests for binaural sensitivity, and all reveal similar ITD minima close to the calculated or measured delay across the mouth (Fig. 4C). One test requires that the time difference between the two ears matches the recorded tuning to ITD; in essence the recorded best ITD should be equal and opposite to the external time difference. In units with significant phase locking with best frequencies below 1 kHz, phase locked responses to either ipsi- or contralateral tonal stimuli at or near best frequency showed mean phase differences of about 280 μs across the mouth that matched recorded ITD minima. ITD sensitivity was also measured at several different frequencies to calculate characteristic delay, or interaural time difference regardless of frequency. Gecko auditory nerve recordings all had characteristic delays of about 240μs. A third test states that click or conduction delays between the ears (194 μs) should match ITDs. Thus the three neural measures yield similar delays, comparable to the measured ITD response minima of 200 μs (Christensen-Dalsgaard et al. 2011). All measures were similar to the median transmission delay of 260 μs found in the laser measurements (Fig. 4C).
Thus, in many respects, the gecko auditory nerve responses resemble responses produced by neural interactions in the avian nucleus laminaris and the mammalian superior olivary nuclei, i.e. after several stages of neural processing (for reviews, see (Klump 2000; Konishi 2003; McAlpine and Grothe 2003; Grothe et al. 2010). An important difference between the gecko data and the binaural responses recorded in birds and mammals is that gecko nerve responses reflect the interaction of ipsi- and contralateral inputs on the motion of the eardrum and therefore the ITD cues are not independent of frequency. Also, in natural, free-field conditions, the interactions of the sound waves on each side of the tympana should simply reflect the strong directionality of the eardrum (Christensen-Dalsgaard and Manley 2005) and unpublished data, Figures 3, 4A and 5). We note that in any animal with coupled ears, the use of dichotic stimulation to characterize binaural processing presents some difficulties, since the underlying assumption, that the ears are stimulated independently, cannot be valid.
Directionality of gecko auditory nerve fiber responses in free field
In order to understand the consequences of coupling on lizard hearing in more natural environment, we investigated the responses to directional sound in auditory nerve fibers in free field (Christensen-Dalsgaard and Carr 2011). Recordings in free field yield strongly directional auditory nerve responses at both low (200–400 Hz) and high frequencies (1–2 kHz) with an ovoidal directivity that resembles the eardrum directivity (example in Fig. 5A). Furthermore, geckos are highly vocal, and the nerve fiber directionality to components of the call is very pronounced Since the auditory nerve fibers show strong directionality, effectively every neuron in the lizard auditory pathway is directional, and the processing of sound direction in the lizard CNS is likely very different from animals with uncoupled ears.
A straightforward assumption is that binaural comparison by EI-type neurons would produce an even more strongly lateralized response. A simplified model for binaural comparison using eardrum vibration data shows very strong lateralization and a clear demarcation of the midline (Christensen-Dalsgaard and Manley 2005; Christensen-Dalsgaard and Manley 2008). We have tested this assumption by modeling the EI response on the actual nerve data simply by comparing individual spike trains from ‘symmetrical’ ipsi- and contralateral directions (symmetrical across the midline, e.g. +30 and −30). Assuming that an ipsilateral spike arriving 1 ms before the contralateral spike inhibits the response in the EI neuron, pairs of individual spike trains can be compared this way. A result is shown in Fig. 5B. Surprisingly, and generally for our sample, the EI processing has very little effect, because the nerve response is already strongly lateralized, and the timing of individual spike trains is so variable that the strong effect seen in averaged eardrum vibration data is reduced. Whether the model data accurately reflects real EI processing in the gecko brain remains to be investigated, but the result suggests that very little CNS processing of the auditory nerve input might be needed. This is supported by results from free field stimulation in the torus semicircularis of Gekko gecko (Manley 1981). Gecko torus units exhibited directivity with activity almost completely suppressed at ipsilateral angles. Manley (1981) pointed out that these responses could have been generated by both neural inhibition and acoustical interactions, but comparison to the auditory nerve data suggest that this putative neural inhibition does not produce a much stronger lateralization than observed peripherally. It is likely, however, that EI processing can extend the dynamic range of directionality (limited by saturation of the firing rate of auditory nerve fibers). It is possible that EE-type processing could be more important in order to evaluate absolute sound levels (see also Bee and Christensen-Dalsgaard, this volume). Fisher information analyses, based on the direction-dependent spike rate, show which directions are most robustly coded by spike-rate changes (Fig. 5D). In most of the units investigated the information maximum is at the frontal directions, supporting the role of auditory nerve directionality in orienting geckos to sound.
