Significance
Brain function requires high-fidelity transmission of information between individual brain cells at synapses, physical contacts that contain a specialized machinery for passing and receiving signals. Synaptic cell adhesion proteins form physical bridges between neurons and are critical for fine-tuning synaptic properties. Because of their roles in synaptic transmission, mutations in these proteins contribute to a wide range of neuropsychiatric diseases. Here, we study the role of two synaptic cell adhesion proteins, LRRTM1 and LRRTM2, in signaling at excitatory synapses utilizing a conditional knockout mouse line. Genetically deleting these proteins in mature neurons dramatically impairs basal synaptic transmission and disrupts long-term potentiation, a prominent form of synaptic plasticity that is critical for learning and memory.
Keywords: synaptic transmission, synaptic plasticity, hippocampus, cell adhesion
Abstract
Leucine-rich repeat transmembrane (LRRTM) proteins are synaptic cell adhesion molecules that influence synapse formation and function. They are genetically associated with neuropsychiatric disorders, and via their synaptic actions likely regulate the establishment and function of neural circuits in the mammalian brain. Here, we take advantage of the generation of a LRRTM1 and LRRTM2 double conditional knockout mouse (LRRTM1,2 cKO) to examine the role of LRRTM1,2 at mature excitatory synapses in hippocampal CA1 pyramidal neurons. Genetic deletion of LRRTM1,2 in vivo in CA1 neurons using Cre recombinase-expressing lentiviruses dramatically impaired long-term potentiation (LTP), an impairment that was rescued by simultaneous expression of LRRTM2, but not LRRTM4. Mutation or deletion of the intracellular tail of LRRTM2 did not affect its ability to rescue LTP, while point mutations designed to impair its binding to presynaptic neurexins prevented rescue of LTP. In contrast to previous work using shRNA-mediated knockdown of LRRTM1,2, KO of these proteins at mature synapses also caused a decrease in AMPA receptor-mediated, but not NMDA receptor-mediated, synaptic transmission and had no detectable effect on presynaptic function. Imaging of recombinant photoactivatable AMPA receptor subunit GluA1 in the dendritic spines of cultured neurons revealed that it was less stable in the absence of LRRTM1,2. These results illustrate the advantages of conditional genetic deletion experiments for elucidating the function of endogenous synaptic proteins and suggest that LRRTM1,2 proteins help stabilize synaptic AMPA receptors at mature spines during basal synaptic transmission and LTP.
Complex signaling between individual neurons of the brain primarily occurs at synapses, which are required for the brain to process information and generate behavior. Synaptic cell adhesion proteins play an important role in defining these synaptic connections. They participate in synaptogenesis by helping to stabilize synapses early in development during the formation of neural circuits and simultaneously contribute to the specification of the diverse properties of synapses (1–3). Because of their central role in synaptic function, mutations in synaptic cell adhesion proteins contribute to a wide range of neuropsychiatric disorders (2, 4–8).
Leucine-rich repeat transmembrane neuronal proteins (LRRTMs) form a family of four ubiquitous, but relatively poorly understood, synaptic adhesion proteins, which are primarily localized to excitatory synapses (8–10). Previous studies have suggested roles for postsynaptic LRRTMs in synaptogenesis, maintenance of AMPA receptor (AMPAR)-mediated transmission in developing synapses, and NMDA receptor (NMDAR)-triggered long-term potentiation (LTP) (8, 10–17). However, many of these previous studies utilized shRNA-mediated knockdown of endogenous LRRTMs and/or overexpression of individual recombinant LRRTMs. These approaches, when carefully controlled, are useful for probing the functions of proteins such as LRRTMs, but also have inherent limitations, which often make interpretation of results difficult. Because shRNA-mediated knockdown does not eliminate the targeted proteins, the remaining pool of protein may be sufficient to provide critical functions. Importantly, remaining endogenous protein may function via heterodimerization and homodimerization with introduced mutant versions of the protein and thereby compensate for and mask critical roles for the imposed mutations. Furthermore, off-target effects are relatively common with shRNA studies (2). Constitutive knockout (KO) mouse lines of individual LRRTM genes have been generated and partially phenotyped (13, 18–20). However, these suffer from the confound that developmental effects or compensations may influence the phenotype observed at mature synapses. Thus, the gold standard for studying the role of proteins in any context, including synaptic proteins in the mammalian brain, is the use of conditional genetic deletion methods, which allow precise temporal and spatial control over complete removal of the proteins of interest (2). Furthermore, LRRTM2, which was studied most intensively in the previous molecular, cellular, and shRNA studies (8, 10–17), has not been analyzed with a genetic KO approach.
Here, we report findings from a mutant mouse line in which the LRRTM1 and LRRTM2 genes have been “floxed” to allow Cre recombinase-mediated genetic double deletion of LRRTM1,2. We focus on the synaptic consequences of genetic deletion of LRRTM1,2 from mature, hippocampal CA1 pyramidal neurons for several reasons. First, LRRTM1 and LRRTM2, but not LRRTM3 and LRRTM4, are robustly expressed in these neurons (9). Second, excitatory synapses on CA1 pyramidal neurons are arguably the most extensively studied and best-understood synapses in the mammalian brain. Third, previous work using shRNA-mediated knockdown of LRRTM1,2 focused on these synapses (16, 17). Fourth, these synapses express robust and extensively studied forms of plasticity; most importantly, they exhibit robust NMDAR-triggered LTP. Fifth, it is possible to use in vivo injection of lentiviruses expressing Cre recombinase to genetically delete floxed alleles from individual CA1 pyramidal neurons in young adult mice, a time at which synaptogenesis has ended. This permits analysis of the functions of LRRTMs at mature synapses, while avoiding any role they have in the development and/or maturation of synapses. Furthermore, the use of lentiviruses allows molecular replacement experiments in which recombinant LRRTMs can be expressed and studied on a null background, thereby avoiding the possibility of dimerization with endogenous LRRTMs.
