Abstract
Background
No-reflow phenomenon is a well-known problem, often accompanying percutaneous coronary intervention (PCI) for ST-segment elevation acute myocardial infarction (STEAMI). This study investigated the value of plasma D-dimer and Endothelin-1 (ET-1) levels on admission in predicting no-reflow after primary PCI and long-term prognosis in STEAMI patients with type 2 diabetes mellitus (T2DM).
Material/Methods
There were 822 patients with STEAMI and T2DM undergoing successful primary PCI included in this study: 418 patients showed normal re-flow after PCI, while 404 patients showed no-reflow phenomenon after PCI. The predictive value of plasma ET-1 and D-dimer level, and other clinical parameters for the no-reflow phenomenon were analyzed.
Results
The high plasma ET-1 and D-dimer levels showed predictive value for the no-reflow phenomenon in STEAMI patients with T2DM. Patients with high D-dimer and ET-1 levels showed higher risk (4.212, with 95%CI of 2.973–5.967 and 2.447 with 95%CI of 1.723–3.476, respectively) of no-reflow phenomenon compared with patients with low plasma D-dimer and ET-1 levels. Sensitivity of high plasma ET-1 and D-dimer levels in predicting no-reflow was 0.766. Both plasma D-dimer and ET-1 were adverse prognosticators for STEAMI patients with a T2DM post PCI (P<0.001).
Conclusions
In conclusion, plasma D-dimer and ET-1 levels on admission independently predict no-reflow after PCI in STEAMI patients with T2DM. When combined, the D-dimer and ET-1 levels as predictive and prognostic values are clinically promising. The plasma D-dimer and ET-1 levels provided a novel marker for treatment selection for the STEAIM patients with a T2DM history.
MeSH Keywords: Acute Coronary Syndrome; Diabetes Mellitus, Type 2; Endothelin-1; No-Reflow Phenomenon; Percutaneous Coronary Intervention
Background
Early restoration of coronary antegrade flow by percutaneous coronary intervention (PCI) plays a critical role in limiting the progression of myocardial necrosis [1,2]. The restoration can enhance the functional recovery of the postischemic myocardium in ST-segment elevation acute myocardial infarction (STEAMI) [1,2]. However, the successful reopening of an occluded coronary artery does not necessarily lead to left ventricular (LV) functional recovery; due to the “no-reflow” phenomenon in some cases [3,4]. This no-reflow phenomenon is commonly evaluated by angiograms, on which it is defined as substantial coronary antegrade flow reduction of less than thrombolysis in myocardial infarction (TIMI) flow grade 3, without mechanical obstruction. The no-reflow phenomenon has been closely correlated with poor LV functional recovery and is a high risk of cardiac mortality in patients with STEAMI [3,4].
Around 10–30% STEAMI patients suffer from no-reflow post emergent percutaneous coronary intervention [3,4]. Although intensively studied, the detailed molecular mechanisms of the no-reflow phenomenon remain unclear [5,6]. Recent studies indicated that plasma Endothelin-1 (ET-1) and D-dimer levels were potential biomarkers of the no-reflow phenomenon or prognosis of STEAMI patients post PCI [7–9]. It is well known that the vascular pathology is common in type 2 diabetes mellitus (T2DM) patients [10–12]. Previous studies have shown that the T2DM condition can enhance the basal level of plasma ET-1 and D-dimer [13,14]. Therefore, it is critical to control the T2DM condition when investigating the predictive values of ET-1 and D-dimer in the non-reflow phenomenon. However, the predictive value of plasma ET-1 and D-dimer levels of the no-reflow phenomenon has not been studied in a T2DM cohort yet. Meanwhile, the clinical value of combing plasma D-dimer and ET-1 levels as a signature has never been studied. In the present study, we evaluated the value of combining plasma ET-1 and D-dimer levels to predict the no-reflow phenomenon in STEAMI patients with a T2DM history.
