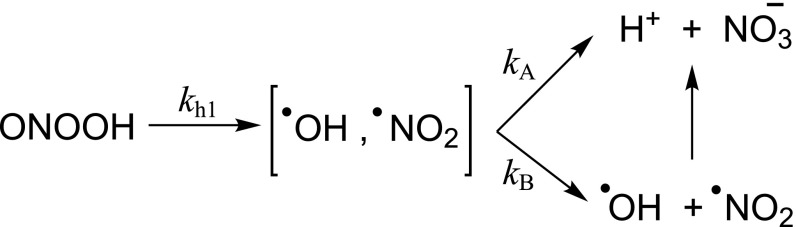Significance
Aerobic life in humans imposes the hazard of excess oxidation in cell and tissue components that may compromise cell function and viability. The formation and accumulation of oxidized products in biomolecules such as proteins and lipids are observed in various pathologies and during the normal aging process. This review article aims to integrate some early and remarkable discoveries in the field, with more recent developments that helped to define a causative role of oxygen radicals, nitric oxide, and peroxynitrite in human physiology and pathology. These aspects of human redox biochemistry contribute to the understanding of the molecular basis of diseases and aging and open avenues for the development of preventive and therapeutic strategies in molecular medicine.
Keywords: free radicals, nitric oxide, peroxynitrite, oxidation, protein tyrosine nitration
Abstract
Oxygen-derived free radicals and related oxidants are ubiquitous and short-lived intermediates formed in aerobic organisms throughout life. These reactive species participate in redox reactions leading to oxidative modifications in biomolecules, among which proteins and lipids are preferential targets. Despite a broad array of enzymatic and nonenzymatic antioxidant systems in mammalian cells and microbes, excess oxidant formation causes accumulation of new products that may compromise cell function and structure leading to cell degeneration and death. Oxidative events are associated with pathological conditions and the process of normal aging. Notably, physiological levels of oxidants also modulate cellular functions via homeostatic redox-sensitive cell signaling cascades. On the other hand, nitric oxide (•NO), a free radical and weak oxidant, represents a master physiological regulator via reversible interactions with heme proteins. The bioavailability and actions of •NO are modulated by its fast reaction with superoxide radical (), which yields an unusual and reactive peroxide, peroxynitrite, representing the merging of the oxygen radicals and •NO pathways. In this Inaugural Article, I summarize early and remarkable developments in free radical biochemistry and the later evolution of the field toward molecular medicine; this transition includes our contributions disclosing the relationship of •NO with redox intermediates and metabolism. The biochemical characterization, identification, and quantitation of peroxynitrite and its role in disease processes have concentrated much of our attention. Being a mediator of protein oxidation and nitration, lipid peroxidation, mitochondrial dysfunction, and cell death, peroxynitrite represents both a pathophysiologically relevant endogenous cytotoxin and a cytotoxic effector against invading pathogens.
The toxicity of endogenously formed oxygen radicals in biological systems was initially substantiated in studies of “oxygen poisoning” parallel to the discovery of the mechanisms of radiation-induced injury (1). (A free radical is a molecule containing an unpaired electron in its external molecular orbital; typically, radicals are reactive and short-lived intermediates.) Indeed, oxygen radicals arising from the radiolysis of water such as hydroxyl radicals (•OH) and superoxide radicals () and, later, peroxyl radicals (ROO•) were identified as key mediators of the actions of ionizing radiation (2). In the 1950s, an increasing body of evidence pointed to the biological formation of oxygen radicals associated with cell redox metabolism starting with the monovalent reduction of molecular oxygen by electron transfer proteins or endogenous reductants to yield :
| [1] |
Oxygen Radicals: From Radiation Chemistry to Metabolism
Seminal works such as refs. 1 and 2 opened the hypothesis that the rate of formation in tissues would increase as a function of oxygen concentration as follows:
| [2] |
where R• represents electron donors present in different cellular and extracellular compartments.
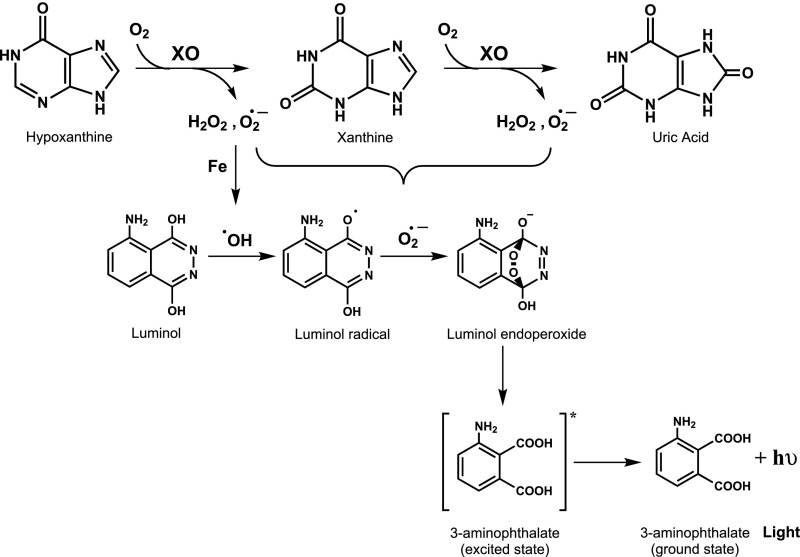
Although these early proposals seemed reasonable, the actual demonstration of the biological formation of oxygen radicals, and in particular , was “reached gradually and as the result of travelling a tortuous path,” as indicated by Irwin Fridovich (3). Indeed, the idea of a continuous and endogenous formation of unstable and potentially toxic intermediates derived from oxidative metabolism was initially difficult to accept. Much of the early mechanistic work on the biochemistry of formation had to do with understanding the enzymology of an enzyme, xanthine oxidase (XO), that participates in the last step of purine catabolism in mammals; XO catalyzes the oxidation of hypoxanthine and xanthine to uric acid utilizing molecular oxygen as cosubstrate (Fig. 1, Upper part).
Fig. 1.
Xanthine oxidase-catalyzed purine oxidation, oxygen radical formation, and luminol chemiluminescence.
Indeed, circumstantial evidence of generation during the aerobic oxidation of xanthine by XO was obtained in experiments in which added sulfite () was oxidized (4). Sulfite oxidation proceeded through a free radical process initiated by XO-derived to yield the sulfur trioxy radical, which, in turn, reacts with molecular oxygen evolving to peroxymonosulfate radical anion (5, 6):
| [3] |
| [4] |
is a strong one-electron oxidant and serves to propagate the free radical chain reaction (5). The additional oxygen consumption secondary to sulfite oxidation in the XO-catalyzed reactions was utilized for the development of an ultrasensitive enzyme assay (4) and also to reveal free radical formation from other enzymatic reactions (7).
Some years later, it was also demonstrated that XO was capable of reducing ferric cytochrome c (cyt c3+) by an -dependent process (reviewed in ref. 3):
| [5] |
(As deducted from Eqs. 3 and 5, can behave both as a one-electron oxidant and reductant.) The biochemical characterization of the XO-dependent reduction of cytochrome c constituted the foundation for the discovery of the catabolizing enzyme, Cu/Zn-containing superoxide dismutase (Cu/Zn-SOD), which competed with the cytochrome c reduction process (8) by the following reaction:
| [6] |
The discovery of an enzyme capable of the enzymatic elimination of at rates approaching the diffusion-controlled limit (k = 2 × 109 M−1⋅s−1) represented a fundamental step to further establish the idea of the endogenous formation of in cells and the need to keep its steady-state levels low, to minimize toxicity due to uncontrolled oxidation reactions on biological targets. [The action of SODs has to be complemented with an array of enzymatic H2O2-detoxifying systems (peroxiredoxins, glutathione peroxide, catalase); otherwise, H2O2 can also promote oxidations, in particular in the presence of transition metal centers that evolve it to •OH via the Fenton reaction.]
Through an extensive analysis of XO enzymology, it was established that molecular oxygen can be reduced either monovalently or divalently, simultaneously yielding and H2O2 (Fig. 1). The XO-dependent formation of reactive species was many years later associated with the ischemia reperfusion syndrome and other disease conditions (revisited in ref. 9). It is important to note that is neither a strong oxidant nor a strong reductant, and thus it has a moderate and selective reactivity with biomolecules compared with other biologically relevant oxidants and radicals (10). Overall, its toxicity mainly relies in the combination of direct reactions with a short number of key biotargets (11) and the formation of secondary reactive species such as peroxynitrite (see below; refs. 12–14).
Xanthine Oxidase and Chemiluminescence: Early Work on Free Radicals
At about the same period that Fridovich and Handler at Duke University were characterizing the formation of free radicals generated during the aerobic oxidation of xanthine by XO (4, 15), related experiments were carried out at the Facultad de Medicina, Universidad de la República in Montevideo, Uruguay, under the leadership of the American biochemist John R. Totter (1914–2001), who arrived at the Department of Biochemistry as a visiting professor under the auspices of the Rockefeller Foundation during the period of 1958–1960; Prof. Totter was an experienced investigator in chemiluminescence and XO enzymology and had previously been a scientist of the Oak Ridge National Laboratory (in Tennessee) and the Atomic Energy Commission (in Washington, DC). (Historical information about John R. Totter can be found at ethw.org/John_Totter and https://ehss.energy.gov/ohre/roadmap/histories/0481/0481toc.html#0481_part.) The arrival of Dr. Totter in Montevideo resulted in the vigorous incorporation of instruments and modern biochemical research in our department, and he initiated studies in the area of free radical and redox biochemistry. Indeed, Totter and a young group of collaborators focused on biochemical studies to characterize the formation of reactive oxygen species generated during the catalytic action of XO through the utilization of chemiluminescence probes, namely luminol (5-amino-2,3-dihydrophthalazine-1,4-dione) and lucigenin [10-methyl-9-(10-methylacridin-10-ium-9-yl)acridin-10-ium dinitrate]. In landmark papers (16, 17), it was shown that luminol and lucigenin chemiexcitation connected to the formation of XO-derived species (Fig. 1); while the exact chemical nature of the reactive species was not precisely disclosed, the works suggested the participation of oxygen-derived radicals, including •OH and . With time, chemiluminescence probes have been extensively used in vitro and in vivo to reveal oxidant formation in biological systems, although caveats regarding their specificity and selectivity must be closely taken into consideration. Luminol chemiexcitation by oxygen radicals involves the formation of a transient electronically excited species (i.e., excited aminophatalate) that decays to the ground state with the emission of light (Fig. 1, Lower part) (18).
