Abstract
Introduction
This study explored D-ribose pharmacokinetics after intravenous (IV) and oral administration to healthy rabbits.
Materials and methods
D-ribose was administered once as 420 mg/kg (N=4) or 840 mg/kg (N=6) dose intravenously, or as an oral dose of 420 mg/kg (N=3) or 840 mg/kg (N=3). Serum was obtained at various time points, up to 210 minutes after administration. Urine was also collected after IV administration. Pharmacokinetic parameters were determined from drug concentration–time data using Kinetica software.
Results
The findings showed that D-ribose follows a dose-dependent kinetic profile. With doubling the IV dose, AUCtotal was significantly increased by threefold, while the clearance was decreased by 44%. The half-life was 1.7-fold longer at the higher dose. Similar nonsignificant trends were also observed at oral administration. D-ribose was rapidly absorbed (Tmax=36–44 minutes) and rapidly disappeared from plasma (within <140 minutes). Additionally, D-ribose was partially (18–37.5%) recovered from urine.
Conclusion
Collectively, D-ribose showed a dose-dependent kinetic profile, where parameters change according to dosing levels. D-ribose clearance seems to follow first-order kinetics at low dose. Thereafter, elimination systems are saturated, and elimination continues in a fast manner. Urine recovery was partial, which could be attributed to the several metabolic pathways that pentose can undergo.
Keywords: D-ribose, single dose, pharmacokinetics, rabbits, oral, intravenous
Introduction
D-ribose is a natural pentose monosaccharide and is a key constituent of newly generated adenosine triphosphate (ATP), guanosine triphosphate (GTP), RNA, DNA, and riboflavin.1 Pharmacological effects of D-ribose and its physiological relation on certain systems have been reported. The beneficial effect on cardiac muscle has been studied in many animal models. These studies have shown a potential protective effect on the heart, by restoring cellular ATP and cardiomyocyte function as in cases of hypoxia.2–4 Clinically, administration of D-ribose as 3.3 mg/kg/min intravenously for 30 minutes to 17 patients with coronary artery disease enhanced the identification ischemic myocardium tissue.5 More recently, it has been reported that when patients (n=11) with diastolic heart failure are given oral D-ribose (5 g/dose) for 6 weeks, a trend toward improvement in tissue Doppler velocity was observed in 64% of patients suggesting improving diastolic function.6 Other studies have shown that D-ribose protects against ischemia-induced renal injury in rats7 and from cisplatin-induced nephrotoxicity in mice,8 and ameliorates the clinical symptoms in fibromyalgia and fatigue syndrome patients at 5 g dose given three times daily.9,10 It has been recently shown that administration of 420 mg/kg D-ribose intravenously to rabbits for 28 days did not induce clinical, behavioral, hematological, pathological, and histological toxic effects.11 Oral administration of D-ribose to healthy people was tolerable with instances of diarrhea occurring at doses higher than 200 mg/kg/h.12
Limited pharmacokinetics data are available about D-ribose. Oral administration of a single dose of D-ribose solution at 2.5, 5, or 10 g to healthy individuals was well tolerated and revealed that D-ribose is rapidly absorbed with an increase in the exposure of more than proportionality with increasing dose.13 The percentage of dose excreted in the urine ranged from 4.15% to 7.2%.13 At the doses up to 200 mg/kg/h, D-ribose absorption in humans was determined to be 87.8–99.8% with minimal first-pass effect.12 The latter study also showed that the urinary loss of D-ribose was 23% of 222 mg/kg/h dose that was administered intravenously over 5 hours.12 This may indicate larger loss at the high dose of administration.
Herein, we assessed the preclinical pharmacokinetics of D-ribose in rabbits after oral and intravenous (IV) administration of two different doses. These routes have been chosen because they are the proposed routes for administration to humans.
Materials and methods
Test material
Natural D-ribose (C5H10O5; molecular weight: 150.13 g/mol, >98% purity, chemical abstracts service number: 50-69-1) was obtained from Heartland Biosciences (Minneapolis, MN, USA) as a spherical white powder. The substance was dissolved in water for injection in the concentration of 8.4%. The 8.4% solution was diluted in 1.63% sodium chloride solution to reach the concentration of test substances of 4.2%. The preparation was made right before the treatment. The final concentrations of the D-ribose in the test substance were 8.4% and 4.2%.
