Significance
As a neurotropic parasite, Toxoplasma predominantly interacts with neurons throughout CNS infection and causes severe damage to the brain. In patients with severe immune dysfunction, dormant encysted bradyzoites can reactivate into fast-replicating tachyzoites and may cause damage to the brain. We report that an endoplasmic reticulum (ER)-associated protein, RTN1-C, is a substrate of ROP18 kinase. ROP18 phosphorylation of RTN1-C triggers ER stress-mediated apoptosis. Phosphorylated RTN1-C enhances GRP78 acetylation via attenuating the activity of histone deacetylases and is associated with neural apoptosis. These results link Toxoplasma’s effector ROP18 to RTN1-C, the CNS predominantly expressed protein, as well as ER stress-induced apoptosis via GRP78 acetylation, which may provide new insight into the preferential invasion of the central nervous system by Toxoplasma.
Keywords: ROP18, RTN1-C, phosphorylation, ER stress, neural apoptosis
Abstract
The neurotropic parasite Toxoplasma gondii is a globally distributed parasitic protozoan among mammalian hosts, including humans. During the course of infection, the CNS is the most commonly damaged organ among invaded tissues. The polymorphic rhoptry protein 18 (ROP18) is a key serine (Ser)/threonine (Thr) kinase that phosphorylates host proteins to modulate acute virulence. However, the basis of neurotropism and the specific substrates through which ROP18 exerts neuropathogenesis remain unknown. Using mass spectrometry, we performed proteomic analysis of proteins that selectively bind to active ROP18 and identified RTN1-C, an endoplasmic reticulum (ER) protein that is preferentially expressed in the CNS. We demonstrated that ROP18 is associated with the N-terminal portion of RTN1-C and specifically phosphorylates RTN1-C at Ser7/134 and Thr4/8/118. ROP18 phosphorylation of RTN1-C triggers ER stress-mediated apoptosis in neural cells. Remarkably, ROP18 phosphorylation of RTN1-C enhances glucose-regulated protein 78 (GRP78) acetylation by attenuating the activity of histone deacetylase (HDAC), and this event is associated with an increase of neural apoptosis. These results clearly demonstrate that both RTN1-C and HDACs are involved in T. gondii ROP18-mediated pathogenesis of encephalitis during Toxoplasma infection.
The obligate intracellular protozoan, Toxoplasma gondii, infects a broad range of hosts, including humans. As a neurotropic parasite, it exhibits a strong predilection for interacting with neurons throughout the CNS (1–3). Toxoplasmic encephalitis (TE) is the most serious outcome of toxoplasmosis due to reactivation of cysts in the brain. In AIDS, cancer, or patients with severe immune dysfunction, dormant encysted bradyzoites can reactivate into fast-replicating tachyzoites and cause severe damage to the brain (4). Identifying essential host factors, particularly those expressed in the nervous system, will represent a major step forward to further understanding of pathogenesis of TE.
Endoplasmic reticulum (ER) stress is triggered by a loss of homeostasis in the ER, resulting in the accumulation of misfolded proteins in the ER lumen (5). Numerous disturbances, including a diverse spectrum of toxins or noxious stimuli, cause accumulation of unfolded proteins in the ER, triggering a well-characterized reaction to “ER stress” termed the unfolded protein response (UPR). One of them, the glucose-regulated protein 78 (GRP78) plays a central role in activating the UPR. When the ER is “stressed” by unfolded proteins, GRP78 binds to the unfolded proteins in the ER, freeing its client proteins to trigger the UPR. However, under prolonged ER stress, the UPR can also induce apoptotic cell death through activation of caspase-12, CHOP, and/or c-JUN NH2-terminal kinase (JNK) (5). Increasing evidence indicates that intracellular pathogens and viruses alike often disturb ER homeostasis and lead to the ER stress response during infection (6, 7). As an obligate intracellular protozoan, T. gondii has been shown previously to cause ER stress and induce apoptotic cell death (2, 8–11).
Rhoptry protein 18 (ROP18), a serine (Ser)/threonine (Thr) kinase secreted by Toxoplasma rhoptries into the parasitophorous vacuole (PV) and host cytosol, was identified as a major virulence factor (12, 13). Previous studies have demonstrated that ROP18 can phosphorylate several targets in the host cell, including immunity-related GTPases (IRGs) (14, 15) and ATF6β (16), to mediate virulence during infection. Our studies revealed that ROP18 phosphorylates the host NF-κB p65 and targets this protein to ubiquitin-dependent degradation, thus inhibiting the NF-κB pathway in infected macrophages (17). In addition, our recent studies have demonstrated that ROP18 can stimulate neural cell death by inducing the ER stress-mediated apoptosis pathway (10). However, host proteins, especially those preferentially expressed in nervous tissues, targeted by ROP18 and its associated molecular mechanism remain unclear.
Herein, we report that an ER-associated protein called reticulon 1-C (RTN1-C) is a substrate that can be phosphorylated by ROP18. ROP18 phosphorylates RTN1-C and triggers ER stress-mediated apoptosis in neural cells. Moreover, phosphorylated RTN1-C enhances GRP78 acetylation by attenuating the activity of histone deacetylase (HDAC), and this event is associated with neural apoptosis. The finding offers clues to gain a better understanding of the neuropathology of TE.
Results
ROP18 Kinase Activity Is Required for Neural Apoptosis.