Importantly, free radical processes in biological systems also yield fractional amounts of electronically excited states, such as singlet molecular oxygen and triplet carbonyls (19); the excited states promote chemical modifications in target molecules and also generate low-level chemiluminescence (20, 21); this last phenomenon has been used to unravel free radical activity in vivo.
After Prof. Totter returned to the United States, one his former associates Eugenio Prodanov continued the characterization of XO enzymology and chemiexcitation processes, and the studies were initially published in Anales de la Facultad de Medicina (22, 23); this prosperous research period finished at about the time of a new publication (24), due to social and political instability in the country, which also caused the exile of Prof. Prodanov to France, which lasted until the mid-1980s. In late 1985, Prodanov, recently returned to the university, invited me (at the time an instructor in the Department of Biochemistry, and an MD and future PhD student) to join him to reinitiate the “Montevideo free radical group,” and I did; after a few years of rebuilding the laboratory and setting up experiments, we accomplished the first international publication of the “new period” (25) with the characterization of the modulatory actions of SOD and cytochrome c on XO-induced chemiluminescence, a quite important milestone to us. This work (25) was among the first to reveal that cytochrome c could elicit a peroxidase-like activity in the presence of H2O2, a process later revealed to have profound consequences in mitochondria and cell redox biology (26). The mechanisms of luminescent probes chemiexcitation continued to be a focus of the group for several years and was later integrated in studies on peroxynitrite-induced chemiluminescence (27).
Intracellular Sources of Superoxide and Oxidative Stress
Seminal work in the 1970s led to the characterization of key intracellular and extracellular sources of , namely the mitochondrial electron transport chain (28, 29) and membrane-bound NADPH oxidase (reviewed in ref. 30), respectively. Mitochondrial formation of [and consequently H2O2, due to enzymatic dismutation by the mitochondrial Mn-containing SOD (11)] is a continuous process due to “electron leakage” to molecular oxygen from the electron transport chain complexes I and III and a series of dehydrogenases (reviewed in refs. 31 and 32). Indeed, most of molecular oxygen in mitochondria (in my view, >99.5%) is reduced by four electrons to water at the terminal oxidase of the respiratory chain, cytochrome aa3. A conservative estimate is that, under physiological conditions, <0.2% of total oxygen consumed by mitochondria is reduced monovalently to . However, as mitochondrial oxygen consumption rates are large, fluxes can be significant [e.g., in the order of 0.5 μM/s in vascular endothelial cells (33)]. Moreover, mitochondrial fluxes of enhance several folds under pathologically relevant conditions, hyperoxia and in the process of aging (33–35). Moderately increased levels of mitochondrial may serve some signaling actions due to the mitochondrial emission of H2O2 (36); however, large fluxes of and H2O2 lead to oxidation of mitochondrial components and cause mitochondrial dysfunction and even signaling of apoptotic cell death (37). Two significant oxidative events triggered by augmented mitochondrial are (i) the inactivation of mitochondrial aconitase, the oxidant-sensitive enzyme of the Krebs cycle, through disruption of its iron–sulfur cluster (15, 38); and (ii) the induction of mitochondrial lipid peroxidation secondary to the reactions of H2O2 with cytochrome c (26, 39), which in turn lead to the cytosolic release of proapoptotic factors. Interestingly, the reaction of mitochondrial with the 4Fe–4S of aconitase (Eq. 7) not only impacts in the metabolic flux of the Krebs cycle but also leads to Fe release that subsequently amplifies oxidative events via Fenton-like reactions (Eq. 8) (40):
| [7] |
| [8] |
While Eq. 8 indicates the formation of hydroxyl radical (•OH), the most powerful oxidizing intermediate reported in biological systems, the actual nature of the proximal oxidant(s) varies depending on the nature of the low–molecular-weight Fe chelator (e.g., organic acids, ATP); indeed, Fe4+=O complexes are formed in variable ratios with “authentic” (•OH) (41).
Another key source of was discovered in immune system cells in the 1970s, mainly in neutrophils and macrophages, the enzyme NADPH oxidase. This is a multicomponent enzyme complex that is assembled and activated in the plasma membrane of phagocytic cells (reviewed in ref. 30). While normally in a resting state, upon appropriate stimulation such as phagocytosis of invading pathogens (bacteria, intracellular parasites), a signaling process permits the translocation of cytosolic components of the enzyme toward the membrane and assembles the active form of the enzyme which triggers production toward the extracellular milieu in a process originally described as the “oxidative burst” (Eq. 9):
| [9] |
In this process, is formed upon the monovalent reduction of molecular oxygen at the expense of reducing equivalents of NADPH; large levels of for relatively short periods of time (15–30 min for neutrophils, 60–90 min for macrophages) and mainly directed to the oxidative killing of invading pathogens. More recently, it has been evidenced that many other cell types contain a similar enzymatic activity, now known as the family of the NOX enzymes (42), but in this case the levels of formed are substantially less and participate in signaling actions in physiological processes (e.g., in vascular endothelial cells NOX activity participates in the modulation of blood pressure).
As new sources of were progressively identified, it became evident that under a wide array of pathophysiologically relevant conditions, and secondary oxidants participate in the disruption of redox balance and promote oxidation reactions that may lead to cellular toxicity. A cutting-edge review in 1982 by Freeman and Crapo (43) elaborated on the possible intracellular and extracellular sources of free radicals and oxidants and their modifying actions on protein, lipid, sugar, and DNA, underscoring the connection oxidative damage and the biology of disease. In 1986, Helmut Sies brought forward the concept of “oxidative stress” as an imbalance between oxidant and antioxidant systems in favor of the latter, a central concept in the area that was refined more recently (reviewed in ref. 31). The updated definition takes into consideration the emergence of additional oxidizing intermediates identified in the late 1980s and early 1990s such as nitric oxide (•NO) and peroxynitrite and the recognition of the role that redox intermediates can play in cell signaling, expanding the breadth of the actions of oxidants from pathology to physiology. While redox signaling involves reversible oxidative modifications (e.g., such thiol oxidation in peroxiredoxins), oxidative damage typically relates to less reversible or irreversible ones (e.g., protein tyrosine nitration).
Endothelial-Derived Vascular Relaxation Factor Is Nitric Oxide: Early Data on Interactions with Superoxide Radicals
In the mid-1980s, the chemical identity of the endothelial-derived vascular relaxation factor (originally described as EDRF by Furchgott, reviewed in ref. 44) was revealed as •NO, a free radical, by Moncada and coworkers (45) and Ignarro et al. (46). Nitric oxide, being a small, neutral, and hydrophobic molecule, is capable of permeating cell membranes and moves through cell compartments to exert paracrine functions in the vascular wall. The diffusion of •NO across tissues is limited by its fast reaction with oxyhemoglobin, and therefore the actions of •NO are mainly local within a tissue; the estimated biological half-life and diffusion distances are on the order of 1–10 s and 50–1,000 μm, respectively. Soon after the characterization of the basic biological actions of •NO in the vasculature, evidence indicated that •NO could be a physiological mediator in other tissues and organs, and its role in neurotransmission and cellular immune responses was appreciated. Most of the biological formation of •NO relies on a tightly controlled enzymatic reaction catalyzed by nitric oxide synthases (NOS), which are present in three isoforms [endothelial NOS (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS)] (47).
Among the different pieces of evidence that characterized the identity of EDRF, it was reported that its stability was shortened by and, conversely, that SOD increased its biological half-life (48–50). These early observations made from a physiological perspective were, without much notice, the basis for the genesis of new concepts in the area of redox and free radical biology and medicine. Indeed, the radical nature of •NO was, at first glance, of little significance for the physiological community. In the late 1980s and early 1990s, a merging of •NO and redox physiology and biochemistry occurred, providing biological context to the observation that •NO and could react with each other at diffusion controlled rates (51). This reaction of •NO with was initially conceived by the physiological community as a way to “regulate” its biological half-life: perceived as an “oxidative inactivation” of •NO, the reaction would simply lead to unreactive products, mainly nitrate, .
Peroxynitrite: A “Stealthy” Biological Oxidant
The discovery of peroxynitrite as a biological oxidant was seeded by combined data from the physiological (48–50) and chemical (reviewed in ref. 52) literature.
First, can react with •NO in aqueous solution to yield peroxynitrite anion (51):
| [10] |
Peroxynitrite anion is in equilibrium with peroxynitrous acid (ONOOH): the pKa is 6.8 at 37 °C (13). Thus, under physiologically relevant pH conditions, both species coexist, a relevant consideration because ONOO− and ONOOH react differently (52). In addition, ONOOH is unstable in aqueous solution and in the absence of molecular targets isomerizes to nitrate (); importantly, an early report proposed that the isomerization of ONOOH involved the intermediacy of •OH and nitrogen dioxide (•NO2) radicals (53), consistent with the ONOOH-dependent nitration of phenolic compounds (54).
The observations regarding the interplay of •NO with and the formation of ONOO−, were integrated into a new biochemical hypothesis of biological oxidative damage and disruption of redox control in the early 1990s with the seminal observations made by Beckman et al. (12), followed by Radi et al. (13, 55) and Ischiropoulos et al. (56, 57), all based at the University of Alabama at Birmingham.
Peroxynitrite was initially proposed as a biologically relevant cytotoxic intermediate by Beckman et al. (12). This landmark paper explored the chemical reactivity of peroxynitrite in the presence of •OH scavengers, and as such attributed the potential toxicity of peroxynitrite to the formation of •OH or •OH-“like” species. The formation of the putative •OH product was due to the proton-catalyzed homolysis of peroxynitrite; the formation of •OH from ONOOH is estimated to occur at ≤30% yields (52), with the rest directly isomerizing to nitrate (Scheme 1).
Scheme 1.
Proton-catalyzed decay of peroxynitrite.