Animals
Healthy adult male V-line (Valencia) rabbits with a weight of 2–2.5 kg were obtained from the Alexandria University, Alexandria, Egypt. Rabbits were selected because they were widely used for pharmacokinetic testing. A total of 16 rabbits were used in the study, which were randomly distributed based on body weights into two groups. Group I (10 rabbits) was used for IV experiments and group II (6 rabbits) was used for oral D-ribose administration. Each animal was administered 10 mL/kg of the prepared test substance to obtain final D-ribose doses of 420 or 840 mg/kg. Four rabbits received 420 mg/kg IV dose, while six rabbits were administered 840 mg/kg D-ribose. Group II encompassed three animals within each subgroup. All experimental protocols were approved by the Animal Care and Use Committee of Jordan University of Science and Technology. Animals’ welfare was insured via following the Institutional Animal Care and Use Committee Guidebook.14
Animals were housed individually in cages and kept in one room that was well-ventilated (>10 air changes per hour) with 100% fresh air (no air circulation). A 12-hour light/12-hour dark photoperiod was maintained. Room temperature and relative humidity were set to be maintained at 22±3°C and 30–70%, respectively. Animals were acclimatized for 1 week before conducting the study.
Administration route and procedure
Rabbits were fasted for ~12 hours prior to dosing the sub-stance, with water being freely offered ad libitum in bottles. D-ribose was administered either IV to group I as a solution by slow injection over 5–10 minutes in the ear vein using 23–25 gauge sterile disposable needles or by oral gavage to group II.
Using 25G syringes, blood samples were collected from the ear veins in volumes of 1.5–2.5 mL at 0, 12, 24, 36, 48, 60, 80, 100, 120, 150, 180, and 210 minutes of D-ribose administration. Feed and water were provided after the end of blood collection. The blood was centrifuged and serum was separated and divided over 3–4 Eppendorf tubes (~300 µL in each) and stored at −20°C until analysis.
Urine samples were also collected for the two subgroups of rabbits that received 840 mg/kg and 420 mg/kg of D-ribose intravenously. Urine collection time periods were at 0–60, 61–120, 121–180, and 181–210 minutes. Samples were divided into 3–5 aliquots and frozen until the assay.
Serum and urine bioanalysis
A simple assay method was adopted from Roe and Rice15 with minor modifications to quantify D-ribose in serum and urine samples. The assay is based on the formation of furfural from D-ribose in 83% acetic acid containing thiourea at 70°C, and the reaction of the furfural with p-bromoaniline acetate to form a pink-colored product. The product was measured using a UV- spectrophotometer (Shimadzu UV-1800; Shimadzu Corporation, Kyoto, Japan) at 515 nm.
The spectrophotometric method was partially validated for both biological matrices, serum and urine, prior to running the animals’ authentic samples. Calibration curves were constructed at eight concentration levels ranging from 1.5625 to 200 µg/mL using 200 µL serum or urine samples. Linear regression analysis was used to fit the data. The accuracy and precision were assessed by analysis of quality control (QC) samples. Six replicates of low QC (LQC=5 µg/mL), medium QC (MQC=15 µg/mL), or high QC (LQC=150 µg/mL) samples were run for 3 days, for a total of n=18 samples at each QC level. Intraday accuracy and precision were determined from the six replicates at 1 of the 3 days, and interday accuracy and precision were calculated from all 18 QC samples. The calculated mean concentration relative to the nominal concentration was used to express accuracy. The coefficient of variation (CV) was used to estimate the precision.
