Significance
Plants synthesize a vast repertoire of steroidal specialized metabolites. These include the well-known class of antinutritional steroidal glycoalkaloids (SGAs), which act as defensive chemicals in the Solanaceae, and the pharmacologically important and widespread steroidal saponins. Here, we uncover an elusive enzymatic step that acts on unsaturated steroidal metabolites. We find that GLYCOALKALOID METABOLISM25 (GAME25) acts at a key branch point in the biosynthesis pathways of steroidal specialized metabolites. The activity of GAME25 not only affects the enormous diversity of SGAs and steroidal saponins, which are produced in hundreds of plant species, but also modulates the molecules’ toxic effects. This work helps explain the extensive structural diversity in specialized metabolism through a relatively simple chemical modification in a single metabolite backbone.
Keywords: steroidal glycoalkaloids, specialized metabolism, structural diversity, antinutritional, tomato
Abstract
Thousands of specialized, steroidal metabolites are found in a wide spectrum of plants. These include the steroidal glycoalkaloids (SGAs), produced primarily by most species of the genus Solanum, and metabolites belonging to the steroidal saponins class that are widespread throughout the plant kingdom. SGAs play a protective role in plants and have potent activity in mammals, including antinutritional effects in humans. The presence or absence of the double bond at the C-5,6 position (unsaturated and saturated, respectively) creates vast structural diversity within this metabolite class and determines the degree of SGA toxicity. For many years, the elimination of the double bond from unsaturated SGAs was presumed to occur through a single hydrogenation step. In contrast to this prior assumption, here, we show that the tomato GLYCOALKALOID METABOLISM25 (GAME25), a short-chain dehydrogenase/reductase, catalyzes the first of three prospective reactions required to reduce the C-5,6 double bond in dehydrotomatidine to form tomatidine. The recombinant GAME25 enzyme displayed 3β-hydroxysteroid dehydrogenase/Δ5,4 isomerase activity not only on diverse steroidal alkaloid aglycone substrates but also on steroidal saponin aglycones. Notably, GAME25 down-regulation rerouted the entire tomato SGA repertoire toward the dehydro-SGAs branch rather than forming the typically abundant saturated α-tomatine derivatives. Overexpressing the tomato GAME25 in the tomato plant resulted in significant accumulation of α-tomatine in ripe fruit, while heterologous expression in cultivated eggplant generated saturated SGAs and atypical saturated steroidal saponin glycosides. This study demonstrates how a single scaffold modification of steroidal metabolites in plants results in extensive structural diversity and modulation of product toxicity.
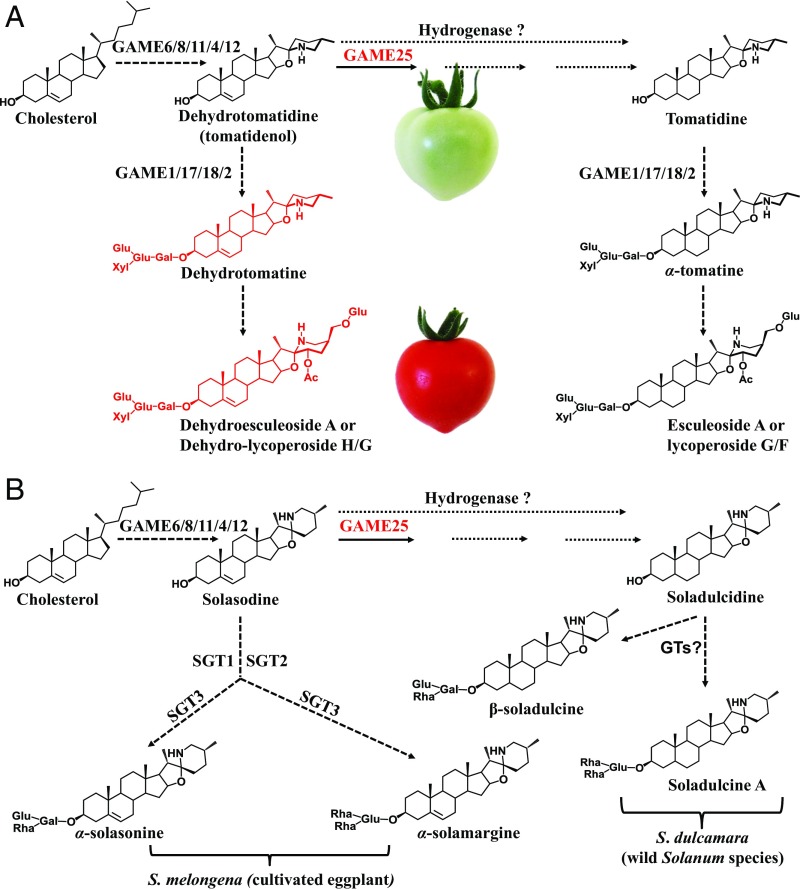
Steroidal glycoalkaloids (SGAs) are nitrogen-containing specialized metabolites present in numerous members of the Solanaceae family. Some well-known representatives of this class include α-tomatine and dehydrotomatine in the tomato (Solanum lycopersicum), α-chaconine and α-solanine in the cultivated potato (Solanum tuberosum), and α-solamargine and α-solasonine in the cultivated eggplant (Solanum melongena) (Fig. 1 and SI Appendix, Fig. S1). SGAs play a protective role against a wide range of plant pathogens and predators, including bacteria, fungi, oomycetes, viruses, insects, and animals (1–4). While beneficial for the plant species that produce them, SGAs are considered antinutritional and toxic to humans (5–7). SGAs are known for their enormous structural diversity, mainly based on the structural variations of the steroidal alkaloid (SA) aglycone, which is either unsaturated (presence of C-5,6 double bond) or saturated (absence of C-5,6 double bond) (Fig. 1 and SI Appendix, Fig. S1). In addition to SGAs, many plants, including Solanum species and monocots, also produce cholesterol-derived steroidal saponins (6). As with SGAs, steroidal saponins can be either saturated (e.g., sarasapogenin) or unsaturated (e.g., diosgenin) in the C-5,6 position (6) (SI Appendix, Fig. S2).
Fig. 1.
The biosynthetic pathway for SGAs in tomato, cultivated eggplant, and other Solanum species. (A) In the tomato plant, the conversion of dehydrotomatidine to tomatidine was previously predicted to be a single-step reaction driven by a hypothetical hydrogenase enzyme (2, 13). SI Appendix, Fig. S3, provides a more detailed SGA pathway schematic. (B) In cultivated eggplant, solasodine, an unsaturated aglycone is glycosylated by STEROL ALKALOID GLYCOSYL TRANSFERASEs (SGTs) to produce unsaturated α-solasonine and α-solamargine SGAs (Left). Cultivated eggplant varieties likely lack a GAME25-like enzyme and therefore do not produce saturated SGAs. Some wild Solanum species (e.g., S. dulcamara) produce a saturated soladulcidine aglycone from solasodine and further glycosylate soladulcidine aglycone to soladulcine A and β-soladulcine (saturated SGAs) (Right). This suggests the presence of GAME25 homologs in S. dulcamara (wild Solanum relative) and other Solanum species producing saturated SGAs starting from solasodine.
Cholesterol serves as the precursor for the biosynthesis of SGAs (8). Recent studies in the tomato and potato plants reported on GLYCOALKALOID METABOLISM (GAME) genes in the core SGA biosynthesis pathway (7, 9–12). The SGA biosynthetic pathway can be divided into two main parts. In the first part, several GAME enzymes form unsaturated SA aglycones from cholesterol (9, 12). The second part results in the generation of glycosylated SAs (i.e., SGAs) through the action of different UDP-glycosyltransferases (7, 10).
Dehydrotomatidine (tomatidenol), solanidine, and solasodine are the first unsaturated SA aglycones formed in the SGA pathway of the cultivated tomato, potato, and eggplant, respectively (Fig. 1 and SI Appendix, Fig. S1). These are then further glycosylated to produce diverse unsaturated SGAs (e.g., dehydrotomatine in tomato, α-chaconine and α-solanine in cultivated potato, and α-solamargine and α-solasonine in cultivated eggplant) (Fig. 1 and SI Appendix, Fig. S1). In the tomato plant, dehydrotomatidine is further hydrogenated at the C-5,6 position to form the saturated tomatidine aglycone (Fig. 1A). Tomatidine also undergoes glycosylation to produce the saturated α-tomatine (Fig. 1A). The major tomato SGAs (i.e., α-tomatine and dehydrotomatine) accumulate predominantly in green tissues (9). As the tomato fruit matures and reaches the ripe, red stage, α-tomatine and dehydrotomatine are mostly converted to diverse saturated and unsaturated SGA derivatives and finally to esculeosides and dehydroesculeosides, respectively (Fig. 1 and SI Appendix, Fig. S3 for detailed tomato SGA pathway). Therefore, dehydrotomatidine and tomatidine are the main SA aglycones responsible for the hundreds of SGA derivatives generated in the tomato plant (Fig. 1 and SI Appendix, Fig. S3). The cultivated eggplant and potato, on the other hand, do not produce saturated SGAs because the hydrogenation step at C-5,6 position does not occur (Fig. 1B and SI Appendix, Fig. S1). However, several wild potato species (e.g., S. demissum, S. chacoense, and S. commersonii) do produce saturated demissidine and its glycosylated form, demissine, from unsaturated solanidine (SI Appendix, Fig. S1). Moreover, some wild Solanum species (e.g., S. dulcamara) produce mostly saturated soladulcidine aglycone-derived SGAs (e.g., soladulcine A and β-soladulcine) from unsaturated solasodine (Fig. 1B).