ONOOH decays with an apparent rate constant of 1.13 s−1 at pH 7.4 and 37 °C in phosphate buffer (t1/2 = 0.6 s). Soon after the report by Beckman et al. (12), the bimolecular reaction of peroxynitrite with thiols was discovered by Radi et al. (13), a process that resulted to be significantly faster than peroxynitrite homolysis. Indeed, using stopped-flow spectrophotometry, it was found that the reaction of peroxynitrite with cysteine and the single thiol group (Cys-34) of BSA had apparent second-order rate constant values of 4,500 and 2,600 M−1⋅s−1, respectively, at pH 7.4 and 37 °C. For example, assuming an intracellular thiol concentration of 5 mM and k2 on the order of 1,350 M−1⋅s−1 [e.g., for glutathione (GSH) (58)] a pseudo–first-order rate constant of 6.75 s−1 is calculated, a larger value than that of proton-catalyzed decay of peroxynitrite. Moreover, the reported reactions in ref. 13 were three orders of magnitude faster than the reaction of the same tested thiols with H2O2. Mechanistic studies on the direct reaction of peroxynitrite with thiols (52) indicate that the thiols are oxidized by two-electron oxidation to yield the corresponding sulfenic acid:
| [11] |
The studies reported in ref. 13 paved the way for an extensive characterization of the reactions and rate constants of peroxynitrite with a large variety of biomolecules and synthetic compounds and helped to establish the preferential fates of peroxynitrite in biological systems (reviewed in ref. 52). Overall, the kinetic assessment of peroxynitrite reactions leads to the conclusion that the formation of •OH in biological systems is a quantitatively marginal process. Still, peroxynitrite-derived •OH and •NO2 may play roles in biomembrane and lipoprotein oxidation, hydrophobic biostructures where the levels of direct reactants with peroxynitrite are scarce and one-electron oxidations propagate by chain reactions involving lipid-derived radicals (59).
Peroxynitrite was also shown to promote the nitration of tyrosine residues in proteins in a process catalyzed by transition metal centers. Indeed, Ischiropoulos et al. (57) found that peroxynitrite led to the nitration of SODs; the hydrophilic tyrosine analog [p-hydroxyphenylacetic acid (p-HPA)] was utilized as a “ nitration probe” to demonstrate the formation of peroxynitrite by macrophages as a result of the cogeneration of •NO and (56). Thus, peroxynitrite started to be visualized as a cytotoxic effector molecule against invading organisms such as bacteria and unicellular parasites that could be phagocytized by macrophages or neutrophils (60–64). These initial works prompted an exploration of the role of peroxynitrite in a variety of inflammatory (65), cardiovascular (66, 67), and neurodegenerative (68) conditions, among other various diseases (reviewed in ref. 52). A thorough view of the contributory role of peroxynitrite to pathology has been reported elsewhere over the last decade (14, 52, 69, 70).
Recent work has also confirmed the formation of heme-peroxynitrite complexes during the interactions of heme compounds with •NO and O2 (71). A notable and biological example of this type of structure involves the oxidation of •NO to by oxyhemoglobin according to the following reaction (Eq. 12):
| [12] |
The intermediate [Hb3+ONOO−] complex readily isomerizes approximately quantitatively to by an “in-cage” recombination of •NO2 with an oxo-ferryl (Fe4+=O) intermediate (72). However, the efficiency of isomerization seems not to be 100%: very small amounts of •NO2 leak out of the cage (71, 73) and a minor percentage of oxy-Hb becomes nitrated (<1% yield) in the presence of •NO. This marginal but continuous “radical leakage” from the [Hb3+ONOO−] complex (a process that may extend to other transition metal-containing centers) likely contributes to hemoglobin tyrosine nitration in normal blood cells (at α-Tyr24, α-Tyr42, β-Tyr130). Peroxynitrite itself can cross the erythrocyte membranes by anion channels (74) and react with oxyHb to mostly isomerize to ; the reaction also yields ∼10% of oxo-ferryl intermediates, •NO2, and protein radicals (75), which can also contribute to hemoglobin nitration. Oxidative modifications of hemoglobin in vivo including tyrosine nitration are greatly enhanced in smokers and type 2 diabetes patients (76, 77), conditions that disrupt vascular •NO and redox metabolism.
Carbon Dioxide Modulation of Redox Biochemistry: The Carbonate Radicals
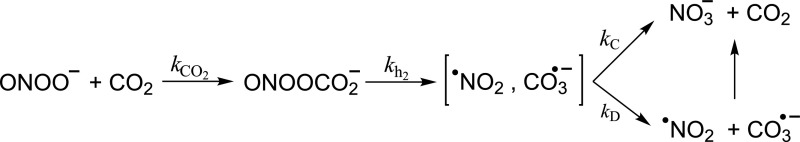
An important observation regarding the biological chemistry of peroxynitrite was made during experiments I carried out to characterize its reactivity with luminol (27). The original idea behind these experiments, based on my earlier experience in chemiluminescence (25, 78), was to find out whether luminol chemiluminescence could be used to reveal peroxynitrite formation in biochemical and cellular systems. Indeed, peroxynitrite induced a potent chemiluminescence response and, notably, in a way enhanced many fold in carbonated buffers (27). The striking observation was rationalized considering that bicarbonate/carbon dioxide () participated in the formation of an intermediate (nitroso-peroxo-carboxylate) that could subsequently promote the first one-electron oxidation step of luminol in the process of chemiexcitation.
Although the details of the reaction chemistry of peroxynitrite with were not fully solved in the first contribution (27), a series of subsequent observations by different groups (reviewed in ref. 52) revealed the actual reactants and products (Scheme 2).
Scheme 2.
Peroxynitrite reaction with carbon dioxide and formation of secondary radicals.
Indeed, a fast reaction of ONOO− with CO2 leads to a transient adduct () that then undergoes homolysis to carbonate radicals () and •NO2 in ∼35% yields (52). The pH-independent rate constant (kCO2) of the reaction is 5.8 × 104 M−1⋅s−1 at pH 7.4 and 37 °C. Considering that CO2 is in equilibrium with and that in biological systems CO2 is usually present in 1–2 mM concentration, it became evident that CO2 constitutes a major reactant for peroxynitrite (79). These works underscored the role that CO2, previously assumed to be a relatively inert molecule in redox biology, could play in the modulation of peroxynitrite-mediated reactions biologically. is a good one-electron oxidant and, among other reactions, promotes the one-electron oxidation of tyrosine to tyrosyl radical, a key step for tyrosine nitration (see below). The final evidence demonstrating the formation of from the reaction of peroxynitrite in carbonated media came from the first direct “visualization” of this radical species in aqueous solution by direct electron paramagnetic resonance studies in experiments performed in collaboration with Ohara Augusto and coworkers (80) at the Universidade de Sao Paulo, Brazil. More recent works support the idea that CO2 can also modulate H2O2 biochemistry (81, 82).
Protein 3-Nitrotyrosine: Footprint and Mediator
Reactive species directly derived from the reduction of molecular oxygen such as , H2O2, and •OH lead to a variety of oxidative modifications in biomolecules. Indeed, due to the transient lifetime and low steady-state concentrations of these species in biological systems, their formation is many times inferred from more stable oxidation products found (e.g., protein carbonyls, F2-isoprostanes, 8-oxo-guanine, for protein, lipid, and DNA oxidation, respectively, among others). During the discovery process that resulted in the recognition of peroxynitrite as a biologically relevant oxidant, work by Ischiropoulos et al. revealed protein 3-nitrotyrosine as characteristic oxidative modification that could potentially serve as a “peroxynitrite footprint” (56, 57) [the biological half-life of peroxynitrite in cells and tissues is estimated to be ∼10–20 ms (79)]. Indeed, peroxynitrite promotes the nitration (i.e., substitution of a hydrogen by a –NO2 group) of tyrosine residues to 3-nitrotyrosine (52). The first tested protein(s) nitrated by addition of peroxynitrite were SODs (57) and the first characterized nitrated protein by X-ray crystallography was the bovine CuZn-SOD (83); during the nitration process of the protein, the observable color of the solution changed to yellow, due to the characteristic absorption of 3-nitrotyrosine in the 420-nm region at pH ≥7.4.
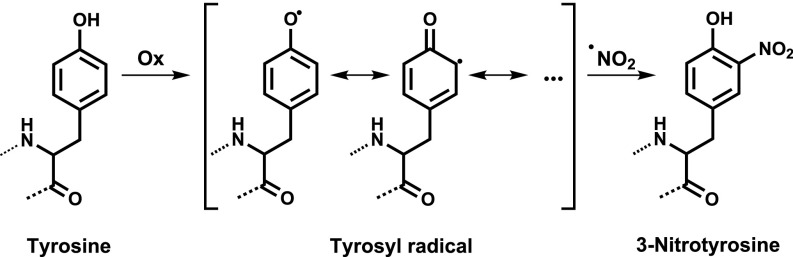
I vividly remember a day in 1991 when Joe S. Beckman came to my area of the laboratory at the University of Alabama at Birmingham with a test tube containing a yellowish solution of peroxynitrite-treated CuZn-SOD, which was immediately followed by an enthusiastic conversation on the possible nature of the protein color change. The nitration of tyrosine residues causes a large change in the pKa of the phenolic hydroxyl group in tyrosine of ≥3 pH units from ∼10.5 to 7–7.5, and this change implies that in the physiological pH region, a significant fraction of 3-nitrotyrosine is deprotonated at the –OH group, and therefore the negatively charged nitrophenolate species is responsible for the yellow color. Protein tyrosine nitration was initially conceived as a specific footprint of peroxynitrite, but it was later found that tyrosine residues could be also nitrated in vivo via peroxynitrite-independent mechanisms, which include the participation of hemeperoxidases such as myeloperoxidase (MPO) and eosinophil peroxidase (EPO) (84). Indeed, one key alternative pathway for protein tyrosine nitration in vivo under acute inflammatory conditions relies on the reactions catalyzed by MPO released by activated neutrophils utilizing as substrates H2O2 and (85). Irrespective of the nature of the nitrating system, the overall mechanism of protein tyrosine nitration in vivo relies on free radical chemistry. Peroxynitrite does not react directly with tyrosine (86), but rather peroxynitrite-derived radicals and other one-electron oxidants (Ox) lead to the intermediate formation of tyrosyl radical (Tyr•), which in turn reacts at near diffusion-controlled rates with •NO2 to yield the stable final product, 3-nitrotyrosine (87) (Fig. 2).
Fig. 2.
Free radicals and the process of tyrosine nitration.
A review of the biologically relevant mechanisms of tyrosine nitration has been presented recently (52). Overall, the presence of protein 3-nitrotyrosine reveals the formation and reactions of •NO-derived oxidants, and the relative relevance of peroxynitrite vs. other nitrating systems can be disclosed experimentally (87). Wisely, the nitration of the probe p-HPA and quantitation of nitro-p-HPA in the extracellular milieu of activated macrophages provided the first estimations of feasible biological fluxes of peroxynitrite (56). The development of antibodies that recognize protein 3-nitrotyrosine (67, 88) was crucial to suggest peroxynitrite formation under pathologically relevant conditions such as atherogenesis, inflammation, sepsis, and neurodegeneration. Later, bioanalytical determination of protein 3-nitrotyrosine in human plasma and tissues allowed a more quantitative view of the role of 3-nitrotyrosine as a disease biomarker and predictor (reviewed in ref. 89). In addition, a large number of studies analyzing the formation of protein 3-nitrotyrosine in preclinical models of disease and the data accumulated in human tissues and fluids have confirmed the role of peroxynitrite in human pathology (52, 69, 70, 90).