Pharmacokinetic and statistical analyses
To obtain the pharmacokinetic parameters, non-compartmental analyses were performed on the measured concentrations of D-ribose using Kinetica software, version 4.4.1 (Thermo Electron Scientific, Waltham, MA, USA). The maximum observed concentration (Cmax) and the time to reach this concentration (Tmax) were determined by visual inspection of oral data. The elimination rate constant (k) was estimated from the slope of the terminal part. The elimination half-life (t1/2) was calculated as 0.693/k. The area under the serum concentration–time curve from time 0 to the last measurable concentration (t) (AUC0−t) was calculated using the log-linear trapezoidal method, and the total AUC (AUCtotal) was calculated as AUC0−t + Clast/k. Total clearance (CL) was calculated as dose/AUCtotal. The volume of distribution at steady state (Vss) and mean residence time (MRT) were also obtained. The amount of D-ribose excreted in the urine, which was calculated from urine volume and the measured urine concentrations (Xu=Cu*V), was used to calculate the renal clearance (CLr =Xu0−t/AUC0−t). The absolute bioavailability (F) was estimated from the AUCtotal data after IV and oral administrations.
Unpaired student’s t-test was used to assess statistical differences in the pharmacokinetic parameters between the two administered doses in each route and between the two routes at the same dose. The analysis was conducted using GraphPad Prism (version 6; GraphPad Software Inc., San Diego, CA, USA). The threshold of significance was p<0.05.
Results
The spectrophotometric assay was found to be linear over D-ribose concentration range of 1.5625–200 µg/mL, with average correlation coefficients (r2) of 0.9981 and 0.9992 for serum and urine standards, respectively. Intraday and interday accuracies for serum were in the range of 97.4–102.1%, while the precision (CV) was in the range of 1.5–5.4% (Table 1). As for urine, the intraday and interday accuracies were in the range of 97–100.2%, and the precision ranged from 1.2% to 8.3% (Table 1). This indicates the accuracy and reproducibility of the method. In addition, the stability study showed that D-ribose samples can be stored frozen at −20°C for at least 2 months without degradation.
Table 1.
Accuracy and precision of quality control samples of D-ribose in serum and urine
| Level | Nominal conc (µg/mL) | Intraday (%)
|
Interday (%)
|
|||
|---|---|---|---|---|---|---|
| Accuracy | CV | Accuracy | CV | |||
| Serum | LQC | 5 | 98.3 | 4.3 | 97.4 | 5.4 |
| MQC | 15 | 100.2 | 3.0 | 99.1 | 3.3 | |
| HQC | 150 | 102.1 | 1.5 | 101 | 2.8 | |
| Urine | LQC | 5 | 97 | 8.3 | 98.2 | 7.3 |
| MQC | 15 | 96.3 | 3.3 | 98.6 | 4.2 | |
| HQC | 150 | 100.2 | 1.2 | 100.2 | 1.9 | |
Abbreviations: conc, concentration; LQC, low quality control; MQC, medium quality control; HQC, high quality control; CV, coefficient of variation.
Figure 1 depicts the serum concentration–time profile of D-ribose after IV administration at doses of 420 mg/kg and 840 mg/kg. After IV administration of D-ribose to rabbits, the results showed a significant threefold increase in AUCtotal from 27.91 mg·min/mL to 100.21 mg·min/mL with a twofold increase in D-ribose dose from 420 mg/kg to 840 mg/kg. The average elimination half-life was significantly increased from 14.46 minutes at the dose of 420 mg/kg to 24.79 minutes at the dose of 840 mg/kg. The total clearance was significantly decreased from 15.3 mL·min/kg with 420 mg/kg dose to 8.53 mL·min/kg for the 840 mg/kg dose. No change in the volume of distribution was observed between the two doses. The pharmacokinetic parameters of IV administration are summarized in Table 2.
Figure 1.
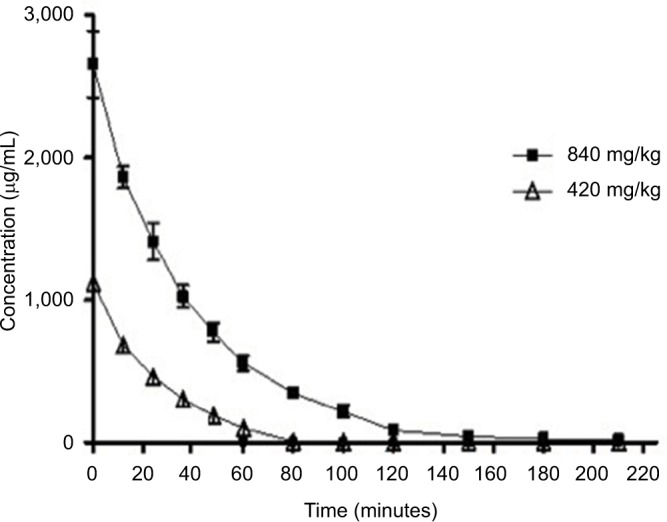
The serum concentration–time profile of D-ribose after IV administration at doses of 840 mg/kg and 420 mg/kg body weight. The values are mean ± SE.
