Significance
Low birthweight is a risk factor for developmental delay, and is also associated with a higher odds of being left-handed. Likewise, compared with singletons, triplet births encompass an increased probability of left-handedness and developmental delay. In a large study, including triplets from two countries, we studied if left-handedness is associated with birthweight and postnatal development. Triplets have naturally low birthweights, being about 1.5 kg lighter than singletons. In both samples of triplets, we showed that left-handedness was associated with lower birthweight. Left-handers had also smaller infant head circumference and delayed motor development compared with right-handers, but these differences were not evident when controlling for birthweight.
Keywords: birthweight, handedness, laterality, prenatal development, triplets
Abstract
The mechanisms behind handedness formation in humans are still poorly understood. Very low birthweight is associated with higher odds of left-handedness, but whether this is due to low birthweight itself or premature birth is unknown. Handedness has also been linked to development, but the role of birthweight behind this association is unclear. Knowing that birthweight is lower in multiple births, triplets being about 1.5 kg lighter in comparison with singletons, and that multiples have a higher prevalence of left-handedness than singletons, we studied the association between birthweight and handedness in two large samples consisting exclusively of triplets from Japan (n = 1,305) and the Netherlands (n = 947). In both samples, left-handers had significantly lower birthweight (Japanese mean = 1,599 g [95% confidence interval (CI): 1,526–1,672 g]; Dutch mean = 1,794 g [95% CI: 1,709–1,879 g]) compared with right-handers (Japanese mean = 1,727 g [95% CI: 1,699–1,755 g]; Dutch mean = 1,903 g [95% CI: 1,867–1,938 g]). Within-family and between-family analyses both suggested that left-handedness is associated with lower birthweight, also when fully controlling for gestational age. Left-handers also had significantly delayed motor development and smaller infant head circumference compared with right-handers, but these associations diluted and became nonsignificant when controlling for birthweight. Our study in triplets provides evidence for the link between low birthweight and left-handedness. Our results also suggest that developmental differences between left- and right-handers are due to a shared etiology associated with low birthweight.
The majority of humans are right-handed, with the prevalence of left-handedness being about 10% in countries that are permissive toward the use of the left hand (1–3). Although the proportion of left-handedness varies between different cultures, a consistent finding is that left-handedness is more common in males compared with females with a difference of about 2–4% points [the male-to-female odds ratio was 1.23 in a metaanalysis of Papadatou-Pastou et al. (4, 5)].
Ultrasound studies have indicated that handedness formation takes place prenatally, as early as 10 wk from gestation, as soon as independent arm movements start to occur (6, 7). Support for the genetic control (i.e., heritability) of handedness exists, but twin studies have suggested that only one-quarter of the variance in handedness is explained by genetic effects (2, 8), thus indicating a substantial role of environmental influences behind the individual differences in handedness. Intrauterine growth and hormonal milieu are prenatal factors that have been studied in relation to handedness (see refs. 9–11 for hormonal theories of handedness).
In singletons, a metaanalysis provided evidence of a higher prevalence of left-handedness in children with a history of preterm birth compared with those born at term (12). Importantly, studies have indicated that very low birthweight (VLBW, <1.5 kg) and extremely low birthweight (ELBW, <1.0 kg) are associated with a greater prevalence of left-handedness (13–15). Besides birthweight, birth order, maternal age, infertility treatment, maternal stress, and birth complications are perinatal factors which have, although inconsistently, been reported to be associated with handedness (16). Gestational age is highly correlated with birthweight and could be additionally considered as an important covariate (12).
In multiples, birthweight is naturally lower compared with singletons, with twins and triplets being about 1.0 and 1.5 kg lighter than singletons, respectively (17). Interestingly, previous studies have indicated a higher prevalence of left-handedness in twins and triplets compared with singletons (8, 18). However, these differences may be due to perinatal factors that differ between multiples and singletons (19). In line with this, we previously reported that the difference in the prevalence of left-handedness between twins and singletons was not evident when controlling for birth- and pregnancy-related factors (20). Furthermore, in twins, the prevalence of left-handedness was significantly higher in those in the lowest decile of birthweight compared with those of higher birthweight. These results suggest that multiple birth itself is not associated with handedness, but the increased odds for left-handedness are rather due to either restricted intrauterine growth or pregnancy/birth-related risks that are more common in twins than singletons (21). Furthermore, together with studies in singletons, the results of Heikkilä et al. (20) suggest that the association between birthweight and handedness is driven by the low end of the birthweight distribution. In line with this, a study of 9,688 singletons found no association between handedness and birthweight when using a cutoff of 2.5 kg (22).
With an average birthweight well below 2.0 kg, triplets have a naturally lower birthweight compared with singletons and twins (17, 20) and, unlike what we have seen among twins and singletons (20), a sizable portion of triplets have a birthweight below the VLBW threshold. Triplets thus offer a unique setting to test the association between birthweight and handedness. Here, by using a continuous measure of birthweight, we showed that lower birthweight was associated with left-handedness in 2,252 triplets in two large samples, from Japan (n = 1,305) and the Netherlands (n = 947). We also showed that compared with right-handers, left-handers had smaller infant head circumferences and more delayed postnatal motor development, but these differences were not evident when controlling for birthweight.
Results
The prevalence of left-handedness was significantly lower in the Japanese than in the Dutch sample (9.6% vs. 13.9%, χ2 = 10.32, P = 0.002) (Table 1 and SI Appendix, Table S1 display handedness classification for self-reported and maternal-reported handedness separately). The prevalence of left-handedness did not differ between males (8.3%) and females (10.9%) in the Japanese sample (P = 0.11), whereas in the Dutch sample significantly more males (16.7%) than females (11.3%) were left-handed (P = 0.02). The number of ambidextrous individuals was 42 (3.2%) in Japan and 35 (3.7%) in the Netherlands; these individuals were excluded from the main analyses.
Table 1.