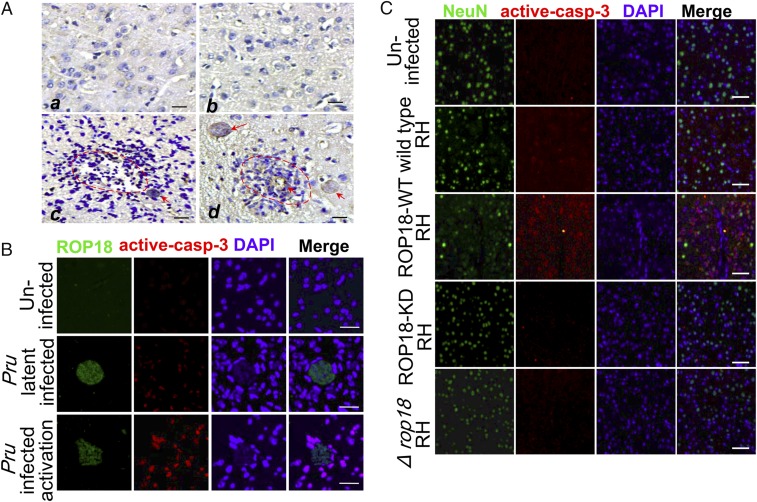
To test the hypothesis that Toxoplasma–CNS host cell interactions occur during acute toxoplasmosis, initial studies were carried out to detect encephalitis in brain tissues. As expected, necrosis and inflammation were present around Toxoplasma cysts (Fig. 1A). More importantly, analyses of immunofluorescent staining of ROP18 and active caspase-3 showed that a high degree of neural apoptosis was present in the cortex of immunosuppressed mice compared with the control, suggesting the ROP18-mediated neural apoptosis in TE (Fig. 1B). To explore whether ROP18 kinase activity plays a pivotal role in the death of neurons during infection, each individual in the group of BALB/c mice was infected i.p. with 1 × 103 tachyzoites from parasites overexpressing wild-type ROP18 reticulon homology (ROP18-WT RH), parasites overexpressing kinase-deficient ROP18 (S394A) RH (ROP18-KD RH), ROP18 knockout Δku80 RH strain (Δrop18 RH), or wild-type RH tachyzoites. Pathology in the brain tissues of animals was examined as soon as obvious clinical manifestations were observed. To detect the neuronal apoptosis in vivo, immunohistochemical staining of active caspase-3 was conducted on the brain sections. Active caspase-3 staining was more pronounced in the hippocampus and cerebral cortex of mice infected with the ROP18-WT RH or wild-type RH tachyzoites compared with the uninfected group. Remarkably, the active caspase-3 staining of the mice infected with the ROP 18-WT RH was significantly increased compared with the ROP18-KD RH– or Δrop18 RH-infected group. The specificity of the staining was confirmed by negative control with IgG and baseline weak staining in the normal brain (SI Appendix, Fig. S1 A and B). Neuronal damage was then examined using immunofluorescent staining of NeuN (a specific neuron marker) and active caspase-3 staining. Consistently, the results demonstrated that the ROP18-WT RH strain induced significant levels of active caspase-3 cleavage–positive neuronal cells, whereas infection with the ROP18-KD strain or Δrop18 RH failed to induce active caspase-3 (Fig. 1C). To investigate whether ROP18 kinase activity is required for neural apoptosis, we infected Neuro2a cells with ROP18-WT, ROP18-KD, or Δrop18 RH tachyzoites and performed flow cytometry analysis. ROP18-WT RH tachyzoites, compared with ROP18-KD or Δrop18 RH tachyzoites, significantly enhanced neural apoptosis (SI Appendix, Fig. S1 C and D). Collectively, these results suggest that ROP18 kinase activity is required for neural apoptosis.
Fig. 1.
ROP18 kinase activity is required for neural apoptosis. (A) Immunohistochemical staining of ROP18 in brain sections of mice infected with the cyst-forming Pru strain reactivated by cyclophosphamide administration. (a and b) Normal brains of the uninfected group are also included. (c and d) Necrosis and inflammatory infiltration are noted in foci of recrudescence (red dotted line), and cysts in TE (red arrows) are evident. (Scale bars, 50 μm.) (B) Immunofluorescence analysis of the brain from mice infected with the cyst-forming T. gondii Pru strain reactivated by cyclophosphamide administration. The cerebral cortex region was double-stained with ROP18 (green) and active caspase-3 (red). DAPI (blue) was used to stain the nuclei. (Scale bars, 20 μm.) (C) Immunofluorescence analysis of the brain from mice infected with ROP18-WT RH, ROP18-KD RH, ∆rop18 RH, and wild-type RH tachyzoites. The cerebral cortex S1 region was double-stained with NeuN (green, a specific marker of neuron cells) and active caspase-3 (red). DAPI (blue) was used to stain the nuclei. (Scale bars, 50 μm.)
Identification of RTN1-C as a ROP18-Interacting Host Protein.
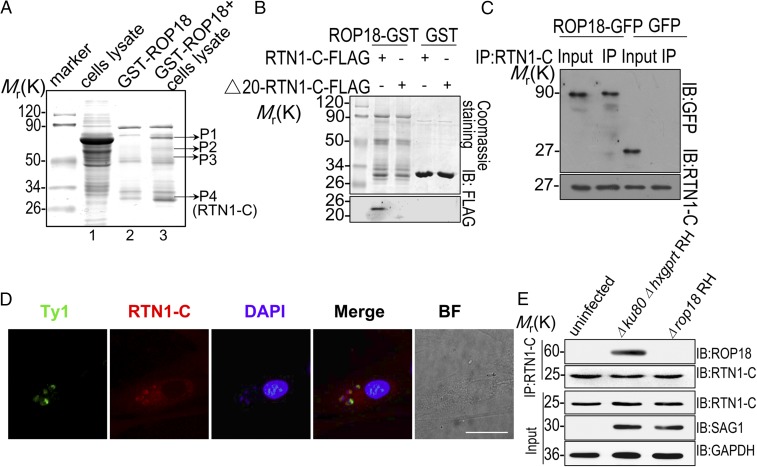
To identify neural binding partners targeted by ROP18, we conducted proteomic screening. To distinguish putative ROP18-binding proteins from nonspecific binding, ROP18-GST was used as an affinity matrix to isolate proteins interacting with active ROP18 from human SH-SY5Y neuroblastoma cell line extracts (Fig. 2A). Silver-stained bands that were enriched in the ROP18-GST pulldown were excised and subjected to protein identification using MALDI-TOF mass spectrometry. Known members of the ROP18 complex were detected, including the transcription factor of the NF-κB family, p65 (RelA), thereby validating this approach (17). In addition, we identified several previously unknown ROP18-binding partners (SI Appendix, Table S1). Among them, RTN1-C, an ER protein preferentially expressed in the CNS, was identified. To demonstrate a direct interaction between ROP18 and RTN1-C, we performed yeast two-hybrid cotransformation and GST pulldown assays. Full-length RTN1-C was cotransformed with ROP1825–251 into yeast cells using β-galactosidase activity (lacZ reporter) as a measure of protein–protein interaction. The results indicated that human RTN1-C is a binding partner for ROP18 (SI Appendix, Fig. S2). According to structure analysis of reticulon proteins, all of the members contain a carboxyl-terminal RH domain, while reticulon amino-terminal domains display little or no similarity to each other. To determine whether ROP18 specifically interacts with RTN1-C, we constructed FLAG-tagged Δ20-RTN1-C (lacking amino acids 1–20 at the N terminus, which displays no sequence similarity to RTN1-A/B and other RTNs) and transfected it into the cells. The GST pulldown assay revealed that FLAG-tagged full-length RTN1-C bound to ROP18 in vitro, whereas FLAG-tagged Δ20-RTN1-C did not (Fig. 2B). Consistently, the immunoprecipitation of RTN1-C revealed that ROP18 binds to the full length of RTN1-C (Fig. 2C) but not to the RTN1-C deficient in 20 N-terminal amino acids (Fig. 3E). Thus, the results strongly suggested the specific interactions of ROP18 with RTN1-C. To address the physiological relevance of this interaction in mammalian cells, we used the overexpressing wild-type ROP18 Ty1-tagged RH strain and infected the primary mouse cortical neurons with the parasites. Interestingly, we found that RTN1-C was recruited around the parasites and codistributed with ROP18, suggesting the colocalization of ROP18 with RTN1-C in the cytosol of the infectious primary neuron cells (Fig. 2D). To determine ROP18 and RTN1-C interaction in infected cells in vivo, SH-SY5Y cells were infected with the Δku80Δhxgprt RH or Δrop18 RH strain, and these cells were then immunoprecipitated for RTN-1C. The results indicated that the host RTN1-C is a binding protein of ROP18 during infection with the virulent type I strain (Fig. 2E). Taken together, we conclude that RTN1-C directly binds to ROP18 both in vitro and in vivo.