The nitration of tyrosine residues in proteins was studied long before any of its relevance in vivo was known. Indeed, seminal studies in the 1960s utilized synthetic nitrating agents such as tetranitromethane (TNM) to explore how chemical modifications in tyrosine residues could impact protein function (91). Despite the fact that the proximal mechanisms of nitration and the selectivity of nitrated tyrosine residues usually differ when comparing nitrating agents such as TNM and peroxynitrite (52), a large amount of the early information turned out to be useful to unravel the role of tyrosine nitration in protein structure and function. Moreover, more recent evidence indicated the protein tyrosine nitration could occur in the gastric lumen by the action of nitrite under the acidic pH conditions of the stomach [e.g., pepsin (92)], indicating that some of the early chemistry of tyrosine nitration may apply to some biologically relevant conditions. The incorporation of a nitro group in tyrosine can cause relevant changes in protein structure and function, depending on the role and location of the tyrosine residue. The nitration of tyrosine usually generates an additional negative charge to the protein and adds a relatively bulky substituent to the protein, which may affect local charge distribution and/or conformation (52). Several examples of loss and gain of protein function by nitration have been established. Moreover, protein tyrosine nitration can elicit autoimmune responses and affect tyrosine phosphorylation cascades and protein turnover (52).
Nitric Oxide Termination of Lipid Peroxidation and Lipid-Derived Radicals in Tyrosine Nitration
The reaction of •NO with typically leads to pro-oxidant events with the intermediacy of peroxynitrite (55), including lipid peroxidation of polyunsaturated fatty acids present in biomembranes and lipoproteins (93). However, •NO can also terminate free radical processes and stop radical chain propagation reactions. The first example of such process was provided with the observation that lipid peroxidation can be inhibited by excess •NO and yield a variety of nitroso- and nitro-fatty acid-derived products (94). Indeed, the oxidation of unsaturated fatty acids in biomembranes and lipoproteins is initiated by a one-electron oxidation leading to the formation of a lipid alkyl radical (L•), which rapidly reacts with molecular oxygen to yield the corresponding lipid peroxyl radical (LOO•), the key species in the propagation of the lipid peroxidation process. Typically, LOO• will cause the oxidation of an adjacent unsaturated fatty acid moiety, although this is a slow reaction; however, •NO will readily react with LOO• to yield oxidized and nitrosated fatty acid intermediates (e.g., LOONO) that then can ultimately evolve to a series of nitrated (e.g., LONO2) fatty acid species. Moreover, nitrated fatty acids, in particular nitroalkenes, are good electrophiles and elicit a wide range of potent biological (and pharmacological) actions (95), including antiinflammatory properties.
On the other hand, LOO• can promote oxidation of amino acids, “connecting” lipid peroxidation and protein oxidation processes in biomembranes and lipoproteins. A specific example of this type of reactivity is exemplified by the one-electron oxidation of tyrosine to tyrosyl radical by LOO•, which promotes tyrosine oxidation and nitration (96). In this context, molecular oxygen becomes a key modulator of tyrosine oxidation yields when LOO• is the proximal oxidant of tyrosine (59). Moreover, this chemistry facilitates the formation of intertwined products arising from the combination reaction of LOO• with Tyr•, the Diels Alder adducts (97).
Superoxide Dismutases, Nitric Oxide, and Peroxynitrite
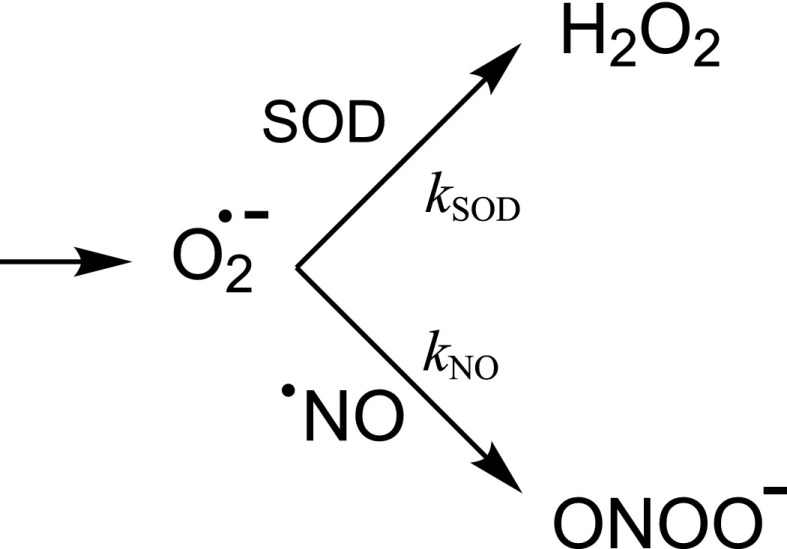
One of the most intriguing kinetic aspects in relation to the formation of peroxynitrite in vivo relies on the fact that the ubiquitous presence of SODs in intracellular and extracellular compartments should largely limit the reaction of with •NO. Indeed, SODs are typically present in significant amounts [e.g., ∼10–20 μM of MnSOD in mammalian mitochondria (33)] and the reaction rate constant of dismutation (Eq. 6) (kSOD, Scheme 3) is close to the diffusion-control limit (∼1–2 × 109 M−1⋅s−1). Thus, at first glance is difficult to conceive the competition for by •NO, usually present at submicromolar concentrations under most physiologically relevant conditions [e.g., 100 nM during eNOS activation (98)].
Scheme 3.
Competition of SOD and nitric oxide for superoxide radical.
However, this competition exists, even under physiological conditions, leading to the formation of basal levels of peroxynitrite and peroxynitrite-derived products, such as tyrosine nitrated proteins. A critically important aspect to consider is that the rate constant for the reaction of with •NO (Eq. 10) (kNO, Scheme 3) to yield peroxynitrite is one order of magnitude higher than that of SOD-catalyzed dismutation. The extremely fast reaction is one of the few examples of reactivity exceeding that with SOD [as an example, the rate constant of with Fe–S-containing dehydratases such as aconitase is 106 to 107 M−1⋅s−1 (99)]. Under low levels of •NO (10–100 nM), most of the “flux” will be channeled through the SOD reaction; still a percentage of will react with other targets, most notably •NO, and therefore a continuous flux of peroxynitrite will be generated under basal physiological conditions. Upon sustained overactivation of the constitutive NOS (eNOS or nNOS) or induction of iNOS, •NO will approach or exceed micromolar levels and will more efficiently compete with SOD, resulting in more significant fluxes of peroxynitrite. A subtle issue is that increases in SOD activity (e.g., induction or overexpression) only partially mitigate peroxynitrite formation rates, due to the concomitant transient increase in •NO levels (secondary to the lowering of the steady-state concentration of !). In other words, it is not kinetically possible to fully impede peroxynitrite formation by SOD in •NO-producing cells.
A notable example of the interplay among , •NO, and SOD is the case of the mammalian MnSOD. Indeed, MnSOD has been shown to react and become inactivated by peroxynitrite secondary to the nitration of critical Tyr34 in vitro and in vivo (52, 100). The rate constant and mechanism leading to the site-specific nitration of Tyr34 have been solved (101). Importantly, due to the metal-catalyzed mechanism of nitration in the active site, peroxynitrite is the only known nitrating agent that results in MnSOD inactivation via nitration. Thus, detection of Tyr34 nitrated MnSOD in vivo reflects intramitochondrial formation or actions of peroxynitrite.
We have extended the observations of peroxynitrite-mediated MnSOD nitration and inactivation to evolutionarily related Fe-containing SODs (not present in mammals), including those of the protozoan parasite Trypanosoma cruzi, the causative agent of Chagas disease (102). Peroxynitrite is a key macrophage-derived cytotoxic agent released to the phagosomes of activated macrophages upon T. cruzi invasion and promotes protein tyrosine nitration in the parasite (64). The reactions of the cytosolic and mitochondrial isoforms of T. cruzi Fe-SODs with peroxynitrite have been disclosed (102), but the biological relevance of these findings in parasite infectivity and virulence demands further investigation.
Thiols, Peroxiredoxins, and Peroxynitrite
The relatively fast reaction of peroxynitrite with low molecular thiols in comparison with H2O2 led to the initial idea that compounds such as GSH, present at millimolar concentrations in mammalian cells, could be preferential targets of peroxynitrite in vivo. However, the later described and much faster reaction with CO2 (and other biomolecules) indicated that typical thiols would be outcompeted by other targets for peroxynitrite. In this context, a notable observation was made in the early 2000s by Nathan and coworkers (103) indicating that a class of thiol-containing antioxidant enzymes in bacteria, the peroxiredoxins, react at large rates with peroxynitrite and reduce it to due to the occurrence of “fast reacting thiols”; in this context, microbial and mammalian peroxiredoxins have been identified as significant biological sinks of peroxynitrite (52, 104). Indeed, different peroxiredoxins react with peroxynitrite with k ranging from 106 to 107 M−1⋅s−1, three to four orders of magnitude faster than with GSH at physiological pH. Peroxiredoxins act as peroxynitrite oxido-reductases by the two-electron reduction of peroxynitrite to (Eq. 11) and have proven to detoxify and protect from the cytotoxic effects of peroxynitrite (52). Redox partners such as thioredoxins or related proteins or thiol compounds reduce the thiol-oxidized peroxiredoxins back to the native state. Moreover, microbial peroxiredoxins (64) and other fast thiol-containing peroxidases (105) have been characterized as virulence factors because of their neutralizing action on peroxynitrite released by activated macrophages and neutrophils.
Recent compilations of the second-order rate constants of H2O2 and peroxynitrite with fast reacting protein thiols show that while in many cases the values reached are similar for both peroxides, this is not always the case, with some proteins reacting at significantly faster rates with peroxynitrite (106). These data support selectivity on intracellular reactions of protein thiols with peroxynitrite (vs. H2O2) and points to the possibility of the existence of “peroxynitrite sensors” or “peroxynitrite relay systems” in signaling processes.
On the other hand, the participation of low–molecular-weight thiols such as GSH in the modulation of peroxynitrite-dependent reactions (e.g., the inhibition of protein tyrosine nitration) rely on one-electron redox processes, including the fast reduction of •NO2 and radical intermediates (e.g., repair of tyrosyl radical back to tyrosine) (52).
