Abstract
Gold-standard diagnostic assessments of autism spectrum disorder (ASD) symptomatology were conducted on 63 children (mean CA: 8.81 years) with 7q11.23 duplication syndrome, one of the copy number variants identified by Sanders et al. (2011a) as associated with ASD. ASD classification rate was 39.6% for the Autism Diagnostic Interview-Revised and 25.4% for the Autism Diagnostic Observation Schedule-2 (ADOS-2). Based on these assessments combined with clinical judgment, 19.0% of children were diagnosed with ASD. Reasons for these discrepancies are discussed, as are differences in rate of diagnosis as a function of sex, age, and ADOS-2 module administered and differences in intellectual and adaptive behavior abilities as a function of presence or absence of ASD diagnosis and ADOS-2 module administered. Implications are addressed.
Keywords: 7q11.23 duplication syndrome, autism spectrum disorders, social anxiety, selective mutism
7q11.23 duplication syndrome (Dup7) is a recently identified neurodevelopmental disorder that has shown a significant association with autism spectrum disorder (ASD) in studies examining common CNVs among children previously diagnosed with ASD (Levy et al., 2011; Sanders et al., 2011a). ASDs are conceptualized as multi-gene disorders (Ronald, Happé, Price, Baron-Cohen, & Plomin, 2006; Zhao et al., 2007), with various pathways contributing to the observed behavioral phenotype (Belmonte & Bourgeron, 2006; Happé, Ronald, & Plomin, 2006; Persico & Bourgeron, 2006). They can be caused by a large number of genetic alterations, some of which result in identified syndromes such as Dup7 (Betancur, 2011; Geschwind, 2011). Given the likely value of studies of Dup7 for refining understanding of the genetic and neural underpinnings of the core and related features of ASD, study of a large group of children with this syndrome who were not ascertained based on ASD symptomatology is critical. The present study takes this approach. Rather than examining the presence of Dup7 among individuals with ASD, the current research constitutes the first systematic study of autism spectrum symptomatology among children with Dup7. In the remainder of the introduction, we briefly review the relevant literature.
The first study to document a significant association between Dup7 and ASD was Sanders et al. (2011a, 2011b). In this study, 4,457 children from 1,174 simplex families of children with ASD were genetically screened, resulting in the identification of four children with Dup7. Findings from exploratory analyses comparing these four children to 20 matched controls with ASD indicated only a few significant differences. The children with Dup7 scored significantly better than the controls with ASD on the ADOS (Lord, Rutter, DiLavore, & Risi, 1999) Social and Communication Total (but almost identically on the ADOS Social Affect Total), significantly worse on the Aberrant Behavior Checklist (ABC; Aman, Singh, Stewart, & Field, 1985) Irritability scale and the ABC Composite, and significantly worse on the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001) Anxious/Depressed scale. It is notable, however, that the participants in the Sanders et al. study were identified as having Dup7 because they already had an ASD diagnosis and were subsequently screened to determine if they had any CNVs. The proportion of children with Dup7 who would be expected to have ASD cannot be discerned based on this approach.
A comprehensive review of the literature regarding the behavioral phenotype of individuals with Dup7 can be found in Mervis et al. (2015); the physical and medical phenotypes are described in Morris et al. (2015). To date, with the exception of these two studies, reports of individuals with Dup7 have been either single-case studies (e.g., Somerville et al., 2005), case series identified by physicians or laboratories (Berg et al., 2007; Dixit et al., 2013; Van der Aa et al. 2009), or reports of children identified based on genetic screening of individuals diagnosed with ASD (e.g., Levy et al., 2011; Malenfant et al., 2012; McGrew, Peters, Crittendon, & Veenstra-Vanderweele, 2012; Sanders et al., 2011a). Among the case studies published in the literature, the presence or absence of an ASD or autistic features was reported for about half of the children and formally evaluated for about one-third (see Mervis et al., 2015 for a summary).
As part of their characterization of the psychological features of a sample of children with Dup7, Mervis and colleagues (2015) described results of parent ratings of ASD-related symptomatology on a screening measure for ASD (the Social Communication Questionnaire; SCQ; Berument et al., 1999; Rutter et al., 2003). The SCQ was completed by parents of 42 children with Dup7, 40 of whom are also represented in the current sample. SCQ raw scores were below the screening cutoff for 66.7% of the sample; scores of those not exceeding cutoff were normally distributed. Fourteen children (33.3%) exceeded the cutoff, often with scores well above the cutoff. Hence, based on a screening measure, it appears that about one-third of children with Dup7 show behavior warranting a comprehensive diagnostic evaluation for ASD.
The current investigation constitutes the first to systematically examine ASD-related symptomatology in a large group of children with Dup7 and demonstrates the extent to which these children meet criteria for ASD and/or show features associated with ASD. Both categorical and dimensional approaches to the characterization of ASD symptomatology were used. Relations of autism spectrum symptomatology to intellectual functioning, chronological age, and other aspects of the behavioral phenotype associated with Dup7 (e.g., anxiety) also were examined. Integration of the results of systematic studies of the behavioral phenotypes of syndromes associated with ASD will contribute to the understanding of the genetic bases for ASD (Budimirovic & Kaufmann, 2011).
Method
Participants
Participants were 63 children with genetically confirmed Dup7 (26 girls, 37 boys) ranging in age from 4 through 17 years (M age = 8.81 years, SD = 3.91). Forty seven of these children were included in the sample reported in Mervis et al. (2015). Genetic testing indicated that all participants’ duplications included the 26 – 28 genes in the classic WS region of chromosome 7q11.23 and did not include any additional genes in that region. Microarray reports were available for 59 participants, including all of the children who were clinically diagnosed with ASD based on the procedures in the present study. Based on these reports, none of the 59 children had an additional deletion or duplication (“second hit”) associated with either intellectual disability or ASD. Participants were recruited through referrals from geneticists and other physicians, from the family support organization for individuals with Dup7 (Duplication Cares), and by parental referral.
Sixty of the participants were probands; the remaining three were identified by cascade testing after a sibling and then a parent was diagnosed with Dup7. The indication(s) for initial genetic testing were available for 56 of the 60 probands. The three most common indicators were developmental delay (55.4%), speech/language delay (19.6%), and ASD (12.5%). Results of genetic testing for Dup7 were available for both biological parents for 47 of the probands. The duplications were de novo for 41 of these children (87.2%) and inherited for six (12.8%; two maternal and four paternal).
Materials
Differential Ability Scales – Second Edition (DAS-II; Elliott, 2007)
The DAS-II (Early Years version for ages 4 – 8 years; School Age version for ages 9 – 17 years) was used to assess intellectual ability. The DAS-II provides a General Conceptual Ability standard score (GCA), which is akin to an IQ score, based on the child’s performance on the six core subtests. The DAS-II also provides standard scores (SSs) for Verbal ability, Nonverbal Reasoning ability, and Spatial ability. Each of these is based on performance on two of the core subtests.
Peabody Picture Vocabulary Test – Fourth Edition (PPVT-4; Dunn & Dunn, 2007)
The PPVT-4 measures single word receptive vocabulary in children aged 2 years, 6 months to adults aged 90 years. The PPVT-4 yields an age-normed SS.