Here, we show that deletion of LRRTM1,2 reduced basal AMPAR-mediated synaptic responses, an effect that was not observed with shRNA-mediated knockdown of LRRTM1,2. Our other results are largely consistent with previous studies using the shRNA-mediated knockdown approach (16, 17). Genetic deletion of LRRTM1,2, like LRRTM1,2 knockdown, blocked LTP, but not long-term depression (LTD). The block of LTP in LRRTM1,2 conditional KO mice (cKO) could be rescued by expressing recombinant LRRTM2 lacking its intracellular domain, but not by LRRTM2 with mutations designed to inhibit binding to presynaptic neurexins. Thus, LRRTMs appear to play a critical role in maintaining a normal complement of AMPARs in the synaptic plasma membrane not only during LTP but also during basal synaptic transmission. Together, these results provide evidence that LRRTMs are critical synaptic cell adhesion proteins for maintaining normal synaptic function and plasticity. They also provide direct evidence for the important advantages of performing conditional genetic deletion experiments when possible.
Results
Generation and Characterization of LRRTM1,2 Floxed Mice.
To study the role of LRRTM1 and LRRTM2 in synaptic function in vivo in the most rigorous manner possible, we generated LRRTM1,2 floxed mice (as described in SI Appendix, SI Materials and Methods and Figs. S1 and S2). As expected from the conditional nature of the floxed alleles, the mice developed and bred normally. To assess whether levels of LRRTM1,2 mRNA were normal in the LRRTM1,2 floxed mice and to confirm that LRRTM1,2 floxed mice were responsive to Cre recombinase, quantitative PCR (qPCR) was performed on disassociated hippocampal cultured neurons. Cultures from LRRTM1,2 floxed mice expressed mRNA transcripts for both LRRTM1 and 2 at levels that were similar to that of WT cultures [LRRTM1: 106.4 ± 6.6%, n = 4, P = not significant (NS); LRRTM2: 112.7 ± 9.3%, n = 4, P = NS; qPCR values were normalized to levels in uninfected WT culture]. Infection with Cre expressing lentivirus did not alter LRRTM1 or LRRTM2 mRNA levels in WT culture (LRRTM1: 109.2 ± 3.1%, n = 3, P = NS; LRRTM2: 95.2 ± 4.7%, n = 3, P = NS), but dramatically decreased LRRTM1 and 2 mRNA in cultures from LRRTM1,2 floxed mice (LRRTM1: 8.3 ± 3.1%, n = 4, P < 0.0001; LRRTM2: 11.1 ± 3.2%, n = 4, P = 0.0001). Furthermore, LRRTM1 and LRRTM2 proteins were undetectable in brain homogenates prepared from LRRTM1 and LRRTM2 floxed mice that were crossed with a mouse line expressing germ-line Cre (SI Appendix, Fig. S3).
LRRTM1,2 Deletion Blocks LTP.
The techniques to genetically delete LRRTM1,2 from young adult CA1 pyramidal neurons and replace it with versions of recombinant LRRTM2 are essentially identical to those used to genetically delete and replace other critical postsynaptic proteins such as neuroligin 1 (21) and synaptotagmins (22). We stereotactically injected lentiviruses encoding Cre recombinase fused to EGFP, a recombinase-dead ΔCre fused to EGFP, or Cre recombinase fused to EGFP and an LRRTM2 variant into the CA1 region of the hippocampus in postnatal day 21 (P21) mice (Fig. 1 A and B). Acute hippocampal slices were prepared 14–21 d later, and whole-cell voltage-clamp recordings were made from visually identified CA1 pyramidal cells (Fig. 1C). Slices were used only when infections were limited to the CA1 hippocampal region, ensuring that effects from the LRRTM manipulations were isolated to the postsynaptic cell.
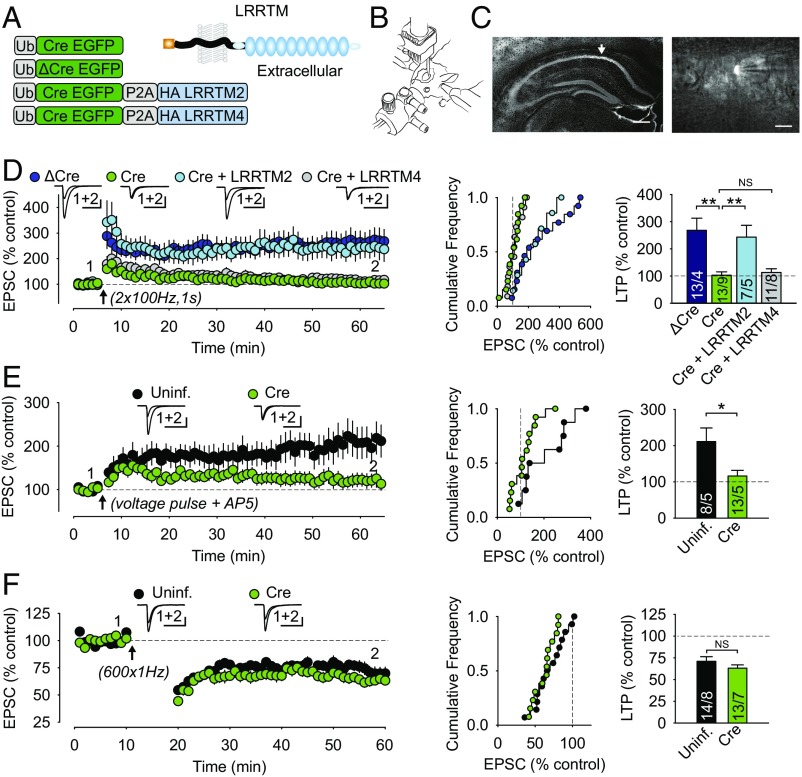
Fig. 1.