A main step in steroidal saponin biosynthesis is formation of an unsaturated steroidal saponin aglycone (SI Appendix, Fig. S1). The aglycone of steroidal saponins is either spirostanol (closed F-ring) or furostanol (open F-ring) (6). Both saponin aglycones undergo either glycosylation to form unsaturated saponin glycosides (e.g., dioscin) or hydrogenation at the C-5,6 position to form saturated saponin aglycones (e.g., sarasapogenin) and their corresponding glycosides (e.g., parillin) (SI Appendix, Fig. S2). Therefore, as with SGAs, unsaturated and saturated aglycone forms of steroidal saponins determine the degree of structural diversity within this metabolite class.
Notably, the unsaturated/saturated SA aglycone and steroidal saponin aglycone pairs differ only in their structures by the presence or absence of the double bond at the C-5,6 position (Fig. 1 and SI Appendix, Figs. S1 and S2). However, the biosynthetic pathway responsible for the formation of saturated steroidal alkaloid and steroidal saponin aglycones from their unsaturated forms in Solanaceae or in any other plant family remains to be identified. For decades, it has been hypothesized that the conversion of dehydrotomatidine to tomatidine in the tomato plant, and solanidine to demissidine in wild potato species, occurs by elimination of the C-5,6 double bond through a single reaction catalyzed by hypothetical hydrogenase enzyme (2, 13–15).
In this study, we present results suggesting that formation of saturated steroidal specialized metabolites from unsaturated steroidal aglycone takes place in multiple steps rather than a single step. We discovered that GLYCOALKALOID METABOLISM25 (GAME25), a member of the short-chain dehydrogenase/reductase (SDR) gene family, is involved in the first among these multiple steps, specifically, the conversion of dehydrotomatidine to tomatidine. Silencing of GAME25 in the tomato plant diverted SGA metabolism within the leaves and during fruit development toward unsaturated dehydrotomatine-derived SGAs rather than the usual formation of saturated α-tomatine–derived SGAs. In vitro, GAME25 exhibited a 3β-hydroxysteroid dehydrogenase/Δ5,4 isomerase activity on various unsaturated steroidal alkaloid and saponin aglycone substrates, but not on their glycosylated forms. Furthermore, overexpression of tomato GAME25 in cultivated eggplant resulted in the formation of saturated SGAs and atypically saturated steroidal saponins. Taken together, GAME25 is a key enzyme in SGA and steroidal saponin metabolism, mediating a significant portion of the structural diversity of these natural product classes in Solanum as well as in saponin-producing plant species.
Results
Expression of GAME25 Correlates with the Accumulation of Typical Green Tissue Steroidal Glycoalkaloids.
A recent report by Cárdenas et al. (11) demonstrated that the GAME9 AP2-type transcription factor is associated with the regulation of SGA biosynthesis in the tomato and potato plants. Transcriptome analysis revealed 27 genes that were up- or down-regulated in GAME9 overexpression and silenced tomato lines, respectively. This concise gene set included a putative 3-β-HYDROXYSTEROID DEHYDROGENASE, a member of the SDR gene family (termed here GAME25, Solyc01g073640). GAME25 displayed expression predominantly in flower buds, young leaves, and in the immature green stage (skin and flesh) of fruit development (SI Appendix, Fig. S4). This expression pattern highly resembled the profile of tomato SGAs (e.g., α-tomatine and dehydrotomatine), which accumulate in the green tissues of the plant (9). Furthermore, the reduced transcript levels of GAME25 during later stages of fruit development correlated with a reduction in α-tomatine and dehydrotomatine content during fruit maturation (SI Appendix, Figs. S3 and S4). The GAME25 expression pattern during tomato fruit maturation was similar to that observed in wild tomato accessions (SI Appendix, Fig. S5A). The known function of some SDR family members in specialized metabolism (16, 17) and the expression profile results showed here suggest that GAME25 might be involved in SGA metabolism in the tomato plant.
SDRs represent one of the largest and most diverse NAD(P)(H)-dependent enzyme superfamilies that have evolved in plants and were recently categorized into 49 subfamilies (16). The 259-aa GAME25 protein sequence shows the characteristics of a classical SDR family member, containing the TGxxxGxG cofactor binding site and the YxxxK catalytic motif (17, 18) (SI Appendix, Fig. S5B). Phylogenetic analysis showed that GAME25 homologs of certain Solanaceae species (i.e., tomato, potato, and Solanum pennellii) formed a subclade distinct from other plant SDRs (SI Appendix, Fig. S6). The closest subclade to the GAME25 proteins in the phylogenic tree contained the 3β-hydroxysteroid dehydrogenase homologs from tomato and Solanum pennellii (3-βHSD, ∼90% amino acid identity with GAME25 subclade proteins), the function of which is unknown. Phylogenetic analysis also showed no homolog for the GAME25 protein in eggplant or capsicum (SI Appendix, Fig. S6). Moreover, GAME25 proteins were clearly separated from the Digitalis lanata 3-βHSD protein (∼75% amino acid identity with the GAME25 proteins), which is involved in the removal of the C-5,6 double bond from steroid derivatives during progesterone and cardenolide biosynthesis (SI Appendix, Fig. S6). The clear separation of the GAME25 subclade suggested a unique catalytic activity of these enzymes that is most likely different from the closely related 3-βHSD subclade members (SI Appendix, Fig. S6 and Dataset S1).
Tomato SGA Metabolism Is Rerouted from the Native, Predominantly Saturated α-Tomatine Branch to the Unsaturated Dehydrotomatine Branch in GAME25-Silenced Leaves.
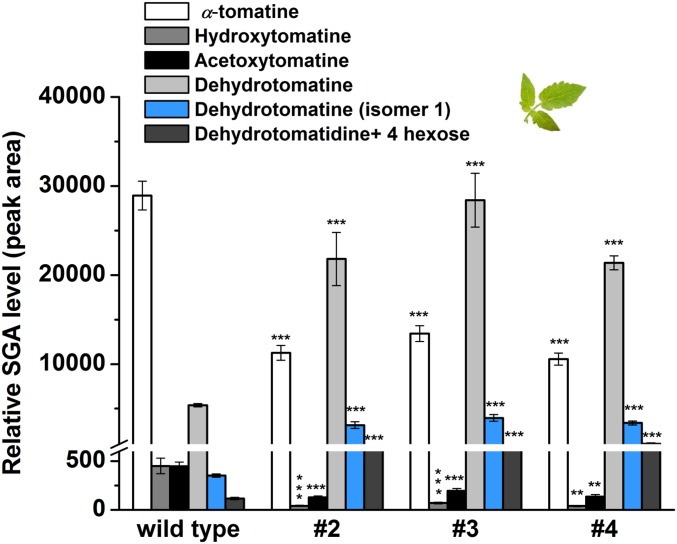
To determine the role of GAME25 in SGA metabolism, we silenced GAME25 in the tomato plant (i.e., GAME25i lines). GAME25 transcript levels were significantly reduced in GAME25i plant leaves and fruit at three developmental stages (green, breaker, and red ripe fruit) (t test, *P value < 0.05) (SI Appendix, Fig. S7). Notably, GAME25i leaves showed a substantial decline in α-tomatine (∼2.5- to 3-fold), hydroxytomatine (∼6- to 10-fold), and acetoxytomatine (∼2- to 3.5-fold) levels compared with wild-type leaves (Fig. 2 and SI Appendix, Fig. S8A; see SI Appendix, Fig. S3 for the detailed tomato SGA pathway). Conversely, we observed considerable increases in dehydrotomatine (∼4- to 6-fold), dehydrotomatine isomer 1 (∼9- to 11-fold), and dehydrotomatidine +4 hexose (∼6- to 9-fold) levels compared with wild-type leaves (Fig. 2 and SI Appendix, Fig. S8A). We noted reduction in α-tomatine and its downstream metabolite levels, yet, accumulation of dehydrotomatine and its isomers in GAME25i lines suggested that either (i) GAME25 is involved in α-tomatine biosynthesis directly from dehydrotomatine glycoside or (ii) the enzyme mediates tomatidine biosynthesis from dehydrotomatidine (i.e., tomatidenol; Fig. 1). We found no accumulation of dehydrotomatidine in GAME25i lines, but this SA aglycone appeared to be converted to its glycosylated derivatives (e.g., dehydrotomatine and dehyrotomatidine +4 hexoses) that did accumulate in leaves (Fig. 2 and SI Appendix, Fig. S3). Rather than acting on glycosylated substrates (e.g., dehydrotomatine), the above findings position GAME25 activity before the dehydrotomatine glycosylation steps, possibly in the conversion of dehydrotomatidine to tomatidine.