Material and Methods
Study design and patient selection
Since 2007, the results of all PCIs were prospectively entered into the Affiliated Hospital of Jining Medical University Database using a uniform case report form. To ensure that we had long-term follow-up data for our study patients, we only included patients treated between January 2007 and June 2014. Meanwhile, we set the following inclusion and exclusion criteria for PCI treatment. Briefly, patients were eligible for PCI treatment if they had symptoms indicative of acute coronary syndrome within the past 24 hours before PCI. The indicators include electrocardiographic evidence of new ischemia or concentrations of cardiac enzymes above the upper limit of normal. Acute coronary syndrome patients were excluded from PCI treatment if they were at high risk of bleeding, if they had major surgeries in the previous 3 month, if they had ischemic stroke in the previous 1 month, if they had undergone PCI or coronary-artery bypass grafting in the previous 3 month, or if they had the class IV heart failure. Within the PCI treated patients, we further set the inclusion criteria for this study: the patients must have had a history of T2DM for at least 1 year, must have been diagnosed as STEAMI, must be younger than 80 years old, shouldn’t have mechanical ventilation and surgery in 3 month post PCI, and blood pressure should be well-controlled. As a retrospective study, we randomly selected 507 patients from the reflow population and 500 patients from the no-reflow population for the primary screening, and 822 patients were finally included (Figure 1). The final cohorts had a comparable number of reflow (418) and no-reflow (404) patients to ensure the representativeness. Informed consent was obtained from all enrolled patients. The local ethics committee approved the study.
Figure 1.
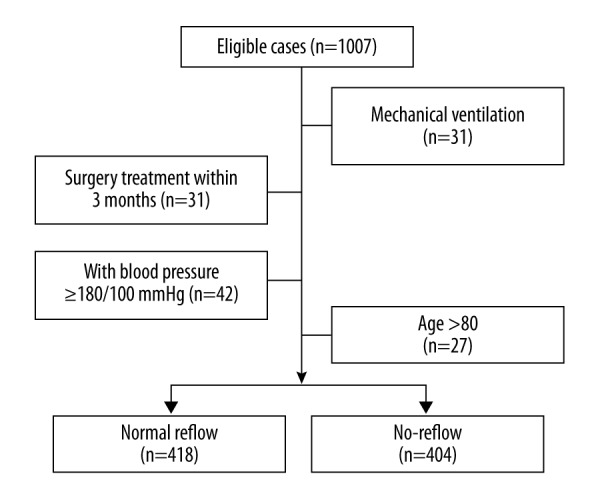
The flow chart of patient selection. We screened 1007 STEAMI patients with a T2DM history after PCI. The patients were excluded from the study due to mechanical ventilation, surgery treatment within 3 months after PCI, poor blood pressure control (≥180/100 mmHg), and older age (>80 years old). STEAMI – ST-segment elevation acute myocardial infarction; T2DM – type 2 diabetes mellitus; PCI – percutaneous coronary intervention.
PCI procedure and angiographic analysis
We performed all PCI procedures using a femoral approach. A bolus of 5000 IU of heparin was given. After conventional wire crossing, a balloon dilatation and stenting implantation were performed whenever possible. Intracoronary nitrates were always given after vessel recanalization. We evaluated the coronary TIMI flow grading [15], the corrected TIMI frame count, the final myocardial blush grade [16], and the thrombus scoring [17] by angiographic analyses. Two independent angiographers did the assessment. The final agreement was 89%, with discordances being resolved by consensus.
Definition of the no-reflow phenomenon
For purposes of this study, the no-reflow phenomenon was diagnosed by 2 independent experienced physicians by angiography during the PCI procedure. We defined the no-reflow phenomenon as a coronary TIMI grade flow ≤2 after vessel reopening.
Laboratory assays
We drew blood samples from a brachial vein in all patients upon admission. Blood was collected in EDTA tubes. After centrifuged (3000 g/min for 10 min), plasma and plasma aliquots were stored at −80°C freezer. Upon thawing from the freezer, samples were applied to experiments immediately. The D-dimer was measured by the human D-dimer ELISA kit (ab196269, Abcam, Cambridge, MA, USA) with a sensitivity of 71 pg/mL. We quantified the ET-1 level by an Endothelin-1 ELISA Kit (ab133030, Abcam, Cambridge, MA, USA) with a sensitivity of 0.41 pg/mL. The C-reactive protein, creatine kinase (CK), CK-MB, and troponin T plasma levels were measured every 4 hours during the first day and every 24 hours in the following 3 days using routine methods. The LV ejection fraction was quantified within 2 hours after the PCI procedure by 2D-echocardiography (Simpson method).