Expressive Vocabulary Test - Second Edition (EVT-2; Williams, 2007)
The EVT-2 measures single word expressive vocabulary in children aged 2 years, 6 months to adults aged 90 years. The EVT-2 yields an age-normed SS. The PPVT-4 and EVT-2 were co-normed, making direct comparison of their SSs straightforward.
Scales of Independent Behavior – Revised (SIB-R; Bruininks et al., 1996)
The SIB-R was administered in interview-format to participants’ parents to assess adaptive behavior. This measure yields a Broad Independence Composite SS as well as SSs in the domains of Motor Skills, Social Interaction & Communication, Personal Living Skills, and Community Living Skills.
Autism Diagnostic Interview – Revised (ADI-R; Lord, Rutter, & Le Couteur, 1994)
The ADI-R is an examiner-based interview of parents and caregivers about their children. This interview measures core diagnostic features of ASDs in the areas of qualitative abnormalities in reciprocal social interaction (RSI); qualitative abnormalities in communication (COM); restricted, repetitive, and stereotyped patterns of behavior (RRB); and age of onset, with items grouped thematically into subdomains. Parents are asked both about current functioning and about functioning in the past, with a focus on the benchmark age of 4 to 5 years. Empirically-derived cutoffs for each domain provide classifications of “non-spectrum” or “autism.” Classifications of “autism” in all domains is required for an overall classification of “autism” on the ADI-R.
Autism Diagnostic Observation Schedule – 2 (ADOS-2; Lord et al., 2012)
The ADOS-2 is a structured, play-like interaction with a trained examiner designed to assess ASD-related impairments in communication, reciprocal social interaction, play, and imagination, and the presence of restricted and repetitive behaviors and interests. It involves structured materials, structured tasks, and structured observations. The examiner selects one of four ADOS modules that best matches the child’s expressive language skills. Immediately following administration, specific behaviors are coded according to clearly articulated operational definitions. A subset of the items scored [including both Social Affect (SA) and Restricted and Repetitive Behavior (RRB) domain items] makes up the “ADOS-2 Algorithm,” empirically derived to distinguish between children with ASDs and children without ASDs. The ADOS-2 yields item scores, an algorithm raw summary score, and an algorithm classification (“non-spectrum”, “autism spectrum”, or “autism”), based on empirically derived cutoffs. (Note that ADOS-2 classification alone is not sufficient to make a diagnosis of an ASD.) Cutoffs in the SA domain only are also available by module (Gotham, Risi, Pickles, & Lord, 2007). The ADOS-2 also yields an ordinal-scale Comparison Score (range = 1 to 10) that describes the child’s severity of autism spectrum-related symptoms compared to that of children with ASD who are the same age and have generally similar language skills (Gotham, Pickles, & Lord, 2009).
Anxiety Disorders Interview Schedule for DSM-IV: Parent Interview Schedule (ADIS-P; Silverman & Albano, 1996)
The ADIS-P is a well-validated semistructured diagnostic interview administered to parents by trained interviewers. This measure is designed to assess for current anxiety and related disorders (including externalizing disorders) in children and adolescents and allows for differential diagnoses among these disorders based on DSM-IV criteria. The diagnoses considered for this study included Generalized Anxiety Disorder, Specific Phobia, Social Phobia, Selective Mutism, and Attention Deficit/Hyperactivity Disorder.
Procedure
A comprehensive battery that included assessment of cognitive and language functioning (DAS-II, PPVT-4, EVT-2), assessment of adaptive functioning (SIB-R), assessment for behavioral disorders (ADIS-P) and evaluation for a possible autism spectrum diagnosis (based on both DSM-IV and DSM-5 criteria) was administered by a multidisciplinary team. The evaluation for possible ASD diagnosis was conducted by a Licensed Psychologist with specialized training in the diagnosis of ASD who had been trained to research reliability on the ADI-R and ADOS-2. Parents were interviewed about ASD-related symptomatology with the ADI-R and about anxiety and other problem behavior using the ADIS-P. The appropriate module of the ADOS– 2 was administered (Mod 1 n = 9, Mod 2 n = 21, Mod 3 n = 33).
Results
Analyses were conducted in SPSS Version 24 (IBM Corp., 2016). Given the small and uneven sample sizes for many of the analyses, nonparametric statistics were used.
Cognitive and Adaptive Functioning
Descriptive statistics for intellectual ability, expressive and receptive language ability, and adaptive functioning are presented in Table 1. Median intellectual ability was in the low average range, and median single-word receptive and expressive vocabulary was in the average range. In contrast, median adaptive functioning overall and by domain was in the mildly impaired range. The standard deviation was > 15 for each of the measures, indicating considerable variability among the participants.
Table 1.
Descriptive statistics for performance on standardized intellectual, language, and adaptive behavior assessments (N = 63).
| Assessment | Mean (SD) | Median | Range |
|---|---|---|---|
| DAS-II | |||
| Verbal Cluster SS | 81.75 (21.51) | 88.00 | 30 – 116 |
| Nonverbal Reasoning Cluster SS | 84.29 (18.77) | 85.00 | 37 – 154 |
| Spatial Cluster SS | 80.35 (19.65) | 85.00 | 32 – 115 |
| GCA (similar to IQ) | 79.38 (19.45) | 83.00 | 33 – 132 |
| PPVT-4 SS | 89.94 (18.83) | 93.00 | 20 – 120 |
| EVT-2 SS | 85.73 (24.14) | 92.00 | 20 – 118 |
| SIB-R | |||
| Motor Skills Cluster SS | 65.54 (21.81) | 63.00 | 6 – 117 |
| Social Inter. & Comm. Skills SS | 71.32 (20.34) | 74.00 | 9 – 109 |
| Personal Living Skills SS | 61.51 (21.90) | 62.00 | 13 – 109 |
| Community Living Skills SS | 63.00 (23.67) | 70.00 | 13 – 98 |
| Broad Independence Composite | 59.24 (23.12) | 58.00 | 5 – 107 |
Note: DAS-II = Differential Ability Scales-II, SS = standard score, PPVT-4 = Peabody Picture Vocabulary Test-4, EVT-2 = Expressive Vocabulary Test-2, SIB-R = Scales of Independent Behavior – Revised.
ASD Symptomatology
ADI-R
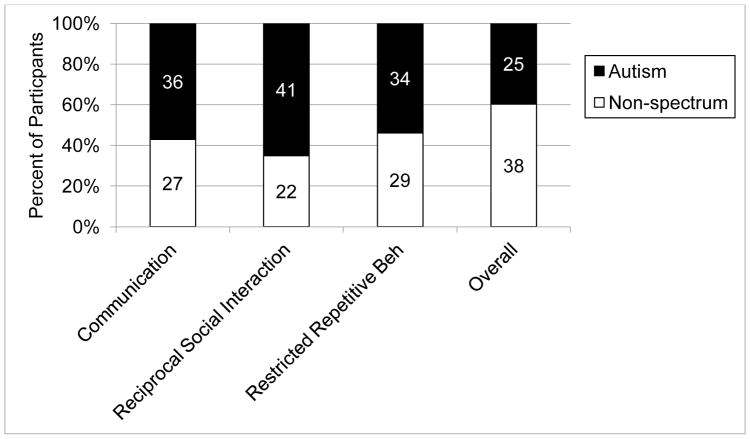
The percentage of children exceeding the diagnostic classification cutoff for autism on the ADI-R and in each domain is indicated in Figure 1. Based on the ADI-R diagnostic algorithm, which heavily weights retrospective accounts of functioning, 25 participants (39.7%) were classified “autism.” Accounts of current and past function were often considerably discrepant. Based on the Wilcoxon Signed Ranks Test, the sum of item abnormalities in COM (z = −4.78, p < .001) and RSI (z = −4.56, p < .001) was significantly lower for current functioning than for past functioning, indicating that parents described milder ASD-related symptomatology currently than at the benchmark age of 4 to 5 years. However, there was no significant difference for the RRB domain (z = −1.14, p = .254). Further examination of the subdomains within COM and RSI indicated significantly lower levels of abnormalities described by parents currently than in the past for all subdomains (ps < .002).