Basic characteristics of the two triplet datasets
| Japanese triplets, n = 1,305 | Dutch triplets, n = 947 | |||
| Handedness | Proportion/mean, SD | N | Proportion/mean, SD | N |
| Left-handed* | 9.6% | 125 | 13.9% | 132 |
| Right-handed | 70.8% | 924 | 75.9% | 719 |
| Ambidextrous | 3.2% | 42 | 3.7% | 35 |
| Missing | 16.4% | 214 | 6.5% | 61 |
| Birthweight, g | 1,706 (426) | 1,293 | 1,888 (476) | 921 |
| Males | 1,734 (435) | 654 | 1,959 (468) | 454 |
| Females | 1,680 (417) | 617 | 1,819 (474) | 467 |
| Gestational age, wk | 33.4 (2.62) | 1,248 | 33.8 (2.60) | 822 |
| Males | 33.4 (2.67) | 638 | 34.0 (2.45) | 411 |
| Females | 33.5 (2.59) | 588 | 33.7 (2.72) | 411 |
After excluding ambidextrous and missing, the prevalence of left-handedness became 11.9% and 15.5% in the triplets of Japan and the Netherlands, respectively. Sex was missing for 22 triplet children in Japanese data.
Males had higher birthweight compared with females (P < 0.001, Table 1) and mean birthweight was smaller in the Japanese compared with the Dutch sample (P < 0.001, Table 1). The birthweight decreased as a function of within-triplet-birth-order in both samples (Fig. 1). The proportion of triplets with a birthweight less than 1,500 g was 30.1% and 21.0% for Japanese and Dutch triplets, respectively. Gestational age did not differ between males and females (P = 0.5) or between Japan and The Netherlands (P = 0.2) (Table 1).
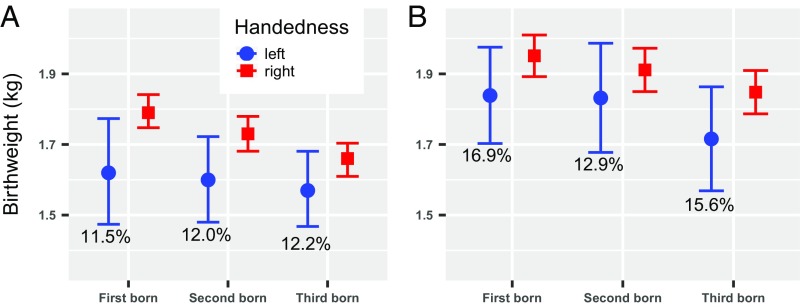
Fig. 1.
Mean birthweight with 95% CI by handedness and birth order in triplets. Right-handers (n = 919) had significantly higher birthweight than left-handers (n = 124; 11.9%) in Japan (A). Right-handers (n = 707) had significantly higher birthweight than left-handers (n = 126; 15.1%) in the Netherlands (B). Percentages indicate the prevalence of left-handers within birth order groups, for P values, see Table 2.
In both samples, left-handers had significantly lower birthweight (Japanese mean = 1,599 g, SD = 411 g; Dutch mean = 1,794 g, SD = 481 g) compared with right-handers (Japanese mean = 1,727 g, SD = 429 g; Dutch mean = 1,903 g, SD = 476 g) (Fig. 1 and Table 2). There was no significant birthweight–country interaction on handedness (P = 0.446), indicating that the effect of birthweight was similar in both countries (combined dataset odds ratio (OR) = 0.60 [95% CI: 0.45, 0.81]; SI Appendix, Table S2). The significant birthweight difference between handedness groups remained when excluding those who were ≤5 y old (OR = 0.54 [95% CI: 0.31, 0.92], in Japan, and OR = 0.65 [95% CI: 0.44, 0.98], in the Netherlands, Ps < 0.05). Additional analyses in the combined dataset indicated that lower birthweight was also related to a higher odds of being ambidextrous, but this association was not statistically significant (OR = 0.71 [95% CI: 0.42, 1.20], P = 0.199). Lower birthweight was also related to a higher odds of non–right-handedness, i.e., when including ambidextrous in the group of left-handers (OR = 0.63 [95% CI: 0.48, 0.82], P = 0.001).
Table 2.
Logistic regression models reporting OR with 95% CI for left-handedness (left- versus right-handed) in Japanese (n = 1,043) and Dutch (n = 833) triplets
| Japanese triplets | Dutch triplets | ||||
| Model | Variable | OR, 95% CIs | P | OR, 95% CIs | P |
| Model 1 | Birthweight, kg | 0.50 (0.31–0.78) | <0.01 | 0.62 (0.43–0.90) | 0.01 |
| Model 2 | First born | (Reference†) | (Reference†) | (Reference†) | (Reference†) |
| Second born | 1.05 (0.67–1.64) | 0.84 | 0.73 (0.46–1.14) | 0.17 | |
| Third born | 1.10 (0.70–1.74) | 0.68 | 0.89 (0.57–1.38) | 0.59 | |
| Model 3 | Sex, boys | (Reference†) | (Reference†) | (Reference†) | (Reference†) |
| Sex, girls | 1.36 (0.94–1.97) | 0.10 | 0.60 (0.42–0.86) | 0.01 | |
| Model 4 | Birthweight, kg | 0.32 (0.16–0.65) | <0.01 | 0.67 (0.39–1.15) | 0.15 |
| Sex, boys | (Reference†) | (Reference†) | (Reference†) | (Reference†) | |
| Sex, girls | 0.28 (0.06–1.20) | 0.09 | 1.13 (0.25–5.16) | 0.88 | |
| Birthweight*Sex | 2.57 (1.06–6.22) | 0.04 | 0.66 (0.29–1.49) | 0.32 | |
| Model 5 | Birthweight, kg | 0.50 (0.31–0.81) | <0.01 | 0.55 (0.36–0.84) | 0.01 |
| First born | (Reference†) | (Reference†) | (Reference†) | (Reference†) | |
| Second born | 1.0 (0.63–1.59) | 1.00 | 0.70 (0.42–1.16) | 0.17 | |
| Third born | 0.98 (0.61–1.58) | 0.94 | 0.85 (0.53–1.36) | 0.49 | |
| Sex, boys | (Reference†) | (Reference†) | (Reference†) | (Reference†) | |
| Sex, girls | 1.26 (0.86–1.85) | 0.24 | 0.59 (0.40–0.87) | 0.01 | |
| Maternal age, y | 0.99 (0.92–1.05) | 0.65 | 0.99 (0.92–1.05) | 0.68 | |
| Infertility treatment | 0.92 (0.55–1.55) | 0.77 | 0.74 (0.47–1.16) | 0.19 | |
Interaction term in the logistic regression model.