Fig. 2.
ROP18 interacts with RTN1-C. (A) Mass spectrometry screening to identify ROP18-binding proteins. ROP18-binding proteins were isolated from human SH-SY5Y neuroblastoma cell line extracts. Silver-stained SDS-polyacrylamide gels of control (lane 2) and ROP18-GST pulldown complexes (lane 3) are presented. Molecular weight markers are indicated. Bands present in the ROP18-GST pulldown were excised (bands shown), and protein determination was performed by MALDI-TOF mass spectrometry. (B) Confirmation of the binding site of RTN1-C and ROP18 by an in vitro GST pulldown assay. ROP18-GST bound to glutathione beads was used as an affinity matrix to absorb FLAG-tagged full-length RTN1-C and its truncate Δ20-RTN1-C–expressed Neuro2a cells. IB, immunoblot. (C) Verifying the interaction of RTN1-C with ROP18 through immunoprecipitation. Neuro2a cells were transfected with ROP18-GFP or control GFP vector for 24 h. Starting fractions (Input) and immunoprecipitates (IP) were analyzed by SDS/PAGE and Western blotting using GFP and RTN1-C antibodies. The result indicates that ROP18 (Upper) and RTN1-C (Lower) were coimmunoprecipitated. (D) Primary cortical neuronal cells grown on glass coverslips were infected with the ROP18-WT-Ty1 RH strain at a multiplicity of infection (m.o.i.) of 1 for 24 h. Double immunofluorescence was performed using mouse monoclonal anti-Ty1 (green) and rabbit polyclonal anti–RTN1-C (red) antibodies. A distinctive pattern of RTN1-C and ROP18 localization was observed around the parasite. BF, bright field. (Scale bar, 5 μm.) (E) ROP18-expressing transgenic strains were used to determine ROP18 and RTN-1C interaction. The SH-SY5Y cells were infected with the indicated parasites at an m.o.i. of ∼1. At 12 h after infection, immunoprecipitation (IP) of RTN-1C from infected cell lysates was detected with rabbit polyclonal ROP18 antibody.
Fig. 3.
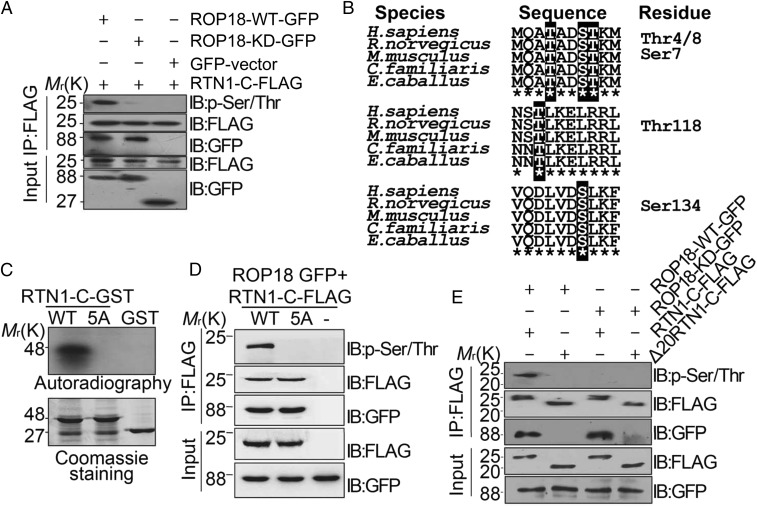
Ser7/134 and Thr4/8/118 on RTN1-C are phosphorylation sites for ROP18 in vitro and in vivo. (A) Neuro2a cells were cotransfected with ROP18-WT-GFP or ROP18-KD-GFP and FLAG-tagged RTN1-C for 24 h, and the cell lysates were immunoprecipitated (IP) with FLAG antibodies. RTN1-C phosphorylation was detected by the phospho-Ser/Thr antibody. IB, immunoblot. (B) Three peptides containing putative phosphorylation sites are indicated with red letters. ClustalW alignment of the amino acid sequences from 1–10, 116–125, and 128–137 of the human RTN1-C with rat, mouse, dog, and horse sequences. (C) ROP18 phosphorylates RTN1-C Ser7/134 and Thr4/8/118 in vitro. Bacterially expressed GST-tagged wild-type RTN1-C and RTN1-C-5A mutant (SS7/134AA + TTT4/8/118AAA) were purified on glutathione-agarose beads and phosphorylated in vitro using [32P] ATP and eukaryotically expressed ROP18 as described (17). Samples were separated by SDS/PAGE gel (Lower) and subsequently incubated with X-ray film (Upper). Of note, in the presence of ROP18, dramatic incorporation of 32P into wild-type RTN1-C, but not RTN1-C-5A, mutant protein was observed. (D) Wild-type or mutant FLAG-tagged RTN1-C-5A was transiently coexpressed with ROP18-GFP in Neuro2a cells for 24 h and immunoblotted with phospho-Ser/Thr antibody and the indicated antibodies, respectively. (E) FLAG-tagged full-length RTN1-C and its truncated form Δ20-RTN1-C were transiently coexpressed with ROP18-WT-GFP or ROP18-KD-GFP in Neuro2a cells for 24 h. Whole-cell extracts were immunoblotted with phospho-Ser/Thr antibody and the indicated antibodies, respectively.
ROP18 Phosphorylates RTN1-C at Ser7/134 and Thr4/8/118.
