Significance
Narcolepsy is a chronic neurological disorder that severely affects the day-to-day life and general well-being of the affected individuals. Unfortunately, treatments available to date are symptomatic therapies with an array of adverse effects. Animal studies showed that orexin peptide can be a mechanistic therapy to treat narcolepsy. However, nonpermeability of orexin to brain–blood barrier limits its use in humans. Here we showed that orexin delivered via intrathecal route at the lumbar level significantly inhibited narcoleptic symptoms in mice without altering sleep/wake profile. Although the outcome of intrathecal treatment may be different in clinical studies, our data indicate the possibility that orexin can be delivered intrathecally in humans, using an implantable, refillable, and programmable miniature pump to treat narcolepsy.
Keywords: non-REM sleep, lumbar spinal cord, slow infusion, orexin knockout mice
Abstract
Narcolepsy–cataplexy is a chronic neurological disorder caused by loss of orexin (hypocretin)-producing neurons, associated with excessive daytime sleepiness, sleep attacks, cataplexy, sleep paralysis, hypnagogic hallucinations, and fragmentation of nighttime sleep. Currently, human narcolepsy is treated by providing symptomatic therapies, which can be associated with an array of side effects. Although peripherally administered orexin does not efficiently penetrate the blood–brain barrier, centrally delivered orexin can effectively alleviate narcoleptic symptoms in animal models. Chronic intrathecal drug infusion through an implantable pump is a clinically available strategy to treat a number of neurological diseases. Here we demonstrate that the narcoleptic symptoms of orexin knockout mice can be reversed by lumbar-level intrathecal orexin delivery. Orexin was delivered via a chronically implanted intrathecal catheter at the upper lumbar level. The computed tomographic scan confirmed that intrathecally administered contrast agent rapidly moved from the spinal cord to the brain. Intrathecally delivered orexin was detected in the brain by radioimmunoassay at levels comparable to endogenous orexin levels. Cataplexy and sleep-onset REM sleep were significantly decreased in orexin knockout mice during and long after slow infusion of orexin (1 nmol/1 µL/h). Sleep/wake states remained unchanged both quantitatively as well as qualitatively. Intrathecal orexin failed to induce any changes in double orexin receptor-1 and -2 knockout mice. This study supports the concept of intrathecal orexin delivery as a potential therapy for narcolepsy–cataplexy to improve the well-being of patients.
Narcolepsy is an underdiagnosed and incurable chronic neurological disorder with disrupted sleep/wake behavior, with such symptoms as excessive daytime sleepiness (EDS) and sleep attacks, and rapid eye movement (REM) sleep-related signs such as hypnagogic hallucinations, sleep paralysis, and cataplexy (sudden weakening of bilateral skeletal muscles often triggered by positive emotions, without impairing consciousness). Deficiency in orexin signaling causes narcoleptic symptoms in experimental models (1–7). Clinical studies in narcolepsy–cataplexy [or type-1 narcolepsy (8)] patients showed undetectable levels of orexin in the cerebrospinal fluid (CSF), and postmortem studies revealed a loss of hypothalamic orexin immunoreactivity as well as mRNA (5, 9).
Only symptoms-oriented therapies are currently available for narcolepsy: methylphenidate, amphetamines, modafinil/armodafinil and pitolisant (an inverse agonist of histamine H3 receptors) are available for EDS and sodium oxybate for both EDS and cataplexy in adults. Antidepressants such as selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), and tricyclic antidepressants are used for cataplexy and to a lesser extent for hypnagogic hallucinations and sleep paralysis (10–12). Although, in some patients, narcoleptic symptoms can be effectively managed, in others these treatments are associated with an array of side effects such as insomnia, constipation, dry mouth, blurred vision, increased anxiety, palpitations, cardiac arrhythmia, allergic reactions, headache, nausea, reduced sexual functions, psychosis, mania, hallucinations, rebound cataplexy, etc. (10, 11, 13).
The neuropeptides orexin-A and orexin-B, also known as hypocretins, are produced from the common precursor preproorexin and act via two G protein-coupled receptors OX1R and OX2R (14, 15). Orexin has widespread projections to the brain areas that are involved in sleep/wake regulation, including specific nuclei in the brainstem, hypothalamus, and basal forebrain (1, 16). Orexin replacement therapy that restores orexin signaling by intracerebroventricular (ICV) administration prevents cataplexy in ligand-deficient mice (17). Since orexin peptide is unable to cross the blood–brain barrier (BBB) upon peripheral administration (6), it needs to be delivered centrally. Highly invasive methods such as ICV administration is nonpractical for human use. Recently, a miniaturized “neural” system has been developed for chronic and local intracerebral drug delivery (18). Although it is effective in rodents, its feasibility and effectiveness in higher animals is not known. On the other hand, intrathecal drug delivery has proven effective in humans for various other conditions, e.g., intrathecal delivery of baclofen for severe pain and spastic paresis (19–22). Here, we adopted intrathecal drug delivery technique to administer orexin peptide centrally in narcoleptic mice.
Results
Intrathecally Injected Contrast Medium Reaches the Brain from the Lumbar Level.