II. Archosaur ears, coupled through sinuses
In crocodilians, the tympanic cavities are connected by sinuses above and below the braincase (Fig. 1B) (Witmer et al. 2008; Witmer and Ridgely 2008; Bierman et al. 2014). The common ancestors of recent archosaurs, crocodiles and dinosaurs lived about 240 million years ago, and may not have had the extensive cranial sinuses found in extant archosaurs (Witmer and Ridgely 2008). However, several bird-like dinosaurs from the late Cretaceous also demonstrate ventral and dorsal intertympanic pathways, with varying degrees of trabeculation (Starck 1994; Clark et al. 2002; Kundrát and Janáček 2007). Cranial sinuses lighten the skull in addition to coupling the ears, and skull pneumaticity appears to have evolved multiple times among the archosaurs (Dufeau 2011; Dufeau and Witmer 2015), although bird skulls have changed more over evolutionary time. A recent series of papers from the Avian Phylogenomics Group has characterized the genomes of extant birds, and the American alligator, saltwater crocodile, and Indian gharial (Zhang et al. 2014). These papers confirm the sister relationship between crocodiles and gharials, the relationship with birds as members of extant Archosauria, and the outgroup status of turtles relative to crocodilians. The crocodilian genome shows a slow rate of genome evolution (Green et al. 2014), as opposed to birds, which have undergone rapid skeletal adaptation including the development of paedomorphic skulls with enlarged eyes and brains and reduced jaws (Lee et al. 2014). Since another paper in this volume, Larsen et al. (2016), discusses the role of avian interaural canals, we will confine this section to a review of coupled ears in crocodilians. Like lizards, the ears of crocodilians are acoustically coupled, not through the buccal cavity, but by air-filled cranial sinuses (Bierman et al. 2014) (Fig. 1B). This coupling generates increased directional cues and appears to modify the available ITDs. The coupling is not as strong as in lizards, and is more dependent on frequency.
Archosaur skulls
The tympanic cavities or middle ears are coupled dorsally via the intertympanic recess, and ventrally, by the quadrate sinus, the pharyngotympanic (Eustachian) recess and the median pharyngeal recess (Witmer and Ridgely 2008; Witmer and Ridgely 2009; Bierman et al. 2014). These paratympanic sinuses widen to form a large midline space below the cranium. If viewed from more anterior plane, the ventral canal is larger than it appears in Figure 1B. This coupling has been hypothesized to generate increased directional cues and modify available ITDs (Dufeau and Witmer 2010; Bierman et al. 2014). Sound transmission through these sinuses would certainly allow the eardrums of alligators to act to some degree like pressure-difference receivers although the strength of the coupling, and its dependence upon frequency, is currently not well understood.
CT-based material has revealed the extent of the paratympanic sinuses that couple the middle ears (Witmer and Ridgely 2009; Dufeau 2011; Bierman et al. 2014; Dufeau and Witmer 2015). In birds, the ventral interaural pathway, or interaural canal, is most prominent (Calford and Piddington 1988), since dorsal connections are via trabeculated bone (Larsen et al. 1992). In alligators, both pathways are patent and well developed, and it has been proposed that the dorsal pathway further couples the middle ear cavities and could amplify the directionality afforded by the ventral interaural pathway (Kundrát and Janáček 2007; Bierman et al. 2014). The effects of coupling are frequency dependent, but at low frequencies, coupled middle ears can increase the physiological range of ITDs by a factor of about 3 in birds, and in simulations (Coles et al. 1980; Hyson et al. 1994; Michelsen and Larsen 2008; Köppl and Carr 2008; Vossen et al. 2010; Bierman et al. 2014).
Laser vibrometry measurements reveal eardrum directionality
In alligators, laser Doppler vibrometry has shown that tympanic membrane motion is directional in response to free field sound stimuli (Bierman et al. 2014) (Fig. 6). The directional sensitivity of the eardrum is largest around 1 kHz, with maximal ipsilateral–contralateral differences from 10 to 18 dB at the best directional frequency, or about 800–1400 Hz. The difference between ipsilateral and contralateral responses at 1 kHz is about 10 dB. The phase of the eardrum vibration transfer function also varies systematically with frequency and direction (Fig. 6D). At 1 kHz, the ipsilateral - contralateral average phase difference corresponds to an average maximal ITD on the eardrum of nearly 300 μs. The directionality was most likely due to coupling via sinuses, not due to head shadowing effects, which were minimal at 1 kHz (Bierman et al. 2014).