Fig. 2.
GAME25 silencing in tomato leaves shifts the SGA pathway to the unsaturated dehydrotomatine branch. SGA levels in leaves of wild-type (nontransformed) and three independent GAME25-RNAi transgenic tomato lines (#2, #3, and #4), as determined by LC–MS. The values represent the means of three biological replicates ±SE (per genotype). Asterisks indicate significant changes from wild-type samples calculated by a Student’s t test (*P value < 0.05; **P value < 0.01; ***P value < 0.001).
GAME25 Silencing Results in Gradual Loss of Saturated SGAs Within the Developing and Ripening Tomato Fruit.
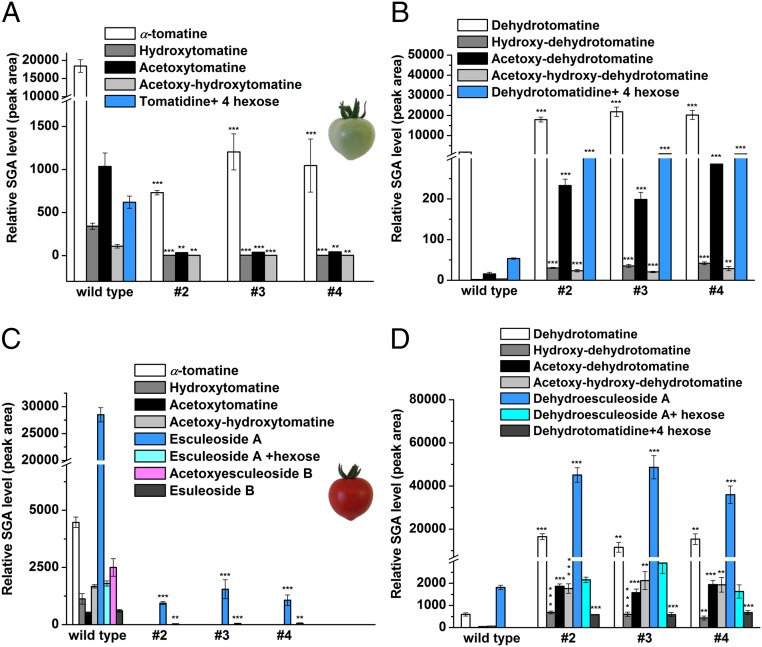
We compared the SGA profile of wild-type and GAME25i tomato fruit through different stages of fruit development and ripening. During the transition from green to red fruit, α-tomatine is typically converted to saturated SGAs (esculeosides and lycoperosides), while dehydrotomatine is converted to dehydroesculeosides and dehydrolycoperosides (unsaturated minor SGAs) (see SI Appendix, Fig. S3 for the detailed tomato SGA pathway). As found in leaves (Fig. 2), GAME25i green fruit displayed a drastic reduction in α-tomatine (∼15- to 25-fold), hydroxytomatine (∼100-fold), and further α-tomatine–derived downstream SGA levels compared with wild-type green fruit (Fig. 3A and SI Appendix, Fig. S8B). Thus, due to GAME25 silencing, α-tomatine and its downstream saturated SGA intermediates were severely affected in green fruit tissue. In contrast, various unsaturated SGAs including dehydrotomatine (∼10- to 12-fold) and hydroxy-dehydrotomatine (∼25-fold) were increased in GAME25i compared with the wild-type tomato green fruit (Fig. 3B and SI Appendix, Fig. S8B). These data suggest that GAME25 silencing in the green tomato fruit results in redirection of biosynthesis toward unsaturated dehydro-SGAs (see SI Appendix, Fig. S3 for detailed tomato SGA pathway).
Fig. 3.
Green and red stage fruit of GAME25-silenced tomato lines display substantially altered SGA metabolism. (A and B) Levels of (A) saturated α-tomatine– and (B) unsaturated dehydrotomatine-derived SGAs in green fruit of the GAME25-silenced tomato lines. (C and D) Levels of the typical (C) saturated SGAs (esculeoside A and derivatives) and (D) unsaturated SGAs (dehydroesculeoside A and derivatives) in GAME25-silenced red stage fruit compared with wild-type red fruit. The values represent means of three biological replicates ±SE (per genotype). Lines #2, #3, and #4 are three independent GAME25i lines. Asterisks indicate significant changes compared with wild-type samples, calculated by a Student’s t test (*P value < 0.05; **P value < 0.01; ***P value < 0.001). LC–MS was used for targeted SGA profiling.
The metabolic shift from the α-tomatine–derived saturated SGA branch to the unsaturated dehydro-SGAs pathway further continued in the GAME25i breaker fruit stage. SGA metabolites that accumulated following GAME25 silencing but not in wild-type fruit included hydroxy-dehydrotomatine (∼20- to 30-fold), acetoxy-hydroxy-dehydrotomatine (∼20- to 25-fold), as well as dehydroesculeoside A (∼20- to 25-fold) and its derivatives (SI Appendix, Figs. S8C and S9A). α-Tomatine (∼20- to 50-fold) and its downstream SGAs were almost absent in the GAME25i breaker fruit (SI Appendix, Figs. S8C and S9B). In parallel, the drastic reduction in α-tomatine levels at the green fruit stage in GAME25i lines resulted in a severe decline of esculeoside A and lycoperoside (∼20- to 25-fold) levels in the red ripe fruit stage compared with levels in wild-type green fruit (Fig. 3C). Moreover, α-tomatine–derived saturated SGAs were not detected in GAME25-silenced red ripe fruit (Fig. 3C and see SI Appendix, Fig. S3 for the detailed tomato SGA pathway). Compared with wild-type green fruit, we did observe a buildup of dehydrotomatine in GAME25i green fruit that resulted in massive accumulation of dehydroesculeoside A (∼20- to 25-fold) and its derivatives in red ripe fruit (Fig. 3D and SI Appendix, Fig. S8D). These data provide additional evidence regarding the role of GAME25 in the conversion of dehydrotomatidine to tomatidine, which we hypothesize to be a bifurcating step between the saturated (α-tomatine) and unsaturated (dehydrotomatine) SGA biosynthesis pathway.
Accumulation of Saturated α-Tomatine and Its Downstream SGAs Due to GAME25 Overexpression in the Tomato Plant.
To further examine the role of GAME25 in SGA biosynthesis, we generated transgenic tomato lines overexpressing the GAME25 gene (GAME25-Ox). GAME25 expression in leaves and fruit tissues (green and red fruit) of transgenic tomato lines was significantly higher than in wild-type tomato plants (t test, **P value < 0.01) (SI Appendix, Fig. S10A). Leaves from GAME25-Ox lines showed higher levels of α-tomatine (∼1.5-fold), α-tomatine (isomer 2) (∼1.5-fold), and acetoxytomatine (∼1.8-fold), with simultaneous reduction of dehydrotomatine (∼1.5-fold), compared with wild-type leaves (SI Appendix, Fig. S10 B and C). GAME25-Ox green tomato fruit displayed reduction in dehydrotomatine levels (∼1.5-fold), whereas no change in α-tomatine content was observed in the same tissues (SI Appendix, Fig. S10D). However, we detected increases in acetoxytomatine (∼1.9-fold) and acetoxy-hydroxytomatine (∼4- to 7-fold) (α-tomatine–derived SGAs) in comparison with wild-type green fruit (SI Appendix, Fig. S10D; see SI Appendix, Fig. S3 for the tomato SGA pathway). Analysis of red fruit from the GAME25-Ox lines showed accumulation of α-tomatine (∼4- to 6-fold) and its downstream saturated SGAs [e.g., acetoxytomatine (∼5- to 7-fold), and acetoxy-hydroxytomatine (∼2- to 3-fold)] compared with wild-type red fruit (SI Appendix, Fig. S10E). Furthermore, GAME25 overexpressing red tomato fruit did not show any change in levels of esculeoside A (acetoxy-hydroxytomatine–derived major SGA), compared with wild-type red fruit.
