Abstract
Sequencing of the 16S ribosomal RNA (rRNA) gene is widely used to survey microbial communities. Specialized 16S rRNA databases have been developed to support this approach including Greengenes, RDP and SILVA. Most taxonomy annotations in these databases are predictions from sequence rather than authoritative assignments based on studies of type strains or isolates. In this work, I investigated the taxonomy annotations and guide trees provided by these databases. Using a blinded test, I estimated that the annotation error rate of the RDP database is ∼10%. The branching orders of the Greengenes and SILVA guide trees were found to disagree at comparable rates with each other and with taxonomy annotations according to the training set (authoritative reference) provided by RDP, indicating that the trees have comparable quality. Pervasive conflicts between tree branching order and type strain taxonomies strongly suggest that the guide trees are unreliable guides to phylogeny. I found 249,490 identical sequences with conflicting annotations in SILVA v128 and Greengenes v13.5 at ranks up to phylum (7,804 conflicts), indicating that the annotation error rate in these databases is ∼17%.
Keywords: Taxonomy, Microbiology, Bioinformatics
Background
Next-generation sequencing of markers such as the 16S ribosomal RNA (rRNA) gene and fungal internal transcribed spacer region has revolutionized the study of microbial communities in environments ranging from the human body (Cho & Blaser, 2012; Pflughoeft & Versalovic, 2012) to oceans (Moran, 2015) and soils (Hartmann et al., 2014). Specialized 16S rRNA sequence databases providing taxonomy annotations include Greengenes (DeSantis et al., 2006), LTP (Yarza et al., 2008), RDP (Maidak et al., 2001) and SILVA (Pruesse et al., 2007). LTP is based on sequences of type strains and isolates classified on the basis of observed traits. The other databases are much larger, containing mostly environmental sequences. In the RDP database, taxonomy annotations of environmental sequences are predicted by the Naive Bayesian Classifier (NBC) (Wang et al., 2007), while those in Greengenes and SILVA are annotated by a combination of database-specific computational prediction methods and manual curation based on predicted phylogenetic trees inferred from multiple sequence alignments (McDonald et al., 2012; Yilmaz et al., 2014).
Microbial taxonomy and phylogeny
The goal of taxonomy has been described as classifying living organisms into a hierarchy of categories (taxa) that are useful (based on biologically informative traits) and monophyletic (consistent with the true phylogenetic tree) (Hennig, 1965), though this point of view is not universally accepted (Benton, 2000). With microbial markers such as 16S rRNA, most organisms are known only from environmental sequencing, and in these cases predictions of taxonomy must necessarily be made from sequence evidence alone. One approach to predicting taxonomy is to infer a tree from available sequences, on the assumption that the tree will be a reasonable approximation to the phylogenetic tree and will therefore correlate with taxonomy (McDonald et al., 2012; Yilmaz et al., 2014). While the question of whether taxonomy should be consistent with phylogeny is controversial, the curators of Greengenes, LTP and SILVA have stated that they consider consistency to be important: “Greengenes is a dedicated full-length 16S rRNA gene database that provides users with a curated taxonomy based on de novo tree inference” (McDonald et al., 2012); “(LTP is based on) comparative sequence analysis of small subunit (SSU) rRNA (which) has been established as the gold standard for reconstructing phylogenetic relationships among prokaryotes for classification purposes” (Yarza et al., 2008); “SILVA predominantly uses phylogenetic classification based on an SSU guide tree…discrepancies are resolved with the overall aim of making classification consistent with phylogeny” (Yilmaz et al., 2014). By contrast, taxonomy annotations for environmental sequences in RDP are predictions made by an algorithm, the NBC, which does not consider phylogeny.
Taxonomy annotation conflicts
If an environmental sequence is annotated as belonging to a taxon which is defined by traits, then this is a prediction which can always be checked in principle, and sometimes can be checked in practice. For example, according to Bergey’s Manual of Systematic Bacteriology (Bergey, 2001), Salmonella has peritrichous flagella, is non-spore-forming, Gram-negative, with cell lengths from 2 to 5 µm and diameters between about 0.7 and 1.5 µm. If an environmental sequence is annotated as Salmonella, it is a prediction that these traits are present. This prediction can be checked if the sequence is later found in a cultured strain; this is what I call a blinded test. Rhodococcus has distinctly different traits, e.g., it is non-motile and Gram-positive. If SILVA annotates a sequence as Salmonella and Greengenes annotates the same sequence as Rhodococcus, then it is certain that at least one of the annotations is objectively wrong, though verification or falsification is not possible until the sequence is found in cells which can be examined for traits. Similarly, if SILVA leaves the genus blank and Greengenes annotates the same sequence as Salmonella, then either SILVA is a false negative or Greengenes is a false positive, and again at least one of the databases is certainly wrong. Many environmental sequences do not belong to named genera. For a given sequence, this can be verified experimentally by finding the sequence in an isolated strain having traits that do not match anything in the standard. Suppose SILVA and Greengenes agree that the genus is blank but give different family names. If the standard defines both families by traits, then at least one of them is certainly wrong. However, in microbial taxonomy, some higher taxa are defined simply as groups of lower taxa without explicitly listing characteristic traits. Such groups are defined based on evidence that the lower taxa are related, and are subject to revision when new evidence suggests that they are polyphyletic. In these cases, the annotation can be regarded as a prediction that the sequence is found below the lowest common ancestor (LCA) node for the group in the true phylogenetic tree. Such a prediction is objectively true or false, but cannot be verified with certainty by any foreseeable method. Regardless of these difficulties, if SILVA and Greengenes disagree on the annotation of a group that is predicted to be monophyletic, then at least one of them must be wrong.
Phylogenetic trees, gene trees and horizontal transfer
The history of vertical (i.e., clonal or sexual) inheritance can be represented as a binary tree. Inheritance can also occur by horizontal transfer of DNA between cells, which has been inferred to occur at a vast range of evolutionary distances ranging from members of the same species to exchanges between different kingdoms (Jain et al., 2002). If a trait is acquired by horizontal transfer, then a graph representing its inheritance is not a tree. If horizontal transfer is rare, then in principle a phylogenetic tree could be constructed by following only vertically inherited traits. Gene content is sometimes highly variable between sets of prokaryotic genomes with otherwise highly similar sequences, which has been interpreted to imply that the optional genes which may be absent are frequently horizontally transferred (Gogarten & Townsend, 2005). If traits used for classification are acquired by horizontal transfer, then it may be impossible to achieve consistency between taxonomy and phylogeny. It is widely believed that the 16S rRNA gene is unlikely to be horizontally transferred and is thus well-suited as a phylogenetic marker (Woese, 1987), though recent evidence suggests that transfer of 16S rRNA genes can occur when sequence identity is as low as ∼80% (Kitahara & Miyazaki, 2013). The evolutionary history of a single gene can be represented as a binary tree if its sequences are inherited as intact units, i.e., providing that rearrangement events such as cross-over recombinations do not occur. If rearrangement events and horizontal transfers are rare for the 16S rRNA gene, as is widely believed to be the case, then its true gene tree is likely to be a good approximation to the true phylogenetic tree based on vertically inherited traits, assuming that the latter tree can be meaningfully defined. However, even if there is good reason to believe that the true gene tree would be an accurate guide to taxonomy, it is very challenging to estimate the gene tree from multiple sequence alignments that span vast evolutionary distances, and it is an open question whether estimated gene trees enable accurate taxonomy prediction in practice.