Figure 1.
ADI-R classifications by domain and overall.
ADOS-2
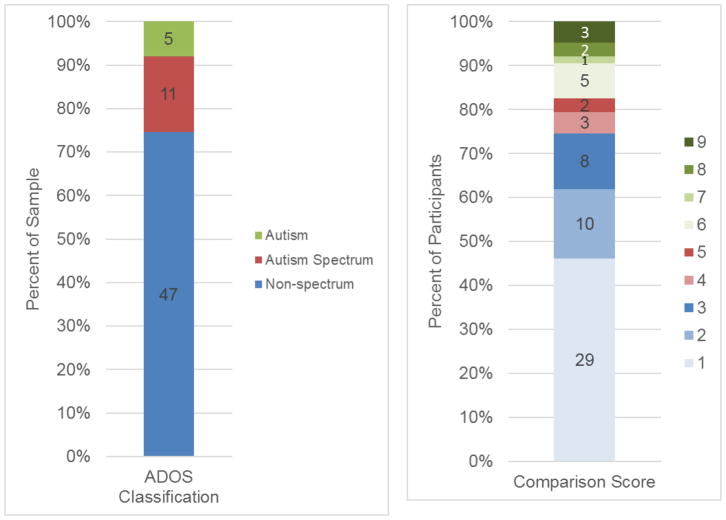
See Figure 2 for the distributions of ADOS-2 classifications and Comparison scores, which provide an index of severity. Based on the SA domain only, 15 children (24%) were classified as showing behavior indicative of an ASD (5 “autism spectrum” and 10 “autism”) and 48 children (76%) were classified “non-spectrum.” Based on the ADOS-2 algorithm, which includes both SA and RRB domains, 16 children (25.4%) were classified as showing behavior indicative of an ASD (5 “autism spectrum”, 11 “autism”) and 47 children (74.6%) were classified as “non-spectrum”. Of the children classified “non-spectrum,” 29 had Comparison scores of 1 (minimal to no evidence), ten had Comparison scores of 2, and eight had Comparison scores of 3.
Figure 2.
Distribution of ADOS-2 classifications and Comparison scores (frequency and percentage).
There were a number of ADOS-2 items on which most children with Dup7 did not evidence impairment. For Module 1 (n = 9), the following items had 2 or fewer children (< 25%) with any difficulty, indicating that difficulty was uncommon: unusual eye contact (n = 2), requesting (n = 2), spontaneous initiation of joint attention (n = 2), and self-injurious behavior (n = 1). For Module 2 (n = 21), the following items had 5 or fewer children (< 25%) with any difficulty: immediate echolalia (n = 4); stereotyped language (n = 4); pointing (n = 4); unusual eye contact (n = 1); facial expressions directed toward others (n = 2); shared enjoyment (n = 1); response to name (n = 1); spontaneous initiation of joint attention (n = 0); response to joint attention (n = 0); amount of social overtures to parent (n = 5); amount of reciprocal social interaction (n = 2); functional play with objects (n = 2); self-injurious behavior (n = 0); overactivity (n = 5); and aggression (n = 5). For Module 3 (n = 33), the following items had 8 or fewer children (< 25%) with any difficulty: overall level of language (n = 7); immediate echolalia (n = 2); stereotyped language (n = 6); offering information (n = 5); gestures (n = 7); language linked with nonverbal communication (n = 2); shared enjoyment (n = 7); amount of social overtures (n = 7); imagination/creativity (n = 8); unusual sensory interests (n = 8); hand and finger and other mannerisms (n = 5); self-injurious behavior (n = 0); repetitive interests and behaviors (n = 6); compulsions and rituals (n = 8); overactivity (n = 5); and aggression (n = 1).
At the same time, there also were some items on which difficulties were commonly observed for children with Dup7. For Module 1, the following items had 5 or more children (> 50%) with at least some difficulty: frequency of vocalization directed toward others (n = 5), intonation of vocalization (n = 5; 3 of the remaining 4 children had too few vocalizations for this item to be coded), gestures (n = 6), response to name (n = 5), giving (n = 7), showing (n = 5), response to joint attention (n = 5), quality of social overtures (n = 8), amount of social overtures to the examiner (n = 5), quality of social response (n = 7), engagement (n = 5), overall quality of rapport (n = 8), functional play with objects (n = 6), imagination/creativity (n = 8), unusual sensory interests (n = 7), overactivity (n = 5), aggression (n = 5), anxiety (n = 6). For Module 2 (n = 21), the following items had 11 or more children with difficulties (> 50%): overall level of language (n = 13); speech abnormalities associated with autism (n = 12); conversation (n = 13); gestures (n = 13); overall quality of rapport (n = 11); imagination/creativity (n = 11); and anxiety (n = 11). For Module 3 (n = 33), the following items had 17 or more children with difficulties (> 50%): asks for information (n = 17); empathy (n = 17); insight (n = 23); overall quality of rapport (n = 17); and anxiety (n = 20).
Mann-Whitney U tests (using exact p-values) were used to examine whether there were significant item-level differences between the Dup7-ASD and Dup7-NS groups for Modules 1 and 3 separately. (This analysis was not conducted for Module 2 because only one child who completed this module was diagnosed with ASD.) For Module 1, the Dup7-ASD and Dup7-NS groups differed significantly on 12 out of 33 total items (36% of items), as follows: overall level of language (z = −2.84, p = .024), frequency of vocalizations directed to others (z = −2.12, p = .048), pointing (z = −2.24, p = .048), shared enjoyment (z = −2.29, p = .048), giving (z = −2.48, p = .024), showing (z = −2.48, p = .024), response to joint attention (z = −2.21, p = .048), amount of social overtures toward the examiner (z = −2.45, p = .024), quality of social response (z = −1.99, p = .047), engagement (z = −1.98, p = .048), overall quality of rapport (z = −2.20, p = .048), functional play (z = −2.26, p = .024), imagination/creativity (z = −2.47, p = .024), and hand and finger and other mannerisms (z = −2.31, p = .048). For Module 3, the Dup7-ASD and Dup7-NS groups differed significantly on 8 out of 29 total items (28%), as follows: unusual eye contact (z = −4.33, p < .001), facial expressions directed toward others (z = −3.43, p = .007), language production and linked nonverbal communication (z = −4.90, p = .007), shared enjoyment (z = −5.17, p < .001), quality of social overtures (z = −4.15, p < .001), amount of social overtures (z = −3.29, p = .017), quality of social response (z = −3.30, p = .003), amount of reciprocal social interaction (z = −4.33, p < .001), and quality of rapport (z = −3.98, p < .001).