Figure 6. Directionality of tympanic membrane vibration in alligators.
A. Cylindrical surface plot of the eardrum vibration transfer function showing eardrum vibration velocity transfer function (color scale, in dB re. 1 mms–1 Pa–1) as a function of direction (x-axis, positive angles are ipsilateral, negative contralateral, the animal facing 0°) and frequency (y-axis).
B. Polar plot of eardrum vibration velocity transfer function at 1 kHz with (red) and without (blue) blockage of the contralateral eardrum in a single alligator. Blockage of the contralateral ear significantly alters ipsilateral eardrum vibration amplitude except when the sound is presented adjacent to the ipsilateral ear (60, 90 and 120°). For all panels, the snout is at 0°, the tail is at 180°, the ipsilateral ear corresponds to positive degrees and the contralateral ear to negative degrees.
C. Polar plots of the averaged eardrum vibration amplitude (transfer functions) to 1 kHz directional sound in 5 alligators.
D. Eardrum transfer function phases relative to the 90° (ipsilateral) direction were converted to delays by multiplying the phase difference by (2π×0.001 s). The delay at 90° was set to 100 μs. (modified from Figure 9, Bierman et al., 2015).
What does this directionality mean for the alligator? Bierman et al (2014) calculated the interaural transmission gain from the ratio of eardrum vibration transfer functions under contralateral and ipsilateral stimulation and found it to be approximately −5 dB (i.e. a ratio of 0.56) at behaviorally relevant frequencies, around 1 kHz (Fig. 6). Arithmetical addition of internal and external sound at the eardrum would result in a directionality of approximately 11 dB, ranging from a value of 0.44 (destructive interference) to 1.56 (constructive interference) (Christensen-Dalsgaard and Manley 2008; Bierman et al. 2014). Since this prediction is close to the actual free field directionality, this suggests that the directionality can largely be ascribed to the coupling between the two ears. Additional sound inputs via the nares and Eustachian tubes may be insignificant (Owen 1843; Bierman et al. 2014).
Auditory brainstem response (ABR) data have also shown directional responses at the auditory nerve level (peak 1 of the ABR), and the neural directionality corresponds to the eardrum directionality (Bierman et al. 2014).
Auditory pathways are similar in birds and crocodilians
Consistent with both the anatomical and laser vibrometry measurements reviewed above, alligators and birds show a larger physiological range of ITDs than would be predicted from their head sizes, presumably as a result of the coupling between their ears (Köppl and Carr 2008; Carr et al. 2009; Bierman et al. 2014). Both crocodilians and birds have well-developed auditory systems, especially in comparison to turtles, the sister group to archosaurs. Crocodilians have large external ears covered by an ear flap, and a well developed inner ear with a long basilar membrane and unidirectional population of hair cells (Düring et al. 1974; Gleich and Manley 2000). Compared to most birds, however, they have a relatively low frequency hearing range (20 – 2,800 Hz) (Beach 1944; Manley 1970; Wever 1978; Rosowski and Saunders 1980; Klinke and Pause M 1980; Strain et al. 1987; Higgs et al. 2002).
Interaural coupling increases the ear’s directionality, and both cochlear microphonic and single unit studies of NL in chicken (Hyson et al. 1994; Köppl and Carr 2008) and barn owl (Carr and Köppl 2004; Palanca-Castan and Köppl 2015b) suggest that coupling can increase the physiological range of ITDs by a factor of about 3 at low frequencies. An increased range of ITDs is consistent with recordings of ITD sensitive neurons in alligator NL, which also show a much larger range of ITDs than would be predicted from the alligator’s head width (Carr et al. 2009; Bierman et al. 2014) (Fig. 7B). A larger range of ITDs makes the job of detecting ITDs easier, since there are more microseconds per degree azimuth. A larger range of ITDs also makes the job of detecting low frequency ITDs easier because low frequencies have intrinsically high temporal dispersion (Hill et al. 1989; Köppl 1997), that is, their temporal coding is less accurate and their resolution is lower.