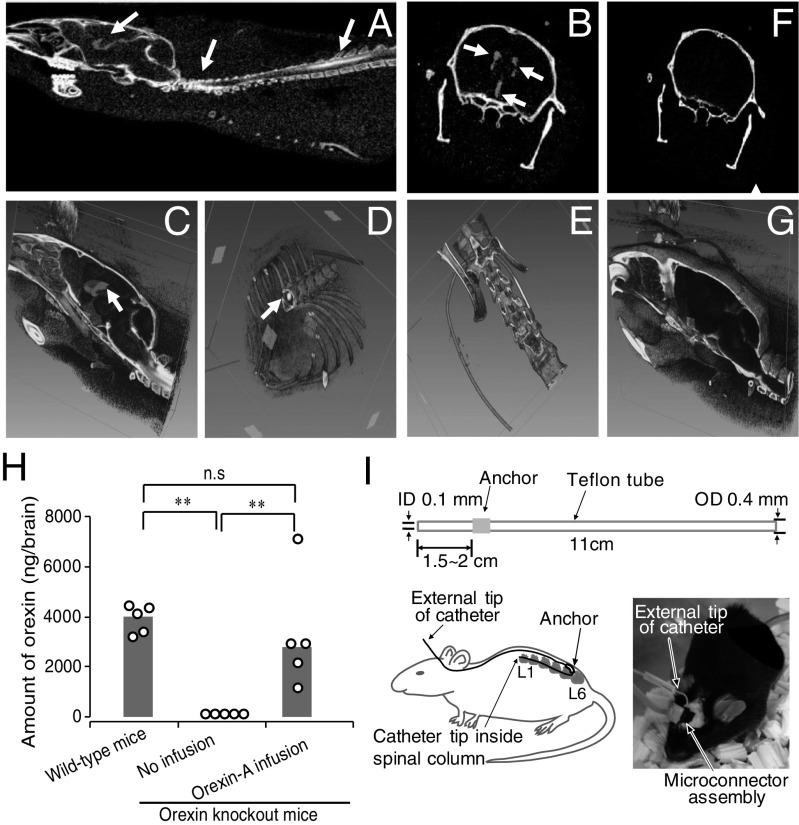
Computed tomographic (CT) scans were performed during the intrathecal infusion to ascertain that the drugs delivered at the lumbar spinal cord level can flow upward to reach the brain. Fig. 1 A–G shows axial cross-sectional images, as well as 3D reconstructed images. A typical example of a mouse with the catheter positioned correctly within the intrathecal space showed that contrast medium was detected in sagittal 2D (Fig. 1A) and 3D (Fig. 1C) cross-sections of the spinal column as well as in the third and lateral ventricles of the brain. Catheter tip was confirmed within the lumbar spinal column (Fig. 1D). A control mouse, whereby the catheter tip was misplaced outside the spinal column (Fig. 1E), did not show contrast medium in ventricles of the brain (Fig. 1 F and G). We then estimated the amount of contrast medium penetrated into cisterna magna and ventricles at 10 min after bolus injection of contrast medium. Based on CT numbers, 6.5% and 5.2% of contrast medium reached the cisterna magna and ventricles, respectively, while at least 67% of contrast medium still remained in the spinal cord level. Although there was an obvious gradient of contrast medium from the spinal cord to brain, the decrease from cisterna magna to ventricles was relatively small (20%). Together, these data suggest that drugs delivered intrathecally at the lumbar level can readily reach the brain to execute its functions.
Fig. 1.
Contrast medium and orexin delivered at lumbar spinal cord level were detected in the brain. (A–D) Representative CT scan of a mouse whereby contrast medium was infused via intrathecal route. Two-dimensional axial cross-sectional sagittal (A) and coronal (B) image showing contrast medium in brain ventricles and spinal cord (arrows). (C and D) The 3D reconstructed image showing contrast medium at brain ventricles and spinal cord (arrows). (E–G) CT scan of a control mouse with a misplaced catheter (E). Two-dimensional coronal (F) and 3D sagittal (G) brain images where no contrast medium was detected. (H) Orexin peptide content in brains of orexin knockout mice with and without orexin-A infusion. Circles represent values of the individual mouse and gray bars show median values. n = 5; **P ≤ 0.01 by using one-way ANOVA, followed by Fisher’s probable least-squares difference (LSD) test. (I) Schematic representation of the construction of the intrathecal catheter. (Upper) Catheter dimensions; (Lower Left) an overview sketch of catheter implantation; and (Lower Right) picture of intact animal after surgery connected to recording leads and infusion system. ID, inside diameter; n.s., not significant; OD, outside diameter.
Orexin Content in Brains of Orexin Knockout Mice After Intrathecal Infusion of Exogenous Orexin-A.
Brain orexin content was measured to estimate the amount of intrathecally injected orexin that reaches the brain. A significant amount of orexin was detected in the brains of orexin-null (orexin knockout) mice intrathecally infused for 6 h with orexin-A (1 nmol/1 µL/h). These orexin levels were similar to the amount of endogenous orexin in wild-type animals (positive control). As expected, orexin was below detection limit in orexin knockout mice (negative control) without infusion (Fig. 1H).
Intrathecal Orexin Infusion Inhibited Cataplexy and SOREM in Orexin Knockout Mice.