Statistical analysis
Comparisons between groups were made by t-test (2-sample) or by one-way ANOVA (more than 2 samples) for continuous variables. The Bonferroni method was used as the post-hoc test. Chi-square test or Fisher’s exact test was used for discrete variables. Correlation analyses were done by Pearson test (continuous variables) or Spearman test (ordinal variables). The mean value of each parameter was selected as the cutoff value of high and low. Receiver operating characteristic (ROC) curve and area under the curve (AUC) were used to evaluate the prediction quality of occurrence of no-reflow. All statistical analyses were performed by SPSS 19.0 (SPSS IBM, Armonk, NY USA). A value of P<0.05 was considered as statistical significance.
Results
General characteristics of patient population
From our electronic medical record system, we screened 1007 STEAMI patients (treated by PCI) with T2DM for more than 1 year. As shown in Figure 1, 185 patients were excluded due to different reasons such as surgery treatment within 3 months after PCI, older age, mechanical ventilation, and poorly controlled blood pressure. Patient baseline features are listed in Table 1. The average age of the 822 patients was 62.5 years. The average HbA1c was 7.7%. The average length of target vessel was 16.9 mm. The mean ET-1 and D-dimer value was 3.8 (pg/mL) and 430.0 (ng/mL) respectively. The median time from diagnosis to PCI was 4.5 hours. Upon PCI procedure, 418 patients showed a normal reflow while 404 patients were identified as no-reflow.
Table 1.
Basic clinical features of the T2DM patients.
| Baseline features | Data |
|---|---|
| Age (year) | 62.5±8.3 |
| Female, n (%) | 379 (46.1) |
| Hypertension (%) | 391 (47.6) |
| Smoker (%) | 457 (55.6) |
| History of ischemic heart disease (%) | 236 (28.7) |
| History of angioplasty (%) | 154 (18.7) |
| Family history (%) | 98 (11.9) |
| Initial TIMI grade (2/3,%) | 246 (29.9) |
| BMI (kg/m2) | 25.9±5.0 |
| Reperfusion time (hour) | 5.8±2.4 |
| Length of target vessel (mm) | 16.9±7.2 |
| CK-MB peak (mmol/L) | 218.9±268.4 |
| TG (mmol/L) | 1.73±0.52 |
| TC (mmol/L) | 5.1±1.4 |
| LDL (mmol/L) | 2.5±0.8 |
| HDL (mmol/L) | 1.0±0.6 |
| HbA1c (%) | 7.7±2.7 |
| PBG (mmol/L) | 14.2±4.3 |
| FBG (mmol/L) | 8.0±1.5 |
| ET-1 (pg/ml) | 3.8±2.6 |
| D-dimer (ng/ml) | 430.0±256.8 |
Correlation of clinical parameters to the no-reflow phenomenon in STEAMI patients with a T2DM history after PCI
Table 2 summarizes the clinical parameters observed in patients with or without the no-reflow phenomenon. Our univariate analysis showed that lower initial TIMI grade (P<0.001) and high-density lipoprotein (HDL) (P=0.005), longer/higher reperfusion time (P<0.001), length of target vessel (P<0.001), CK-MB peak (P=0.002), postprandial blood glucose (PBG) (P=0.002), fasting blood glucose (FBG) (P=0.016), D-dimer (P<0.001), and ET-1 (P<0.001) were correlated with the no-reflow phenomenon. Then we performed the logistic regression analysis to identify the independent predictor of no-reflow. In Table 3, we included all the clinical parameters of the univariate analysis in the multivariate analysis. Smoking, initial TIMI grade, reperfusion time, length of the target vessel, HDL, ET-1, and D-dimer showed significance in the multivariate analysis. Interestingly, D-dimer and ET-1 showed the highest odds ratio (OR) (4.212 with 95%CI of 2.973–5.967 and 2.447 with 95%CI of 1.723–3.476, respectively) than other factors. This data suggested that the D-dimer and ET-1 on admission have very promising predictive value for the no-reflow phenomenon after PCI in STEAMI patients with a T2DM history. Pearson correlation test showed that there was a very weak correlation between the ET-1 and D-dimer levels (r=0.164, P<0.05).