Taxonomy prediction from a sequence-based tree
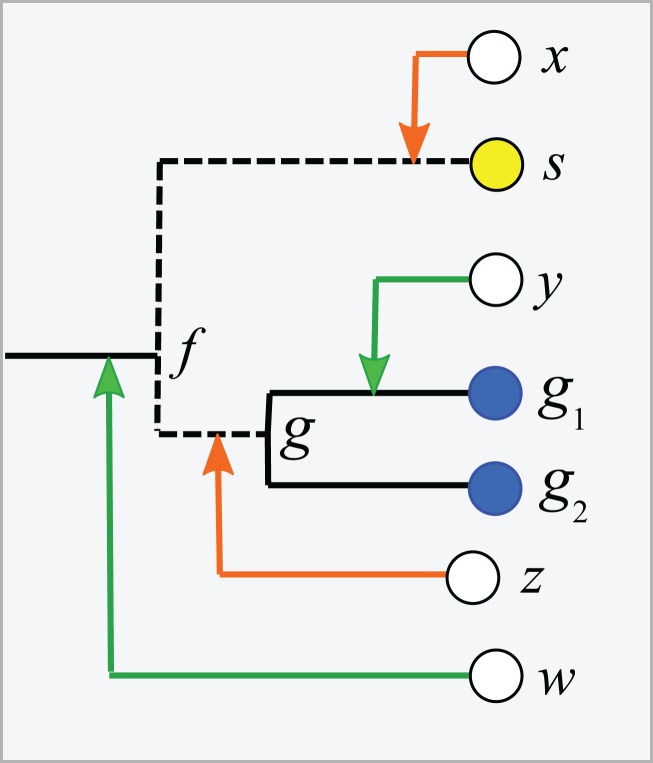
Consider a binary tree with authoritative taxonomies for a small subset of its leaves. The goal is to predict taxonomies for the remaining leaves which represent organisms known only from sequence, based on the assumption that taxonomy is consistent with the tree. The authoritative tree is the implied binary tree for the subset of sequences with authoritative taxonomies, i.e., classifications based on direct observations of characteristic traits. Note that the annotations of this subset tree are considered to be authoritative, but not necessarily the branching order. The LCA of a taxon is the lowest node above all its leaves. If the branching order is correct and the taxonomy is consistent with the tree, then the subtree under the LCA for a taxon is pure, i.e., contains no other taxa at the same rank (this is the definition of consistency). Purity is closely related to monophyly; here I am making a distinction between consistency with a predicted tree which may have branching order errors (purity) and the true, but almost always unknown, phylogeny (monophyly). I will use upper-case letters to represent taxon names and lower-case letters for tree nodes and sequences. The lowest pure taxon (LPT) for a node is the taxon at lowest rank for which all the leaves in its subtree belong to the same taxon. For example, the LPT for node n is family F if, and only if, the leaves in the subtree under n belong to two or more genera in F. If F contains exactly one genus G, then F cannot be the LPT of any node because if F is pure then G will also be pure; G is lower, and the LPT would then be G rather than F. A pure edge connects two nodes having the same LPT. For example, consider the tree shown in Fig. 1. Sequences s, g1 and g2 are type strains with authoritative taxonomy; w, x, y and z are sequences obtained from environmental samples. The authoritative tree (black edges) connects s, g1 and g2. Genus is the lowest rank; s belongs to a singleton genus S (i.e., it is the only authoritatively named sequence for that genus); g1 and g2 belong to the same genus G. S and G belong to the same family, F. The LCA for S is s (a singleton leaf is always its own LCA), and the LCA for G is g. The LPTs of g1, g2 and g are G, the LPT of s is S, and the LPT of f is F. Pure edges are shown by continuous lines; impure edges are dashed lines. In general, if a tree is consistent with taxonomy, then all impure edges join an LCA node for some taxon t to a node which has an LPT at a higher rank than t, and conversely every LCA node has one impure edge which connects it to a higher node.
Figure 1. Example tree showing join nodes and impure edges.
Leaves s, g1 and g2 are sequences with authoritative taxonomies. Sequences w, x, y and z are environmental sequences. Sequence s belongs to genus S, which is a singleton (i.e., has only one authoritative sequence); sequences g1 and g2 belong to genus G. The LCA for G is g, the LCA for S is s. The inferred positions of w, x, y and z in the tree are indicated by arrows. An arrowhead is a join node, i.e., the new node created by adding a new sequence to the tree. Black edges are the authoritative tree, i.e., the binary tree for leaves with authoritative taxonomies. Dashed lines are impure edges, continuous lines are pure edges (see main text).
The clade extension problem
Adding a new sequence (q) of unknown taxonomy introduces a new node into the tree, its join node. Assume the tree is correct and the taxonomy is consistent with the tree. Then, if the join node is below the LCA for taxon t, q certainly belongs to t. In Fig. 1, join nodes are indicated by arrow-heads. Sequence y certainly belongs to G because its join node is below g, the LCA of G. Also, w certainly belongs to a novel genus because it joins a family edge (which is above two or more genera by definition), and a new genus must therefore be introduced because it is impossible for the join node or any node above it to be a pure LCA of any known genus. The environmental sequence x joins the tree at the impure edge joining s and f, so it is above the LCA for S, but the subtree under the join node contains only S. The tree therefore cannot resolve whether x belongs to S or to a novel genus because both possibilities are consistent with the available data. If x belongs to S, its join node will be the new LCA for S, expanding the clade by moving its LCA higher in the tree. Otherwise, x belongs to a novel singleton genus, call it V, and x will be the LCA of V if it is added to the tree. Thus, s is the only sequence which unambiguously belongs to S—if a new sequence q differs from s, then the join node is above the LCA for S and q could belong to a different genus. This ambiguity could be resolved by introducing a sequence identity threshold. For example, if x is 97% identical to s, then it might be reasonable to assign x to S, but identity thresholds are only approximate guides and this prediction is uncertain even if the tree is assumed to be correct. Thus, an environmental sequence can be assigned to a singleton taxon only by considering sequence similarity, which is unreliable even under the idealized assumptions that the tree is correct and consistent with taxonomy. This is significant in practice because roughly half of named genera currently have only a single sequence in authoritative databases. A similar problem arises in non-singleton taxa. The join node for z is above the LCA for G, but the subtree below the join node contains only G and z could therefore belong to G (which would expand the clade by moving the LCA for G up to the join node for z), or to a novel genus, in which case z would be a new singleton LCA. In general, if an environmental sequence q joins the tree at an impure edge, then the tree is consistent with two incompatible predictions: (1) assigning q to a new taxon, or (2) extending an existing clade to include q.