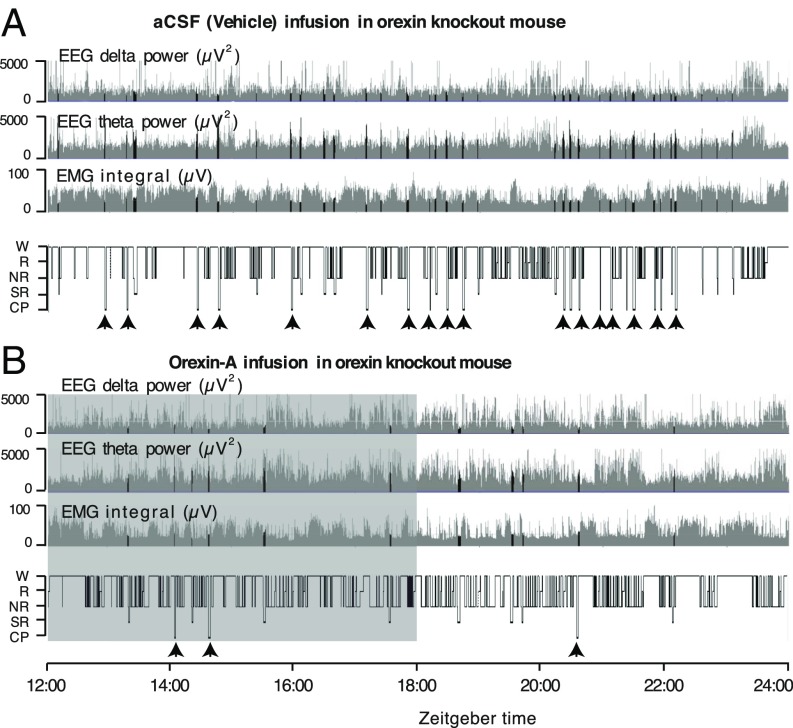
We measured dark-phase video/electroencephalogram (EEG)/electromyogram (EMG) in mice equipped with the intrathecal catheter. Typical examples of EEG delta power, EEG theta power, EMG integral, and hypnograms are shown during/after the intrathecal infusion of artificial CSF (aCSF) (1 µL/h) and orexin-A (1 nmol/1 µL/h, for 6 h in the beginning of dark phase) into an orexin knockout mouse (Fig. 2, respectively). Whereas the hypnogram during vehicle infusion was characterized by the presence of a number of cataplexy and SOREM episodes (Materials and Methods provides definitions), the hypnogram during orexin-A infusion was largely devoid of cataplexy and SOREM. The corresponding increase in theta power and a decrease in EMG integral and delta power are apparent on the transition from wake to REM or during SOREM (Fig. 2A); intrusions of REM-like states are decreased during/after orexin-A infusion (Fig. 2B). We also observed one orexin knockout mouse whereby cataplexy/SOREM episodes were completely abolished after orexin-A infusion (hypnogram not shown). Although the infusion of orexin-A was performed only during the initial 6 h of dark phase, as evident in the representative hypnogram (Fig. 2B), the cataplexy and SOREM remain suppressed even in the second half of dark phase, i.e., long after the infusion of orexin-A was over.
Fig. 2.
Intrathecally delivered orexin-A suppressed cataplexy and SOREM in orexin knockout mice. (A and B) Typical examples of EEG delta power (0.5–4 Hz), EEG theta power (4–10 Hz), EMG integral, and hypnograms of an orexin knockout mouse during aCSF (vehicle) (A) and orexin-A (B) infusion with chocolate provided in the cage. Hypnograms represent concatenated 10-s epochs of EEG/EMG activity, scored as wake, REM, NREM (gray), and SOREM and cataplexy (black). Twelve hours per mouse, starting at the beginning of the dark phase, are shown. The shaded area in B shows the duration of orexin-A infusion. CP, cataplexy; NR, NREM sleep; R, REM sleep; SR, SOREM; W, wake.
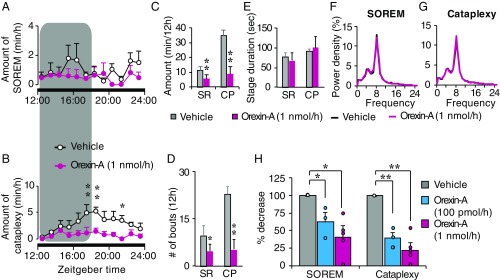
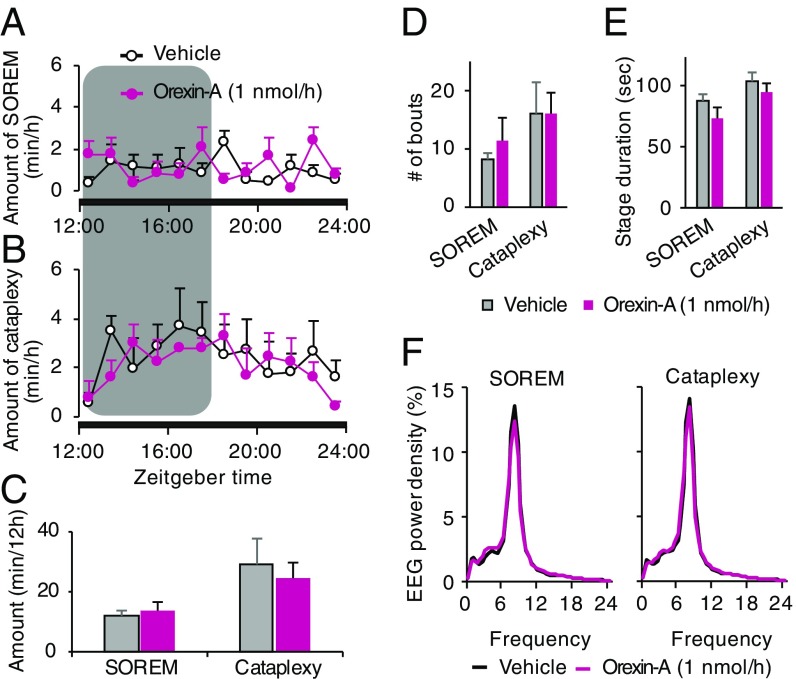
Time-course data show that orexin knockout mice spend less time in cataplexy and SOREM after intrathecal orexin-A infusion compared with vehicle infusion, and these episodes were effectively suppressed throughout the dark period (Fig. 3 A and B). Orexin knockout mice showed a significantly reduced total amount of cataplexy and SOREM after orexin-A infusion during the 12-h dark phase, compared with vehicle (Fig. 3C). Further, orexin-A infusion resulted in significant reduction of cataplexy and SOREM episode numbers compared with vehicle. The number of cataplectic attacks over a 12-h dark phase after vehicle infusion ranged between 19 and 30 per mouse; the number was significantly reduced to 0–14 per mouse after orexin-A infusion. There was no difference in the mean duration of individual cataplexy and SOREM episodes (Fig. 3E). No apparent difference was observed in EEG power spectral densities of non-REM (NREM) and REM sleep between the vehicle and orexin-A infusion in orexin knockout mice (Fig. 3 F and G).