Tomato GAME25 Overexpression in Cultivated Eggplant (S. melongena) Results in Newly Produced Saturated SGAs and Steroidal Saponins.
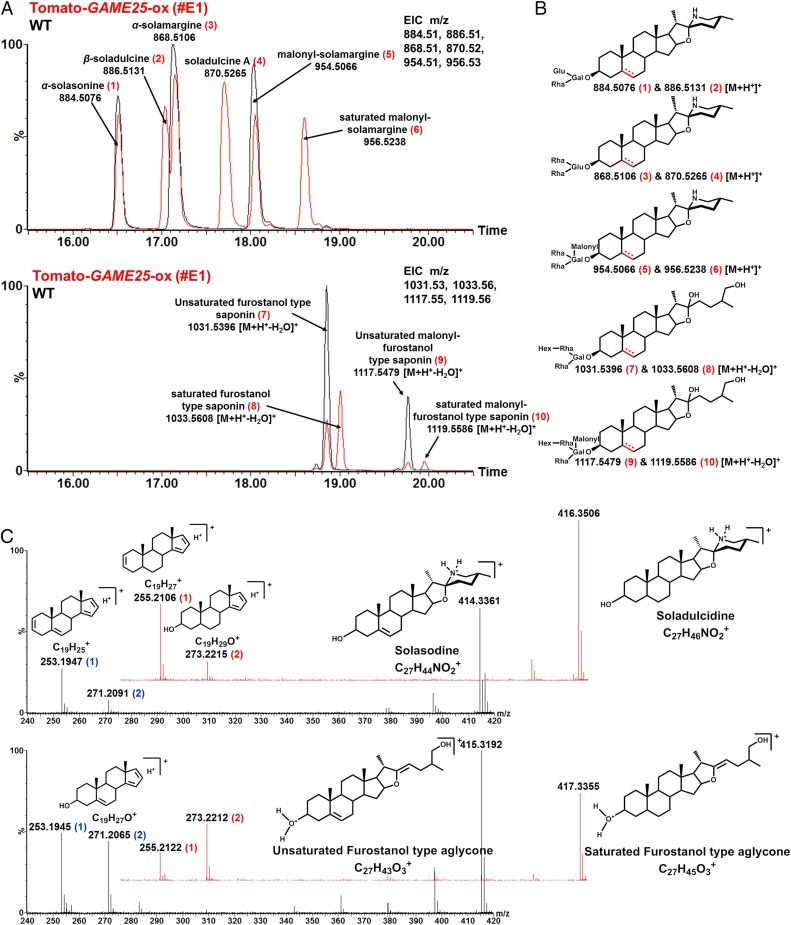
Unlike in the tomato plant, saturated SGAs are normally absent in cultivated eggplant, suggesting the absence of GAME25 activity in this species. This is further supported by the absence of a GAME25 homolog in cultivated eggplant (SI Appendix, Fig. S6). In cultivated eggplant, α-solasonine, α-solamargine, and malonyl-solamargine are the major unsaturated SGAs (with a C-5,6 double bond) (19) derived from the solasodine aglycone (Fig. 4A, Upper). Moreover, cultivated eggplant also produces unsaturated furostanol-type steroidal saponin glycosides from the unsaturated furostanol-type saponin aglycone (Fig. 4A, Lower). To investigate the impact of tomato GAME25 activity in cultivated eggplant, we generated transgenic eggplant lines overexpressing the tomato GAME25 gene (SI Appendix, Fig. S11). Specifically, we wanted to assess whether GAME25 can shift SGA metabolism from predominantly unsaturated SGAs to saturated SGAs that are not naturally present in this plant. In transgenic eggplant leaves, GAME25 overexpression resulted in reduced levels of the unsaturated SGAs, α-solasonine, α-solamargine, and malonyl-solamargine (Fig. 4A, Upper) as well as of unsaturated furostanol saponin glycosides (Fig. 4A, Lower). Conversely, we observed major accumulation of β-soladulcine, soladulcine A, and the saturated form of malonyl-solamargine (Fig. 4A, Upper). Both β-soladulcine and soladulcine A (lacking the C-5,6 double bond) are SGAs derived from saturated soladulcidine aglycone and are typically found in S. dulcamara, a wild Solanum relative (Fig. 1B). Thus, S. dulcamara likely contains an active GAME25 homolog that mediates the formation of the above-mentioned saturated SGAs. Moreover, saturated furostanol-type steroidal saponin glycosides (Fig. 4A, Lower) were detected in GAME25-overexpressing eggplant lines, which are normally undetectable in cultivated eggplant.
Fig. 4.
Overexpression of tomato GAME25 results in accumulation of new saturated SGAs and steroidal saponins in cultivated eggplant. (A) Comparison of SGA (Upper) and steroidal saponin (Lower) profile of wild-type (WT, nontransformed) and GAME25-overexpressing transgenic eggplant line #E1 (GAME25-ox). (B) Structures of detected SGAs and saponins. Chemical structures were putatively assigned by calculating elemental compositions from the accurate mass and interpretation of mass fragmentation patterns. Loss of water from steroidal saponins in positive ionization mode is typical for furostanol-type compounds. Presence or absence of a double bond at the C-5,6 position in SGAs and saponins is marked in red. (C) Comparison of mass fragmentation of steroidal SA and steroidal saponin aglycones. (Upper) Overlays of mass spectra of saturated SA aglycones (red) and unsaturated SA aglycones (black). (Lower) Overlays of mass spectra of saturated steroidal saponin aglycones (red) and unsaturated steroidal saponin aglycones (black). Characteristic fragment structures are depicted. The fragments following the loss of the side chain of SGAs or saponins were identical: m/z 253.19 and 271.21 (in blue) for unsaturated compounds and m/z 255.21 and 273.22 (in red) for saturated compounds, respectively. For simplicity, only #E1 is shown here as the representative transgenic line. EIC, extracted ion chromatogram; m/z, mass to charge; Gal, galactosyl; GAME25-ox, GAME25 overexpression (#E1); Glu, glucosyl; Hex, hexosyl; M, molecular mass; Rha, rhamnosyl; WT, wild type. Metabolite analysis was done by LC–MS. Lines #E1 and #E2 are two independent transgenic GAME25-Ox lines (SI Appendix, Fig. S11). Line #E2 showed a similar LC–MS profile as that observed for #E1.
The chemical structures of unsaturated and saturated SGAs as well as of the steroidal saponins identified here are shown in Fig. 4B. Metabolites were identified based on accurate mass-derived elemental composition and mass fragmentation pattern. Loss of C-3 sugar moieties in unsaturated SGAs leads to the formation of the fragment ion m/z 414.3 that corresponds to solasodine, an unsaturated steroidal aglycone backbone (Fig. 4C, Upper). Further loss of the E and F ring from the solasodine backbone results in the characteristic fragment ion m/z 271.2, which loses a water molecule to form fragment m/z 253.19 (Fig. 4C, Upper). Due to the absence of a C-5,6 double bond in saturated SGAs, all fragment ions showed a mass shift of plus 2 Da, i.e., m/z 416.3 (soladulcidine, saturated aglycone backbone), m/z 273.2, and m/z 255.2 after loss of the E/F rings and dehydration, respectively (Fig. 4C, Upper). Similarly, unsaturated furostanol-type steroidal saponins showed aglycone fragment ions with a mass of m/z 415.3, 271.2, and 253.19, whereas saturated furostanol-type steroidal saponins displayed m/z 417.3, 273.2, and 255.2 fragment ions after MS-fragmentation analysis (Fig. 4C, Lower).
Insect Cells Expressing GAME25 Convert Dehydrotomatidine to Tomatid-4-En-3-One.