cKO of LRRTM1,2 in vivo blocks LTP. (A, Left) Schematics of the lentiviral constructs used are shown. (A, Right) The structure of LRRTM2, where balls represent individual LRR domains, is shown. (B) Illustration depicting a mouse on a stereotaxic instrument used for injecting lentiviruses into the CA1 region of the hippocampus at P21. (C, Left) Image of an infected hippocampal slice. (Scale bar: 500 µm.) (C, Right) Epifluorescent image of an infected CA1 pyramidal cell being patched. (Scale bar: 50 µm.) (D–F) Summary of effects of LRRTM1,2 KO on NMDAR-dependent LTP (D); NMDAR-independent LTP, induced by repetitive l-type Ca2+-channel activation (E); and NMDAR-dependent LTD (F). Each image shows, from left to right, a summary time course of experiments with sample EPSC traces from before and after LTP or LTD induction (Insets); cumulative frequency plot of the magnitude of EPSC change for all individual experiments; and summary of the magnitude of EPSC change for the different molecular manipulations. Experimental “n” values are provided in the individual bars and give number of cells from which recordings were made/number of animals from which slices were prepared. [Scale bars: 50 pA, 50 ms (D–F).] All values plotted with error bars are mean ± SEM. *P < 0.05; **P < 0.01; NS, not significant. Uninf., uninfected.
We first assessed NMDAR-dependent LTP, which was triggered by using a standard tetanic stimulation protocol (21, 23, 24). While cells expressing recombinase dead ΔCre exhibited robust LTP, cells infected with the Cre virus did not, on average, express LTP (Fig. 1D). The block in LTP due to the LRRTM1,2 deletion was rescued by simultaneous expression of full-length LRRTM2 (Fig. 1D), suggesting functional redundancy of LRRTM1,2 in supporting LTP. We did not attempt to rescue with LRRTM1 because previous studies and in-laboratory experience suggest that LRRTM1 constructs traffic poorly to the plasma membrane, largely accumulating in the endoplasmic reticulum (25). We also attempted to rescue LTP with LRRTM4, which is not believed to be expressed in hippocampal pyramidal neurons, but has been suggested to bind to AMPARs and to support chemically induced LTP in cultured dentate gyrus neurons (19, 26). Surprisingly, LRRTM4 expression could not rescue LTP, even though it traffics to the plasma membrane in cultured neurons (SI Appendix, Fig. S4). These results suggest that LRRTMs may support LTP at different synapses through different molecular mechanisms or interactions.
Although LRRTMs are not thought to influence NMDAR level or function, it is conceivable that some unappreciated effect on NMDARs may account for the block of LTP following LRRTM1,2 deletion. To test this possibility and to provide further evidence that LRRTM1,2 function downstream of the Ca2+ influx is necessary to trigger LTP, we assayed LTP induced by repetitive activation of L-type Ca2+ channels via voltage pulses during NMDAR blockade and without presynaptic stimulation (21, 27–29). Similar to NMDAR-dependent LTP, this form of LTP was impaired in cells lacking LRRTM1,2 (Fig. 1E), suggesting that deletion of LRRTM1,2 blocks LTP independent of the source of Ca2+ required for triggering LTP. We also assayed NMDAR-dependent LTD and found that it was unaffected by the LRRTM1,2 deletion (Fig. 1F). Together, these data suggest that LRRTM1,2 proteins play a specific role in LTP and not a general role in all forms of activity-dependent AMPAR trafficking that contribute to LTP and LTD.
LRRTM2 Extracellular Domain Rescues LTP and May Require Neurexin Interaction.
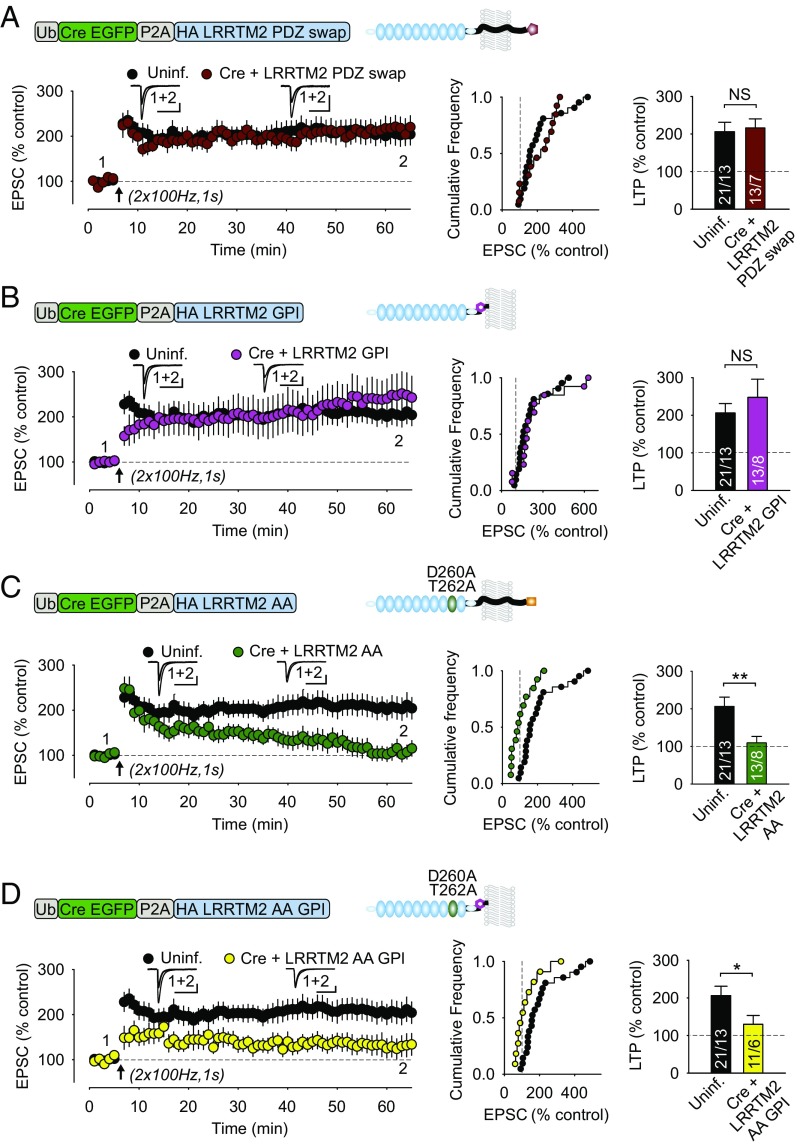