Fig. 3.
Intrathecal orexin-A infusion suppressed cataplexy and SOREM in orexin knockout mice. (A and B) Hourly plots of SOREM and cataplexy during vehicle and orexin-A infusion within the 12-h dark phase. The shaded area on graphs represents the duration of orexin-A infusion. (C) Total amounts of time spent in SOREM and cataplexy within the 12-h dark phase, during vehicle and orexin-A infusion. (D and E) The number of bouts (D) and stage duration (E) of SOREM and cataplexy during a vehicle and orexin-A infusion. (F and G) EEG power densities of SOREM (F) and cataplexy (G) episodes during vehicle and orexin-A infusion. Values are presented as mean ± SEM; n = 4; *P ≤ 0.05, **P ≤ 0.01 by using two-way ANOVA (A, B, F, and G) and paired t test (C–E). (H) Dose-dependent changes in SOREM and cataplexy in orexin knockout mice during/after vehicle, orexin-A (100 pmol/1 µL/h and 1 nmol/1 µL/h, blue bars and red bars, respectively). Circles represent individual animal values. Values are mean ± SEM; n = 3–5; *P ≤ 0.05, **P ≤ 0.01 by using one-way ANOVA, followed by Scheffé’s post hoc test. CP, cataplexy; orexin-A, colored lines and bars; SR, SOREM; vehicle, black lines and gray bars.
Similar observations were made when a lower dose (100 pmol/1 µL/h) of orexin-A was infused, although the extent of cataplexy and SOREM suppression was lower. The amounts of cataplexy and SOREM over a 12-h dark phase decreased in a dose-dependent manner after infusion of two different concentrations of orexin-A (100 pmol/h and 1 nmol/h) (Fig. 3H).
Sleep/Wake Profile of Orexin Knockout Mice Did Not Change During or After Intrathecal Orexin-A Infusion.
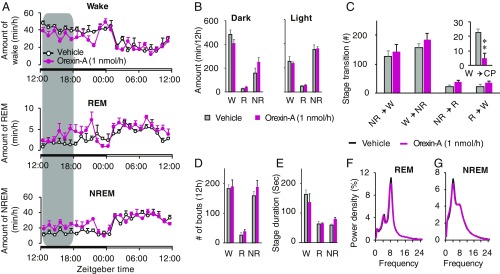
Sleep/wake profile was quantified to evaluate the effect of intrathecal orexin-A administration in orexin knockout mice. Time course of wake, REM, and NREM sleep amounts in orexin knockout mice showed no detectable changes during/after a vehicle and orexin-A infusion (Fig. 4A) during both dark and light phases. The total amount of each stage was also comparable between the vehicle and orexin-A infusion (Fig. 4B). Stage transition from wake to NREM, NREM to wake, NREM to REM, and REM to wake remain unchanged (Fig. 4C). The frequency and mean duration of wake, REM, and NREM sleep episodes did not change significantly after orexin-A infusion (Fig. 4 D and E). The power density of REM and NREM sleep was indistinguishable between vehicle and orexin-A (Fig. 4 F and G).
Fig. 4.
Intrathecally delivered orexin-A did not alter sleep/wake stages in orexin knockout mice. (A) Hourly plots of wake (W), REM (R), and NREM (NR) sleep during a vehicle and orexin-A infusion are shown. (B) Total amounts of time spent in the wake, REM, and NREM sleep in dark and light phases. (C) Stage transitions from NR to W, W to NR, NR to R, R to W, and W to cataplexy (CP) during a vehicle and orexin-A infusion. (D and E) Changes in the number of bouts (D) and stage duration (E) of the wake, REM, and NREM sleep, during vehicle and orexin-A infusion. (F and G) EEG power densities of REM and NREM episodes during a vehicle and orexin-A infusion. The shaded area on graphs represents the duration of orexin-A infusion, while nonshaded area represents aCSF infusion. Values are mean ± SEM; n = 4; *P ≤ 0.05, **P ≤ 0.01 by using two-way ANOVA (A, B, F, and G) and paired t test (C–E). Orexin-A, colored lines and bars; vehicle, black lines and gray bars.
Intrathecal Infusion of Orexin-A in OX1R/OX2R Double Knockout Mice Had No Effect on Cataplexy, SOREM, and Sleep/Wake Behavior.
Typical examples of EEG delta power, EEG theta power, EMG integral, and hypnograms are shown in SI Appendix, Fig. S1 after the intrathecal infusion of vehicle and orexin-A into OX1R/OX2R double knockout (OXR-DKO) mice. The hypnograms during vehicle and orexin-A infusion in OXR-DKO mice did not show differences in the amount of cataplexy and SOREM (SI Appendix, Fig. S1). The hourly amounts of cataplectic arrests and SOREM over a 12-h dark phase were comparable between the vehicle and orexin-A infusion in OXR-DKO mice (Fig. 5 A and B). Total amounts of cataplexy and SOREM also remain unchanged between vehicle and orexin-A administration (Fig. 5C). Mean stage duration and the number of bouts of cataplexy and SOREM episodes (Fig. 5 D and E), and EEG power spectral density of cataplexy and SOREM episodes, remain unchanged between the vehicle and orexin-A treatment (Fig. 5F).
Fig. 5.
Intrathecal orexin-A infusion in OXR-DKO mice did not inhibit SOREM and cataplexy. Hourly plots of SOREM (A), cataplexy (B), and the total amount of time spent in SOREM and cataplexy (C) over a 12-h dark phase during a vehicle and orexin-A infusion are shown. The number of bouts (D) and stage duration (E) of SOREM and cataplexy during/after a vehicle and orexin-A infusion are shown. (F) EEG power densities of SOREM and cataplexy episodes during/after a vehicle and orexin-A infusion. Values presented are mean ± SEM; n = 5. Orexin-A, colored lines and bars; vehicle, black lines and gray bars.