Impure taxa
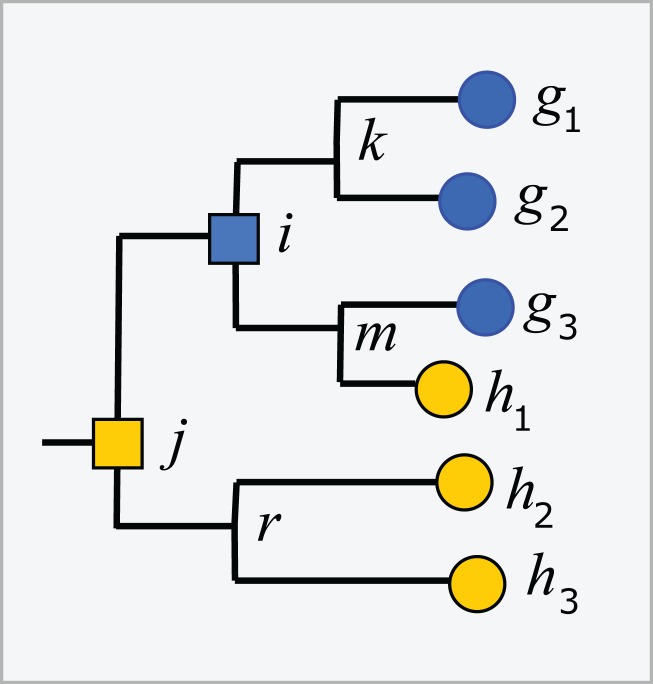
Trees inferred from large sequence sets are unreliable guides to phylogeny due to incorrect branching orders (Huelsenbeck, Rannala & Masly, 2000; Philippe et al., 2011). Naive assumptions that the tree is correct and the taxonomy is consistent with the tree are violated by branching order errors and also by taxa which are found to overlap by sequence such as Escherichia and Shigella (Escobar-Páramo et al., 2003), which may be regarded as errors in the taxonomy. A conflict between the tree and the taxonomy occurs when taxa at the same rank overlap (Fig. 2). An overlapped leaf is below LCAs for two or more taxa at the same rank. Conversely, a taxon t is consistent with the tree if, and only if, the subtree under the LCA for t is pure. In Fig. 2, i is the LCA for G and j is the LCA for H; neither is pure because i is above h1 and j is above all members of both G and H. If an environmental sequence q joins the tree at an edge where taxa overlap, i.e., the edge is above leaves belonging to two or more taxa at a given rank but below the LCAs for those taxa, the prediction at that rank is ambiguous. For example, consider cases where the join node for q is in i–k, i–m, h1–m or g3–m. Here, q could plausibly belong to G, H or a novel genus, depending on the underlying explanation of the tree conflict. If q joins the tree below a node for which taxon t is pure, then it is plausible that q belongs to t, though this purity heuristic is not reliable because the branching order may be incorrect. For example, suppose h1 in Fig. 2 is an environmental sequence rather than a known strain, then the subtree under i is pure at genus rank and h1 would be incorrectly assigned to G by the purity heuristic.
Figure 2. Example tree showing conflicts with taxonomy.
Leaves g1, g2 and g3 belong to genus G, h1, h2 and h3 belong to a different genus H. The tree is not consistent with the taxonomy because G and H overlap. The LCA for G is i, which is above h1, and the LCA for H is j, which is above all members of both G and H. Overlaps are common in practice (Fig. 5).
Taxonomic nomenclatures
At least three prokaryotic taxonomy nomenclatures are widely used. The Greengenes nomenclature is based on the NCBI Taxonomy database (Sayers et al., 2011), RDP uses Bergey’s Manual (Bergey, 2001), while LPT and SILVA are based on Bergey’s Manual and LSPN (Parte, 2014). These nomenclatures differ mainly in revisions to resolve conflicts with sequence-based phylogenies and add new candidate groups identified in environmental sequences. For example, Escherichia and Shigella overlap as noted above, which Greengenes resolves by leaving their sequences unclassified at ranks below Enterobacteriaceae. SILVA, RDP and LTP deal with this issue differently by retaining well-established species names such as Escherichia coli and introducing a combined genus named Escherichia–Shigella. An example of an environmental candidate group is JS1 (Webster et al., 2004), which has been adopted in the SILVA nomenclature but not in RDP or Greengenes at the time of writing.
Methods
Database versions
Unless otherwise stated, I used these databases: Greengenes v13.5, LTP v128, RDP downloaded on 15th January 2017, SILVA v128, the RDP 16S rRNA training set v16, and the BLAST 16S rRNA reference database (Sayers et al., 2011) downloaded 17th January 2017 (BLAST16S).
Common nomenclature
For a given pair of databases, I identified their common nomenclature as the set of taxon names appearing in both databases.
Nomenclature hierarchy conflicts
Most well-established taxa are found in all nomenclatures, and in these cases a biologist would presumably expect a taxon name to be placed within the same group at a higher rank. To investigate this for a given pair of databases, I identified cases where a taxon name and its parent name were both in the common nomenclature in at least one of the databases, but the databases nevertheless disagreed on the parent name.
Conflicting annotations of the same sequence
For a given pair of databases, I identified sequences that were present in both databases and were annotated as belonging to different taxa where at least one of the taxon names was in the common nomenclature.
Conflicts between the LTP taxonomy and the LTP guide tree
For each taxon in the LTP nomenclature, I identified its LCA in the LTP tree (i.e., the lowest node above all members of the taxon), and then the overlapped taxa and leaves at each rank.
Blinded testing of taxonomy predictions
If a sequence that was previously known only from environmental samples is found in a newly sequenced isolate strain, it can provide a blinded test of taxonomy prediction analogous to CASP (Moult, 2005), where protein structures predicted from sequence are compared to newly solved structures determined by sequence-independent methods such as X-ray crystallography. In principle, predictions for environmental sequences in an older version of Greengenes or SILVA could be assessed by finding identical sequences of isolates deposited more recently, but this approach is stymied by a lack of data specifying which sequences were used as references and the deposition dates of new isolate sequences. With RDP, it is straightforward because some of the older references (called training sets) for the RDP Naive Bayesian Classifier are deposited in public archives, and new sequences are readily identifiable as those not found in an older training set. If a training set is considered to be an authoritative reference, this enables predictions for new, authoritatively classified sequences to be assessed using an old training set as a reference, which is effectively equivalent to a blinded test. I implemented this test using versions 9 and 16 of the RDP 16S rRNA training sets, which were the oldest and newest, respectively, available for download at the time this work was performed. Version 9 contains 10,049 sequences; version 16 has 13,212 and therefore contains more than 3,000 new sequences. I assessed predictions by NBC on these new sequences using v9 as a reference and the v16 annotations as the truth standard.
Prediction performance metrics for the blinded test
I used the following metrics (Edgar, 2014, 2018) to measure prediction accuracy using a reference database (A) with a query set (S) containing sequences with known taxonomy. I distinguish two types of false positive. An over-classification error is a false positive where an incorrect name is predicted at a rank which in fact is novel, i.e., the true name is not present in the reference database. A misclassification error is a false positive where the taxon is known but the wrong name is predicted. An under-classification error is a false negative; i.e., case where no name is predicted but the taxon is known (present in A). Let N be the number of sequences in S, K be the number of query sequences with known names, i.e., names which are present in the reference database A, and L = N − K be the number of novel test sequences, i.e., sequences in S with names that are not present in A. Let TP be the number of names which are correctly predicted, MC the number of misclassification errors, OC the number of over-classification errors, and UC the number under-classification errors. The rate for each type of error is defined as the number of errors divided by the number of opportunities to make that error: OCR = OC/L (over-classification rate), UCR = UC/K (under-classification rate) and MCR = MC/K (misclassification rate). The true positive rate is TPR = TP/K, i.e., the number of correct names divided by the number of opportunities to correctly predict a name. Accuracy is calculated as Acc = TP/(K + OC), i.e., the number of correct predictions divided by the number of predictions for which correctness can be determined. Metrics are calculated separately for each rank.