Reference group (OR = 1.0 with missing confidence interval).
Fig. 1 also shows the mean birthweight of the right- and left-handed participants as a function of birth order (see SI Appendix, Fig. S1 for birthweight distributions of left- and right-handed participants in the two datasets). Birth order was not significantly associated with handedness (Fig. 1 and Table 2). The association between birthweight and handedness remained in both samples after controlling for birth order, sex, maternal age and infertility treatment status (Japanese sample OR = 0.50 [95% CI: 0.31, 0.81]; Dutch sample OR = 0.55 [95% CI: 0.36, 0.84]). Maternal age and infertility treatment status were not significantly associated with handedness (Table 2). There was a significant sex by birthweight interaction on handedness only in the Japanese data (Table 2).
In the Japanese sample, gestational age did not show a significant association with handedness (see model 1 in SI Appendix, Table S3). When birthweight was added to the model, birthweight was a significant (P < 0.01) predictor of handedness, while the association between gestational age and handedness remained nonsignificant (P = 0.30). In the Dutch sample, both gestational age and birthweight, when analyzed separately, were significantly associated with handedness (P = 0.01 for both), but neither of these associations remained significant when modeled simultaneously (SI Appendix, Table S3). The correlation of gestational age and birthweight was 0.79 and 0.74 in the Japanese and Dutch datasets, respectively (SI Appendix, Figs. S2 and S3). Zygosity was not associated with handedness (SI Appendix, Table S3).
For within-triplet analyses, the number of handedness-discordant triplet-member pairs were 102 and 104 in the Japanese and Dutch samples, respectively. The results turned out to be similar to those of the between-family analyses: overall OR of handedness on birthweight was 0.63 (95% CI: 0.32, 1.24); (OR = 0.67 [95% CI: 0.22, 1.99] and OR = 0.51 [95% CI 0.21, 1.23] in the Japanese and Dutch samples, respectively; SI Appendix, Table S4). The results, however, were not statistically significant.
Looking at general motor development, as indicated by the first unrotated principal component of five motor development items, left-handers had significantly delayed motor development compared with right-handers in the Japanese triplets (n = 386) (Table 3). A similar trend was observed in the Dutch triplets, but the difference was not statistically significant in this smaller sample including only nine left-handers (n = 65) (Table 3). In the Japanese sample, turning, sitting and walking alone were achieved significantly later in left-handers than right-handers (SI Appendix, Table S5). In the Dutch sample, four out of five individual milestones were achieved later in left-handers, but none of the differences were statistically significant (SI Appendix, Table S5). Higher birthweight was associated with earlier motor development in both samples (Table 3 and SI Appendix, Table S5 for individual items). Moreover, no significant differences in motor development between left- and right-handers were evident after controlling for birthweight (Table 3 and SI Appendix, Table S5).
Table 3.
Regression analysis of motor development milestones and head circumference by handedness and birthweight
| Model 1 | Model 2 | Model 3 | ||||||||||
| Left-handers | Right-handers | Handedness | Birthweight | Handedness | Birthweight | |||||||
| Sample/measure | M, SD | N | M, SD | N | Beta | P | Beta | P | Beta | P | Beta | P |
| Japan | ||||||||||||
| Motor development* | 0.57 (2.02) | 47 | −0.14 (1.72) | 339 | 0.71 | 0.049 | −2.11 | <0.001 | 0.28 | 0.270 | −2.10 | <0.001 |
| Head circumference | 29.3 (2.23) | 60 | 30.13 (2.39) | 496 | −0.83 | 0.012 | 4.57 | <0.001 | −0.01 | 0.978 | 4.54 | <0.001 |
| The Netherlands | ||||||||||||
| Motor development* | 0.12 (1.82) | 9 | 0.004 (1.89) | 56 | 0.27 | 0.715 | −1.01 | 0.032 | 0.13 | 0.833 | −0.85 | 0.085 |
First unrotated component of the five motor development milestones (turning, sitting, crawling, standing alone, walking alone). Higher values indicate more delayed motor development. All models included sex as a covariate. Models 1 and 2 had only one covariate of importance, handedness, and birthweight, respectively. Model 3 included both independent variables.
Left-handers had significantly smaller infant head circumferences than right-handers (Table 3). Higher birthweight was positively associated with infant head circumference, but handedness was not related to infant head circumference after controlling for birthweight (Table 3).
Discussion
This is a study to analyze triplet data to explore the effects of birthweight and other perinatal factors on handedness. We found that triplets, who naturally have a low birthweight, show an increased prevalence of left-handedness as a function of lower birthweight. Furthermore, birthweight was a stronger explanatory variable for left-handedness than birth order or gestational age. Results were similar in two separate datasets, from Japan and the Netherlands. Within-triplet results, fully controlling for gestational age, were also similar to between-family results. However, within-family analyses remained nonsignificant, likely due to the low number of triplets discordant for handedness.
Our finding of the association between birthweight and the increased proportion of left-handedness among triplets is in line with singleton studies of VLBW and ELBW individuals (14, 15), a metaanalysis of preterm singletons (12), and a large twin–singleton study which found birthweight to be a significant explanatory variable of handedness (2). Additionally, in our previous study (20), we found that differences in handedness between singletons and twins were not evident after controlling for birthweight.