Intrathecal orexin-A infusion in OXR-DKO mice failed to induce changes in the wake, REM, and NREM sleep (SI Appendix, Fig. S2A). Total amounts of wake, REM, and NREM sleep during dark phase were unchanged between the vehicle and orexin-A administrations (SI Appendix, Fig. S2B). Similar negative observations were made for the number of bouts, stage episode duration, stage transition between stages, and EEG power density of REM and NREM sleep (SI Appendix, Fig. S2 C–F).
Discussion
Cataplexy, EDS, and other symptoms associated with narcolepsy can be challenging to treat. The currently available drugs for narcolepsy had unavoidable, sometimes severe side effects in some patients, even though a life-long, constant treatment is needed. Decisions regarding which drugs to use need to take into account a variety of factors that may affect adherence, efficacy, and tolerability (10, 11, 13). Replacement strategy by using small-molecule orexin receptor agonists could provide mechanistic therapy for the pathophysiology of narcolepsy (23). Synthetic orexin peptide could also potentially be considered as replacement therapy. However, although centrally delivered orexin inhibits cataplexy in ligand-deficient mice (17), the peripheral administration of orexin is virtually ineffective (6, 24), due to its nonpermeability to BBB. Hence, the peptide needs to be administered centrally through such means as intrathecal drug delivery system in humans. This approach could be valuable in case both the narcoleptic symptoms and the adverse effects of standard treatment are severe and problematic (13).
In 1984, Penn and Kroin introduced intrathecal administration of baclofen in humans to alleviate spasticity in severe cases (25). Since then, intrathecal drug delivery has become an important treatment option for individuals with severe spasticity, dyskinetic cerebral palsy, stiff-man syndrome, and chronic pain (20, 22, 26). Because the GABA analog baclofen has relatively low BBB penetrability (27), a high dose is needed orally, imparting unwanted side effects. Such drugs can be administered via an intrathecal route that allows the placement of medication in close proximity to target receptors so that a much lower dose is needed. By using continuous intrathecal delivery, a steady drug concentration can be maintained within the brain (21). In a long-term (>10 y) clinical study where the drug was administrated intrathecally, patients reported a high level of treatment and life satisfaction (19). However, it cannot be ignored that unlike baclofen, which acts at spinal cord level, orexin needs to penetrate to deep and higher brain structures.
In our experiments in mice, we showed that orexin delivered intrathecally at the lumbar level was readily available in the brain. Although there is currently no clear theory about the flow of CSF within the central nervous system (28–30), our CT scan analysis clearly showed the flow of contrast medium from the spinal cord to the brain. Our data showed that intrathecally delivered orexin-A flows to the brain and can act on the receptors to activate downstream neuronal networks. The observed decrease in the amount of contrast medium from spinal cord to cisterna magna in the current study is in agreement with a previous study showing that, while drugs administered intrathecally at the lumbar level can readily be detected in the cisterna magna in humans, their concentration decreases from lumbar to cisternal levels (31). This suggests the possibility that even less drug may be reaching orexin receptor sites deeply seated in the brain after delivery at lumbar level.
Intrathecally delivered orexin-A inhibited cataplexy and SOREM in a murine model of narcolepsy (orexin knockout mice). These observations are in accordance with the previous studies where cataplectic behavior was suppressed by ICV orexin administration in narcoleptic mice (17). Our results are in contrast to the observations made by Schatzberg et al. (32), in which orexin was delivered intrathecally in an orexin ligand-deficient narcoleptic dog. In this study, cataplexy was not adequately controlled even at a very high dose. A number of possible reasons for the failure of treatment in this study were suggested by the authors: (i) insufficient backflow of orexin through foramen magnum, preventing from reaching to the critical structures (although the brain orexin content was not estimated in this study); (ii) there may be an alteration in orexin receptor function; (iii) there may be a reduction in the number of orexin receptors in this animal, because a later study showed that sporadic ligand-deficient narcoleptic dogs showed a significant decrease in OX1R, without a change in OX2R levels (33). On the other hand, ligand-deficient mouse models show normal expression of OX1R and OX2Rs at least during early adulthood (1, 33). Moreover, since ICV orexin administration was effective to reduce cataplexy in the ligand-deficient mouse, the downstream orexin signaling pathway, including the orexin receptors, intracellular signaling, postsynaptic neuronal networks, and neurotransmitter pathways required for orexin-mediated arousal and suppression of cataplexy, all appear to remain functional even under permanent orexin deficiency (17). The degree to which cataplexy was suppressed after orexin-A administration varied between animals ranging from 0 to 14 cataplectic attacks/12 h. This may be in part due to variations in the amount of orexin detected in the brain after infusion (990 ∼ 6,735 ng/mL). After 1 nmol/1 µL/h orexin-A infusion, we could achieve complete suppression of cataplexy/SOREM only in 20% of animals, suggesting that a higher dose of orexin-A may be required for complete inhibition of the cataplexy and SOREM. As expected, OXR-DKO mice did not respond to intrathecally administered orexin-A, demonstrating the specificity of the therapeutic effect.
We showed that slow continuous infusion of orexin-A via intrathecal route did not appreciably alter the sleep/wake profile of animals. These observations are in contrast with previous reports where a robust increase in arousal was observed in mice (17, 34) and rats (35) upon ICV administration of orexin. These latter studies used bolus injections, thus rapidly and transiently increased the concentration of peptide at the vicinity of orexin receptors, resulting in an acute increase in wakefulness. In contrast, slow infusion in the current study maintains a low but steady peptide concentration that minimized acute alternations in sleep/wake amounts. It can also be argued that cataplexy is sensitive to the presence of orexin even at low concentrations. In our study, the concentration of orexin may not be sufficiently high to inhibit cataplexy completely and to induce changes in sleep/wake amounts.