Conflicts between the RDP taxonomy and SSU guide trees
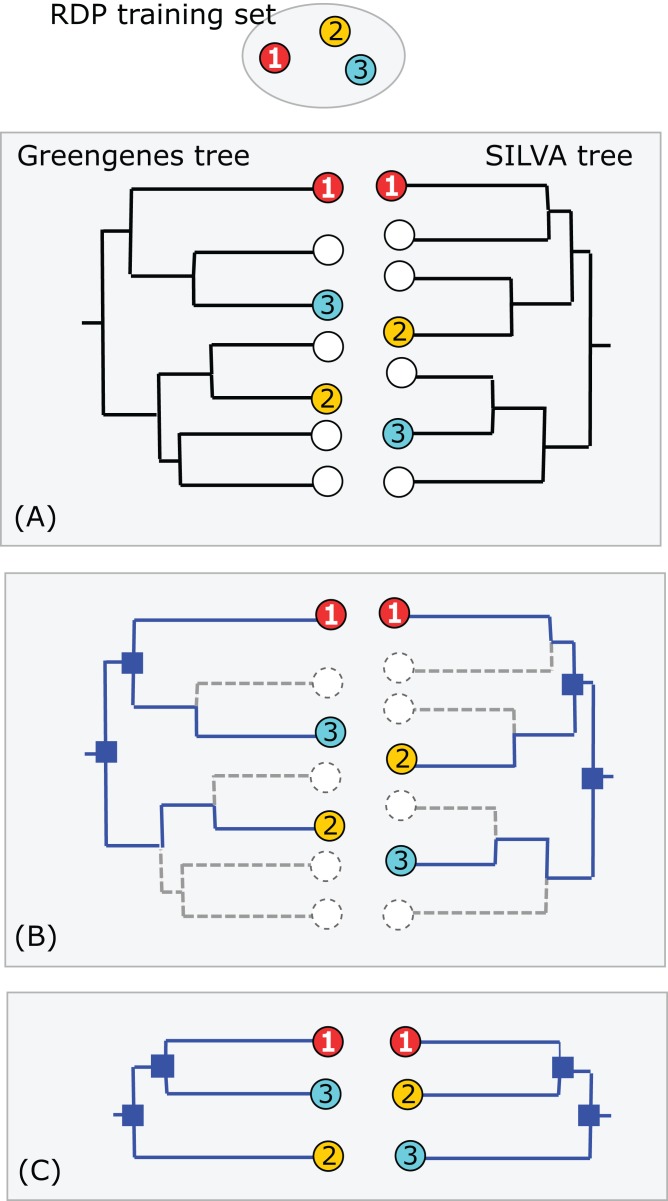
Greengenes and SILVA use sequence-based trees to guide predictions of taxonomy for environmental sequences. Conflicts between a phylogenetic tree and a taxonomic hierarchy arise when taxa at the same rank overlap in the tree (see “Background”). I used RDP training set v16 as a reference to assess the Greengenes guide tree from 2011 (http://greengenes.lbl.gov/Download/Trees/16S_all_gg_2011_1.tree.gz) and the SILVA guide tree from release 108 (dated August 2011). This assesses older guide trees of approximately the same age by comparison with each other and with a more recent authoritative reference. As with the RDP blinded test, using an older version of the tree ensures that some newer isolate sequences are blinded, i.e., have authoritative taxonomies in the reference but predicted taxonomies in the trees. Using an independent reference minimizes bias due to manual adjustment of the trees to achieve consistency with reference sequences. I identified the common subset of sequences, i.e., sequences found in all three databases: SILVA, Greengenes and the training set. For each guide tree, I extracted the binary tree for the common subset of sequences (Fig. 3). The leaves in this subset tree were assigned taxonomy annotations according to the RDP training set while preserving the branching order specified by the guide tree. For each taxon in the RDP nomenclature, I identified its LCA in this subset tree (i.e., the lowest node above all members of the taxon), and then the overlapped taxa and leaves at each rank.
Figure 3. Assessment of Greengenes and SILVA trees by RDP taxonomy.
(A) Identical sequences (the common subset) are identified in all three databases (Greengenes, SILVA and the RDP training set). (B) Edges specifying the branching order of the common subset are identified in each tree. (C) The binary trees implied by those edges are extracted and compared. In this example, the branching order is different. If the three leaves belong to the same taxon, there is no conflict; otherwise one or both trees have a conflict with the RDP taxonomy. Circled numbers represent sequences found in all three databases.
Consistency between SSU guide trees
A taxon that is impure according to an SSU guide tree implies one of the following: (1) the taxon is monophyletic and there are branching order errors in the tree, (2) the taxon is polyphyletic and the branching order correctly indicates the overlap, or (3) the taxon is in fact polyphyletic, but overlaps indicated by the branching order are different from those in the true phylogenetic tree. If case (2) is common, then subtrees of two independently constructed guide trees should often be consistent with each other despite conflicts with taxonomy. To investigate whether this is the case in practice, I sought a suitable strategy for comparing two guide trees with taxonomy annotations for the same set of sequences. Published metrics for tree comparison include Robinson–Foulds (Robinson & Foulds, 1981) and Branch Score Distance (Kuhner & Felsenstein, 1994), but these are not suitable here as the connection between their numerical values and taxonomic consistency is unclear, noting that branching errors which preserve purity are less important than those which cause monophyletic taxa to become impure in the predicted tree. It is more informative to measure the degree to which the trees agree on pure and overlapping taxa. For example, if trees X and Y agree that taxa s and t overlap, this suggests that both trees may be correctly implying that s and t overlap in the true phylogenetic tree, while if s and t are pure in X but overlap in Y, this suggests that the branching order of X is better than Y. I therefore defined the Taxonomy Concurrence Score (TCS) of trees X and Y as follows. Let LCAX(t) be the LCA of taxon t in tree X and let SX(n) be the set of taxon names at a given rank found in the subtree under node n. Then, t is pure in X if, and only if, t is the only taxon in SX(LCAX(t)). If t is impure, then there are two or more taxa in this set. Trees X and Y concur with respect to t if the same set of taxon names is found under the LCA for t in both trees, i.e., if SX(LCAX(t)) = SY(LCAY(t)). Thus, the trees concur if t is pure in both trees, or the same set of overlapping taxon names is found under the LCA of t. An alternative design would be to require that the same set of sequences is found, but this would be excessively restrictive as it would treat a single misplaced sequence as equivalent to many disagreements. By considering names rather than sequences, the trees concur if they both imply that, say, s and t overlap with each other even if they place some sequences of s and t differently. TCS at a given rank is calculated as the fraction of non-singleton taxa where the trees concur. Singleton taxa are excluded because they are pure in all possible trees and are therefore uninformative.
High-identity outliers
The lowest common rank (LCR) (Edgar, 2018) is the lowest rank shared by a pair of sequences. Note that this is the name of a rank, not a taxon name at that rank. For example, if two sequences belong to different genera in the same family, then their LCR is family. If a pair of sequences has an anomalously high identity considering its LCR, this indicates a possible classification error or annotation error, though it could also be explained by unusually high sequence conservation or by horizontal transfer. For example, if two 16S rRNA sequences are 99% identical and are annotated as belonging to different orders in the same class, this is likely to be a problem in the annotations.