To establish which domains of LRRTM2 are required for its function in LTP, we tested the ability of four different mutant LRRTM2 constructs to rescue LTP in the absence of endogenous LRRTM1,2. Initially, we hypothesized that, in the absence of dimerization with endogenous LRRTMs, the intracellular PDZ motif might be required to scaffold it in the proper location at the synapse. To test this hypothesis, we swapped the C-terminal PDZ ligand (PYKECEV) of LRRTM2 for that of the human beta-2 adrenergic receptor (β2AR: STNDSLL), since this PDZ ligand is known to mediate β2AR plasma membrane trafficking, but its binding partners are different from that of LRRTM2. Specifically, the LRRTM2 PDZ ligand interacts with PSD-95, whereas the PDZ ligand of human β2AR does not (12, 30, 31). However, the LRRTM2 PDZ swap construct rescued LTP (Fig. 2A), suggesting that LRRTM2 interaction with PSD-95 is not required. As it is formally possible that the β2AR PDZ motif scaffolds LRRTM2 to other components of the synapse, we next tested whether any of the intracellular interactions of LRRTM2 are required to rescue LTP by creating a plasma membrane GPI-anchored LRRTM2 construct that lacks the intracellular domain of LRRTM2. This LRRTM2-GPI construct also rescued LTP (Fig. 2B), suggesting that only the extracellular portion of LRRTM2 is required for its function in LTP.
Fig. 2.
Molecular replacement strategy reveals that LRRTM2 extracellular LRR domains and binding to neurexins are involved in LTP. Each image shows schematics of rescue constructs used in illustrated experiments. Results are shown as in Fig. 1. (A) Full-length LRRTM2 containing swap of C-terminal PDZ ligand with that for β2AR. (B) Extracellular portion of LRRTM2 anchored to the plasma membrane using GPI. (C) Full-length LRRTM2 containing two point mutations (D260A and T262A) that were designed to interfere with binding to neurexins. (D) GPI-anchored LRRTM2, as in B, containing neurexin binding mutations. (Scale bars: 50 pA, 50 ms.) Experimental n values are shown in bars and give cells per animals. All values plotted with error bars are mean ± SEM. *P < 0.05; **P < 0.01; NS, not significant. Uninf., uninfected.
Major binding partners of the extracellular domains of LRRTMs are presynaptic neurexins, interactions with which are thought to be essential for the roles of LRRTMs both in synapse formation and in LTP (2, 8, 10–12, 14, 17, 32). To test the importance of neurexin interactions in LTP, we introduced a mutation of two key residues into LRRTM2 (D260A and T262A), which were designed to interrupt this interaction (14). This double mutation was introduced into both full-length LRRTM2 (LRRTM2 AA) and into GPI-anchored LRRTM2 (LRRTM2 AA GPI). In marked contrast to the rescue of LTP by LRRTM2 PDZ swap and LRRTM2 GPI, neither of the neurexin-binding mutant forms of LRRTM2 was capable of rescuing LTP (Fig. 2 C and D). Finally, we examined whether full-length LRRTM2 AA trafficked to the plasma membrane and found that it was expressed on the surface of cultured neurons approximately as effectively as WT LRRTM2, LRRTM2 PDZ swap, and LRRTM2GPI (SI Appendix, Fig. S4).
LRRTM1,2 Deletion Decreases AMPAR-Mediated Transmission.
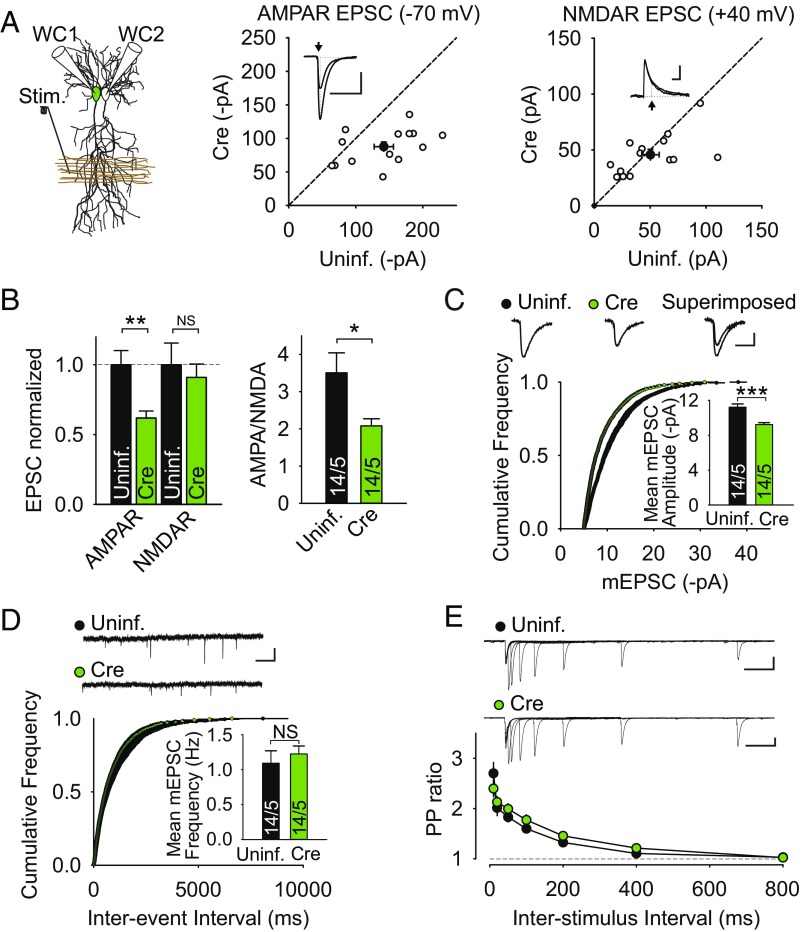
The results thus far are consistent with results of experiments in which shRNA-mediated knockdown of LRRTM1,2 was the main manipulation (17), although the interpretation of the rescue experiments can now be firmer (Discussion). One confusing result from previous work was that while LTP was impaired by LRRTM1,2 knockdown in young adult mice (17), there was no detectable effect on basal synaptic responses at this age, even though the same manipulation at P0 during early postnatal development caused a clear decrease in AMPAR-mediated excitatory postsynaptic currents (AMPAR EPSCs) (16). We therefore examined whether genetic deletion of LRRTM1,2 in young adult mice affected basal excitatory synaptic transmission. EPSCs were simultaneously measured in pairs of neighboring Cre-infected and uninfected neurons at −70 mV to measure AMPAR EPSCs and at +40 mV to measure NMDAR EPSCs (Fig. 3A). Deletion of LRRTM1,2 caused a significant decrease in AMPAR EPSCs, while NMDAR EPSCs were unaffected; this resulted in a decreased AMPAR/NMDAR ratio (Fig. 3 A and B). Consistent with these observations, the amplitude of AMPAR-mediated miniature EPSCs (mEPSCs) was significantly decreased in cells lacking LRRTM1,2, whereas the mEPSC frequency was unaltered (Fig. 3 C and D). To determine if there were changes in the probability of presynaptic release due to the deletion of LRRTM1,2, we also assessed paired-pulse ratios (33) at multiple interstimulus intervals, but found no change (Fig. 3E).