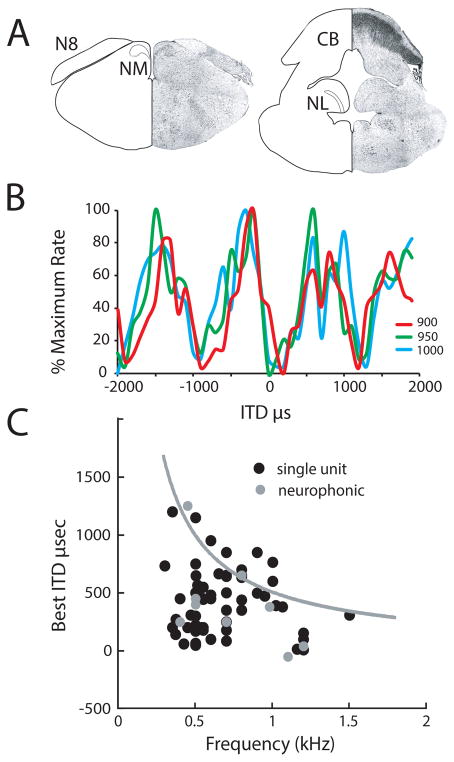
Figure 7. ITD responses within alligator NL reveal a wide range of best ITDs.
A. Left: Schematic cross section and matching photomicrograph through the alligator brainstem at the level of the 8th nerve, showing the cochlear nucleus magnocellularis (NM) and the ascending nerve root to cochlear nucleus angularis (NA). Right: Schematic crosssection and matching photomicrograph through the alligator brainstem at the level of the nucleus laminaris (NL) extending into the IVth ventricle.
B. Interaural delay curves plot the response of an NL neuron against changing ITDs in alligator at 3 frequencies around best frequency. This single-unit was recorded in the right NL and had a best ITD of −162 μs, i.e. in the contralateral hemifield.
C. Best ITDs were distributed throughout the contralateral hemifield. Like all animals described so far, best ITDs showed less scatter with increasing best frequency for all single units (black circles) and neurophonic recordings (gray circles). The gray line follows ITD values corresponding to 0.5 cycle, the “pi-limit.” Data for B and C modified from (Carr et al. 2009).
How are these large ITDs represented in the alligator’s auditory system? Crocodilians and birds share a similar organization of their central auditory system, as far as is known (Leake 1974; Carr and Soares 2006; Carr et al. 2009), and we will draw on studies of both birds and crocodilians for this discussion of neural circuits and sound localization. Birds have been far more intensively studied than crocodilians (for reviews see (Carr 1993; Burger and Rubel 2008; Grothe et al. 2010); Larsen article in this volume), but both bird and crocodilian auditory systems have parallel ascending pathways that may be specialized for encoding timing and loudness. In both birds and crocodilians, the auditory nerve enters the brain and divides to form an ascending branch that largely terminates in the nucleus angularis (NA), and the descending branch in the nucleus magnocellularis (NM) (Leake 1974) (Fig. 7A). The nucleus magnocellularis projects bilaterally to the nucleus laminaris (NL), which encodes ITD, and projects to the superior olive, to the lemniscal nuclei, and to the central nucleus of the auditory midbrain (Grothe et al. 2004; Carr et al. 2009). We will review ITD coding in the next section.
A wide range of ITDs in crocodilians
Like birds, the neurons in the crocodilian auditory first- and second order nuclei display specializations that appear to be related to the encoding of temporal information. These include robust endbulb synapses from the auditory nerve to the nucleus magnocellularis (Leake 1974), and the few and/or short dendrites and thick axons of the magnocellular and laminaris neurons (Carr and Soares 2002). In alligators, precisely timed spikes in the first-order nucleus magnocellularis (NM) encode the timing of sounds, and NM neurons project to neurons in the nucleus laminaris (NL) that detect interaural time differences (Carr et al. 2009). In vivo recordings from NL neurons show that the arrival time of phase-locked spikes differs between the ipsilateral and contralateral inputs. When this disparity is nullified by their best ITD, the neurons respond maximally (Fig. 7B). Thus crocodilian NL neurons act as coincidence detectors, and employ similar algorithms for ITD detection to birds (Bierman and Carr 2015). Nevertheless, the range of best ITDs represented in alligator NL was much larger than in birds, however, and extended from 0 to 1000 μs contralateral, with a median ITD of 450 μs (Fig. 7C, (Carr et al. 2009). This very large range of ITDs may reflect the increased directionality of the coupled ears at the low sound frequencies heard by crocodilians. More fundamentally, however, the directionality of the coupled ear produces strongly lateralized responses, also at these low frequencies. These lateralized responses are not ideal for a coincidence detector, because one of its inputs will be weak at almost all directions and the ITD comparison therefore difficult. Thus, the presence of strong interaural coupling could limit coincidence detection.