Table 2.
Univariant analysis of predictors of the no-reflow in T2DM patients after PCI.
| Features | No-reflow (n= 418) | Normal reflow (n= 404) | P value |
|---|---|---|---|
| Age (> 62 year) | 218 (54.0) | 231 (55.3) | 0.708 |
| Female, n (%) | 182 (45.0) | 197 (47.1) | 0.550 |
| Hypertension (%) | 199 (49.3) | 192 (45.9) | 0.340 |
| Smoker (%) | 208 (51.5) | 249 (59.6) | 0.020 |
| History of ischemic heart disease (%) | 110 (27.2) | 126 (30.1) | 0.356 |
| History of angioplasty (%) | 77 (19.1) | 77 (18.4) | 0.815 |
| Family history | 53 (13.1) | 45 (10.8) | 0.298 |
| Initial TIMI grade (2/3,%) | 255 (63.1) | 321 (76.8) | <0.001 |
| BMI (kg/m2) | 26.2±5.0 | 25.6±4.9 | 0.083 |
| Reperfusion time (hour) | 6.1±2.3 | 5.5±2.5 | <0.001 |
| Length of target vessel (mm) | 20.2±6.8 | 13.5±6.0 | <0.001 |
| CK-MB peak (mmol/L) | 247.8±345.8 | 189.1±145.8 | 0.002 |
| TG (mmol/L) | 1.8±0.5 | 1.7±0.5 | 0.113 |
| TC (mmol/L) | 5.2±1.4 | 5.0±1.4 | 0.161 |
| LDL (mmol/L) | 2.5±0.8 | 2.4±0.8 | 0.285 |
| HDL (mmol/L) | 1.0±0.6 | 1.1±0.5 | 0.005 |
| HbA1c (%) | 7.8±2.6 | 7.6±2.7 | 0.272 |
| PBG (mmol/L) | 14.7±4.3 | 13.8±4.2 | 0.002 |
| FBG (mmol/L) | 8.1±1.4 | 7.8±1.6 | 0.016 |
| ET-1 (pg/ml) | 4.4±2.9 | 3.5±2.3 | <0.001 |
| D-dimer (ng/ml) | 533.0±244.0 | 323.4±224.4 | <0.001 |
Table 3.
Multivariant analysis of predictors of no-reflow in T2DM patients after PCI.
| Features | OR | 95% CI | P value | |
|---|---|---|---|---|
| Lower bound | Upper bound | |||
| Smoking (yes vs. no) | 1.455 | 1.031 | 2.055 | 0.033 |
| Initial TIMI grade | 2.146 | 1.463 | 3.147 | <0.001 |
| Reperfusion time | 2.198 | 1.55 | 3.118 | <0.001 |
| Length of target vessel | 1.685 | 1.158 | 2.452 | <0.001 |
| HDL | 0.607 | 0.43 | 0.858 | 0.005 |
| ET-1 (pg/ml) | 2.447 | 1.723 | 3.476 | <0.001 |
| D-dimer (ng/ml) | 4.212 | 2.973 | 5.967 | <0.001 |
The sensitivity and specificity of ET-1 and D-dimer in predicting the no-reflow phenomenon in STEAMI patients with a T2DM history after PCI
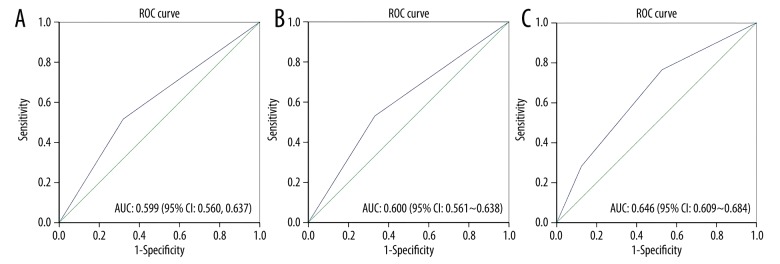
To further evaluate the value of using ET-1 and D-dimer as predictive markers for the no-reflow phenomenon after PCI in T2DM patients, we performed the ROC analysis. As shown in Figure 2A, ET-1 showed good specificity (0.618) and sensitivity (0.517). The AUC of ET-1 was 0.599 with a 95%CI of 0.560–0.637. A similar value of D-dimer was seen (Figure 2B). Then we combined the ET-1 and D-dimer to form a predictive signature. When both ET-1 and D-dimer were high, the sensitivity of predicting no-reflow was 0.766, indicating the signature was particularly promising in screening for the no-reflow phenomenon.
