Significance
Our innate circadian clocks control myriad aspects of behavior and physiology. Disruption of our clocks by shift work, jet lag, or inherited mutation leads to metabolic dysregulation and contributes to diseases, including diabetes and cancer. A central step in clock control is phosphorylation of the PERIOD 2 (PER2) protein. Here we conclusively identify the elusive PER2 priming kinase and find it to be the well-known circadian kinase, casein kinase 1 (CK1). Surprisingly, different forms of CK1 have differing abilities to phosphorylate the PER2 priming site, adding to the complexity of circadian regulation. These insights into the phosphoregulation of PER2 will be of broad interest to circadian biologists, computational modelers, and those seeking to pharmacologically manipulate the circadian clock.
Keywords: circadian rhythms, protein kinase, cell regulation
Abstract
Multisite phosphorylation of the PERIOD 2 (PER2) protein is the key step that determines the period of the mammalian circadian clock. Previous studies concluded that an unidentified kinase is required to prime PER2 for subsequent phosphorylation by casein kinase 1 (CK1), an essential clock component that is conserved from algae to humans. These subsequent phosphorylations stabilize PER2, delay its degradation, and lengthen the period of the circadian clock. Here, we perform a comprehensive biochemical and biophysical analysis of mouse PER2 (mPER2) priming phosphorylation and demonstrate, surprisingly, that CK1δ/ε is indeed the priming kinase. We find that both CK1ε and a recently characterized CK1δ2 splice variant more efficiently prime mPER2 for downstream phosphorylation in cells than the well-studied splice variant CK1δ1. While CK1 phosphorylation of PER2 was previously shown to be robust to changes in the cellular environment, our phosphoswitch mathematical model of circadian rhythms shows that the CK1 carboxyl-terminal tail can allow the period of the clock to be sensitive to cellular signaling. These studies implicate the extreme carboxyl terminus of CK1 as a key regulator of circadian timing.
The PERIOD (PER) proteins are the central regulators of the metazoan circadian (daily) clock. per was originally discovered in Drosophila as a gene whose mutation could lead to a short or long period, initiating the modern era of molecular circadian biology (1). In all metazoan clocks, PER expression oscillates at both the mRNA and protein levels, with posttranslational control of PER protein stability tightly regulated by phosphorylation (2, 3). PER phosphorylation also plays a critical role in a process known as temperature compensation, a defining feature of circadian rhythms that confers stable clock timing independent of ambient temperature (4–6). Due to its central role in controlling circadian period, pharmacological modification of PER2 phosphorylation is being pursued to alter circadian rhythms and ameliorate jet lag and seasonal affective disorder (7, 8). However, the complex process by which phosphorylation controls PER stability is still not well understood.
Characterization of circadian rhythm mutants has provided key insight into the role that phosphorylation plays in the clock mechanism. Mutations that alter phosphorylation of PER cause dramatic changes in the circadian period of Drosophila, mice, hamsters, and even humans (9–14). An inherited polymorphism of Ser-662 (S662G) in humans enhances PER2 degradation, shortens circadian period, and causes familial advanced sleep phase (FASP) syndrome (11). Despite the importance of the FASP site, it is still unclear how it gets phosphorylated. Two casein kinase 1 (CK1) genes, CSNK1D and CSNK1E, encode kinases (CK1δ and CK1ε, respectively) that phosphorylate PER2 at two key regions that control a “phosphoswitch” mechanism regulating PER2 stability (5, 15). While CK1δ is more abundant than CK1ε and may play a major role in regulating rhythms in most tissues (16–18), the fact that a single amino acid change in CK1ε (CK1εtau, R178C) in hamsters and mice shortens period by 2 and 4 h in the heterozygote and homozygote, respectively, and loses temperature compensation indicates a major role for CK1ε in rhythms as well (12, 14, 19, 20).
Multiple studies and recent reviews have concluded that an additional but currently unidentified “priming” kinase is required to phosphorylate the FASP site before the downstream serines can be phosphorylated by CK1δ and/or CK1ε (4, 11, 13, 21). This is because members of the CK1 family preferentially phosphorylate primed sites—that is, sites where a phosphorylated residue drives recognition of a downstream serine in the +3 position (22). There are four serine residues with this spacing immediately downstream of S659 in mPER2 (S662 in humans) (Fig. 1A). Based on in vitro assays using peptide substrates, it appears that the downstream serines are phosphorylated by CK1δ/ε only after S659 is primed. The importance of this S659 priming site is illustrated by mice with a phosphomimetic mutation (Ser to Asp) at the FASP site in a human PER2 transgene, which leads to a longer circadian period, contrasted with the shorter period observed with the Ser to Gly FASP polymorphism (13). The Nemo-like kinase was recently identified as a priming kinase for Drosophila PER, but the identity of the mammalian priming kinase responsible for phosphorylation of S659 in mPER2 remains a major open question in the field (23, 24).
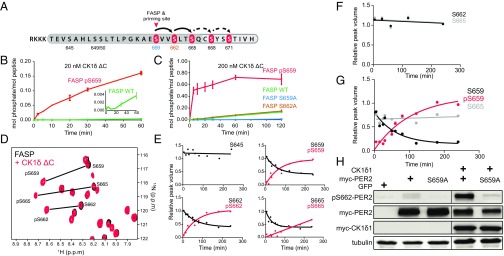
Fig. 1.