The relatively low frequency crocodilian hearing range is relevant to discussion of how ITDs are coded. The response of NL neurons has been described as a cross-correlation of narrow-band inputs from the ipsi- and contralateral ears (Batra and Yin 2004; Fischer and Pena 2009). The accuracy of a place code decreases at low frequencies, however, because the ITD curves broaden, so even a large change in ITD leads to only a small change in spike rate. Also, with low best frequency ITD functions, the response maxima often lie outside the physiological range of ITDs, so that the resolution of place code of best ITDs decreases (Harper and McAlpine 2004; Palanca-Castan and Köppl 2015b; Palanca-Castan and Köppl 2015a).
Conclusions
The degree of coupling varies among reptiles. Lizard ears in general show the strongest coupling of any tetrapod (Christensen-Dalsgaard 2005; Christensen-Dalsgaard and Manley 2005; Christensen-Dalsgaard and Manley 2008; Christensen-Dalsgaard et al. 2011), with very directional ears over a large frequency band. In consequence all auditory responses in their brains within this frequency range should be directional, without a requirement for computation of sound source location. Lizards should, of course, still require binaural comparisons to differentiate between loud sounds at a poor location and quiet sounds at a good location.
Crocodilians and birds have coupled ears with weaker interaural transmission than lizards (Bierman and Carr 2015). The effects of their coupling on CNS computation of sound source direction vary with frequency. Coupling may improve the processing of low frequency directional signals, while higher frequency signals appear to be progressively uncoupled, at least in birds (Moiseff and Konishi 1981).
The increased directionality of the coupled ears in lizards and crocodilians creates an effectively “larger” head and larger physiological range of ITDs. This real physiological range is relevant to current theories of sound localization. Small early mammals are assumed to have relied upon a population coding strategy (Grothe and Pecka 2014), which requires comparisons of firing rates in ITD sensitive populations on each side of the brain. Archosaurs, however, use a place code or map for ITDs, as seen in chickens and barn owls. The relative advantages of the two coding strategies depend both upon head size and frequency range. The presence of strong acoustical coupling could bias lizards towards a population coding strategy, since the strongly lateralized inputs from the auditory nerve would not provide coincidence detectors with sufficient information.
Despite notable differences in peripheral structure and physiology reviewed here, the auditory brainstem circuits in lizards and archosaurs are structurally similar (Yan et al. 2010; Tang et al. 2012; Bierman and Carr 2015), and are also organized along similar lines to the ascending auditory systems of amphibians and mammals (for reviews, see Grothe et al. 2004; Grothe et al. 2010).
Contributor Information
Catherine E. Carr, Department of Biology, University of Maryland College Park, College Park, Maryland USA 20742
Jakob Christensen-Dalsgaard, Department of Biology, University of Southern Denmark, DK-5230 Odense, Denmark.
Hilary Bierman, Department of Biology, University of Maryland College Park, College Park, Maryland USA 20742.
