The origin of Neotropical diversity has intrigued scientists since Humboldt and Wallace. Species radiations in this region have been associated with major biogeographic events such as Quaternary climatic changes (1), Neogene geological changes such as the Andean uplift, closure of the Panamanian isthmus, Miocene Marine incursions, and the formation of the Amazon river basin (2). Less attention has been devoted to the importance of regional biotic interchange in shaping extant Neotropical diversity patterns. In PNAS, Antonelli et al. (3) marshal an impressive array of species occurrence and phylogenetic data from six major clades (i.e., angiosperms, ferns, squamates, birds, mammals, and frogs) to test the amount of immigration among Neotropical regions. This study finds that Amazonia is by far the greatest contributor of lineages to other regions, which has direct implications for our current understanding of the processes that underlie the origin and maintenance of diversity in the world’s most species-rich region. These results shed light on the biogeographic connections among Neotropical regions over time and identify which regions have experienced the most interchange and which have been more isolated.
Many new phylogenetic studies have contributed to our understanding of global diversity patterns and, in general, have provided support for the “Out of the Tropics” (4) and “Tropical Conservatism” (5) hypotheses, with older tropical clades giving rise to younger temperate lineages (6, 7). Tropical wet forest biomes are well known as the source of global biodiversity, due to their large time-integrated area, high productivity, more intense biotic interactions, and relatively stable climate (8). These factors promote large cumulative average population sizes, which, in turn, promote speciation while decreasing the risk of extinction (8). However, very few studies have looked at the patterns of diversity and dispersal among tropical regions. The factors that influence speciation and extinction of resident lineages may or may not be closely aligned with the factors that influence immigration among those regions. For example, all else being equal, regions that are larger and older (e.g., Amazonia) should have higher cumulative average population sizes of resident taxa and a higher probability that resident species will undergo long-distance dispersal to another region compared with smaller and younger regions (e.g., Caatinga). If a simple relationship exists between factors that promote population sizes within regions and factors that promote emigration of lineages, we would predict that disparate clades would show similar patterns, even in taxa as different as ferns and frogs. On the other hand, successful immigration from a new region may instead be linked to particular traits (e.g., dispersal ability) or be contingent on priority effects (i.e., lineages already present in a region) (9), confounding predictions of interchange among regions based on factors like time, area, productivity, and climate stability. If idiosyncratic traits, historical contingency, or priority effects predominate, then we would predict very different patterns for distinct clades. Antonelli et al. (3) find congruent patterns among six major clades, suggesting that traits (including dispersal ability) are less important than broad biogeographic factors like time and area.
Is It Easier to Move or to Evolve?
Biome conservatism and phylogenetic niche conservatism have emerged as consensus explanations for understanding patterns of phylogenetic clustering within regions in global analyses. For example, in a study of Southern Hemisphere plants, very few biome transitions were found between Mediterranean, desert, alpine, and rain forest biomes (10). The adaptations involved in thriving in such disparate conditions include costly physiological tradeoffs, making it unlikely that desert-adapted organisms could survive in a rainforest, and vice versa (10). The same applies to transitions from the tropics into temperate regions, as these involve the evolution of cold tolerance traits (11).
The analysis presented by Antonelli et al. (3) indicates that biome switching within the Neotropics is much more common than expected, providing additional support for the idea that it is easier to move than to evolve (11). One important take away here is to consider the appropriate spatial scale to best evaluate biome conservatism. Antonelli et al. (3) focus on the Neotropics and compare dispersal among bioregions that experience less extreme differences in temperature and rainfall than those associated with immigration between tropical and temperate regions, for instance. Furthermore, microhabitats and microclimates in dry habitats like gallery forest around rivers can sustain forest-adapted lineages in dry regions. Interestingly, Antonelli et al. (3) found that drought-adapted lineages are more likely to evolve from wet-adapted lineages than vice versa, with few transitions being observed from dry biomes to wet regions such as the Amazon and the Atlantic forest. The dynamic nature of forest expansion and contraction at different times in the past (12, 13) has contributed to complex patterns of interchange of wet-adapted vs. dry-adapted taxa earlier in time, causing strong selective pressures for some wet-adapted lineages to evolve drought tolerance (14). It is possible that some wet-forest taxa might have evolved key traits associated with survival in dry microhabitats within wet regions before their immigration into dry regions. Future analyses considering traits and specifically evaluating the rates of character evolution and dispersal in the Neotropics would allow for an explicit test of this hypothesis.