Clinical diagnostic status. Based on a combination of the ADI-R, ADOS-2, and clinical judgment (including developmental and medical history), 12 participants (19.0%) met clinical criteria for diagnosis of DSM-IV ASD (either PDD-NOS or Autistic Disorder) and DSM-5 ASD by a Licensed Psychologist. All 12 diagnosed with ASD were classified “AS” or “autism” on the ADOS-2; 9 were classified “autism” on the ADI-R. Of the remaining 51 participants, 16 were classified “autism” on the ADI-R and 4 were classified “AS” or “autism” on the ADOS-2.
Of the 12 children who met clinical criteria for ASD, 10 had de novo Dup7, one inherited his duplication from a parent, and the origin of one child’s duplication was unknown. The indication(s) for genetic testing was available for 11 of the 12 children. For the child who inherited his duplication, the only indicator given for genetic testing was macrocephaly. For the child for whom the origin of her duplication was unknown, the indicators for genetic testing were developmental delay and hypotonia. For four children with de novo Dup7, concern regarding ASD was the only indicator; for a fifth child, the indicators were concern regarding ASD, speech/language delay, and macrocephaly. ASD was not indicated as a concern for the remaining four children with de novo Dup7 for whom indications for genetic testing were available. The indicators for these children included developmental delay, speech/language delay, macrocephaly, and delayed puberty. Two additional children who had been referred for genetic testing due to concerns regarding ASD were classified “non-spectrum.”
Relation between ADI-R classification and ADOS-2 Comparison score
The distribution of ADOS-2 Comparison scores for children classified “autism” on the ADI-R was significantly higher (worse) than the distribution for children classified “non-spectrum” [z = −4.30, p <.001].
Potential Correlates of ASD Symptomatology: Relations to Age, Sex, Intellectual Functioning, and Adaptive Behavior
Relations between ASD status and potential correlates of ASD symptomatology are reported in Table 2 for each of the four measures of ASD status (ADI-R classification, ADOS-2 classification, ADOS-2 Comparison score, and ASD clinical diagnosis). Notably, sex was related to ASD status across all measures, with p-values ranging from .051 for clinical diagnosis to .005 for ADI-R classification. Age was not significantly related to ASD status; it is possible that relations to age are obscured by a curvilinear pattern as the children administered Module 2 were unlikely to show ASD-related symptomatology. Children with and without ASD status differed significantly in overall intellectual functioning (DAS-II GCA) based on the ADI-R, ADOS classification, and ADOS comparison score but not based on clinical diagnosis and differed significantly in overall adaptive behavior and in Social Interaction & Communication Skills and Personal Living Skills across all ASD dependent measures. Other aspects of intellectual functioning (e.g., nonverbal reasoning, spatial abilities, receptive vocabulary) showed group differences as a function of ASD status based on the ADI-R but not based on the other ASD-related dependent measures, while verbal functioning and expressive vocabulary were significantly related to ADOS comparison score but not to the other measures.
Table 2.
Relations of ASD status to age, sex, intellectual abilities, expressive and receptive language, and adaptive behavior as a function of ASD measure.
| Variable | ADI-R | ADOS classification | ADOS Comparison Score | Clinical Diagnosis | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Statistic | p | Statistic | p | Statistic | p | Statistic | p | |
| Chronological Age | z = 0.27 | .784 | z = −0.33 | .742 | rho(63) = .06 | .670 | z = −1.58 | .115 |
| Sex | Fisher’s Exact 1 sided | .005** | Fisher’s Exact 1−sided | .029* | z = −2.20 | .027* | Fisher’s Exact 1-sided | .051 |
| DAS-II GCA (“IQ”) | z = −2.99 | .003** | z = −2.18 | .029* | rho(63) = −.28 | .029* | z = −1.66 | .098 |
| DAS-II Verbal Cluster SS | z = −1.72 | .085 | z = −1.87 | .062 | rho(63) = −.26 | .036* | z = −1.54 | .123 |
| DAS-II Nonverbal Cluster SS | z = −2.35 | .019* | z = −1.55 | .121 | rho(63) = −.24 | .056 | z = −1.69 | .091 |
| DAS-II Spatial SS | z = −3.20 | .001** | z = −1.93 | .054 | rho(63) = −.21 | .094 | z = −2.99 | .062 |
| PPVT-4 SS | z = −3.11 | .002** | z = −1.79 | .073 | rho(63) = −.23 | .065 | z = −1.56 | .119 |
| EVT-2 SS | z = −1.24 | .216 | z = −1.33 | .185 | rho(63) = −.25 | .050* | z = −1.25 | .210 |
| SIB-R Broad Independ. SS | z = −3.21 | .001** | z = −2.93 | .003** | rho(63) = −.30 | .019* | z = −2.70 | .007** |
| SIB-R Motor Skills SS | z = −2.75 | .006** | z = −2.38 | .017* | rho(63) = −.24 | .064 | z = −2.44 | .015* |
| SIB-R Social & Comm. SS | z = −2.92 | .003** | z = −2.68 | .007** | rho(63) = −.30 | .016* | z = −2.28 | .022* |
| SIB-R Personal Living SS | z = −3.23 | .001** | z = −3.29 | .001** | rho(63) = −.36 | .004** | z = −2.54 | .011* |
| SIB-R Comm. Living SS | z = −2.43 | .015* | z = −2.41 | .016* | rho(63) = −.25 | .052 | z = −2.78 | .006** |
Note: Negative Z-values indicate that the distribution of SSs was higher for the NS group than for the ASD group.
A significant sex difference indicates that boys had more difficulty than girls.
p < .05;
p < .01.
Relations to Anxiety and Attention Problems
There were no significant differences in proportion of children diagnosed with Social Phobia, Specific Phobia, Separation Anxiety, Generalized Anxiety, Selective Mutism, or Attention Deficit/Hyperactivity Disorder based on ASD clinical diagnosis status (Fisher’s exact p-value ranged from .265 to .653) or ADOS classification (ASD vs non-spectrum; Fisher’s exact p-value ranged from .166 to 1.00). ADOS Comparison scores were not significantly correlated with any anxiety or attention-related diagnoses (rho p-values ranged .161 to .782). However, participants classified “autism” on the ADI-R were significantly more likely than those classified non-spectrum to be diagnosed with separation anxiety (Fisher’s exact p = .023 2-sided) or social phobia (Fisher’s exact p = .023 2-sided) and were more likely to be diagnosed with ADHD of some kind (Fisher’s exact p = .038 2-sided).
Examination of Module Effects: Relations to Sex and Cognitive, Language, and Adaptive Functioning
ASD prevalence as a function of ADOS-2 module (expressive language level) and sex
See Table 3 for the proportion of children diagnosed with ASD as a function of ADOS-2 module. There was a significant effect of module on rate of diagnosis. ASD diagnosis was least likely for children administered Module 2; rate of ASD diagnosis was only 4.7%. The rate of diagnosis for children administered Module 1 did not differ significantly from the rate for Module 3.
Table 3.