Fig. 3.
LRRTM1,2 deletion reduces AMPAR-mediated synaptic transmission. (A, Left) Schematic of simultaneous dual whole-cell (WC) recording from neighboring CA1 pyramidal cells during stimulation (Stim.) of inputs in stratum radiatum. (A, Center and Right) Graphs show scatter plots of amplitudes of simultaneously recorded AMPAR and NMDAR EPSCs (n = 14 pairs from 5 LRRTM1,2 cKO mice). Filled circles are means ± SEMs. Arrow next to representative EPSC pairs indicate time at which AMPAR and NMDAR EPSC amplitudes were measured. (Scale bars: 50 pA, 50 ms.) (B) Summary graphs of normalized AMPAR and NMDAR EPSCs (Left) and AMPAR/NMDAR ratios (Right) from cells in A. (C) Cumulative probability plot of mEPSC amplitudes and summary bar graphs of mean mEPSC amplitude from uninfected and Cre-expressing cells. Sample mEPSC traces are shown above graphs. (Scale bars: 5 pA, 5 ms.) n in bar graphs represents cells/mice. (D) Cumulative probability plot of mEPSC interevent interval and summary bar graphs of mean mEPSC frequency from uninfected and Cre-expressing cells. Sample mEPSC traces are shown above graphs. (Scale bars: 10 pA, 1 s.) “n” in bar graphs represents cells/mice. (E) Summary graph of the paired-pulse (PP) ratios at different interstimulus intervals (ISIs) for uninfected and Cre-expressing cells. Data are means ± SEM (n = 17/10 uninfected cells/mice and n = 17/8 infected cells/mice at all ISIs except for 10 and 800 ms, where n = 7/3 uninfected cells/mice and n = 10/3 infected cells/mice). Sample traces are shown above graphs. (Scale bars: 50 pA, 100 ms.) All values plotted with error bars are mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant. Uninf., uninfected.
LRRTM1,2 Proteins Are Essential for Maintaining AMPARs in Dendritic Spines.
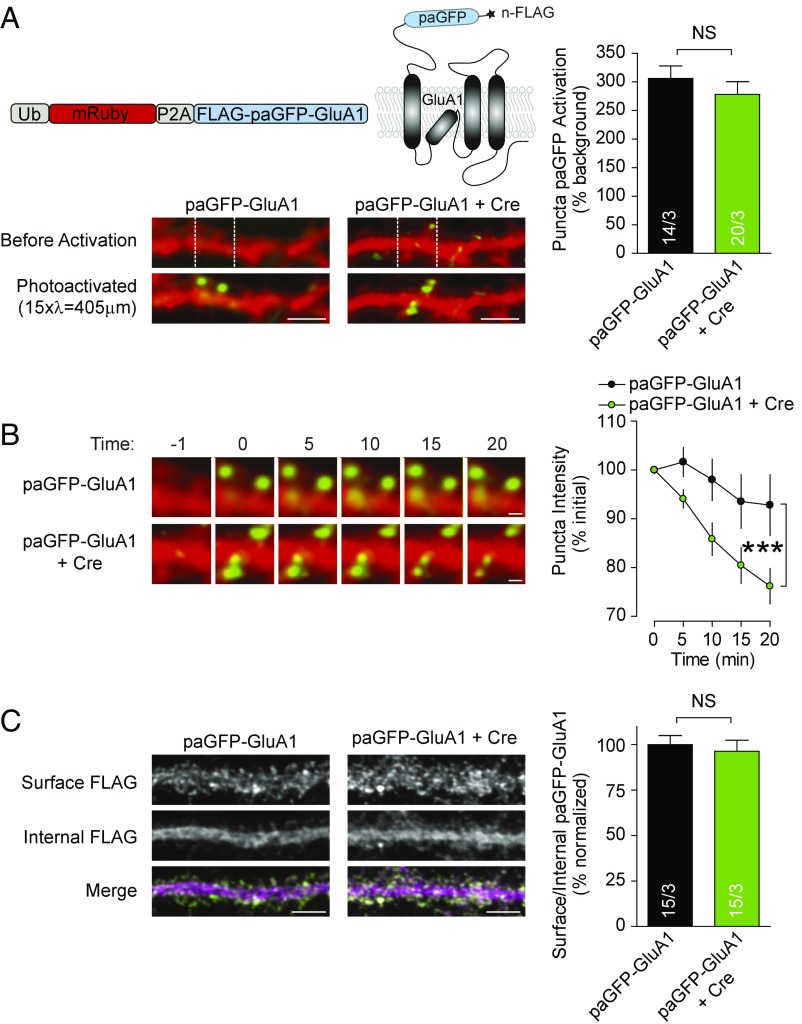
Our results thus far are all consistent with the hypothesis that LRRTMs are important for the maintenance of AMPARs at synapses during both basal synaptic transmission and following the increase in AMPAR numbers during LTP. Although it has been suggested that the major AMPAR subunit GluA1 can biochemically interact with LRRTMs (13, 26) and that LRRTM1,2 knockdown reduces the levels of AMPARs at synapses in cultured neurons (13, 17), the role of LRRTM1,2 in stabilizing GluA1 at the synapse has not been tested directly. To directly test whether the LRRTM1,2 deletion alters GluA1 stability at synapses, we expressed a photoactivatable GFP-tagged version of GluA1 (paGFP-GluA1) in cultured neurons and activated GFP in small regions of secondary dendrites in control and Cre-treated cultured neurons prepared from LRRTM1,2 floxed mice (Fig. 4 A, Left). We first quantified the amount of activated paGFP-GluA1 in visually identified spines immediately upon photoactivation. We found that the LRRTM1,2 deletion did not detectably alter levels of paGFP-GluA1 in spines (Fig. 4 A, Right). However, when we tracked the stability of paGFP signal in spines over 20 min, we found that that LRRTM1,2 deletion resulted in a faster loss of the paGFP-GluA1 spine signal (Fig. 4B).