Literature cited
- Batra R, Yin TCT. Cross correlation by neurons of the medial superior olive: a reexamination. JARO. 2004;5:238–252. doi: 10.1007/s10162-004-4027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach FA. Responses of captive alligators to auditory stimulation. American Naturalist. 1944;78:481–505. [Google Scholar]
- Bierman HS, Carr CE. Sound localization in the alligator. Hearing Research. 2015 doi: 10.1016/j.heares.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierman HS, Thornton JL, Jones HG, et al. Biophysics of directional hearing in the American alligator (Alligator mississippiensis) J Exp Biol. 2014;217:1094–1107. doi: 10.1242/jeb.092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger RM, Rubel EW. Encoding of Interaural Timing for Binaural Hearing. In: Basbaum Allan I, Kaneko Akimichi, Shepherd Gordon M, Westheimer Gerald., editors. The Senses: A Comprehensive Reference, Vol 3, Audition, Peter Dallos and Donata Oertel. San Diego: Academic Press; 2008. pp. 613–630. [Google Scholar]
- Calford M, Piddington R. Avian interaural canal enhances interaural delay. J Comp Physiol A. 1988;162:503–510. [Google Scholar]
- Carr CE. Processing of temporal information in the brain. Annu Rev Neurosci. 1993;16:223–243. doi: 10.1146/annurev.ne.16.030193.001255. [DOI] [PubMed] [Google Scholar]
- Carr CE, Köppl C. Coding interaural time differences at low best frequencies in the barn owl. J Physiol Paris. 2004;98:99–112. doi: 10.1016/j.jphysparis.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Carr CE, Soares D. 2.21 Shared Features of the Auditory System of Birds and Mammals. Evolution of Nervous Systems. 2006:1–15. [Google Scholar]
- Carr CE, Soares D. Evolutionary convergence and shared computational principles in the auditory system. Brain Behav Evol. 2002;59:294–311. doi: 10.1159/000063565. [DOI] [PubMed] [Google Scholar]
- Carr CE, Soares D, Smolders J, Simon JZ. Detection of interaural time differences in the alligator. J Neurosci. 2009;29:7978–7990. doi: 10.1523/JNEUROSCI.6154-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J. Directional hearing in nonmammalian tetrapods. Sound Source Localization. 2005:67–123. doi: 10.1007/0-387-28863-5. [DOI] [Google Scholar]
- Christensen-Dalsgaard J, Carr CE. Directionality of gecko auditory nerve fibers with free field stimulation. Assoc Res Otolaryngol Abs 2011 [Google Scholar]
- Christensen-Dalsgaard J, Manley GA. Acoustical coupling of lizard eardrums. JARO. 2008;9:407–416. doi: 10.1007/s10162-008-0130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Manley GA. Directionality of the lizard ear. J Exp Biol. 2005;208:1209–1217. doi: 10.1242/jeb.01511. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Tang Y-Z, Carr CE. Binaural processing by the gecko auditory periphery. J Neurophys. 2011;105:1992–2004. doi: 10.1152/jn.00004.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Norell MA, Rowe T. Cranial Anatomy of Citipati Osmolskae (Theropoda, Oviraptorosauria), and a Reinterpretation of the Holotype of Oviraptor Philoceratops 2002 [Google Scholar]
- Coles R, Lewis DB, Hill KG, et al. Directional Hearing in the Japanese Quail (Coturnix Coturnix Japonica) II. Cochlear Physiology. J Exp Biol. 1980;86:153–170. [Google Scholar]
- Dufeau D. The Evolution of Cranial Pneumaticity in Archosauria: Patterns of Paratympanic Sinus Development. Ohio University; 2011. [Google Scholar]
- Dufeau D, Witmer LM. Acoustic resonance of the middle-ear in Alligator - implications for behavioral correlations. Integrative and Comparative Biology. 2010;50(Supplement 1) [Google Scholar]
- Dufeau DL, Witmer LM. Ontogeny of the Middle-Ear Air-Sinus System in Alligator mississippiensis (Archosauria: Crocodylia) PLoS ONE. 2015;10:e0137060–25. doi: 10.1371/journal.pone.0137060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Düring M, Karduck A, Richter H-G. The fine structure of the inner ear in caiman crocodilus. Anatomy embryol. 1974;145:41–65. doi: 10.1007/BF00519125. [DOI] [PubMed] [Google Scholar]
- Fischer BJ, Pena JL. Bilateral matching of frequency tuning in neural cross-correlators of the owl. Biol Cyber. 2009;100:521–531. doi: 10.1007/s00422-009-0312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher NH. Acoustic systems in biology. Oxford University Press; USA: 1992. [Google Scholar]