Phosphorylation of the mPER2 priming site by CK1δ is required for downstream phosphorylation of CK1 consensus sites. (A) Schematic diagram of the FASP serine cluster phosphorylation in a synthetic peptide. RKKK, polybasic motif for kinase assay. Dark gray shading indicates minimal FASP peptide used in this study, with phosphorylation sites shown in red; arrows demonstrate the potential for processive phosphorylation of CK1 consensus sites. Additional downstream phosphorylation sites in PER2 (not included in the minimal FASP peptide) are shown in light gray shading with dashed arrows. (B and C) Time course of representative peptide phosphorylation assays with 20 nM (B) or 200 nM (C) CK1δ ΔC. Error bars represent SD for two biological replicates per assay. The experiment was repeated more than three times, with essentially identical results. (D) Zoom-in view of overlaid 15N/1H heteronuclear single quantum correlation spectra of 200 µM 15N mPER2 FASP peptide alone (black) or 4 h after addition of 200 nM CK1δ ΔC (red) at 30 °C. NMR spectra were acquired after kinase reactions were quenched with 20 mM EDTA. (E) Quantification of peak volumes for indicated serines in the 15N FASP peptide from NMR time course (unphosphorylated shown in black; phosphorylated shown in red). Data points are fit to a one-phase exponential curve, except for S665 phosphorylation, which is fit to a linear regression. See SI Appendix, Table S1 for more details. (F and G) Quantification of peak volumes for indicated serine residues in 15N FASP S659A (F) and S662A (G) peptides, as in E. (H) Phosphorylation of S662 is dependent on the S659 site. Full-length wild-type and FASP mutant mPER2 were expressed with or without CK1δ1. Lysates were probed with indicated antibodies. The gel was spliced, as indicated, for clarity.
This priming kinase theory is attractive from a systems biology perspective because its action could precisely control PER’s degradation rate, which is a key factor in regulating the timing of the mammalian circadian clock (25). Thus, it opens up the possibility of a signaling mechanism to regulate circadian period through posttranslational modifications of a single site. The delay in PER degradation caused by FASP priming and subsequent downstream site phosphorylation also was shown to be enhanced at higher temperatures, providing a mechanism for how the circadian clock can keep a 24-h period despite increasing reaction rates with increasing temperature (5). However, and despite much effort, the identity of the priming kinase has yet to be discovered.
Here, we show that CK1δ/ε itself is the priming kinase. Using an NMR-based assay that quantitatively probes phosphorylation with site-specific resolution, we demonstrate that phosphorylation of mPER2 S659 by CK1δ/ε is necessary and sufficient for the rapid phosphorylation of downstream consensus sites. In cells, CK1ε and a recently discovered splice variant of CK1δ (CK1δ2) that resembles CK1ε at the extreme carboxyl terminus are more-efficient priming kinases than CK1δ1. Interestingly, our previously proposed phosphoswitch model recapitulates salient features of the model, including the PER2 degradation pattern when CK1δ/ε is introduced as the priming kinase (5). Our modeling work suggests a robust yet fragile design to PER phosphorylation that allows the period of the circadian clock to be robust to environmental variations (e.g., changes in temperature) but also allows for regulatory changes in the CK1 carboxyl terminus to have a large effect on circadian period. This model makes the prediction that the CK1 tail preferentially controls phosphorylation on the FASP site, a prediction that is experimentally verified. Together, this presents a mechanism for regulation of circadian period that is surprisingly divergent from that used in Drosophila.
Results
Processive Phosphorylation of the mPER2 FASP Region Is Dependent on S659 Phosphorylation.
Phosphorylation of the FASP serine cluster of mPER2 by CK1δ/ε following a pSxxS consensus motif is thought to occur processively, but only after phosphorylation of an upstream priming site (S659 on mPER2) by an unidentified kinase, as illustrated in Fig. 1A. To identify the priming kinase and assess the activity of CK1δ/ε on primed peptides, we synthesized a peptide of the mPER2 FASP region containing three potential phosphorylation sites, complete with its native upstream sequence and an amino-terminal basic tag to facilitate peptide kinase assays (Fig. 1A and SI Appendix, Fig. S1A). As predicted, kinase reactions with limiting concentrations of CK1δ ΔC [a constitutively active form of recombinant CK1δ lacking its carboxyl-terminal autoregulatory domain (26)] led to efficient phosphorylation of a primed FASP peptide that was synthetically phosphorylated on S659 (pS659). However, we noted that the unprimed wild-type peptide (FASP-WT) was also a potential substrate for CK1δ ΔC, albeit with >50-fold lower efficiency (Fig. 1B). Since CK1δ/ε is found stably associated with PER1 and PER2 complexes in vivo (2, 27, 28), we reasoned that increasing the kinase concentration would better mimic the in vivo setting where kinase and substrate are found in a stoichiometric complex. Increasing the concentration of CK1δ ΔC by only 10-fold led to phosphorylation of the unprimed FASP-WT peptide, but not the S659A mutant (Fig. 1C), suggesting that CK1δ ΔC may be capable of acting as the priming kinase when its local concentration is increased relative to the mPER2 substrate. We observed that phosphorylation of the S662A mutant occurred at a rate similar to FASP-WT (Fig. 1C), suggesting that phosphorylation of the priming site at S659 by CK1δ ΔC is the rate-limiting step in regulation of the FASP region.