Abbreviations: IV, intravenous; SE, standard error.
Table 2.
Pharmacokinetic parameters of D-ribose in rabbits following a single intravenous or oral dose administration
| Intravenous (IV)
|
Oral
|
||||
|---|---|---|---|---|---|
| 420 mg/kg (n=4) | 840 mg/kg (n=6) | 420 mg/kg (n=3) | 840 mg/kg (n=3) | ||
| AUCtotal (mg·min)/mL | 27.91±4.25 | 100.21±14.7* | AUCtotal (mg·min)/mL | 8.11±3.32** | 25.36±11.92** |
| k (min−1) | 0.0486±0.006 | 0.0283±0004* | k (min−1) | 0.0583±0.026 | 0.0306±0.01 |
| t1/2 (min) | 14.46±1.87 | 24.79±3.22* | t1/2 (min) | 13.44±5.23 | 24.52±8.89 |
| MRT (min) | 22.64±1.88 | 38.31±3.03* | MRT (min) | 44.53±13.9** | 54.63±6.25** |
| CL (mL·min/kg) | 15.3±2.05 | 8.53±1.25* | CL (mL·min/kg) | 60.32±31.4** | 40.93±25.2** |
| Vss (mL/kg) | 344.47±38.3 | 326.69±51.1 | Vss (mL/kg) | 2,438.03±577** | 2,266.43±1,510.4** |
| Cmax (µg/mL) | 184.6±52.1 | 369.10 ±137.6 | |||
| Tmax (min) | 36.0±12.0 | 44.0±13.9 | |||
| F (%) | 29.1±0.12 | 25.3±0.19 | |||
Notes:
Significant difference from 420 mg/kg IV dose;
significant difference from the corresponding IV dose. Data are presented as mean ± SD.
Abbreviations: AUCtotal, total area under the serum concentration–time curve from zero to infinite time; k, elimination rate constant; t1/2, elimination half-life; MRT, mean residence time; CL, clearance; Vss, volume of distribution at steady state; Cmax, maximum observed concentration; Tmax, time to reach maximum concentration; F, absolute bioavailability; IV, intravenous.
Figure 2 shows the serum concentration–time profile after oral administration of D-ribose. Oral data showed that D-ribose is rapidly absorbed (Tmax=36–44 minutes) and eliminated from the body. No significant changes in pharmacokinetic parameters were observed when oral D-ribose dose was increased from 420 mg/kg to 840 mg/kg. However, a trend toward increased AUCtotal, Cmax, t1/2, and MRT, as well as decreased clearance, was noticed with a twofold increase in D-ribose dose. The pharmacokinetic parameters of D-ribose after oral administration are listed in Table 3.
Figure 2.
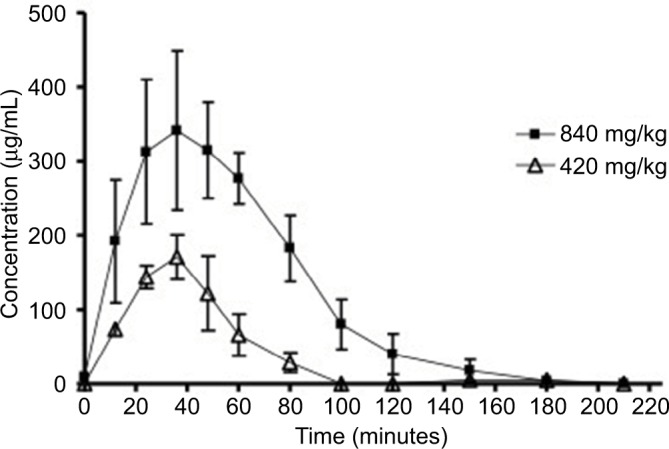
The serum concentration–time profile of D-ribose after oral administration at doses of 840 mg/kg and 420 mg/kg body weight. The values are mean ± SE.