Figure 2.
The ROC curve of ET-1, D-dimer, and ET-1/D-dimer combination. ROC curves were built to evaluate plasma ET-1 and D-dimer level in predicting the non-reflow phenomenon of STEAMI patients with T2DM after PCI. (A) The AUC of plasma ET-1 in predicting the non-reflow phenomenon was 0.599 with 95% CI of 0.560–0.637. (B) The AUC of plasma D-dimer in predicting the non-reflow phenomenon was 0.600 with 95% CI from 0.561–0.638. (C) The AUC of combining plasma ET-1 and D-dimer (double high, single high, double negative) in predicting the non-reflow phenomenon was 0.646 with 95% CI of 0.609–0.684. ROC – receiver operating characteristic; ET-1 – Endothelin-1; STEAMI – ST-segment elevation acute myocardial infarction; T2DM – type 2 diabetes mellitus; PCI – percutaneous coronary intervention; AUC – area under the curve.
The prognostic value of ET-1 and D-dimer in STEAMI patients with a T2DM history after PCI
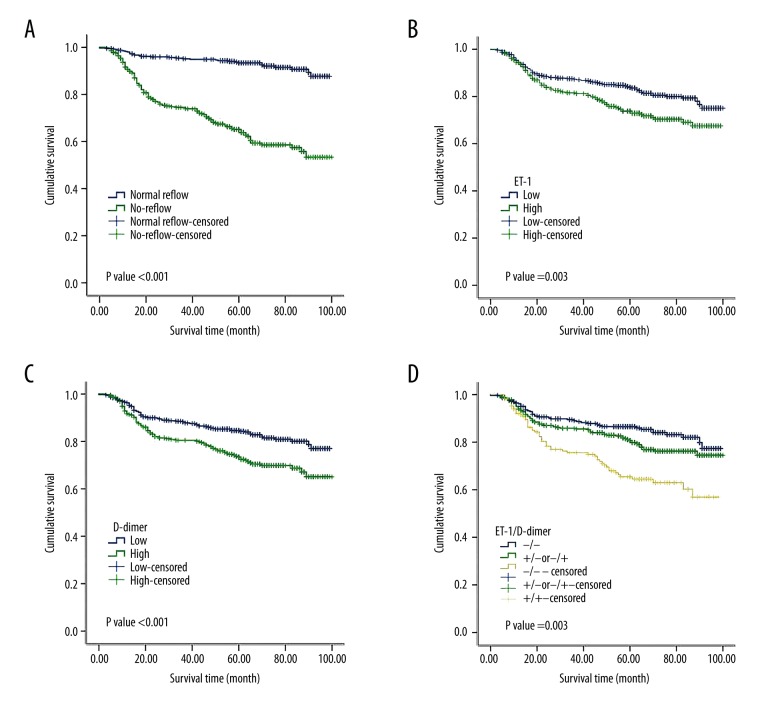
Using the Kaplan-Meier analysis, we investigated the prognostic value of the no-reflow phenomenon, ET-1 level, and D-dimer level in the T2DM patients after PCI. As expected, the patients with no-reflow had a poor prognosis (Figure 3A). The ET-1 and D-dimer levels also showed prognostic significance, with high ET-1 and D-dimer indicating poor prognosis (Figure 3B, 3C). Again, we combined the ET-1 and D-dimer status as a signature and found that patients with both high ET-1 and D-dimer had the worst prognosis (Figure 3D). These data highly suggested the clinical value of combing ET-1 and D-dimer levels as a signature in predicting long-term cardiovascular death in STEAMI patients with a T2DM history after PCI.
Figure 3.