Results
Annotation errors in the full RDP database
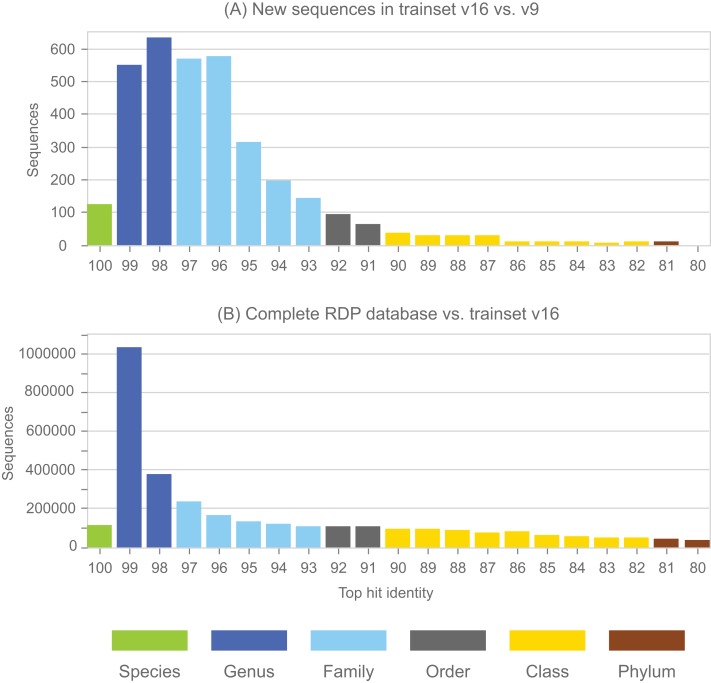
Annotations in the full RDP database are generated by the NBC. Performance metrics for NBC predictions on new sequences in RDP training set v16 using v9 as a reference are shown in Table 1. The top-hit identity distribution (Edgar, 2018) of the blinded test is shown in Fig. 4, together with the distribution of the full RDP database against training set v16 for comparison. The full distribution is strongly peaked at 99% identity, while the blinded distribution has a higher frequency of low-identity sequences. The blinded test is therefore more challenging than annotating the full database, and the average genus accuracy of the RDP annotations is therefore probably higher than the ∼85% observed in the blinded test. Given these results, a rough estimate is that ∼10% sequences in RDP have incorrect genus annotations.
Table 1. Accuracy metrics for blinded RDP prediction test.
| Rank | TPR (%) | MCR (%) | OCR (%) | UCR (%) | Acc (%) |
|---|---|---|---|---|---|
| Phylum | 99.5 | 0.1 | 41.1 | 0.1 | 98.8 |
| Class | 99.1 | 0.4 | 90.2 | 0.4 | 83.5 |
| Order | 97.8 | 0.7 | 84.2 | 0.7 | 92.9 |
| Family | 96.9 | 0.7 | 71.7 | 0.7 | 92.8 |
| Genus | 91.8 | 2.6 | 41.9 | 2.6 | 84.8 |
Notes:
See main text for definition of metrics. The low accuracy at class rank is striking; this is because there are many sequence with novel classes in v16 compared to v9 of the training set, and many of these (90%) are over-classified, i.e., falsely predicted to have class names which are known in v9.
Figure 4. Top-hit identity distribution for the blinded RDP prediction test compared with the full RDP database.
Panel (A) shows the top-hit identity distribution (THID) for new sequences in RDP trainset v16 compared to v9 as a reference. Panel (B) shows the THID for the full RDP database against trainset v16 as a reference.
Nomenclature hierarchy disagreements between Greengenes and SILVA
I found 46 taxon names placed into different parent taxa by Greengenes v13.5 and SILVA v128, considering only names present in both databases. For example, family Brevibacteriaceae is in order Actinomycetales according to Greengenes (e.g., accession 1109545), but this family is in order Micrococcales according to SILVA (JAJB01000072.80.1512). Order Micrococcales is also found in Greengenes (825086), so there is an unambiguous disagreement between the nomenclature hierarchies. A complete list is given in Table S1.
Annotation conflicts between Greengenes and SILVA
I found 784,242 identical sequences in SILVA and Greengenes, of which 732,048 (93%) had taxonomy annotations from the common nomenclature in one or both databases. Of the sequences with common nomenclature annotations, I found that 249,490 (34%) disagreed (Table 2). About 59,637 (24%) of these conflicts were due to a rank which was blank (unspecified) in one database, which implies a false positive by one database or a false negative by the other. An example of a conflict between a pair of names in the common nomenclature is Greengenes 4366627 which has the same sequence as SILVA LOSM01000005.1106908.1108461. Greengenes assigns this sequence to family Pseudoalteromonadaceae while SILVA assigns it to family Vibrionaceae. Pseudoalteromonadaceae is found in SILVA (FJ889568.1.1501) and Greengenes contains Vibrionaceae (3059893). A small fraction of the conflicts (440, or 0.2%) reflected nomenclature hierarchy disagreements (see previous section); the rest were conflicts in taxon names for which both databases agreed on the parent group. Conflicts were found at all ranks: 7,804 at phylum, 23,082 class, 110,308 order, 45,712 family and 62,584 genus, counting only the highest conflicted rank. An example of a phylum conflict in a sequence classified to genus level by both databases is Greengenes 851746 which is assigned to genus Streptococcus in phylum Firmicutes and has the same sequence as SILVA JZIA01000003.3571786.3573313, which is assigned to genus Xanthomonas in phylum Proteobacteria. Assuming that a given named taxon is required to have the same definition (e.g., the same characteristic traits) in all nomenclatures where it appears, then all conflicts are necessarily due to annotation errors in one or both databases. The lower bound on the sum of the number of errors in both databases is obtained when all conflicts are due to an error in one database but not the other. This follows because cases where both databases make the same error (so there is no disagreement, but the annotations are nevertheless wrong), or a given sequence has errors in both databases, can only add to this sum. Thus, a lower bound for the sum of the annotation error rates of SILVA and Greengenes is (number of identical sequences with common nomenclature conflicts)/(number of identical sequences annotated by the common nomenclature) = 249,490/732,048 = 34%.
Table 2. Conflicts between Greengenes and SILVA annotations of identical sequences.
| Rank | Conflicts | Blank vs. name | Hierarchy |
|---|---|---|---|
| Phylum | 7,804 (1.1%) | 92 | 0 |
| Class | 23,082 (3.2%) | 489 | 0 |
| Order | 110,308 (15.1%) | 2,777 | 4 |
| Family | 45,712 (6.2%) | 20,093 | 0 |
| Genus | 62,584 (8.5%) | 36,186 | 203 |
| Total | 249,490 (34.1%) | 59,637 | 207 |
Notes:
Columns are: Rank, the highest rank where there was a disagreement between names in the common nomenclature; Conflicts, the number of disagreements (and as a percentage of annotations in the common nomenclature); Blank, the number of conflicts where the rank was not named in one of the databases; Hierarchy, conflicts where the databases disagreed on the parent name in cases where both the rank and its parent are in the common nomenclature in both databases. The number of conflicts is seen to be largest at Order rank. The reason for this is not clear; possibly, the impact of branching order errors is most severe at intermediate levels of the tree.
Conflicts between the LTP taxonomy and the LTP guide tree
Result for genus and family are shown in Table 3; higher ranks are not annotated in LTP. At genus rank, roughly one-third of non-singleton genera overlap (430/1,328 = 32%) and at family rank almost one-half overlap (155/326 = 48%). A large majority of leaves (∼300/12,953 = 98%) are overlapped at both family and genus rank, i.e., are in the subtrees under LCAs for two or more taxa at the same rank.
Table 3. Conflicts between the tree and taxonomy annotations in LTP.
| Genus | Family | |
|---|---|---|
| Singletons | 1,206 | 79 |
| Non-singletons | 1,328 | 326 |
| Pure taxa | 898 (68%) | 171 (52%) |
| Impure taxa | 430 (32%) | 155 (48%) |
| Pure leaves | 273 | 295 |
| Impure leaves | 12,680 | 12,658 |
Notes:
LTP does not annotate ranks above genus.