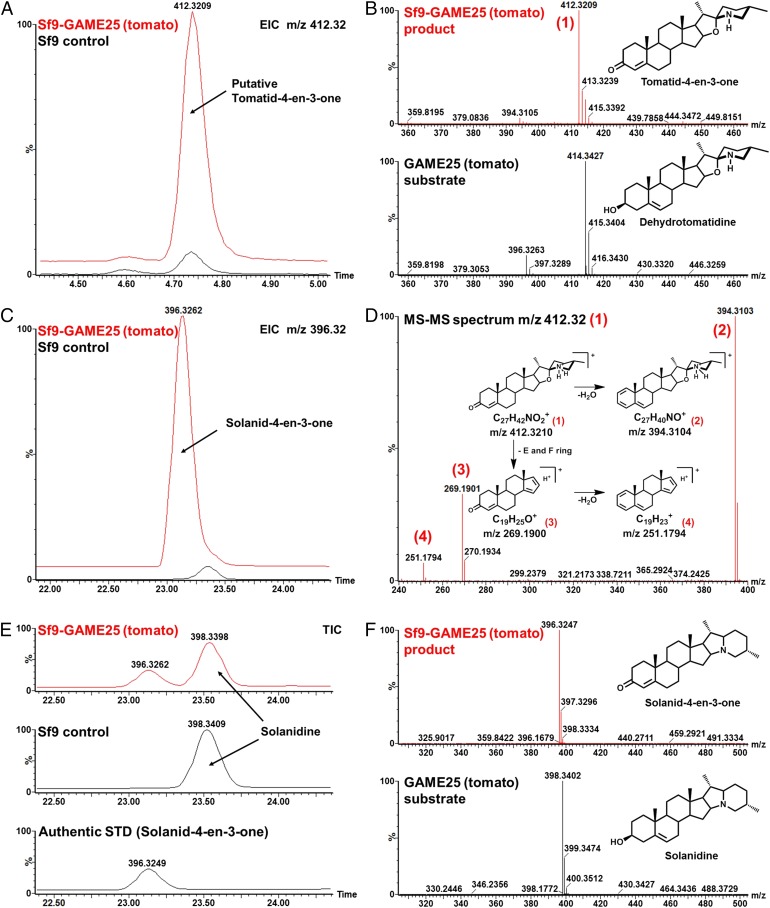
We examined the potential role of GAME25 in the conversion of dehydrotomatidine to tomatidine by expressing either the recombinant tomato or potato enzymes in Sf9 insect cells and testing microsomal fractions for their activity (SI Appendix, Fig. S12). We performed enzymatic assays in the presence of NAD+ as a cofactor and with dehydrotomatidine, solanidine, and solasodine (unsaturated SA aglycones) as substrates. Surprisingly, assays with either enzyme did not result in the formation of the expected reaction products, tomatidine, demissidine, or soladulcidine (saturated SA aglycones). However, an assay with each recombinant GAME25 enzyme (either tomato or potato) and dehydrotomatidine resulted in the formation of a compound with the mass m/z 412.3 (M + H+) (Fig. 5 A and B for tomato GAME25 assay and SI Appendix, Fig. S13 A and B for the potato GAME25 assay). MS-MS fragmentation pattern analysis of the newly formed compounds (Fig. 5 A, B, and D) showed three major fragment ions derived from two parallel fragmentation routes. Loss of the carbonyl oxygen and formation of an additional double bond led to a fragment ion with m/z 394.3 (Fig. 5D). Loss of the E and F rings of the steroidal skeleton led to the fragment ion m/z 269.2, which was then dehydrated to form fragment m/z 251.17 (Fig. 5D). The newly formed compound was putatively assigned as tomatid-4-en-3-one (Fig. 5 A and B).
Fig. 5.
Activity of recombinant tomato GAME25 produced in insect cells. (A) Overlay of extracted ion chromatograms of m/z 412.32 Da, [M + H+]+ (mass of the GAME25 reaction product), and the control reaction obtained with dehydrotomatidine as a substrate. (B) Mass spectra and structures of the detected product (Upper) and substrate (Lower) of the GAME25 enzymatic reaction with dehydrotomatidine as substrate. (C) Overlay of extracted ion chromatograms of m/z 396.32 Da, [M + H+]+ (mass of the GAME25 reaction product), and the control reaction with solanidine as substrate. (D) Mass fragmentation spectrum of the GAME25 enzymatic reaction product (with dehydrotomatidine as substrate), including the interpretation of the detected mass fragments. The fragmentation pattern corresponds to the tomatid-4-en-3-one (proposed structure of the GAME25 product). (E) Chromatograms of the GAME25 enzymatic reaction (Upper), control reaction (Middle), both with solanidine as substrate, and the solanid-4-en-3-one authentic standard (Lower). The newly formed product (at retention time 23.2 min) coeluted with the solanid-4-en-3-one commercial authentic standard. Comparison of MS-MS spectra between the newly formed product and authentic standard solanid-4-en-3-one was similar and is provided in SI Appendix, Fig. S17. Thus, this newly formed GAME25 product was assigned as solanid-4-en-3-one. (F) Mass spectra and structures of the detected product (Upper) and substrate (Lower) of the GAME25 enzymatic reaction with solanidine as substrate. Analysis of enzyme assay reactions was carried out by LC–MS. The control reaction was performed using protein extracts from nontransfected Sf9 insect cell microsomes. EIC, extracted ion chromatogram; m/z, mass to charge; STD, metabolite standard; TIC, total ion chromatogram.
Using solanidine as a substrate, the GAME25 enzyme assays (either with tomato or potato GAME25 enzymes) resulted in the formation of a new product with an apparent molecular ion of m/z 396.3 (M + H+) (Fig. 5 C, E, and F for the tomato GAME25 assay and SI Appendix, Fig. S13 C and D for the potato GAME25 assay). We identified the compound as solanid-4-en-3-one by comparing retention time and mass spectrum to the authentic, commercially available, solanid-4-en-3-one standard and MS-MS analysis (Fig. 5 C, E, and F and see SI Appendix, Fig. S17 for MS-MS analysis of solanid-4-en-3-one standard and solanid-4-en-3-one product after GAME25 assay). Either the recombinant tomato or potato GAME25 enzymes also successfully converted solasodine, the cultivated eggplant aglycone, to the putative solasod-4-en-3-one compound (SI Appendix, Fig. S13 E and F for potato GAME25 assay and SI Appendix, Fig. S14 for tomato GAME25 assay).
The tomato and potato recombinant GAME25 enzymes showed no activity on glycosylated SA substrates (i.e., α-tomatine, dehydrotomatine, α-solanine, α-chaconine, and α-solamargine). These results suggest that GAME25 catalyzes the oxidation of the 3β-hydroxyl group (3β-hydroxysteroid dehydrogenase activity) and the isomerization of the double bond from the C-5,6 to the C-4,5 position (3-oxosteroid Δ5,4 isomerase activity) in SA aglycone substrates to form the 3-oxo-Δ5,4 SA intermediates identified here (tomatid-4-en-3-one, solanid-4-en-3-one, or solasod-4-en-3-one). Thus, GAME25 possesses a previously uncharacterized 3β-hydroxysteroid dehydrogenase/Δ5,4 isomerase activity.
Recombinant Tomato GAME25 Expressed in Escherichia coli Confirms 3β-Hydroxysteroid Dehydrogenase and Δ5,4 Isomerase Activity.
The observed 3β-hydroxysteroid dehydrogenase/Δ5,4 isomerase activity of the recombinant tomato and potato GAME25 enzymes is rather uncommon as other SDR family enzymes participating in specialized metabolism typically possess only 3β-hydroxysteroid dehydrogenase activity (20–26). To confirm that the Δ5,4 isomerization observed here was a result of GAME25 activity and not due to activity of an endogenous enzyme of the insect cell microsomes, we expressed tomato GAME25 in E. coli and purified the enzyme for activity assays (SI Appendix, Fig. S15). Enzyme assay with the recombinant GAME25 enzyme using solanidine as a substrate and NAD+ as a cofactor resulted in the formation of the same solanid-4-en-3-one product that we observed in the insect cell enzyme assay (SI Appendix, Fig. S16A). We confirmed the identity of the product by comparing retention time, mass spectrum, and MS-MS fragments with an authentic solanid-4-en-3-one standard (SI Appendix, Fig. S17). Thus, this result provided substantial evidence that the recombinant GAME25 enzymes possess both 3β-hydroxysteroid dehydrogenase and Δ5,4 isomerase activities.
The Recombinant Tomato GAME25 Converts Diosgenin, a Spirostanol-Type Saponin Aglycone, to Diosgen-4-En-3-One.
Like SGAs, steroidal saponins display two structural forms, saturated or unsaturated C-5,6 (SI Appendix, Fig. S2). Our observation that new saturated furostanol-type saponins were formed as a result of GAME25 overexpression in cultivated eggplant suggested the potential role of GAME25 in elimination of the C-5,6 double bond, not only from SA substrates but also from steroidal saponins. To examine this possibility, we performed assays with the recombinant enzyme and diosgenin [(M + H+, m/z 415.3), a major spirostanol-type steroidal saponin aglycone produced by Dioscorea species]. Interestingly, GAME25 activity resulted in the formation of a novel compound with the molecular ion m/z 413.3 (M + H+), representing oxidation of the 3β-hydroxyl group and isomerization of the double bond from the C-5,6 position to the C-4,5 position (SI Appendix, Fig. S18A). While the unsaturated diosgenin substrate produced three major fragment ions with m/z 415.3, 271.2, and 253.2, the newly formed compound (m/z 413.3) showed fragment ions with m/z 413.3, 269.2, and 251.2, respectively (SI Appendix, Fig. S18B) and thus putatively was assigned as diosgen-4-en-3-one based on mass fragmentation spectra analysis (SI Appendix, Fig. S18). Our results suggest that recombinant GAME25 can catalyze the oxidation of the 3β-hydroxyl group and the isomerization of the double bond from the C-5,6 to the C-4,5 position in steroidal saponin aglycones to form the 3-oxo-Δ5,4 saponin intermediate.