ASD prevalence as a function of ADOS-2 module and sex.
| Module 1 | Module 2 | Module 3 | p (module) | |
|---|---|---|---|---|
| Full Sample | 3/9 (33.3%) | 1/21 (4.8%) | 8/33 (24.2%) | 0.07 |
| Girls | 0/3 (0%) | 1/10 (10.0%) | 1/13 (7.7%) | 1.00 |
| Boys | 3/6 (50%) | 0/11 (0%) | 7/20 (35.0%) | 0.02 |
| p (girls v. boys) | 0.46 | 0.48 | 0.10 |
To consider the possibility of a sex effect, the proportion of children diagnosed with ASD as a function of module was considered separately for boys and girls. For boys, the rate of ASD diagnosis was significantly lower for Module 2 than for either Module 1 or Module 3; the difference in diagnostic rate between Modules 1 and 3 was not significant. For girls, there was no significant effect of module, with low rates of ASD across modules.
Significant differences in cognitive functioning and adaptive behavior as a function of ASD clinical diagnostic status and ADOS-2 Module
See Table 4 for a summary of significant differences between those with ASD diagnoses and those without in intellectual functioning, expressive and receptive vocabulary, and adaptive behavior. Significant differences favoring the non-spectrum group were found for more than half of the measures for children who completed Module 1. Similarly, the only child who was diagnosed with ASD after completion of Module 2 earned the lowest or next lowest SS on more than half the measures considered. In contrast, for children who completed Module 3, only one significant difference was found between children diagnosed with ASD and children classified as non-spectrum.
Table 4.
Differences in standardized assessment performance as a function of ASD clinical diagnostic status and ADOS-2 module.a
| Assessment | Module 1 (3 ASD, 6 NS) | Module 2 (1 ASD, 20 NS) | Module 3 (8 ASD, 25 NS) |
|---|---|---|---|
| DAS-II GCA (“IQ”) | z = −2.20, p = .028* | + | z = −0.74, p = .462 |
| DAS-II Verbal SS | z = −2.36, p = .018* | + | z = −0.04, p = .966 |
| DAS-II Nonverbal Reasoning SS | z = −1.55, p = .167 | z = −1.07, p = .283 | |
| DAS-II Spatial SS | z = −0.78, p = .435 | + | z = −1.18, p = .239 |
| PPVT-4 SS | z = −2.07, p = .038* | z = −0.57, p = .570 | |
| EVT-2 SS | z = −1.06, p = .291 | z = −0.46, p = .643 | |
| SIB-R Broad Independence SS | z = −1.23, p = .195 | + | z = −1.70, p = .089 |
| SIB-R Motor Skills SS | z = −0.26, p = .795 | + | z = −1.45, p = .147 |
| SIB-R Social Int. & Comm. SS | z = −2.07, p = .039* | + | z = −1.64, p = .101 |
| SIB-R Personal Living SS | z = −2.07, p = .039* | + | z = −2.21, p = .027* |
| SIB-R Community Living SS | z = −1.29, p = .197 | + | z = −1.51, p = .130 |
Note: ASD = Autism spectrum disorder, NS = Non-spectrum, DAS-II = Differential Ability Scales-II, GCA = General Conceptual Ability, SS = standard score, PPVT-4 = Peabody Picture Vocabulary Test-4, EVT-2 = Expressive Vocabulary Test-2, SIB-R = Scales of Independent Behavior-Revised, Int. = Interaction, Comm. = Communication.
Negative z-values indicate that the distribution of SSs was higher for the NS group than for the ASD group.
p < .05.
Child with ASD earned the lowest or next lowest SS.
Discussion
Careful characterization of ASD-related symptomatology using gold-standard ASD assessment measures combined with clinical judgment indicated that 19% of children with Dup7 showed symptomatology consistent with an ASD clinical diagnosis. This rate indicates an elevated risk in comparison to the general population but also makes clear that the diagnosis of Dup7 by itself is not strongly indicative of the presence of an ASD. Few girls with Dup7 were diagnosed with ASD, and for children with ASD diagnoses severity was higher for boys than for girls, consistent with findings in the general population (Fombonne, 2009; Werling & Geschwind, 2013). It is notable that reliance on retrospective parental report alone resulted in a considerably higher rate (39.7%) of identified ASD-related symptomatology. The history of language delays coupled with extreme social anxiety and/or shyness characteristic of individuals with Dup7 may have contributed to parental recollections of socio-communicative and restricted and repetitive behavior challenges that may sometimes not be indicative of ASD symptomatology.
There is clear evidence for elevated risk of ASD symptomatology in children with Dup7 based both on parental and clinician observation, suggesting that genes in this region likely do confer risk for an ASD. However, the presence of ASD is nowhere near universal among children with Dup7 and the proportion who meet gold-standard criteria for ASD is considerably lower than might be expected based on the published literature. Prior research about ASD symptomatology in children with Dup7 has consisted mainly of case studies (individual and family) and the identification of the presence of Dup7 among individuals diagnosed with ASD. The current sample constitutes the first study of children with Dup7 that involved a large number of children who were not specifically included due to the presence of an ASD. In a study with overlapping participants, Mervis and colleagues (2015) found that 33.3% of children with Dup7 exceeded the screening cutoff on the SCQ, a parental-report measure. A similar rate of elevations was observed in the current study based on semistructured parent interview (ADI-R), with a considerably lower rate of ASD symptoms based on clinician observation (ADOS-2) or clinical diagnosis. Dykens and colleagues (2017) reported a similar pattern regarding rates of ASD-related symptomatology when comparing questionnaire screener and direct observation for children with Präder Willi syndrome. On this basis, they argued that direct observation is imperative for determining if a child has an ASD. We concur that ASD diagnoses of children with Dup7 should be made based on comprehensive clinical evaluation that includes direct observation. Furthermore, the assessment process needs to take into account other characteristics of the syndrome, including selective mutism and/or pronounced social anxiety.
7q11.23 CNV as a Risk Factor for ASD Symptomatology
The findings of the present study clearly indicate that 7q11.23 duplication is a risk factor for ASD symptomatology. It is notable that overlaps in symptomatology with the autism spectrum also have been observed in children with WS, who have a deletion of the same genes duplicated in Dup7. Klein-Tasman et al. (2007) found that almost half of their sample of young children with WS with limited or no expressive language met or exceeded the ADOS Module 1 cutoff. Furthermore, ADOS ratings indicated that the WS group had greater difficulties than a cognitive ability-matched comparison group of children with developmental disabilities of mixed etiologies without ASD (Klein-Tasman et al., 2009). Lincoln and colleagues (2007) conducted comprehensive clinical assessments of young children with WS with limited language using gold-standard instruments; based on these assessments, 20% of the children with WS were clinically diagnosed with an ASD. This pattern also has been found for children with WS with more advanced language, with > 25% meeting or exceeding the ASD cutoff for ADOS Module 2 or 3 (van der Fluit, 2014). High rates of socio-communicative difficulties and restricted and repetitive behavior also are evident based on parent report of children with WS ages 4 through 16 years (Klein-Tasman, Li-Barber, & Magargee, 2011). In fact, while children with WS are generally portrayed as highly social in contrast to the shyness and social anxiety seen for children with Dup7, fundamental challenges with social communication and restricted and repetitive behaviors are evident for both children with Dup7 and children with WS.
The parallel increase in incidence of ASD for both syndrome groups suggests strongly that alteration in gene dosage (both increased dosage and decreased dosage) of one or more genes in the WS region of 7q11.23 is related to ASD symptomatology. WS and Dup7 both are associated with differential methylation patterns relative to those found for typically developing children, and the set of differently methylated genes is enriched for genes associated with ASD (Strong et al., 2015), providing further support for this position.