As an independent quantitative approach to study phosphorylation of the mPER2 FASP peptide by CK1δ, we developed an NMR-based kinase assay that utilizes uniformly 15N-labeled FASP to monitor site-specific detection of phosphorylated residues. Addition of CK1δ ΔC led to kinase-dependent perturbations of FASP peptide chemical shifts that only occurred in the presence of ATP, consistent with phosphorylation (Fig. 1D and SI Appendix, Fig. S1 B–E). By quantifying peak intensities over time after quenching the kinase assay with EDTA, we observed a progressive loss of intensity for unphosphorylated serines 659, 662, 665 and surrounding peaks, followed by a concomitant increase in intensity for new downfield peaks that correspond to phosphorylated serines (Fig. 1E), as seen in other NMR-based kinase assays (29, 30). CK1δ ΔC specifically targets S659 and downstream serines for phosphorylation, because a representative serine upstream of the priming site did not exhibit chemical shifts indicative of phosphorylation. We also observed similar results for kinase assays monitored in real time by NMR (SI Appendix, Fig. S1F). Peaks for phosphorylated S659 and S662 arise at essentially indistinguishable rates, consistent with earlier observations that phosphorylation of the priming site appears to be rate limiting, while phosphorylation of the consensus site downstream at S662 occurs nearly instantaneously after the priming site (SI Appendix, Table S1). Phosphorylation of S665 was noticeably slower, perhaps due to its location near the carboxyl terminus of our minimal FASP peptide (Fig. 1E and SI Appendix, Fig. S1F).
To test the role of priming phosphorylation on S659 for CK1δ ΔC activity on downstream sites, we examined activity of the kinase on the 15N FASP S659A mutant. In the absence of the priming site, we found no detectable phosphorylation anywhere on the FASP peptide (Fig. 1F). This provides clear evidence that CK1δ ΔC requires S659 for priming phosphorylation of the mPER2 FASP region in vitro to elicit phosphorylation of downstream consensus sites at S662 and S665. We then used our NMR assay to examine the processivity of phosphorylation at these downstream consensus sites by CK1δ ΔC. If phosphorylation of the priming site at S659 establishes the pSxxS CK1δ consensus motif, then loss of the second site with the S662A mutant should allow us to identify whether phosphorylation proceeds in a processive manner down the FASP peptide. Although phosphorylation of the priming site in the S662A mutant occurred with a similar rate to FASP-WT, phosphorylation of the downstream site at S665 was abolished (Fig. 1G). These data demonstrate that the kinase phosphorylates S659 independently of S662 and uses a processive mechanism to phosphorylate target downstream serines in the mPER2 FASP region.
To test whether phosphorylation of S662 of mPER2 requires priming phosphorylation in full-length protein in cellular system, as an independent approach, we used a newly generated affinity-purified pS662-mPER2 antibody (Materials and Methods and SI Appendix, Fig. S2). As seen in Fig. 1H, mutation of the priming site (S659) abrogated phosphorylation of S662, confirming the processive nature of FASP phosphorylation in cells.
CK1δ Protein Is Sufficient to Phosphorylate S659 of mPER2.
To facilitate identification of the mPER2 priming kinase, we generated affinity-purified phosphoepitope-specific antibodies recognizing pS659 of mPER2 (Materials and Methods). The pS659-PER2 antibody was highly selective for immobilized pS659-FASP peptide, recognizing the peptide even at a dilution of 1:32,000 (Fig. 2A). The pS659-PER2 antibody also recognized pS659, but not S659A, in full-length and truncated mPER2 in immunoblots (Fig. 2B). Confirming the phosphorylation dependency of the antibody, treatment of lysates with alkaline phosphatase specifically decreased binding of the pS659-PER2 antibody (SI Appendix, Fig. S3).
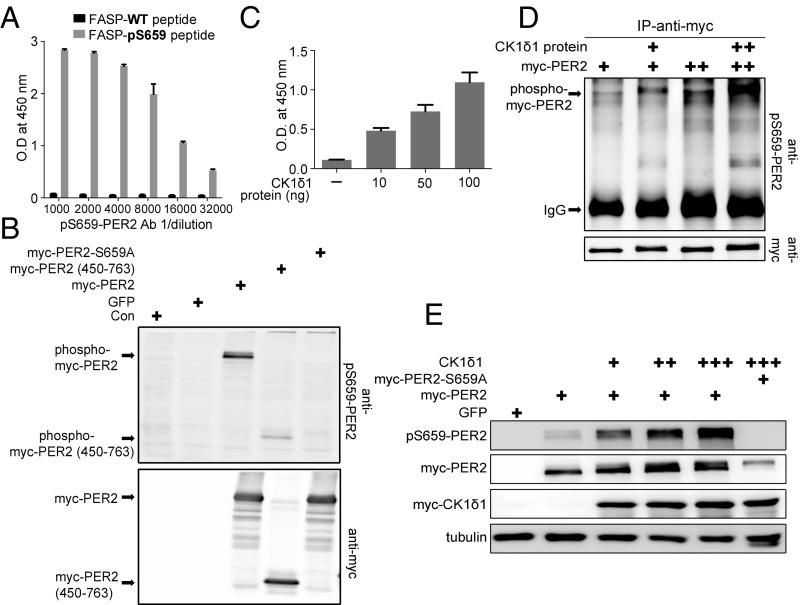
Fig. 2.