In the current study, birthweight seemed to dominate over gestational age and other perinatal covariates which have been associated with handedness in some but not all studies (16). The high correlation between birthweight and gestational age (r > 0.7 in our datasets) indicates that these variables cannot be included in regression models simultaneously. If birthweight was the most important pregnancy/birth-related factor determining handedness, the associations between gestational age and handedness could also reflect the effects of birthweight on handedness.
Without contrasting with singletons (i.e., our triplets-only sample), based on the birthweight distribution, an association between continuous birthweight and an increased proportion of left-handedness became evident due to the remarkable fraction of triplets having a birthweight less than 1.5 kg (Japanese: 30.1%; Dutch: 21.0%), a VLBW threshold in singletons.
We did not have nontriplet participants in our study. However, a Dutch family study combining 27,071 twins and their nontwin siblings reported the prevalence of left-handedness (including ambidextrous persons) to be 17.6% and 15.2% among the male and female participants, respectively (2). A study of Japanese adolescent twins (n > 2,000) reported a prevalence of 8.4% and 7.2% for left-handedness in boys and girls, respectively (23). In line with our study in triplets, sex differences in these samples including twins were evident only in the Netherlands (2), but not Japan (23). However, studies in Japanese singletons have also indicated a higher prevalence of left-handedness in males (4). We note that these studies have used different handedness measures and classifications in different birth cohorts, thus not allowing for direct comparisons between studies. Nevertheless, they suggest a somewhat higher prevalence of left-handedness in the Netherlands than Japan; a similar observation was made in our study of triplets from these countries. This difference between countries may be due to cultural differences [i.e., European countries being more permissive toward left-handedness (16)], but genetic, epigenetic, prenatal healthcare, or other reasons cannot be ruled out. The mean age at the time of handedness classification was also higher in the Dutch sample.
Handedness has commonly been linked with cerebral lateralization of language. However, the correspondence between lateralization of language and motor functions is far from unity (24–26): The proportions of typical left hemispheric language dominance in right- and non–right-handers are about 90% and 70–80%, respectively (25–27). Our study did not include a measure of language lateralization, but studies in singletons have suggested atypical language lateralization to be more common in prematurely born children (28, 29).
In singletons, a metaanalysis indicated a higher prevalence of left-handedness in children with a history of preterm birth compared with those born at term (12). Further, other metaanalyses have shown that lower birthweight is associated with poorer motor development, coordination disorders (30, 31), and poorer language development (32) across childhood. Taken together, low birthweight seems to have global effects on cognitive and motor development, including handedness.
Many previous studies have found neurological deficits to relate to VLBW, preterm birth, and multiples (21, 33–39). A metaanalysis indicated that non–right-handedness is more common in those with intellectual disabilities compared with those with no intellectual disability (40). Similarly, another metaanalysis showed that low birthweight is a risk factor of intellectual disability (41). Despite the significant group differences between left- and right-handers in cognitive abilities (42) and despite the higher prevalence of non–right-handedness in some disorders such as schizophrenia (43) and deafness (44), we note that left-handedness itself is not a deficit. Indeed, a recent systematic review and metaanalysis indicated negligible differences in intelligence between left-handers and right-handers in the general population (45). Although both poorer cognitive ability (35, 37, 38) and left-handedness have been linked to low birthweight, this does not imply a causal link between cognitive ability and left-handedness.
It has been proposed that left-handedness has a pathological (46, 47) origin in some individuals, resulting from left hemisphere damage during the prenatal or perinatal period. Indeed, lower birthweight increases the risk of perinatal brain injury (48) and is known to have extensive effects on brain development (49), including smaller brain size even in the absence of neonatal focal brain injury (38). The prevalence of left-handedness is elevated in some neurodevelopmental disorders with notable brain alterations, such as autism (50), Down’s syndrome (51), and schizophrenia (43). According to Satz’s model (46, 47), lower cognitive abilities and left-handedness result independently from brain damage. While the shared and independent mechanisms behind the associations between birthweight, handedness, and postnatal development are not well known, studies have shown that both handedness and VLBW are related to alterations in white-matter tracts including inter- and intrahemispheric connections (37, 52).
Our results are in line with the hypothesis of pathological left-handedness, but the theory of pathological left-handedness has met with some criticism (see ref. 53). A difficulty lies in the original definition, which assumes that brain damage causes a deviation from “natural handedness.” The mechanism behind the birthweight–handedness association may also be due to genetic effects. Genes related to growth factors (54, 55) have been linked with handedness, and birthweight is also influenced by genetic effects (56) [self-regulation of multiples is an additional cause of low birthweight (57)]. Our within-family analyses controlled for familial effects and the results were in line with results from the between-family analyses. Familial effects included parental handedness and genetic effects (controlling fully for maternal and paternal genes and, in part, those of the offspring: Monozygotic triplet sets are genetically identical, whereas di- and trizygotic triplet sets share about half of their segregating genes). In addition to genetics, epigenetic changes are suggested to be linked to low birthweight and variation in handedness (16).
Our results showed that there were significant differences in postnatal motor development and infant head circumference between left- and right-handers. Left-handers achieved motor milestones later than right-handers in both samples, but the differences were nonsignificant in the Dutch sample. We note that there were only nine left-handers with motor development data in the Dutch sample, but the magnitude of mean differences was generally similar in both samples (with the exception of sitting, which indicated nonsignificantly later development of right-handers in the Dutch sample; SI Appendix, Table S5). Higher birthweight was consistently associated with earlier motor development in both samples. Importantly, the effects of handedness in the Japanese data were not significant when controlling for birthweight, indicating that handedness itself is not an independent predictor of motor development in early childhood. We observed a similar pattern of results with regard to infant head circumference, a proxy measure of brain development available only in the Japanese sample (i.e., smaller head circumference in left-handers compared with right-handers, but no difference after controlling for birthweight). Birthweight was an independent predictor of head circumference. Together, the analyses on motor development and head circumference suggest that developmental differences observed between left- and right-handers are at least in part due to a shared etiology associated with low birthweight. Thus, handedness is associated with birthweight rather than pathology. In line with this, cognitive deficits of low-birthweight infants have also been suggested to be due to small birthweight itself (35, 37, 38).