Fig. 4.
Diffusion of photoactivatable GFP-tagged GluA1 out of spines is greater in spines lacking LRRTM1,2. (A, Upper Left) Schematic of construct that expresses soluble mRuby and paGFP tagged GluA1. (A, Lower Left) Representative images show secondary dendrites in disassociated hippocampal culture prepared from LRRTM1,2 floxed mice before and after photoactivation of paGFP-GluA1 in the demarcated region. Cells expressed paGFP-GluA1 alone or with Cre recombinase. (A, Right) Bar graphs show quantification of the photoactivation in spines. (Scale bars: 5 µm.) (B, Left) Representative images show montages of photoactivated dendritic regions during the course of an experiment. (B, Right) Quantification of puncta intensity over time in photoactivated regions. In Left Upper, note that the paGFP-GluA1 puncta do not change, while in Left Lower, the puncta decrease. (Scale bars: 1 µm.) (C, Left) Representative images of primary dendrites showing surface (nonpermeabilized) or internal (postpermeabilization) FLAG epitope staining in cells expressing paGFP-GluA1. (C, Right) Quantification of the ratio of surface to internal staining. (Scale bars: 5 µm.) Experimental n values are shown in bars and give cells/cultures. All values plotted with error bars are mean ± SEM. ***P < 0.001; NS, not significant.
A limitation of the use of paGFP-GluA1 is that, formally, we will detect paGFP-GluA1 in the spine plasma membrane as well as any intracellular paGFP-GluA1 in the spine. To determine if deletion of LRRTM1,2 might have somehow affected the relative proportion of surface paGFP-GluA1, and internal paGFP-GluA1, we first stained nonpermeabilized neurons expressing paGFP-GluA1 containing an N-terminal, extracellular FLAG epitope with FLAG antibodies and then stained the same neurons for the FLAG epitope after permeabilization using a different secondary antibody (Fig. 4A). This experiment allowed us to measure the ratio of surface to internal paGFP-GluA1, but showed no difference between LRRTM1,2 KO and control cells (Fig. 4C). Thus, the decay of paGFP-GluA1 signal in spines in LRRTM1,2 KO neurons is probably not due to a change in the relative proportion of intracellular paGFP-GluA1, but more likely due to the destabilization of spine paGFP-GluA1 in the absence of LRRTMs.
Discussion
LRRTMs first received attention because they robustly promoted synaptogenesis in the so-called artificial synapse-formation assay in which LRRTMs that were expressed in nonneuronal cells dramatically induced synapse formation by cocultured neurons and also because their overexpression in cultured neurons increased the density of excitatory, but not inhibitory, synapse (2, 8, 32). For LRRTM1 and 2, these synaptogenic effects require binding to presynaptic neurexins that lack splice site 4 (2, 8, 10, 12, 14, 32). While these results pointed to a potential important role for LRRTM1,2 in synapse development, such overexpression studies do not permit conclusions about the function of LRRTM1,2 in vivo (2). Similarly, constitutive KOs of LRRTM1 alone (11, 18), LRRTM3 alone (20), and LRRTM4 alone (19) have been generated and partially phenotyped, revealing modest effects on excitatory synapse structure in the CA1 region for LRRTM1 and synapse structure and function in the dentate gyrus for LRRTM3,4. However, because these were constitutive KOs, they revealed effects on the development and/or maturation of synapses as well as potential compensatory adaptations during development. Furthermore, no rescue experiments were performed in these previous studies to probe structure–function relationships.