Abbreviation: SE, standard error.
Table 3.
Urinary excretion data of D-ribose in rabbits after a single-dose intravenous administration
| Dose | CLr (mL·min/kg) | Xu∞ (mg) | UR (ratio) |
|---|---|---|---|
| 420 mg/kg (n=4) | 2.78±0.96 | 75.48±20.7 | 0.180±0.05 |
| 840 mg/kg (n=6) | 3.29±1.83 | 315.13±147.3 | 0.375±0.18 |
Note: Data are presented as mean ± SD.
Abbreviations: Xu∞, cumulative D-ribose amount excreted in urine; CLr, renal clearance; UR, percentage of D-ribose excreted unchanged in the urine.
In comparison between the two routes of administration at a given dose, oral D-ribose showed significant 3–4-fold lower AUCtotal, 4–5-fold higher clearance, twofold increase in MRT, and sevenfold increase in the volume of distribution as compared to the corresponding IV dose. For instance, the clearance of orally given 840 mg D-ribose was 40.93 mL·min/kg compared to 8.53 mL·min/kg for 840 mg IV dose. Mean half-life was not different between the oral and IV administration at both doses of 420 and 840 mg/kg. The differences in AUCtotal values between groups are presented in Figure 3.
Figure 3.
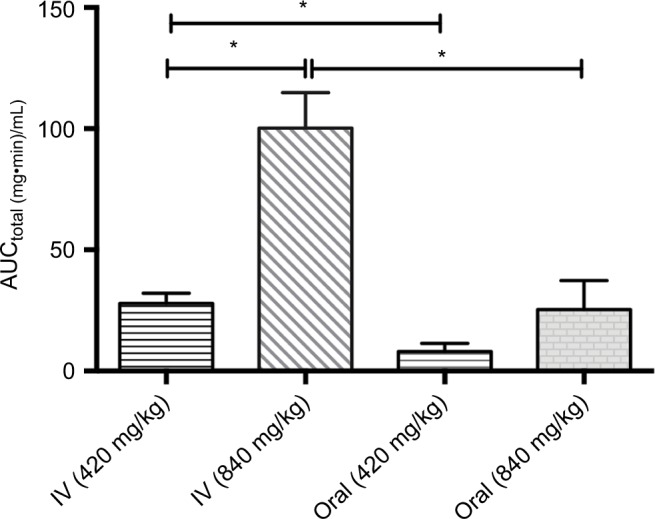
The total area under the serum concentration–time curve (AUCtotal) values for the doses given orally or intravenously to the rabbits. Data are presented as mean ± SD. *p<0.05.
Abbreviation: IV, intravenous.
D-ribose was recovered in the urine collected up to 210 minutes of its IV administration. The percentage of D-ribose excreted unchanged in the urine (UR) was 18% and 37.5% for the 420 and 840 mg/kg IV doses, respectively. This resulted in the mean renal clearance of 2.78 mL·min/kg and 3.29 mL·min/kg for the previous two doses, respectively. Urinary excretion data of D-ribose are summarized in Table 3.
Discussion
This study evaluated the pharmacokinetics of D-ribose in rabbits after single IV or oral dose administration. To our knowledge, this is the first comprehensive investigation assessing the preclinical kinetic profile of D-ribose. As indicated by results of the current study, D-ribose seems to follow a dose-dependent kinetic profile. The exposure of D-ribose increased with doubling the dose, while the clearance decreased.