Tempo and Mode of Regional Biotic Interchanges
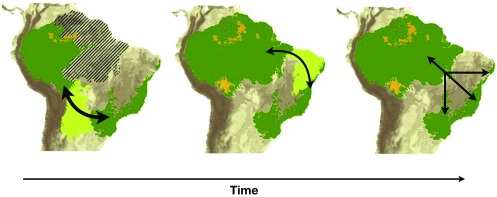
Antonelli et al. (3) find that about half of the dispersal events among regions are between biomes with contrasting climates and vegetation cover (i.e., wet Amazonian forests and dry Brazilian Cerrado). Consistent directionality of biotic interchange was recovered across taxonomic groups, although no general temporal pattern was found. If wind direction, past climates, and vegetation types were stable for geologically relevant periods of time, one would expect to find biases in the predominant direction of passive dispersal despite lineage-specific differences in dispersal and establishment capability. Past climatic reconstructions suggest that climate has strongly varied over geological time in Eastern and Western Amazonia, with more intense drying in the East (12). These reconstructions have direct implications for past vegetation cover and suggest that more stable and longer-lasting forest corridors connected the Amazon and the Atlantic Forest through the south, while less stable and shorter-lasting forest corridors connected these biomes in the north (12) (Fig. 1).
Fig. 1.
Current and potential past distributions of South American tropical forest based on speleothem data (12). Dark green shows extant tropical forests, and light green shows possible forest corridors at different times. Arrows on the maps indicate possible dispersal routes between the Atlantic Forest and the Amazon, while the x-axis indicates the time sequence suggested by various authors (e.g., refs. 19 and 20). Southern forest corridors are thought to have been more stable and to have lasted longer (∼50 kya), while the more recent northern corridor is thought to have been less stable and to have lasted for a shorter time (5–10 kya) (12). Adapted with permission from ref. 12, Springer Nature: Nature Communications, copyright (2013).
The fact that the extent of tropical forest cover may have been spatially and temporally dynamic in different parts of the Neotropics may help explain the lack of generalities found in terms of the timing of biotic interchanges. This lack of relationship may be associated with the fact that biotic exchanges across biomes likely occurred through different routes that may have been more widely used at different times. Connections between the Amazon and the Atlantic Forest, for instance, likely occurred through three different forest corridors (i.e., north, central, and south corridors; Fig. 1) that connected these biomes at very different windows of time (12, 15, 16). Therefore, lumping of all of the transitions between any two biomes without considering past forest corridors and the actual route of the transitions obscures finer scale temporal patterns of dispersal. Dynamic global vegetation models based on geobiochemistry and ecophysiology interpreted in the light of pollen core data are allowing for robust paleovegetation reconstructions (17). Moreover, empirical studies and statistical modeling offer new insights into dispersal dynamics, especially with respect to propagule characteristics and distance effects (18).
The Importance of Sampling in Historical Biogeography
It is important to consider that the phylogenies included in the analysis by Antonelli et al. (3) include a small percentage of all species from each clade, making one wonder how increased sampling within clades might change the overall patterns observed. In other words, as noted by the authors themselves, the high number of shifts in major biome types could be partially accounted for by the large taxonomic and geographic scale of this analysis (3). Moreover, most phylogenies only contain extant species and do not account for extinct taxa. Despite the difficulties involved, the importance of incorporating fossil data into phylogenies has been extensively documented (19). An important alternative hypothesis for the scenario proposed by Antonelli et al. (3) is that Amazonia experienced low extinction rates compared with the other Neotropical regions and, for that reason alone, taxa are more likely to be reconstructed as having an Amazonian origin. Indeed, comparisons of speciation and extinction rates of tropical and temperate lineages of birds, reptiles, amphibians, and mammals all estimated low extinction rates for the tropics (8). Within the Neotropics, Amazonia would be expected to have the lowest extinction rates due to its larger time-integrated area, higher productivity, and relatively stable climate compared with all other regions (8). Ongoing efforts to document the Neotropical fossil record should provide key insights into this question.
While studies like that of Antonelli et al. (3) exemplify the new knowledge we gain when pooling together large, cross-taxonomic datasets to test general biogeographic hypotheses, data derived from careful systematic research combining monographic work, densely sampled phylogenies, and multiple fossils have the potential of bringing many additional insights. These kinds of studies are still scarce for the Neotropics; however, they are critical for the identification of cryptic species and comparable evolutionary units across taxa and would allow us to make detailed inferences about species movements based on species-pair comparisons.
Perspectives and Conclusions
The approach proposed by Antonelli et al. (3) could be applied to other regions across the globe, contributing to our understanding of global biodiversity patterns. Future studies should aim for full taxon sampling in ecologically important groups in order to obtain a more complete view of biome history. New analyses based on detailed species-level phylogenies will allow us to investigate the mechanisms that have driven current biogeographic patterns and should be integrated with paleoclimatic reconstructions of Neotropical biomes. These studies are urgent because a good understanding of the history of biomes can both inform conservation decisions and help predict the effects of future environmental changes at a time when biodiversity is being impacted on an unprecedented rate. The effects of changing global climate will only increase the importance of dispersal events for species maintenance (20).
The Amazon basin, Central Africa’s Congo, and the Island of Borneo each contain large areas of contiguous rainforest harboring record totals of biodiversity and carbon storage. Recent bad news from these biodiversity hotspots regarding road building and habitat destruction (21) underscores the significance of Amazonia as a global reservoir of species richness. Antonelli et al. (3) provide an additional argument for why large areas of the Amazon must be conserved. Indeed, given that this region serves as the primary source of lineages means that destroying the Amazon will impoverish the “species pump” that contributes to the diversity in all other Neotropical regions.