Columns are: Singletons, the number of singleton taxa; Non-singletons, the number of non-singleton taxa; Pure taxa, the number of non-singleton taxa which are pure; Impure taxa, the number of non-singleton taxa which are impure; Pure leaves, the number of pure leaves, i.e., the number of leaves found under the LCA for exactly one taxon; Impure leaves, the number of leaves which are not pure.
Common subset of SILVA, Greengenes and the RDP training set
Using the method sketched in Fig. 3, I found 7,216 identical sequences in SILVA, Greengenes and the RDP training set. Extracting the subtrees for these sequences from the SILVA and Greengenes guide trees gave subset trees with 7,216 leaves with taxonomy annotations according to the RDP training set.
Concurrence of Greengenes and SILVA guide trees
Results are shown in Table 4. This shows the concurrence between the subset of the Greengenes and SILVA guide trees for sequences found in Greengenes, SILVA and the RDP training set, using taxonomy annotations from the training set. These subset trees are directly comparable because they contain the same set of sequences with taxonomy annotations from an independent database. The results show comparable rates of disagreement with the training set taxonomy. For example, 58 genera are pure according to the Greengenes tree but not SILVA, while 74 genera are pure according to SILVA but not Greengenes. If one tree (call it X) was substantially better than the other (Y), then there would be more taxa that are impure in Y but not in X than vice versa, but this is not the case here, which indicates that the Greengenes and SILVA trees have comparable accuracy, as might be expected. There are 74 genera which both trees agree are impure and have the same set of overlapping names. These cases could be explained by taxa which are truly polyphyletic, or by branching order errors which are reproduced in both trees. Reproduced errors may occur by chance, noting that noise which is unbiased when measured by branching order will have a strong tendency to merge neighboring taxa, and will therefore be strongly biased when measured by taxonomy. Reproduced errors may also occur due to systematic effects such as long branch attraction, which is known to occur in a wide range of tree inference algorithms including neighbor-joining, parsimony, maximum likelihood and Bayesian methods (Bergsten, 2005).
Table 4. Concurrence between Greengenes, SILVA and RDP training set.
| Rank | Pure both | Pure GG only | Pure SILVA only | Impure, same set | Impure, diff. set | TCS |
|---|---|---|---|---|---|---|
| Phylum | 37 (78.7%) | 0 (0.0%) | 3 (6.4%) | 3 (6.4%) | 4 (8.5%) | 40 (85.1%) |
| Class | 65 (79.3%) | 2 (2.4%) | 2 (2.4%) | 6 (7.3%) | 7 (8.5%) | 71 (86.6%) |
| Order | 89 (65.0%) | 7 (5.1%) | 3 (2.2%) | 12 (8.8%) | 26 (19.0%) | 101 (73.7%) |
| Family | 198 (61.3%) | 20 (6.2%) | 13 (4.0%) | 31 (9.6%) | 61 (18.9%) | 229 (70.9%) |
| Genus | 1,449 (82.5%) | 58 (3.3%) | 74 (4.2%) | 74 (4.2%) | 101 (5.8%) | 1,523 (86.7%) |
Notes:
Concurrence for identical sequences found in all three databases determined by the method shown in Fig. 3.
Columns are: Pure both, the number of non-singleton taxa which are pure in the subset trees for both Greengenes and SILVA; Pure GG only, the number which are pure only in the Greengenes subset tree; Pure SILVA only, similarly for SILVA; Impure, same set, the number of taxa where the trees agreed on the set of overlapping names; Impure, diff. set, the number where the trees disagreed on the set of overlapping names; TCS, taxonomy concurrence score, i.e., the fraction of taxa where the trees concur that the taxon was pure or had the same set of overlapping names under its LCA.
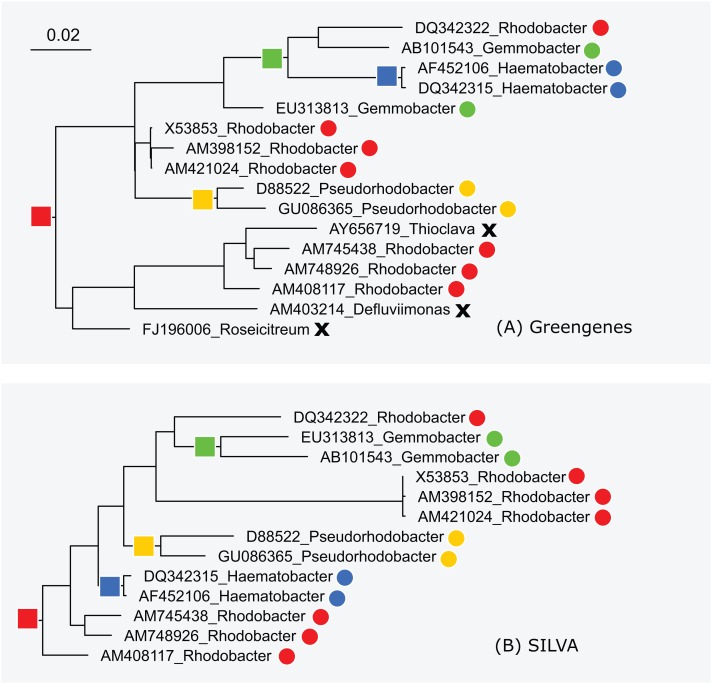
Rhodobacter subtrees
The subtrees for genus Rhodobacter in Greengenes and SILVA are shown in Fig. 5. After manually reviewing many taxa, I selected Rhodobacter as a representative example with different branching orders. As is typically the case, groups of highly similar sequences are concurrent between the trees. For example, Rhodobacter sequences X54853, AM398152 and AM421024 have pair-wise identities >99% and are placed together in both trees. When sequence similarity is lower, there is often radical disagreement in the branching order. For example, the two Heamobacter sequences AF452106 and DQ342315 are placed inside the Gemmobacter subtree by Greengenes, while both Haemobacter and Gemmobacter are pure according to SILVA. With the LTP tree, the subtree under its Rhodobacter LCA node contains 390 sequences from 122 genera as shown in Fig. S1.
Figure 5. Subtrees for Rhodobacter in (A) Greengenes and (B) SILVA.
Leaves are labeled with their sequence identifiers and genus names according to the RDP training set. Colored dots indicate genera found in both subtrees; black crosses indicate genera found in the Greengenes subtree only.
High-identity outliers
The outliers with highest pair-wise identity at each LCR for BLAST16S and the RDP training set are given in Tables 5 and 6 respectively. The 10 pairs with highest identity at each rank for all these databases and also LTP are given in Table S2. As an example, sequences gi|645320505 and gi|265678369 in BLAST16S are 100% identical and have LCR = phylum, which almost certainly implies an annotation error.