Here, by taking advantage of a LRRTM1,2 floxed mouse line, we were able to study the consequences of genetic deletion of LRRTM1,2 from mature excitatory synapses on CA1 pyramidal neurons and to also perform rescue experiments on the impairment of LTP caused by LRRTM1,2 deletion with six different constructs. We replicated a number of key results from previous studies that used shRNA-mediated knockdown of LRRTM1,2 (16, 17). At a time when replicability in science is a topic of great concern and debate, we think it is important to test previous conclusions rigorously with the best available methodology. Importantly, our results not only firmed up previous conclusions, but also allowed several conclusions about the role of LRRTMs in LTP at excitatory synapses on CA1 pyramidal neurons. First, the fact that LRRTM2 alone rescued the impairment of LTP caused by deletion of both LRRTM1 and LRRTM2 suggests that LRRTM1 is functionally redundant with LRRTM2. Second, the intracellular domain of LRRTM2, including its PDZ ligand, is not necessary for its role in LTP. This conclusion was supported by previous rescue experiments (17), but because shRNA knockdown was used, it was conceivable that the rescue LRRTM2 construct lacking its intracellular domain might have dimerized with endogenous LRRTM1 or 2 that provided the requisite intracellular domain. Third, we now demonstrate that LRRTM4 cannot rescue LTP. LRRTM4 not only has a different intracellular domain than LRRTM2 (8), which we now know is unimportant for LTP, but mediates presynaptic differentiation through binding to heparan sulfate proteoglycans and perhaps protein phosphatase sigma (8, 13, 19). This strongly suggests that at excitatory synapses on CA1 pyramidal neurons, LRRTM1,2 binding to presynaptic neurexins may be critical for its function in LTP. Fourth, consistent with this conclusion, placing two point mutations in LRRTM2 that were designed to disrupt neurexin binding prevented its rescue of LTP. Again, while the same result was generated previously (17), because shRNA knockdown of LRRTM1,2 was used, we could not rule out dimerization with remaining endogenous LRRTM1,2.
An important result that was not observed in previous work using shRNA-mediated knockdown of LRRTM1,2 (16) was that the LRRTM1,2 genetic deletion caused a clear decrease in basal AMPAR-mediated synaptic transmission at mature synapses on CA1 pyramidal neurons. This was demonstrated in two ways: (i) a decrease in evoked AMPAR EPSCs, but not NMDAR EPSCs, during simultaneous paired recordings from a Cre-expressing and an adjacent control CA1 pyramidal neuron; and (ii) a decrease in the amplitude, but not frequency, of mEPSCs. This decrease in basal AMPAR-mediated synaptic transmission due to ablation of LRRTM1,2 was not observed with shRNA-mediated knockdown of these proteins, likely because the knockdown did not reduce LRRTM1,2 levels sufficiently at the synapses that were assayed. These results suggest that the presence of LRRTM1,2 is not necessary for the maintenance of functional synapses on CA1 pyramidal neurons: If that was the case, we would expect LRRTM1,2 deletion to decrease evoked NMDAR EPSCs as well as mEPSC frequency. Importantly, the observations of a decrease in basal AMPAR-mediated transmission and lack of LTP following LRRTM1,2 deletion supports the parsimonious hypothesis that LRRTM1,2 proteins are critical scaffolds at excitatory synapses on CA1 pyramidal neurons for maintaining a normal complement of AMPARs in the postsynaptic density. To further test this hypothesis using a complementary assay, we expressed GluA1 fused to paGFP in cultured hippocampal neurons and found that it was less stable in dendritic spines in the absence of LRRTM1,2.
The detailed molecular mechanisms by which LRRTM1,2 protein interaction with AMPARs and presynaptic neurexins maintains a full complement of AMPARs at excitatory synapses on CA1 pyramidal neurons remains unknown. Rigorous biochemical studies involving purified proteins will be necessary to elucidate the regions of LRRTM1,2 that are necessary for its reported direct interaction with AMPAR subunits (13, 26). If key LRRTM1,2 residues for this interaction can be found, it will allow rescue experiments with appropriate mutant LRRTM2 constructs to be performed to directly test the hypothesis that direct interactions between LRRTM2 and AMPAR subunits are required for maintaining AMPARs in the postsynaptic density. Similarly, the critical features of the LRRTM1,2–neurexin interaction that are necessary for LTP remain mysterious. Perhaps this interaction plays some unexpected signaling role in LTP in addition to being required for maintaining additional synaptic AMPARs. It is also important to note that excitatory synapses on different cell types use different sets of LRRTMs for unknown reasons, but such a diversity of LRRTM expression is likely functionally important. For example, in dentate gyrus granule cells, LRRTM3,4, not LRRTM1,2, are thought to be critical for maintaining normal basal synaptic transmission (19, 20). Given the importance of these families of proteins in synaptic plasticity and the genetic contributions to neuropsychiatric disorders, further detailed mechanistic work is warranted and will be facilitated by the types of approaches used in this study.
Materials and Methods
All animal procedures conformed to the NIH Guide for the Care and Use of Laboratory Animals (34) and were approved by the Stanford University Administrative Panel on Laboratory Animal Care (APLAC-10322). All genetic manipulations for electrophysiological assays were performed in vivo by using stereotaxic injections of lentiviruses into the CA1 region of the hippocampus of young adult mice (P21). Two to 3 wk later, acute hippocampal slices were prepared, and standard whole-cell patch-clamp recording techniques were used to assess synaptic function (21). See SI Appendix, SI Materials and Methods for details on all procedures used in this study.
Supplementary Material
Acknowledgments
This work was supported by a Brain and Behaviour Foundation Young Investigator Grant (to T.J.S.); NIH Grant P50 MH086403 (to R.M. and T.C.S.); German Research Foundation Grants SPP1365/KA3423/1-1 (to N.B. and H.K.) and KA3423/3-1 (to H.K.); German Federal Ministry of Education and Research Grant ERA-NET-Neuron SynPathy (to N.B. and A.M.C.); Natural Sciences and Engineering Research Council Discovery Grant RGPIN-2015-05994 (to T.J.S.); and Canadian Institutes of Health Research Grants 84241 and FDN-143206 (to A.M.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1803280115/-/DCSupplemental.