A limitation of our study was that we investigated the association between birthweight and handedness, while not controlling for any neurological disorders of focal brain injury in prenatal or early postnatal development. Further, we did not control for maternal stress and birth complications. With regard to within-family analyses our sample size was limited. Our data included triplet individuals with different zygosities (monozygotic, dizygotic, and trizygotic). When we analyzed birthweight effects in within-family analysis, zygosity-specific analyses were not feasible, due to the small number of observations. In between-family analyses, we did not find any main effect of zygosity on handedness. An earlier twin study compared the intelligence in handedness-discordant MZ twin pairs with regard to relative birthweight and found that left-handers had lower intelligence, but only when a cotwin with lower birthweight was left-handed (58). In our sample of triplets, the number of handedness-discordant MZ pairs was too small to conduct similar analyses with regard to motor development.
We had some limitations with regard to our handedness classification. First, we included hand preference only, not any hand skill measures. Secondly, we measured only direction (left- versus right-handed) but not the degree of handedness (consistent versus inconsistent, i.e., how strongly individuals prefer to use one hand over the other). It has been suggested that the degree rather than the direction of handedness is more closely linked to behavioral abnormalities and brain pathology (e.g., refs. 59 and 60). Our handedness classification was based on simply asking if the person was left-handed, right-handed, or ambidextrous. For younger participants, handedness was based on maternal report and not on self-report. Some of our participants were younger than 5 y; handedness is more stable after 3–5 y of age. Together, these factors may have resulted in a misclassification of handedness in some individuals. Despite these limitations, we showed that the association between birthweight and handedness was similar in both datasets and even when restricting analyses to those who were older than 5 y.
Strengths of our study include two large samples of triplets, both of which had data on birthweight and handedness and important covariates. Because the participants were all triplets with naturally small sizes, they probably had a more homogeneous birthweight distribution than singletons, being naturally near to the singleton definition of VLBW. In comparison with triplets, singleton VLBW or ELBW children may have more varied etiologies behind their smaller birthweights.
In conclusion, studies of singletons and twins suggest that birthweight within the normal range may not be related to handedness; however, as our triplet study demonstrates, the prevalence of left-handedness increases with decreasing birthweights at the lower tail of the distribution, resembling earlier results with VLBW singletons. Although VLBW is known to be associated with other adversities during fetal life, we do not yet confidently know the extent to which an increased prevalence of left-handedness would be accompanied by adversities. In our study, low birthweight appeared to be the most important prenatal factor explaining the variation in handedness, and differences in motor and brain development between left- and right-handers were not evident when controlling for birthweight. Owing to the importance of low birthweight, future studies should utilize more triplets.
Materials and Methods
The participants of the current study were from two large triplet datasets from Japan and the Netherlands with available information on handedness, birthweight, and other perinatal factors. In the Netherlands, the study was approved by the Central Ethics Committee on Research Involving Human Subjects of the VU University Medical Center, Amsterdam, an Institutional Review Board certified by the US Office of Human Research Protections. In Japan, the study was approved by the ethical review board of Osaka City University. In both studies, information about the study was provided and returning the questionnaire was considered as a consent to participate. Data will be available upon request (Dutch data from D.I.B. and Japanese data from Y.Y.).
Triplets from Japan.
The participants of this study were recruited from the West Japan Twins and Higher Order Multiple Births Registry (61). Mothers and their triplet children were also enrolled from several other sources, such as various Japanese Mother’s Organizations for Higher Order Multiple Births and referrals from public health nurses. Data were collected through a mailed questionnaire sent to the mothers asking for information recorded in medical records (for further details, see SI Appendix, SI Supplementary Methods). Mothers were asked to indicate if their child was left-handed, right-handed, or ambidextrous. The birth years of the Japanese triplets ranged from 1978 to 2012. Zygosity was based on a series of survey questions.
Triplets from the Netherlands.
The Netherlands Twin Register (NTR) recruits twins, triplets, and higher-order multiples a few weeks to months after birth (62). In 2008, a survey combined surveys 1 and 2 (SI Appendix, SI Methods) and was sent to mothers of all NTR triplets. Mothers were asked to indicate if their child was left-handed, right-handed, or ambidextrous. If handedness was not available from maternal reports, handedness was supplemented with self-reported data obtained at age 14. Participants were asked to indicate if they were left-handed, right-handed, or ambidextrous. The birth years of the NTR triplets varied from 1970 to 2006. Triplet zygosity was assessed by DNA markers for 2.6% of the sample, on chorionicity for 9%, on blood group assessments for 2%, on a series of survey questions for 70.4%, and on a one-question survey item on zygosity for 14.5%.
Handedness Classification.
Our study had three classes of handedness in both datasets: left-handers, right-handers, and ambidextrous. We used a dichotomous handedness classification of left- versus right-handers. The majority of self-reported ambidextrous individuals do write with their right hand (3, 26), but many studies consider ambidextrous to be non–right-handed (43, 63–65). To make clear distinctions between left- and right-handedness, we excluded the small class of ambidextrous individuals (3.2% in Japan and 3.7% in the Netherlands) from the main analyses. In additional analyses, we did include ambidextrous individuals when using handedness with three classes (left-handed, right-handed, or ambidextrous) or when contrasting right-handers and non–right-handers (26). Handedness classification was based on either maternal report or self-reported handedness in general (i.e., not in relation to any specific activity, such as writing).
Motor Milestone Measures and Infant Head Circumference.