Survival curves of STEAMI patients with T2DM post PCI. (A) The patients with the no-reflow phenomenon showed a significantly shorter survival time than the patients without the no-reflow phenomenon (P<0.001 in the log-rank test). (B) The patients with high plasma ET-1 level on admission showed a significantly shorter survival time than the patients with low plasma ET-1 level on admission (P=0.003 in the log-rank test). (C) The patients with high plasma D-dimer level on admission showed a significantly shorter survival time than the patients with low plasma D-dimer level on admission (P<0.001 in the log-rank test). (D) The patients with both high levels of plasma ET-1 and D-dimer showed the shortest survival time compared with the other patients (P<0.001 in the log-rank test). STEAMI – ST-segment elevation acute myocardial infarction; T2DM – type 2 diabetes mellitus; PCI – percutaneous coronary intervention; ET-1 – Endothelin-1.
Discussion
The no-reflow phenomenon is defined as the occurrence of areas with extremely low tissue flow after reopening the target vessel. Current knowledge suggests that the no-reflow phenomenon is caused by the damage to microvascular integrity established both during ischemia and during reperfusion. Several factors, such as plasma ET-1 level, TIMI thrombus grade, and female gender, have shown predictive value for the no-reflow phenomenon [18,19]. Niccoli and colleagues reported that ET-1 was an independent predictor of no-reflow after a successful PCI in acute myocardial infarction [9]. However, its predictive value needs to be further validated in more populations and patients with different conditions. More importantly, the significance of combining different plasma markers has not been studied yet. In this study, we combined the plasma ET-1 and D-dimer levels to predict the no-reflow phenomenon in T2DM patients after PCI for their acute coronary syndrome.
Several mechanisms supported the notion that high ET-1 can be a potential predictive marker for the no-reflow phenomenon. First, ET-1 is one of the most potent vasoconstrictors in the human body [20]. It is synthesized and released by the vascular endothelium in the injured regions and targets on the small-resistance coronary arteries. It has been shown that ET-1 level increases in ischemia condition [21]. In kidney ischemia/reperfusion injury, ET-1 deletion from endothelial cells protects kidney function [22]. Second, ET-1 also interacts with polymorphonuclear (PMN) leukocytes, which are associated with the no-reflow phenomenon [23,24]. It is well known that ET-1 can enhance PMN leukocytes adherence to the endothelium, thus favoring PMN plugging [23]. Third, ET-1 can promote microvascular compression by increasing its permeability and edema [25]. Therefore, it is possible that the release of ET-1 from ischemia injured endothelium can cause intense and sustained microvascular constriction, thus potentiating the no-reflow phenomenon.
Another plasma marker that was measured in this study was D-dimer. D-dimer is a fibrin degradation product that presents in the blood. The prognostic value of D-dimer has been documented in patients with acute coronary syndrome [26,27]. A high D-dimer level was associated with increased in-hospital cardiovascular mortality and 6-month all-cause mortality in patients with STEMI undergoing primary PCI [7]. In another patient cohort, high plasma D-dimer level on admission was found to be an independent predictor of both angiographic and electrocardiographic no-reflow [8]. However, the D-dimer level didn’t show prognostic value [8].
In the present study, we investigated the no-reflow predictive value of ET-1 and D-dimer in T2DM patients. It has been well-acknowledged that atherothrombotic complications are the main cause of mortality in T2DM patients and high glucose increases platelet reactivity and activation of coagulation factors with associated hypofibrinolysis [28]. All these facts suggest that the predictive value of ET-1 and D-dimer might be different in patients with and without T2DM background, which could influence ET-1 and D-dimer basal levels. Therefore, our study has a special clinical significance for the T2DM population, which were not studied before. Another special value of our study is that the ET-1 and D-dimer, 2 potential plasma markers, were combined to form a predictive signature. Interestingly, we noticed that patients with high ET-1 and D-dimer on admission had a significant higher possibility of getting no-reflow after PCI than other patients. These data strongly supported the potential of using ET-1 and D-dimer levels on admission to predict the no-reflow phenomenon in STEAMI patients with a T2DM history after PCI.
To understand if there is significant correlation between ET-1 and D-dimer levels, we performed the Pearson correlation analysis between these 2 variables. A very weak correlation between these 2 variables was observed. Nevertheless, our observational study didn’t directly clarify any causal relationship between ET-1 and D-dimer. Thus, more experimental studies are needed to further explore if elevated D-dimer level can cause elevation of ET-1 level or vice versa.