Nitric Oxide, Peroxynitrite, and Mitochondria
Early work showed that sustained •NO production by cells leads to alterations in cellular respiration (107–109). This property of •NO led to the postulation that some of the cytotoxic effects of immunostimulated macrophages toward target cells were due to the inhibition of the mitochondrial electron transport chain. In fact, long incubation times of cells with •NO lead to inactivation of mitochondrial electron transport complexes I and II (110). At the time, I found these observations intriguing, because due to the modest reactivity of •NO with biomolecules it was not obvious to expect that •NO per se would permanently interfere in the activity of the electron transport complexes. Thus, we postulated that this •NO-dependent process was not due to •NO itself, but rather to peroxynitrite, likely to be formed intramitochondrially. Indeed, utilizing intact mitochondria preparations, we found that exposure to peroxynitrite caused patterns of electron transport flow inhibition that fully recapitulated the data observed in cells (111). Moreover, peroxynitrite caused inactivation of NADH dehydrogenase and succinate dehydrogenase, without affecting cytochrome oxidase. Interestingly, the resistance of cytochrome oxidase to peroxynitrite is in part due to its capacity to act as a peroxynitrite reductase (112). On the other hand, pure •NO was found to cause reversible inhibition of complex IV by binding to the heme group of cytochrome oxidase (in competition with molecular oxygen). The contribution of several groups during the mid-1990s (reviewed in refs. 111 and 113) and more current data support the idea that enhanced •NO levels reaching mitochondria cause mitochondrial dysfunction through a sequence of events: (i) reversible inhibition of respiration at complex IV, (ii) enhanced “electron leakage” and formation of mitochondrial , (iii) formation of peroxynitrite, (iv) oxidation, nitration, and S-nitrosation of mitochondrial complexes I and II, and (v) inactivation of complexes I and II. This sequence of events contributes to alteration in electrochemical gradients, mitochondrial redox and bioenergy homeostasis, opening of the permeability transition pore, and subsequent apoptotic signaling. To note, peroxynitrite arising from extramitochondrial sites can also reach mitochondria and cause oxidative damage. As mitochondria are main sources of CO2, some of the peroxynitrite will react with it and through the secondary radicals (Scheme 2) trigger the one-electron oxidation of ubiquinol to ubisemiquinone (114), a process that further promotes mitochondrial formation.
Overall, mitochondria constitute a “sink” of •NO due the continuous formation of . This characteristic explains why under basal conditions mitochondria already have a significant level of nitrated proteins and that key mitochondrial proteins, including MnSOD, become significantly nitrated under pathologically relevant conditions (115).
Biological Quantitation of Peroxynitrite
A major issue in the field of free radical and redox biology relates to the “quantities” of oxidants generated in biological systems and how this generation can be recapitulated in experiments in which oxidants are administered to cells and tissues. A key distinction to make has to do with the distinct issue of formation rates vs. steady-state concentrations, which most of the time is underappreciated (116). For example, formation rates by different cell types ranging from bacteria (117) to vascular endothelial cells (33) are on the order of micromolar per second and largely increase during the disruption of redox homeostasis or NADPH oxidase activation in inflammatory cells. However, due to the high concentration of SOD (5–20 μM) and dismutation rate constant, steady-state concentrations are estimated to be on the nanomolar level. Nitric oxide formation rates after NOS activation or induction are also in the micromolar per second range. In this context, indirect measurements in early work estimated peroxynitrite formation rates of ∼5 μM⋅s−1 inside the phagosome of activated macrophages, a value fully compatible with the toxic effects of added peroxynitrite over intracellular pathogens [e.g., Trypanosoma cruzi, Escherichia coli (60, 61)]. The translation of concentrations and effects of cellular fluxes of peroxynitrite vs. addition of pure peroxynitrite (either bolus or infusion) is not evident, but mathematical approximations and experimental evidence permit to affirm that the initial estimations of peroxynitrite formation rates and its correlation with cellular effects were reasonable (52). Recent experiments confirm the role of peroxynitrite in intracellular pathogen killing (64), and studies with probes for peroxynitrite such as boron-based compounds have confirmed the estimation of formation rates in different cell types (118, 119). Because of the different systems that intracellularly consume or decompose peroxynitrite, the resulting steady-state concentrations can be estimated in the nanomolar range (79).
Nitric Oxide-Derived Oxidants and Disease
Formation of peroxynitrite and, in general, of •NO-derived oxidants is associated to a variety of frequent disease processes, including atherosclerosis, hypertension, neurodegeneration, cancer, inflammation, and sepsis (52, 69, 70). The •NO/ interactions can mediate pathophysiological effects by (i) blunting the salutary actions of •NO, (ii) the oxidation and nitration reactions promoted by peroxynitrite, or by a combination of both effects. It is largely documented that protein tyrosine nitration constitutes a good oxidative biomarker of disease progression. Moreover, peroxynitrite and protein tyrosine nitration participate also in the normal aging process (120, 121). In addition to peroxynitrite, other •NO-derived oxidants participate in disease conditions that involve inflammation. In fact, neutrophil or eosinophil activation and degranulation lead to release of hemeperoxidases (MPO, EPO) that promote the formation of a chlorinating, brominating, and nitrating species (85, 87). Disclosing the relative contribution of the peroxynitrite-dependent and independent pathways on protein tyrosine nitration is relevant for the development of appropriate therapeutics, for example, to neutralize either the peroxynitrite or MPO-pathways (87, 122). Hemeperoxidases can further contribute to the biochemistry of •NO-derived oxidants by (i) catalyzing peroxynitrite-dependent nitration reactions (52) and (ii) consuming •NO by their compounds I and II, converting it to (123); overall, hemeperoxidases act as catalytic sinks of •NO at sites of inflammation, limiting its bioavailability and redirecting its chemistry toward nitration.
Selected examples of protein tyrosine nitration and its association to functional changes that may be relevant in disease conditions have been reported recently (52). As a relevant example, it was early shown that endogenous formation of peroxynitrite and protein tyrosine nitration participate in apoptosis of motoneurons in culture (124); later work, in an animal model of ALS, demonstrated the formation of protein-derived radicals and 3-nitrotyrosine in motoneurons of the spinal cord during disease progression (125). Interestingly, peroxynitrite-dependent motor neuron apoptosis can be prevented by cell-permeable tyrosine-containing peptides that spare key proteins from nitration (126) such as HSP90. Indeed, nitro-HSP90 was identified as an inductor of motor neuron cell death (127).
Redox-Based Therapeutics to Neutralize Peroxynitrite
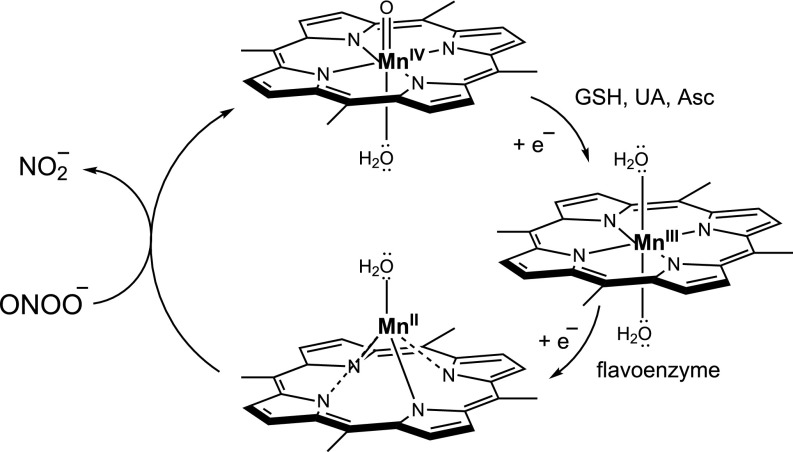
As the evidence grew in regards to the participation of peroxynitrite as a pathogenic mediator, strategies were conceived and developed to cope with it. One obvious possibility is to inhibit the formation of peroxynitrite by decreasing the formation of •NO and/or . Indeed, NOS and NADPH oxidase inhibitors have been successfully utilized to prevent the formation of peroxynitrite and attenuate oxidative damage (64, 128). Alternatively, pharmacologically directed to decompose peroxynitrite or scavenge peroxynitrite-derived radicals have proven to be successful in vitro and in vivo. I will comment on a series of examples of molecules that interfere with the effects of peroxynitrite by direct or indirect reactions. Organometallic compounds of the Mn-porphyrin (MnP) type have been characterized as peroxynitrite decomposition catalysts, among other possible redox functions (129–131). MnP are administered in the Mn3+ state and are reduced intracellularly to the Mn2+ by various enzymatic and nonenzymatic systems, including complexes of the mitochondrial electron transport chain (132). Once in the Mn2+ state, they can readily react with peroxynitrite anion with rate constants on the order of 106 to 107 M−1⋅s−1, reducing it to and leaving MnP in the 4+ state of compound; the latter goes back to the 3+ state by low–molecular-weight compounds including GSH, uric acid (UA), or ascorbate (Asc) (52), and then the catalytic cycle of peroxynitrite decomposition reinitiates (Fig. 3).
Fig. 3.
MnP-catalyzed peroxynitrite decomposition.
Notably, MnTBAP, a MnP largely applied to attenuate oxidative stress in cell and animal models of disease and typically quoted as a “SOD mimetic,” lacks significant SOD activity in vivo and its direct “antioxidant” actions are likely due to peroxynitrite decomposition (133). MnP have been shown to neutralize peroxynitrite-dependent processes in many disease conditions, decreasing oxidation and nitration of cell/tissue components and resulting in improved biological outcome. The safe levels of MnP that can reach cells and organelles [including mitochondria (134)] are in the micromolar range, a value that can compete well with other prevalent reactions of peroxynitrite, including the one with CO2 (79). MnP represent a class of compounds useful in preclinical disease models and that keep promise for application in some clinical conditions. In the case of molecules that efficiently scavenge peroxynitrite-derived radicals, a notable example is uric acid (52). Indeed, enhanced levels of endogenous uric acid or uric acid administration decreases the toxic effect of peroxynitrite in vitro and in vivo and largely decreases protein tyrosine nitration (135). Similarly, expansion of the mitochondrial ubiquinol pool (114) or administration of mitochondria-targeted ubiquinol (mitoQ) (136) results in protection against peroxynitrite-induced mitochondrial dysfunction by interception of radical intermediates arising from peroxynitrite in vitro and in vivo. Other approaches to intercept peroxynitrite-derived radicals include the use of cell-permeable tyrosine-containing peptides (126). Finally, recent studies have shown that organic Se compounds can activate redox-sensitive signaling pathways (such as Nrf-2 and FoxO3) and result in up-regulation of cytosolic and mitochondrial antioxidant enzyme systems (including MnSOD and peroxirexodins) that decrease intracellular concentrations of peroxynitrite and protect cells from peroxynitrite-dependent toxicity (137).