The Presence of the C-5,6 Double Bond in SGAs Inhibits Fungal Growth and Pathogenicity.
α-Tomatine in green tomato tissues is known to affect the growth of pathogenic fungi, including Botrytis cinerea, Fusarium oxysporum, and Colletotrichum gloeosporioides (2). In contrast, the role of unsaturated dehydrotomatine in phytopathogenicity has not been previously examined primarily because it is typically produced in small amounts in tomato tissues. As silencing of GAME25 in the tomato plant redirected SGA metabolism toward formation of dehydro-SGAs, we examined the effects of dehydro-SGAs on fungal growth and pathogenicity. Analysis of GAME25i leaf extracts showed mycelial growth inhibition of the pathogenic fungi C. gloeosporioides and B. cinerea compared with wild-type saturated SGA-containing extracts (SI Appendix, Fig. S19 A, B, E, and F). In addition, C. gloeosporioides and B. cinerea fungal conidia germination was severely reduced upon treatment with GAME25i extracts compared with treatment with wild-type extracts (SI Appendix, Fig. S19 C, D, and G–J).
Discussion
GAME25 Is a Key Branch Point Enzyme That Determines the Diversity of SGAs Produced in Solanum Species and Modulates Their Level of Toxicity.
The presence or absence of a double bond at the C-5,6 position within the core SGA scaffold is a major source of structural diversity among SGAs produced by Solanum species. In tomato, both dehydrotomatidine and tomatidine SA aglycones are highly toxic to plant cells, and it is therefore likely that they undergo glycosylation to prevent self-toxicity (9). Studies in animal models demonstrated that SGAs lacking the C-5,6 double bond (e.g., α-tomatine) are much less toxic to animals and humans compared with those SGAs that contain this double bond (e.g., α-chaconine and α-solanine from potato) (2). These prior findings also suggest that dehydrotomatine is likely a more toxic SGA compared with α-tomatine. Although less toxic to humans and animals, α-tomatine is a highly active molecule involved in a range of host-plant resistance mechanisms in tomato plants (2, 4, 5). However, the contribution of dehydrotomatine, typically produced at lower levels in tomato, to plant resistance against pathogens remains unclear. In the present study, severe growth and conidia germination inhibition of the pathogenic fungi B. cinerea and C. gloeosporioides by extracts enriched with dehydro-derivatives (due to GAME25 silencing) suggest enhanced toxicity of these compounds compared with wild-type samples containing mainly saturated α-tomatine and related metabolites (SI Appendix, Fig. S19). Thus, in Solanum plants, α-tomatine and dehydrotomatine SGAs may act synergistically against pathogens and might have coevolved to exert a combined effect against a broad range of disease-causing pathogens. As demonstrated here, GAME25 catalyzes the first step in the conversion of dehydrotomatidine to tomatidine in which the double bond at the C-5,6 position is reduced. This reaction, of the C-5,6 double bond removal in SA aglycones, is therefore a key branch point that not only determines the diversity of SGAs produced in hundreds of Solanum species but also modulates the toxic effects of this metabolite class to the plant itself, other animals, and likely also as a plant defense against pathogens and herbivores.
Production of Tomatidine from Dehydrotomatidine in the Tomato Plant Involves a Yet-Unknown Plant SDR-Type GAME25 Enzyme Activity.
To date, the biosynthesis of dehydrotomatine and α-tomatine was hypothesized to occur through several different pathways (1, 2, 14). In one scenario, dehydrotomatidine was proposed to be derived from cholesterol (contains a C-5,6 double bond), while tomatidine was predicted to be synthesized from cholestanol (lacking the C-5,6 double bond) (1, 2). Thus, conversion of cholesterol to cholestanol was thought to be responsible for the formation of tomatidine. An alternative suggested pathway is that the cholesterol-derived teneimine intermediate (possessing a double bond) is partitioned, leading to formation of both tomatidine (through the action of a hypothetical hydrogenase that reduces the double bond) and dehydrotomatidine (1, 2, 14). In a third hypothesis, tomatidine was proposed to be partly dehydrogenated to form dehydrotomatidine by a hypothetical dehydrogenase (1, 2). Finally, the formation of tomatidine from dehydrotomatidine was hypothesized as a single-step hydrogenation reaction (2, 13, 14). In the present study, functional characterization of GAME25 provided strong evidence that the formation of tomatidine from dehydrotomatidine [i.e., the reduction of the Δ5 (C-5,6 position) bond in the SA aglycones] is likely carried out in multiple steps and that the GAME25 catalyzes the first of these. In vitro enzyme assays with the recombinant tomato and potato GAME25 enzyme demonstrated the enzyme’s dual activity, namely, oxidation of the 3β-hydroxyl group (3β-hydroxysteroid dehydrogenase activity) and isomerization of the double bond from the C-5,6 position to the C-4,5 position (3-oxosteroid Δ5,4 isomerase activity) on both SA and steroidal saponin aglycones (Fig. 5 and SI Appendix, Figs. S13, S14, and S18). These results also suggest that formation of the saturated steroidal saponin aglycone (by removal of the C-5,6 double bond) is likely not a single-step reaction, but rather that GAME25 catalyzes the first step, as we observed for SA biosynthesis.
In plants, SDR enzymes catalyze NAD(P)(H)-dependent oxidation/reduction reactions involving a wide range of primary or specialized metabolites (16–18, 20, 23–26). Members of this family have been reported to participate in the metabolism of various specialized metabolites including cardiac glycosides (i.e., cardenolides) in Digitalis spp. (3-βHSD), tropane-like alkaloids (SDR65C), terpenoids (SDR110C, SDR114C), benzylisoquinoline alkaloids in poppy (NOS), oryzalexin diterpenoids in rice (MSI and MI1-3), and phenolics (SDR108E) (20, 23–26). GAME25 is a unique plant SDR enzyme that exhibits dual activity, with the ability to oxidize the 3β-hydroxyl group (3β-hydroxysteroid dehydrogenase activity) and isomerization of the double bond from the C-5,6 to the C-4,5 position (3-oxosteroid Δ5,4 isomerase activity) in steroidal substrates. Most, if not all, 3-βHSD enzymes participating in specialized metabolism in plants (i.e., members of the 3-βHSD and SDR family) merely possess 3β-hydroxysteroid dehydrogenase activity and not Δ5,4 isomerase activity (21–25). For example, in Digitalis, the oxidation (of the 3β-hydroxyl group) and isomerization (C-5,6 to the C-4,5 position) steps, required during the conversion of pregnenolone to progesterone, are carried out successively by two separate enzymes, 3-βHSD (3β-hydroxysteroid dehydrogenase) and 3-KSI (Δ5-3-ketosteroid isomerase) (20–22). Phylogenetic analysis of SDR family proteins from plants involved in specialized metabolism suggested that the GAME25 proteins of the Solanaceae family have undergone significant diversification compared with other SDR proteins. Moreover, the dual enzyme activity of GAME25 proteins reported here suggests that it could have evolved from the classical monofunctional SDRs. Interestingly, in mammalian steroid hormone metabolism, the conversion of pregnenolone to progesterone includes a 3-βHSD enzyme that, similar to GAME25, possesses dual dehydrogenase and isomerase activities (27, 28).
The Absence of GAME25 Activity in Cultivated Potato and Eggplant Underlies the Lack of Saturated SGAs in These Plants.