As noted above, ROP18 is a key Ser/Thr kinase that phosphorylates host proteins to modulate acute virulence, and its kinase activity is essential for neural apoptosis. To further clarify whether RTN1-C is the phosphorylated substrate of ROP18, we overexpressed ROP18-WT-GFP or ROP18-KD-GFP with FLAG-tagged RTN1-C in Neuro2a cells. Immunoprecipitation was performed using a FLAG antibody followed by phosphorylated Ser/Thr immunoblotting analysis. The wild-type ROP18 phosphorylated RTN1-C at Ser/Thr residues, while the kinase-deficient ROP18 did not (Fig. 3A). To identify the regulatory phosphorylation sites on RTN1-C modified by ROP18 kinase, we utilized bioinformatics analysis with NetPhos 2.0 Server (www.cbs.dtu.dk/services/NetPhos-2.0/) and ScanProsite (https://prosite.expasy.org/scanprosite/) software due to the failure of the mass spectrometry analyses of phosphorylated residues of RTN1-C using immunoprecipitation. Given the possible phosphorylated sites identified by bioinformatics, we focused on the five putative phosphorylated sites with the highest scores, that is, Ser7/134 and Thr4/8/118, as indicated in Fig. 3B. To determine which of these residues was phosphorylated, we generated a GST-tagged mutated version of the full-length RTN1-C containing single, double, triple, quadruple, or quintuple mutations where Ser or Thr was mutated to the nonphosphorylatable residue alanine. The recombinant wild-type RTN1-C or its mutant proteins were then incubated with eukaryotically expressed ROP18, and in vitro phosphorylation assays were performed. The results revealed that 32P failed to be incorporated into the RTN1-C-5A mutant proteins in which both Ser7/134 and Thr4/8/118 were replaced with alanine (SI Appendix, Fig. S3). To further confirm this result, we generated the nonphosphorylatable RTN1-C-5A mutation and repeated the in vitro phosphorylation experiment. The result showed that Ser7/134 and Thr4/8/118 of RTN1-C are the main substrates of ROP18, while phosphorylation of the RTN1-C-5A mutant proteins by ROP18 was significantly reduced, as verified by 32P incorporation in vitro (Fig. 3C). To extend these studies into cells, we cotransfected FLAG-tagged wild-type RTN1-C or the nonphosphorylatable RTN1-C-5A mutation with ROP18-GFP into Neuro2a cells. ROP18 greatly enhanced the basal phosphorylation of RTN1-C, and this increase was abolished by the nonphosphorylatable RTN1-C-5A mutation (Fig. 3D). As mentioned above, RTN1-C bound to ROP18 through the first 20 N-terminal amino acids. Therefore, we sought to address whether this interaction affects RTN1-C phosphorylation. Consequently, the absence of the 20 amino acids at the N terminus of RTN1-C eliminated its phosphorylation by ROP18, suggesting that the ROP18 and RTN1-C interaction is required for its subsequent phosphorylation (Fig. 3E). Our results strongly indicated that the interaction of RTN1-C with ROP18 mediates its phosphorylation by ROP18 at Ser7/134 and Thr4/8/118 residues.
ROP18 Phosphorylation of RTN1-C Induces ER Stress-Associated Apoptosis.
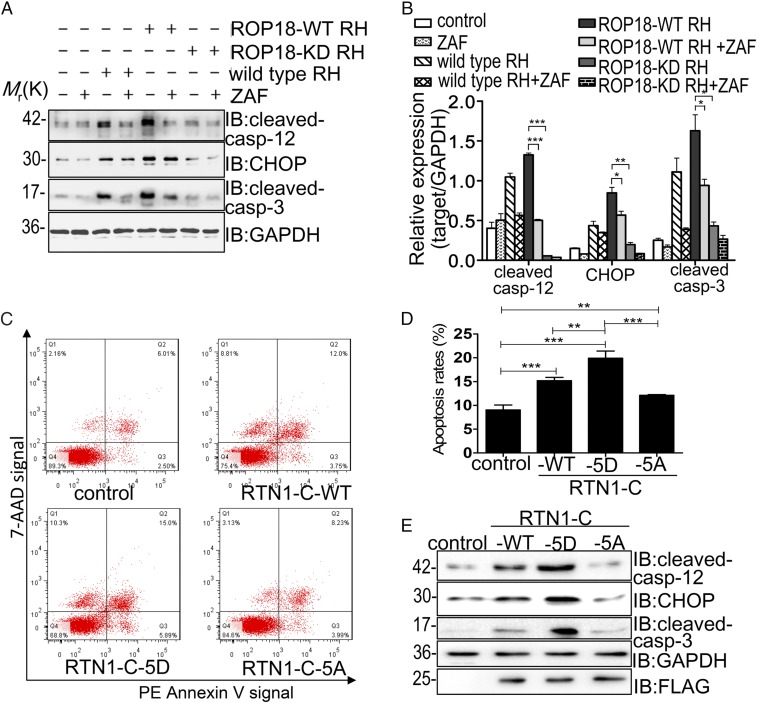
To explore whether ROP18 kinase activity is essential for activating ER stress-mediated apoptosis, Neuro2a cells transfected with ROP18-WT-GFP or ROP18-KD-GFP were treated with or without 5 μmol/L Z-ATAD-FMK, a specific caspase-12 inhibitor, and immunoblotting analyses were performed. The expression levels of cleaved caspase-12, CHOP, and cleaved caspase-3 increased significantly in the ROP18-WT-GFP group compared with the ROP18-KD-GFP and GFP groups, suggesting a pivotal role of kinase activity of ROP18 in the activation of ER stress-associated apoptosis. After Z-ATAD-FMK pretreatment, the expression levels of these related proteins were notably decreased in the ROP18-WT-GFP group compared with the group that was not pretreated, confirming that ER stress-associated apoptosis was dependent on ROP18 kinase activity (SI Appendix, Fig. S4). Additional experiments employing the ROP18-WT RH strain or ROP18-KD RH strain consistently revealed that the ROP18-WT-RH strain significantly induced high levels of these related proteins in the ER stress-mediated apoptosis pathway compared with ROP18-KD RH and wild-type RH parasites. In addition, these up-regulations were blocked by Z-ATAD-FMK treatment (Fig. 4 A and B). These data confirmed in vivo that ROP18 kinase activity is required for ER stress-associated apoptosis. To examine whether ROP18-mediated phosphorylation of RTN1-C is essential for neuronal apoptosis, plasmids expressing FLAG-tagged wild-type nonphosphorylatable RTN1-C-5A or phospho-mimicking RTN1-C (RTN1-C-5D) were transfected into Neuro2a cells. Our quantitative analysis indicated that wild-type (RTN1-C-WT) and phospho-mimicking RTN1-C (RTN1-C-5D) significantly enhanced neural apoptosis (Fig. 4C). Importantly, the apoptosis level of nonphosphorylatable RTN1-C-5A–overexpressing cells was significantly decreased compared with RTN1-C-5D–overexpressing cells, suggesting that ROP18-dependent RTN1-C phosphorylation induced apoptosis (Fig. 4D). We next examined the levels of these related proteins in the ER stress-mediated apoptosis pathway in all three FLAG-tagged RTN1-C (wild-type, RTN1-C-5D, and RTN1-C-5A) overexpressing cells. In RTN1-C-5A–transfected cells, the levels of cleaved caspase-12, CHOP, and cleaved caspase-3 were significantly decreased, whereas these signaling proteins were increased in cells transfected with wild-type RTN1-C and RTN1-C-5D mutant (Fig. 4E). Moreover, the absence of the 20 amino acids at the N terminus of RTN1-C eliminated ROP18-dependent ER stress-mediated neuronal apoptosis (SI Appendix, Fig. S5). Collectively, all these results provided evidence that ROP18-dependent RTN1-C phosphorylation at Ser7/134 and Thr4/8/118 sites promoted ER stress-associated apoptosis of Neuro2a cells.