Age in months of achieving motor milestones were reported by parents and included five questions that were available in both samples: turning, sitting, crawling, standing alone, and walking alone. We excluded individuals with ±3 SD separately for each question in each sample. In addition to the five individual items, a single latent variable indicating general motor development was used. This variable was derived using a principal component analysis of the five individual measures (SI Appendix, SI Methods). Infant head circumference (in centimeters) was available only for Japanese triplets. Values outside ±3 SD were excluded: 556 individuals with head circumference data were included in the analyses.
Statistical Analyses.
Birthweight (in kilograms) was continuous in all analyses. Sex, birth order and gestational age, maternal age, and infertility treatment were included as covariates. The models were run by the logistic procedure of Stata (Stata release 13; Stata Corp.), with the cluster option to correct for the bias in variation caused by the relatedness of triplet members. We used conditional logistic models in within-triplet analyses to investigate whether the between-family birthweight–handedness relationship could be confirmed in within-family comparisons. Linear regression was used to investigate the associations of handedness with motor development measures and head circumference using sex as a covariate. The linear regression models were repeated adding birthweight as another adjusting covariate. Clustered family data were also taken into account in these analyses.
Supplementary Material
Acknowledgments
We warmly thank all triplet families for their participation. Japan: West Japan Twins and Higher Order Multiple Births Registry is supported by Grant-in-Aid for Scientific Research (B) (Grant 15H05105) from the Japan Society for the Promotion of Science. NTR: We gratefully acknowledge the Netherlands Organization for Scientific Research (NWO) for Grant NWO 480-15-001/674: Netherlands Twin Registry Repository: researching the interplay between genome and environment and the Royal Netherlands Academy of Science Professor Award (PAH/6635) (to D.I.B.). Data collection and zygosity typing were made possible by multiple grants from the NWO: 575-25-006, 480-04-004, 904-61-090, 904-61-193, 400-05-717, 311-60008, SPI 56-464-14192, and the Avera Institute for Human Genetics.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5832.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1719567115/-/DCSupplemental.
References
- 1.Medland SE, et al. Special twin environments, genetic influences and their effects on the handedness of twins and their siblings. Twin Res. 2003;6:119–130. doi: 10.1375/136905203321536245. [DOI] [PubMed] [Google Scholar]
- 2.Medland SE, et al. Genetic influences on handedness: Data from 25,732 Australian and Dutch twin families. Neuropsychologia. 2009;47:330–337. doi: 10.1016/j.neuropsychologia.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perelle IB, Ehrman L. An international study of human handedness: The data. Behav Genet. 1994;24:217–227. doi: 10.1007/BF01067189. [DOI] [PubMed] [Google Scholar]
- 4.Papadatou-Pastou M, Martin M, Munafò MR, Jones GV. Sex differences in left-handedness: A meta-analysis of 144 studies. Psychol Bull. 2008;134:677–699. doi: 10.1037/a0012814. [DOI] [PubMed] [Google Scholar]
- 5.Vuoksimaa E, Kaprio J. Sex differences in left-handedness are also evident in Scandinavia and in twins: Comment on Papadatou-Pastou, Martin, Munafò, and Jones (2008) Psychol Bull. 2010;136:344–347. doi: 10.1037/a0018972. [DOI] [PubMed] [Google Scholar]
- 6.McCartney G, Hepper P. Development of lateralized behaviour in the human fetus from 12 to 27 weeks’ gestation. Dev Med Child Neurol. 1999;41:83–86. doi: 10.1017/s0012162299000183. [DOI] [PubMed] [Google Scholar]
- 7.Hepper PG, McCartney GR, Shannon EA. Lateralised behaviour in first trimester human foetuses. Neuropsychologia. 1998;36:531–534. doi: 10.1016/s0028-3932(97)00156-5. [DOI] [PubMed] [Google Scholar]
- 8.Vuoksimaa E, Koskenvuo M, Rose RJ, Kaprio J. Origins of handedness: A nationwide study of 30,161 adults. Neuropsychologia. 2009;47:1294–1301. doi: 10.1016/j.neuropsychologia.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryden MP, McManus IC, Bulman-Fleming MB. Evaluating the empirical support for the Geschwind-Behan-Galaburda model of cerebral lateralization. Brain Cogn. 1994;26:103–167. doi: 10.1006/brcg.1994.1045. [DOI] [PubMed] [Google Scholar]
- 10.McManus IC, Bryden MP. Geschwind’s theory of cerebral lateralization: Developing a formal, causal model. Psychol Bull. 1991;110:237–253. doi: 10.1037/0033-2909.110.2.237. [DOI] [PubMed] [Google Scholar]
- 11.Vuoksimaa E, Eriksson CJ, Pulkkinen L, Rose RJ, Kaprio J. Decreased prevalence of left-handedness among females with male co-twins: Evidence suggesting prenatal testosterone transfer in humans? Psychoneuroendocrinology. 2010;35:1462–1472. doi: 10.1016/j.psyneuen.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domellöf E, Johansson AM, Rönnqvist L. Handedness in preterm born children: A systematic review and a meta-analysis. Neuropsychologia. 2011;49:2299–2310. doi: 10.1016/j.neuropsychologia.2011.04.033. [DOI] [PubMed] [Google Scholar]
- 13.O’Callaghan MJ, et al. Handedness in children with birthweights below 1000 g. Lancet. 1987;1:1155. doi: 10.1016/s0140-6736(87)91719-3. [DOI] [PubMed] [Google Scholar]
- 14.O’Callaghan MJ, Burn YR, Mohay HA, Rogers Y, Tudehope DI. Handedness in extremely low birth weight infants: Aetiology and relationship to intellectual abilities, motor performance and behaviour at four and six years. Cortex. 1993;29:629–637. doi: 10.1016/s0010-9452(13)80286-9. [DOI] [PubMed] [Google Scholar]