Klein-Tasman and colleagues (2007) state that the phenotype of the children with WS classified as ASD based on their performance on the ADOS is consistent with Wing and Gould’s (1979) “active but odd” ASD subtype. The pattern most commonly seen for children with Dup7 who were clinically diagnosed with ASD contrasts significantly and generally fits with the aloof subtype given the social withdrawal that is commonly seen. Further research directly contrasting these syndromes is needed. Assessment of very rare children with smaller deletions or duplications of 7q11.23 will be important for narrowing the possible set of 7q11.23 genes associated with ASD symptomatology. Crespi and Procyshyn (2017) argue that GTF2I is a prime candidate, but additional research is needed to address this assertion.
Module and Age Effects
The rate of ASD diagnosis among children with Dup7 was similar for children with very limited or no expressive language (Module 1: 33%) and children with fluent language (Module 3: 24%). In contrast, the rate for children with phrase speech was significantly and considerably lower (Module 2: 5%). Several factors likely contribute to this U-shaped ASD diagnosis rate.
First, the most severely affected children cognitively who were diagnosed with ASD were included within the Module 1 sample, and these children scored significantly lower than the other children who completed Module 1 on the majority of the intellectual and adaptive behavior measures. It is possible that one or more of these participants may have a second genetic hit associated with ASD that was not apparent on their microarray report. These children were 4 years old at the time of their assessment; at the present time (at least 3 years later), they have not developed phrase speech, and it is possible they never will. The lack of phrase speech by age 7 years is quite rare among children with Dup7, characterizing only 1 of 35 participants in the present study. Thus, the children diagnosed with ASD based on assessment with Module 1 had more severe cognitive limitations than expected for children with Dup7 and several years later have unexpectedly limited language abilities relative to others with this syndrome.
The rate of ASD clinical diagnosis for children who completed Module 2 (phrase speech) was very low (1 of 21; ~5%). This rate was considerably lower than the rate for either children with limited or no expressive language (3 of 9) or children with fluent expressive language (8 of 33). Intellectual and adaptive abilities were considerably more limited in the child with ASD than in the children classified non-spectrum.
For Module 3, the pattern of abilities of children classified ASD relative to those classified non-spectrum was very different than for Module 1 or 2: Children diagnosed with ASD who had completed Module 3 had very similar IQs and language abilities to peers classified non-spectrum, with SSs typically in the low average to average range. Although adaptive behavior skills appeared to be somewhat more limited for the ASD group, the only significant difference was for personal living skills. The children with ASD also showed similar rates of social phobia, selective mutism, and disruptive behavior disorders as peers classified non-spectrum.
The considerably higher rate of ASD diagnosis for children who completed Module 3 than for children who completed Module 2 is consistent with the idea that some socio-communicative difficulties may not be clearly evident until the level of demands increases. This idea is represented within the DSM-5 (American Psychiatric Association, 2013) criteria for ASD, which state that symptoms “must be present in the early developmental period (but may not become fully manifest until social demands exceed limited capacities).” (p. 59). Due to the developmental nature of ASD, the specific behaviors indicative of the core impairments of ASD vary with age and language level, reflecting an increase in expectations regarding socio-communicative capabilities once language becomes fluent. On the ADOS-2, this change in the behaviors indicative of core impairments between children who have phrase speech and children who have fluent language is reflected in large differences in the proportion of the assessment that takes place within the context of play with toys (much higher in Module 2 than in Module 3) and in the proportion that assesses conversation, reporting of events, and insight into the nature of social relationships and emotions (much higher in Module 3 than in Module 2). Thus, difficulty transitioning from the more concrete socio-communicative demands of Module 2 to the more abstract socio-communicative demands of Module 3 may underlie the significant increase in rate of ASD diagnosis found in the present study for children with fluent language compared to children with phrase speech.
Furthermore, within the group of children who completed Module 3, the rate of ASD diagnosis varied significantly with age; a relatively low rate of diagnosis was seen for children < 10 years old (8%) and a much higher rate for children ≥ 10 years old (35%). There are changes in ADOS-2 expectations for children with fluent language as chronological age increases, as socio-communicative behaviors are coded relative to chronological age: Expectations are higher for older children than for younger children and for children with more advanced nonverbal abilities than for children with lower nonverbal abilities. Therefore, a plateau in socio-communicative development once a child has fluent language may result in an increase in evident ASD symptomatology (and therefore in rate of ASD diagnosis) as the gap from same-aged peers increases. This increase in ASD diagnosis rate for older children with fluent language relative to younger children with fluent language also is consistent with prior findings that children with Asperger syndrome or milder symptoms of ASD (e.g., DSM-IV diagnoses of PDD-NOS rather than autism) and children with intellectual abilities in the normal range are diagnosed with ASD on average at a considerably later age (Daniels & Mandell, 2014; Howlin, & Asgharian, 1999; Jónsdóttir, Saemundsen, Antonsdóttir, Sigurdardóttir, & Ólason, 2011; Mandell, Novak, & Zubritsky, 2005; Wiggins, Baio, & Rice, 2006).
Picci and Scherf (2015) describe a “second hit” model whereby early abnormalities in neural development set up vulnerabilities to the neural system, and then later in development there are additional challenges to the system including both neural developmental changes and increased and more sophisticated social and independence demands. These challenges reveal ASD-related difficulties in adapting to new demands. Both the significant increase in rate of ASD diagnoses between children who completed Module 2 and children who completed Module 3 and the significant increase in rate of ASD diagnoses among children who completed Module 3 between younger children (< 10 years old) and older children are consistent with this model. Longitudinal research will be critical to address the possibility that ASD symptomatology may become more prominent with age in individuals with Dup7 and to determine if there are early indicators of those symptoms in the preschool years that are not captured by the diagnostic measures used.
Item-Level Findings
Examination of item-level performance may provide additional insight into the nature of the socio-communicative challenges of children with Dup7. For children administered Module 1 or 2, some basic communicative building blocks such as modulation of eye contact and initiation of joint attention generally did not show difficulties. However, other basic socio-communicative skills consistently showed difficulties for children with limited language (e.g., giving, showing, responding to joint attention). Echolalia was not commonly observed, but unusual intonation or stereotyped language was evident across modules for more than half of the participants. While children administered Module 1 often had difficulties with shared enjoyment, such difficulties were rare for children administered Module 2 or 3. Across modules overall rapport showed challenges for more than half of the children. Examination of item-level performance also indicated that relative to children classified non-spectrum, children with an ASD diagnosis showed significantly more difficulty with shared enjoyment, amount of social overtures, quality of social response, and quality of rapport, indicating that difficulties in these areas are especially prominent when ASD is present.
ASD Diagnosis as a Function of Sex
The preponderance of ASD diagnoses was among the boys; only two of 26 girls but ten of 37 boys met criteria for a gold-standard clinical diagnosis of ASD. This finding is consistent with the general population finding that it is much more common for boys to meet criteria for ASD than girls (Fombonne, 2009; Werling & Geschwind, 2013). However, there is also discussion that there may be under-identification of ASD in girls using current diagnostic methods for a variety of reasons, including that girls with ASD generally show more prosocial behavior than boys (see Halliday et al., 2015 for a review). Continued examination of sex differences in ASD-related symptomatology and underlying cognitive and social processing in Dup7 is warranted.