Fig. 4.
ROP18 phosphorylation of RTN1-C induces ER stress-associated apoptosis (A) After Neuro2a cells were treated with or without Z-ATAD-FMK (ZAF) for 6 h, the cells were infected with ROP18-WT RH or ROP18-KD RH tachyzoites at a multiplicity of infection of ∼3 for 24 h. Then, cell lysates were assessed by Western blotting using the indicated antibodies. GAPDH was included as a loading control. IB, immunoblot. (B) Quantitative data of A. The presented figures are from a representative study, and the quantitative data are expressed as the mean ± SD based on different assays (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001 for ROP18-WT RH vs. ROP18-KD RH, ROP18-WT RH + ZAF, or RH group. (C) Neuro2a cells were transfected with FLAG-tagged RTN1-C-WT, RTN1-C-5A, or RTN1-C-5D for 24 h. Then, apoptotic cells were detected by flow cytometry after annexin V-phycoerythrin (PE)/7-aminoactinomycin D (7-AAD) staining. The plots were obtained from a representative measurement. (D) Quantitative data of C are expressed as the mean ± SD based on three different assays (n = 3). **P < 0.01 vs. negative controls; ***P < 0.001 vs. negative controls. (E) Representative Western blot analysis of cleaved caspase-12, CHOP, and cleaved caspase-3 in Neuro2a cells after transfection with FLAG-tagged wild-type RTN1-C or its mutants (RTN1-C-5D and RTN1-C-5A).
ROP18-Dependent RTN1-C Phosphorylation Down-Regulates HDAC Activity to Induce Apoptosis.
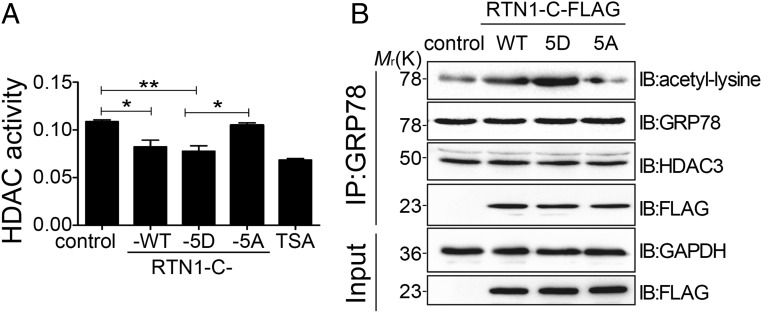
Considering that ROP18-dependent RTN1-C phosphorylation induces ER stress-associated apoptosis, we next decided to clarify how phosphorylated RTN1-C induces ER stress and its subsequent apoptosis. All three FLAG-tagged RTN1-C (wild-type, RTN1-C-5D, and RTN1-C-5A)–overexpressing Neuro2a cell extracts were analyzed for HDAC activity, and data were compared with the effect elicited by a specific inhibitor of HDAC [trichostatin A (TSA)]. Colorimetric HDAC activity assays revealed that phospho-mimicking RTN1-C-5D reduced HDAC activity and nonphosphorylatable RTN1-C-5A abolished the inhibition of HDAC activity, suggesting attenuation of HDAC activity by phosphorylated RTN1-C (Fig. 5A). Recent studies have identified GRP78, a chaperone protein primarily localized in the ER, as a molecular target of the HDAC inhibitor. Inhibition of HDACs led to GRP78 acetylation, which dissociated GRP78 from PERK, and this was associated with the activation of the UPR (18–20). Immunoprecipitation assay confirmed that inhibition of HDAC activity by TSA could also enhance GRP78 acetylation (SI Appendix, Fig. S6A). As we confirmed above that ROP18-dependent RTN1-C phosphorylation inhibited HDAC activity, we subsequently addressed whether this phosphorylation induces acetylation of GRP78. Neuro2a cells expressing all three FLAG-tagged RTN1-C proteins (wild-type, RTN1-C-5D, and RTN1-C-5A) were immunoprecipitated for GRP78, followed by immunoblotting with the antibody that can recognize antiacetylated lysine residues. Our results confirmed that HDAC3, a member of class I HDACs, interacted with GRP78 in vivo (Fig. 5B). Moreover, phospho-mimicking RTN1-C-5D overexpression compared with wild-type RTN1-C and control was found to lead to significant GRP78 acetylation. In contrast, nonphosphorylatable RTN1-C-5A dramatically reduced GRP78 acetylation, demonstrating that ROP18 phosphorylation of RTN1-C induces GRP78 acetylation (Fig. 5B). Thus, we concluded that ROP18-dependent RTN1-C phosphorylation enhanced GRP78 acetylation via attenuating HDAC activity. Recent investigations have demonstrated the capacity of GRP78 acetylation to promote dissociation of its client protein PERK and to activate the UPR. The onset of ER stress and initiation of the UPR have evolved for normal ER function. However, following prolonged ER stress or when the adaptive response fails, UPR-mediated signals can elicit apoptosis. Therefore, we hypothesized that ER stress-associated apoptosis induced by ROP18-phosphorylated RTN1-C is a consequence of GRP78 acetylation. To confirm this hypothesis, we analyzed phosphorylated RTN1-C–mediated apoptosis in the presence of anacardic acid (AA), which inhibits acetylation. Interestingly, when cells are pretreated with AA, the phosphorylated RTN-1C protein did not induce apparent apoptosis compared with the untreated phosphorylated RTN-1C group (SI Appendix, Fig. S6 B and C). Therefore, ROP18-mediated phosphorylation of RTN1-C enhanced GRP78 acetylation by attenuating HDAC activity, and this event is associated with an increase in neural cell apoptosis.
Fig. 5.
ROP18 phosphorylation of RTN1-C down-regulates HDAC activity to induce apoptosis. (A) Neuro2a cells were transfected with FLAG-tagged wild-type RTN1-C or its mutants for 24 h. Then, HDAC activity was measured using total lysates (100 mg) from these cells. As a positive control, Neuro2a cells were treated with 400 nM TSA for 12 h. *P < 0.05; **P < 0.01. (B) Neuro2a cells were transfected with FLAG-tagged wild-type RTN1-C or its mutants for 24 h. Then, cell lysates were immunoprecipitated (IP) for GRP78, and immunoblotting (IB) was performed for acetyl-lysine and the indicated antibodies, respectively.
Knockout of RTN1-C Results in Resistance to Neural Apoptosis Induced by ROP18.