Our demonstration of intrathecal orexin delivery as a treatment for narcolepsy/cataplexy in mice could provide implications for human therapy. However, the stability of orexin peptide during the long-term storage in the reservoir kept at body temperature could be an issue. Although intrathecal orexin administration could be beneficial for human narcoleptic patients, the pump placement requires a commitment for periodic small surgery and reservoir fills, including pump and catheter replacement, and the procedure may be associated with complications such as infection and mechanical injury (25, 36). Nevertheless, we achieved definitive inhibition of behavioral cataplexy via intrathecal orexin delivery. The amount of peptide needed was small. Intrathecal orexin delivery in humans could be performed via an implantable, refillable, and programmable pump so that orexin flow rate can be programmed and controlled.
Materials and Methods
Animals.
Male wild-type, orexin knockout and OXR-DKO mice in the C57BL/6J background (23) (13–20 wk) were used. C57BL/6J mice were obtained from Oriental Bio Services. Mice were housed in an insulated soundproofed recording room maintained at an ambient temperature of 23 ± 0.5 °C with a relative humidity of 50 ± 5% on an automatically controlled 12-h light/dark cycle (light on at 07:00), and they had ad libitum access to food and water. The experimental protocols were approved by the University of Tsukuba Animal Ethics Committee (approval no. 16086), and every effort was made to minimize the number of animals used as well as any pain and discomfort.
Preparation and Implantation of the Intrathecal Catheter.
We used 11-cm-long Teflon tubing [inside diameter (ID) 0.1 mm; outside diameter (OD) 0.4 mm] as an intrathecal catheter. A sleeve of polyethylene tube (ID 0.6 mm) was placed at about 1.5 ∼ 2.0 cm from the tip of the catheter tube to anchor the catheter in position (Fig. 1I). Surgeries were performed under anesthesia using pentobarbital (50 mg/kg, intraperitoneally). Under aseptic conditions, mice were chronically implanted with the intrathecal catheter by using protocols modified from Wu et al. (37). Briefly, the mouse was placed on the surgical table in prone position. A longitudinal (2.0 cm) skin incision was made above vertebrae L4–L6. The mouse was held firmly by the pelvic girdle in one hand and a hole was made in the muscles, using a 24-gauge needle to access vertebra. The catheter (filled with normal saline) was guided slowly between L5 and L6 spinal processes (slightly lateral from midline) at an angle of about 20–30° above the vertebral column. Tail and hindlimb reflexes were observed on dura penetration. The catheter was then pushed upward slowly and gently through the intervertebral space and dura up to the upper lumbar level. The catheter was then fixed by suturing the anchoring sleeve to the superficial muscles. The patency of the catheter was verified by the gravitational pull of saline into the catheter. The mouse was then fixed into the stereotaxic instrument. A longitudinal incision was made in the skin over the skull. The other end of the implanted catheter was guided under the skin and taken out at the posterior surface of the skull (Fig. 1A). During the same surgery, mice were then chronically implanted with EEG and EMG electrodes for sleep/wake recordings, and the whole assembly was then fixed to the skull with self-curing dental acrylic resin, as previously described (38, 39). The motor functions of mice were examined 1 d after implantation by observing whether there was any hind body paralysis, walking dysfunction, weakened extension withdrawal reflex of the hind limb, and reduced toe spread. The mice that showed motor dysfunction were discontinued from the study. The placement of the catheter was confirmed at the end of the experiment by postmortem examination by opening the spinal column.
Video-EEG/EMG Recording and Analysis.
After 8–10 d of postoperative recovery, the mice were placed in experimental cages for a 4-d habituation/acclimatization period and connected with counterbalanced recording leads. At the same time, intrathecal catheters of mice were connected with the infusion pump and aCSF (1 µL/h) was continuously infused as a vehicle at least for 72 h before the start of data acquisition. Vigilance states were monitored for a period of 24 h, under continuous infusion of aCSF and served as a control for the same animal. The following day, infusion of orexin-A (1 μL/h for 6 h; Nacalai Tesque) was started. Video-EEG/EMG was recorded continuously for 24 h. Chocolate was provided in cages 1 h before the onset of the dark period to increase the cataplectic events. The observed gradual increase in cataplexy during the initial 6 h may be due to the time at which chocolate was provided (Fig. 3B). Cortical EEG and EMG signals were recorded and analyzed by using SleepSign software (Kissei Comtec), based on previously described standard criteria (40, 41). Cataplexy was identified based on criteria explained by Scammell et al. (42). Briefly, both video and EEG/EMG recordings were used to quantify the cataplexy and SOREM. The defining features of murine cataplexy are: (i) an abrupt episode of nuchal atonia lasting at least 10 s; (ii) the mouse is immobile during the episode; (iii) theta activity dominates the EEG during the episode; and (iv) at least 40 s of wakefulness precedes the episode. SOREM was characterized as the appearance of REM sleep within 60 s of NREM sleep onset. Spectral analysis of EEG by fast Fourier transformation (FFT) was performed, and EEG power densities of each 0.5-Hz bin were averaged by calculating the percentage of each bin with respect to the total power in a range of 0.5–24.5 Hz.
CT Scan Analysis.
Mice (C57BL/6J) were anesthetized with isoflurane and set in the micro-CT equipment (Latheta LCT-200, Hitachi-Aloka Medical) with a holder (48 mm in diameter), and then iodized contrast agent (iopamiron, Bayer) was intrathecally administrated (25 µL/5 min) through the preoperated catheter (as explained in Preparation and Implantation of the Intrathecal Catheter). The CT scan image data from each mouse were analyzed by visualization software (Hitachi-Aloka), which displayed the data as 2D axial cross-sectional images. The 3D data were reconstructed from sliced CT images by summing those images along the z axis by using VGStudio MAX software (Volume Graphics).