Phosphorylation of the priming site by CK1δ1. (A) pS659 mPER2 antibody specifically recognizes phosphorylated peptides in ELISA. Error bars represent SD (n = 3). (B) pS659 mPER2 antibody specifically recognizes mPER2, but not mPER2 S659A. The immunoblot is representative of multiple replicates. (C) Purified CK1δ1 phosphorylates FASP peptide at S659. Peptide phosphorylation was assessed by phospho-S659 PER2 antibody ELISA. Error bars represent SD (n = 3 biological replicates per point). (D) CK1δ1 directly phosphorylates mPER2. Myc-tagged mPER2 was immunoprecipitated (IP) from HEK293 cells and incubated with 500 ng of recombinant CK1δ1. Phosphorylation of S659 was assessed by immunoblotting with the pS659 antibody. (E) CK1δ1 phosphorylates mPER2 in cells. mPER2 expression plasmid (1 µg) was cotransfected with 10 ng (+), 25 ng (++), and 50 ng (+++) of CK1δ1 expression plasmid, and lysates were probed for S659 phosphorylation. The immunoblot is representative of three independent experiments.
As noted by Fustin et al. (31), CK1δ has two splice variants, CK1δ1 and CK1δ2. Using the in vitro peptide kinase ELISA, we confirmed that CK1δ1 can phosphorylate the FASP-WT peptide at S659 in a concentration-dependent manner (Fig. 2C). Next, we tested whether CK1δ1 could phosphorylate the FASP priming site of mPER2 in full-length protein (Fig. 2 D and E). Myc-tagged mPER2 expressed in HEK293 cells was immunoprecipitated and then incubated with recombinant full-length CK1δ1 protein. Priming-site phosphorylation was assessed by SDS/PAGE and immunoblotting. As noted in Fig. 2D, mPER2 in cells is phosphorylated on S659 to some extent by endogenous kinases. However, addition of increasing amounts of recombinant CK1δ1 protein was sufficient to increase the phosphorylation of S659 in full-length mPER2.
CK1δ/ε Is the Priming Kinase in Cells.
Having established that CK1δ1 could phosphorylate the priming site in the peptide and in the immunopurified protein, we asked whether CK1δ1 could phosphorylate the mPER2 priming site in intact cells (Fig. 2E). mPER2 was expressed alone or with increasing amounts of ectopic CK1δ1. In the absence of added CK1δ1, mPER2 was phosphorylated on S659, and the amount of phosphorylation increased markedly with coexpression of CK1δ1. Conversely, mPER2 with a S659A mutation (Fig. 2E, last lane) was not recognized by the pS659 antibody and displayed a mobility shift in the presence of ectopic CK1δ1, consistent with phosphorylation at the β-TrCP phosphodegron and other sites (15).
The preceding assays demonstrate that CK1δ1 is sufficient to phosphorylate the mPER2 priming site on peptides in vitro and in cells in the context of the full-length protein. To test whether CK1δ and/or CK1ε kinase is necessary for priming phosphorylation in cells, we performed knockdown and inhibitor assays. Knockdown of total CK1δ only partially inhibited the priming-site phosphorylation (Fig. 3A). Knockdown of CK1ε, a kinase generally present in much lower abundance than CK1δ isoforms, also gave rise to a marked decrease in priming-site phosphorylation, and knockdown of both CK1δ and CK1ε together eliminated priming phosphorylation. Confirming that CK1ε can phosphorylate the priming site, purified CK1ε protein could phosphorylate the FASP peptide in vitro (Fig. 3B), and overexpression of CK1ε drove phosphorylation of the S659 site in cells (Fig. 3C). Lastly, as an independent test, we found that the dual CK1δ/ε inhibitor PF670462 (7) and, to a lesser extent, the pan-CK1 inhibitor D4476 also markedly reduced mPER2 priming phosphorylation (Fig. 3D). We conclude that CK1δ and CK1ε are both necessary and sufficient to phosphorylate the mPER2 FASP priming and downstream sites in vitro and in cells.
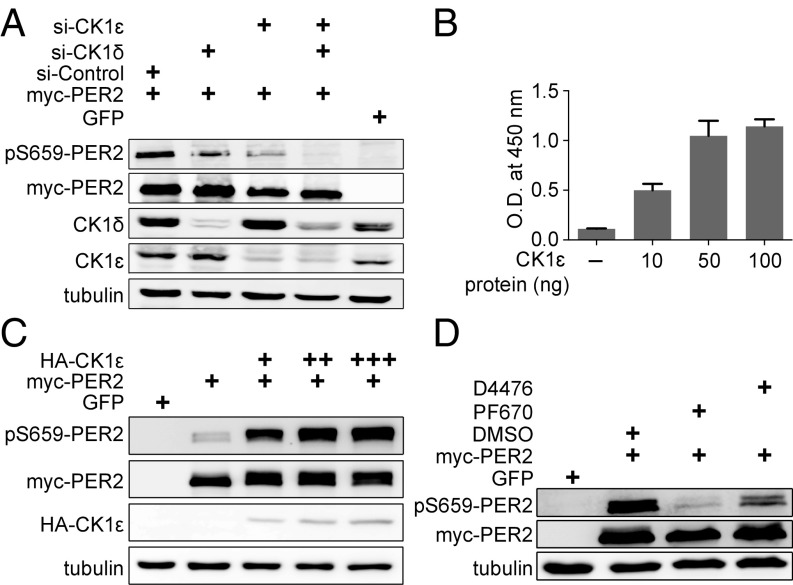
Fig. 3.