The pathway from the unsaturated SA aglycone solanidine to the saturated SA aglycone demissidine and its glycosylated form (i.e., demissine) in wild potato species corresponds to the tomato pathway in which the C-5,6 double bond is eliminated from dehydrotomatidine toward tomatidine and glycosylated α-tomatine (SI Appendix, Figs. S1 and S3). The domesticated potato does not accumulate saturated demissidine or demissine SGAs. The presence of a GAME25 homolog (SI Appendix, Fig. S6) but the absence of saturated SGAs in cultivated potato tubers suggests that these SGAs were lost during the domestication process, possibly through altered GAME25 gene activity. Moreover, in vitro, the recombinant potato GAME25 enzyme shows 3β-hydroxysteroid dehydrogenase/Δ5,4 isomerase activity on diverse unsaturated steroidal metabolites (SI Appendix, Fig. S13). Therefore, even if GAME25 is considered active in potato (in vivo), additional enzymes required for the formation of saturated SA aglycone might be absent or inactive in the domesticated potato. In the case of the cultivated eggplant, it is likely that GAME25 activity is the single factor that is responsible for the lack of saturated SGA production, as overexpression of tomato GAME25 resulted in accumulation of saturated SGAs (Fig. 4). In addition to saturated SGAs, novel saturated steroidal saponins were formed in GAME25-Ox eggplant transgenic lines, underscoring GAME25’s crucial role in steroidal saponin biosynthesis in Solanum plants (Fig. 4). Thus, GAME25-like enzymes might be involved in eliminating the C-5,6 double bond from unsaturated steroidal saponin aglycone substrates in numerous plant species, including those outside the Solanaceae family.
The Pathway to Saturated Steroidal Specialized Metabolites in Solanum and Other Species.
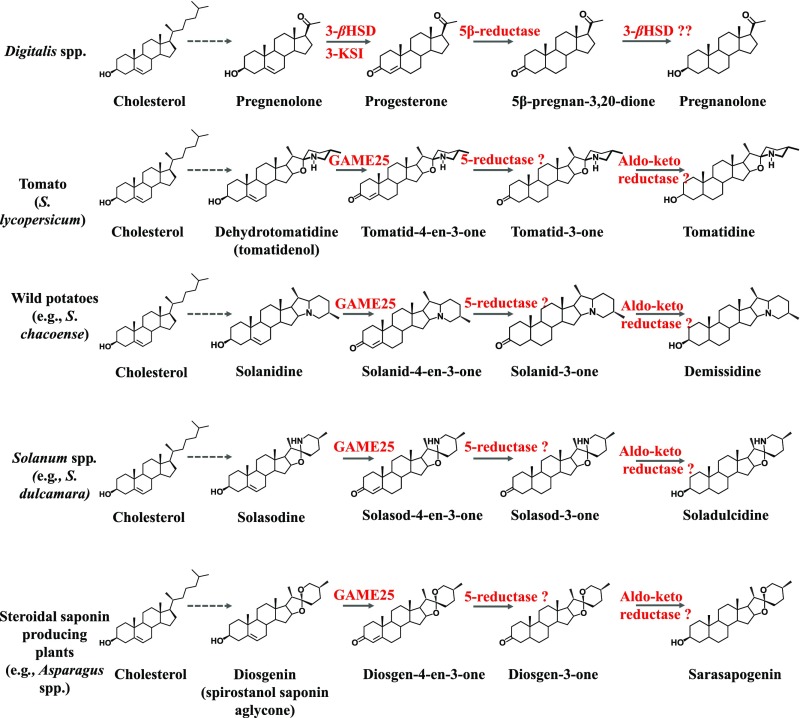
During cardenolide biosynthesis in Digitalis species, pregnenolone is converted to pregnanolone. Both pregnenolone and pregnanolone are steroid derivatives that differ only by the presence or absence of the double bond at the C-5,6 position (Fig. 6). The conversion of pregnenolone to pregnanolone (removal of C-5,6 double bond) occurs in the four following steps: (i) oxidation of (3β-hydroxyl group) pregnenolone by the 3βHSD enzyme, followed by (ii) isomerization of the double bond from the C-5,6 to the C-4,5 position by the 3-KSI enzyme to form progesterone; (iii) conversion of progesterone to 5β-pregnan-3,20-dione (removal of C-4,5 bond) by 5β-progesterone reductase (5β-POR), (iv) which is then converted to pregnanolone by a 3βHSD enzyme (Fig. 6) (20–22, 27, 28). Thus, the conversion of pregnenolone to pregnanolone resembles the formation of tomatidine from dehydrotomatidine in tomato, or demissidine from solanidine in wild potato plants, in which the C-5,6 double bond is also removed (Fig. 6). Assays assessing recombinant GAME25 activity clearly showed that GAME25 is not sufficient to catalyze the entire Δ5 reduction in unsaturated SA aglycones and that additional enzymes are required to eliminate the C-5,6 double bond and form saturated products. The additional enzymatic steps required after GAME25 activity resemble the Digitalis (iii) and (iv) reactions described above. Thus, we expected that the Digitalis 5β-POR reductase homolog in tomato (Solyc10g049620, termed here GAME35) might act downstream to the pair of reactions catalyzed by GAME25. In this case, Digitalis 5β-POR would catalyze reduction of various 3-oxo-Δ5,4 SA aglycone intermediates (GAME25 enzyme products; e.g., solanid-4-en-3-one). Enzyme assays with the purified recombinant GAME35 protein (homolog of the Digitalis 5β-POR) showed that it is not active on the solanid-4-en-3-one substrate (SI Appendix, Figs. S15 and S16B). Thus, a different reductase enzyme is likely required to carry out this second removal of C-4,5 bond reaction.
Fig. 6.
GAME25 enzymes play a key role in the formation of steroidal specialized metabolites in a proposed sequence of three reactions. A proposed three-step reaction sequence for the conversion of dehydrotomatidine to tomatidine in tomato, solanidine to demissidine in wild potatoes (e.g., S. chacoense), and solasodine to soladulcidine in certain Solanum species (e.g., S. dulcamara). Given our results, we propose a three-step reaction for the conversion of unsaturated steroidal saponin aglycone to saturated steroidal saponin aglycone. GAME25, a 3β-hydroxysteroid dehydrogenase/isomerase, performs the first step in this reaction sequence, producing 3-oxo-Δ5,4 steroidal alkaloid/saponin aglycone derivatives from the respective unsaturated steroidal alkaloid/saponin aglycone substrates, which are further converted to saturated products by successive actions of putative 5-reductases and aldo-keto reductases, respectively. This multistep conversion partly resembles steroid metabolism in species such as Digitalis spp. that produce cardiac glycosides (cardenolides). Dashed arrows indicate multistep reactions.
Based on the intermediates produced by GAME25 and the further requirement of analogous enzymatic reactions, we propose that the conversion of dehydrotomatidine to tomatidine, and likewise the conversion of other SAs, requires a three-step reaction sequence with GAME25 catalyzing the first step, converting dehydrotomatidine to tomatid-4-en-3-one (Fig. 6). Tomatid-4-en-3-one is subsequently reduced to tomatidine by the successive action of a putative 5-reductase and an aldo-keto reductase, which remain to be identified (Fig. 6). We also predict a similar three-step conversion in wild potato species producing saturated demissidine from the solanidine aglycone (Fig. 6). In addition, tomato GAME25 overexpression in cultivated eggplant generated saturated SGAs naturally produced by certain Solanum species (e.g., S. dulcamara). Based on these findings, we anticipate similar reactions in Solanum species that produce a saturated soladulcidine aglycone from solasodine (e.g., S. dulcamara; Fig. 6). Our in vivo and in vitro results support a multireaction sequence in the biosynthesis of saturated steroidal saponins (Fig. 6). Hence, evolution of structural diversity in steroidal alkaloids and saponins by elimination of the C-5,6 double bond and its underlying enzymatic base is likely conserved in a wide range of plant families that are rich in steroidal specialized metabolites.
Characterization of GAME25 activity in the SGAs biosynthesis pathway is a significant step toward resolving the entire core SGA pathway in Solanaceae species. This work further contributes to the understanding of how a large portion of structural diversity in SGAs and steroidal saponin-producing species is generated. Nevertheless, the enzymes completing the elimination of the C-5,6 double bond succeeding GAME25 still remain to be identified. The dramatic shift from saturated to unsaturated SGAs in GAME25-silenced tomato plants, including the dominance of dehydro-esculeosides in ripening fruit, make this genetic material an excellent resource for future investigation. Further genetic and biochemical analyses will enable linking the structural information on steroidal specialized metabolites to the potency of these molecules with respect to plant pathogens and herbivores. Ripe fruit accumulating α-tomatine (instead of the typical esculeosides) as a result of GAME25 overexpression will also be of value for carrying out similar interaction studies. The presence of the double bond at the C-5,6 position is not merely an issue of structural variation as ample evidence suggests its relevance in determining the level of toxicity of these molecules to humans. SGAs, primarily the unsaturated ones prevalent in potato tubers, are renowned antinutritionals, and their levels in the cultivated potato are tightly regulated. The current work has implications for commercial farming. Together with the previously reported structural and regulatory genes, overexpression of GAME25 could be a valuable strategy to reduce the levels of these substances in commercial potato varieties.
Materials and Methods
Plant Extract Preparation and Targeted Profiling of Steroidal Metabolites.