Acknowledgments
We thank all participants of the Spring 2018 University of California, Berkeley Integrative Biology 290 graduate seminar “Neotropical Geology and Biodiversity” for fruitful discussions. L.G.L. thanks the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for funding her sabbatical visit to the University of California, Berkeley (2017/12797-1) and a collaborative FAPESP/National Science Foundation/NASA grant on the “Assembly and Evolution of the Amazonian Biota and Its Environment” (2012/50260).
Footnotes
The authors declare no conflict of interest.
See companion article on page 6034.
References
- 1.Haffer J. Speciation in amazonian forest birds. Science. 1969;165:131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- 2.Hoorn C, et al. Amazonia through time: Andean uplift, climate change, landscape evolution, and biodiversity. Science. 2010;330:927–931. doi: 10.1126/science.1194585. [DOI] [PubMed] [Google Scholar]
- 3.Antonelli A, et al. Amazonia is the primary source of Neotropical biodiversity. Proc Natl Acad Sci USA. 2018;115:6034–6039. doi: 10.1073/pnas.1713819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jablonski D, et al. Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient. Proc Natl Acad Sci USA. 2013;110:10487–10494. doi: 10.1073/pnas.1308997110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiens JJ, Donoghue MJ. Historical biogeography, ecology and species richness. Trends Ecol Evol. 2004;19:639–644. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Pyron RA, Wiens JJ. A large-scale phylogeny of Amphibia including over 2800 species, and a revised classification of extant frogs, salamanders, and caecilians. Mol Phylogenet Evol. 2011;61:543–583. doi: 10.1016/j.ympev.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Kerkhoff AJ, Moriarty PE, Weiser MD. The latitudinal species richness gradient in New World woody angiosperms is consistent with the tropical conservatism hypothesis. Proc Natl Acad Sci USA. 2014;111:8125–8130. doi: 10.1073/pnas.1308932111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fine PVA. Ecological and evolutionary drivers of geographic variation in species diversity. Annu Rev Ecol Evol Syst. 2015;46:369–392. [Google Scholar]
- 9.Fukami T. Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annu Rev Ecol Evol Syst. 2015;46:1–23. [Google Scholar]
- 10.Crisp MD, et al. Phylogenetic biome conservatism on a global scale. Nature. 2009;458:754–756. doi: 10.1038/nature07764. [DOI] [PubMed] [Google Scholar]
- 11.Donoghue MJ, Edwards EJ. Biome shifts and niche evolution in plants. Annu Rev Ecol Evol Syst. 2014;45:547–572. [Google Scholar]
- 12.Cheng H, et al. Climate change patterns in Amazonia and biodiversity. Nat Commun. 2013;4:1411. doi: 10.1038/ncomms2415. [DOI] [PubMed] [Google Scholar]
- 13.Leite YLR, et al. Neotropical forest expansion during the last glacial period challenges refuge hypothesis. Proc Natl Acad Sci USA. 2016;113:1008–1013. doi: 10.1073/pnas.1513062113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon MF, et al. Recent assembly of the Cerrado, a neotropical plant diversity hotspot, by in situ evolution of adaptations to fire. Proc Natl Acad Sci USA. 2009;106:20359–20364. doi: 10.1073/pnas.0903410106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Por FD. Sooretama: The Atlantic Rain Forest of Brazil. SPB Academic; The Hague: 1992. [Google Scholar]
- 16.Batalha-Filho H, Fjeldsa J, Fabre PH, Miyaki CY. Connections between the Atlantic and the Amazonian forest avifaunas represent distinct historical events. J Ornithol. 2013;154:41–50. [Google Scholar]
- 17.Sato H, Cowling SA. Glacial Amazonia at the canopy-scale: Using a biophysical model to understand forest robustness. Quat Sci Rev. 2017;171:38–47. [Google Scholar]
- 18.Muñoz J, Felicísimo AM, Cabezas F, Burgaz AR, Martínez I. Wind as a long-distance dispersal vehicle in the Southern Hemisphere. Science. 2004;304:1144–1147. doi: 10.1126/science.1095210. [DOI] [PubMed] [Google Scholar]
- 19.Marshall CR. The fossil record and estimating divergence times between lineages: Maximum divergence times and the importance of reliable phylogenies. J Mol Evol. 1990;30:400–408. doi: 10.1007/BF02101112. [DOI] [PubMed] [Google Scholar]
- 20.Trakhtenbrot A, Nathan R, Perry G, Richardson DM. The importance of long-distance dispersal in biodiversity conservation. Divers Distrib. 2005;11:173–181. [Google Scholar]
- 21.Laurance WF, Goosem M, Laurance SG. Impacts of roads and linear clearings on tropical forests. Trends Ecol Evol. 2009;24:659–669. doi: 10.1016/j.tree.2009.06.009. [DOI] [PubMed] [Google Scholar]