CK1δ and CK1ε are required for mPER2 priming phosphorylation in cells. (A) Knockdown of CK1δ and/or CK1ε impairs mPER2 phosphorylation at S659. The immunoblot is representative of duplicate experiments. (B) CK1ε phosphorylates the FASP peptide on S659, as assessed by ELISA. Error bars represent SD (n = 3 biological replicates per point). (C) CK1ε phosphorylates mPER2 in cells. mPer2 (1 µg plasmid) was cotransfected with 10 ng (+), 25 ng (++), and 50 ng (+++) of CK1ε expression plasmid, and lysates were probed for S659 phosphorylation. The experiment was performed twice, with identical results. (D) Inhibitors of CK1δ/ε block phosphorylation of the priming site in cells. PF670 (1 µM) and D4476 (5 µM) were added to cells expressing myc-mPER2. The experiment was performed three times, with similar results.
The Role of the CK1δ/ε Carboxyl Terminus in Priming-Site Phosphorylation.
Fustin et al. (31) suggested that CK1δ1 and CK1δ2 have differing activities in phosphorylation of the priming site. CK1δ2 differs from CK1δ1 only in the last 16 amino acids due to inclusion of a penultimate exon in CK1δ2 that alters the reading frame (Fig. 4A). In fact, CK1δ2 more closely resembles CK1ε at the extreme carboxyl terminus. We assessed the relative priming activity of the three kinases in cells. CK1δ2 and CK1ε are more active than CK1δ1 in phosphorylating the priming site, as illustrated in the immunoblot in Fig. 4B and as presented quantitatively in Fig. 4C. Consistent with this, Fustin et al. (31) found that overexpression of CK1δ2, more active on the FASP site, lengthens the circadian period in cells, whereas specific knockdown of CK1δ1 shortens period. This suggests a previously unappreciated role for the extreme carboxyl terminus of CK1 in influencing the activity of the kinase. Confirming this, truncation of CK1δ1 at amino acid 400, removing the last 16 residues, increased the activity of the kinase on the priming site to that of CK1δ2 and CK1ε (Fig. 4 B and C). Thus, the activity of CK1δ/ε as a priming kinase is tightly regulated by the extreme carboxyl terminus of the protein.
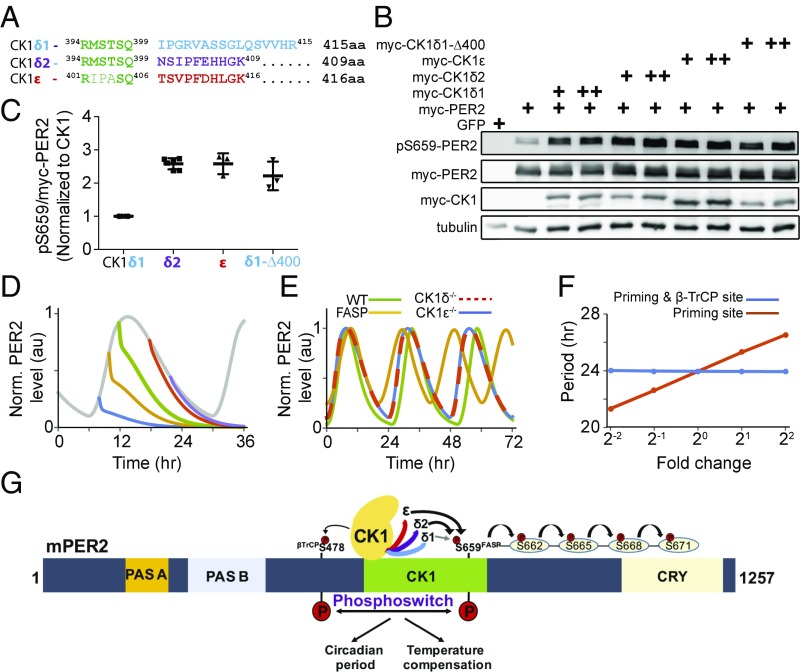
Fig. 4.
The extreme carboxyl terminus of CK1 regulates priming phosphorylation. (A) Alignment of the carboxyl termini of CK1δ1, CK1δ2, and CK1ε. (B) CK1δ2 and CK1ε are more-active priming kinases than the CK1δ1. mPer2 plasmid (1 µg) was cotransfected with 5 ng (+) and 10 ng (++) of indicated CK1 isoforms, and lysates were probed with indicated antibodies. The experiment was performed multiple times, with similar results. (C) Quantitative analysis of the priming kinase activity of CK1 isoforms. The ratio of pS659 to myc-PER2 was calculated after normalization to CK1 expression, and the value of CK1δ1 was taken as 1 to express fold change. n = 6 lanes for CK1δ1 and CK1δ2; n = 3 for CK1ε and CK1δ1-D400. (D) The identified priming kinases recapitulate the phosphoswitch model. Here, translation rates of the model are exponentially decreased with a half-life (∼0.13 h) after cycloheximide treatment at various timings. (E) The model accurately simulates various CK1 mutation phenotypes (13, 18, 33). (F) Phosphorylation of the FASP site has a profound impact on period. Period is robust to global changes in CK1 phosphorylation activity but sensitive to isolated change of the phosphorylation rate on the priming site. Here, the model parameters, kri (i = 1, 2) and/or kpo, are changed in the same magnitudes (SI Appendix, Table S3). (G) CK1δ2 and CK1ε are more-active priming kinases than CK1δ1. Diagrammatic representation of the mPER2 protein shows PAS-A and PAS-B domains and the CK1 and CRY binding regions.
Revising the Phosphoswitch Mathematical Model.