Final Reflection
About 60 y have passed since the realization that free radicals and oxidants could be formed endogenously. In this context, my three decades of research have mainly concentrated on biochemical mechanisms that lead to the enzymatic and nonenzymatic formation of these species, their reactions with biomolecules, and their influence in physiological and pathological processes. Our studies on the /•NO interplay and the oxidative chemistry of peroxynitrite have contributed to the understanding of conditions of mitochondrial dysfunction and alterations of cell redox homeostasis that play roles in human disease. In this sense, redox-based therapeutics represent promising tools to restore cell and tissue homeostasis via direct detoxification of reactive intermediates and/or by triggering cytoprotective and antioxidant signaling pathways. My research path benefitted from bridging and integrating scientific knowledge generated over decades and by fostering synergistic interactions between redox biochemistry and molecular medicine.
Acknowledgments
I gratefully acknowledge the assistance of Natalia Rios to the artwork and the recollections of Mario Calcagno and Claudio Scazzocchio in relation to the days of John R. Totter in Montevideo. I thank all of the co-investigators and collaborators who have jointly contributed to our research program at the Universidad de la República in Montevideo, Uruguay, and the funding agencies that provided the long-term support to our work, including the Howard Hughes Medical Institute and the National Institutes of Health.
Footnotes
The author declares no conflict of interest.
References
- 1.Gerschman R, Gilbert DL, Nye SW, Dwyer P, Fenn WO. Oxygen poisoning and x-irradiation: A mechanism in common. Science. 1954;119:623–626. doi: 10.1126/science.119.3097.623. [DOI] [PubMed] [Google Scholar]
- 2.Daniels M, Scholes G, Weiss J. After-effect in aqueous solutions of deoxyribonucleic acid irradiated with x-rays. Nature. 1953;171:1153–1154. doi: 10.1038/1711153b0. [DOI] [PubMed] [Google Scholar]
- 3.Fridovich I. Superoxide radical and superoxide dismutase. Acc Chem Res. 1972;10:321–326. [Google Scholar]
- 4.Fridovich I, Handler P. Xanthine oxidase. III. Sulfite oxidation as an ultra sensitive assay. J Biol Chem. 1958;233:1578–1580. [PubMed] [Google Scholar]
- 5.Neta P, Huie RE. Free-radical chemistry of sulfite. Environ Health Perspect. 1985;64:209–217. doi: 10.1289/ehp.8564209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed GA, Curtis JF, Mottley C, Eling TE, Mason RP. Epoxidation of (+/−)-7,8-dihydroxy-7,8-dihydrobenzo[a]pyrene during (bi)sulfite autoxidation: Activation of a procarcinogen by a cocarcinogen. Proc Natl Acad Sci USA. 1986;83:7499–7502. doi: 10.1073/pnas.83.19.7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridovich I, Handler P. Detection of free radicals generated during enzymic oxidations by the initiation of sulfite oxidation. J Biol Chem. 1961;236:1836–1840. [PubMed] [Google Scholar]
- 8.McCord JM, Fridovich I. The reduction of cytochrome c by milk xanthine oxidase. J Biol Chem. 1968;243:5753–5760. [PubMed] [Google Scholar]
- 9.Cantu-Medellin N, Kelley EE. Xanthine oxidoreductase-catalyzed reactive species generation: A process in critical need of reevaluation. Redox Biol. 2013;1:353–358. doi: 10.1016/j.redox.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sawyer DT, Valentine J. How super is superoxide? Acc Chem Res. 1981;14:393–400. [Google Scholar]
- 11.Fridovich I. Superoxide radical and superoxide dismutases. Annu Rev Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA. Apparent hydroxyl radical production by peroxynitrite: Implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J Biol Chem. 1991;266:4244–4250. [PubMed] [Google Scholar]
- 14.Radi R. Peroxynitrite, a stealthy biological oxidant. J Biol Chem. 2013;288:26464–26472. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fridovich I. With the help of giants. Annu Rev Biochem. 2003;72:1–18. doi: 10.1146/annurev.biochem.72.081902.140918. [DOI] [PubMed] [Google Scholar]
- 16.Totter JR, De Dugros EC, Riveiro C. The use of chemiluminescent compounds as possible indicators of radical production during xanthine oxidase action. J Biol Chem. 1960;235:1839–1842. [PubMed] [Google Scholar]
- 17.Totter JR, Medina VJ, Scoseria JL. Luminescence during the oxidation of hypoxanthine by xanthine oxidase in the presence of dimethylbiacridylium nitrate. J Biol Chem. 1960;235:238–241. [PubMed] [Google Scholar]
- 18.Merényi G, Lind J, Eriksen TE. Luminol chemiluminescence: Chemistry, excitation, emitter. J Biolumin Chemilumin. 1990;5:53–56. doi: 10.1002/bio.1170050111. [DOI] [PubMed] [Google Scholar]
- 19.Di Mascio P, Medeiros MH. Singlet molecular oxygen generated in dark biological process. Free Radic Biol Med. 2014;75(Suppl 1):S28–S29. doi: 10.1016/j.freeradbiomed.2014.10.755. [DOI] [PubMed] [Google Scholar]
- 20.Boveris A, et al. Organ chemiluminescence: Noninvasive assay for oxidative radical reactions. Proc Natl Acad Sci USA. 1980;77:347–351. doi: 10.1073/pnas.77.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cadenas E, Sies H. Low-level chemiluminescence as an indicator of singlet molecular oxygen in biological systems. Methods Enzymol. 1984;105:221–231. doi: 10.1016/s0076-6879(84)05029-1. [DOI] [PubMed] [Google Scholar]
- 22.Blanco PR, De Angelis WJ, Demicheli de Díaz G, Prodanov E. Two-phase method for the study of xanthine oxidase activity, using triphenyltetrazolium chloride. An Fac Med Univ Repub Montev Urug. 1965;50:114–120. [PubMed] [Google Scholar]
- 23.Blanco PR, Oyamburo GM, Prodanov E, Garciamoreira C. Xanthine oxidase. Some kinetic characteristics of the enzymatic oxidation of hypoxanthine detected by the chemoluminescence of luminol. An Fac Med Univ Repub Montev Urug. 1963;48:349–354. [PubMed] [Google Scholar]
- 24.Oyamburo GM, Prego CE, Prodanov E, Soto H. Xanthine oxidase. Study of the enzyme-catalyzed oxidation of hypoxanthine through the chemiluminescence of luminol. Biochim Biophys Acta. 1970;205:190–195. doi: 10.1016/0005-2728(70)90249-5. [DOI] [PubMed] [Google Scholar]
- 25.Radi RA, Rubbo H, Prodanov E. Comparison of the effects of superoxide dismutase and cytochrome c on luminol chemiluminescence produced by xanthine oxidase-catalyzed reactions. Biochim Biophys Acta. 1989;994:89–93. doi: 10.1016/0167-4838(89)90066-6. [DOI] [PubMed] [Google Scholar]
- 26.Kagan VE, et al. Cytochrome c acts as a cardiolipin oxygenase required for release of proapoptotic factors. Nat Chem Biol. 2005;1:223–232. doi: 10.1038/nchembio727. [DOI] [PubMed] [Google Scholar]
- 27.Radi R, Cosgrove TP, Beckman JS, Freeman BA. Peroxynitrite-induced luminol chemiluminescence. Biochem J. 1993;290:51–57. doi: 10.1042/bj2900051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loschen G, Azzi A, Richter C, Flohé L. Superoxide radicals as precursors of mitochondrial hydrogen peroxide. FEBS Lett. 1974;42:68–72. doi: 10.1016/0014-5793(74)80281-4. [DOI] [PubMed] [Google Scholar]
- 29.Boveris A, Cadenas E. Mitochondrial production of superoxide anions and its relationship to the antimycin insensitive respiration. FEBS Lett. 1975;54:311–314. doi: 10.1016/0014-5793(75)80928-8. [DOI] [PubMed] [Google Scholar]
- 30.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 31.Sies H, Berndt C, Jones DP. Oxidative stress. Annu Rev Biochem. 2017;86:715–748. doi: 10.1146/annurev-biochem-061516-045037. [DOI] [PubMed] [Google Scholar]
- 32.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quijano C, Castro L, Peluffo G, Valez V, Radi R. Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: Direct measurements and formation of hydrogen peroxide and peroxynitrite. Am J Physiol Heart Circ Physiol. 2007;293:H3404–H3414. doi: 10.1152/ajpheart.00761.2007. [DOI] [PubMed] [Google Scholar]
- 34.Turrens JF, Freeman BA, Crapo JD. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- 35.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 36.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic Biol Med. 2016;100:14–31. doi: 10.1016/j.freeradbiomed.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 37.Murphy MP. Understanding and preventing mitochondrial oxidative damage. Biochem Soc Trans. 2016;44:1219–1226. doi: 10.1042/BST20160108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner PR, Raineri I, Epstein LB, White CW. Superoxide radical and iron modulate aconitase activity in mammalian cells. J Biol Chem. 1995;270:13399–13405. doi: 10.1074/jbc.270.22.13399. [DOI] [PubMed] [Google Scholar]
- 39.Radi R, Sims S, Cassina A, Turrens JF. Roles of catalase and cytochrome c in hydroperoxide-dependent lipid peroxidation and chemiluminescence in rat heart and kidney mitochondria. Free Radic Biol Med. 1993;15:653–659. doi: 10.1016/0891-5849(93)90169-u. [DOI] [PubMed] [Google Scholar]
- 40.Vasquez-Vivar J, Kalyanaraman B, Kennedy MC. Mitochondrial aconitase is a source of hydroxyl radical. An electron spin resonance investigation. J Biol Chem. 2000;275:14064–14069. doi: 10.1074/jbc.275.19.14064. [DOI] [PubMed] [Google Scholar]
- 41.Yamazaki I, Piette LH. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2-reactive species in oxygen toxicity in biology. J Biol Chem. 1990;265:13589–13594. [PubMed] [Google Scholar]
- 42.Lambeth JD, Neish AS. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu Rev Pathol. 2014;9:119–145. doi: 10.1146/annurev-pathol-012513-104651. [DOI] [PubMed] [Google Scholar]
- 43.Freeman BA, Crapo JD. Biology of disease: Free radicals and tissue injury. Lab Invest. 1982;47:412–426. [PubMed] [Google Scholar]