- 15.Powls A, Botting N, Cooke RW, Marlow N. Handedness in very-low-birthweight (VLBW) children at 12 years of age: Relation to perinatal and outcome variables. Dev Med Child Neurol. 1996;38:594–602. doi: 10.1111/j.1469-8749.1996.tb12124.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz J, Metz GAS, Güntürkün O, Ocklenburg S. Beyond the genome-Towards an epigenetic understanding of handedness ontogenesis. Prog Neurobiol. 2017;159:69–89. doi: 10.1016/j.pneurobio.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Blickstein I, Keith L, Keith D, Teplica D. Multiple Pregnancy. 2nd Ed. Taylor & Francis; London: 2005. pp. 3–9. [Google Scholar]
- 18.Williams CS, Buss KA, Eskenazi B. Infant resuscitation is associated with an increased risk of left-handedness. Am J Epidemiol. 1992;136:277–286. doi: 10.1093/oxfordjournals.aje.a116493. [DOI] [PubMed] [Google Scholar]
- 19.Coren S. Twinning is associated with an increased risk of left-handedness and inverted writing hand posture. Early Hum Dev. 1994;40:23–27. doi: 10.1016/0378-3782(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 20.Heikkilä K, et al. Higher prevalence of left-handedness in twins? Not after controlling birth time confounders. Twin Res Hum Genet. 2015;18:526–532. doi: 10.1017/thg.2015.53. [DOI] [PubMed] [Google Scholar]
- 21.Dudenhausen JW, Maier RF. Perinatal problems in multiple births. Dtsch Arztebl Int. 2010;107:663–668. doi: 10.3238/arztebl.2010.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nicholls ME, Johnston DW, Shields MA. Adverse birth factors predict cognitive ability, but not hand preference. Neuropsychology. 2012;26:578–587. doi: 10.1037/a0029151. [DOI] [PubMed] [Google Scholar]
- 23.Ooki S, Asaka A. Comparison of obstetric and birthweight characteristics between the two largest databases of Japanese twins measured in childhood. Twin Res Hum Genet. 2005;8:63–68. doi: 10.1375/1832427053435436. [DOI] [PubMed] [Google Scholar]
- 24.Groen MA, Whitehouse AJ, Badcock NA, Bishop DV. Associations between handedness and cerebral lateralisation for language: A comparison of three measures in children. PLoS One. 2013;8:e64876. doi: 10.1371/journal.pone.0064876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazoyer B, et al. Gaussian mixture modeling of hemispheric lateralization for language in a large sample of healthy individuals balanced for handedness. PLoS One. 2014;9:e101165. doi: 10.1371/journal.pone.0101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szaflarski JP, et al. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 27.Szaflarski JP, et al. Left-handedness and language lateralization in children. Brain Res. 2012;1433:85–97. doi: 10.1016/j.brainres.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kwon SH, et al. Adaptive mechanisms of developing brain: Cerebral lateralization in the prematurely-born. Neuroimage. 2015;108:144–150. doi: 10.1016/j.neuroimage.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mürner-Lavanchy I, et al. Delayed development of neural language organization in very preterm born children. Dev Neuropsychol. 2014;39:529–542. doi: 10.1080/87565641.2014.959173. [DOI] [PubMed] [Google Scholar]
- 30.de Kieviet JF, Piek JP, Aarnoudse-Moens CS, Oosterlaan J. Motor development in very preterm and very low-birth-weight children from birth to adolescence: A meta-analysis. JAMA. 2009;302:2235–2242. doi: 10.1001/jama.2009.1708. [DOI] [PubMed] [Google Scholar]
- 31.Edwards J, et al. Developmental coordination disorder in school-aged children born very preterm and/or at very low birth weight: A systematic review. J Dev Behav Pediatr. 2011;32:678–687. doi: 10.1097/DBP.0b013e31822a396a. [DOI] [PubMed] [Google Scholar]
- 32.Zimmerman E. Do infants born very premature and who have very low birth weight catch up with their full term peers in their language abilities by early school age? J Speech Lang Hear Res. 2018;61:53–65. doi: 10.1044/2017_JSLHR-L-16-0150. [DOI] [PubMed] [Google Scholar]
- 33.Blickstein I, Goldman RD, Mazkereth R. Adaptive growth restriction as a pattern of birth weight discordance in twin gestations. Obstet Gynecol. 2000;96:986–990. doi: 10.1016/s0029-7844(00)01079-6. [DOI] [PubMed] [Google Scholar]
- 34.Farina A, Vesce F, Garutti P, Jorizzo G, Bianciotto A. Evaluation of intrauterine growth pattern of twins by linear discriminant analysis of the values of biparietal diameter, femur length and abdominal circumference1. Gynecol Obstet Invest. 1999;48:14–17. doi: 10.1159/000010126. [DOI] [PubMed] [Google Scholar]
- 35.Pyhälä R, et al. Neurocognitive abilities in young adults with very low birth weight. Neurology. 2011;77:2052–2060. doi: 10.1212/WNL.0b013e31823b473e. [DOI] [PubMed] [Google Scholar]
- 36.Schieve LA, et al. Population impact of preterm birth and low birth weight on developmental disabilities in US children. Ann Epidemiol. 2016;26:267–274. doi: 10.1016/j.annepidem.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sølsnes AE, et al. Limited microstructural and connectivity deficits despite subcortical volume reductions in school-aged children born preterm with very low birth weight. Neuroimage. 2016;130:24–34. doi: 10.1016/j.neuroimage.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 38.Sølsnes AE, et al. Cortical morphometry and IQ in VLBW children without cerebral palsy born in 2003-2007. Neuroimage Clin. 2015;8:193–201. doi: 10.1016/j.nicl.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yokoyama Y, et al. Motor development of triplets: A Japanese prospective cohort study. Twin Res Hum Genet. 2011;14:185–191. doi: 10.1375/twin.14.2.185. [DOI] [PubMed] [Google Scholar]