We previously proposed a phosphoswitch model in which the PER2 protein switches between two fates (5). When the β-TrCP site is phosphorylated by CK1δ/ε, PER2 is rapidly degraded. Based on previous experimental data (32), we also proposed a priming kinase that phosphorylates the FASP site, stabilizes PER2, and allows for sequential phosphorylation of PER2 downstream from the FASP site by CK1. With the identification of CK1 as the priming kinase, we asked whether the phosphoswitch model was still predictive and accurate. We modified our previous phosphoswitch model by incorporating the finding that CK1ε/δ1/δ2 functions as the priming kinase (see SI Appendix, Materials and Methods for details). This revised model continues to accurately simulate the unusual kinetics of PER2 degradation where, during phases when PER2 accumulates in the cell, its degradation curve contains a plateau (Fig. 4D). Furthermore, the model successfully reproduces the negligible period change of CK1ε−/− mutant mice (33), the longer period of CK1δ−/− mutant mice (18, 33), and the shorter period of FASP humans and mice (11, 13) (Fig. 4E). CK1 phosphorylation of PER2 is temperature compensated in vitro by balancing the higher dissociation constant and higher phosphorylation rate at higher temperatures, which is proposed as a temperature compensation mechanism of circadian period (4, 6). Consistent with this finding, when the dissociation constant of CK1 and phosphorylation rates for both the priming site and the β-TrCP site of PER2 increase together, the period is nearly constant in the model (SI Appendix, Fig. S4).
Discussion
Regulation of PER2 abundance controls circadian timing, yet understanding the phosphorylation of the key control point has been elusive. Here, we show by multiple approaches that CK1δ/ε can function as the PER2 priming kinase, resolving a long-standing question in circadian biology. Additionally, we find that CK1δ1 and CK1ε differ markedly in their ability to phosphorylate the priming site. CK1δ2, which has residues at the extreme carboxyl terminus that more closely resemble CK1ε, displays increased priming kinase activity like CK1ε, suggesting that control of splicing can also regulate priming and, hence, clock timing (31). These studies highlight the role of the CK1δ/ε carboxyl terminus (Fig. 4G). Prior studies demonstrated that while CK1ε does not require its carboxyl terminus to bind to PER proteins, inclusion of the tail can significantly enhance the interaction (34, 35). Because CK1δ/ε is also regulated by phosphorylation and dephosphorylation of the carboxyl terminus, this also suggests a mechanism for cellular signaling pathways to regulate PER2 priming rates (26, 36–40). For example, changes in phosphorylation of the CK1δ/ε carboxyl terminus by cell cycle-dependent kinases has recently been shown to alter CK1δ activity on PER2 (41). Other signaling pathways that impinge on CK1δ/ε carboxyl-terminal phosphorylation, such as metabotropic glutamate and Wnt signaling, could further influence priming and, hence, alter the clock period (42, 43).
CK1δ/ε has the additional feature of being temperature compensated—that is, the activity of the kinase on selected substrates is relatively constant as temperature increases (4). A molecular mechanism for this has recently been proposed, involving key residues adjacent to the substrate recognition motif (6). Since CK1 phosphorylation of PER2 is temperature compensated in vitro and CK1 is involved in both priming and degradation, how does the time required for PER2 degradation lengthen at higher temperatures, a property we showed is essential for the temperature compensation of circadian period (5)? The answer may lie in a better understanding of how the carboxyl terminus of the CK1δ/ε family members regulates temperature compensation on different substrates. The regulation of a fraction of the CK1ε/δ1/δ2 kinase pool (in which each member has a different tail sequence and, thus, priming phosphorylation rate), may provide a mechanism for temperature-compensated regulation of circadian period. Additionally, CK1 may have different Q10 on the priming and β-TrCP sites in PER2 so that temperature compensation of the circadian clock utilizes the differential phosphorylation of the priming and β-TrCP sites to regulate the phosphoswitch controlling mPER2 stability (5). This is consistent with the data from Ueda and coworkers (4), who reported different Q10 for different forms of recombinant CK1. However, it is unfortunately challenging to study temperature compensation in vitro because of the rapid inactivating autophosphorylation of full-length CK1δ/ε in the presence of ATP. In contrast, in vivo, the net phosphorylation of the carboxyl terminus of CK1δ/ε is highly dynamic, balancing active autophosphorylation and phosphorylation by other kinases with active dephosphorylation by multiple phosphatases (36, 37, 42). In the phosphoswitch model, when the phosphorylation rate for the priming site is increased, which we now show strongly depends on the CK1 carboxyl terminus (Fig. 4 B and C), the period changes dramatically (Fig. 4F). On the other hand, when the phosphorylation rates for both the priming site and the β-TrCP site increase equally, the period is nearly constant (Fig. 4F). This shows that the CK1 phosphorylation of PER2 follows a “robust yet fragile” paradigm that is emerging in systems biology, where cellular systems are robustly resistant to environmental changes yet can still react to specific signaling pathways.
The differential activity of CK1δ and CK1ε on priming phosphorylation must also be viewed in the context of their relative protein abundance. Knockout studies have demonstrated that CK1δ plays a major role in regulation of the circadian clock in vivo (17, 33). Quantitative mass spectrometry studies have recently found that CK1δ protein was over 20-fold more abundant than CK1ε in liver and that CK1δ is more readily detectable in PER-containing macromolecular circadian complexes (16, 27, 28). However, we found that CK1ε is significantly more active than CK1δ1 as a priming kinase, which may explain why the tau mutation of CK1ε has a semidominant phenotype (19). Together, the data here and from Fustin et al. (31) indicate that dynamic posttranscriptional splicing and posttranslational phosphorylation of CK1δ and CK1ε can regulate priming and, hence, PER2 activity, providing important flexibility to regulate clock speed.