Measurement of Orexin Content in Brain.
Orexin content was measured in three groups of five mice (12–20 wk) each: positive control (C57BL/6J: without orexin-A infusion); negative control (orexin knockout mice: without orexin-A infusion); and a drug-treated group (orexin knockout mice: with intrathecal orexin-A infusion). All animals were killed 1–2 h before the onset of the dark phase. In the drug-treated group, the infusion of orexin-A was started 4 h after light onset (at 11:00 h). After 6 h of infusion (at 17:00 h), mice were killed with pentobarbital, decapitated at the foramen magnum, and brains were quickly removed. The orexin content was measured using a modified protocol (1, 43, 44). Briefly, the brains were weighed and cut into six to eight pieces, cooled on dry ice, and stored at −80 °C until used. Brains were boiled for 3 min in 7 mL of Milli-Q water. After cooling on ice, acetic acid and HCl were added to final concentrations of 1 M and 20 mM (10 mL), respectively. Polytron homogenization at 10,000 rpm (Kinematica, using a 7.5-mm rotor) for 3 min was done, followed by two centrifugation steps: 1,300 × g for 15 min and then 20,000 × g for 60 min. The supernatant was collected for estimation of orexin content. Orexin-A was measured with commercially available 125I Radioimmunoassay (RIA) kits (Phoenix Pharmaceuticals) following the manufacturer’s instructions. Orexin-A concentrations were determined against a known standard curve (1–128 pg). The detection limit of the assay was 2.2 pg/mL with less than 4.3% of the intraassay variability. For measuring samples, 100 μL of each supernatant was diluted 10 times with 900 μL RIA buffer. A total of 300 μL of samples was incubated with 100 μL of provided rabbit anti–orexin-A antibody at 4 °C for 24 h. Then, 100 μL of 125I–orexin-A was introduced and samples were incubated at 4 °C for 5 d. On the third day, 100 μL of provided secondary antibody (goat anti-rabbit IgG) and 100 μL of provided normal rabbit serum were added to the mix and incubated at room temperature for 90 min. Finally, samples were centrifuged at 1,700 × g for 20 min at 4 °C and supernatants were immediately removed. The level of radioactivity left in the pellet was measured with an RIA counter.
Statistical Analysis.
All data were expressed as the mean ± SEM unless otherwise mentioned. The time course data of the wake, NREM, REM sleep, SOREM, and cataplexy were statistically analyzed by repetitive measures two-way ANOVA followed by Bonferroni’s post hoc test. Multiple group comparisons were performed by using one-way ANOVA followed by Fisher’s probable least-squares difference test where the sample size was similar in all groups (Fig. 1H), and Scheffé’s post hoc test was applied to uneven sample size across groups (Fig. 3H). Paired t test was applied for comparisons between two groups. In all cases, P ≤ 0.05 was considered as significant. Statistical analysis was performed using GraphPad Prism6.
Supplementary Material
Acknowledgments
This study was supported in part by the World Premier International Research Center Initiative, Ministry of Education, Culture, Sports, Science, and Technology, Japan (M.Y.) and the Japan Society for Promotion of Science KAKENHI Grants 17H06095 (to M.Y.) and 16H01881 (to Y.U.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1722686115/-/DCSupplemental.
References
- 1.Chemelli RM, et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 2.Hara J, et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 3.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 4.Nishino S, Ripley B, Overeem S, Lammers GJ, Mignot E. Hypocretin (orexin) deficiency in human narcolepsy. Lancet. 2000;355:39–40. doi: 10.1016/S0140-6736(99)05582-8. [DOI] [PubMed] [Google Scholar]
- 5.Thannickal TC, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujiki N, Yoshida Y, Ripley B, Mignot E, Nishino S. Effects of IV and ICV hypocretin-1 (orexin A) in hypocretin receptor-2 gene mutated narcoleptic dogs and IV hypocretin-1 replacement therapy in a hypocretin-ligand-deficient narcoleptic dog. Sleep. 2003;26:953–959. doi: 10.1093/sleep/26.8.953. [DOI] [PubMed] [Google Scholar]
- 7.Willie JT, et al. Distinct narcolepsy syndromes in orexin receptor-2 and orexin null mice: Molecular genetic dissection of non-REM and REM sleep regulatory processes. Neuron. 2003;38:715–730. doi: 10.1016/s0896-6273(03)00330-1. [DOI] [PubMed] [Google Scholar]
- 8.Sateia MJ. International classification of sleep disorders-third edition: Highlights and modifications. Chest. 2014;146:1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 9.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 10.Thorpy MJ. Update on therapy for narcolepsy. Curr Treat Options Neurol. 2015;17:347. doi: 10.1007/s11940-015-0347-4. [DOI] [PubMed] [Google Scholar]
- 11.Thorpy MJ, Dauvilliers Y. Clinical and practical considerations in the pharmacologic management of narcolepsy. Sleep Med. 2015;16:9–18. doi: 10.1016/j.sleep.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Inocente C, et al. Pitolisant, an inverse agonist of the histamine H3 receptor: An alternative stimulant for narcolepsy-cataplexy in teenagers with refractory sleepiness. Clin Neuropharmacol. 2012;35:55–60. doi: 10.1097/WNF.0b013e318246879d. [DOI] [PubMed] [Google Scholar]