The increase in D-ribose exposure observed in this study was not proportionate to the dose. Gross et al12 have shown that the steady-state concentrations (Css) of D-ribose achieved after continuous IV infusion of 83.3 and 166.7 mg/kg/h were 7.8 and 45.3 mg/100 mL, respectively. Also, oral administration of the same previous doses to healthy individuals resulted in Css of 4.8 and 32.6 mg/100 mL.12 Thus, doubling the dose has resulted in more than fivefold increase in the Css concentration. Similar finding has been demonstrated by Thompson et al13 who documented that administering oral doses of 2.5, 5, or 10 g of D-ribose to healthy subjects resulted in AUC0−t values of 6.78, 48.3, and 149 µg·h/mL, respectively, suggesting that a fourfold increase in D-ribose dose yielded more than 22-fold increase in AUC0−t. In our study, this nonproportionate increase in exposure was significant with IV route, but the same trend, although not statistically significant, was also observed via oral administration. The nonsignificant finding of oral data may be attributed in part to the small sample size (n=3) and to the inter-rabbit variability observed. This large increase in exposure may be explained partially by the saturable metabolism of D-ribose at higher doses.16 This finding might have clinical value as exposing subjects to higher doses of D-ribose might result in nonproportional increase in the pharmacological effects and perhaps an adverse effect profile. For instance, higher episodes of diarrhea were observed when D-ribose was given orally at 200 mg/kg/h versus doses of 83.3 or 166.7 mg/kg/h.12
Previous studies have shown that the levels of D-ribose fall down rapidly after IV administration in normal and diabetic human subjects.17 The time of D-ribose elimination in human to the near zero levels was about 110 minutes after a 3–20 g IV infusion dose given over 15 minutes.16 This is in accordance with our current study results, where it took about 150 minutes for D-ribose to reach near zero levels after 840 mg/kg IV bolus. The half-life of D-ribose was relatively short ranging from 13 to 25 minutes, which is in agreement with the finding of Thompson et al13 who reported half-lives over 12–20 minutes after a 2.5–15 g of D-ribose oral solution.
As shown in the results of this study, D-ribose plasma clearance is also dose dependent. A significant decrease in total clearance was shown with doubling the IV dose, and the same trend was also observed with oral administration. This suggests that D-ribose follows first-order kinetics at a low dose; thereafter, elimination systems seem to be saturated. The decrease in clearance was associated with a prolonged half-life from ~14.5 to 24.8 minutes with doubling the dose. These results are also in agreement with a previous observation that clearance values of 240, 115, and 83.2 L/h were documented with oral dosing of 2.5, 5, or 10 g, respectively.13 Thus, there appears to be no concerns about systemic accumulation.
The F of D-ribose was low (mean F=25–29%), which may inform about the higher utilization and/or incomplete intestinal absorption of D-ribose when it is orally given. Four- to fivefold higher clearance was observed with oral versus IV dosing which may have contributed to 3–4-fold lower exposure of oral D-ribose. The increase in clearance with the oral dose was accompanied with an increase in volume of distribution. These two changes may contribute, in part, to the similar half-life of D-ribose observed via oral and IV routes.
While the metabolism via the pentose phosphate pathway accounts for the primary route of D-ribose elimination,16,18 it could also be excreted by the kidney. Renal elimination of D-ribose is mediated largely by filtration with minimal contribution to reabsorption at high doses.16 Results of this study showed that D-ribose was partially (18–38%) recovered from urine. Previous studies have shown that an average of 21% of D-ribose was recovered in the urine after a 15-minute D-ribose IV infusion,16 while 4.15–16.6% was recovered after oral dosing.12,13 In our study, doubling the dose has yielded a little increase in the fraction of D-ribose excreted in the urine and the renal clearance, which may be explained, in part, by saturation of reabsorption sites at higher doses and an increase in urinary excretion of D-ribose. However, considering the high metabolic uptake and turnover of pentose sugars, D-ribose excretion as an unchanged molecule is unlikely after typical supplemental doses. Studying urinary excretion for lower doses of D-ribose is a recommended future direction.
In conclusion, D-ribose showed a dose-dependent kinetic profile, where kinetic variables change according to the administered dosing level. It rapidly disappeared from the plasma (within less than 140 minutes). Urine recovery was partial, which could be attributed to the several metabolic pathways that pentose can undergo.