Relation between Anxiety and ASD
Anxiety-related symptomatology is also elevated for children with Dup7. Mervis et al. (2015) reported that 50% of children with Dup7 in their sample met DSM-IV criteria for Social Phobia and 29% met DSM-IV criteria for Selective Mutism. Elevated rates of these anxiety disorders may place children at risk for parental observations of abnormal social reciprocity. This possibility is supported by the finding that ASD classification on the parent interview (ADI-R) was related significantly to social and separation anxiety diagnoses, suggesting that some of the ASD-related symptomatology reported by parents may be reflections of anxiety. Symptoms of anxiety were commonly shown during the ADOS-2, with the anxiety item endorsed by the examiner for six of nine children who completed Module 1 (66.7%), 11 of 21 children who completed Module 2 (52.4%), and 20 of 33 children who completed Module 3 (60.1%), Nevertheless, the presence or absence of an anxiety disorder (including social phobia and selective mutism) was not significantly related to ASD clinical diagnostic status in this sample, indicating the importance of careful clinical evaluation for the differentiation of anxiety disorder and ASD.
Limitations
A number of limitations of this investigation should be kept in mind. First, as Dup7 is a rare condition that was not identified until 2005, there may be some ascertainment factors that could contribute to the pattern of findings observed. In particular, children with anxiety disorders or speech disorders who do not evidence clear intellectual disability are not routinely evaluated with genetic microarrays, the method most likely to identify Dup7. And children who do not have any apparent difficulties would not be expected to have had any kind of genetic testing. In the present study, cascade testing (testing of parents for Dup7 and then testing of the proband’s siblings if a parent was found to have Dup7) was offered to the families and in most cases was completed, resulting in the identification of some siblings who had not come to medical or psychological attention previously. These siblings were included in the study. Nevertheless, there remain many individuals with Dup7 who are undiagnosed. Second, the number of children administered Module 1 was relatively small. Further phenotypic characterization of both children with Dup7 who have very limited or no expressive language and of adolescents with this syndrome is warranted to provide a stable estimation of the phenotype and developmental trajectory. Third, while the available cross-sectional data suggest there may be developmental effects on ASD symptomatology and diagnosis in this syndrome, longitudinal data will be crucial to allow more definitive conclusions regarding how the phenotype associated with Dup7 may vary across development. Finally, further examination of patterns of behavior of children with Dup7 in comparison to children who have ASD diagnoses but do not have Dup7 across development would be beneficial.
Summary
This is the first comprehensive characterization of the ASD symptomatology of a substantial sample of children with Dup7. An elevated rate of ASD in children with Dup7 was indeed evident in comparison to the general population, with 19% meeting criteria for clinical diagnosis of ASD. Girls with Dup7 appear to be at low risk for ASD diagnosis. Preliminary evidence that rates of ASD are higher in children with fluent language than in children with phrase speech and higher in older children with fluent language than in younger children with fluent language was also found. Further research regarding the developmental trajectory of children with Dup7 is warranted.
Acknowledgments
Funding: This study was funded by a grant from the Simons Foundation (SFARI award #238896) and by NINDS R01 NS35102.
Footnotes
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington, VT: University of Vermont; 2001. [Google Scholar]
- Aman MG, Singh NN, Stewart AW, Field CJ. Psychometric characteristics of the Aberrant Behavior Checklist. American Journal of Mental Deficiency. 1985;89:492–502. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, DC: Author; 2013. [Google Scholar]
- Belmonte MK, Bourgeron T. Fragile X syndrome and autism at the intersection of genetic and neural networks. Nature Neuroscience. 2006;9:1221–1225. doi: 10.1038/nn1765. nn1765. [DOI] [PubMed] [Google Scholar]
- Berg JS, Brunetti-Pierri N, Peters SU, Kang SH, Fong CT, Salamone J, … Cheung SW. Speech delay and autism spectrum behaviors are frequently associated with duplication of the 7q11.23 Williams-Beuren syndrome region. Genetics in Medicine. 2007;9:427–441. doi: 10.1097/gim.0b013e3180986192. doi:10.1097GIM.0b013e3180986192. [DOI] [PubMed] [Google Scholar]
- Berg JS, Potocki L, Bacino CA. Common recurrent microduplication syndromes: diagnosis and management in clinical practice. American Journal of Medical Genetics Part A. 2010;152:1066–107. doi: 10.1002/ajmg.a.33185. [DOI] [PubMed] [Google Scholar]
- Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism Screening Questionnaire: Diagnostic validity. British Journal of Psychiatry. 1999;175:444–451. doi: 10.1192/bjp.175.5.444. [DOI] [PubMed] [Google Scholar]
- Betancur C. Etiological heterogeneity in autism spectrum disorders: More than 100 genetic and genomic disorders and still counting. Brain Research. 2011;1380:42–77. doi: 10.1016/j.brainres.2010.11.078. [DOI] [PubMed] [Google Scholar]
- Bruininks RH, Woodcock RW, Weatherman RF, Hill BK. Scales of Independent Behavior – Revised. Itasca, IL: Riverside Publishing; 1996. [Google Scholar]
- Budimirovic DB, Kaufmann WE. What can we learn about autism from studying fragile X syndrome? Developmental Neuroscience. 2011;33:379–394. doi: 10.1159/000330213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi BJ, Procyshyn TL. Williams syndrome deletions and duplications: Genetic windows to understanding anxiety, sociality, autism, and schizophrenia. Neuroscience and Biobehavioral Reviews. 2017;79:14–26. doi: 10.1016/j.neubiorev.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Daniels AM, Mandell DS. Explaining differences in age at autism spectrum disorder diagnosis: A critical review. Autism: The International Journal of Research and Practice. 2014;18:583–597. doi: 10.1177/1362361313480277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit A, McKee S, Mansour S, Mehta SG, Tanteles GA, Anastasiadou V, … Sarkar A. 7q11.23 microduplication: A recognizable phenotype. Clinical Genetics. 2013;83:155–161. doi: 10.1111/j.1399-0004.2012.01862.x. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test-4. Minneapolis, MN: Pearson; 2007. [Google Scholar]
- Dykens EM, Roof E, Hunt-Hawkins H, Dankner N, Lee EB, Shivers CM, … Kim SJ. Diagnoses and characteristics of autism spectrum disorders in children with Prader-Willi syndrome. Journal of Neurodevelopmental Disorders. 2017;9 doi: 10.1186/s11689-017-9200-2. 18-017-9200-2 eCollection 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott CD. Differential Ability Scales. 2. San Antonio, TX: Psychological Corporation; [Google Scholar]
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric Research. 2009;65:591–598. doi: 10.1203/PDR.0b013e31819e7203. [DOI] [PubMed] [Google Scholar]
- Geschwind DH. Genetics of autism spectrum disorders. Trends in Cognitive Sciences. 2011;15:409–416. doi: 10.1016/j.tics.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39:693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37:613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, … Szatmari P. Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Molecular Autism. 2005;6(36) doi: 10.1186/s13229-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nature Neuroscience. 2006;9:1218–1220. doi: 10.1038/nn1770. nn1770. [DOI] [PubMed] [Google Scholar]