- 44.Furchgott RF. Endothelium-derived relaxing factor: Discovery, early studies, and identification as nitric oxide. In: Jornvall H, editor. Nobel Lectures in Physiology or Medicine 1996–2000. World Scientific Publishing Company; Singapore: 2003. pp. 152–169. [Google Scholar]
- 45.Palmer RMJ, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 46.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marletta MA. Nitric oxide synthase: Aspects concerning structure and catalysis. Cell. 1994;78:927–930. doi: 10.1016/0092-8674(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 48.Gryglewski RJ, Palmer RM, Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 49.Rubanyi GM, Vanhoutte PM. Superoxide anions and hyperoxia inactivate endothelium-derived relaxing factor. Am J Physiol. 1986;250:H822–H827. doi: 10.1152/ajpheart.1986.250.5.H822. [DOI] [PubMed] [Google Scholar]
- 50.Ignarro LJ, Byrns RE, Buga GM, Wood KS, Chaudhuri G. Pharmacological evidence that endothelium-derived relaxing factor is nitric oxide: Use of pyrogallol and superoxide dismutase to study endothelium-dependent and nitric oxide-elicited vascular smooth muscle relaxation. J Pharmacol Exp Ther. 1988;244:181–189. [PubMed] [Google Scholar]
- 51.Blough NV, Zafiriou OC. Reaction of superoxide with nitric oxide to form peroxonitrite in alkaline aqueous solution. Inorg Chem. 1985;24:3502–3504. [Google Scholar]
- 52.Ferrer-Sueta G, et al. Biochemistry of peroxynitrite and protein tyrosine nitration. Chem Rev. 2018;118:1338–1408. doi: 10.1021/acs.chemrev.7b00568. [DOI] [PubMed] [Google Scholar]
- 53.Mahoney LR. Evidence for the formation of hydroxyl radicals in the isomerization of pernitrous acid to nitric acid in aqueous solution. J Am Chem Soc. 1970;92:5262–5263. [Google Scholar]
- 54.Halfpenny E, Robinson PL. The nitration and hydroxylation of aromatic compounds by pernitrous acid. J Chem Soc. 1952;1952:939–946. [Google Scholar]
- 55.Radi R, Beckman JS, Bush KM, Freeman BA. Peroxynitrite-induced membrane lipid peroxidation: The cytotoxic potential of superoxide and nitric oxide. Arch Biochem Biophys. 1991;288:481–487. doi: 10.1016/0003-9861(91)90224-7. [DOI] [PubMed] [Google Scholar]
- 56.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 57.Ischiropoulos H, et al. Peroxynitrite-mediated tyrosine nitration catalyzed by superoxide dismutase. Arch Biochem Biophys. 1992;298:431–437. doi: 10.1016/0003-9861(92)90431-u. [DOI] [PubMed] [Google Scholar]
- 58.Koppenol WH, Moreno JJ, Pryor WA, Ischiropoulos H, Beckman JS. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol. 1992;5:834–842. doi: 10.1021/tx00030a017. [DOI] [PubMed] [Google Scholar]
- 59.Bartesaghi S, et al. Tyrosine oxidation and nitration in transmembrane peptides is connected to lipid peroxidation. Arch Biochem Biophys. 2017;622:9–25. doi: 10.1016/j.abb.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Zhu L, Gunn C, Beckman JS. Bactericidal activity of peroxynitrite. Arch Biochem Biophys. 1992;298:452–457. doi: 10.1016/0003-9861(92)90434-x. [DOI] [PubMed] [Google Scholar]
- 61.Denicola A, Rubbo H, Rodríguez D, Radi R. Peroxynitrite-mediated cytotoxicity to Trypanosoma cruzi. Arch Biochem Biophys. 1993;304:279–286. doi: 10.1006/abbi.1993.1350. [DOI] [PubMed] [Google Scholar]
- 62.Evans TJ, et al. Cytokine-treated human neutrophils contain inducible nitric oxide synthase that produces nitration of ingested bacteria. Proc Natl Acad Sci USA. 1996;93:9553–9558. doi: 10.1073/pnas.93.18.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Groote MA, et al. Periplasmic superoxide dismutase protects Salmonella from products of phagocyte NADPH-oxidase and nitric oxide synthase. Proc Natl Acad Sci USA. 1997;94:13997–14001. doi: 10.1073/pnas.94.25.13997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvarez MN, Peluffo G, Piacenza L, Radi R. Intraphagosomal peroxynitrite as a macrophage-derived cytotoxin against internalized Trypanosoma cruzi: Consequences for oxidative killing and role of microbial peroxiredoxins in infectivity. J Biol Chem. 2011;286:6627–6640. doi: 10.1074/jbc.M110.167247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulligan MS, Hevel JM, Marletta MA, Ward PA. Tissue injury caused by deposition of immune complexes is l-arginine dependent. Proc Natl Acad Sci USA. 1991;88:6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White CR, et al. Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci USA. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beckmann JS, et al. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 68.Beckman JS, Carson M, Smith CD, Koppenol WH. ALS, SOD and peroxynitrite. Nature. 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 69.Szabó C, Ischiropoulos H, Radi R. Peroxynitrite: Biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–680. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 70.Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharma SK, et al. A six-coordinate peroxynitrite low-spin iron(III) porphyrinate complex-the product of the reaction of nitrogen monoxide (·NO(g)) with a ferric-superoxide species. J Am Chem Soc. 2017;139:17421–17430. doi: 10.1021/jacs.7b08468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO*-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 73.Wade RS, Castro CE. Reactions of oxymyoglobin with NO, NO2, and NO2− under argon and in air. Chem Res Toxicol. 1996;9:1382–1390. doi: 10.1021/tx9600457. [DOI] [PubMed] [Google Scholar]
- 74.Denicola A, Souza JM, Radi R. Diffusion of peroxynitrite across erythrocyte membranes. Proc Natl Acad Sci USA. 1998;95:3566–3571. doi: 10.1073/pnas.95.7.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Romero N, et al. Reaction of human hemoglobin with peroxynitrite. Isomerization to nitrate and secondary formation of protein radicals. J Biol Chem. 2003;278:44049–44057. doi: 10.1074/jbc.M305895200. [DOI] [PubMed] [Google Scholar]
- 76.Chen HJ, Chen YC. Reactive nitrogen oxide species-induced post-translational modifications in human hemoglobin and the association with cigarette smoking. Anal Chem. 2012;84:7881–7890. doi: 10.1021/ac301597r. [DOI] [PubMed] [Google Scholar]
- 77.Chen HJ, Yang YF, Lai PY, Chen PF. Analysis of chlorination, nitration, and nitrosylation of tyrosine and oxidation of methionine and cysteine in hemoglobin from type 2 diabetes mellitus patients by nanoflow liquid chromatography tandem mass spectrometry. Anal Chem. 2016;88:9276–9284. doi: 10.1021/acs.analchem.6b02663. [DOI] [PubMed] [Google Scholar]
- 78.Radi R, Rubbo H, Thomson L, Prodanov E. Luminol chemiluminescence using xanthine and hypoxanthine as xanthine oxidase substrates. Free Radic Biol Med. 1990;8:121–126. doi: 10.1016/0891-5849(90)90084-v. [DOI] [PubMed] [Google Scholar]
- 79.Ferrer-Sueta G, Radi R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. ACS Chem Biol. 2009;4:161–177. doi: 10.1021/cb800279q. [DOI] [PubMed] [Google Scholar]
- 80.Bonini MG, Radi R, Ferrer-Sueta G, Ferreira AM, Augusto O. Direct EPR detection of the carbonate radical anion produced from peroxynitrite and carbon dioxide. J Biol Chem. 1999;274:10802–10806. doi: 10.1074/jbc.274.16.10802. [DOI] [PubMed] [Google Scholar]
- 81.Trindade DF, Cerchiaro G, Augusto O. A role for peroxymonocarbonate in the stimulation of biothiol peroxidation by the bicarbonate/carbon dioxide pair. Chem Res Toxicol. 2006;19:1475–1482. doi: 10.1021/tx060146x. [DOI] [PubMed] [Google Scholar]
- 82.Liochev SI, Fridovich I. CO2, not HCO3−, facilitates oxidations by Cu,Zn superoxide dismutase plus H2O2. Proc Natl Acad Sci USA. 2004;101:743–744. doi: 10.1073/pnas.0307635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Smith CD, et al. Crystal structure of peroxynitrite-modified bovine Cu,Zn superoxide dismutase. Arch Biochem Biophys. 1992;299:350–355. doi: 10.1016/0003-9861(92)90286-6. [DOI] [PubMed] [Google Scholar]
- 84.van der Vliet A, Eiserich JP, O’Neill CA, Halliwell B, Cross CE. Tyrosine modification by reactive nitrogen species: A closer look. Arch Biochem Biophys. 1995;319:341–349. doi: 10.1006/abbi.1995.1303. [DOI] [PubMed] [Google Scholar]
- 85.Brennan M-L, et al. A tale of two controversies: Defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J Biol Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 86.Alvarez B, Ferrer-Sueta G, Freeman BA, Radi R. Kinetics of peroxynitrite reaction with amino acids and human serum albumin. J Biol Chem. 1999;274:842–848. doi: 10.1074/jbc.274.2.842. [DOI] [PubMed] [Google Scholar]
- 87.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc Natl Acad Sci USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ye YZ, Strong M, Huang ZQ, Beckman JS. Antibodies that recognize nitrotyrosine. Methods Enzymol. 1996;269:201–209. doi: 10.1016/s0076-6879(96)69022-3. [DOI] [PubMed] [Google Scholar]
- 89.Batthyány C, et al. Tyrosine-nitrated proteins: Proteomic and bioanalytical aspects. Antioxid Redox Signal. 2017;26:313–328. doi: 10.1089/ars.2016.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brito C, et al. Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol. 1999;162:3356–3366. [PubMed] [Google Scholar]
- 91.Sokolovsky M, Riordan JF, Vallee BL. Tetranitromethane. A reagent for the nitration of tyrosyl residues in proteins. Biochemistry. 1966;5:3582–3589. doi: 10.1021/bi00875a029. [DOI] [PubMed] [Google Scholar]
- 92.Rocha BS, et al. Pepsin is nitrated in the rat stomach, acquiring antiulcerogenic activity: A novel interaction between dietary nitrate and gut proteins. Free Radic Biol Med. 2013;58:26–34. doi: 10.1016/j.freeradbiomed.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 93.Darley-Usmar VM, Hogg N, O’Leary VJ, Wilson MT, Moncada S. The simultaneous generation of superoxide and nitric oxide can initiate lipid peroxidation in human low density lipoprotein. Free Radic Res Commun. 1992;17:9–20. doi: 10.3109/10715769209061085. [DOI] [PubMed] [Google Scholar]