Materials and Methods
A detailed description of reagents, methods, and mathematical models, including the various parameters analyzed, equations applied, and the Mathematica code, can be found in SI Appendix, Materials and Methods. The rabbit polyclonal antibody against pS659 and pS662 of mouse PER2 were developed at Abfrontier (Young In Frontier Co.) using the phosphopeptides GKAEpSVVSLT-Cys and KAESVVpSLTSQ-Cys, respectively.
Supplementary Material
Acknowledgments
We thank Dae Wook Kim for assistance with the mathematical model. This work was funded by the National Medical Research Council of Singapore Grant NMRC/CIRG/1465/2017 (to D.M.V.); the National Research Foundation of Korea grant and the POSCO TJ Park Foundation Science Fellowship (to J.K.K.); the National Institutes of Health Grants R01 GM107069 and R01 GM121507, as well as funds from the NIH Office of the Director under Award S10OD018455 (to C.L.P.); and the National Science Foundation Grant DMS-1714094 (to D.B.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The NMR chemical shift assignments for the mPER2 FASP peptide have been deposited in the Biological Magnetic Resonance Bank database (accession no. 27306).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721076115/-/DCSupplemental.
References
- 1.Konopka RJ, Benzer S. Clock mutants of Drosophila melanogaster. Proc Natl Acad Sci USA. 1971;68:2112–2116. doi: 10.1073/pnas.68.9.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee C, Etchegaray JP, Cagampang FR, Loudon AS, Reppert SM. Posttranslational mechanisms regulate the mammalian circadian clock. Cell. 2001;107:855–867. doi: 10.1016/s0092-8674(01)00610-9. [DOI] [PubMed] [Google Scholar]
- 3.Edery I, Zwiebel LJ, Dembinska ME, Rosbash M. Temporal phosphorylation of the Drosophila period protein. Proc Natl Acad Sci USA. 1994;91:2260–2264. doi: 10.1073/pnas.91.6.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Isojima Y, et al. CKIε/δ-dependent phosphorylation is a temperature-insensitive, period-determining process in the mammalian circadian clock. Proc Natl Acad Sci USA. 2009;106:15744–15749. doi: 10.1073/pnas.0908733106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou M, Kim JK, Eng GWL, Forger DB, Virshup DM. A Period2 phosphoswitch regulates and temperature compensates circadian period. Mol Cell. 2015;60:77–88. doi: 10.1016/j.molcel.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 6.Shinohara Y, et al. Temperature-sensitive substrate and product binding underlie temperature-compensated phosphorylation in the clock. Mol Cell. 2017;67:783–798.e20. doi: 10.1016/j.molcel.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Badura L, et al. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J Pharmacol Exp Ther. 2007;322:730–738. doi: 10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- 8.Kim JK, et al. Modeling and validating chronic pharmacological manipulation of circadian rhythms. CPT Pharmacometrics Syst Pharmacol. 2013;2:e57. doi: 10.1038/psp.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kloss B, et al. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iε. Cell. 1998;94:97–107. doi: 10.1016/s0092-8674(00)81225-8. [DOI] [PubMed] [Google Scholar]
- 10.Price JL, et al. double-time is a novel Drosophila clock gene that regulates PERIOD protein accumulation. Cell. 1998;94:83–95. doi: 10.1016/s0092-8674(00)81224-6. [DOI] [PubMed] [Google Scholar]
- 11.Toh KL, et al. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science. 2001;291:1040–1043. doi: 10.1126/science.1057499. [DOI] [PubMed] [Google Scholar]
- 12.Meng Q-J, et al. Setting clock speed in mammals: The CK1ε tau mutation in mice accelerates circadian pacemakers by selectively destabilizing PERIOD proteins. Neuron. 2008;58:78–88. doi: 10.1016/j.neuron.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, et al. Modeling of a human circadian mutation yields insights into clock regulation by PER2. Cell. 2007;128:59–70. doi: 10.1016/j.cell.2006.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowrey PL, et al. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science. 2000;288:483–492. doi: 10.1126/science.288.5465.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eide EJ, et al. Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation. Mol Cell Biol. 2005;25:2795–2807. doi: 10.1128/MCB.25.7.2795-2807.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narumi R, et al. Mass spectrometry-based absolute quantification reveals rhythmic variation of mouse circadian clock proteins. Proc Natl Acad Sci USA. 2016;113:E3461–E3467. doi: 10.1073/pnas.1603799113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Etchegaray JP, et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol Cell Biol. 2009;29:3853–3866. doi: 10.1128/MCB.00338-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee H, Chen R, Lee Y, Yoo S, Lee C. Essential roles of CKIδ and CKIε in the mammalian circadian clock. Proc Natl Acad Sci USA. 2009;106:21359–21364. doi: 10.1073/pnas.0906651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ralph MR, Menaker M. A mutation of the circadian system in golden hamsters. Science. 1988;241:1225–1227. doi: 10.1126/science.3413487. [DOI] [PubMed] [Google Scholar]
- 20.Tosini G, Menaker M. The tau mutation affects temperature compensation of hamster retinal circadian oscillators. Neuroreport. 1998;9:1001–1005. doi: 10.1097/00001756-199804200-00009. [DOI] [PubMed] [Google Scholar]