- 40.Papadatou-Pastou M, Tomprou DM. Intelligence and handedness: Meta-analyses of studies on intellectually disabled, typically developing, and gifted individuals. Neurosci Biobehav Rev. 2015;56:151–165. doi: 10.1016/j.neubiorev.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 41.Huang J, Zhu T, Qu Y, Mu D. Prenatal, perinatal and neonatal risk factors for intellectual disability: A systemic review and meta-analysis. PLoS One. 2016;11:e0153655. doi: 10.1371/journal.pone.0153655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somers M, Shields LS, Boks MP, Kahn RS, Sommer IE. Cognitive benefits of right-handedness: A meta-analysis. Neurosci Biobehav Rev. 2015;51:48–63. doi: 10.1016/j.neubiorev.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Hirnstein M, Hugdahl K. Excess of non-right-handedness in schizophrenia: Meta-analysis of gender effects and potential biases in handedness assessment. Br J Psychiatry. 2014;205:260–267. doi: 10.1192/bjp.bp.113.137349. [DOI] [PubMed] [Google Scholar]
- 44.Papadatou-Pastou M, Sáfár A. Handedness prevalence in the deaf: Meta-analyses. Neurosci Biobehav Rev. 2016;60:98–114. doi: 10.1016/j.neubiorev.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Ntolka E, Papadatou-Pastou M. Right-handers have negligibly higher IQ scores than left-handers: Systematic review and meta-analyses. Neurosci Biobehav Rev. 2018;84:376–393. doi: 10.1016/j.neubiorev.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 46.Satz P. Pathological left-handedness: An explanatory model. Cortex. 1972;8:121–135. doi: 10.1016/s0010-9452(72)80013-3. [DOI] [PubMed] [Google Scholar]
- 47.Satz P, Orsini DL, Saslow E, Henry R. The pathological left-handedness syndrome. Brain Cogn. 1985;4:27–46. doi: 10.1016/0278-2626(85)90052-1. [DOI] [PubMed] [Google Scholar]
- 48.Volpe JJ. Brain injury in premature infants: A complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Farajdokht F, et al. Very low birth weight is associated with brain structure abnormalities and cognitive function impairments: A systematic review. Brain Cogn. 2017;118:80–89. doi: 10.1016/j.bandc.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Markou P, Ahtam B, Papadatou-Pastou M. Elevated levels of atypical handedness in autism: Meta-analyses. Neuropsychol Rev. 2017;27:258–283. doi: 10.1007/s11065-017-9354-4. [DOI] [PubMed] [Google Scholar]
- 51.Lewin J, Kohen D, Mathew G. Handedness in mental handicap: Investigation into populations of down’s syndrome, epilepsy and autism. Br J Psychiatry. 1993;163:674–676. doi: 10.1192/bjp.163.5.674. [DOI] [PubMed] [Google Scholar]
- 52.Perlaki G, et al. White-matter microstructure and language lateralization in left-handers: A whole-brain MRI analysis. Brain Cogn. 2013;82:319–328. doi: 10.1016/j.bandc.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 53.McManus IC. Pathologic left-handedness: Does it exist? J Commun Disord. 1983;16:315–344. doi: 10.1016/0021-9924(83)90016-3. [DOI] [PubMed] [Google Scholar]
- 54.Arning L, et al. Handedness and the X chromosome: The role of androgen receptor CAG-repeat length. Sci Rep. 2015;5:8325. doi: 10.1038/srep08325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shore R, et al. The handedness-associated PCSK6 locus spans an intronic promoter regulating novel transcripts. Hum Mol Genet. 2016;25:1771–1779. doi: 10.1093/hmg/ddw047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parent-offspring data. Am J Epidemiol. 2007;165:734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- 57.Muhlhausler BS, Hancock SN, Bloomfield FH, Harding R. Are twins growth restricted? Pediatr Res. 2011;70:117–122. doi: 10.1203/PDR.0b013e31821f6cfd. [DOI] [PubMed] [Google Scholar]
- 58.Segal NL. Origins and implications of handedness and relative birth weight for IQ in monozygotic twin pairs. Neuropsychologia. 1989;27:549–561. doi: 10.1016/0028-3932(89)90059-6. [DOI] [PubMed] [Google Scholar]
- 59.Corballis MC, Hattie J, Fletcher R. Handedness and intellectual achievement: An even-handed look. Neuropsychologia. 2008;46:374–378. doi: 10.1016/j.neuropsychologia.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Crow TJ, Crow LR, Done DJ, Leask S. Relative hand skill predicts academic ability: Global deficits at the point of hemispheric indecision. Neuropsychologia. 1998;36:1275–1282. doi: 10.1016/s0028-3932(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 61.Yokoyama Y, Sugimoto M, Ooki S. Analysis of factors affecting birthweight, birth length and head circumference: Study of Japanese triplets. Twin Res Hum Genet. 2005;8:657–663. doi: 10.1375/183242705774860150. [DOI] [PubMed] [Google Scholar]
- 62.Boomsma DI, et al. Netherlands twin register: A focus on longitudinal research. Twin Res. 2002;5:401–406. doi: 10.1375/136905202320906174. [DOI] [PubMed] [Google Scholar]
- 63.Annett M. Handedness as a continuous variable with dextral shift: Sex, generation, and family handedness in subgroups of left- and right-handers. Behav Genet. 1994;24:51–63. doi: 10.1007/BF01067928. [DOI] [PubMed] [Google Scholar]
- 64.Bailey LM, McKeever WF. A large-scale study of handedness and pregnancy/birth risk events: Implications for genetic theories of handedness. Laterality. 2004;9:175–188. doi: 10.1080/13576500342000013. [DOI] [PubMed] [Google Scholar]
- 65.Johnston DW, Nicholls ME, Shah M, Shields MA. Nature’s experiment? Handedness and early childhood development. Demography. 2009;46:281–301. doi: 10.1353/dem.0.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.