- Gans E, Willis KL, Bierman HS, Carr CE. The interaural canal of the barn owl, Tyto alba. Integrative and Comparative Biology. 2012;52(Supplement 1) [Google Scholar]
- Gleich O, Manley GA. The Hearing Organ of Birds and Crocodilia. In: Dooling RJ, Fay RR, Popper AN, editors. Comparative hearing: Birds and reptiles. Springer; 2000. pp. 70–138. [Google Scholar]
- Green RE, Braun EL, Armstrong J, et al. Three crocodilian genomes reveal ancestral patterns of evolution among archosaurs. Science. 2014;346:1254449. doi: 10.1126/science.1254449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Carr CE, Casseday J, et al. In: The evolution of central pathways and their neural processing patterns. Manley GA, Popper AN, Fay RR, editors. Springer; New York: 2004. pp. 289–359. [Google Scholar]
- Grothe B, Pecka M. The natural history of sound localization in mammals - a story of neuronal inhibition. Front Neural Circuits. 2014;8:116. doi: 10.3389/fncir.2014.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grothe B, Pecka M, McAlpine D. Mechanisms of sound localization in mammals. Physiol Rev. 2010;90:983–1012. doi: 10.1152/physrev.00026.2009. [DOI] [PubMed] [Google Scholar]
- Harper NS, McAlpine D. Optimal neural population coding of an auditory spatial cue. Nature. 2004;430:682–686. doi: 10.1038/nature02768. [DOI] [PubMed] [Google Scholar]
- Higgs DM, Brittan-Powell EF, Soares D, et al. Amphibious auditory responses of the American alligator (Alligator mississipiensis) Journal of Comparative and Physiological Psychology. 2002;188:217–223. doi: 10.1007/s00359-002-0296-8. [DOI] [PubMed] [Google Scholar]
- Hill KG, Stange G, Mo J. Temporal synchronization in the primary auditory response in the pigeon. Hearing Research. 1989;39:63–73. doi: 10.1016/0378-5955(89)90082-8. [DOI] [PubMed] [Google Scholar]
- Hyson RL, Overholt EM, Lippe WR. Cochlear microphonic measurements of interaural time differences in the chick. Hearing Research. 1994;81:109–118. doi: 10.1016/0378-5955(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Klinke R, Pause M. Discharge properties of primary auditory fibres in Caiman crocodilus: comparisons and contrasts to the mammalian auditory nerve. Exp Brain Res. 1980;38:137–150. doi: 10.1007/BF00236735. [DOI] [PubMed] [Google Scholar]
- Klump GM. Comparative Hearing: Birds and Reptiles. Springer; New York, New York, NY: 2000. Sound Localization in Birds; pp. 249–307. [Google Scholar]
- Konishi M. Coding of auditory space. Annu Rev Neurosci. 2003;26:31–55. doi: 10.1146/annurev.neuro.26.041002.131123. [DOI] [PubMed] [Google Scholar]
- Köppl C. Phase locking to high frequencies in the auditory nerve and cochlear nucleus magnocellularis of the barn owl, Tyto alba. J Neurosci. 1997;17:3312–3321. doi: 10.1523/JNEUROSCI.17-09-03312.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köppl C, Carr CE. Maps of interaural time difference in the chicken’s brainstem nucleus laminaris. Biol Cyber. 2008;98:541–559. doi: 10.1007/s00422-008-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundrát M, Janáček J. Cranial pneumatization and auditory perceptions of the oviraptorid dinosaur Conchoraptor gracilis (Theropoda, Maniraptora) from the Late Cretaceous of Mongolia. Naturwissenschaften. 2007;94:769–778. doi: 10.1007/s00114-007-0258-7. [DOI] [PubMed] [Google Scholar]
- Larsen ON, Dooling RJ, Ryals BM. Roles of Intracranial Air Pressure in Bird Audition. 1992. pp. 11–14. [Google Scholar]
- Leake PA. Central Projections of the Statoacoustic Nerve in Caiman crocodilus. Brain Behav Evol. 1974;10:170–196. doi: 10.1159/000124311. [DOI] [PubMed] [Google Scholar]
- Lee M, Cau A, Naish D, Dyke GJ. Sustained miniaturization and anatomical innovation in the dinosaurian ancestors of birds. Science. 2014;345:558–562. doi: 10.1126/science.1240064. [DOI] [PubMed] [Google Scholar]
- Manley GA. A review of the auditory physiology of reptiles. Progress in Sensory Physiology. 1981;2:49–134. [Google Scholar]
- Manley GA. Frequency sensitivity of auditory neurons in the caiman cochlear nucleus. Zeitschrift für vergleichende Physiologie. 1970;66:251–256. [Google Scholar]