Table 5. Outliers with high pair-wise identity in the BLAST16S database.
| %Id | LCR | Accessions | Highest distinct taxa | Species |
|---|---|---|---|---|
| 100.0 | Phylum | gi|645320505 | c:Betaproteobacteria | Neisseria flava |
| gi|265678369 | c:Gammaproteobacteria | Moraxella caviae | ||
| 97.1 | Phylum | gi|559795246 | c:Bacilli | Lactobacillus rogosae |
| gi|645321781 | c:Clostridia | Lachnospira pectinoschiza | ||
| 99.9 | Class | gi|558508609 | o:Pseudonocardiales | Prauserella marina |
| gi|631251240 | o:Streptomycetales | Streptomyces parvus | ||
| 99.6 | Class | gi|645319747 | o:Pseudomonadales | Pseudomonas halophila |
| gi|343198728 | o:Oceanospirillales | Halovibrio denitrificans | ||
| 100.0 | Order | gi|636559468 | f:Bacillaceae | Bacillus racemilacticus |
| gi|219846253 | f:Sporolactobacillaceae | Sporolactobacillus laevolacticus | ||
| 100.0 | Order | gi|645320480 | f:Rhodobiaceae | Afifella marina |
| gi|343200250 | f:Bradyrhizobiaceae | Rhodopseudomonas julia | ||
| 100.0 | Family | gi|636560543 | g:Obesumbacterium | Obesumbacterium proteus |
| gi|631251787 | g:Hafnia | Hafnia alvei | ||
| 100.0 | Family | gi|1212229262 | g:Feifantangia | Feifantangia zhejiangensis |
| gi|1137647790 | g:Xanthomarina | Xanthomarina gelatinilytica | ||
| 100.0 | Genus | gi|343198889 | s:aurantiaca | Stigmatella aurantiaca |
| gi|343201672 | s:erecta | Stigmatella erecta | ||
| 100.0 | Genus | gi|219857540 | s:florum | Mesoplasma florum |
| gi|219846097 | s:grammopterae | Mesoplasma grammopterae |
Notes:
Columns are: %Id, identity; LCR, lowest common rank; Accessions, database identifiers; Highest distinct taxa, names at the highest rank where the sequences are assigned to different taxa; Species, species names according to the database.
Table 6. Outliers with high pair-wise identity in the RDP training set.
| %Id | LCR | Accessions | Highest distinct taxa | Genus |
|---|---|---|---|---|
| 98.1 | Phylum | AB680539 | c:Betaproteobacteria | Aquaspirillum |
| Y10109 | c:Alphaproteobacteria | Magnetospirillum | ||
| 99.9 | Class | Y10755 | o:Xanthomonadales | Xanthomonas |
| AB021399 | o:Pseudomonadales | Pseudomonas | ||
| 99.6 | Class | AJ007800 | o:Caulobacterales | Asticcacaulis |
| AJ227812 | o:Sphingomonadales | Sphingomonas | ||
| 99.8 | Order | AY345990 | f:Entomoplasmataceae | Entomoplasma |
| FR733691 | f:Spiroplasmataceae | Spiroplasma | ||
| 99.1 | Order | AJ006086 | f:Planococcaceae | Solibacillus |
| EF114311 | f:Bacillaceae_1 | Bacillus | ||
| 100.0 | Family | GU256437 | g:Nitrospirillum | Nitrospirillum |
| KC109787 | g:Azospirillum | Azospirillum | ||
| 99.9 | Family | FR733721 | g:Nocardia | Nocardia |
| X79289 | g:Rhodococcus | Rhodococcus |
Note:
Columns as for Table 5.
Discussion
Challenges in microbial taxonomy
Microbial taxonomy presents unique challenges. The number of known 16S rRNA sequences is rapidly increasing. Characteristic traits are currently unknown for most environmental sequences, many of which do not belong to named genera (Yarza et al., 2008). Multiple nomenclatures are in common use, each of which is subject to revisions. From this perspective, microbial taxonomy appears to be a moving target. However, updates to a taxonomic classification standard, e.g., Bergey’s Manual, preserve classification criteria as far as practically possible because the meaning of a name such as Salmonella should not change over time. Occasionally, classification criteria (characteristic traits) for a taxon are revised; e.g., the description of a genus might change in a new edition of Bergey’s Manual. Also, groups believed to be polyphyletic may be re-assigned. However, these issues account for only a small minority of annotation discrepancies.
Taxonomy annotation error rates
The taxonomy annotation error rates of SILVA, RDP and Greengenes have previously been estimated to be in the range 0.2–2.5% (Kozlov et al., 2016). The results presented here show that this is a substantial underestimate. A lower bound of 34% was obtained for the sum of annotation error rates in Greengenes and SILVA. I believe that this bound is robust because it is obtained from sequences classified by their common nomenclature, so any disagreement is necessarily due to an error in one or both annotations. Guide trees in SILVA and Greengenes conflict with each other and with taxonomy at comparable rates, which strongly suggests that many or most of these conflicts are due to branching order errors in the predicted trees compared with the true gene tree. These trees are therefore not suitable as authoritative guides to phylogeny. The number of conflicts between the SILVA and Greengenes trees and an independent type-strain-based reference (the RDP training set) is similar, and neither tree contains substantially more pure taxa according to the RDP training set. These results imply that the annotation error rate of both databases is similar, as might be expected. If the error rates are similar and the sum is close to its lower bound of 34%, then the annotation error rates of Greengenes and SILVA are both approximately 17%. If one database is somewhat better (say, its error rate is 15%) then the error rate of the other must be correspondingly higher (19%) so that the sum remains at least 34%. It seems very unlikely that either database could have an error rate as low as 10% as this would imply that the other has an error rate of at least 24%, and a difference of this magnitude should be noticeable in the numbers of pure taxa. Noting that the sum may be somewhat higher than its lower bound due to systematic errors reproduced in both databases, a reasonable rough estimate is that one in five sequences in Greengenes and SILVA have incorrect taxonomy annotations. The blinded test suggests that the annotation rate of RDP is ∼10% and is therefore likely to be lower than Greengenes or SILVA, though the uncertainties in these estimates are too great to support a firm conclusion that annotations in any one of these databases are better or worse than another. However, while keeping this caveat in mind, it is striking that a pessimistic estimate for the RDP database of 15% on the blinded test is less than a conservative estimate for SILVA and Greengenes of a 17% error rate from disagreements between annotations. This suggests that the accuracy of fully automated annotations generated by the RDP Naive Bayesian Classifier, which does not explicitly consider phylogeny, is quite likely to be better than the accuracy of annotations in SILVA and Greengenes, which were generated by a combination of automated and manual methods guided by alignments and trees. Predictions generated by the RDP Classifier have several advantages: they are based on a documented reference, can easily be reproduced, and report a bootstrap confidence value. By contrast, annotations in SILVA and Greengenes do not report confidence and are not reproducible because there is a manual curation step and references are not documented.
Supplemental Information
The LTP subtree under its Rhodobacter LCA node, which contains 390 sequences from 122 genera.
Complete list of the 46 taxon names placed into different parent taxa by Greengenes v13.5 and SILVA v128, considering only names present in both databases.
The ten outliers with highest pair-wise identity at each LCR for BLAST16S, the RDP training set and LTP.
Funding Statement
The author received no funding for this work.
Additional Information and Declarations
Competing Interests
The author declares that he has no competing interests.
Author Contributions
Robert Edgar conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The data are provided in the Supplemental Files.