- Howlin P, Asgharian A. The diagnosis of autism and Asperger syndrome: Findings from a survey of 770 families. Developmental Medicine and Child Neurology. 1999;41:834–839. doi: 10.1017/s0012162299001656. [DOI] [PubMed] [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp; 2016. [Google Scholar]
- Jónsdóttir SL, Saemundsen E, Antonsdóttir IS, Sigurdardóttir S, Ólason D. Children diagnosed with autism spectrum disorder before or after the age of 6 years. Research in Autism Spectrum Disorders. 2011;5:175–184. doi: http://dx.doi.org/10.1016/j.rasd.2010.03.007. [Google Scholar]
- Klein-Tasman BP, Li-Barber KT, Magargee ET. Honing in on the social phenotype in Williams syndrome using multiple measures and multiple raters. Journal of Autism and Developmental Disorders. 2011;41:341–351. doi: 10.1007/s10803-010-1060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Tasman BP, Phillips KD, Lord C, Mervis CB, Gallo FG. Overlap with the autism spectrum in young children with Williams syndrome. Journal of Developmental and Behavioral Pediatrics. 2009;30:289–299. doi: 10.1097/DBP.0b013e3181ad1f9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Tasman BP, Mervis CB, Lord CE, Phillips KD. Socio-communicative deficits in young children with Williams syndrome: Performance on the Autism Diagnostic Observation Schedule. Child Neuropsychology. 2007;13:444–467. doi: 10.1080/09297040601033680. [DOI] [PubMed] [Google Scholar]
- Lincoln AJ, Searcy YM, Jones W, Lord C. Social interaction behaviors discriminate young children with autism and Williams syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2007;46:323–331. doi: 10.1097/chi.0b013e31802b9522. [DOI] [PubMed] [Google Scholar]
- Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, … Wigler M. Rare de novo and transmitted copy-number variation in autistic spectrum disorders. Neuron. 2011;70:886–897. doi: 10.1016/j.neuron.2011.05.015. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, Risi S. Autism Diagnostic Observation Schedule (ADOS) manual. Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule. 2. Los Angeles, CA: Western Psychological Services; 2012. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Malenfant P, Liu X, Hudson ML, Qiao Y, Hrynchak M, Riendeau N, … Holden JJ. Association of GTF2i in the Williams-Beuren syndrome critical region with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42:1459–1469. doi: 10.1007/s10803-011-1389-4. [DOI] [PubMed] [Google Scholar]
- Mandell DS, Novak MM, Zubritsky CD. Factors associated with age of diagnosis among children with autism spectrum disorders. Pediatrics. 2005;116:1480–1486. doi: 10.1542/peds.2005-0185. 116/6/1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrew SG, Peters BR, Crittendon JA, Veenstra-Vanderweele J. Diagnostic yield of chromosomal microarray analysis in an autism primary care practice: Which guidelines to implement? Journal of Autism and Developmental Disorders. 2012;42:1582–1591. doi: 10.1007/s10803-011-1398-3. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Klein-Tasman BP, Huffman MJ, Velleman SL, Pitts CH, Henderson DR, … Osborne LR. Children with 7q11.23 duplication syndrome: Psychological characteristics. American Journal of Medical Genetics Part A. 2015;167:1436–1450. doi: 10.1002/ajmg.a.37071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Mervis CB, Paciorkowski AP, Abdul-Rahman O, Dugan SL, Rope AF, … Osborne LR. 7q11.23 duplication syndrome: Physical characteristics and natural history. American Journal of Medical Genetics Part A. 2015;167:2916–2935. doi: 10.1002/ajmg.a.37340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne LR, Mervis CB. Rearrangements of the williams-beuren syndrome locus: Molecular basis and implications for speech and language development. Expert Reviews in Molecular Medicine. 2007;9(15):1–16. doi: 10.1017/S146239940700035X. S146239940700035X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico AM, Bourgeron T. Searching for ways out of the autism maze: Genetic, epigenetic and environmental clues. Trends in Neurosciences. 2006;29:349–358. doi: 10.1016/j.tins.2006.05.010. S0166-2236(06)00111-1. [DOI] [PubMed] [Google Scholar]
- Picci G, Scherf KS. A two-hit model of autism: Adolescence as the second hit. Clinical Psychological Science. 2015;3:349–371. doi: 10.1177/2167702614540646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald A, Happe F, Price TS, Baron-Cohen S, Plomin R. Phenotypic and genetic overlap between autistic traits at the extremes of the general population. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1206–1214. doi: 10.1097/01.chi.0000230165.54117.41. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. Social Communication Questionnaire. Los Angeles: Western Psychological Services; 2003. [Google Scholar]
- Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, … State MW. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011a;70:863–885. doi: 10.1016/j.neuron.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SJ, Ercan-Sencecek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, … State MW. Supplemental materials. Neuron. 2011b:70. doi: 10.1016/j.neuron.2011.05.002. Retrieved from http://www.sciencedirect.com/science/article/pii/S0896627311003746. [DOI]
- Silverman WK, Albano AM. The Anxiety Disorders Interview Schedule for DSM-IV: Parent Interview Schedule. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Somerville MJ, Mervis CB, Young EJ, Seo EJ, del Campo M, Bamforth S, … Osborne LR. Severe expressive-language delay related to duplication of the Williams-Beuren locus. The New England Journal of Medicine. 2005;353:1694–1701. doi: 10.1056/NEJMoa051962. 353/16/1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong E, Butcher DT, Singhania R, Mervis CB, Morris CA, De Carvalho D, … Osborne LR. Symmetrical dose-dependent DNA-methylation profiles in children with deletion or duplication of 7q11.23. American Journal of Human Genetics. 2015;97:216–227. doi: 10.1016/j.ajhg.2015.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Aa N, Rooms L, Vandeweyer G, van den Ende J, Reyniers E, Fichera M, … Kooy RF. Fourteen new cases contribute to the characterization of the 7q11.23 microduplication syndrome. European Journal of Medical Genetics. 2009;52:94–100. doi: 10.1016/j.ejmg.2009.02.006. [DOI] [PubMed] [Google Scholar]
- van der Fluit F. Autism Spectrum Disorder symptomatology in verbal children with Williams syndrome. Dissertation. 2014 doi: 10.1007/s10803-018-3555-4. Retrieved from UWM Digital Commons (Number 772). http://dc.uwm.edu/etd/772. [DOI] [PMC free article] [PubMed]
- Werling DM, Geschwind DH. Understanding sex bias in autism spectrum disorder. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4868–4869. doi: 10.1073/pnas.1301602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins LD, Baio J, Rice C. Examination of the time between first evaluation and first autism spectrum diagnosis in a population-based sample. Journal of Developmental and Behavioral Pediatrics. 2006;27(2 Suppl):S79–87. doi: 10.1097/00004703-200604002-00005. 00004703-200604002-00005. [DOI] [PubMed] [Google Scholar]
- Williams KT. Expressive Vocabulary Test-2. Minneapolis, MN: Pearson; 2007. [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of Autism and Developmental Disorders. 1979;9:11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Zhao X, Leotta A, Kustanovich V, Lajonchere C, Geschwind DH, Law K, … Wigler M. A unified genetic theory for sporadic and inherited autism. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12831–12836. doi: 10.1073/pnas.0705803104. 0705803104. [DOI] [PMC free article] [PubMed] [Google Scholar]