- 21.Shanware NP, et al. Casein kinase 1-dependent phosphorylation of familial advanced sleep phase syndrome-associated residues controls PERIOD 2 stability. J Biol Chem. 2011;286:12766–12774. doi: 10.1074/jbc.M111.224014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flotow H, et al. Phosphate groups as substrate determinants for casein kinase I action. J Biol Chem. 1990;265:14264–14269. [PubMed] [Google Scholar]
- 23.Chiu JC, Ko HW, Edery I. NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell. 2011;145:357–370. doi: 10.1016/j.cell.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko HW, et al. A hierarchical phosphorylation cascade that regulates the timing of PERIOD nuclear entry reveals novel roles for proline-directed kinases and GSK-3β/SGG in circadian clocks. J Neurosci. 2010;30:12664–12675. doi: 10.1523/JNEUROSCI.1586-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Alessandro M, et al. Stability of wake-sleep cycles requires robust degradation of the PERIOD protein. Curr Biol. 2017;27:3454–3467.e8. doi: 10.1016/j.cub.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graves PR, Roach PJ. Role of COOH-terminal phosphorylation in the regulation of casein kinase I delta. J Biol Chem. 1995;270:21689–21694. doi: 10.1074/jbc.270.37.21689. [DOI] [PubMed] [Google Scholar]
- 27.Aryal RP, et al. Macromolecular assemblies of the mammalian circadian clock. Mol Cell. 2017;67:770–782.e6. doi: 10.1016/j.molcel.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duong HA, Robles MS, Knutti D, Weitz CJ. A molecular mechanism for circadian clock negative feedback. Science. 2011;332:1436–1439. doi: 10.1126/science.1196766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mylona A, et al. Opposing effects of Elk-1 multisite phosphorylation shape its response to ERK activation. Science. 2016;354:233–237. doi: 10.1126/science.aad1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Theillet F-X, et al. Site-specific NMR mapping and time-resolved monitoring of serine and threonine phosphorylation in reconstituted kinase reactions and mammalian cell extracts. Nat Protoc. 2013;8:1416–1432. doi: 10.1038/nprot.2013.083. [DOI] [PubMed] [Google Scholar]
- 31.Fustin J-M, et al. Two Ck1δ transcripts regulated by m6A methylation code for two antagonistic kinases in the control of the circadian clock. Proc Natl Acad Sci USA. 2018;115:5980–5985. doi: 10.1073/pnas.1721371115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vanselow K, et al. Differential effects of PER2 phosphorylation: Molecular basis for the human familial advanced sleep phase syndrome (FASPS) Genes Dev. 2006;20:2660–2672. doi: 10.1101/gad.397006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etchegaray J-P, Yu EA, Indic P, Dallmann R, Weaver DR. Casein kinase 1 delta (CK1δ) regulates period length of the mouse suprachiasmatic circadian clock in vitro. PLoS One. 2010;5:e10303. doi: 10.1371/journal.pone.0010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dahlberg CL, Nguyen EZ, Goodlett D, Kimelman D. Interactions between casein kinase Iε (CKIε) and two substrates from disparate signaling pathways reveal mechanisms for substrate-kinase specificity. PLoS One. 2009;4:e4766. doi: 10.1371/journal.pone.0004766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vielhaber E, Eide E, Rivers A, Gao ZH, Virshup DM. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol Cell Biol. 2000;20:4888–4899. doi: 10.1128/mcb.20.13.4888-4899.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cegielska A, Gietzen KF, Rivers A, Virshup DM. Autoinhibition of casein kinase I epsilon (CKIε) is relieved by protein phosphatases and limited proteolysis. J Biol Chem. 1998;273:1357–1364. doi: 10.1074/jbc.273.3.1357. [DOI] [PubMed] [Google Scholar]
- 37.Rivers A, Gietzen KF, Vielhaber E, Virshup DM. Regulation of casein kinase I epsilon and casein kinase I delta by an in vivo futile phosphorylation cycle. J Biol Chem. 1998;273:15980–15984. doi: 10.1074/jbc.273.26.15980. [DOI] [PubMed] [Google Scholar]
- 38.Gallego M, Eide EJ, Woolf MF, Virshup DM, Forger DB. An opposite role for tau in circadian rhythms revealed by mathematical modeling. Proc Natl Acad Sci USA. 2006;103:10618–10623. doi: 10.1073/pnas.0604511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hirano A, Fu Y-H, Ptáček LJ. The intricate dance of post-translational modifications in the rhythm of life. Nat Struct Mol Biol. 2016;23:1053–1060. doi: 10.1038/nsmb.3326. [DOI] [PubMed] [Google Scholar]
- 40.Giamas G, et al. Phosphorylation of CK1δ: Identification of Ser370 as the major phosphorylation site targeted by PKA in vitro and in vivo. Biochem J. 2007;406:389–398. doi: 10.1042/BJ20070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eng GWL, Edison, Virshup DM. Site-specific phosphorylation of casein kinase 1 δ (CK1δ) regulates its activity towards the circadian regulator PER2. PLoS One. 2017;12:e0177834. doi: 10.1371/journal.pone.0177834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swiatek W, et al. Regulation of casein kinase Iε activity by Wnt signaling. J Biol Chem. 2004;279:13011–13017. doi: 10.1074/jbc.M304682200. [DOI] [PubMed] [Google Scholar]
- 43.Liu F, Virshup DM, Nairn AC, Greengard P. Mechanism of regulation of casein kinase I activity by group I metabotropic glutamate receptors. J Biol Chem. 2002;277:45393–45399. doi: 10.1074/jbc.M204499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.