Acknowledgments
The authors would like to thank the staff of Philadelphia Biomed Product Development Centre, Amman, Jordan, and Ascent Medical Technology Fund II, LP, USA, for their financial and technical support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Berg JM, Tymoczko JL, Stryer L. Biochemistry. 3rd ed. New York: W. H. Freeman; 1998. [Google Scholar]
- 2.Zimmer HG. Normalization of depressed heart function in rats by ribose. Science. 1983;220:81–82. doi: 10.1126/science.6402820. [DOI] [PubMed] [Google Scholar]
- 3.Zimmer HG, Ibel H. Ribose accelerates the repletion of the ATP pool during recovery from reversible ischemia of the rat myocardium. J Mol Cell Cardiol. 1984;16:863–866. doi: 10.1016/s0022-2828(84)80010-3. [DOI] [PubMed] [Google Scholar]
- 4.Hudson T. D-ribose in chronic fatigue syndrome, fibromyalgia, and cardiac disease. J Nat Med. 2010;2:1–3. [Google Scholar]
- 5.Perlmutter NS, Wilson RA, Angello DA, Palac RT, Lin J, Brown BG. Ribose facilitates thallium-201 redistribution in patients with coronary artery disease. J Nucl Med. 1991;32:193–200. [PubMed] [Google Scholar]
- 6.Bayram M, Cyr JA, Abraham WT. D-ribose aids heart failure patients with preserved ejection fraction and diastolic dysfunction: a pilot study. Ther Adv Cardiovasc Dis. 2015;9:56–65. doi: 10.1177/1753944715572752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishiyama J, Ueki M, Asaga T, Chujo K, Maekawa N. Protective action of D-ribose against renal injury caused by ischemia and reperfusion in rats with transient hyperglycemia. Tohoku J Exp Med. 2009;219:215–222. doi: 10.1620/tjem.219.215. [DOI] [PubMed] [Google Scholar]
- 8.Ueki M, Ueno M, Morishita J, Maekawa N. D-ribose ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. Tohoku J Exp Med. 2013;229:195–201. doi: 10.1620/tjem.229.195. [DOI] [PubMed] [Google Scholar]
- 9.Teitelbaum JE, Johnson C, Cyr J. The use of D-ribose in chronic fatigue syndrome and fibromyalgia: a pilot study. J Altern Complement Med. 2006;12:857–862. doi: 10.1089/acm.2006.12.857. [DOI] [PubMed] [Google Scholar]
- 10.Teitelbaum J, Jandrain J, McGrew R. Treatment of chronic fatigue syndrome and fibromyalgia with D-ribose – an open-label, multicenter study. Open Pain J. 2012;5:32–37. [Google Scholar]
- 11.Ismail ZB, Abu-Baker N, Alzoubi K, et al. Evaluation of alpha-D-ribofuranose (D-ribose) toxicity after intravenous administration to rabbits. Hum Exp Toxicol. 2012;31:820–829. doi: 10.1177/0960327111432505. [DOI] [PubMed] [Google Scholar]
- 12.Gross M, Reiter S, Zollner N. Metabolism of D-ribose administered continuously to healthy persons and to patients with myoadenylate deaminase deficiency. Klin Wochenschr. 1989;67:1205–1213. doi: 10.1007/BF01716208. [DOI] [PubMed] [Google Scholar]
- 13.Thompson J, Neutel J, Homer K, Tempero K, Shah A, Khankari R. Evaluation of D-ribose pharmacokinetics, dose proportionality, food effect, and pharmacodynamics after oral solution administration in healthy male and female subjects. J Clin Pharmacol. 2014;54:546–554. doi: 10.1002/jcph.241. [DOI] [PubMed] [Google Scholar]
- 14.Office of Laboratory Animal Welfare . Institutional Animal Care and Use Committee Guidebook. 2nd edition. National Institutes of Health; Bethesda, MD, USA: 2002. [Google Scholar]
- 15.Roe JH, Rice EW. A photometric method for the determination of free pentoses in animal tissues. J Biol Chem. 1948;173:507–512. [PubMed] [Google Scholar]
- 16.Segal S, Foley J. The metabolism of D-ribose in man. J Clin Invest. 1958;37:719–735. doi: 10.1172/JCI103658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bierman EL, Baker EM, Plough IC, Hall WH. Metabolism of D-ribose in diabetes mellitus. Diabetes. 1959;8:455–458. doi: 10.2337/diab.8.6.455. [DOI] [PubMed] [Google Scholar]
- 18.Hiatt HH. Studies of ribose metabolism. III. The pathway of ribose carbon conversion to glucose in man. J Clin Invest. 1958;37:651–654. doi: 10.1172/JCI103649. [DOI] [PMC free article] [PubMed] [Google Scholar]