Conclusions
A prolonged decrease of coronary microvascular perfusion often occurs even after flow is restored in STEAMI patients. This “no-reflow” phenomenon worsens patients’ prognosis after PCI. Our data suggested the value of plasma ET-1 and D-dimer levels on admission in predicting the no-reflow phenomenon in STEAMI patients with a T2DM history. Therefore, our study provides the reference for treatment selection in STEAMI patients with the T2DM condition.
Acknowledgement
We thank the start-up funding from the Affiliated Hospital of Jining Medical University.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Heusch G. Improving the outcome of primary PCI: Protection from reperfusion injury. Cardiovasc Res. 2017;113(10):e33–34. doi: 10.1093/cvr/cvx120. [DOI] [PubMed] [Google Scholar]
- 2.Oldroyd KG. Complete immediate revascularization of the patient with ST-segment-elevation myocardial infarction is the new standard of care. Circulation. 2017;135(17):1571–73. doi: 10.1161/CIRCULATIONAHA.117.025265. [DOI] [PubMed] [Google Scholar]
- 3.Caixeta A, Ybarra LF, Latib A, et al. Coronary artery dissections, perforations, and the no reflow phenomenon. John Wiley & Sons, Ltd; 2017. pp. 248–66. [Google Scholar]
- 4.Rezkalla SH, Stankowski RV, Hanna J, Kloner RA, et al. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. 2017;10(3):215–23. doi: 10.1016/j.jcin.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Dai W, Hale S, Kloner RA. Delayed therapeutic hypothermia protects against the myocardial no-reflow phenomenon independently of myocardial infarct size in a rat ischemia/reperfusion model. Int J Cardiol. 2017;236:400–4. doi: 10.1016/j.ijcard.2017.01.079. [DOI] [PubMed] [Google Scholar]
- 6.Long R, You Y, Li W, et al. Sodium tanshinone IIA sulfonate ameliorates experimental coronary no-reflow phenomenon through down-regulation of FGL2. Life Sci. 2015;142:8–18. doi: 10.1016/j.lfs.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Akgul O, Uyarel H, Pusuroglu H, et al. Predictive value of elevated D-dimer in patients undergoing primary angioplasty for ST elevation myocardial infarction. Blood Coagul Fibrinolysis. 2013;24(7):704–10. doi: 10.1097/MBC.0b013e3283610396. [DOI] [PubMed] [Google Scholar]
- 8.Erkol A, Oduncu V, Turan B, et al. The value of plasma D-dimer level on admission in predicting no-reflow after primary percutaneous coronary intervention and long-term prognosis in patients with acute ST segment elevation myocardial infarction. J Thromb Thrombolysis. 2014;38(3):339–47. doi: 10.1007/s11239-013-1044-3. [DOI] [PubMed] [Google Scholar]
- 9.Niccoli G, Lanza GA, Shaw S, et al. Endothelin-1 and acute myocardial infarction: A no-reflow mediator after successful percutaneous myocardial revascularization. Eur Heart J. 2006;27(15):1793–98. doi: 10.1093/eurheartj/ehl119. [DOI] [PubMed] [Google Scholar]
- 10.Tsimerman G, Roguin A, Bachar A, et al. Involvement of microparticles in diabetic vascular complications. Thromb Haemost. 2011;106(2):310–21. doi: 10.1160/TH10-11-0712. [DOI] [PubMed] [Google Scholar]
- 11.Hoffmann B, Prisco A, Widlansky M, Greene A. Hyperglycemia-induced alterations of the vascular endothelium in type 2 diabetes mellitus. FASEB J. 2015 Abstract Number: 802.4. [Google Scholar]
- 12.Ebara S, Marumo M, Yamabata C, et al. Inverse associations of HDL cholesterol and oxidized HDL with d-dimer in patients with type 2 diabetes mellitus. Thromb Res. 2017;155:12–15. doi: 10.1016/j.thromres.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Coban E, Sari R, Ozdogan M, Akcit F, et al. Levels of plasma fibrinogen and d-dimer in patients with impaired fasting glucose. Exp Clin Endocrinol Diabetes. 2005;113(1):35–37. doi: 10.1055/s-2004-830524. [DOI] [PubMed] [Google Scholar]