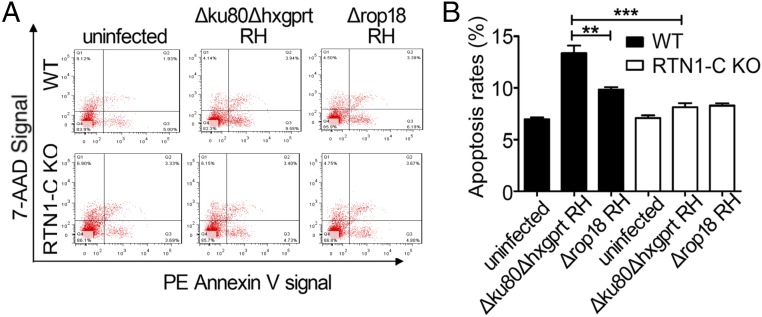
Given the correlation between the ROP18–RTN1-C interaction and neural apoptosis, we hypothesized that RTN1-C–deficient Neuro2a cells are resistant, at least in part, to apoptosis induced by ROP18. To test this possibility, we knocked out RTN1-C in Neuro2a cells using CRISPR/Cas9 genome editing (SI Appendix, Fig. S7). Wild-type or RTN1-C–deficient Neuro2a cells were infected with Δku80Δhxgprt RH or Δrop18 RH parasites. Flow cytometry analysis revealed that WT Neuro2a cells infected with Δku80Δhxgprt RH parasites exhibited a significantly increased apoptotic population compared with Δrop18 RH. In sharp contrast, RTN1-C–deficient Neuro2a cells infected with Δku80Δhxgprt RH parasites exhibited few apoptotic cells, suggesting that the ROP18–RTN1-C interaction is essential for neural cell apoptosis (Fig. 6). Taken together, all of these results confirmed that RTN1-C phosphorylation by ROP18 in neural cells is responsible for apoptosis during toxoplasmosis.
Fig. 6.
Knockdown of RTN1-C rescues neural apoptosis induced by ROP18. (A) WT or RTN1-C KO Neuro2a cells were infected with ∆ku80∆hxgprt RH or ∆rop18 RH parasites at a multiplicity of infection of ∼3 for 24 h. Then, cell apoptosis was detected by flow cytometry after annexin V-phycoerythrin (PE)/7-aminoactinomycin D (7-AAD) staining. The plots are obtained from a representative measurement. (B) Quantitative data of A are expressed as the mean ± SD based on three different assays (n = 3). **P < 0.01; ***P < 0.001.
Discussion
In intermediate mammalian hosts, including humans, T. gondii exists as two distinct forms, the tachyzoite and bradyzoite. The tachyzoite is the rapidly replicating and disease-causing form. However, it can convert into the slowly replicating bradyzoite due to host immune responses and many other factors. It then finally develops into cysts in a variety of tissues, particularly the brain. In patients with severe immune suppression, such as AIDS, dormant encysted bradyzoites can be reactivated into fast-replicating tachyzoites. Uncontrolled tachyzoite replication may cause severe and potentially life-threatening encephalitis. It is well known that encephalitis caused by T. gondii can be lethal, due to tissue inflammation, apoptosis, and/or necrosis, as well as other complex pathological processes (2, 8, 10, 11, 21). However, the mechanism of TE is still unclear despite a variety of hypotheses that have been proposed. The CNS is most commonly involved during development of toxoplasmosis, and uncontrolled parasite replication may cause life-threatening brain damage characterized by brain abscesses and necrosis. It is essential to explore the reasons for the preferential tropism of Toxoplasma parasites to these tissues and the mechanisms underlying neuropathology in patients with toxoplasmosis (3). Populations of T. gondii in North America and Europe are dominated by three clonal lineages known as types I, II and III. Type I strains are uniformly lethal with an infectious dose of a single viable organism in mice, and type II strains have an intermediate virulence, while type III strains are considered avirulent (22–24). A large of number of aspects of the parasite have been investigated to explain pathogenesis and virulence. Among them, ROP18 has been identified as a major molecule contributing to strain-specific differences in virulence (12, 13). High expression of ROP18 is closely associated with high virulence of type I and type II strains, while low expression is associated with avirulence in type III strains (12, 13, 24). Nevertheless, the causative mechanism of ROP18 as a key factor in neural tropism in Toxoplasma infections remains a mystery.
Previous studies suggested that Toxoplasma-mediated apoptosis might play a pivotal role in neurodegeneration and neuropathology in the process of TE (8). Our previous investigations demonstrated that ROP18 could induce the apoptosis of neural cells (10). However, the host proteins that are targeted by ROP18 and the exact molecular mechanisms involved are unclear. Here, we demonstrate that RTN1-C, a protein that is preferentially expressed in the CNS, is a specific substrate of the ROP18 kinase. RTNs are a group of membrane proteins localized on the ER that regulate ER structure and functions. These proteins have also been implicated in different signaling pathways and are associated with the pathogenesis of neurodegenerative disorders (25). RTN1, initially considered to be a neuroendocrine-specific protein, was actually demonstrated to be expressed in neurons of various brain regions (26). Remarkably, as an isoform of RTN1, RTN1-C is dominantly expressed in the CNS. The RTN1-C isoform could be detected in both human and mouse brain tissues and was preferentially expressed in neurons and cells of neuroendocrine tissues (27–30). Structural analyses reveal that all RTNs share a C-terminal conserved RH domain, whereas the N-terminal region of RTNs represents the variable portion that is likely responsible for the specific biological function of each isoform. On the basis of the results obtained here, we found that ROP18 was strongly bound to RTN1-C via its N-terminal 20 amino acids, suggesting that ROP18 specially interacts with RTN1-C, but not RTN1-A/B or other RTNs. A better understanding of the interaction between RTN1-C and ROP18 might offer insights into the mechanism of neural tropism of Toxoplasma infection.