- 94.Rubbo H, et al. Nitric oxide regulation of superoxide and peroxynitrite-dependent lipid peroxidation. Formation of novel nitrogen-containing oxidized lipid derivatives. J Biol Chem. 1994;269:26066–26075. [PubMed] [Google Scholar]
- 95.Schopfer FJ, Cipollina C, Freeman BA. Formation and signaling actions of electrophilic lipids. Chem Rev. 2011;111:5997–6021. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartesaghi S, et al. Lipid peroxyl radicals mediate tyrosine dimerization and nitration in membranes. Chem Res Toxicol. 2010;23:821–835. doi: 10.1021/tx900446r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shchepin R, et al. Tyrosine-lipid peroxide adducts from radical termination: Para coupling and intramolecular Diels-Alder cyclization. J Am Chem Soc. 2010;132:17490–17500. doi: 10.1021/ja106503a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147:S193–S201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hausladen A, Fridovich I. Measuring nitric oxide and superoxide: Rate constants for aconitase reactivity. Methods Enzymol. 1996;269:37–41. doi: 10.1016/s0076-6879(96)69007-7. [DOI] [PubMed] [Google Scholar]
- 100.MacMillan-Crow LA, Crow JP, Kerby JD, Beckman JS, Thompson JA. Nitration and inactivation of manganese superoxide dismutase in chronic rejection of human renal allografts. Proc Natl Acad Sci USA. 1996;93:11853–11858. doi: 10.1073/pnas.93.21.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Demicheli V, et al. Mechanism of the reaction of human manganese superoxide dismutase with peroxynitrite: Nitration of critical tyrosine 34. Biochemistry. 2016;55:3403–3417. doi: 10.1021/acs.biochem.6b00045. [DOI] [PubMed] [Google Scholar]
- 102.Martinez A, et al. Structural and molecular basis of the peroxynitrite-mediated nitration and inactivation of Trypanosoma cruzi iron-superoxide dismutases (Fe-SODs) A and B: Disparate susceptibilities due to the repair of Tyr35 radical by Cys83 in Fe-SODB through intramolecular electron transfer. J Biol Chem. 2014;289:12760–12778. doi: 10.1074/jbc.M113.545590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bryk R, Griffin P, Nathan C. Peroxynitrite reductase activity of bacterial peroxiredoxins. Nature. 2000;407:211–215. doi: 10.1038/35025109. [DOI] [PubMed] [Google Scholar]
- 104.Trujillo M, et al. Trypanosoma brucei and Trypanosoma cruzi tryparedoxin peroxidases catalytically detoxify peroxynitrite via oxidation of fast reacting thiols. J Biol Chem. 2004;279:34175–34182. doi: 10.1074/jbc.M404317200. [DOI] [PubMed] [Google Scholar]
- 105.Alegria TG, et al. Ohr plays a central role in bacterial responses against fatty acid hydroperoxides and peroxynitrite. Proc Natl Acad Sci USA. 2017;114:E132–E141. doi: 10.1073/pnas.1619659114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Trujillo M, Alvarez B, Radi R. One- and two-electron oxidation of thiols: Mechanisms, kinetics and biological fates. Free Radic Res. 2016;50:150–171. doi: 10.3109/10715762.2015.1089988. [DOI] [PubMed] [Google Scholar]
- 107.Drapier JC, Pellat C, Henry Y. Generation of EPR-detectable nitrosyl-iron complexes in tumor target cells cocultured with activated macrophages. J Biol Chem. 1991;266:10162–10167. [PubMed] [Google Scholar]
- 108.Brown GC, Bolaños JP, Heales SJ, Clark JB. Nitric oxide produced by activated astrocytes rapidly and reversibly inhibits cellular respiration. Neurosci Lett. 1995;193:201–204. doi: 10.1016/0304-3940(95)11703-y. [DOI] [PubMed] [Google Scholar]
- 109.Bolaños JP, Peuchen S, Heales SJ, Land JM, Clark JB. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem. 1994;63:910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- 110.Radi R, Cassina A, Hodara R, Quijano C, Castro L. Peroxynitrite reactions and formation in mitochondria. Free Radic Biol Med. 2002;33:1451–1464. doi: 10.1016/s0891-5849(02)01111-5. [DOI] [PubMed] [Google Scholar]
- 111.Radi R, Rodriguez M, Castro L, Telleri R. Inhibition of mitochondrial electron transport by peroxynitrite. Arch Biochem Biophys. 1994;308:89–95. doi: 10.1006/abbi.1994.1013. [DOI] [PubMed] [Google Scholar]
- 112.Pearce LL, Pitt BR, Peterson J. The peroxynitrite reductase activity of cytochrome c oxidase involves a two-electron redox reaction at the heme a(3)-Cu(B) site. J Biol Chem. 1999;274:35763–35767. doi: 10.1074/jbc.274.50.35763. [DOI] [PubMed] [Google Scholar]
- 113.Moncada PS. Nitric oxide and oxygen: Actions and interactions in health and disease. Redox Biol. 2015;5:421. doi: 10.1016/j.redox.2015.09.034. [DOI] [PubMed] [Google Scholar]
- 114.Schöpfer F, et al. Oxidation of ubiquinol by peroxynitrite: Implications for protection of mitochondria against nitrosative damage. Biochem J. 2000;349:35–42. doi: 10.1042/0264-6021:3490035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Castro L, Demicheli V, Tórtora V, Radi R. Mitochondrial protein tyrosine nitration. Free Radic Res. 2011;45:37–52. doi: 10.3109/10715762.2010.516254. [DOI] [PubMed] [Google Scholar]
- 116.Winterbourn CC. Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol. 2008;4:278–286. doi: 10.1038/nchembio.85. [DOI] [PubMed] [Google Scholar]
- 117.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu Rev Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zielonka J, et al. Boronate probes as diagnostic tools for real time monitoring of peroxynitrite and hydroperoxides. Chem Res Toxicol. 2012;25:1793–1799. doi: 10.1021/tx300164j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rios N, et al. Sensitive detection and estimation of cell-derived peroxynitrite fluxes using fluorescein-boronate. Free Radic Biol Med. 2016;101:284–295. doi: 10.1016/j.freeradbiomed.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 120.Viner RI, Ferrington DA, Williams TD, Bigelow DJ, Schöneich C. Protein modification during biological aging: Selective tyrosine nitration of the SERCA2a isoform of the sarcoplasmic reticulum Ca2+-ATPase in skeletal muscle. Biochem J. 1999;340:657–669. [PMC free article] [PubMed] [Google Scholar]
- 121.van der Loo B, et al. Enhanced peroxynitrite formation is associated with vascular aging. J Exp Med. 2000;192:1731–1744. doi: 10.1084/jem.192.12.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Eiserich JP, et al. Formation of nitric oxide-derived inflammatory oxidants by myeloperoxidase in neutrophils. Nature. 1998;391:393–397. doi: 10.1038/34923. [DOI] [PubMed] [Google Scholar]
- 123.Abu-Soud HM, Hazen SL. Nitric oxide modulates the catalytic activity of myeloperoxidase. J Biol Chem. 2000;275:5425–5430. doi: 10.1074/jbc.275.8.5425. [DOI] [PubMed] [Google Scholar]
- 124.Estévez AG, et al. Nitric oxide and superoxide contribute to motor neuron apoptosis induced by trophic factor deprivation. J Neurosci. 1998;18:923–931. doi: 10.1523/JNEUROSCI.18-03-00923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cassina P, et al. Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: Prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ye Y, et al. Prevention of peroxynitrite-induced apoptosis of motor neurons and PC12 cells by tyrosine-containing peptides. J Biol Chem. 2007;282:6324–6337. doi: 10.1074/jbc.M610800200. [DOI] [PubMed] [Google Scholar]
- 127.Franco MC, et al. Nitration of Hsp90 induces cell death. Proc Natl Acad Sci USA. 2013;110:E1102–E1111. doi: 10.1073/pnas.1215177110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zielonka J, et al. Mitigation of NADPH oxidase 2 activity as a strategy to inhibit peroxynitrite formation. J Biol Chem. 2016;291:7029–7044. doi: 10.1074/jbc.M115.702787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lee J, Hunt JA, Groves JT. Manganese porphyrins as redox-coupled peroxynitrite reductases. J Am Chem Soc. 1998;120:6053–6061. [Google Scholar]
- 130.Ferrer-Sueta G, Batinić-Haberle I, Spasojević I, Fridovich I, Radi R. Catalytic scavenging of peroxynitrite by isomeric Mn(III) N-methylpyridylporphyrins in the presence of reductants. Chem Res Toxicol. 1999;12:442–449. doi: 10.1021/tx980245d. [DOI] [PubMed] [Google Scholar]
- 131.Batinic-Haberle I, et al. SOD therapeutics: Latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid Redox Signal. 2014;20:2372–2415. doi: 10.1089/ars.2012.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Valez V, et al. Peroxynitrite formation in nitric oxide-exposed submitochondrial particles: Detection, oxidative damage and catalytic removal by Mn-porphyrins. Arch Biochem Biophys. 2013;529:45–54. doi: 10.1016/j.abb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Batinić-Haberle I, et al. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: Comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Spasojević I, et al. Mn porphyrin-based superoxide dismutase (SOD) mimic, MnIIITE-2-PyP5+, targets mouse heart mitochondria. Free Radic Biol Med. 2007;42:1193–1200. doi: 10.1016/j.freeradbiomed.2007.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hooper DC, et al. Uric acid, a natural scavenger of peroxynitrite, in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1998;95:675–680. doi: 10.1073/pnas.95.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miquel E, et al. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic Biol Med. 2014;70:204–213. doi: 10.1016/j.freeradbiomed.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 137.Fiuza B, et al. Impact of SIN-1-derived peroxynitrite flux on endothelial cell redox homeostasis and bioenergetics: Protective role of diphenyl diselenide via induction of peroxiredoxins. Free Radic Res. 2015;49:122–132. doi: 10.3109/10715762.2014.983096. [DOI] [PubMed] [Google Scholar]