- 14.el-Mesallamy H, Suwailem S, Hamdy N. Evaluation of C-reactive protein, endothelin-1, adhesion molecule(s), and lipids as inflammatory markers in type 2 diabetes mellitus patients. Mediators Inflamm. 2007;2007:73635. doi: 10.1155/2007/73635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.TIMI Study Group. The Thrombolysis in myocardial infarction (TIMI) trial. Phase I findings. N Engl J Med. 1985;312(14):932–36. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 16.van ‘t Hof AW, Liem A, Suryapranata H, et al. Angiographic assessment of myocardial reperfusion in patients treated with primary angioplasty for acute myocardial infarction: Myocardial blush grade. Zwolle Myocardial Infarction Study Group. Circulation. 1998;97(23):2302–6. doi: 10.1161/01.cir.97.23.2302. [DOI] [PubMed] [Google Scholar]
- 17.Gibson CM, de Lemos JA, Murphy SA, et al. Combination therapy with abciximab reduces angiographically evident thrombus in acute myocardial infarction: a TIMI 14 substudy. Circulation. 2001;103(21):2550–54. doi: 10.1161/01.cir.103.21.2550. [DOI] [PubMed] [Google Scholar]
- 18.Eitel I, Nowak M, Stehl C, et al. Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am Heart J. 2010;159(5):882–90. doi: 10.1016/j.ahj.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Celik T, Balta S, Ozturk C, et al. Predictors of no-reflow phenomenon in young patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Angiology. 2016;67(7):683–89. doi: 10.1177/0003319715605977. [DOI] [PubMed] [Google Scholar]
- 20.Bourque SL, Davidge ST, Adams MA. The interaction between endothelin-1 and nitric oxide in the vasculature: new perspectives. Am J Physiol Regul Integr Comp Physiol. 2011;300(6):R1288–95. doi: 10.1152/ajpregu.00397.2010. [DOI] [PubMed] [Google Scholar]
- 21.Ziv I, Fleminger G, Djaldetti R, et al. Increased plasma endothelin-1 in acute ischemic stroke. Stroke. 1992;23(7):1014–16. doi: 10.1161/01.str.23.7.1014. [DOI] [PubMed] [Google Scholar]
- 22.Arfian N, Emoto N, Vignon-Zellweger N, et al. ET-1 deletion from endothelial cells protects the kidney during the extension phase of ischemia/reperfusion injury. Biochem Biophys Res Commun. 2012;425(2):443–49. doi: 10.1016/j.bbrc.2012.07.121. [DOI] [PubMed] [Google Scholar]
- 23.Sato Y, Hogg JC, English D, van Eeden SF. Endothelin-1 changes polymorphonuclear leukocytes’ deformability and CD11b expression and promotes their retention in the lung. Am J Respir Cell Mol Biol. 2000;23(3):404–10. doi: 10.1165/ajrcmb.23.3.4057. [DOI] [PubMed] [Google Scholar]
- 24.Boros M, Massberg S, Baranyi L, et al. Endothelin 1 induces leukocyte adhesion in submucosal venules of the rat small intestine. Gastroenterology. 1998;114(1):103–14. doi: 10.1016/s0016-5085(98)70638-9. [DOI] [PubMed] [Google Scholar]
- 25.del Zoppo GJ, Hallenbeck JM. Advances in the vascular pathophysiology of ischemic stroke. Thromb Res. 2000;98(3):73–81. doi: 10.1016/s0049-3848(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 26.Gorog DA. Prognostic value of plasma fibrinolysis activation markers in cardiovascular disease. J Am Coll Cardiol. 2010;55(24):2701–9. doi: 10.1016/j.jacc.2009.11.095. [DOI] [PubMed] [Google Scholar]
- 27.Mueller C. Biomarkers and acute coronary syndromes: An update. Eur Heart J. 2014;35(9):552–56. doi: 10.1093/eurheartj/eht530. [DOI] [PubMed] [Google Scholar]
- 28.Alzahrani SH, Ajjan RA. Coagulation and fibrinolysis in diabetes. Diab Vasc Dis Res. 2010;7(4):260–73. doi: 10.1177/1479164110383723. [DOI] [PubMed] [Google Scholar]