During host cell invasion, T. gondii forcibly invaginates and modifies the host-cell plasma membrane, thereby creating the parasitophorous vacuole membrane (PVM). ROP18 is an active Ser/Thr protein kinase that, after secretion into the host cell, traffics into cells and inserts into the host membrane to form the PVM. Previous findings have confirmed that part of the host ER is fused with the PV during T. gondii infection (16, 31). Colocalization of the ER with the PVM suggests that the mechanism triggering up-regulation of the UPR might be a key outcome of direct protein–protein interactions between the PVM and host proteins. However, the molecular mechanisms by which Toxoplasma effectors initiate the ER stress-associated pathway remain unexplored. Our results here indicate that ROP18 kinase activity is closely involved in induction of neural apoptosis. We also demonstrate that RTN1-C is phosphorylated at Ser7/134 and Thr4/8/118 by ROP18 kinase, leading to the activation of ER stress-mediated apoptosis in neural cells. These findings suggest that ROP18 kinase induces neural apoptosis by phosphorylating RTN1-C to activate subsequent ER stress-mediated apoptosis. Despite ROP18-phosphorylated, RTN1-C mediated neural apoptosis, we also found that nonphosphorylatable RTN1-C-5A mutant and Δrop18 RH parasites could still induce neural apoptosis compared with control or uninfected cells. Therefore, even though the ROP18/RTN1-C–dependent mechanism activates ER stress-associated neural apoptosis, there might be other factors that contribute to neuropathology of TE. In this study, we also demonstrated that GRP78 could form a complex with HDAC3 and RTN1-C. The UPR is controlled by GRP78 and three different ER transmembrane sensor proteins: PERK, IRE1, and ATF6. In normal and nonstressed cells, GRP78 binds to ER transmembrane sensor proteins and maintains them in an inactive form. During ER stress, it was shown that an increase in unfolded protein substrates could lead to the sequestration of GRP78, releasing the sensors to initiate UPR signals (5, 7). If the ER stress is not relieved, the injured cells may spiral into the process of apoptosis (32). Although the function of HDAC was primarily associated with transcriptional regulation through deacetylation of histones, these enzymes have been recognized as generalized protein deacetylases rather than HDACs due to the fact that HDACs modulate numerous nonhistone substrates. For example, HDAC6 functions as an Hsp90 deacetylase, and HDAC6-regulated Hsp90 acetylation is important for Hsp90 chaperone function. In addition, at least 50 nonhistone proteins of known biological function have been identified (33). Accumulating evidence suggests that HDACs play a role in regulating the UPR through acetylation of GRP78 (19, 34). Additionally, HDAC inhibition induces the acetylation of GRP78 and activates the UPR and ER stress (18, 19, 34, 35). Interestingly, in the presence of AA, which inhibits GRP78 acetylation, phosphorylated RTN1-C protein failed to induce apoptosis. Taken together, we conclude that ROP18-phosphorylated RTN1-C leads to GRP78 acetylation by attenuating HDAC activity, and prolonged ER stress induced ER stress-associated apoptosis in neural cells. Different strains of Toxoplasma have likely evolved specific effector molecules to modulate the immune responses of different hosts. Previous investigation has shown that IFN-γ–induced IRGs are an important phosphorylated substrate of ROP18 and crucial for thwarting innate immunity in IFN-γ–activated mouse cells, although these effectors appear to play little role in human cells due to the absence of IRGs in human cells (36). Our results here, however, demonstrated that ROP18 of T. gondii type I strains is involved in ER stress-associated apoptosis in neural cells through targeting the binding partner RTN1-C in murine models as well as in humans.
TE causes complex neuropathogenesis, including inflammation, apoptosis, and necrosis (1, 2, 8–11, 21). Based on our findings, we propose a mechanism of apoptosis caused by T. gondii ROP18. Following attachment to the host cell, ROP18 is secreted by T. gondii and released into the cytosol of the host cell before the entry of the parasite into the PV. Shortly afterward, these proteins traffic to the host ER. Then, the ROP18 binds to the N-terminal portion of RTN1-C and specifically phosphorylates RTN1-C. RTN1-C phosphorylated by ROP18 regulates the induction of ER stress by inhibition of HDAC activity. The attenuation of HDAC activity leads to GRP78 acetylation and subsequent activation of the UPR. Finally, the UPR and prolonged ER stress lead to apoptosis in neural cells, which is an important factor leading to pathological damage and TE (SI Appendix, Fig. S8).
In the light of our results, we consider that RTN-1C is an important candidate for the pathogenesis of TE encephalitis. Furthermore, the ER is increasingly considered as an attractive potential therapeutic target under the premise that maintaining ER function and dampening ER stress may ameliorate neuropathology during Toxoplasma infection. Our results do not merely hint at the long-term puzzle as to why the nervous system is Toxoplasma’s primary target but offer a detailed understanding that could lead to novel approaches to disease control.
Materials and Methods
Animals.
The 3- to 4-wk-old female BALB/c mice used in this study were obtained from the Experimental Animal Center of Anhui Province. The mouse model of TE was established as previously described (11).
Parasites.
The ROP18 wild-type overexpressing transgenic RH strain (ROP18-WT RH) and ROP18 kinase-deficient (S394A) overexpressing transgenic RH strain (ROP18-KD RH) were constructed as previously described (17). Briefly, the 5′-UTR-TUB promoter wild-type ROP18-Ty1-HXGPRT 3′-UTR fragment or the 5′-UTR TUB promoter kinase-deficient ROP18-Ty1-HXGPRT 3′-UTR fragment was transfected into the Δku80Δhxgprt RH parasites by electroporation. The Δku80Δhxgprt RH strain (parental; Δku80Δhxgprt RH) and ROP18 knockout Δku80 RH strain (Δrop18 RH) were kindly provided by John Boothroyd, Stanford University School of Medicine, Stanford, CA (37). Stable integrants were selected in media with mycophenolic acid and xanthine and cloned by limiting dilution. The wild-type ROP18-Ty1 RH strain (overexpressing wild-type ROP18 RH strain) and the kinase-deficient ROP18-Ty1 RH strain (38) (overexpressing kinase-deficient ROP18 RH strain) were kindly provided by J. F. Dubremetz, Universite de Montpellier, Montpellier, France. All parasite strains and cell lines were routinely assessed for mycoplasma contamination, and no contamination was detected. Other materials and methods are described in SI Appendix.
Ethics Statement.
Ethical permission was obtained from the Institutional Review Board (IRB) of the Institute of Biomedicine at Anhui Medical University, which records and regulates all research activities in the school. The IRB of the Anhui Medical University approved both animal and human protocols. The approval from the IRB includes the permission to use mice under CO2 anesthesia and all experimental procedures were performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (39).
Supplementary Material
Acknowledgments
We thank Dr. John Boothroyd and Dr. J. F. Dubremetz, who kindly provided the strains of T. gondii and plasmids, and Dr. Geoff Hide, who provided comments. This work was supported by the National Key R&D Program of China (Grant 2017YFD0500400), the National Natural Science Foundation of China (Grants 81672046, 81471983, 81271864, and 31772445), the Academic and Technology Leaders Reserve Candidate Fund of Anhui Province (Grant 2016H080), and the Key Project of Outstanding Young Talent Support Program of Anhui Province University (Grant gxyqZD2016047).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1801118115/-/DCSupplemental.