Preparation of plant extracts and the profiling of steroidal metabolites in various tomato (leaves, green fruit, breaker fruit, and red fruit) and eggplant leaf tissue were performed as described previously (9, 11). Detailed liquid chromatography–mass spectrometry (LC–MS) methods are provided in SI Appendix, SI Materials and Methods.
Protein Expression and in Vitro Enzyme Assay.
The detailed steps for tomato/potato GAME25 and tomato GAME35 protein expression and recombinant protein enzyme assay are in SI Appendix, SI Materials and Methods.
Fungal Inhibition Assay.
B. cinerea (B05.10) and C. gloeosporioides (Cg14) fungal inhibition activity of the GAME25i and wild-type methanolic extracts was determined by the disk diffusion method (9). Details of the fungal inhibition assay are in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Adelis Foundation; the Leona M. and Harry B. Helmsley Charitable Trust; the Jeanne and Joseph Nissim Foundation for Life Sciences; the Tom and Sondra Rykoff Family Foundation Research; and the Raymond Burton Plant Genome Research Fund for supporting the A.A. laboratory activity. The work also was supported by Israel Science Foundation Grant 1805/15 and European Research Council (SAMIT-FP7) personal grants (to A.A.). The research in the A.A. laboratory was supported by the European Union Seventh Framework Program FP7/2007–2013 Grant 613692–TriForC. A.A. is the incumbent of the Peter J. Cohn Professorial Chair.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1804835115/-/DCSupplemental.
References
- 1.Friedman M, McDonald G, Filadelfi-keszi M. Potato glycoalkaloids: Chemistry analysis safety and plant physiology. Crit Rev Plant Sci. 1997;16:55–132. [Google Scholar]
- 2.Friedman M. Tomato glycoalkaloids: Role in the plant and in the diet. J Agric Food Chem. 2002;50:5751–5780. doi: 10.1021/jf020560c. [DOI] [PubMed] [Google Scholar]
- 3.Friedman M. Potato glycoalkaloids and metabolites: Roles in the plant and in the diet. J Agric Food Chem. 2006;54:8655–8681. doi: 10.1021/jf061471t. [DOI] [PubMed] [Google Scholar]
- 4.Milner SE, et al. Bioactivities of glycoalkaloids and their aglycones from Solanum species. J Agric Food Chem. 2011;59:3454–3484. doi: 10.1021/jf200439q. [DOI] [PubMed] [Google Scholar]
- 5.Roddick JG. Steroidal glycoalkaloids: Nature and consequences of bioactivity. Adv Exp Med Biol. 1996;404:277–295. doi: 10.1007/978-1-4899-1367-8_25. [DOI] [PubMed] [Google Scholar]
- 6.Eich E. Solanaceae and Convolvulaceae–Specialized Metabolites: Biosynthesis Chemotaxonomy Biological and Economic Significance: A Handbook. Springer; Berlin: 2008. [Google Scholar]
- 7.Itkin M, et al. Biosynthesis of antinutritional alkaloids in solanaceous crops is mediated by clustered genes. Science. 2013;341:175–179. doi: 10.1126/science.1240230. [DOI] [PubMed] [Google Scholar]
- 8.Sonawane PD, et al. Plant cholesterol biosynthetic pathway overlaps with phytosterol metabolism. Nat Plants. 2016;3:16205. doi: 10.1038/nplants.2016.205. [DOI] [PubMed] [Google Scholar]
- 9.Itkin M, et al. GLYCOALKALOID METABOLISM1 is required for steroidal alkaloid glycosylation and prevention of phytotoxicity in tomato. Plant Cell. 2011;23:4507–4525. doi: 10.1105/tpc.111.088732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cárdenas PD, et al. The bitter side of the nightshades: Genomics drives discovery in Solanaceae steroidal alkaloid metabolism. Phytochemistry. 2015;113:24–32. doi: 10.1016/j.phytochem.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Cárdenas PD, et al. GAME9 regulates the biosynthesis of steroidal alkaloids and upstream isoprenoids in the plant mevalonate pathway. Nat Commun. 2016;7:10654. doi: 10.1038/ncomms10654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Umemoto N, et al. Two cytochrome P450 monooxygenases catalyze early hydroxylation steps in the potato steroid glycoalkaloid biosynthetic pathway. Plant Physiol. 2016;171:2458–2467. doi: 10.1104/pp.16.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Friedman M, Levin CE. Dehydrotomatine content in tomatoes. J Agric Food Chem. 1998;46:4571–4576. [Google Scholar]
- 14.Laurila J, Laakso I, Valkonen JP, Hiltunen R, Pehu E. Formation of parental-type and novel glycoalkaloids in somatic hybrids between Solanum brevidens and S. tuberosum. Plant Sci. 1996;118:145–155. [Google Scholar]
- 15.Ginzberg I, Tokuhisa JG, Veilleux RE. Potato steroidal glycoalkaloids: Biosynthesis and genetic manipulation. Potato Res. 2009;52:1–15. [Google Scholar]
- 16.Moummou H, Kallberg Y, Tonfack LB, Persson B, van der Rest B. The plant short-chain dehydrogenase (SDR) superfamily: Genome-wide inventory and diversification patterns. BMC Plant Biol. 2012;12:219. doi: 10.1186/1471-2229-12-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kavanagh KL, Jörnvall H, Persson B, Oppermann U. Medium- and short-chain dehydrogenase/reductase gene and protein families: The SDR superfamily: Functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci. 2008;65:3895–3906. doi: 10.1007/s00018-008-8588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kallberg Y, Oppermann U, Jörnvall H, Persson B. Short-chain dehydrogenases/reductases (SDRs) Eur J Biochem. 2002;269:4409–4417. doi: 10.1046/j.1432-1033.2002.03130.x. [DOI] [PubMed] [Google Scholar]
- 19.Wu SB, Meyer RS, Whitaker BD, Litt A, Kennelly EJ. A new liquid chromatography-mass spectrometry-based strategy to integrate chemistry, morphology, and evolution of eggplant (Solanum) species. J Chromatogr A. 2013;1314:154–172. doi: 10.1016/j.chroma.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Herl V, Frankenstein J, Meitinger N, Müller-Uri F, Kreis W. Δ 5-3β-hydroxysteroid dehydrogenase (3 β HSD) from Digitalis lanata. Heterologous expression and characterisation of the recombinant enzyme. Planta Med. 2007;73:704–710. doi: 10.1055/s-2007-981537. [DOI] [PubMed] [Google Scholar]
- 21.Meitinger N, Geiger D, Augusto TW, Maia de Pádua R, Kreis W. Purification of Δ(5)-3-ketosteroid isomerase from Digitalis lanata. Phytochemistry. 2015;109:6–13. doi: 10.1016/j.phytochem.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 22.Meitinger N, et al. The catalytic mechanism of the 3-ketosteroid isomerase of Digitalis lanata involves an intramolecular proton transfer and the activity is not associated with the 3β-hydroxysteroid dehydrogenase activity. Tetrahedron Lett. 2016;57:1567–1571. [Google Scholar]
- 23.Chen X, Facchini PJ. Short-chain dehydrogenase/reductase catalyzing the final step of noscapine biosynthesis is localized to laticifers in opium poppy. Plant J. 2014;77:173–184. doi: 10.1111/tpj.12379. [DOI] [PubMed] [Google Scholar]
- 24.Kitaoka N, Wu Y, Zi J, Peters RJ. Investigating inducible short-chain alcohol dehydrogenases/reductases clarifies rice oryzalexin biosynthesis. Plant J. 2016;88:271–279. doi: 10.1111/tpj.13249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ringer KL, Davis EM, Croteau R. Monoterpene metabolism. Cloning, expression, and characterization of (-)-isopiperitenol/(-)-carveol dehydrogenase of peppermint and spearmint. Plant Physiol. 2005;137:863–872. doi: 10.1104/pp.104.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okamoto S, et al. A short-chain dehydrogenase involved in terpene metabolism from Zingiber zerumbet. FEBS J. 2011;278:2892–2900. doi: 10.1111/j.1742-4658.2011.08211.x. [DOI] [PubMed] [Google Scholar]
- 27.Gavidia I, Tarrío R, Rodríguez-Trelles F, Pérez-Bermúdez P, Seitz HU. Plant progesterone 5β-reductase is not homologous to the animal enzyme. Molecular evolutionary characterization of P5betaR from Digitalis purpurea. Phytochemistry. 2007;68:853–864. doi: 10.1016/j.phytochem.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Herl V, Fischer G, Müller-Uri F, Kreis W. Molecular cloning and heterologous expression of progesterone 5β-reductase from Digitalis lanata Ehrh. Phytochemistry. 2006;67:225–231. doi: 10.1016/j.phytochem.2005.11.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.