References
- Benton (2000).Benton MJ. Stems, nodes, crown clades, and rank-free lists: is Linnaeus dead? Biological Reviews. 2000;75(4):633–648. doi: 10.1111/j.1469-185x.2000.tb00055.x. [DOI] [PubMed] [Google Scholar]
- Bergey (2001).Bergey DH. Bergey’s Manual of Systematic Bacteriology. Hoboken: Wiley; 2001. [Google Scholar]
- Bergsten (2005).Bergsten J. A review of long-branch attraction. Cladistics. 2005;21(2):163–193. doi: 10.1111/j.1096-0031.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- Cho & Blaser (2012).Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nature Reviews Genetics. 2012;13(4):260–270. doi: 10.1038/nrg3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSantis et al. (2006).DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, Huber T, Dalevi D, Hu P, Andersen GL. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology. 2006;72(7):5069–5072. doi: 10.1128/aem.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar (2014).Edgar RC. Taxonomy benchmark tests, USEARCH manual v8.1. 2014. https://drive5.com/usearch/manual8.1 https://drive5.com/usearch/manual8.1
- Edgar (2018).Edgar RC. Accuracy of taxonomy prediction for 16S rRNA and fungal ITS sequences. PeerJ. 2018;6:e5030. doi: 10.7717/peerj.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar-Páramo et al. (2003).Escobar-Páramo P, Giudicelli C, Parsot C, Denamur E. The evolutionary history of Shigella and enteroinvasive Escherichia coli revised. Journal of Molecular Evolution. 2003;57(2):140–148. doi: 10.1007/s00239-003-2460-3. [DOI] [PubMed] [Google Scholar]
- Gogarten & Townsend (2005).Gogarten JP, Townsend JP. Horizontal gene transfer, genome innovation and evolution. Nature Reviews Microbiology. 2005;3(9):679–687. doi: 10.1038/nrmicro1204. [DOI] [PubMed] [Google Scholar]
- Hartmann et al. (2014).Hartmann M, Niklaus PA, Zimmermann S, Schmutz S, Kremer J, Abarenkov K, Lüscher P, Widmer F, Frey B. Resistance and resilience of the forest soil microbiome to logging-associated compaction. ISME Journal. 2014;8(1):226–244. doi: 10.1038/ismej.2013.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig (1965).Hennig W. Phylogenetic systematics. Annual Review of Entomology. 1965;10(1):97–116. doi: 10.1146/annurev.en.10.010165.000525. [DOI] [Google Scholar]
- Huelsenbeck, Rannala & Masly (2000).Huelsenbeck JP, Rannala B, Masly JP. Accommodating phylogenetic uncertainty in evolutionary studies. Science. 2000;288(5475):2349–2350. doi: 10.1126/science.288.5475.2349. [DOI] [PubMed] [Google Scholar]
- Jain et al. (2002).Jain R, Rivera MC, Moore JE, Lake JA. Horizontal gene transfer in microbial genome evolution. Theoretical Population Biology. 2002;61(4):489–495. doi: 10.1006/tpbi.2002.1596. [DOI] [PubMed] [Google Scholar]
- Kitahara & Miyazaki (2013).Kitahara K, Miyazaki K. Revisiting bacterial phylogeny: natural and experimental evidence for horizontal gene transfer of 16S rRNA. Mobile Genetic Elements. 2013;3(1):e24210. doi: 10.4161/mge.24210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov et al. (2016).Kozlov AM, Zhang J, Yilmaz P, Glöckner FO, Stamatakis A. Phylogeny-aware identification and correction of taxonomically mislabeled sequences. Nucleic Acids Research. 2016;44(11):5022–5033. doi: 10.1093/nar/gkw396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhner & Felsenstein (1994).Kuhner MK, Felsenstein J. A simulation comparison of phylogeny algorithms under equal and unequal evolutionary rates. Molecular Biology and Evolution. 1994;11:459–468. doi: 10.1093/oxfordjournals.molbev.a040126. [DOI] [PubMed] [Google Scholar]
- Maidak et al. (2001).Maidak BL, Cole JR, Lilburn TG, Parker CT, Saxman PR, Farris RJ, Garrity GM, Olsen GJ, Schmidt TM, Tiedje JM. The RDP-II (ribosomal database project) Nucleic Acids Research. 2001;29(1):173–174. doi: 10.1093/nar/29.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald et al. (2012).McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, Andersen GL, Knight R, Hugenholtz P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME Journal. 2012;6(3):610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran (2015).Moran MA. The global ocean microbiome. Science. 2015;350(6266):aac8455. doi: 10.1126/science.aac8455. [DOI] [PubMed] [Google Scholar]
- Moult (2005).Moult J. A decade of CASP: progress, bottlenecks and prognosis in protein structure prediction. Current Opinion in Structural Biology. 2005;15(3):285–289. doi: 10.1016/j.sbi.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Parte (2014).Parte AC. LPSN—list of prokaryotic names with standing in nomenclature. Nucleic Acids Research. 2014;42(D1):D613–D616. doi: 10.1093/nar/gkt1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflughoeft & Versalovic (2012).Pflughoeft KJ, Versalovic J. Human microbiome in health and disease. Annual Review of Pathology. 2012;7(1):99–122. doi: 10.1146/annurev-pathol-011811-132421. [DOI] [PubMed] [Google Scholar]
- Philippe et al. (2011).Philippe H, Brinkmann H, Lavrov DV, Littlewood DT, Manuel M, Wörheide G, Baurain D. Resolving difficult phylogenetic questions: why more sequences are not enough. PLOS Biology. 2011;9(3):e1000602. doi: 10.1371/journal.pbio.1000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruesse et al. (2007).Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glockner FO. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Research. 2007;35(21):7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson & Foulds (1981).Robinson DF, Foulds LR. Comparison of phylogenetic trees. Mathematical Biosciences. 1981;53(1–2):131–147. doi: 10.1016/0025-5564(81)90043-2. [DOI] [Google Scholar]
- Sayers et al. (2011).Sayers EW, Barrett T, Benson DA, Bolton E, Bryant SH, Canese K, Chetvernin V, Church DM, DiCuccio M, Federhen S, Feolo M, Fingerman IM, Geer LY, Helmberg W, Kapustin Y, Landsman D, Lipman DJ, Lu Z, Madden TL, Madej T, Maglott DR, Marchler-Bauer A, Miller V, Mizrachi I, Ostell J, Panchenko A, Phan L, Pruitt KD, Schuler GD, Sequeira E, Sherry ST, Shumway M, Sirotkin K, Slotta D, Souvorov A, Starchenko G, Tatusova TA, Wagner L, Wang Y, Wilbur WJ, Yaschenko E, Ye J. Database resources of the National Center for Biotechnology Information. Nucleic Acids Research. 2011;40(suppl_1):D13–D25. doi: 10.1093/nar/gkq1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2007).Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;73(16):5261–5267. doi: 10.1128/aem.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster et al. (2004).Webster G, Parkes RJ, Fry JC, Weightman AJ. Widespread occurrence of a novel division of bacteria identified by 16S rRNA gene sequences originally found in deep marine sediments. Applied and Environmental Microbiology. 2004;70(9):5708–5713. doi: 10.1128/aem.70.9.5708-5713.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese (1987).Woese C. Bacterial evolution background. Microbiology. 1987;51:221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarza et al. (2008).Yarza P, Richter M, Peplies J, Euzeby J, Amann R, Schleifer KH, Ludwig W, Glöckner FO, Rosselló-Móra R. The all-species Living Tree project: a 16S rRNA-based phylogenetic tree of all sequenced type strains. Systematic and Applied Microbiology. 2008;31(4):241–250. doi: 10.1016/j.syapm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Yilmaz et al. (2014).Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glöckner FO. The SILVA and ‘all-species Living Tree Project (LTP)’ taxonomic frameworks. Nucleic Acids Research. 2014;42(D1):D643–D648. doi: 10.1093/nar/gkt1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The LTP subtree under its Rhodobacter LCA node, which contains 390 sequences from 122 genera.
Complete list of the 46 taxon names placed into different parent taxa by Greengenes v13.5 and SILVA v128, considering only names present in both databases.
The ten outliers with highest pair-wise identity at each LCR for BLAST16S, the RDP training set and LTP.
Data Availability Statement
The following information was supplied regarding data availability:
The data are provided in the Supplemental Files.