References
- 1.Cabral CM, et al. Neurons are the primary target cell for the brain-tropic intracellular parasite Toxoplasma gondii. PLoS Pathog. 2016;12:e1005447. doi: 10.1371/journal.ppat.1005447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parlog A, Schlüter D, Dunay IR. Toxoplasma gondii-induced neuronal alterations. Parasite Immunol. 2015;37:159–170. doi: 10.1111/pim.12157. [DOI] [PubMed] [Google Scholar]
- 3.Wohlfert EA, Blader IJ, Wilson EH. Brains and brawn: Toxoplasma infections of the central nervous system and skeletal muscle. Trends Parasitol. 2017;33:519–531. doi: 10.1016/j.pt.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubey JP. Toxoplasmosis of Animals and Humans. 2nd Ed CRC Press; Boca Raton, FL: 2010. [Google Scholar]
- 5.Madeo F, Kroemer G. Intricate links between ER stress and apoptosis. Mol Cell. 2009;33:669–670. doi: 10.1016/j.molcel.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Inácio P, et al. Parasite-induced ER stress response in hepatocytes facilitates Plasmodium liver stage infection. EMBO Rep. 2015;16:955–964. doi: 10.15252/embr.201439979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fung TS, Liu DX. Coronavirus infection, ER stress, apoptosis and innate immunity. Front Microbiol. 2014;5:296. doi: 10.3389/fmicb.2014.00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dincel GC, Atmaca HT. Increased expressions of ADAMTS-13 and apoptosis contribute to neuropathology during Toxoplasma gondii encephalitis in mice. Neuropathology. 2016;36:211–226. doi: 10.1111/neup.12263. [DOI] [PubMed] [Google Scholar]
- 9.Zhou J, et al. Toxoplasma gondii prevalent in China induce weaker apoptosis of neural stem cells C17.2 via endoplasmic reticulum stress (ERS) signaling pathways. Parasit Vectors. 2015;8:73. doi: 10.1186/s13071-015-0670-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wan L, et al. T. gondii rhoptry protein ROP18 induces apoptosis of neural cells via endoplasmic reticulum stress pathway. Parasit Vectors. 2015;8:554. doi: 10.1186/s13071-015-1103-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang YH, et al. Activated microglia contribute to neuronal apoptosis in Toxoplasmic encephalitis. Parasit Vectors. 2014;7:372. doi: 10.1186/1756-3305-7-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeij JP, et al. Polymorphic secreted kinases are key virulence factors in toxoplasmosis. Science. 2006;314:1780–1783. doi: 10.1126/science.1133690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor S, et al. A secreted serine-threonine kinase determines virulence in the eukaryotic pathogen Toxoplasma gondii. Science. 2006;314:1776–1780. doi: 10.1126/science.1133643. [DOI] [PubMed] [Google Scholar]
- 14.Hakimi MA, Olias P, Sibley LD. Toxoplasma effectors targeting host signaling and transcription. Clin Microbiol Rev. 2017;30:615–645. doi: 10.1128/CMR.00005-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fentress SJ, et al. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe. 2010;8:484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto M, et al. ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. 2011;208:1533–1546. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du J, et al. Toxoplasma gondii virulence factor ROP18 inhibits the host NF-κB pathway by promoting p65 degradation. J Biol Chem. 2014;289:12578–12592. doi: 10.1074/jbc.M113.544718. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Kahali S, Sarcar B, Prabhu A, Seto E, Chinnaiyan P. Class I histone deacetylases localize to the endoplasmic reticulum and modulate the unfolded protein response. FASEB J. 2012;26:2437–2445. doi: 10.1096/fj.11-193706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kahali S, et al. Activation of the unfolded protein response contributes toward the antitumor activity of vorinostat. Neoplasia. 2010;12:80–86. doi: 10.1593/neo.91422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao R, et al. Treatment with panobinostat induces glucose-regulated protein 78 acetylation and endoplasmic reticulum stress in breast cancer cells. Mol Cancer Ther. 2010;9:942–952. doi: 10.1158/1535-7163.MCT-09-0988. [DOI] [PubMed] [Google Scholar]
- 21.Dincel GC, Atmaca HT. Role of oxidative stress in the pathophysiology of Toxoplasma gondii infection. Int J Immunopathol Pharmacol. 2016;29:226–240. doi: 10.1177/0394632016638668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dubey JP, Hotea I, Olariu TR, Jones JL, Dărăbuş G. Epidemiological review of toxoplasmosis in humans and animals in Romania. Parasitology. 2014;141:311–325. doi: 10.1017/S0031182013001509. [DOI] [PubMed] [Google Scholar]
- 23.Dubey JP, Tiao N, Gebreyes WA, Jones JL. A review of toxoplasmosis in humans and animals in Ethiopia. Epidemiol Infect. 2012;140:1935–1938. doi: 10.1017/S0950268812001392. [DOI] [PubMed] [Google Scholar]
- 24.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10:766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Sano F, Bernardoni P, Piacentini M. The reticulons: Guardians of the structure and function of the endoplasmic reticulum. Exp Cell Res. 2012;318:1201–1207. doi: 10.1016/j.yexcr.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Roebroek AJ, et al. Cloning and expression of alternative transcripts of a novel neuroendocrine-specific gene and identification of its 135-kDa translational product. J Biol Chem. 1993;268:13439–13447. [PubMed] [Google Scholar]
- 27.Steiner P, et al. Reticulon 1-C/neuroendocrine-specific protein-C interacts with SNARE proteins. J Neurochem. 2004;89:569–580. doi: 10.1111/j.1471-4159.2004.02345.x. [DOI] [PubMed] [Google Scholar]
- 28.Wildasin K. Role of reticulon proteins in Alzheimer’s disease. Lancet Neurol. 2004;3:576. doi: 10.1016/s1474-4422(04)00874-9. [DOI] [PubMed] [Google Scholar]
- 29.Di Sano F, Fazi B, Tufi R, Nardacci R, Piacentini M. Reticulon-1C acts as a molecular switch between endoplasmic reticulum stress and genotoxic cell death pathway in human neuroblastoma cells. J Neurochem. 2007;102:345–353. doi: 10.1111/j.1471-4159.2007.04479.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang YS, Strittmatter SM. The reticulons: A family of proteins with diverse functions. Genome Biol. 2007;8:234. doi: 10.1186/gb-2007-8-12-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldszmid RS, et al. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J Exp Med. 2009;206:399–410. doi: 10.1084/jem.20082108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scroggins BT, et al. An acetylation site in the middle domain of Hsp90 regulates chaperone function. Mol Cell. 2007;25:151–159. doi: 10.1016/j.molcel.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kahali S, Sarcar B, Chinnaiyan P. The emerging role of histone deacetylases (HDACs) in UPR regulation. Methods Enzymol. 2011;490:159–174. doi: 10.1016/B978-0-12-385114-7.00010-6. [DOI] [PubMed] [Google Scholar]
- 35.Fazi B, et al. Acetylation of RTN-1C regulates the induction of ER stress by the inhibition of HDAC activity in neuroectodermal tumors. Oncogene. 2009;28:3814–3824. doi: 10.1038/onc.2009.233. [DOI] [PubMed] [Google Scholar]
- 36.Niedelman W, et al. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 2012;8:e1002784. doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci USA. 2011;108:9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Hajj H, et al. ROP18 is a rhoptry kinase controlling the intracellular proliferation of Toxoplasma gondii. PLoS Pathog. 2007;3:e14. doi: 10.1371/journal.ppat.0030014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.