- 13.Mignot EJ. A practical guide to the therapy of narcolepsy and hypersomnia syndromes. Neurotherapeutics. 2012;9:739–752. doi: 10.1007/s13311-012-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 16.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mieda M, et al. Orexin peptides prevent cataplexy and improve wakefulness in an orexin neuron-ablated model of narcolepsy in mice. Proc Natl Acad Sci USA. 2004;101:4649–4654. doi: 10.1073/pnas.0400590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagdeviren C, et al. Miniaturized neural system for chronic, local intracerebral drug delivery. Sci Transl Med. 2018;10:eaan2742. doi: 10.1126/scitranslmed.aan2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormick ZL, et al. Intrathecal versus oral baclofen: A matched cohort study of spasticity, pain, sleep, fatigue, and quality of life. PM R. 2016;8:553–562. doi: 10.1016/j.pmrj.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Penn RD, Mangieri EA. Stiff-man syndrome treated with intrathecal baclofen. Neurology. 1993;43:2412. doi: 10.1212/wnl.43.11.2412. [DOI] [PubMed] [Google Scholar]
- 21.Mathur SN, Chu SK, McCormick Z, Chang Chien GC, Marciniak CM. Long-term intrathecal baclofen: Outcomes after more than 10 years of treatment. PM R. 2014;6:506–513 e1. doi: 10.1016/j.pmrj.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Eek MN, et al. Intrathecal baclofen in dyskinetic cerebral palsy: Effects on function and activity. Dev Med Child Neurol. 2017;60:94–99. doi: 10.1111/dmcn.13625. [DOI] [PubMed] [Google Scholar]
- 23.Irukayama-Tomobe Y, et al. Nonpeptide orexin type-2 receptor agonist ameliorates narcolepsy-cataplexy symptoms in mouse models. Proc Natl Acad Sci USA. 2017;114:5731–5736. doi: 10.1073/pnas.1700499114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.John J, Wu MF, Siegel JM. Systemic administration of hypocretin-1 reduces cataplexy and normalizes sleep and waking durations in narcoleptic dogs. Sleep Res Online. 2000;3:23–28. [PMC free article] [PubMed] [Google Scholar]
- 25.Penn RD, Kroin JS. Intrathecal baclofen alleviates spinal cord spasticity. Lancet. 1984;1:1078. doi: 10.1016/s0140-6736(84)91487-9. [DOI] [PubMed] [Google Scholar]
- 26.Saval A, Chiodo AE. Effect of intrathecal baclofen concentration on spasticity control: Case series. J Spinal Cord Med. 2008;31:394–397. doi: 10.1080/10790268.2008.11760742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knutsson E, Lindblom U, Mårtensson A. Plasma and cerebrospinal fluid levels of baclofen (Lioresal) at optimal therapeutic responses in spastic paresis. J Neurol Sci. 1974;23:473–484. doi: 10.1016/0022-510x(74)90163-4. [DOI] [PubMed] [Google Scholar]
- 28.Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: Evaluation of the evidence. Fluids Barriers CNS. 2014;11:26. doi: 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Louveau A, et al. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210–3219. doi: 10.1172/JCI90603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kroin JS, Ali A, York M, Penn RD. The distribution of medication along the spinal canal after chronic intrathecal administration. Neurosurgery. 1993;33:226–230, discussion 230. [PubMed] [Google Scholar]
- 32.Schatzberg SJ, et al. The effect of hypocretin replacement therapy in a 3-year-old Weimaraner with narcolepsy. J Vet Intern Med. 2004;18:586–588. doi: 10.1892/0891-6640(2004)18<586:teohrt>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 33.Mishima K, et al. Hypocretin receptor expression in canine and murine narcolepsy models and in hypocretin-ligand deficient human narcolepsy. Sleep. 2008;31:1119–1126. [PMC free article] [PubMed] [Google Scholar]
- 34.Cun Y, et al. Orexin A attenuates the sleep-promoting effect of adenosine in the lateral hypothalamus of rats. Neurosci Bull. 2014;30:877–886. doi: 10.1007/s12264-013-1442-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hagan JJ, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen-Pfeffer JL, et al. Intracerebroventricular delivery as a safe, long-term route of drug administration. Pediatr Neurol. 2017;67:23–35. doi: 10.1016/j.pediatrneurol.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 37.Wu WP, Xu XJ, Hao JX. Chronic lumbar catheterization of the spinal subarachnoid space in mice. J Neurosci Methods. 2004;133:65–69. doi: 10.1016/j.jneumeth.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 38.Kaushik MK, Aritake K, Takeuchi A, Yanagisawa M, Urade Y. Octacosanol restores stress-affected sleep in mice by alleviating stress. Sci Rep. 2017;7:8892. doi: 10.1038/s41598-017-08874-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaushik MK, Kaul SC, Wadhwa R, Yanagisawa M, Urade Y. Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction. PLoS One. 2017;12:e0172508. doi: 10.1371/journal.pone.0172508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang ZL, et al. Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci USA. 2001;98:9965–9970. doi: 10.1073/pnas.181330998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohtoh S, Taguchi Y, Matsumoto N, Huang ZL, Urade Y. Algorithm for sleep scoring in experimental animals based on fast Fourier transform power spectrum analysis of the electroencephalogram. Sleep Biol Rhythms. 2008;6:163–171. [Google Scholar]
- 42.Scammell TE, Willie JT, Guilleminault C, Siegel JM. International Working Group on Rodent Models of Narcolepsy A consensus definition of cataplexy in mouse models of narcolepsy. Sleep. 2009;32:111–116. [PMC free article] [PubMed] [Google Scholar]
- 43.Nishino S, et al. Low cerebrospinal fluid hypocretin (Orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol. 2001;50:381–388. doi: 10.1002/ana.1130. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura M, Kanbayashi T, Sugiura T, Inoue Y. Relationship between clinical characteristics of narcolepsy and CSF orexin-A levels. J Sleep Res. 2011;20:45–49. doi: 10.1111/j.1365-2869.2010.00870.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.