- McAlpine D, Grothe B. Sound localization and delay lines--do mammals fit the model? Trends in Neurosci. 2003;26:347–350. doi: 10.1016/S0166-2236(03)00140-1. [DOI] [PubMed] [Google Scholar]
- Michelsen A, Larsen ON. Pressure difference receiving ears. Bioinspiration & biomimetics. 2008;3:11001. doi: 10.1088/1748-3182/3/1/011001. [DOI] [PubMed] [Google Scholar]
- Moiseff A, Konishi M. The owl’s interaural pathway is not involved in sound localization. J Comp Physiol A. 1981;144:299–304. [Google Scholar]
- Owen R. On the Communications between the Tympanum and Palate in the Crocodilian Reptiles.[Abstract] Abstracts of the Papers Communicated to the Royal Society of …. 1843 doi: 10.2307/111072. [DOI]
- Palanca-Castan N, Köppl C. In Vivo Recordings from Low-frequency Nucleus Laminaris in the Barn Owl. Baltimore: 2015a. pp. 452–453. [DOI] [PubMed] [Google Scholar]
- Palanca-Castan N, Köppl C. Change in the coding of interaural time difference along the tonotopic axis of the chicken nucleus laminaris. Front Neural Circuits. 2015b;9:43. doi: 10.3389/fncir.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosowski JJ, Saunders JC. Sound transmission through the avian interaural pathways. Journal of Comparative and Physiological Psychology. 1980;136:183–190. doi: 10.1007/BF00657532. [DOI] [Google Scholar]
- Saunders JC, Duncan RK, Doan DE, Werner YL. The middle ear of reptiles and birds. In: Dooling RJ, Fay RR, Popper AN, editors. Comparative hearing: Birds and reptiles. Springer; 2000. pp. 70–138. [Google Scholar]
- Starck JM. Comparative anatomy of the external and middle ear of palaeognathous birds. Advances in Anatomy, Embryology and Cell Biology. 1994;131:1–137. doi: 10.1007/978-3-642-79592-3. [DOI] [PubMed] [Google Scholar]
- Strain GM, Tucker TA, Graham MC, O’Malley NA. Brain-stem auditory evoked potentials in the alligator. Effects of temperature and hypoxia. Electroencephalography and Clinical Neurophysiology. 1987;67:68–76. doi: 10.1016/0013-4694(87)90165-9. [DOI] [PubMed] [Google Scholar]
- Tang Y-Z, Christensen-Dalsgaard J, Carr CE. Organization of the auditory brainstem in a lizard, Gekko gecko. I. Auditory nerve, cochlear nuclei, and superior olivary nuclei. J Comp Neurol. 2012;520:1784–1799. doi: 10.1002/cne.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossen C, Christensen-Dalsgaard J, van Hemmen JL. Analytical model of internally coupled ears. J Acoust Soc Am. 2010;128:909–918. doi: 10.1121/1.3455853. [DOI] [PubMed] [Google Scholar]
- Werner YL, Montgomery LG, Seifan M, Saunders JC. Effects of age and size in the ears of gekkotan lizards: auditory sensitivity, its determinants, and new insights into tetrapod middle-ear function. Pflugers Arch. 2008;456:951–967. doi: 10.1007/s00424-008-0462-0. [DOI] [PubMed] [Google Scholar]
- Werner YL, Wever EG. The function of the middle ear in lizards:Gekko gecko andEublepharis macularius (Gekkonoidea) J Exp Zool. 1972;179:1–16. doi: 10.1002/jez.1401790102. [DOI] [Google Scholar]
- Wever EG. The reptile ear: its structure and function. Princeton, NJ: Princeton University Press; 1978. [Google Scholar]
- Witmer LM, Ridgely R, Dufeau D, Semones M. Using CT to peer into the past: 3D visualization of the brain and ear regions of birds, crocodiles, and nonavian dinosaurs. Anatomical Imaging. 2008:67–87. [Google Scholar]
- Witmer LM, Ridgely RC. The paranasal air sinuses of predatory and armored dinosaurs (archosauria: theropoda and ankylosauria) and their contribution to cephalic structure. Anatom Rec. 2008;291:1362–1388. doi: 10.1002/ar.20794. [DOI] [PubMed] [Google Scholar]
- Witmer LM, Ridgely RC. New insights into the brain, braincase, and ear region of tyrannosaurs (Dinosauria, Theropoda), with implications for sensory organization and behavior. Anatom Rec. 2009;292:1266–1296. doi: 10.1002/ar.20983. [DOI] [PubMed] [Google Scholar]
- Yan K, Tang Y-Z, Carr CE. Calcium-binding protein immunoreactivity characterizes the auditory system of Gekko gecko. J Comp Neurol. 2010;518:3409–3426. doi: 10.1002/cne.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Jarvis ED, Gilbert MTP. Avian genomes. A flock of genomes. Introduction. Science. 2014;346:1308–1309. doi: 10.1126/science.346.6215.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]