References
- 1.Siddiqui TJ, Craig AM. Synaptic organizing complexes. Curr Opin Neurobiol. 2011;21:132–143. doi: 10.1016/j.conb.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Südhof TC. Synaptic neurexin complexes: A molecular code for the logic of neural circuits. Cell. 2017;171:745–769. doi: 10.1016/j.cell.2017.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang S, Lee H, Kim E. Synaptic adhesion molecules and excitatory synaptic transmission. Curr Opin Neurobiol. 2017;45:45–50. doi: 10.1016/j.conb.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Francks C, et al. LRRTM1 on chromosome 2p12 is a maternally suppressed gene that is associated paternally with handedness and schizophrenia. Mol Psychiatry. 2007;12:1129–1139, 1057. doi: 10.1038/sj.mp.4002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Betancur C, Sakurai T, Buxbaum JD. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009;32:402–412. doi: 10.1016/j.tins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa I, et al. International Molecular Genetic Study of Autism Consortium (IMGSAC) Polymorphisms in leucine-rich repeat genes are associated with autism spectrum disorder susceptibility in populations of European ancestry. Mol Autism. 2010;1:7. doi: 10.1186/2040-2392-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres VI, Vallejo D, Inestrosa NC. Emerging synaptic molecules as candidates in the etiology of neurological disorders. Neural Plast. 2017;2017:8081758. doi: 10.1155/2017/8081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roppongi RT, Karimi B, Siddiqui TJ. Role of LRRTMs in synapse development and plasticity. Neurosci Res. 2017;116:18–28. doi: 10.1016/j.neures.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Laurén J, Airaksinen MS, Saarma M, Timmusk T. A novel gene family encoding leucine-rich repeat transmembrane proteins differentially expressed in the nervous system. Genomics. 2003;81:411–421. doi: 10.1016/s0888-7543(03)00030-2. [DOI] [PubMed] [Google Scholar]
- 10.de Wit J, Ghosh A. Control of neural circuit formation by leucine-rich repeat proteins. Trends Neurosci. 2014;37:539–550. doi: 10.1016/j.tins.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linhoff MW, et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko J, Fuccillo MV, Malenka RC, Südhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64:791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wit J, et al. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siddiqui TJ, Pancaroglu R, Kang Y, Rooyakkers A, Craig AM. LRRTMs and neuroligins bind neurexins with a differential code to cooperate in glutamate synapse development. J Neurosci. 2010;30:7495–7506. doi: 10.1523/JNEUROSCI.0470-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko J, Soler-Llavina GJ, Fuccillo MV, Malenka RC, Südhof TC. Neuroligins/LRRTMs prevent activity- and Ca2+/calmodulin-dependent synapse elimination in cultured neurons. J Cell Biol. 2011;194:323–334. doi: 10.1083/jcb.201101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soler-Llavina GJ, Fuccillo MV, Ko J, Südhof TC, Malenka RC. The neurexin ligands, neuroligins and leucine-rich repeat transmembrane proteins, perform convergent and divergent synaptic functions in vivo. Proc Natl Acad Sci USA. 2011;108:16502–16509. doi: 10.1073/pnas.1114028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soler-Llavina GJ, et al. Leucine-rich repeat transmembrane proteins are essential for maintenance of long-term potentiation. Neuron. 2013;79:439–446. doi: 10.1016/j.neuron.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takashima N, et al. Impaired cognitive function and altered hippocampal synapse morphology in mice lacking Lrrtm1, a gene associated with schizophrenia. PLoS One. 2011;6:e22716. doi: 10.1371/journal.pone.0022716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siddiqui TJ, et al. An LRRTM4-HSPG complex mediates excitatory synapse development on dentate gyrus granule cells. Neuron. 2013;79:680–695. doi: 10.1016/j.neuron.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 20.Um JW, et al. LRRTM3 regulates excitatory synapse development through alternative splicing and neurexin binding. Cell Rep. 2016;14:808–822. doi: 10.1016/j.celrep.2015.12.081. [DOI] [PubMed] [Google Scholar]
- 21.Jiang M, et al. Conditional ablation of neuroligin-1 in CA1 pyramidal neurons blocks LTP by a cell-autonomous NMDA receptor-independent mechanism. Mol Psychiatry. 2017;22:375–383. doi: 10.1038/mp.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu D, et al. Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature. 2017;544:316–321. doi: 10.1038/nature21720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahmad M, et al. Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron. 2012;73:260–267. doi: 10.1016/j.neuron.2011.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jurado S, et al. LTP requires a unique postsynaptic SNARE fusion machinery. Neuron. 2013;77:542–558. doi: 10.1016/j.neuron.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Francks C, et al. LRRTM1 protein is located in the endoplasmic reticulum (ER) in mammalian cells. Mol Psychiatry. 2007;12:1057. [Google Scholar]
- 26.Schwenk J, et al. High-resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron. 2012;74:621–633. doi: 10.1016/j.neuron.2012.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Kullmann DM, Perkel DJ, Manabe T, Nicoll RA. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992;9:1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- 28.Wyllie DJ, Nicoll RA. A role for protein kinases and phosphatases in the Ca(2+)-induced enhancement of hippocampal AMPA receptor-mediated synaptic responses. Neuron. 1994;13:635–643. doi: 10.1016/0896-6273(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 29.Kato HK, Watabe AM, Manabe T. Non-Hebbian synaptic plasticity induced by repetitive postsynaptic action potentials. J Neurosci. 2009;29:11153–11160. doi: 10.1523/JNEUROSCI.5881-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao TT, Deacon HW, Reczek D, Bretscher A, von Zastrow M. A kinase-regulated PDZ-domain interaction controls endocytic sorting of the β2-adrenergic receptor. Nature. 1999;401:286–290. doi: 10.1038/45816. [DOI] [PubMed] [Google Scholar]
- 31.Hu LA, et al. β 1-adrenergic receptor association with PSD-95. Inhibition of receptor internalization and facilitation of β 1-adrenergic receptor interaction with N-methyl-D-aspartate receptors. J Biol Chem. 2000;275:38659–38666. doi: 10.1074/jbc.M005938200. [DOI] [PubMed] [Google Scholar]
- 32.Ko J. The leucine-rich repeat superfamily of synaptic adhesion molecules: LRRTMs and Slitrks. Mol Cells. 2012;34:335–340. doi: 10.1007/s10059-012-0113-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 34.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.